Abstract
Background
Epilepsy is the most common chronic neurological disease in the United States, and 70% of diagnoses occur in late adolescence or adulthood. A disease of the brain, epilepsy can affect sleep. Lack of adequate or high-quality sleep can cause decrements in quality of life. Adults living with epilepsy are at especially high risk for sleep alterations, and these changes in sleep can worsen their epilepsy. The purpose of the study was to describe the perceived effect of epilepsy on sleep in adults who developed epilepsy in late adolescence or adulthood. Research questions included: 1. In adults with epilepsy, what is the perceived change in ability to sleep well due to having epilepsy?; 2. In adults with epilepsy, is there a significant relationship between perceived changes in ability to sleep well and perceived changes in overall life due to having epilepsy?; and 3. In adults with epilepsy, is there a significant relationship between perceived changes in ability to sleep well due to having epilepsy and total Life Changes in Epilepsy Scale (LCES) scores?
Methods
174 adults with epilepsy were recruited. Utilizing data collected via the LCES, a quantitative descriptive/correlational design was utilized. Analyses were carried out to answer each research question.
Results
The mean score for the sleep item of the LCES was 2.76 (SD 1.31), indicating an overall negative change in ability to sleep well. There was a statistically significant, strong positive relationship between the sleep and overall life changes items of the LCES (Pearson r 0.476, p<0.0000), and also between the sleep item and total LCES scores (Pearson r 0.620, p<0.0000).
Implications
Findings from this study contribute to the extant literature by revealing epilepsy-related changes in sleep as perceived by adults living with epilepsy specifically due to having epilepsy. Based on findings rendered from this sample, having epilepsy can lead to perceived negative changes in a person’s ability to sleep well, and these negative changes are significantly correlated with negative overall life changes. Recommendations for clinical practice and research can be made based on current results.
Introduction
Epilepsy, a chronic condition characterized by unprovoked seizures, is the most common chronic neurologic condition worldwide, with 1 in 26 people diagnosed with the disease. Though epilepsy affects people across the lifespan, 70% of new epilepsy diagnoses occur in late adolescence or in adulthood (Epilepsy Foundation, 2013). Adults with epilepsy experience decrements in quality of life, particularly in the areas of social functioning, somatic health, and well-being (Unger & Buelow, 2009; World Health Organization, 2012; Miller, 2014).
A disease of the brain, epilepsy can affect sleep and sleep cycles (Bazil, 2003). Sleep begins with a non-rapid eye movement phase (NREM) and then transitions into a cyclic pattern of NREM and rapid eye movement (REM) sleep. Each cycle of NREM and REM sleep recurs approximately every 90 minutes, with the average cycle lasting 90–110 minutes (Aschoff, 1965; Bowman, 2003). Stages of sleep are often referred to as ‘sleep architecture’ to reflect the structure and timing of events (Bowman & Moshenin, 2003; Vena et al., 2004). More importantly, sleep is controlled by a complex relationship between the central (brain and spinal cord) and peripheral (links rest of body to central system) nervous systems, and circadian rhythm functions (internal clock) (Guyton & Hall, 1997). The central and peripheral nervous systems work with the circadian rhythms within the body through complex circuits of neurons (signals) that produce changes in breathing, heart rate, and immune function during sleep. Epilepsy, which is characterized by bursts of electrical activity in the brain, can negatively affect the brain’s ability to engage in appropriate sleep architecture, placing those living with epilepsy at a higher risk of altered sleep (Bazil, 2003).
In addition to being at risk for sleep disruptions caused by the pathophysiology of epilepsy, persons with epilepsy are also at risk for sleep disturbances caused by anti-epileptic drugs (AEDs). While the emergence of newer AED formulations that support decreased sedative effects provide additional options for treatment with less chance of effects on sleep, many AEDs, especially when used in combination with other AEDs or medications for other conditions, are known to disrupt the sleep cycle in some fashion—some contribute to insomnia, while others cause somnolence (Bazil, 2003).
While a lack of adequate and high-quality sleep can lead to negative effects in all people, persons living with epilepsy succumb to particularly adverse effects of loss of sleep. These negative effects present in persons with epilepsy in a variety of ways dependent on seizure type and control. However, sleep deprivation—lack of adequate sleep or poor quality sleep—is a common trigger for seizures (Epilepsy Foundation, 2013). Thus, if a person with epilepsy has difficulty achieving adequate and high-quality sleep, the result can be increased frequency of seizure events, decreased ability to participate fully in activities of daily living, and ultimately a lower quality of life.
The National Sleep Foundation (2014) describes the association with sleep difficulties in persons with epilepsy as a two-sided phenomenon—epilepsy can disturb sleep patterns, and those sleep pattern disruptions can exaggerate epilepsy. Enhancing sleep quality in chronic illnesses such as epilepsy is emerging as a national priority of the National Institutes of Health due to the vast number of negative consequences to health and quality of life. Therefore, it is critical to begin to investigate this complex issue of poor sleep in this population. Based on Institute of Medicine (IOM) (2012) recommendations, there is a particular need to focus on quality of life issues, such as sleep, from a patient-centered perspective in persons with epilepsy. While evidence exists to suggest that poor sleep is a problem for persons living with epilepsy (Piperdou et al., 2008; Chen et al., 2011), there are no published studies that report perceived changes in sleep in late adolescent or adult-onset epilepsy. Thus, while it is known that sleep alterations are a common co-morbidity in persons with lifelong epilepsy, it is not known how sleep is affected in persons with adult-onset epilepsy or how adults with epilepsy perceive that their sleep has been affected due to epilepsy. Measuring changes in ability to sleep well from this patient-centered perspective is in line with IOM recommendations, and also allows for assessment in changes in sleep caused specifically by epilepsy.
Investigating perceived changes in sleep in persons who are diagnosed with epilepsy in their late teenage or early adulthood years is important to begin pinpointing the potential effect of epilepsy on sleep in the population most affected by new-onset epilepsy. Perceived changes in sleep in this population may vary from those in persons who have had epilepsy most of their lives. In addition, existing studies do not report alterations in sleep due to having epilepsy; rather, existing literature describes overall changes in sleep in persons with epilepsy without specifying sleep alterations perceived by participants as epilepsy-related. In order to improve sleep-related outcomes in persons who develop epilepsy near or in their adult years, the presence of problems with sleep in this population, as well as the degree to which those problems exist and affect other aspects of life, must be investigated.
The Life Changes in Epilepsy Scale (LCES) was developed based on existing qualitative data and the literature, as well as Lazarus and Folkman’s (1984) Theory of Stress, Coping, and Adaptation. The LCES is designed to measure perceived life changes in three areas of life (social functioning, somatic health, and subjective well-being) since epilepsy onset in adults with epilepsy (Miller et al., 2015). The initial, 35-item version of the LCES was recently tested for content validity (Miller et al., 2015) as well as other psychometric properties, in which the scale demonstrated evidence of acceptable internal consistency reliability and construct and criterion-related validity (Miller et al., In Review). One item of the LCES targets respondents’ perceived changes in sleep since epilepsy onset.
The purpose of the study was to, using the LCES, describe the perceived effect of epilepsy on sleep in adults who developed epilepsy in late adolescence or adulthood. The following research questions were posed:
In adults with epilepsy, what is the perceived change in ability to sleep well due to having epilepsy?
In adults with epilepsy, is there a significant relationship between perceived changes in ability to sleep well and perceived overall life changes due to having epilepsy?
In adults with epilepsy, is there a significant relationship between perceived changes in ability to sleep well due to having epilepsy and total Life Changes in Epilepsy Scale (LCES) scores?
Methods
Sample and Setting
Following approval of the study by the Institutional Review Board, 184 adults with epilepsy were recruited to take part in the study. Participants were recruited via an epilepsy clinic at a large neuroscience center utilizing at-a-distance recruitment techniques (Miller, Bakas, Buelow, & Habermann, 2013). The following inclusion criteria were used for recruitment: 1) diagnosis of epilepsy at or after age 16; 2) age 18 years or older; 3) prescribed at least one anti-epileptic drug (AED); 4) English-speaking; 5) access to a telephone; and 6) cognitive ability to engage in answering questions via phone. While participants were required to be at least 18 years of age at the time of the study, given the focus on adults in the research questions, we chose to include those diagnosed with the condition at or after age 16. The rationale for this decision was based on the fact that those diagnosed with epilepsy at age 16 or 17 are developmentally able to perceive changes in their lives due to epilepsy even if they were not technically adults at the time of diagnosis (Centers for Disease Control and Prevention, 2015). Potential participants who met the first five inclusion criteria and were interested in participating were screened using the Six-Item Cognitive Screener (Callahan et al., 2002) to ensure adequate cognitive capacity.
Measures
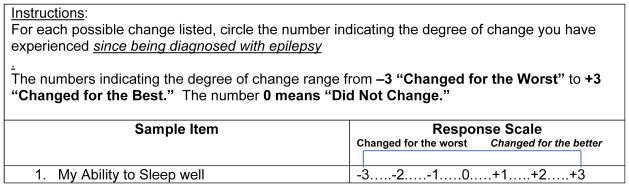
Demographic data were collected, and the LCES (Miller et al., 2015) was administered to all participants. Responses to 35 items of the LCES are measured on a 7-point scale (Changed for the Worst= −3 to Changed for the Best=+3) (Miller et al., 2015). One item of the LCES is specifically related to respondents’ sleep (See Figure 1), and asks respondents to indicate perceived changes in their ability to sleep well. A 36th item of the LCES was added as a criterion item in order to test for criterion-related validity of the scale; this item asks respondents to report the perceived change in their life overall due to having epilepsy. Respondents’ answers to each of the LCES items are recoded 1–7 to prevent negative numbers for analysis. An item score of 1 indicates changed for the worst, 4 indicates no change, and 7 indicates changed for the best. Scale scores can range from 35–245. Lower item-level and total scores on the LCES indicate more negative life changes, while higher scores indicate more positive life changes.
Figure 1.
Sleep Item of the LCES
Procedures
Participants who met inclusion criteria and were interested in participating were enrolled in the study. Instruments were administered via phone, with data collectors recording respondents’ answers on paper forms. Participants were provided with copies of the instruments to allow them to follow along with the data collector. Recorded answers were then entered into an electronic filing system, and double-checked for accuracy prior to analysis.
Data were analyzed using SPSS for Windows. Demographic data were analyzed using descriptive statistics (mean, median, mode, range, and standard deviations). Participants’ LCES scores were also analyzed descriptively by item, and total LCES scores were calculated. Given the specific purpose of the study to examine changes in ability to sleep well, the sleep item of the LCES was analyzed descriptively (mean, median, mode, range, and standard deviations), and was also correlated, using a Pearson r correlation test, with total LCES scores, as well as the criterion item of the LCES.
Results
Sample
Following recruitment, 10 participants were lost to follow-up, resulting in a total sample of 174. The sample consisted of 63 males and 111 females, with a mean age of 45.6 years and a range of 18–74 years. The mean age of epilepsy onset was 33.4 years (SD 13.94), and the mean length of time since diagnosis was 12.2 years (SD 11.5). Table 1 depicts other characteristics of the sample.
Table 1.
Additional Demographic Characteristics of the Sample (N=174)
| Variable | Frequency |
|---|---|
|
| |
| Ethnicity | |
| • White | • n=136 |
| • African American | • n=22 |
| • Hispanic | • n=1 |
| • Pacific Islander | • n=1 |
| • Other | • n=4 |
| • Not reported | • n=8 |
|
| |
| Seizure Type | |
| • Complex Partial | • n=33 |
| • Tonic Clonic | • n=32 |
| • Combination | • n=31 |
| • Other | • n=17 |
| • Unknown | • n=61 |
|
| |
| Employment Status | |
| • Employed Full-Time | • n=38 |
| • Employed Part-Time | • n=13 |
| • Homemaker | • n=10 |
| • Retired | • n=16 |
| • Unemployed | • n=41 |
| • Disability | • n=47 |
| • Other | • n=9 |
|
| |
| Seizure Frequency | |
| • Daily | • 7 |
| • Weekly | • 22 |
| • Monthly | • 22 |
| • Bi-Monthly | • 28 |
| • Bi-Annually | • 19 |
| • <1/year | • 67 |
| • Other | • 9 |
Research Question 1
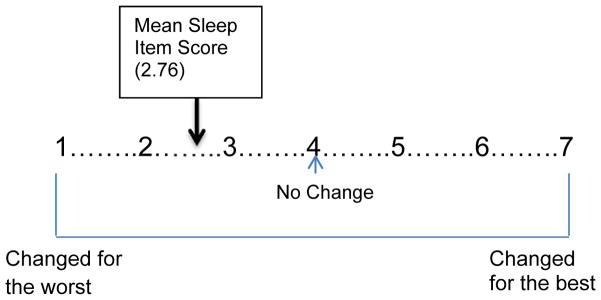
The mean score for the sleep item of the LCES was 2.76 (SD 1.31). Therefore, the sample experienced negative perceived changes in their ability to sleep well due to having epilepsy (Figure 2).
Figure 2.
Mean Sleep Item Score (recoded to 1–7)
Research Question 2
There was a statistically significant, strong positive relationship between the sleep and overall life changes items of the LCES (Pearson r 0.476, p<0.0000). Therefore, negative perceived changes in ability to sleep well were correlated with negative perceived changes in overall health.
Research Question 3
There was a statistically significant, strong positive relationship between the sleep item of the LCES and total LCES scores (Pearson r 0.620, p<0.0000). Thus, negative perceived changes in ability to sleep well were correlated with lower total LCES scores.
Discussion
Findings from this study contribute to the extant literature by revealing epilepsy-related changes in sleep as perceived by adults living with epilepsy specifically due to having epilepsy. Based on findings rendered from this sample, having epilepsy can lead to perceived negative changes in a person’s ability to sleep well, and these negative changes are significantly correlated with negative overall life changes.
The finding that participants experienced negative changes in their ability to sleep well is in agreement with existing literature. For example, Chen and colleagues (2011) found that, compared to control subjects, persons with epilepsy have poorer sleep quality and excessive daytime sleepiness; their sleep quality and sleepiness were correlated with partial epilepsy, poor seizure control, and multi-AED use. Current findings are in accord with those of Chen and colleagues (2011), though findings from the current study reflect perceived changes in sleep due to having epilepsy and were generated from a sample of participants diagnosed with epilepsy in late adolescence or adulthood. Chen and colleagues’ (2011) sample included participants with lifelong epilepsy, and thus did not allow for the capture of perceived changes in sleep since epilepsy onset.
There is evidence that persons with epilepsy have a higher-than-normal incidence of actual sleep disorders, such as sleep apnea and restless leg syndrome, especially in those who experience nocturnal seizures (Grigg-Damberger & Foldvary-Schaefer, 2012). It is unclear if the participants in this sample reported such decrements in their ability to sleep well due to the presence of actual sleep disorders (diagnosed or undiagnosed) or due to AED side effects or problems directly related to the pathophysiology of epilepsy.
In this sample, there was a clear relationship between the LCES sleep item and total LCES scores, as well as between the LCES sleep item and criterion item (which asks participants to rate how their lives have changed overall as a result of epilepsy). Given that disruptions in sleep lead to negative effects on the central nervous, immune, cardiovascular, respiratory, and digestive systems (National Sleep Foundation, 2015), it is unsurprising that negative changes in ability to sleep well were associated more global life changes. It is well-known that persons with epilepsy report lower levels of quality of life when compared to those without epilepsy.
Based on current and prior research results, undesirable changes in sleep may significantly contribute to the documented downturns in quality of life in persons with epilepsy. Based on current results, recommendations for clinical practice and future research can be made. Given the high incidence of new-onset epilepsy in adults, nurses practicing in all settings will find themselves commonly caring for patients who have, among other diagnoses, epilepsy. Epilepsy is a complex condition affecting various portions of a person’s life (Unger & Buelow, 2009; Miller, 2014) and, based on these and prior results, ability to sleep well is chief among them. It is important for both bedside and advanced practice nurses to assess the sleep status of persons with epilepsy. This assessment could be achieved via the LCES, a short, easy-to-administer tool assessing sleep and other important areas of life changes in person with epilepsy. Alternatively, nurses caring for persons with epilepsy could simply ask patients about their ability to sleep well. Those who indicate negative changes in ability to sleep well via the LCES or other assessment method could be further evaluated for sleep quality using a tool such as the Pittsburgh Sleep Quality Index (Buysse et al., 1989). Based on individuals’ specific problems with sleep, interventions should be developed to promote sleep hygiene. In addition, given the role that AEDs play in altering the sleep of persons with epilepsy, nurses may consider advocating with prescribing providers to alter AEDs to promote sleep. For example, simply changing the schedule of AEDs could be of benefit. A person who experiences insomnia may have improved sleep by taking a sedative AED just prior to bed, rather than in the morning.
A qualitative study that would allow for a more in depth view of the experiences of changes in sleep in adults with adult-onset epilepsy may be warranted. Such an investigation could uncover the specific, context-dependent experiences of sleep alterations in this population, while also elucidating potential causes and strategies used to manage these problems. Coupled with results from the current study, findings rendered from a qualitative descriptive or phenomenological study could inform the development of interventions aimed at curtailing negative life changes in this population, including their ability to sleep well. Additional quantitative work, in which statistical analyses could provide further information on the effect of specific types of changes in sleep (insomnia versus excessive somnolence, for example) on quality of life may also be useful.
Limitations exist which lessen the generalizability of current results. First, the LCES is not sensitive to the types of changes experienced in sleep. That is, the tool does not capture if a person is experiencing insomnia, excessive somnolence, or other types of problems sleeping well. Thus, the types of problems with sleep experienced by this sample—and whether insomnia or excessive somnolence is the more common problem—is not known. The LCES is designed to detect changes in broad areas of quality of life, of which sleep is one aspect. In the clinical setting, it is important for further assessment to occur in individuals who indicate negative changes in ability to sleep well. Second, the diversity of the sample is somewhat limited. Future studies regarding this topic should involve a more diverse sample, especially in terms of geographic location and race/ethnicity.
Table 2.
Additional Demographic Characteristics of the Sample (N=174)
| Variable | Frequency |
|---|---|
|
| |
| Ethnicity | |
| • White | • n=136 |
| • African American | • n=22 |
| • Hispanic | • n=1 |
| • Pacific Islander | • n=1 |
| • Other | • n=4 |
| • Not reported | • n=8 |
|
| |
| Seizure Type | |
| • Complex Partial | • n=33 |
| • Tonic Clonic | • n=32 |
| • Combination | • n=31 |
| • Other | • n=17 |
| • Unknown | • n=61 |
|
| |
| Employment Status | |
| • Employed Full-Time | • n=38 |
| • Employed Part-Time | • n=13 |
| • Homemaker | • n=10 |
| • Retired | • n=16 |
| • Unemployed | • n=41 |
| • Disability | • n=47 |
| • Other | • n=9 |
|
| |
| Seizure Frequency | |
| • Daily | • 7 |
| • Weekly | • 22 |
| • Monthly | • 22 |
| • Bi-Monthly | • 28 |
| • Bi-Annually | • 19 |
| • <1/year | • 67 |
| • Other | • 9 |
Acknowledgments
This work was supported by NIH grant KL2TR000163
Contributor Information
Wendy R. Miller, Email: wrtruebl@iu.edu, Assistant Professor, Indiana University School of Nursing, Department of Science of Nursing Care, 1033 East Third Street, Sycamore Hall, Room 441, Bloomington, IN 47405 USA, p: 812-797-4646, f: 812-855-6986.
Julie Otte, Assistant Professor, Indiana University School of Nursing, Department of Science of Nursing Care, Indianapolis, IN USA.
Madona Pleuger, Clinical Nurse Specialist, Barrow Neurological Institute, St. Joseph’s Hospital and Medical Center, Phoenix, AZ USA.
References
- Aschoff J. Circadian rhythms in man. Science. 1965;148:1427–1432. doi: 10.1126/science.148.3676.1427. [DOI] [PubMed] [Google Scholar]
- Bazil CW. Epilepsy and sleep disturbance. Epilepsy & Behavior. 2003;4(Suppl 2):S39–S45. doi: 10.1016/j.yebeh.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Bowman TJ. Normal sleep patterns. In: Bowman TJ, editor. Review of sleep medicine. Boston: Butterworth & Heinemann; 2003. pp. 166–201. [Google Scholar]
- Bowman TJ, Moshenin V. In: Review of sleep medicine. Bowman TJ, editor. Boston: Butterworth & Heinemann; 2003. pp. 3–18. [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index (PSQI): A new instrument for psychiatric research and practice. Psychiatry Research. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Callahan C, Unverzagt F, Hui SL, Perkins AJ, Hendrie HC. Six-item screener to identify cognitive impairment. Medical Care. 2002;40(9):771–781. doi: 10.1097/00005650-200209000-00007. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Teenagers. 2015 Retrieved May 8, 2015 from http://www.cdc.gov/ncbddd/childdevelopment/positiveparenting/adolescence2.html.
- Chen NC, Tsai MH, Chang CC, Lu CH, Chang WN, Lai SL, Tseng YL, Chuant YC. Sleep quality and daytime sleepiness in patients with epilepsy. Acta Neurological Taiwan. 2011;20(4):249–256. [PubMed] [Google Scholar]
- Epilepsy Foundation of America. Epilepsy statistics. 2013 Retrieved December 1, 20014 from www.epilepsy.com.
- Grigg-Damberger M, Foldvary-Schaefer N. Primary sleep disorders in people with epilepsy: What we know, don’t know, and need to know. Sleep Medicine Clinics. 2012;7:75–89. [Google Scholar]
- Guyton AC, Hall JE. Human physiology and mechanisms of disease. 6. Philadelphia: Saunders; 1997. [Google Scholar]
- Institute of Medicine. Epilepsy Across the Spectrum: Promoting Health and Understanding. The National Academies Press; Washington, DC: 2012. [PubMed] [Google Scholar]
- Miller W, Bakas T, Buelow J, Habermann B. Research involving participants with chronic diseases: Overcoming recruitment obstacles. Clinical Nurse Specialist. 2013;27(6):307–313. doi: 10.1097/NUR.0b013e3182a8725a. [DOI] [PubMed] [Google Scholar]
- Miller W. Patient-centered outcomes in older adults with epilepsy. Seizure. 2014;23(8):592–597. doi: 10.1016/j.seizure.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W, Bakas T, Weaver M, Buelow J, Sabau D. The life changes in epilepsy scale: development and establishment of content and face validity. Clinical Nurse Specialist. 2015;29(2):95–99. doi: 10.1097/NUR.0000000000000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W, Weaver M, Bakas T, Buelow J, Sabau D. Psychometric testing of the life changes in epilepsy scale. Journal of Nursing Measurement. doi: 10.1891/1061-3749.25.1.41. In Review. [DOI] [PubMed] [Google Scholar]
- National Sleep Foundation. Epilepsy and sleep. 2015 Retrieved February 1, 2015 from http://sleepfoundation.org/sleep-disorders-problems/disease-and-sleep/epilepsy.
- Piperdou C, Karlovasitou A, Triantafyllou N, Terzoudi A, Constantinidis T, Vadikolias K, Heliopoulos I, Vassilopoulos D, Balogiannis S. Influence of sleep disturbance on quality of life of patients with epilepsy. Seizure. 2008;17(7):588–594. doi: 10.1016/j.seizure.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Unger WR, Buelow JM. Hybrid concept analysis of self-management in adults newly diagnosed with epilepsy. Epilepsy & Behavior. 2009;14(1):89–95. doi: 10.1016/j.yebeh.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Vena C, Cunningham M, Clark J, McMillan S. Continuing education: Sleep-wake disturbances in people with cancer part I: an overview of sleep, sleep regulation, and effects of disease and treatment. Oncology Nursing Forum. 2004;31:735–736. doi: 10.1188/04.ONF.735-746. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Epilepsy fact sheets. 2012 Retrieved January 2, 2015, from http://www.who.int/mediacentre/factsheets/fs999/en/