Abstract
Background
Many patients evaluated for acute coronary syndrome (ACS) in emergency departments (EDs) continue to experience troubling symptoms after discharge—regardless of their ultimate medical diagnosis. However, comprehensive understanding of common postED symptom trajectories is lacking.
Objectives
To identify common trajectories of symptom severity in the six months after an ED visit for potential ACS.
Methods
This was a secondary analysis of data from a larger observational, prospective study conducted in five U.S. emergency departments. Patients (N = 1005) who had electrocardiogram and biomarker testing ordered, and were identified by the triage nurse as potentially having ACS, were enrolled. Symptom severity was assessed in the hospital after initial stabilization and by telephone at 30 days and six months using the validated 13-item ACS Symptom Checklist. Growth mixture modeling was used for the secondary analysis. The eight most commonly reported symptoms (chest discomfort, chest pain, chest pressure, lightheadedness, shortness of breath, shoulder pain, unusual fatigue, and upper back pain) were modeled across the three study timepoints. Models with increasing numbers of classes were compared, and final model selection was based on a combination of interpretability, theoretical justification, and statistical fit indices.
Results
The sample was 62.6% male with a mean age of 60.2 years (SD = 14.17), and 57.1% ruled out for ACS. Between two and four distinct trajectory classes were identified for each symptom. The seven different types of trajectories identified across the eight symptoms were labeled “tapering off,” “mild/persistent,” “moderate/persistent,” “moderate/worsening,” “moderate/improving,” “late onset, “and “severe/improving.” Trajectories differed on age, sex, and diagnosis.
Discussion
Research on the individual nature of symptom trajectories can contribute to patient-centered, rather than disease-centered, care. Further research is needed to verify the existence of multiple symptoms trajectories in diverse populations, and to assess the antecedents and consequences of individual symptom trajectories.
Keywords: acute coronary syndrome, growth mixture model, health trajectories, hospital emergency service, longitudinal studies, symptom assessment
Over 5.5 million patients are evaluated annually for acute coronary syndrome (ACS) in U.S. emergency departments (EDs). The need for rapid diagnosis and treatment of ACS has driven efforts to increase awareness of ACS symptoms among the lay public and health professionals (Mozaffarian et al., 2015). While this disease-centered approach to ACS symptoms is valuable in reducing morbidity and mortality, the impact of these symptoms on patient-centered outcomes, such as functional status and quality of life, remains unknown.
Purpose
The purpose of this study was to explore trajectories of severity of common symptoms (chest discomfort, chest pain, chest pressure, lightheadedness, shortness of breath, shoulder pain, unusual fatigue, and upper back pain) experienced by patients during and after ED evaluation for ACS over three timepoints (ED presentation, 30 days, and six months). This timeframe was chosen because a significant portion of patients are readmitted within 30 days after discharge, and many report symptoms up to six months later (Barnason, Zimmerman, Nieveen, Schulz, & Young, 2012; Vashi et al., 2013). The goals of this exploratory investigation were to increase understanding of these symptom trajectories, generate further testable hypotheses, and inform education for patients and healthcare providers about symptom trajectories after hospital discharge.
Background
Symptoms Associated with ACS
A variety of physical symptoms may trigger suspicion for ACS, including “classic” symptoms of chest pain or discomfort, discomfort in other areas of the upper body, shortness of breath, sweating, nausea, and lightheadedness, as well as less typical symptoms of fatigue, palpitations, indigestion, and pain in other areas (Canto et al., 2012; DeVon, Ryan, Ochs, & Shapiro, 2008; El-Menyar et al., 2011; Go et al., 2014; McSweeney, O’Sullivan, Cody, & Crane, 2004). These symptoms are distressing both for patients ruled in and ruled out for ACS. All patients with such symptoms require treatment, support, and education regardless of their ultimate medical diagnoses.
Significance of Post-ED Symptoms
The great majority of patients ruled in and ruled out for ACS are eventually discharged home—either from the ED or from inpatient units—but little is known about their symptoms after discharge (Barnason et al., 2012). This knowledge gap results in periods where patients are undersupported, undereducated, and undertreated (Naylor, Aiken, Kurtzman, Olds, & Hirschman, 2011). Unmet needs, including lack of education and unmanaged symptoms, are associated with high rates of repeat ED use for both patients with and without an ACS diagnosis (LeClerc, Wells, Craig, & Wilson, 2002; Vashi et al., 2013). These patients' symptoms burdens are also associated with decreased quality of life (Karlson, Währbogrm, Sjöland, Lindqvist, & Herlitz, 1998).
Current evidence suggests that myocardial infarction (MI) patients—a subset of ACS patients—are especially vulnerable to readmission: Nearly 20% of MI patients are readmitted within 30 days of discharge. However, only 10% of these readmissions result in a primary diagnosis of MI (Dharmarajan et al., 2013). This evidence suggests that post-ED symptoms, in addition to reducing quality of life, may reflect a variety of disease processes requiring intervention.
Symptom Trajectories
The role of symptoms in patients’ quality of life and functional status has been identified as a key area of inquiry to support high-quality, patient-centered healthcare (Rumsfeld et al., 2013). However, most ACS symptom research to date has been cross-sectional (Arslanian-Engoren et al., 2006). Because patients frequently experience ongoing or recurrent symptoms following an ED visit or hospitalization, a truly patient-centered approach requires understanding individual symptom trajectories. A symptom trajectory is defined as a pattern of symptoms over time (Henly, Wyman, & Gaugler, 2011). Focusing research on individuals’ symptom trajectories, rather than on symptoms at a single point in time, is a patient-centered approach and is highly relevant to clinical situations (Henly, Wyman, & Findorff, 2011).
Trajectories of both fatigue and vital exhaustion have been identified in ACS patients after discharge (Fennessy et al., 2010; Smith, Kupper, Denollet, & de Jonge, 2011). Different symptom trajectories have been associated with differences in disease-related outcomes: Severe and increasing vital exhaustion trajectories have been associated with a greater incidence of cardiac events than mild and decreasing trajectories in one study using latent class growth analysis (Smith et al., 2011). In another study using regression analysis, symptom trajectories also varied by sex, with women often experiencing severe but improving fatigue, in contrast to men’s moderate, persistent fatigue (Fennessy et al., 2010).
Psychological symptoms, such as anxiety and depression, have also been shown to persist over time (Doyle, McGee, Delaney, Motterlini, & Conroy, 2011; Kaptein, de Jonge, van den Brink, & Korf, 2006; Murphy et al., 2014). The findings have not been consistent across studies, however. Some found that symptoms varied between individuals, but were largely stable over time (Doyle et al., 2011; Martens, Smith, Winter, Denollet, & Pedersen, 2008; van Beek et al., 2012), while others found worsening depression trajectories and improving anxiety trajectories within individuals (Kaptein et al., 2006; Lane, Carroll, Ring, Beevers, & Lip, 2002; Murphy et al., 2014; Tisminetzky, Bray, Miozzo, Aupont, & McLaughlin, 2011).
Though the evidence on post-ACS symptom trajectories is promising, the spectrum of potential ACS-related symptoms is diverse, and patients who rule in as well as those who rule out are affected. To our knowledge, post-ED trajectories of multiple physical symptoms potentially related to ACS have not yet been studied.
Growth Mixture Modeling of Symptom Trajectories
Existing research suggests that potential ACS symptoms vary among individuals based on combinations of characteristics such as age, sex, and diagnosis (Arslanian-Engoren et al., 2006; Canto et al., 2012; DeVon, Ryan, Rankin, & Cooper, 2010). However, this body of evidence includes findings that are, at times, conflicting and of unclear clinical significance. Variable-centered techniques, such as regression and chi-square analysis, have been used to test for specific hypothesized group differences and interactions, but cannot reveal previously unknown subgroups of individuals. Neither cross-sectional models nor standard growth models are able to effectively model the complex heterogeneity of potential ACS symptoms experienced by patients over time. As a result, this phenomenon remains poorly understood despite considerable research.
Growth mixture modeling (GMM) is used to analyze change over time and identify groups of individuals who change in similar ways (Muthén, 2004). Unlike traditional growth curve modeling, GMM does not rely on the assumption that all individuals belong to a single population. Instead, parameters are allowed to vary across unobserved subpopulations, which are also known as “latent classes” (Muthén, 2004). These groups are “latent” in that they are revealed by identifying similar patterns of change in the data rather than defined by known baseline characteristics (Muthén, 2004). One important parameter of GMM models is the number of groups—which is determined by comparing the fit of models with different numbers of groups. Rather than a single mean trajectory, GMM models may include multiple mean trajectories representing the different patterns of change in these subgroups. GMM is uniquely suited to exploring heterogeneity in diverse populations where multiple change patterns may exist, as is the case with potential ACS patients.
Conceptual Framework
A modified version of the theory of unpleasant symptoms served as the organizing framework for this study (Lenz, Pugh, Milligan, Gift, & Suppe, 1997). This theory relates influencing factors, symptoms, and performance outcomes. Symptoms are conceptualized as multidimensional, including quality, intensity, distress, and timing. Timing is especially important to the conceptualization of symptom trajectories, so for the present study, timing is extended to include not only initial occurrence, but also change over time (Brant, Beck, & Miaskowski, 2010).
Methods
Design, Setting, and Sample
The present investigation was a secondary analysis of data from a larger observational, prospective study. Detailed methods of the parent study are published elsewhere (DeVon, Burke, Nelson, Zerwic, & Riley, 2014). In the parent study, participants were recruited from the EDs of four academic medical centers and one community hospital located in the Midwest, Pacific Northwest, and Western U.S. The convenience sample consisted of 1,005 patients who presented with symptoms suggestive of ACS. Patients were initially approached if they had an abnormal electrocardiogram or troponin T level and were identified by the triage nurse as potentially having ACS. Other inclusion criteria were symptoms on presentation to the ED, age greater than 21 years, English speaking, telephone access, and intact cognition. One site, located in the Southwestern U.S., also included Spanish speakers. Exclusion criteria included heart failure exacerbation (B-type natriuretic peptide > 500 ng/mL), referral to the ED from a dialysis center, and referral for cardiac dysrhythmia. Data collection occurred during the initial ED visit with follow-up phone calls at 30 days and six months. The respective Institutional Review Boards at each site approved the study.
Data Collection
The EDs were staffed with trained research assistants (RAs) between eight and 16 hours per day (between 0700 and 2300). Initial data collection occurred as soon as possible after arrival. After the patients’ initial stabilization (i.e., vital signs were stable and patients were treated with analgesics), RAs obtained written informed consent and administered questionnaires to collect symptom, clinical, and demographic data.
Measures
Demographic and clinical characteristics
Baseline participant characteristics were assessed using the ACS Patient Information Questionnaire, which includes both demographic and clinical items. It was designed to capture all relevant data as outlined in the standardized reporting guidelines for ED patients with potential ACS, which are supported by the Society for Academic Medicine, the American College of Emergency Physicians, the American Heart Association, and the American College of Cardiology (Hollander et al., 2004). Final diagnosis was based on the clinical judgment of the attending physicians (who were blinded to study data) and was abstracted from the medical record by trained RAs.
Symptoms
Symptoms were assessed using the validated 13-item ACS Symptom Checklist, which was derived from the original Symptoms of Acute Coronary Syndrome Index (DeVon & Zerwic, 2003). Each symptom is analyzed individually with no summary score. Symptom severity was scored from 0 = none to 10 = worst. After initial stabilization, participants were instructed to rate their current symptoms. At 30 days and six months, participants were instructed to rate symptoms experienced in the past week. Due to the subjective nature of symptom experiences, self-report is the most appropriate method of assessment. However, it is important to note that self-report of symptom severity may be influenced by patient factors such as prior symptom experiences.
Although data were collected for 13 symptoms as part of the parent study, only those symptoms reported by at least 25% of participants at any study timepoint (chest discomfort, chest pain, chest pressure, lightheadedness, shortness of breath, shoulder pain, unusual fatigue, and upper back pain) were included in this secondary data analysis. The decision to limit included symptoms in this manner reflects both sample size limitations and the desire to generate a parsimonious, clinically useful set of models. Because symptom trajectories have not previously been described in this population, symptoms were analyzed individually for this exploratory study.
Time
Time was coded in months for the analysis (0 = baseline, 1 = 30 days, and 6 = six months).
Statistical Analysis
Model specification
Preliminary analyses, including calculation of descriptive statistics, were carried out using SPSS (v.22). Growth mixture models were estimated using Mplus software (v.7.11) with a robust maximum-likelihood estimator. Cases with missing symptom data for all three study time points (n = 3) were excluded from the analysis. Cases with partial data were retained, and missing data were handled using a full-information maximum-likelihood method (Muthén, 2004).
For each of the eight symptoms, cases with a nonzero score (indicating symptom presence) at any point during the study period were included in that symptom’s models. This choice reflects the focus on trajectories of symptom severity rather than probability of symptom occurrence. The resulting samples for each individual symptom model ranged from n = 725 to n = 816.
Linear models (i.e., with the rate of change constant across timepoints) were estimated because the data comprised three timepoints. Initially, models with growth factor variances fixed to zero (i.e., latent class growth analysis [LCGA]) were estimated (Nagin & Odgers, 2010). LCGA is considered a special case of GMM, and produces simpler models (Berlin, Parra, & Williams, 2014; Jung & Wickrama, 2008). In subgroups identified using LCGA, the same model is used to describe change for each member, and differences among the members of a subgroup are regarded as error. In GMM, individual trajectories vary around class-specific mean trajectories for change. After LCGA models were estimated, more flexible GMMs with intercept variances freed were estimated. Models with between one and five latent classes were considered for each symptom. Both sample size limitations and clinical utility limit the interpretability of additional classes.
Model selection
For each symptom, models with increasing numbers of classes were systematically compared. Criteria were interpretability, parsimony, lowest adjusted Bayesian Information Criterion (BIC), significant Vo-Lo-Mendell-Rubin likelihood ratio test (VLMR-LRT), and class size greater than 5% of the sample based on most likely class membership (Wang & Bodner, 2007). Bootstrap likelihood ratio tests (BLRT) were also computed for promising models (Nylund, Asparouhov, & Muthén, 2007). Models with higher entropy and greater average posterior probability within each class were favored if other criteria were not decisive (Zaslavsky et al., 2014).
Covariates
Patient characteristics (age, sex, and diagnosis) were treated as potential covariates using a revised three-step procedure (model estimation, class assignment based on posterior probabilities, and logistic regression accounting for misclassification) (Vermunt, 2010). The procedure was implemented by adding the variables of interest into the original model as auxiliary variables and using the “r3step” command in Mplus. The largest class for each symptom was used as the reference class. Each potential covariate was analyzed individually. There was no missing data for age or sex. Five cases had missing data for diagnosis and were excluded from further analysis based on the assumption that data were missing at random. This assumption is supported by the similarity of patients with complete and incomplete data based on available information.
Results
Baseline Sample Characteristics
Demographic and clinical characteristics of the sample are shown in Table 1. The majority of the 1,002 included participants who were White (70.5%), male (62.6%), ruled out for ACS (57.1%), and had a mean age of 60.2 years (SD = 14.17).
TABLE 1.
Sample Characteristics
| Characteristic | M | (SD) | n | Missing |
|---|---|---|---|---|
| Age (years) | 60.2 | (14.17) | 1002 | 0 |
| BMI (kg/m2) | 30.0 | (7.31) | 983 | 19 |
|
|
||||
| n | (%) | |||
|
|
||||
| Sex | 1002 | 0 | ||
| Male | 627 | (62.6) | ||
| Female | 375 | (37.4) | ||
| Study Site | 1002 | 0 | ||
| A | 121 | (12.1) | ||
| B | 121 | (12.1) | ||
| C | 439 | (43.8) | ||
| D | 51 | (5.1) | ||
| E | 270 | (26.9) | ||
| Race/ethnicity | 998 | 4 | ||
| White | 706 | (70.5) | ||
| Black | 132 | (13.2) | ||
| Hispanic | 59 | (5.9) | ||
| Other | 101 | (10.1) | ||
| Education | 1000 | 2 | ||
| HS or less | 335 | (33.5) | ||
| Some college | 330 | (32.9) | ||
| College degree | 205 | (20.5) | ||
| Graduate degree | 130 | (13.0) | ||
| Insurance | 988 | 14 | ||
| Private | 418 | (41.7) | ||
| Government | 438 | (43.7) | ||
| Uninsured | 132 | (13.2) | ||
| Diagnosis | 997 | 5 | ||
| Non-ACS | 572 | (57.1) | ||
| UA | 101 | (10.1) | ||
| NSTEMI | 222 | (22.2) | ||
| STEMI | 102 | (10.2) | ||
Note. n = 1002. ACS = acute coronary syndrome; BMI = body mass index; HS = high school; NSTEM = non-ST-segment elevation myocardial infarction; SD = standard deviation; STEMI = ST-segment elevation myocardial infarction; UA = unstable angina.
Individual Symptom Severity
The most common symptoms experienced in the hospital after stabilization were chest discomfort (49.2%), chest pressure (47.8%), unusual fatigue (46.5%), chest pain (42.3%) shortness of breath (37.8%), lightheadedness (35.6%), upper back pain (26.8%), and shoulder pain (25.8%). Mean symptom severity ranged from 1.71–3.32 after stabilization, 1.41–2.35 at 30 days, and 1.42–3.24 at six months (Table 2). Because baseline characteristics of patients with complete versus missing data were similar, missing symptom data at any timepoint was considered to be missing at random (i.e., not related to the missing values).
TABLE 2.
Symptom Severity at Each Time Point
| Baseline
|
30 days
|
6 months
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Symptom | M | (SD) | n | M | (SD) | n | M | (SD) | n |
| Chest discomfort | 3.02 | (3.25) | 810 | 1.79 | (2.57) | 545 | 1.53 | (2.43) | 393 |
| Chest pain | 2.87 | (3.35) | 759 | 1.53 | (2.59) | 494 | 1.42 | (2.63) | 345 |
| Chest pressure | 2.90 | (3.15) | 782 | 1.41 | (2.35) | 519 | 1.54 | (2.58) | 368 |
| Lightheadedness | 2.15 | (2.95) | 775 | 2.00 | (2.71) | 509 | 1.90 | (2.81) | 358 |
| Shortness of breath | 2.55 | (3.25) | 784 | 2.38 | (2.83) | 515 | 2.37 | (3.07) | 368 |
| Shoulder pain | 1.71 | (2.82) | 737 | 2.10 | (3.04) | 470 | 2.39 | (3.18) | 321 |
| Unusual fatigue | 3.32 | (3.50) | 811 | 2.35 | (3.20) | 548 | 2.23 | (3.24) | 397 |
| Upper back pain | 1.82 | (2.86) | 717 | 1.80 | (2.89) | 454 | 2.02 | (3.04) | 303 |
Note. Findings are reported for participants who reported the symptom during the study period. SD = standard deviation.
Final Model Selection
Model description and fit indices for each symptom’s final model are shown in Table 3. Fit statistics for rejected models are not included here due to the large number of models tested, but are available (see Table, Supplemental Digital Content1). For the majority of symptoms, LCGA models with intercept and slope variance fixed to 0 were selected over less restrictive models based on the selection criteria defined above. For two symptoms (shortness of breath and shoulder pain), GMM models with intercept variance freely estimated were selected. Each symptom’s final model included between two and four classes; no symptom was best modeled by a single trajectory class. Models with greater than four classes generally included at least one class too small to interpret (i.e., less than 5% of the sample).
TABLE 3.
Final Trajectory Model for Each Symptom
| Symptom | na | Modelb | aBIC | pc | Entropy |
|---|---|---|---|---|---|
| Chest discomfort | 816 | 3-class LCGA | 8225.488 | < .0009 | .643 |
| Chest pain | 768 | 2-class LCGA | 7747.768 | < .0025 | .714 |
| Chest pressure | 790 | 2-class LCGA | 7952.736 | < .0001 | .640 |
| Lightheadedness | 782 | 2-class LCGA | 7859.931 | < .0001 | .897 |
| Shortness of breath | 791 | 3-class GMM, intercept variances estimated | 8038.124 | < .0001 | .941 |
| Shoulder pain | 744 | 4-class GMM, intercept variances estimated | 6650.923 | < .0001 | .985 |
| Unusual fatigue | 819 | 3-class LCGA | 8684.698 | < .0001 | .612 |
| Upper back pain | 725 | 4-class LCGA | 6449.754 | < .0001 | .988 |
Note. aBIC = adjusted Bayesian information criterion; GMM = growth mixture model; LCGA = latent class growth analysis.
Total cases included.
Complete model comparison information is available as Supplemental Digital Content 1.
p-value is associated with the Vuong-Lo-Mendell-Rubin likelihood ratio test .
Symptom Trajectories
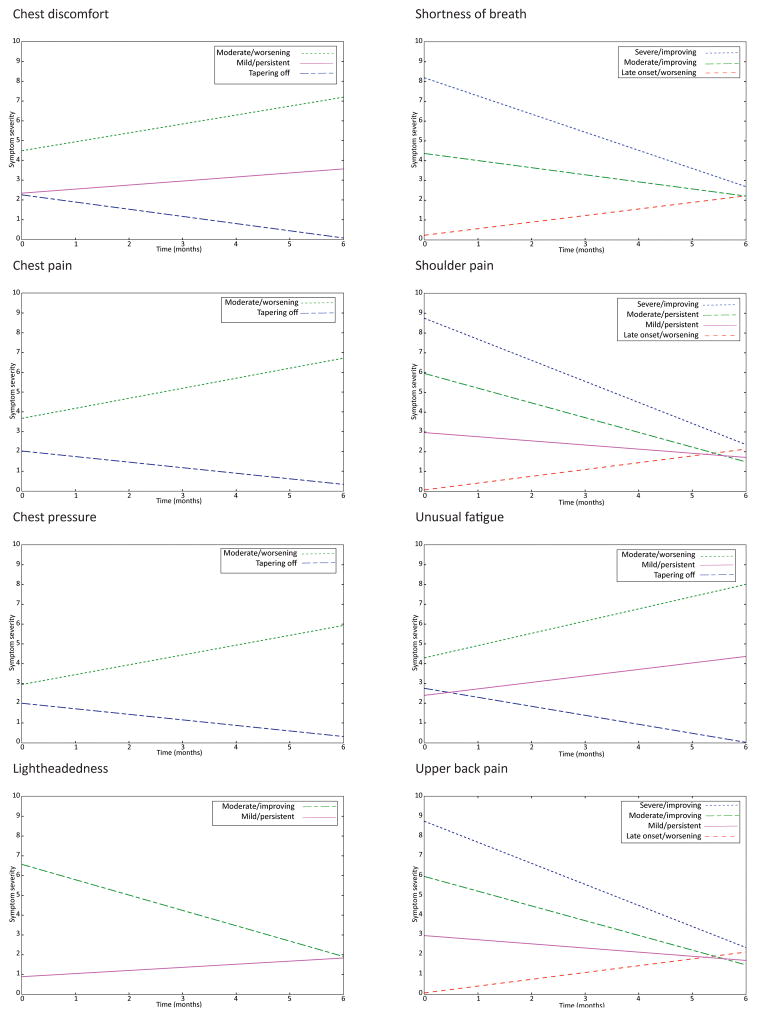
Parameter estimates by class for each symptom’s final model are shown in Table 4, and the identified trajectory classes for each symptom are illustrated in Figure 1. For four of the eight symptoms (chest discomfort, chest pain, chest pressure, and unusual fatigue), the majority of participants were assigned to a class with an intercept less than 3.0 and a negative slope; a trajectory labeled “tapering off.” Another common trajectory, defined by intercept close to 0 with a positive slope, was labeled “late-onset/worsening” and was common in participants with upper back pain (66.7%), shoulder pain (69.3%), and shortness of breath (61.0%). A high percentage of participants with lightheadedness (78.2%), and smaller percentages of patients with chest discomfort (20.4%), shoulder pain (11.8%), and unusual fatigue (16.7%), exhibited a “mild/persistent” trajectory with an intercept less than 3.0 and a relatively flat slope. A “moderate/worsening” trajectory, with an intercept between 2.5 and 5.0 and a positive slope, was found in a minority of patients with chest discomfort (10.2%), chest pain (15.2%), chest pressure (20.0%), and unusual fatigue (17.9%). A “severe/improving” trajectory, characterized by intercept greater than 8.0 and a negative slope, was identified in a small number of participants with shortness of breath (17.4%) and upper back pain (7.4%). Other, uncommon trajectories identified included initially moderate symptoms that either decreased (improving) or remained stable (persistent).
TABLE 4.
Trajectory Parameter Estimates by Class for Each Symptom Model
| Symptom/class | (%)a | Mean intercept | Mean slopeb | Interpretation |
|---|---|---|---|---|
| Chest discomfort | ||||
| Class 1 | (20.4) | 2.34** | 0.20* | Mild/persistent |
| Class 2 | (69.4) | 2.26** | −0.36** | Tapering off |
| Class 3 | (10.2) | 4.49** | 0.45** | Moderate/worsening |
| Chest pain | ||||
| Class 1 | (84.8) | 2.02** | −0.28** | Tapering off |
| Class 2 | (15.2) | 3.67** | 0.51** | Moderate/worsening |
| Chest pressure | ||||
| Class 1 | (80.0) | 2.00** | −0.28** | Tapering off |
| Class 2 | (20.0) | 2.95** | 0.50** | Moderate/worsening |
| Lightheadedness | ||||
| Class 1 | (78.2) | 0.89** | 0.18** | Mild/persistent |
| Class 2 | (21.8) | 6.58** | −0.76** | Moderate/improving |
| Shortness of breathc | ||||
| Class 1 | (17.4) | 8.19** | −0.91** | Severe/improving |
| Class 2 | (21.6) | 4.36** | −0.36** | Moderate/persistent |
| Class 3 | (61.0) | 0.29** | 0.33** | Late onset/worsening |
| Shoulder paind | ||||
| Class 1 | (11.8) | 2.96** | −0.07** | Mild/persistent |
| Class 2 | (11.1) | 5.90** | −0.49** | Moderate/persistent |
| Class 3 | (7.7) | 8.57** | −1.04** | Severe/improving |
| Class 4 | (69.3) | 0.06** | 0.39** | Late-onset/worsening |
| Unusual fatigue | ||||
| Class 1 | (65.4) | 2.75** | −0.45** | Tapering off |
| Class 2 | (17.9) | 4.29** | 0.62** | Moderate/worsening |
| Class 3 | (16.7) | 2.40** | 0.33** | Mild/persistent |
| Upper back pain | ||||
| Class 1 | (66.8) | 0.07** | 0.34** | Late onset/worsening |
| Class 2 | (11.8) | 5.96** | −0.74** | Moderate/improving |
| Class 3 | (14.1) | 2.97** | −0.21* | Mild/persistent |
| Class 4 | (7.4) | 8.75** | −1.06** | Severe/improving |
Percentage of sample in each class.
Change in symptom severity/month.
Intercept variances were: 2.90 (Class 1), 3.29 (Class 2), and 0.70 (Class 3).
Intercept variances were: 0.67 (Class 1), 0.88 (Class 2), 1.23 (Class 3), and 0.07 (Class 4).
p < .05.
p < .001.
FIGURE 1.
Symptom trajectory groups following emergency department visits for possible acute coronary syndrome. Symptom severity over six months was self-reported using a scale that ranged from 0 = none to 10 = worst.
Age, Sex, and Diagnosis as Predictors of Group Membership
Because prior research suggests that ACS symptoms vary based on age, sex, and diagnosis, these characteristics were treated as potential covariates. For each of the eight symptoms studied, trajectory classes differed significantly on at least one patient characteristic (Table 5). Age was a significant predictor of trajectory membership for chest discomfort, lightheadedness, shortness of breath, shoulder pain, and upper back pain. “Late-onset” and “tapering off” trajectories were most commonly predicted by older age. Female sex predicted more severe symptom trajectories for shoulder pain, unusual fatigue, and upper back pain. Diagnosis was a significant predictor of trajectory membership for every symptom except unusual fatigue. Non-ST-segment elevation myocardial infarction (NSTEMI) diagnosis predicted “tapering off” or “late onset” trajectories for most symptoms (chest discomfort, chest pain, chest pressure, shortness of breath, shoulder pain, and upper back pain). ST-segment elevation myocardial infarction (STEMI) diagnosis was also predicted “late onset” trajectories for upper back pain, shoulder pain, and shortness of breath.
TABLE 5.
Characteristics Associated With Symptom Trajectory Groups
| Age (years)
|
Sex (female)
|
DX: UA
|
DX: NSTEMI
|
DX: STEMI
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Symptom/trajectory group | M | (SD) | n | (%) | n | (%) | n | (%) | n | (%) |
| Chest discomfort | ||||||||||
| Tapering off | 59.8 | (14.08) | 257 | (38.4) | 64 | (9.6) | 154 | (23.1) | 66 | (9.9) |
| Mild/persistent | 55.9 | (12.77) | 38 | (41.8) | 12 | (13.2) | 13* | (14.3) | 7 | (7.7) |
| Moderate/worsening | 53.5** | (14.76) | 25 | (44.6) | 4 | (7.1) | 6 | (10.7) | 1 | (1.8) |
| Chest pain | ||||||||||
| Tapering off | 59.6 | (14.24) | 261 | (37.5) | 65 | (9.4) | 160 | (23.1) | 68 | (9.8) |
| Moderate/worsening | 56.1 | (14.24) | 34 | (47.9) | 10 | (14.1) | 6* | ( 8.5) | 2 | (2.8) |
| Chest pressure | ||||||||||
| Tapering off | 59.5 | (14.12) | 267 | (38.3) | 68 | (9.8) | 157 | (22.6) | 65 | (9.4) |
| Moderate/worsening | 57.4 | (13.75) | 45 | (48.9) | 14 | (15.2) | 9* | (9.8) | 3 | (3.3) |
| Lightheadedness | ||||||||||
| Mild/persistent | 60.0 | (14.11) | 219 | (35.2) | 58 | (9.4) | 149 | (24.1) | 70 | ( 11.3) |
| Moderate/improving | 57.8* | (15.17) | 82 | (51.6) | 12 | (7.6) | 19** | (12.0) | 4* | (2.5) |
| Shortness of breath | ||||||||||
| Late onset | 60.9 | (14.28) | 165 | (34.1) | 54 | (11.2) | 126 | (26.2) | 62 | (12.9) |
| Moderate/persistent | 50.8 | (14.36) | 81 | (46.8) | 14 | (8.1) | 19** | (11.0) | 7** | (4.1) |
| Severe/improving | 57.1** | (14.36) | 61 | (45.5) | 7** | (5.2) | 18** | (13.4) | 4** | (3.0) |
| Shoulder pain | ||||||||||
| Late onset | 60.2 | (13.94) | 182 | ( 35.3) | 58 | (11.3) | 125 | (24.3) | 59 | (11.5) |
| Moderate/persistent | 58.8 | (13.73) | 43 | (51.8) | 10 | (10.8) | 10* | (12.1) | 2* | (2.4) |
| Severe/improving | 58.0 | (15.55) | 30* | (52.6) | 5 | (8.9) | 10 | (17.9) | 3 | (5.4) |
| Mild/persistent | 56.5* | (13.56) | 35 | (39.8) | 8 | (9.2) | 13* | (14.9) | 2* | (2.3) |
| Unusual fatigue | ||||||||||
| Tapering off | 60.2 | (14.25) | 236 | (36.2) | 62 | (9.6) | 153 | (23.6) | 67 | (10.3) |
| Moderate/worsening | 59.1 | (13.18) | 57** | (57.0) | 10 | (10.0) | 14 | (14.0) | 5 | (5.0) |
| Mild/persistent | 59.1 | (13.41) | 32 | (47.7) | 9 | (13.4) | 10 | (14.9) | 6 | (9.0) |
| Upper back pain | ||||||||||
| Late onset | 59.7 | (13.69) | 168 | (34.6) | 48 | (10.0) | 131 | (27.3) | 53 | (11.0) |
| Moderate/improving | 55.4* | (15.58) | 39 | (45.9) | 11 | (12.9) | 5** | (5.9) | 4* | (4.7) |
| Mild/persistent | 59.9 | (13.75) | 46 | (45.1) | 11 | (10.8) | 16* | (15.7) | 6 | (5.9) |
| Severe/improving | 60.2 | (15.23) | 36** | 2 | (9.6) | 8 | (15.1) | 0** | (0.0) | |
Note. Italic font signifies largest class for each symptom. DX = cardiac diagnosis; NSTEMI = non-ST-segment elevation myocardial infarction; STEMI = ST-segment elevation myocardial infarction; UA = unstable angina.
Signficant vs. largest class at p < .05.
Significant vs. largest class at p < .01.
Discussion
Increased Understanding of Symptom Trajectories
The findings of this secondary data analysis add support to existing evidence that patients experience ongoing symptoms after an ED visit for potential ACS (Barnason et al., 2012). The analysis also reveals that individuals vary in both their initial symptom severity and rate and direction of change—a finding that has not been previously described in this population. Because the mean symptom severity scores for the entire sample fail to capture this heterogeneity, growth mixture modeling is a valuable analytic tool for generating a more nuanced understanding. The identification of multiple, distinct classes of patients with different symptom trajectories is an initial step toward understanding how the symptom experience varies both among and within patients, and thereby improving patient-centered care and symptom management.
The examination of the individual trajectories within the symptom models is also revealing. The “tapering off” trajectory was common for chest symptoms and fits the expectation that symptoms generally improve over time after an acute period. The “late-onset/worsening” trajectory (observed for shortness of breath, shoulder pain, and upper back pain) and the “moderate/worsening” trajectory (seen in chest discomfort, chest pain, chest pressure, and unusual fatigue) are more concerning. These patterns suggest that some patients experience unexpected new or worsening symptoms after their initial ED visit. Such patients may require more intensive follow-up to ensure symptom management and prevent unnecessary readmissions (Karlson et al., 1998). Trajectories that are mild or moderate and persistent (found in chest discomfort, lightheadedness, shortness of breath, shoulder pain, unusual fatigue, and upper back pain) are also noteworthy. Such unresolved symptoms reduce quality of life and may signify chronic, stable disease that requires treatment (Kim, Kim, & Hwang, 2015).
The finding that patient characteristics differ among symptom trajectories is clinically important. The same characteristics (younger age, female sex, and less likelihood of NSTEMI diagnosis) tended to predict membership in smaller, less typical classes across different symptoms regardless of the morphology of these trajectories. Patients with these characteristics may, therefore, be less likely to receive appropriate education and treatment than their peers with more common symptom trajectories.
Generating Hypotheses for Future Research
The unique approach of including all patients with similarly distressing symptoms, rather than including only patients with a confirmed ACS diagnosis, is patient-centered rather than disease-centered. Future patient-centered studies may build on our findings by evaluating distal outcomes of different symptom trajectories (e.g., health service use and quality of life) and testing interventions to reduce symptom burden based on differing symptom trajectories. The statistical techniques used in the present study may also be used to identify symptom trajectories in other clinical and disease contexts.
Informing Patient Education
Patients presenting to the ED with symptoms suggestive of ACS, but who go on to be ruled out, is a population that has been understudied in previous research. These patients utilize significant healthcare resources, but may be undertreated in the current disease-focused model of care. Our findings support the notion that follow-up is important for these patients, as well as for patients who ruled in for ACS. All patients should be encouraged to track and report their ongoing and recurrent symptoms to ensure that they receive adequate medical treatment and symptom management plans.
Prediction of symptom trajectories based on information observable at initial presentation is also important to patient education. Patient characteristics associated with late-onset, persistent, or worsening trajectories may be seen as “red flags” for identifying at-risk patients. These patients may be targeted for individualized education, postdischarge support, and symptom management plans.
Limitations
Because symptom severity data were collected at only three timepoints, only linear models could be estimated. Based on our findings that distinct trajectory classes can be identified, future studies should be designed to allow comparison of model fit for higher-order and piecewise models by including more timepoints. Such models may represent symptom trajectories with greater nuance and accuracy. This study is also limited by the available sample: though large, the sample includes a higher proportion of patients who ruled in for ACS than other studies of this population. The parent study’s primary aims required an adequate number of patients ruled in for ACS, and the resulting recruitment decisions led to underrepresentation of ruled out patients relative to the population of interest. Because multiple models were estimated in this analysis, there is the potential for overfitting to the sample. Therefore, these results should be verified in other samples. Finally, analysis of antecedents and consequences of symptom trajectories and the interaction of multiple symptoms were beyond the scope of this exploratory analysis, but may generate clinically useful information in future studies.
Conclusions and Implications
Evidence of individuals’ distinct symptom trajectories in the six months following an ED visit for potential ACS has the potential to improve patient education and clinical assessment of ongoing symptoms. Continued research on the individual nature of symptom trajectories may thus support patient-centered care. Symptom trajectory research can also contribute to the further development of symptom theory by expanding empirical evidence about the temporal aspects of symptom experiences. Future studies are needed to verify the existence of multiple symptoms trajectories in diverse populations, to assess the antecedents and consequences of individual symptom trajectories, and to examine relationships among different symptoms.
Supplementary Material
Acknowledgments
The authors acknowledge funding was provided by NIH: R01 NR012012 (DeVon, PI) and Sigma Theta Tau International, Beta Mu Chapter.
Footnotes
The authors have no conflicts of interest to report.
Contributor Information
Elizabeth P. Knight, Clinical Assistant Professor, University of Arizona College of Nursing, Tucson, AZ.
Kimberly Shea, Assistant Professor, University of Arizona College of Nursing, Tucson, AZ.
Anne G. Rosenfeld, Professor, University of Arizona College of Nursing, Tucson, AZ.
Sarah Schmiege, Assistant Professor, University of Colorado- Denver College of Nursing.
Chiu-Hsieh Hsu, Associate Professor, University of Arizona College of Public Health, Tucson, AZ.
Holli A. DeVon, Associate Professor, University of Illinois at Chicago College of Nursing.
References
- Arslanian-Engoren C, Patel A, Fang J, Armstrong D, Kline-Rogers E, Duvernoy CS, Eagle KA. Symptoms of men and women presenting with acute coronary syndromes. American Journal of Cardiology. 2006;98:1177–1181. doi: 10.1016/j.amjcard.2006.05.049. [DOI] [PubMed] [Google Scholar]
- Barnason S, Zimmerman L, Nieveen J, Schulz P, Young L. Patient recovery and transitions after hospitalization for acute cardiac events: An integrative review. Journal of Cardiovascular Nursing. 2012;27:175–191. doi: 10.1097/JCN.0b013e318239f5f5. [DOI] [PubMed] [Google Scholar]
- Berlin KS, Parra GR, Williams NA. An introduction to latent variable mixture modeling (Part 2): Longitudinal latent class growth analysis and growth mixture models. Journal of Pediatric Psychology. 2014;39:188–203. doi: 10.1093/jpepsy/jst085. [DOI] [PubMed] [Google Scholar]
- Brant JM, Beck S, Miaskowski C. Building dynamic models and theories to advance the science of symptom management research. Journal of Advanced Nursing. 2010;66:228–240. doi: 10.1111/j.1365-2648.2009.05179.x. [DOI] [PubMed] [Google Scholar]
- Canto JG, Rogers WJ, Goldberg RJ, Peterson ED, Wenger NK, Vaccarino V, … Zheng ZJ. Association of age and sex with myocardial infarction symptom presentation and in-hospital mortality. JAMA. 2012;307:813–822. doi: 10.1001/jama.2012.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVon HA, Zerwic JJ. The symptoms of unstable angina: Do women and men differ? Nursing Research. 2003;52:108–118. doi: 10.1097/00006199-200303000-00007. [DOI] [PubMed] [Google Scholar]
- DeVon HA, Burke LA, Nelson H, Zerwic JJ, Riley B. Disparities in patients presenting to the emergency department with potential acute coronary syndrome: It matters if you are Black or White. Heart & Lung. 2014;43:270–277. doi: 10.1016/j.hrtlng.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVon HA, Ryan CJ, Ochs AL, Shapiro M. Symptoms across the continuum of acute coronary syndromes: Differences between women and men. American Journal of Critical Care. 2008;17:14–24. [PMC free article] [PubMed] [Google Scholar]
- DeVon HA, Ryan CJ, Rankin SH, Cooper BA. Classifying subgroups of patients with symptoms of acute coronary syndromes: A cluster analysis. Research in Nursing & Health. 2010;33:386–397. doi: 10.1002/nur.20395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmarajan K, Hsieh AF, Lin Z, Bueno H, Ross JS, Horwitz LI, … Krumholz HM. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309:355–363. doi: 10.1001/jama.2012.216476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle F, McGee H, Delaney M, Motterlini N, Conroy R. Depressive vulnerabilities predict depression status and trajectories of depression over 1 year in persons with acute coronary syndrome. General Hospital Psychiatry. 2011;33:224–231. doi: 10.1016/j.genhosppsych.2011.03.008. [DOI] [PubMed] [Google Scholar]
- El-Menyar A, Zubaid M, Sulaiman K, AlMahmeed W, Singh R, Alsheikh-Ali AA, Al Suwaidi J. Atypical presentation of acute coronary syndrome: A significant independent predictor of in-hospital mortality. Journal of Cardiology. 2011;57:165–171. doi: 10.1016/j.jjcc.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Fennessy MM, Fink AM, Eckhardt AL, Jones J, Kruse DK, VanderZwan KJ, … Zerwic JJ. Gender differences in fatigue associated with acute myocardial infarction. Journal of Cardiopulmonary Rehabilitation and Prevention. 2010;30:224–230. doi: 10.1097/HCR.0b013e3181d0c493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, … Turner MB. Heart disease and stroke statistics—2014 update: A report from the American Heart Association. Circulation. 2014;129:399–410. doi: 10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- Henly SJ, Wyman JF, Findorff MJ. Health and illness over time: The trajectory perspective in nursing science. Nursing Research. 2011;60(Suppl 3):S5–S14. doi: 10.1097/NNR.0b013e318216dfd3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henly SJ, Wyman JF, Gaugler JE. Health trajectory research: A call to action for nursing science. Nursing Research. 2011;60(Suppl 3):S79–S82. doi: 10.1097/NNR.0b013e31821cc240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander JE, Blomkalns AL, Brogan GX, Diercks DB, Field JM, Garvey JL, … Jackson RE. Standardized reporting guidelines for studies evaluating risk stratification of ED patients with potential acute coronary syndromes. Academic Emergency Medicine. 2004;11:1331–1340. doi: 10.1197/j.aem.2004.08.033. [DOI] [PubMed] [Google Scholar]
- Jung T, Wickrama K. An introduction to latent class growth analysis and growth mixture modeling. Social and Personality Psychology Compass. 2008;2:302–317. doi: 10.1111/j.1751-9004.2007.00054.x. [DOI] [Google Scholar]
- Kaptein KI, de Jonge P, van den Brink RHS, Korf J. Course of depressive symptoms after myocardial infarction and cardiac prognosis: A latent class analysis. Psychosomatic Medicine. 2006;68:662–668. doi: 10.1097/01.psy.0000233237.79085.57. [DOI] [PubMed] [Google Scholar]
- Karlson BW, Währbogrm P, Sjöland H, Lindqvist J, Herlitz J. Impact of a chest pain clinic on recurrency of symptoms and readmissions among patients early discharged from hospital after acute myocardial infarction was ruled out. European Journal of Emergency Medicine. 1998;5:29–36. [PubMed] [Google Scholar]
- Kim HM, Kim J, Hwang SY. Health-related quality of life in symptomatic postmyocardial infarction patients with left ventricular dysfunction. Asian Nursing Research. 2015;9:47–52. doi: 10.1016/j.anr.2014.11.004. [DOI] [PubMed] [Google Scholar]
- Lane D, Carroll D, Ring C, Beevers DG, Lip GYH. The prevalence and persistence of depression and anxiety following myocardial infarction. British Journal of Health Psychology. 2002;7:11–21. doi: 10.1348/135910702169321. [DOI] [PubMed] [Google Scholar]
- LeClerc CM, Wells DL, Craig D, Wilson JL. Falling short of the mark: Tales of life after hospital discharge. Clinical Nursing Research. 2002;11:242–263. doi: 10.1177/10573802011003002. [DOI] [PubMed] [Google Scholar]
- Lenz ER, Pugh LC, Milligan RA, Gift A, Suppe F. The middle-range theory of unpleasant symptoms: An update. Advances in Nursing Science. 1997;19:14–27. doi: 10.1097/00012272-199703000-00003. [DOI] [PubMed] [Google Scholar]
- Martens EJ, Smith ORF, Winter J, Denollet J, Pedersen SS. Cardiac history, prior depression and personality predict course of depressive symptoms after myocardial infarction. Psychological Medicine. 2008;38:257–264. doi: 10.1017/S0033291707001377. [DOI] [PubMed] [Google Scholar]
- McSweeney JC, O’Sullivan P, Cody M, Crane PB. Development of the McSweeney Acute and Prodromal Myocardial Infarction Symptom Survey. Journal of Cardiovascular Nursing. 2004;19:58–67. doi: 10.1097/00005082-200401000-00010. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, … Turner MB. Heart disease and stroke statistics—2015 update: A report from the American Heart Association. Circulation. 2015;131:e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- Murphy B, Ludeman D, Elliott P, Judd F, Humphreys J, Edington J, … Worcester M. Red flags for persistent or worsening anxiety and depression after an acute cardiac event: A 6-month longitudinal study in regional and rural Australia. European Journal of Preventive Cardiology. 2014;21:1079–1089. doi: 10.1177/2047487313493058. [DOI] [PubMed] [Google Scholar]
- Muthén B. Growth mixture modeling and related techniques for longitudinal data. In: Kaplan D, editor. The SAGE Handbook of quantitative methodology for the social sciences. Thousand Oaks, CA: Sage; 2004. pp. 345–368. [Google Scholar]
- Nagin DS, Odgers CL. Group-based trajectory modeling (nearly) two decades later. Journal of Quantitative Criminology. 2010;26:445–453. doi: 10.1007/s10940-010-9113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor MD, Aiken LH, Kurtzman ET, Olds DM, Hirschman KB. The importance of transitional care in achieving health reform. Health Affairs. 2011;30:746–754. doi: 10.1377/hlthaff.2011.0041. [DOI] [PubMed] [Google Scholar]
- Nylund KL, Asparouhov T, Muthén BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Structural Equation Modeling. 2007;14:535–569. doi: 10.1080/10705510701575396. [DOI] [Google Scholar]
- Rumsfeld JS, Alexander KP, Goff DC, Graham MM, Ho PM, Masoudi FA, … Stroke Council. Cardiovascular health: The importance of measuring patient-reported health status: A scientific statement from the American Heart Association. Circulation. 2013;127:2233–2249. doi: 10.1161/CIR.0b013e3182949a2e. [DOI] [PubMed] [Google Scholar]
- Smith ORF, Kupper N, Denollet J, de Jonge P. Vital exhaustion and cardiovascular prognosis in myocardial infarction and heart failure: Predictive power of different trajectories. Psychological Medicine. 2011;41:731–738. doi: 10.1017/S0033291710001133. [DOI] [PubMed] [Google Scholar]
- Tisminetzky M, Bray BC, Miozzo R, Aupont O, McLaughlin TJ. Identifying symptom profiles of depression and anxiety in patients with an acute coronary syndrome using latent class and latent transition analysis. International Journal of Psychiatry in Medicine. 2011;42:195–210. doi: 10.2190/PM.42.2.g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beek MHCT, Mingels M, Voshaar RCO, van Balkom AJLM, Lappenschaar M, Pop G, Speckens AEM. One-year follow up of cardiac anxiety after a myocardial infarction: A latent class analysis. Journal of Psychosomatic Research. 2012;73:362–368. doi: 10.1016/j.jpsychores.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Vashi AA, Fox JP, Carr BG, D’Onofrio G, Pines JM, Ross JS, Gross CP. Use of hospital-based acute care among patients recently discharged from the hospital. JAMA. 2013;309:364–371. doi: 10.1001/jama.2012.216219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermunt JK. Latent class modeling with covariates: Two improved +three-step approaches. Political Analysis. 2010;18:450–469. doi: 10.1093/pan/mpq025. [DOI] [Google Scholar]
- Wang M, Bodner TE. Growth mixture modeling: Identifying and predicting unobserved subpopulations with longitudinal data. Organizational Research Methods. 2007;10:635–656. doi: 10.1177/1094428106289397. [DOI] [Google Scholar]
- Zaslavsky O, Cochrane BB, Herting JR, Thompson HJ, Woods NF, LaCroix A. Application of person-centered analytic methodology in longitudinal research: Exemplars from the Women’s Health Initiative clinical trial data. Research in Nursing & Health. 2014;37:53–64. doi: 10.1002/nur.21575. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.