Abstract
Objectives
To review evidence regarding sensory and cognitive interactions in older adults published since 2009, the approximate date of the most recent reviews on this topic.
Design
Following an electronic database search of articles published in English since 2009 on measures of hearing and cognition or vision and cognition in older adults, a total of 437 articles were identified. Screening by title and abstract for appropriateness of topic and for articles presenting original research in peer-reviewed journals reduced the final number of articles reviewed to 34. These articles were qualitatively evaluated and synthesized with the existing knowledge base.
Results
Additional evidence has been obtained since 2009 associating declines in vision, hearing, or both with declines in cognition among older adults. The observed sensory-cognitive associations are generally stronger when more than one sensory domain is measured and when the sensory measures involve more than simple threshold sensitivity.
Conclusions
Evidence continues to accumulate supporting a link between decline in sensory function and cognitive decline in older adults.
INTRODUCTION
When the topic of cognitive effort or cognitive energy has been raised in an auditory context in recent years, it has frequently been in the context of listening effort, or the mental effort expended when listening to speech in challenging conditions. Of course, as audiologists, hearing researchers, or members of the hearing-aid industry, the focus is naturally placed on the auditory aspects of the target signal. It has long been recognized, however, that in most everyday listening situations, perception of the speech stimulus is a multi-sensory process involving at least the senses of hearing and vision (e.g., Summerfield, 1987; Massaro, 1987; Grant, 2002; Altieri et al. 2011). The relative role of these two senses in auditory-visual speech communication is known to vary with the acoustical signal-to-noise ratio such that the perceiver makes little use of visual information in quiet listening conditions, but relies heavily on this same information in acoustically challenging conditions (Sumby & Pollack, 1954; Grant et al., 2007; Altieri et al., 2011). One could think of the use of additional visual information for speech perception in acoustically challenging conditions as a built-in system to reduce listening effort and enhance recognition performance. Given the general bias toward auditory sensation in our own field, it is not too surprising that the links between sensory function and cognition have focused on hearing as well. However, if one considers the target signal to be an auditory-visual speech stimulus, then the associations between vision deficits and cognition take on added importance for those working in our field.
How do the senses interact with cognition? Schneider and Pichora-Fuller (2000) and Schneider et al. (2010), building on the hypotheses put forward by Lindenberger and Baltes (1994), reviewed four conceptual models for such sensory-cognitive interactions drawn from the literature, with a special focus on age-related changes in older adults. These conceptual models were: (1) cognition influences sensory processing; (2) a common-cause mechanism; (3) an information-degradation process; and (4) a sensory-deprivation model. As they noted, the first model garnered little support from the literature on aging. In the other three models, associations between sensory-processing deficits and cognitive function are posited, with differences among the three models attributed to aspects of the relative timing or duration of sensory and cognitive declines. In the common-cause model, for example, it is presumed that some underlying systemic change occurs in aging, such as detrimental vascular changes which, in turn, cause concomitant processing deficits in various senses as well as cognition. The sensory and cognitive losses occur essentially simultaneously when initiated by the presumed underlying common cause.
For both the information-degradation and sensory-deprivation models, a sensory-processing deficit precedes the measured cognition dysfunction. The difference between the two is basically the duration of the sensory input degradation. Information-degradation occurs as soon as the sensory input is degraded and this leads to immediate decline in measured cognitive function using stimuli applied through that sensory system. Application of masking noise, earplugs, or temporary threshold shift from noise exposure would be examples of nearly instantaneous information degradation that could negatively affect cognitive function when assessed auditorily. Of course, more slowly developing permanent hearing loss would also lead to information degradation. There are many examples, dating back at least to Rabbitt (1968), of recall for auditory stimuli being negatively affected by the addition of a masking noise, with the noise level adjusted so as not to affect the repetition of the auditory stimuli presented individually rather than in recall strings. Analogous effects have been observed for degraded vision dating back to at least Dickinson and Rabbitt (1991). As noted, age-related hearing loss (inaudibility) or vision loss would represent other possible forms of information-degradation (e.g., Rabbitt, 1991; Pichora-Fuller et al., 1995).
The auditory-deprivation model is very similar to the information-degradation model except that it is generally believed that the degradation of the sensory input must be long-term so that the brain's plasticity reallocates resources on a more permanent basis to compensate for the loss of sensory input. Auditory deprivation in human listeners was the topic of an earlier Eriksholm workshop (Arlinger et al., 1996). Often underlying neuroanatomical changes are presumed to have taken place following a prolonged period of sensory deprivation (e.g., Peele, Troiani, Grossman & Wingfield, 2011; Eckert, Cute, Vaden, Kuchinsky & Dubno, 2012; Lin et al., 2014). In principle, such neuroanatomical changes might also occur from repeated or prolonged exposures to masking noise, earplug usage, or temporary threshold shift, as well as from the development of sensorineural hearing loss. In practice, however, hearing loss is the most likely and most prevalent long-term form of auditory sensory deprivation. Although the focus in these examples for each model has been placed on the auditory system, historically the visual system has been the sensory system studied in the research leading to the development of each of these models of sensory-cognitive interactions in older adults.
Models of sensory-cognitive interactions have been of special interest to those studying age-related changes in older adults. It has been well established for many decades that, on average, older adults experience declines in sensory function [e.g., ISO (2000) for hearing; Owsley et al. (1983) for vision], with signal detection being the most frequently studied phenomenon. Further, older adults also experience declines in processing-based measures of cognitive function [See review by Salthouse (2010)]. As a result, it is probably only natural to wonder how these parallel declines in ability with advancing age might be related. Of course, the population of older adults is also of particular interest to audiologists and the hearing-aid industry because at least 2/3 of hearing aids produced are provided to adults over 60 years of age (Skafte, 2000; Strom, 2006).
MATERIALS AND METHODS
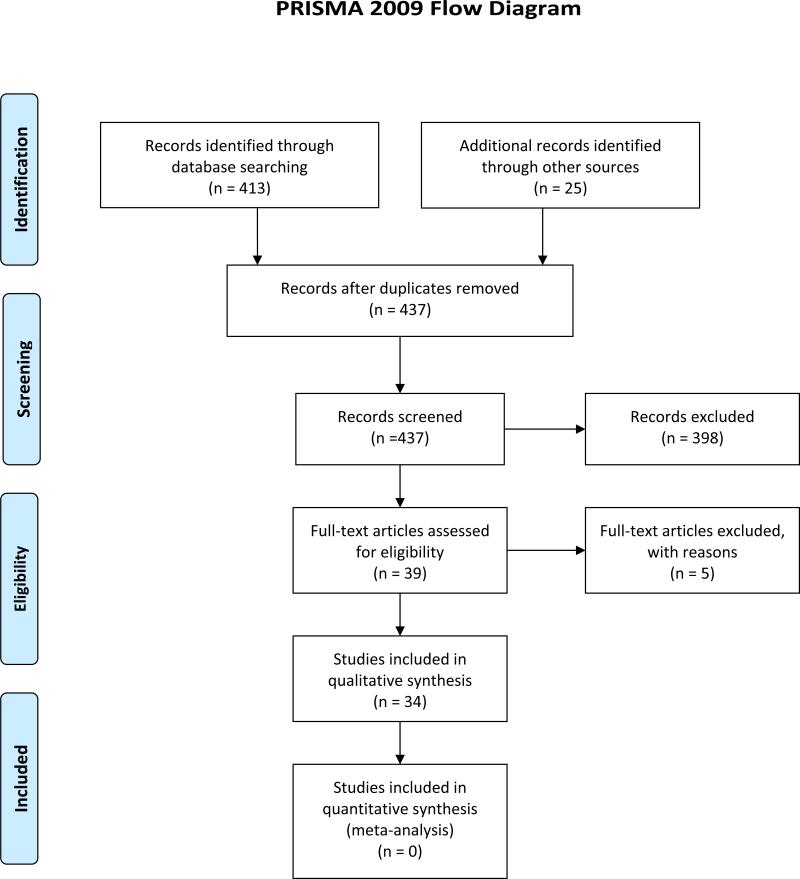
As noted, reviews of the sensory-cognitive interactions, with a special focus on the auditory system, have been provided previously by Schneider and Pichora-Fuller (2000) and Schneider et al. (2010) with others including additional sensory systems (Anstey et al., 2008) or special emphases on such interactions in cognitive impairments, such as Alzheimer's Disease (Albers et al., 2015). As an update to these recent reviews, a literature search was conducted via Medline using keywords of “aging+hearing+cognition” or “aging+vision+cognition”. Given the workshop focus on listening effort, especially as it pertains to speech perception, the focus of the literature search was on the two senses central to speech perception: hearing and vision. As shown in Figure 1, a total of 413 articles were originally identified through this database search that were published in English since 2009. In addition, another 25 articles on these topics were identified from other recently published reviews resulting in a total of 438 articles. One of these proved to be a duplicate and was removed. The titles and abstracts of the remaining 437 articles were screened by the authors. Screening by title and abstract for appropriateness of topic and for articles presenting original research in peer-reviewed journals reduced the number of articles reviewed to 39. Review of the full articles resulted in five articles being excluded due to being on an incorrect topic. The remaining 34 articles on aging, hearing or vision and cognition were the focus of this review. (The citations for these 35 articles have been marked with an asterisk in the reference list.) Although the focus here is on hearing and vision, driven primarily by the importance of these two senses to the speech-perception process, it should be noted that age-related declines in olfaction have also been identified as potential markers for age-related decline in cognition (e.g., Mesholam et al., 1998; Graves et al., 1999; Kovacs, 2004; Devanand et al., 2010; Wang et al., 2010). Moreover, age-related changes to motor systems, as well as sensory systems, have been conjectured to precede the development of Alzheimer's Disease (Albers et al., 2015). Here, however, the focus is on recent studies of age-related changes in vision or hearing in relation to age-related changes in cognition in otherwise healthy older adults. This focus for this workshop appears appropriate given the critical roles of hearing, vision and cognition in speech perception, including the effort required to achieve a desired level of speech-perception performance.
Figure 1.
PRISMA (Moher et al., 2009) flow diagram for electronic literature search for this review.
RESULTS AND DISCUSSION
Most of the 34 research articles identified through this search could be grouped into various topical clusters, such as vision and cognition, hearing and cognition, and so on. As a consequence, the results are presented and discussed here as clusters of papers on specific subtopics of the general theme of sensory-cognitive interactions.
Vision and Cognition
For vision, two smaller-scale laboratory-based research studies were conducted since 2009 and examined the effect of poor visual acuity on cognitive function (Jefferis et al., 2012; Killen et al., 2013). In both cases, the Mini Mental Status Exam (MMSE; Folstein et al., 1975), a screening test for dementia, was the cognitive measure of interest, although Killen et al. (2013) also examined cognitive tests that did not depend on vision. In both studies, poor visual acuity was related to poor performance on the MMSE, especially on the visual items of the MMSE (Jefferis et al., 2012). No effect of poor visual acuity was observed for either the non-visual items of the MMSE (Jefferis et al., 2012) or the vision-independent cognitive measures used by Killen et al. (2013). Thus, when the visual information is degraded by poor visual acuity, performance on the visual measures of cognitive function suffers (See Phillips, this issue, pp. XXXX). Some forms of visual acuity deficits, such as refractory problems, can be remedied, but others, such as those resulting from macular degeneration or retinopathy are less easily remedied. In such cases, it is important to include non-visual measures of cognitive function and to also recognize that the peripheral visual deficit may limit performance on a variety of everyday cognitive tasks making use of visual stimuli (e.g., memory for to-do lists, written directions, written instructions), as well as the processing of the visual information in speech.
Two recent large-scale studies of older adults found, as in prior studies, an association between simple measures of visual acuity and cognitive function (Clay et al., 2009; Ong et al., 2012). Clay et al. (2009) studied 842 older adults and found that the well-established associations between age and memory span, as well as age and fluid intelligence, were mediated by a combination of visual acuity and processing speed. Ong et al. (2012), in a study of 1,179 60-80 year olds, found higher percentages of cognitive impairment, measured with a 10-item MMSE-like screener, among those with poor visual acuity and eye health (retinopathy).
Hearing and Cognition
With regard to recent auditory studies of older adults, several smaller-scale studies have focused on the effect of the presence of age-related hearing loss, or compensatory procedures to accomodate such loss, on the performance of auditory cognitive tasks (Heinrich & Schneider, 2011; Verhaegen et al. 2014; Dupuis et al., 2015). Heinrich and Schneider (2011) examined the effect of the use of different stimulus presentation levels on paired-associate memory performance for word pairs presented auditorily. To compensate for the presence of age-related hearing loss, one might choose to adjust the presentation level based on the severity of an older individual's hearing loss. Doing so, however, runs the risk of further degrading the input due to level-dependent forms of sensory degradation, such as broader tuning or greater masking effects (e.g., Studebaker et al, 1999; Dubno, Horwitz & Ahlstrom, 2006). As Heinrich and Schneider (2011) demonstrated, use of higher presentation levels also reduces the measured memory performance.
Verhaegen et al. (2014) studied three groups, each comprised of 16 adults: young with normal hearing, young with impaired hearing, and older with impaired hearing. The hearing loss of the two groups with impaired hearing was matched from 500-2000 Hz (but not at the higher frequencies). The authors demonstrated that the performance of the two groups with impaired hearing was reduced, relative to that of the young group with normal hearing, on auditory measures of verbal short-term memory. Had the young group with matched peripheral hearing loss not been included, one might have concluded that aging leads to poor auditory-based verbal short-term memory.
Finally, Dupuis et al. (2015) demonstrated, in a sample of 301 older adults, that poor visual (and hearing) acuity led to reduced performance on the Montreal Cognitive Assessment, a widely used cognitive screener. This was true even after modifying the cognitive scores based on the sensory deficits. At a minimum, the foregoing summary of recent research suggests that the clinician and the researcher must be cognizant of sensory loss when assessing cognitive function, especially for stimuli in the same sensory modality used for stimulus presentation in the cognitive tasks (see also Phillips, this issue, pp.XXXX)
The presence of hearing loss, in many older adults, likely degrades the auditory input used frequently to assess cognitive function. This is consistent with the information-degradation model described previously. Another recent study examined the effect of such degradation in more detail. Piquado et al. (2010) studied the recall of a list of words in which one particular word in the list was masked by noise. They demonstrated that the introduction of the masking noise not only reduced recall accuracy for the masked word, but also words in the list that immediately preceded the masked word. They suggested that the decline in subsequent recall performance for the words preceding the masked word reflects the effects of the increased cognitive effort needed for the recognition of the degraded word.
Over the past five years, there have been numerous reports of epidemiological studies examining the association between hearing loss and cognitive function in older adults. These were likely precipitated by an impressive series of studies published by Frank Lin and colleagues (Lin et al., 2011a, 2011b, 2011c, 2013). In each of these large-scale studies, a significant association was observed between measured hearing loss and cognitive function. The association was observed even when controlling for a number of potential confounding variables. This was true, moreover, for a wide range of cognitive measures used across the various studies, including many visually based measures of cognition. In addition, the same general pattern emerged in both cross-sectional and longitudinal datasets. Across the series of published analyses, datasets ranged in size from several hundred to several thousand older adults. It should be kept in mind, however, that because of the large samples employed, relatively small correlations achieve statistical significance. When individual correlations were reported, for example, they were often in the range of 0.1 < r < 0.2, indicating that 1-4% of the variance in cognitive function was explained by hearing loss. Subsequent reports from similar epidemiological studies in the U.S. (Surprenant & DiDonato, 2014; Bush et al., 2015) and Australia (Kiely et al., 2012) have observed comparable associations between hearing loss and cognition.
Ronnberg et al. (2014) analyzed data from perhaps the largest dataset addressing the association between hearing loss and cognitive function. These investigators analyzed the data from 138,098 individuals in the UK BioBank dataset. It should be noted, however, that the measure of hearing loss used here was one based on the digit-triplet test, which is a hearing screening test based on a speech-in-noise threshold, rather than the more conventional pure-tone-audiometry measure of hearing loss. Nonetheless, when groups were formed on the basis of their performance on the digit-triplet task, those with poorer performance also performed worse on two measures of visuospatial memory (a short-term working-memory and a long-term episodic-memory task). Interestingly, for those with poor hearing ability, those who reported using hearing aids did better than those without hearing aids on the visuospatial short-term working-memory task (but not the long-term episodic-memory task).
Hearing, Vision and Cognition
Although some recent studies also addressed dual-sensory loss, exclusively auditory and visual loss of sensitivity, the focus of two such studies was only tangentially related to cognitive function (Heyl & Wahl, 2012; Kiely et al., 2013). As noted previously, however, Dupuis et al. (2015) looked at the effect of uni-sensory and dual sensory loss on a cognitive screening test, the Montreal Cognitive Assessment (See also Phillips, this issue, pp. XXXX).
There were, however, two Swedish longitudinal studies which included auditory, visual and cognitive measures obtained from older adults. Sternang et al. (2010) reported the results from 1,057 45-90 year-old participants in a longitudinal study. They observed significant associations between hearing loss, visual acuity, and memory, but the associations were generally weaker than many prior correlations reported from cross-sectional studies. The second Swedish study, Ronnberg et al. (2011), drew data from the same longitudinal dataset, but focused on the data from 160 older adults wearing hearing aids tested at one of the longitudinal measurement intervals. Hearing loss, but not visual acuity, was negatively correlated (r ~0.40-0.45) with long-term memory, but not short-term memory, in this group of older adults wearing hearing aids. The mean duration of hearing aid use was about two years for these participants. Comparing the results across Ronnberg et al. (2014) and Ronnberg et al. (2011), a tentative conclusion would be that hearing loss has negative impact on both short-term and long-term memory (importantly, when measured with visual or visuospatial tasks) and intervention with hearing aids reduces or eliminates the linkage of hearing loss to short-term memory performance.
The foregoing brief review of recent studies of sensory-cognition interactions in older adults generally supports the earlier literature in providing supporting evidence for associations between sensory decline and cognitive decline in older adults. Because most of the studies reported were cross-sectional designs, had restricted measures of sensory function, cognitive function, or both, the evidence accumulated from these studies doesn't argue convincingly in favor of one model of sensory-cognitive interaction over another. That is, the recent evidence reviewed could be used to support the sensory-deprivation, information-degradation, or common-cause models in that it just confirms a link between sensory function and cognitive function in older adults. The recent literature has added greatly to the support of a link between declines in sensory and cognitive processing with advancing age, but the exact nature of that linkage remains elusive.
There are also implications for speech perception and the use of hearing aids. Almost by definition, older adults provided with hearing aids have had long-standing hearing loss. Many will also have visual impairments of varying type and degree. The possible linkage of these acuity deficits in hearing and vision with cognitive function should make the clinician aware of possible cognitive-processing deficits that are greater than those experienced by older adults of the same age without such deficits in sensory acuity. Of course, because speech is a complex auditory-visual stimulus, the higher-level processing of that stimulus may also be impoverished.
Including Measures other than Simple Threshold Acuity
Historically, the vast majority of studies of the linkages between sensory function and cognition in older adults have focused exclusively on measures of threshold sensitivity. Although the work of Gates and colleagues (Gates et al., 2008, 2010) might be considered to be an exception, the primary measures of auditory function in these studies were various forms of degraded speech perception. Given the key cognitive contributions to degraded speech perception, it is not too surprising to find a link between performance on such measures and cognition. In that regard, the measures of the perception of degraded speech are not likely to be considered “pure” measures of sensory processing.
Another exception to this can be found in a series of recent reports from our research group (Humes et al., 2009; Craig et al., 2010; Fogerty et al., 2010; Humes et al., 2010; Busey et al., 2010; Humes et al., 2013). Across this series of studies, rigorous criterion-free psychophysical measures of threshold sensitivity and temporal processing were obtained from samples of 150-250 young, middle-age, and older adults in hearing, vision and touch in each study, with the exact sample sizes for each age group varying somewhat from study to study. Measures of temporal processing included temporal acuity (i.e., gap detection in hearing and touch, flicker fusion in vision), temporal-order identification, and temporal masking (forward and backward masking of identification performance). Briefly, aging had a significant negative impact on the vast majority of psychophysical measures in all three senses (Humes et al., 2013). Performance on the forty psychophysical tasks was captured well by eight underlying sensory factors, with some weak to moderate correlations among the set of eight sensory factors. When the common variance across the eight sensory factors was captured by a single higher-order factor, global sensory processing, this factor appeared to mediate the well-known correlation between age and cognition. This is, of course, in general agreement with the various models of sensory-cognitive interaction in aging described previously. Here, however, an interesting twist was that global sensory processing appeared to mediate the link between age and cognition not just for older adults, but for young and middle-aged adults as well (Humes et al., 2013; Humes, 2015). That is, this linkage between sensory-processing, as measured in multiple senses and across multiple tasks, and cognition was independent of age.
Although our earlier study had obtained parallel measures of threshold sensitivity and temporal processing in hearing, vision, and touch, given the focus here on auditory and visual processing alone, we wondered whether the measures of threshold sensitivity and temporal processing for touch were needed for accurate prediction of cognitive function in our earlier study. Briefly, the accuracy of the predictions for cognitive function were found to be identical with or without the tactile measures and the same pattern of correlations and partial correlations was observed among age, sensory processing, and cognitive function as reported in Humes et al. (2013). Specifically, when sensory processing in hearing and vision was partialled out, the correlation between age and cognition declined from a moderate and significant value (r = −0.55) to a non-significant zero correlation (r = −0.08). A strong correlation (r=0.70) between sensory processing and cognition was maintained, however, whether or not one controlled for age. When only auditory or visual measures were considered, however, the amount of variance explained in the cognitive measures was lower (total variance accounted for dropped from about 70% to 56%).
It was also clear from the analyses presented in Humes et al. (2013) that many of the temporal-processing measures within each sense were redundant. For example, the auditory temporal-order measures were strongly correlated with the auditory temporal-masking measures and likewise for the visual measures. Elimination of the tactile measures, as well as all the temporal-masking measures (tactile, auditory, and visual), pared the set of auditory and visual sensory-processing measures down to 16 variables. When these variables were analyzed with principal-components factor analysis for the 195 middle-aged and older adults in the dataset from Humes et al. (2013), five sensory-processing factors emerged (accounting for 72.1% of the total variance): (1) visual temporal order; (2) visual flicker fusion (a temporal-resolution task); (3) auditory temporal order; (4) auditory threshold; and (5) auditory gap detection. These five (orthogonal) sensory-processing factors, together with educational level and age, a total of seven predictors, were then used to predict cognitive function (a global cognitive-processing factor from 15 subtests of the Wechsler Adult Intelligence Scale, WAIS-III). A total of 56.2% of the variance could be explained by 6 of the 7 predictor variables. The results from this step-wise multiple-regression analysis are summarized in Table 1. Note that, as expected, age and education level explain significant portions of the variance, accounting for 18% of the variance in combination, but that sensory-processing factors, especially measures of temporal processing in hearing and vision, account for an additional 38.2% of the variance in cognitive function in these 195 middle-aged and older adults.
Table 1.
Results of regression analyses using only auditory and visual sensory measures, together with education level and age, to predict the global cognitive processing factor score for the older adults from Humes et al. (2013). Step-wise regression was used and the significant predictors are listed in the left column. The middle column shows the percentage of total variance accounted for by each of those significant predictors and the standardized Beta coefficient in the subsequent regression equation is provided in the right column.
| Predictor Variable | % Variance Accounted For | Standardized Beta |
|---|---|---|
| Visual Temporal Order | 24.9 | −0.12 |
| Education Level | 14.7 | 0.32 |
| Auditory Temporal Order | 7.7 | −0.23 |
| Auditory Gap Detection | 4.3 | −0.18 |
| Age | 3.3 | −0.23 |
| Visual Flicker Fusion | 1.3 | −0.13 |
Figure 2 plots the global cognitive-processing factor score from the WAIS-III for subgroups of these same 195 middle-aged and older adults from Humes et al. (2013). The subgroups were formed on the basis of the number of the five sensory-processing factor scores that were abnormal (> 1 SD worse than mean). Two cognitive-processing factor scores are shown in this figure; an uncorrected score and one that has been corrected for the effects of age and education level. In both cases, the same trend emerges. The greater the number of sensory processes that were abnormal, the lower the global cognitive-processing performance. For both the corrected and uncorrected cognitive factor scores, the subgroups with only one or fewer abnormal sensory-processing factor scores had significantly higher cognitive scores than those with two or more abnormal sensory-processing scores. This confirms the importance of testing multiple senses and sampling multiple processes in a battery of sensory-processing measures if one wishes to obtain the best possible accounting of individual differences in cognitive function among middle-aged and older adults. It could be important to assess multiple senses and multiple processes because the link to cognition is via modality-general, rather than modality-specific, processes (e.g., Daneman & Carpenter, 1980; Just & Carpenter, 1992; Kane et al., 2004). In this sense, impact of aging on multiple senses could be thought of as either multiple degradations of input to modality-general processes or more robust indications of the failure of modality-general processes. On the other hand, in a common-cause hypothesis, each additional sensory system or process impacted could indicate more widespread sensory and cortical declines. Further research is needed to both confirm these findings and to further explain them.
Figure 2.
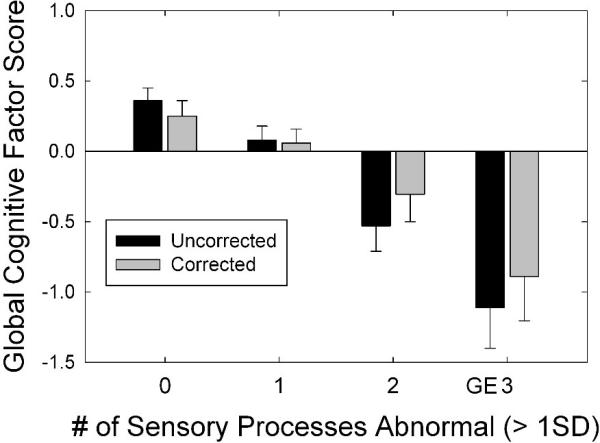
The Global Cognitive Factor Score plotted as a function of the number of sensory measures that were “abnormal” (by more than on standard deviation from the mean) from the reanalysis of auditory and visual data alone obtained from older adults by Humes et al. (2013). Bars represent mean factor scores and the associated error bars depict 1 standard error.
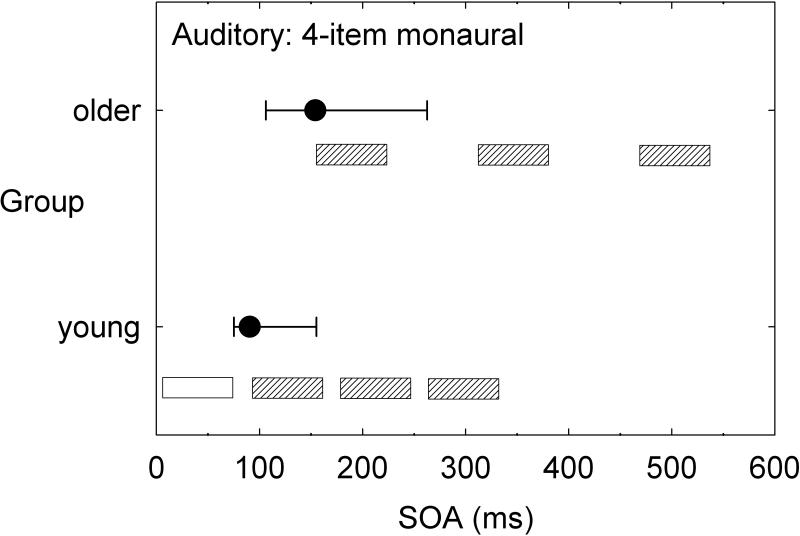
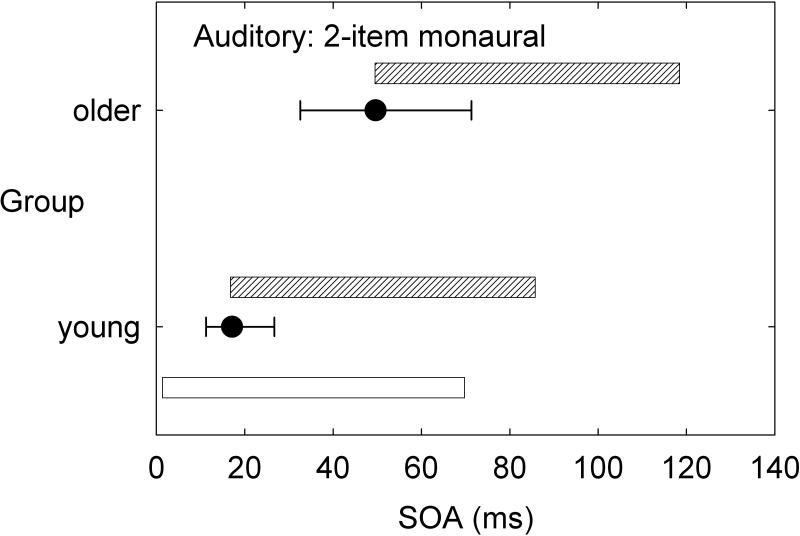
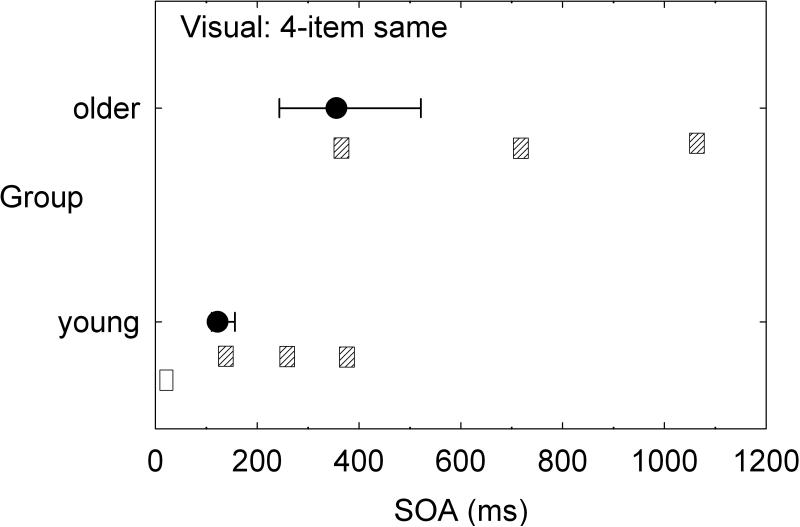
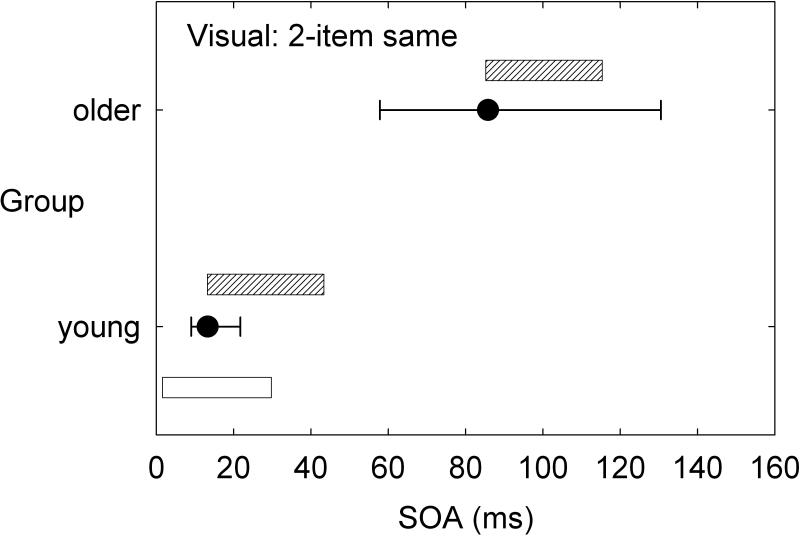
Although speech perception was not assessed in detail in the aforementioned series of multi-sensory studies by our research group, there would appear to be strong implications for the processing of speech. To illustrate, consider just two tasks: two-item same-location (ear) temporal-order identification and four-item same-location (ear) temporal-order identification. Figure 3 and Figure 4 provide the group data from the dataset of Humes et al. (2013) for the young (N=53) and older (N=141) adults for the auditory and visual modalities, respectively. These data make use of the dataset from Humes et al. (2013), but were not analyzed in this fashion previously. In each panel, the median (filled circle) and interquartile range (error bars) are provided for the measured stimulus onset asynchrony (SOA) yielding 50% correct performance. To put these SOA values in perspective, relative to the duration of the stimuli used, the unfilled and cross-hatched horizontal bars in each panel illustrate the temporal arrangement of the stimulus sequence at the median threshold for each group. The unfilled horizontal bar is the initial stimulus and starts at 0 ms, with a duration of 40 ms in hearing and 30 ms in vision. The same pattern of results appears in both hearing (Figure 3) and vision (Figure 4) when results are compared across tasks and groups. Consider first the two-item temporal-order tasks in the top panel of each figure. Whereas young adults can achieve 50% accuracy with two vowels or two letters that physically overlap in time by about 50% of the stimulus duration, older adults require a physical separation between the two stimuli to achieve the same level of performance. When the task is made more difficult by stringing together four stimuli in each sequence (lower panel in each figure), both groups require much more temporal separation between each stimulus in the sequence to achieve the same 50% accuracy. Notice that the young adults have processed the full four-item sequence in the same amount of time that the older adults have only processed one (vision, Figure 4) or two (hearing, Figure 3) of the stimuli in the four-item sequence. Natural speech is comprised of fast and complex temporal sequences of (correlated) auditory and visual stimuli. Relative to young adults, it would appear that older adults would lag well behind in the online processing of running speech, for both the auditory and the visual information. This has been supported by the work of Piquado et al. (2012) which demonstrated age-related recall differences for narratives when all subjects had to process the narratives continuously, but no age-group differences when the speech input was self-paced. Further, if aging introduces a differential lag in auditory and visual processing of sequential stimuli, this could negatively impact the integration of information across both senses and reduce the correlation between them expected for speechreading.
Figure 3.
Medians (filled circles) and interquartile ranges (error bars) for the measured stimulus onset asynchrony (SOA) obtained from young and older adults on the auditory two-item (bottom) and four-item (top) temporal-order identification tasks [data from Humes et al. (2013)]. Results are shown relative to the temporal parameters of the stimulus sequence with the white rectangle indicating the initial stimulus in the sequence for both subject groups and tasks and the grey rectangles representing the location of the subsequent stimuli in the sequence based on the median SOAs measured for each task and group.
Figure 4.
Medians (filled circles) and interquartile ranges (error bars) for the measured stimulus onset asynchrony (SOA) obtained from young and older adults on the visual two-item (bottom) and four-item (top) temporal-order identification tasks [data from Humes et al. (2013)]. Results are shown relative to the temporal parameters of the stimulus sequence with the white rectangle indicating the initial stimulus in the sequence for both subject groups and tasks and the grey rectangles representing the location of the subsequent stimuli in the sequence based on the median SOAs measured for each task and group.
Sensory Function, Cognition and Speech Understanding
The focus of another recently completed study from our research group was on individual differences in the understanding of amplified speech by the 98 older adults (Humes et al. 2013). The main findings that emerged are summarized briefly here as follows. First, using the procedures and tasks in this study, it was possible to obtain reliable estimates of performance from older adults on measures of non-speech auditory perception, visually based cognitive-linguistic processing, and speech understanding. Second, as a group, the older adults in this study were outperformed by the group of young adults on about 25% of the measures. About half the time, however, these differences were in the cognitive domain and seldom were age-group differences observed in aided speech-understanding. The latter observation is likely due to the use of optimal spectral shaping in this study to minimize the influence of stimulus inaudibility on speech-understanding performance. This suggests, however, that neither the differences in age nor the presence of cochlear pathology between the young and older adults were critical for recognizing or identifying the spectrally shaped speech stimuli. That is, when the audibility of speech was fully restored, the older adults with varying degrees of underlying cochlear pathology had speech-understanding performance comparable to that of young adults with no cochlear pathology. Third, individual differences in aided speech-understanding performance among the 98 older adults were well explained (accounting for 55-60% of the total variance) by 5-6 predictor variables included in this study, with significant and roughly equal contributions from visually based measures of cognitive-linguistic processing, non-speech auditory measures, and age. Of course, the critical importance of cognitive-linguistic factors to the perception of amplified speech underscores the likely importance of cognitive energy and listening effort to everyday speech understanding in older adults. As noted previously, the importance of cognitive factors in the prediction of aided speech-in-noise performance has been a common finding in recent years [see reviews by Houtgast & Festen (2008), Akeroyd (2008), and Humes & Dubno (2010)].
After completion of this project (Humes et al. 2013), it occurred to us that these same data could offer some insights about the association between performance in multiple senses and cognition in another relatively large (N=98) sample of older adults. That is, rather than use the various predictor measures, including a measure of cognitive-linguistic speed of processing and a battery of working-memory measures, to predict speech-understanding in competition, perhaps speech-understanding measures, as well as several of the other non-cognitive predictor measures, could be used to predict cognitive function. There were three separate and weak-to-moderately correlated (0.19 < r < 0.44) measures of cognitive function included in the study by Humes et al. (2013): (1) the Mini-Mental Status Exam (MMSE); (2) a computer-based battery of three common working-memory tests (Lewandowsky et al., 2010); and (3) A Quick Test of cognitive processing (AQT; Wiig et al. 2002). The AQT was designed to tap several abilities including verbal-processing speed, automaticity of naming, working memory, and the ability to shift attention between dimensions of multidimensional visual stimuli. It has been used to screen for dementia in older adults as well. With each of these three cognitive measures as separate dependent variables, a set of nine potential predictors were evaluated: five factor scores from the factor analysis of the non-speech psychophysical measures, age, two factor scores from the factor analysis of the large set of speech-understanding measures, and the text-recognition threshold (TRT; Zekveld et al., 2007). The latter is a visual sentence-recognition task using the visual presentation of the text of a sentence on the computer screen while varying the width of black vertical bars obscuring various portions of the text. For each of the three multiple-regression analyses (one each for the MMSE, AQT, and Working-Memory tests), 26.4-31.5% of the variance in the cognitive dependent variable was explained by two or three predictor variables. Moreover, one predictor that always emerged was the visually based TRT and another was always an auditory measure, although not always the same one (a measure of temporal processing in two cases and speech-in-noise in the other case). Thus, this pattern of findings confirms the importance of including performance measures from multiple senses, in this case auditory and visual measures, when predicting cognitive function in older adults. Also, in at least two of the three analyses, this pattern of findings supports the importance of auditory temporal processing as a predictor of cognitive function in older adults. It is important to note, moreover, that hearing threshold was one of the potential predictors in each regression analysis and this factor was never identified as a significant predictor (and this was not due to collinearity of hearing loss with another predictor variable). In summary, although the individual-differences study by Humes et al. (2013) was designed initially to gain a better understanding of the sensory and cognitive factors that might explain individual differences in aided speech understanding in a group of 98 older adults, the dataset also sheds light on the association of sensory and speech-understanding measures with cognition. The pattern of findings in this separate sample of older adults is in good agreement with the findings from our larger scale study of sensory processing in hearing, vision, and touch in older adults. In particular, declines in more than one sense and for more complex stimuli and tasks predict declines in cognitive function among older adults.
Humes et al. (2013) were certainly not the only ones to explore the sensory and cognitive factors underlying speech-understanding difficulties of older adults since 2009. Benichov et al. (2012), in a study of 53 adults aged 19-89 years, found that pure-tone hearing loss, age, and cognitive function were all significant predictors of word-recognition performance, but that the relative roles of these factors varied with the predictive context of the preceding portion of the sentence. In particular, in the three lowest predictability contexts, audiometric hearing loss was a significant contributor, but not at the highest level of predictability. Cognition and age explained significant amounts of variance in word-recognition performance at all levels of predictability.
Glyde et al. (2013) studied spatial benefit for speech-in-noise perception in 80 listeners ranging in age from 7-89 years. Neither age nor cognition was related to spatial benefit for speech-in-noise perception. However, the spatial benefit declined as hearing loss increased, despite the use of appropriately amplified speech for those listeners with impaired hearing. In another recent study, Moore et al. (2014), in a sample of 40,655 40-69 year-old adults from the UK BioBank, found that digit-triplet recognition in noise was independently and negatively related to both age and cognitive function. Finally, Fullgrabe, Moore and Stone (2015) measured auditory temporal processing, cognition, and speech perception in noise in 20 older adults and 9 young adults with normal hearing. The best predictions of speech-perception in noise were found for a combination of temporal-processing measures, particularly measures of temporal fine structure and cognition.
CONCLUSIONS
A review of over thirty recently published peer-reviewed articles provides additional evidence associating declines in vision, hearing, or both with declines in cognition among older adults. The observed associations were generally stronger when more than one sensory domain was measured and when the sensory measures involved more than simple threshold sensitivity. This observation could be due to underlying common mechanisms or neuroanatomical sites impacted across these senses, but could also simply be due to higher-level sensory-processing measures that draw more on cognitive resources to perform the more complex tasks. More research is needed to further explore these possibilities. Evidence continues to accumulate supporting a link between decline in sensory function and cognitive decline in older adults, but more research is needed, especially longitudinal studies, to better determine the nature of the link. Declines in both sensory processing and cognition result in declines in speech understanding among older adults, even for amplified speech. Further, such declines in sensory processing and cognition, regardless of whether these functions are causally linked, will ultimately increase the demands placed on the listener's processing resources. As a result, listening effort is likely to increase to achieve the level of performance desired by the listener. Of course, this is true for sensory loss, independent of cognitive decline, as well as cognitive decline, independent of sensory loss. To the extent that sensory and cognitive declines are linked, however, one would predict that even more processing resources would be needed to compensate for such combined declines to achieve the desired performance level.
Short Summary.
This paper reviews the current state of knowledge regarding interactions among hearing, vision and cognition in older adults. Evidence continues to accumulate supporting a link between decline in sensory function and cognitive decline in older adults.
ACKNOWLEDGEMENTS
L.E.H. coordinated the electronic literature search performed by L.A.Y. and wrote the paper. Both authors screened and analyzed, independently, the articles identified at each stage and then discussed the results. L.A.Y. also took primary responsibility for the compilation and accuracy of the references. Finally, this work was supported, in part, by research grant R01 AG008293-21 from the National Institute on Aging.
Footnotes
Conflicts of Interest and Source of Funding:
No conflicts
References
(*=articles among 34 identified through online literature search)
Additional References (among 34 identified in online search, but not reviewed further in article due to not aligning with subtopic clusters):
- Akeroyd MA. Are individual differences in speech reception related to individual differences in cognitive ability? A survey of twenty experimental studies with normal and hearing-impaired adults. Int J Audiol. 2008;47(sup2):S53–S71. doi: 10.1080/14992020802301142. [DOI] [PubMed] [Google Scholar]
- *.Albers MW, Gilmore GC, Kaye J, et al. At the interface of sensory and motor dysfunctions and Alzheimer's disease. Alzheimers Dement. 2015;11:70–98. doi: 10.1016/j.jalz.2014.04.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altieri N, Pisoni DB, Townsend JT. Some behavioral and neurobiological constraints on theories of audiovisual speech integration: a review and suggestions for new directions. Seeing Perceiving. 2011;24:513–539. doi: 10.1163/187847611X595864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstey KJ, Lipnicki DM, Low LF. Cholesterol as a risk factor for dementia and cognitive decline: a systematic review of prospective studies with meta-analysis. Am J Geriatr Psychiatry. 2008;16:343–354. doi: 10.1097/JGP.0b013e31816b72d4. [DOI] [PubMed] [Google Scholar]
- Arlinger S, Gatehouse S, Bentler RA, Byrne D, Cox RM, et al. Report of the Eriksholm Workshop on auditory deprivation and acclimatization. Ear Hear. 1996;17:87S–98S. doi: 10.1097/00003446-199617031-00009. [DOI] [PubMed] [Google Scholar]
- *.Benichov J, Cox LC, Tun PA, et al. Word recognition within a linguistic context: Effects of age, hearing acuity, verbal ability and cognitive function. Ear Hear. 2012;32:250. doi: 10.1097/AUD.0b013e31822f680f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Busey T, Craig J, Clark C, et al. Age-related changes in visual temporal order judgment performance: Relation to sensory and cognitive capacities. Vision Res. 2010;50:1628–1640. doi: 10.1016/j.visres.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Bush AL, Lister JJ, Lin FR, et al. Peripheral hearing and cognition: evidence from the Staying Keen in Later Life (SKILL) Study. Ear Hear. 2015 Jan 13; doi: 10.1097/AUD.0000000000000142. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Clay OJ, Edwards JD, Ross LA, et al. Visual function and cognitive speed of processing mediate age-related decline in memory span and fluid intelligence. J Aging Health. 2009;21:547–566. doi: 10.1177/0898264309333326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig JC, Rhodes R, Busey TA, et al. Aging and tactile temporal order. Atten Percept Psychophys. 2010;72:226–235. doi: 10.3758/APP.72.1.226. [DOI] [PubMed] [Google Scholar]
- Daneman MR, Carpenter PA. Individual differences in integrating information between and within sentences. J Exp Psychol: Learn, Mem. Cog. 1980;9:561–584. [Google Scholar]
- Devanand DP, Tabert MH, Cuasay K, et al. Olfactory identification deficits and MCI in a multi-ethnic elderly community sample. Neurobiol Aging. 2010;31:1593–1600. doi: 10.1016/j.neurobiolaging.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson CVM, Rabbitt PMA. Simulated visual impairment: Effects on text comprehension and reading speed. Clin Vis Sci. 1991;6:301–308. [Google Scholar]
- Dubno JR, Horwitz AR, Ahlstrom JB. Spectral and threshold effects on recognition of speech at higher-than-normal levels. J Acoust Soc Am. 2006;120:310–320. doi: 10.1121/1.2206508. [DOI] [PubMed] [Google Scholar]
- *.Dupuis K, Pichora-Fuller MK, Chasteen, et al. Effects of hearing and vision impairments on the Montreal Cognitive Assessment. Aging Neuropsychol Cogn. 2015;22:413–437. doi: 10.1080/13825585.2014.968084. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Cute SL, Vaden KI, Kuchinsky SE, Dubno JR. Auditory cortex signs of age-related hearing loss. J Assoc Res Otolaryngol. 2012;13:703–713. doi: 10.1007/s10162-012-0332-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogerty D, Humes LE, Kewley-Port D. Auditory temporal-order processing of vowel sequences by young and elderly listeners. J Acoust Soc Am. 2010;127:2509–2520. doi: 10.1121/1.3316291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- *.Fullgrabe C, Moore BC, Stone MA. Age-group differences in speech identification despite matched audiometrically normal hearing: Contributions from auditory temporal processing and cognition. Front Aging Neurosci. 2015;6 doi: 10.3389/fnagi.2014.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates GA, Anderson ML, Feeney MP, McCurry SM, Larson EB. Central auditory dysfunction in older persons with memory impairment or alzheimer dementia. Arch Otolaryngol Head Neck Surgery. 2008;134:771–777. doi: 10.1001/archotol.134.7.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates GA, Gibbons L, McCurry S, Crane P, Feeney MP, Larson E. Executive dysfunction and presbycusis in older persons with and without dementia. Cog Behav Neurol. 2010;23:218–23. doi: 10.1097/WNN.0b013e3181d748d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Glyde H, Cameron S, Dillon H, et al. The effects of hearing impairment and aging on spatial processing. Ear Hear. 2013;34:15–28. doi: 10.1097/AUD.0b013e3182617f94. [DOI] [PubMed] [Google Scholar]
- Grant KW. Measures of auditory-visual integration for speech understanding: a theoretical perspective (L). J Acoust Soc Am. 2002;112:30–33. doi: 10.1121/1.1482076. [DOI] [PubMed] [Google Scholar]
- Grant KW, Tufts JB, Greenberg S. Integration efficiency for speech perception within and across sensory modalities by normal-hearing and hearing impaired individuals. J Acoust Soc Am. 2007;121:1164–1176. doi: 10.1121/1.2405859. [DOI] [PubMed] [Google Scholar]
- Graves AB, Bowen JD, Rajaram L, et al. Impaired olfaction as a marker for cognitive decline Interaction with apolipoprotein E ε4 status. Neurology. 1999;53:1480. doi: 10.1212/wnl.53.7.1480. [DOI] [PubMed] [Google Scholar]
- *.Heinrich A, Schneider BA. The effect of presentation level on memory performance. Ear Hear. 2011;32:524–532. doi: 10.1097/AUD.0b013e31820a0281. [DOI] [PubMed] [Google Scholar]
- *.Heyl V, Wahl H. Managing daily life with age-related sensory loss: cognitive resources gain in importance. Psychol Aging. 2012;27:510. doi: 10.1037/a0025471. [DOI] [PubMed] [Google Scholar]
- *.Humes LE. Age-related changes in cognitive and sensory processing: Focus on middle-aged adults. Am J Audiol. 2015:1–4. doi: 10.1044/2015_AJA-14-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Humes LE, Busey TA, Craig J, et al. Are age-related changes in cognitive function driven by age-related changes in sensory processing? Atten Percept Psychophys. 2013;75:508–524. doi: 10.3758/s13414-012-0406-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes LE, Busey TA, Craig JC, et al. The effects of age on sensory thresholds and temporal gap detection in hearing, vision and touch. Atten Percept Psychophys. 2009;71:860–871. doi: 10.3758/APP.71.4.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes LE, Dubno JR. Factors affecting speech understanding in older adults. In: Gordon-Salant S, Frisina RD, Popper AN, Fay RR, editors. The Aging Auditory System. Springer; New York: 2010. pp. 211–258. [Google Scholar]
- Humes LE, Kewley-Port D, Fogerty D, et al. Measures of hearing threshold and temporal processing across the adult lifespan. Hear Res. 2010;264:30–40. doi: 10.1016/j.heares.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Humes LE, Kidd GR, Lentz JJ. Auditory and cognitive factors underlying individual differences in aided speech-understanding among older adults. Front Syst Neurosci. 2013;7:55. doi: 10.3389/fnsys.2013.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtgast T, Festen J. On the auditory and cognitive functions that may explain an individual's elevation of the speech reception threshold in noise. Int J Audiol. 2008;47:287–295. doi: 10.1080/14992020802127109. [DOI] [PubMed] [Google Scholar]
- International Standards Organization . Acoustics—Statistical Distribution of Hearing Thresholds as a Function of Age, ISO 7029. ISO; Geneva: 2000. [Google Scholar]
- *.Jefferis JM, Collerton J, Taylor J, et al. The impact of visual impairment on Mini-Mental State Examination Scores in the Newcastle 85+ study. Age Ageing. 2012;41:565–568. doi: 10.1093/ageing/afs042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Carpenter PA. A capacity theory of comprehension—individual differences in working memory. Psychol Rev. 1992;99:122–149. doi: 10.1037/0033-295x.99.1.122. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Hambrick DZ, Tuholski SW, et al. The generality of working memory capacity: a latent-variable approach to verbal and visuospatial memory span and reasoning. J Exp Psychol: Gen. 2004;133:189–217. doi: 10.1037/0096-3445.133.2.189. [DOI] [PubMed] [Google Scholar]
- *.Kiely KM, Anstey KJ, Luszcz M. Dual sensory loss and depressive symptoms: the importance of hearing, daily functioning, and activity engagement. Front Hum Neurosci. 2013;7:837. doi: 10.3389/fnhum.2013.00837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Kiely KM, Gopinath B, Mitchell P, et al. Cognitive, health, and sociodemographic predictors of longitudinal decline in hearing acuity among older adults. J Gerontol A Biol Sci Med Sci. 2012;67:997–1003. doi: 10.1093/gerona/gls066. [DOI] [PubMed] [Google Scholar]
- *.Killen A, Firbank MJ, Collerton D, et al. The assessment of cognition in visually impaired older adults. Age Ageing. 2013;42:98–102. doi: 10.1093/ageing/afs157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs T. Mechanisms of olfactory dysfunction in aging and neurodegenerative disorders. Ageing Res Rev. 2004;3:215–232. doi: 10.1016/j.arr.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Lewandowsky S, Oberauer K, Yang L-X, et al. A working memory test battery for MATLAB. Behav Res Methods. 2010;42:571–585. doi: 10.3758/BRM.42.2.571. [DOI] [PubMed] [Google Scholar]
- *.Lin FR. Hearing loss and cognition among older adults in the United States. J Gerontol A Biol Sci Med Sci. 2011;66:1131–1136. doi: 10.1093/gerona/glr115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Lin FR, Ferrucci L, Metter EJ, et al. Hearing loss and cognition in the Baltimore Longitudinal Study of Aging. J Neuropsychol. 2011;25:763–770. doi: 10.1037/a0024238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Lin FR, Metter EJ, O'Brien RJ, et al. Hearing loss and incident dementia. Arch Neurol. 2011;68:214–220. doi: 10.1001/archneurol.2010.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Lin FR, Yaffe K, Xia J, et al. Hearing loss and cognitive decline in older adults. JAMA Intern Med. 2013;173:293–299. doi: 10.1001/jamainternmed.2013.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FR, Ferrucci L, An Y, Goh JO, Doshi J, Metter EJ, Davatzikos C, Kraut MA, Resnick SM. Association of hearing impairment with brain volume changes in older adults. NeuroImage. 2014;90:84–92. doi: 10.1016/j.neuroimage.2013.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberger U, Baltes PB. Sensory functioning and intelligence in old age: a strong connection. Psychol Aging. 1994;9(3):339–355. doi: 10.1037//0882-7974.9.3.339. [DOI] [PubMed] [Google Scholar]
- Massaro DW. Speech perception by ear and eye. In: Dodd B, Campbell R, editors. Hearing by Eye: The Psychology of Lip-Reading. Lawrence Erlbaum; Hillsdale, NJ: 1987. pp. 53–83. [Google Scholar]
- Mesholam RI, Moberg PJ, Mahr RN, et al. Olfaction in neurodegenerative disease—a meta-analysis of olfactory functioning in Alzheimer's and Parkinson's diseases. Arch Neurol. 1998;55:84–90. doi: 10.1001/archneur.55.1.84. [DOI] [PubMed] [Google Scholar]
- *.Moore DR, Edmondson-Jones M, Dawes P, et al. Relation between speech-in-noise threshold, hearing loss and cognition from 40–69 years of age. PLoS ONE. 2014;9(9):e107720. doi: 10.1371/journal.pone.0107720. doi:10.1371/journal.pone.0107720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Ong SY, Cheung CY, Li X, et al. Visual impairment, age-related eye diseases, and cognitive function: the Singapore Malay Eye study. Arch Ophthalmol. 2012;130:895–900. doi: 10.1001/archophthalmol.2012.152. [DOI] [PubMed] [Google Scholar]
- Owsley C, Sekuler R, Siemsen D. Contrast sensitivity throughout adulthood. Vision Res. 1983;23:689–699. doi: 10.1016/0042-6989(83)90210-9. [DOI] [PubMed] [Google Scholar]
- Peelle JE, Troiani V, Grossman M, Wingfield A. Hearing loss in older adults affects neural systems supporting speech comprehension. J Neurosci. 2011;31:12638–12643. doi: 10.1523/JNEUROSCI.2559-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichora-Fuller MK, Schneider BA, Daneman M. How young and old listen to and remember speech in noise. J Acoust Soc Am. 1995;97:593–608. doi: 10.1121/1.412282. [DOI] [PubMed] [Google Scholar]
- *.Piquado T, Cousins KAQ, Wingfield A, et al. Effects of degraded sensory input on memory for speech: Behavioral data and a test of biologically constrained computational models. Brain Res. 2010;1365:48–65. doi: 10.1016/j.brainres.2010.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piquado T, Benichov JI, Brownell H, Wingfied A. The hidden effect of hearing acuity on speech recall, and compensatory effects of self-paced listening. International Journal of Audiology. 2012;51:576–583. doi: 10.3109/14992027.2012.684403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitt PM. Channel capacity, intelligibility and immediate memory. Q J Exp Psychol. 1968;20:241–248. doi: 10.1080/14640746808400158. [DOI] [PubMed] [Google Scholar]
- Rabbitt P. Mild hearing loss can cause apparent memory failures which increase with age and reduce with IQ. Acta Otolaryngol. 1991;111(S476):167–176. doi: 10.3109/00016489109127274. [DOI] [PubMed] [Google Scholar]
- *.Ronnberg J, Danielsson H, Rudner M, et al. Hearing loss is negatively related to episodic and semantic long-term memory but not to short-term memory. J Speech Lang Hear Res. 2011;54:705–726. doi: 10.1044/1092-4388(2010/09-0088). [DOI] [PubMed] [Google Scholar]
- *.Ronnberg J, Hygge S, Keidser G, et al. The effect of functional hearing loss and age on long-and short-term visuospatial memory: evidence from the UK biobank resource. Front aging neurosci. 2014;6 doi: 10.3389/fnagi.2014.00326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Major Issues in Cognitive Aging. Oxford University Press; London: 2010. [Google Scholar]
- Schneider BA, Pichora-Fuller MK. Implications of perceptual processing for cognitive aging research. In: Craik FIM, Salthouse TA, editors. The Handbook of Aging and Cognition. 2nd ed. Lawrence Erlbaum Associates; New York: 2000. [Google Scholar]
- Schneider BA, Pichora-Fuller MK, Daneman M. Effects of senescent changes in audition and cognition on spoken language comprehension. In: Frisina RD, Gordon-Salant S, editors. The Aging Auditory System. Springer; New York: 2010. pp. 167–210. [Google Scholar]
- Skafte MD. The 1999 hearing instrument market—the dispenser's perspective. Hearing Rev. 2000;7:8–40. [Google Scholar]
- *.Sternang O, Jonsson B, Wahlin A, et al. Examination of the common cause account in a population-based longitudinal study with narrow age cohort design. Gerontology. 2010;56:553–563. doi: 10.1159/000279754. [DOI] [PubMed] [Google Scholar]
- Strom KE. The HR 2006 dispenser survey. Hearing Rev. 2006;13(6) [Google Scholar]
- Studebaker GA, Sherbecoe RL, McDaniel DM, Gwaltney CA. Monosyllabic word recognition at higher-than-normal speech and noise levels. J Acoust Soc Am. 1999;105:2431–2444. doi: 10.1121/1.426848. [DOI] [PubMed] [Google Scholar]
- Sumby WH, Pollack I. Visual contribution to speech intelligibility in noise. J Acoust Soc Am. 1954;26:212–215. [Google Scholar]
- Summerfield Q. Some preliminaries to a comprehensive account of audio-visual speech perception. In: Dodd B, Campbell R, editors. The Psychology of Lip-Reading. LEA; Hillsdale, NJ: 1987. pp. 3–50. [Google Scholar]
- *.Surprenant AM, DiDonato R. Community-dwelling older adults with hearing loss experience greater decline in cognitive function over time than those with normal hearing. Evid Based Nurs. 2014;17:60–61. doi: 10.1136/eb-2013-101375. [DOI] [PubMed] [Google Scholar]
- *.Verhaegen C, Collette F, Majerus S. The impact of aging and hearing status on verbal short-term memory. Aging Neuropsychol Cogn. 2014;21:464–482. doi: 10.1080/13825585.2013.832725. [DOI] [PubMed] [Google Scholar]
- Wang J, Eslinger PJ, Doty RL, et al. Olfactory deficit detected by fMRI in early Alzheimer's disease. Brain Res. 2010;1357:184–194. doi: 10.1016/j.brainres.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiig EH, Nielsen NP, Minthon L, et al. A Quick Test of Cognitive Speed (AQT) Pearson; San Antonio, TX: 2002. [Google Scholar]
- Zekveld AA, George EL, Kramer SE, et al. The development of the text reception threshold test: a visual analogue of the speech reception threshold test. J Speech Lang Hear Res. 2007;50:576–584. doi: 10.1044/1092-4388(2007/040). [DOI] [PubMed] [Google Scholar]
- Gygi B, Shafiro V. Auditory and cognitive effects of aging on perception of environmental sounds in natural auditory scenes. J Speech, Lang Hear Res. 2013;56(5):1373–1388. doi: 10.1044/1092-4388(2013/12-0283). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter MS, Bhorade A, Gordon M, Hollingsworth HH, Baum MC. Cognitive, visual, auditory, and emotional factors that affect participation in older adults. The Am J Occup Ther. 2010;64(4):570–579. doi: 10.5014/ajot.2010.09089. [DOI] [PubMed] [Google Scholar]
- Rovner BW, Casten RJ, Massof RW, Leiby BE, Tasman WS. Psychological and cognitive determinants of vision function in age-related macular degeneration. Arch Ophthalmol. 2011;129(7):885–890. doi: 10.1001/archophthalmol.2011.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura S, Yasue M, Sakurai T, Sumigaki C, Uchida Y, Nakashima T, Toba K. Effect of cerumen impaction on hearing and cognitive functions in Japanese older adults with cognitive impairment. Geriatrics & Gerontology Int. 2014;14(Suppl):256–61. doi: 10.1111/ggi.12251. [DOI] [PubMed] [Google Scholar]
- Zekveld AA, Kramer SE, Festen JM. Cognitive load during speech perception in noise: the influence of age, hearing loss, and cognition on the pupil response. Ear Hear. 2011;32(4):498–510. doi: 10.1097/AUD.0b013e31820512bb. [DOI] [PubMed] [Google Scholar]