Abstract
Introduction
Hormonal contraception with DMPA may increase HIV acquisition risk, but observational human studies are inconclusive, and animal models can help investigate this risk. Here we test the impact of a low DMPA dose, designed to resemble human contraceptive use, on SHIV acquisition risk in pigtail macaques (Macaca nemestrina).
Methods
Macaques metabolize DMPA faster than humans. We previously identified a per-weight DMPA dose and administration frequency that achieves long-lasting suppression of ovulation in macaques. Eight macaques were given 1.5 mg/kg DMPA monthly, while eleven were untreated controls. For comparison, women receive 150 mg (approximately 2 mg/kg) every 3 months. We exposed monkeys to 20 sub-optimal SHIV challenges, designed to slowly infect half of controls and allow increased infection in the DMPA group.
Results
It took a median 5.5 viral challenges to infect DMPA-treated macaques, and 9 challenges for controls (p=0.27; exact conditional logistic regression). The exact odds ratio was 2.2 (CI 0.6 – 8.3). Ovulation was suppressed, and the vaginal epithelium was thinned after DMPA-treatment in all animals (mean 30 and 219 micrometers in DMPA-treated and control macaques, respectively, p=0.03, t-test using the Satterthwaite degrees-of-freedom approximation).
Conclusions
SHIV infections in DMPA treated macaques were 2.2 times those of controls, but this was not statistically significant. The result is remarkably similar to studies of human DMPA use, which have shown HIV risk increases of a similar magnitude and of variable significance. Taken together with previous studies of higher DMPA doses in macaques, the results suggest a dose-dependent effect of DMPA on SIV or SHIV acquisition.
Keywords: HIV, acquisition risk, hormonal contraception, macaque model
Introduction
The injectable hormonal contraceptive DMPA (depot medroxyprogesterone acetate, brand name Depo-Provera® [Pfizer, New York, NY]) has been associated with increased HIV acquisition risk in observational studies (recently reviewed 1-4), although the statistical significance and magnitude of the risk increase remains under discussion. Ongoing research focuses on determining underlying biological mechanisms5, characterizing potentially safer alternatives such as Net-en 6, and identifying other risk factors like young age and co-STIs 7,8. In addition, a planned randomized clinical trial may clarify the issue9, but results are not expected for several years. In the meantime, animal model studies can uniquely and relatively quickly contribute to the comprehensive scientific evaluation of DMPA.
Early macaque model studies clearly demonstrated increased infection risk during high-dose injectable DMPA use, or other routes of high-dose progestin administration10,11. These studies provided proof-of-concept data for the potential of progestins to increase risk, and further work allowed identification of associated biological mechanisms contributing to increased HIV transmission, such as the thinning of vaginal epithelial walls10,11. Early studies done with high progestin doses, e.g., 200 mg progestin implants, were reported to enhance vaginal SIV transmission to 7.7 fold in rhesus macaques10. Later studies frequently used single, 30 mg DMPA intra-muscular doses to facilitate vaginal SIV infection for biomedical research studies (e.g., recently used in our research group12, and reviewed by Veazey et al 13), although the associated risk increase with this dose (4.3mg/kg in an average weight (7kg) macaque) has not been quantitated to our knowledge. Taken together, the studies contributed greatly to HIV risk factor and contraceptive research, but also earned the criticism that high doses of progestins may not be comparable to human contraceptive use and may overestimate infection risk.
Here we asked the question whether a DMPA dose modeled after human Depo-Provera® use is associated with increased vaginal infection risk in a pigtail macaque model of SHIV infection. The goal of the research is to develop a realistic animal model that would allow further investigation of current research questions around safe use of contraception for women at risk of HIV infection.
In women, the standard contraceptive dose of 150 mg is given every 3 months, and equates to 2 mg/kg in an average weight woman (75kg) living in the United States14. Macaques, however, metabolize DMPA faster than women15. In particular, after intramuscular DMPA injection of comparable doses in macaques, MPA appearance in blood peaks faster and at higher levels than in humans, but it also declines faster and disappears within one month, while it remains detectable for much longer in humans15. In earlier work by us and our colleagues16-18, various DMPA doses were tested in pigtail macaques (1, 3, 15, 30 mg per macaque, and 0.5, 1.5, and 2.5 mg/kg of body weight). We determined resulting blood MPA levels over time, and their effectiveness in suppressing ovulation, their ability to induce thinning of the vaginal epithelium, and their effects on other putative HIV susceptibility factors. We showed that a low, monthly DMPA dose of 1.5 mg/kg can achieve suppression of ovulation over two months 18, while it also achieves constantly detectable MPA levels, and is associated with significant vaginal epithelial thinning. Other slightly lower or higher doses were also associated with changes in these parameters16-18. In summary, we selected 1.5 kg/mg DMPA given monthly for our current study. The study was designed to determine associated SHIV risk in a model of repeated, low-dose, vaginal SHIV exposure19, given to realistically mimic the human situation where not every HIV exposure leads to infection.
Methods
Animals; Ethics statement
We purchased twelve female pigtail macaques (Macaca nemestrina), aged 5 to 15 years (Supplemental Table 1), and weighing 5 – 8 kg. They were housed at CDC in an AAALAC-accredited facility with veterinary oversight. Housing conditions, enrichment, and all experimental procedures were reviewed and approved by the CDC IACUC (Institutional Animal Care and Use Committee). All recommendations of the Guide for the Care and Use of Laboratory Animals20 were followed, including those for enrichment and minimization of suffering. All sample collections were performed under anesthesia with Ketamine, often together with Telazol.
Study Design
Eight macaques were placed in a DMPA-treated group, four served as real-time untreated controls. Data from seven additional untreated macaques were included as historical controls21. Study phases were: I: Daily visual menstrual cycle observation for up to four weeks; II: Baseline sample collection weekly for 3 weeks (blood and vaginal swab collection); III: Commencement of DMPA injections (see below) two weeks prior to the first virus administration, then repeated virus and DMPA administrations (see below) with continuing weekly blood draws. Phase III ended when an animal was infected, or continued until 20 virus challenges were completed. IV. Continued DMPA injections and monitoring of infections for up to 12 weeks (weekly blood draws, vaginal swabs, biopsy collection).
Virus; Repeat-Low dose model; Infection determination
To determine SHIV acquisition risk, we administered 20 weekly vaginal SHIVSF162P322 exposures at 10 TCID50, lower than our standard virus dose and designed to cause infection in only half of control macaques, thus allowing detection of increased infection in the DMPA group21. The virus stock was produced at CDC, using an inoculation stock obtained through the NIH AIDS Reagent program (Germantown, MD). As previously described21, an in-house real-time PCR assay was used to determine infection when blood plasma levels reached at least 50 copies/ mL in two consecutive blood samples. Infection or lack thereof was confirmed with an in-house assay for proviral DNA integration in PBMCs, and with detection of anti-HIV 1/2 antibodies as described21.
DMPA administration; menstrual cycle determination
We purchased Depo-Provera® from Pfizer (New York, NY) and injected suspensions of 1.5 mg/kg DMPA into the gluteal muscle every 28 days as described 18. Menstrual cycling was monitored by visual observation and plasma progesterone18. Visual observations were recorded on every weekday throughout, and included menstrual blood and perineal tumescence (“sex skin swelling”, documented in 23). Plasma progesterone and MPA were measured at the Wisconsin National Primate Research Center, as previously described 18. Vaginal pH was recorded after infection or at study end before biopsy collection, and did not differ in study groups (supplemental Table 1, and data not shown).
Epithelial analyses
We obtained biopsy punches from three separate vaginal sites from each animal and measured the thicknesses of the superficial non-nucleated epithelial layer and the combined underlying nucleated epithelial cell layers, as previously described in detail 18. In brief, after H&E staining of sectioned, slide-mounted tissues, we used the ScanScope imaging system (Aperio, Vista, CA) and HALO image analysis software's epithelial thickness algorithm (Indica Labs, Corrales, NM) to measure epithelial thickness at 50 micron intervals along the entire length of appropriately oriented tissue sections from the three punches. A board-certified veterinary pathologist delineated the nucleated and non-nucleated cell layers, as previously described18,24.
Statistics
GraphPad Prism software version 5.03 (San Diego, CA) was used to construct Kaplan-Meier survival curves and other graphs, to calculate descriptive statistics, and to perform parametric and non-parametric statistical tests to assess treatment group differences. We performed exact conditional logistic regression in SAS version 9.3 software to test for group differences in risk for infection using repeat-low dose infection data 25. For survival analyses, uninfected macaques were right censored at the maximal exposure number (20 exposures).
Results
Infection risk during DMPA treatment
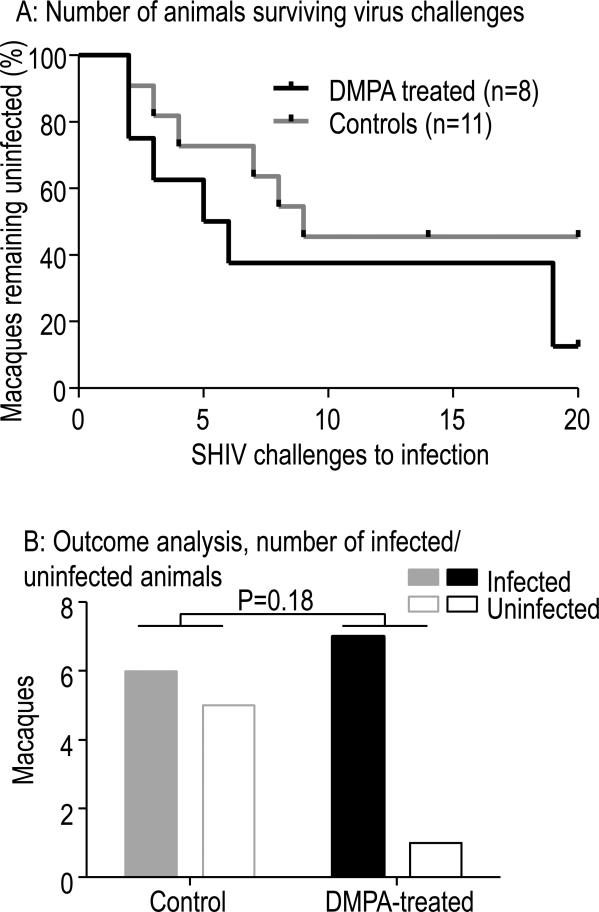
The study question was whether DMPA treatment at a dose of 1.5 mg/kg increases risk of vaginal SHIV infection. We used a model of repeated virus exposures at very low, sub-infectious virus dose (10 TCID50 of SHIVSF162P3) and hypothesized that untreated controls would be infected after many exposures or remain uninfected, while DMPA-treated animals would experience rapid infection. Of eight female, DMPA-treated pigtail macaques five were infected within the first six challenges, two after 19 challenges, and the eighth macaque remained uninfected after 20 virus challenges. Overall, it took a median 5.5 challenges to infect DMPA-treated macaques. Two of the four real-time control animals became infected, as did four of seven control animals that had previously been subjected to the same SHIV exposure regimen21. In all, a median of nine viral challenges were required for infection of controls. The difference in challenges to infection were not statistically significant (p=0.18, log-rank test). These results are graphically displayed in Fig. 1A with Kaplan-Meier survival curves depicting how many animals remained uninfected after each challenge. We opted to display the number of virus challenges rather than menstrual cycles19 during challenges because DMPA abrogates cycling (see below). SHIV infection in DMPA-treated macaques were 2.2 (95% CI 0.6 – 8.3) those in control animals, though this was not statistically significant (p=0.27, exact conditional logistic regression). Seven of eight DMPA-treated macaques became infected, compared to six of 11 controls (Fig. 1B, p=0.18, Fisher's exact test). Thus, DMPA treatment at 1.5 mg/kg did not significantly increase SHIV infection risk whether analyzed at the exposure or individual level.
Fig. 1.
A: Kaplan Meier survival analyses showing the number of macaques remaining uninfected after vaginal SHIV challenges in the presence or absence of DMPA. Eight DMPA treated macaques required a median 5.5 challenges, while four real time controls and seven historical controls required a median 9. The difference was not statistically significant different (p=0.18, log-rank test). Five controls and one DMPA-treated macaque remained uninfected. B: Bar graphs show different outcomes for control (grey) or DMPA-treated (black) macaques. Analyses were performed for the number of animals infected or uninfected per group. P values refer to Fisher's exact test (two-tailed).
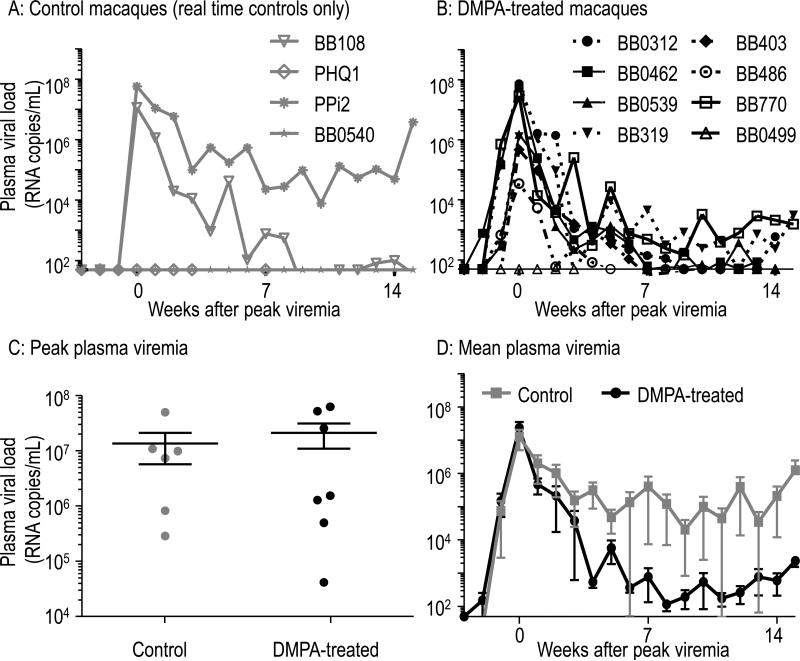
The course of resulting SHIV infections is described in Fig. 2. Fig. 2A shows plasma viral RNA levels in the two control animals infected in real time. Infection data of historic control infections were previously published with similar results21. In Fig. 2B, the infection course of seven DMPA-treated, infected macaques is shown while they continued to receive DMPA for at least 12 weeks post infection. The uninfected, treated animal BB0499 is also shown. At peak viremia, the median log10 transformed values were 7.0 and 6.2 RNA copies per milliliter of plasma in DMPA or untreated animals, respectively (Fig. 2C). This was not statistically significantly different (p=0.94, Mann-Whitney U test). A comparison of treatment groups for total viremia over the course of 15 weeks post infection by assessing differences in the distributions of area under the viral load curves (Fig. 2D) was not statistically significant (p=0.83, Mann-Whitney U test).
Fig. 2.
Course of SHIV infection in controls or DMPA-treated macaques. A, B: Figures show plasma viremia (RNA copies/mL, determined by in-house real-time PCR assay) in both SHIV-infected control or DMPA-treated macaques. DMPA treatment was continued after infection. Three macaques remained uninfected (B0499, PHQ1, BB0540). The detection limit was 49 copies/mL. Viremia was aligned at peak. C: Comparison of peak viremia in infected animals. The black line represents median values. The difference in distribution of peak values was not statistically significant (p=0.94, Mann-Whitney U test). D: Mean viremia is shown of infected animals, including data from four infected historic controls.
DMPA treatment at 1.5 mg/kg suppressed menstrual cycling and ovulation
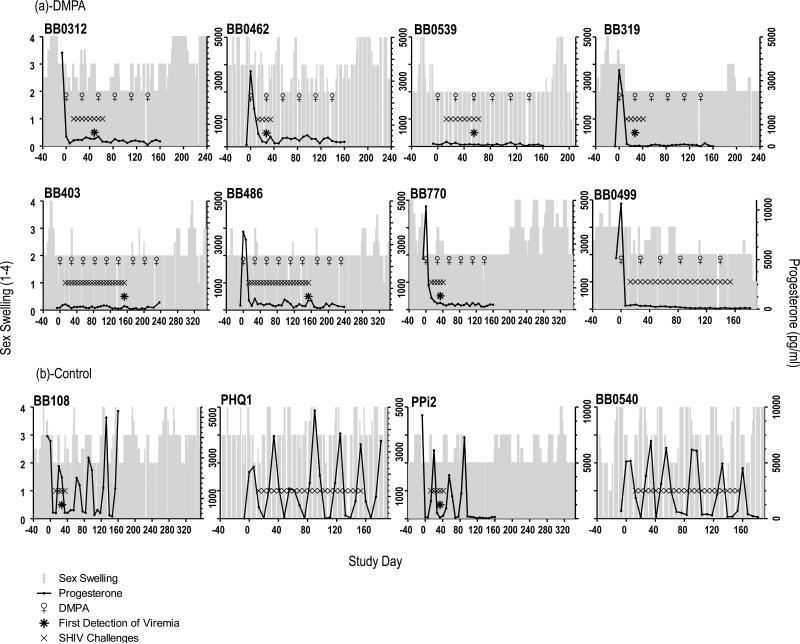
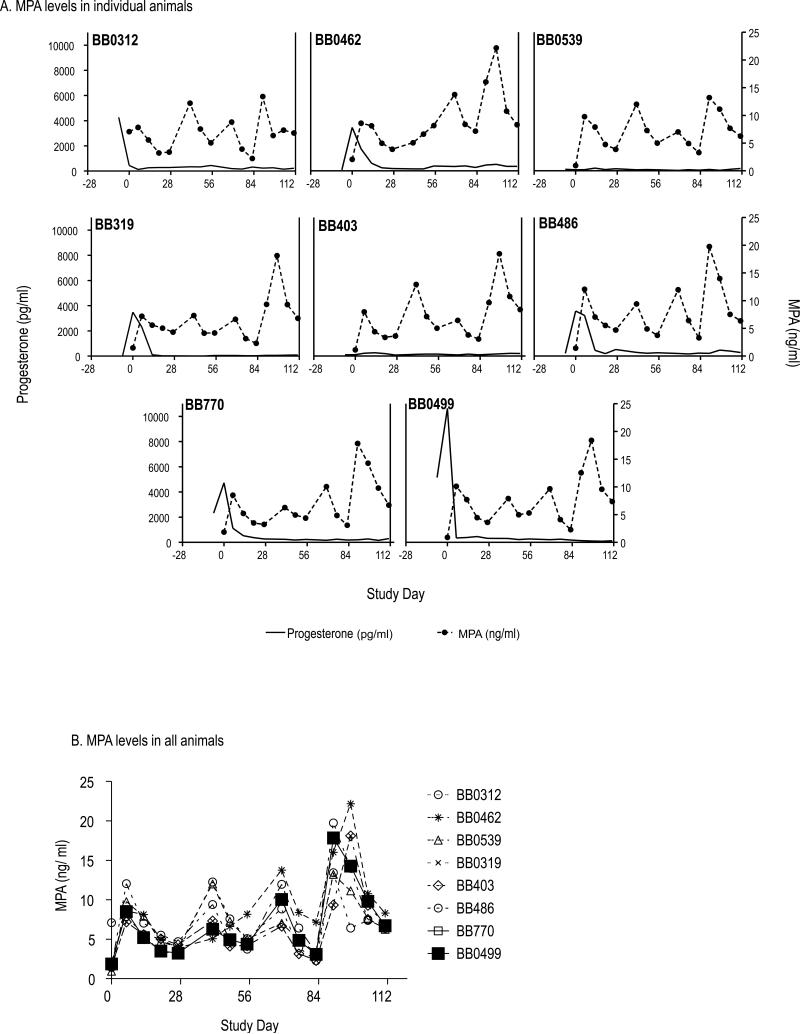
DMPA prevents pregnancy by suppressing ovulation and menstrual cycling, among other mechanisms that contribute to preventing fertilization, such as changes in mucus properties. We examined the effect of monthly 1.5 mg/kg DMPA injections on menstrual cycling. Fig. 3A and B show cycling information for all twelve macaques throughout all of their study phases (baseline, virus challenge, and post infection), for up to 320 study days. At study start and before treatment, all but two animals had plasma progesterone peaks recorded, a sign of recent ovulation as progesterone is produced by the corpus luteum after ovulation. The remaining two animals BB0539 and BB403 (Fig. 3A) likely had no progesterone peak recorded because we only took blood samples for three weeks. Nonetheless, the two animals had evidence of active menstrual cycles at study start based on the swelling of their “sex skin” (perineal tumescence), i.e., the color and size changes of the genital area associated with rising estrogen during menstrual cycling in this species (previously documented in 23). When DMPA injections started, all eight treated animals had cessation of progesterone fluctuations, indicating lack of cycling. In contrast, control animals continued to cycle (Fig. 3B). Thus, the DMPA treatments were effective in suppressing ovulation, as we had previously shown in three similarly treated animals over two months18. As expected, suppression occurred shortly after MPA levels became measureable in plasma (Fig. 4A). The figure shows the relationship of endogenous progesterone (left y-axis) and plasma MPA (right y-axis) for each treated animal. The uninfected, DMPA-treated macaque BB0499 had measurable MPA levels of comparable magnitude as other animals (Fig. 4B), indicating that drug absorption following intramuscular DMPA injection was normal.
Fig. 3.
Menstrual cycles of the twelve study animals. Results of plasma progesterone measurements (black lines) and visual observations of sex skin swellings (grey bars) are shown, relative to the administration of DMPA (♀), SHIV exposure (x symbol), and first detection of infection (* asterisks). A: DMPA treatment abrogated cycling in all animals. B. All controls macaques had menstrual cycles during virus exposures.
Fig. 4.
A: DMPA treatment led to detectable MPA levels in all treated animals. MPA levels in plasma are shown (right y-axis), as are endogenous progesterone levels (left y-axis). DMPA was injected on days 0, 28, 56, and 84. B. MPA levels are shown over time. MPA increased in all treated animals.
DMPA treatment at 1.5 mg/kg caused vaginal epithelial thinning
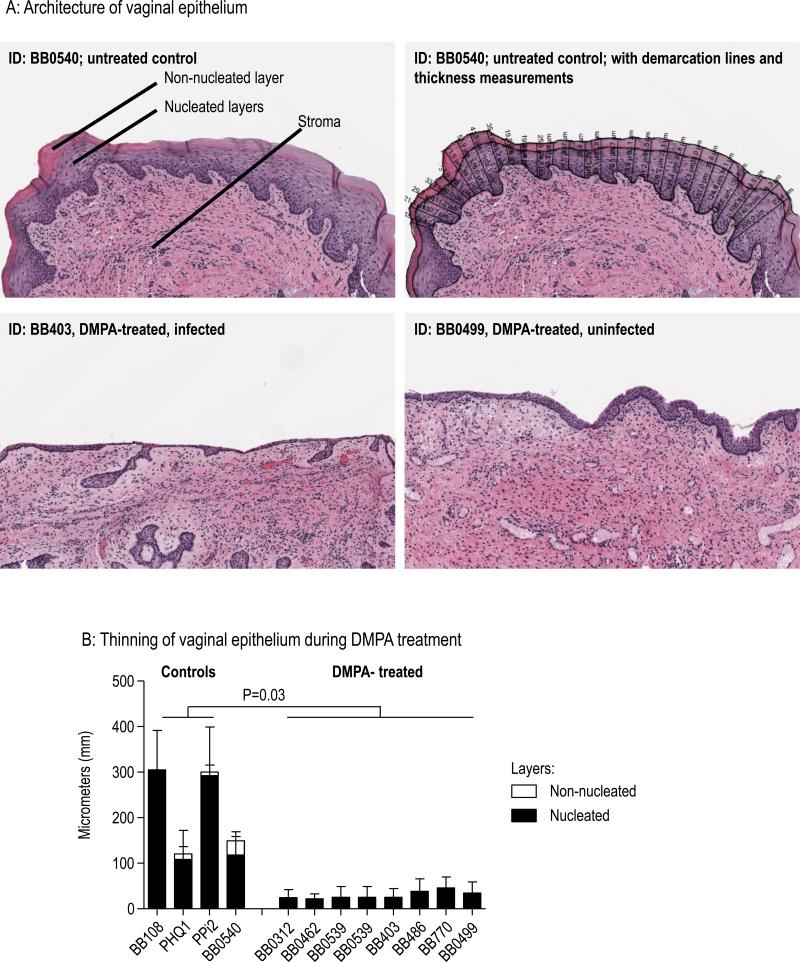
DMPA treatment may increase vaginal HIV infection risk by thinning the vaginal epithelium10, among other mechanisms, thus limiting its barrier function and permitting HIV easier access to underlying target cells. We measured epithelial thinning in biopsy tissues taken shortly after infection in each animal, or at the end of vaginal virus challenges in uninfected animals. Fig. 5 shows that all DMPA-treated macaques had extensive thinning of the epithelium. Fig. 5A displays examples of the vaginal epithelial architecture. The top images are from an untreated animal (BB0540). The top left image illustrates the nucleated and non-nucleated epithelial layers, and the underlying stroma. The top right image shows the same tissue after manual delineation of the epithelial layers and automated thickness measurements at 50 micron intervals. The bottom images are from DMPA-treated animals, and show complete loss of the non-nucleated layer, as well as marked thinning of the nucleated layers, leaving the underlying stroma close to the vaginal lumen. For the four control animals, mean total epithelial thickness (all epithelial layers combined) was 219 (Standard Deviation 97.6) micrometers, and differences between controls were likely due to the random time points within the menstrual cycle at time of biopsy (Fig. 5B). In contrast, the epithelium of DMPA-treated animals had a mean thickness of 30 (Standard Deviation 8.4) micrometers, and none of the animals had the superficial non-nucleated cell layer, as was previously reported for this and other DMPA doses16-18. The difference in thicknesses between study groups was statistically significant (p=0.03, Satterthwaite t-test for groups with unequal variances).
Fig. 5.
DMPA treatment thins the vaginal epithelium. A: Top left: Untreated control animal BB0540 illustrating superficial non-nucleated, and underlying nucleated vaginal epithelial layers, and stroma. Top right: Automated measurements of non-nucleated and nucleated layer thicknesses by image analysis software after manual delineation of epithelial layers. Bottom: Loss of superficial non-nucleated layer and marked thinning of nucleated layers in DMPA-treated, infected (left) and uninfected (right) animals. B: Quantitative analysis of the vaginal epithelial measurements indicating significant epithelial thinning and loss of non-nucleated cell layers in DMPA-treated animals. Data are from combined measurements of the three biopsy sites. Thickness of the epithelium (sum of nucleated and non-nucleated layers) was analyzed. Mean values and standard deviations are shown, the p-value refers to the Satterthwaite t-test for unequal variances.
Discussion
In summary, we here show that SHIV infections in macaques treated with a human-equivalent dose of DMPA were 2.2-times those of untreated controls, though this difference was not statistically significant. This finding was obtained despite robust suppression of ovulation and induction of putative HIV susceptibility factors such as vaginal thinning, and likely also increases in vaginal pH18, as we have previously shown in different pigtail macaques treated with this DMPA dose 18.
Our goal was to develop a realistic animal model for further HIV prevention research such as PrEP regimen testing or risk comparisons with other contraceptive products. We expected to find a risk increase associated with DMPA injections, based on earlier work in rhesus macaques reporting that progesterone implants enhanced SIV transmission to 7.7-fold over placebo-treated macaques10. The ubiquitous use of DMPA in macaque models to facilitate otherwise uncertain vaginal virus transmission also contributed to our expectations (reviewed by Veazey 13, and also recently employed by our research team12). However, we did not observe a statistically significant treatment group difference, and we conclude that this is likely a result of the comparatively low DMPA dose employed here, which was modeled as a human-equivalent dose.
The non-significant odds ratio of 2.2 was nonetheless perhaps not surprising, as it was in the range of the most recent data from human observational studies. In meta-analyses of epidemiologic evidence concerning DMPA and HIV risk, Polis et al described “persisting uncertainty whether DMPA increases risk of HIV acquisition”3, with some studies finding no association, others between 1.5 and 2.2 times the risk 2. Similarly, Ralph et al., recently determined a pooled HR of 1.40 (95% CI 1·16-1·69) in the general population, with heterogeneity between studies, and higher among women at high risk of HIV infection 1, while Morrison et al determined a HR of 1.5 relative to no hormonal contraception 4. Observational studies in humans may under- or overestimate risk increases during DMPA use due to study confounding by potentially different sexual behavior or condom use with and without DMPA contraception. Future results from randomized clinical trials with expected minimized confounding conditions will further clarify how closely the macaque data resemble the human situation. In all, our results are in the range of current data on human DMPA use with regards to magnitude and significance of findings. Although animal model data are often best understood as proof-of-concept studies, and do not necessarily allow quantitative comparisons to human data, we can conclude that use of a human-like DMPA dose resulted in a human-like outcome with regards to SHIV infection risk in this model.
Two- to three-fold risk differences can be difficult to detect in animal models due to limitations in animal group sizes and resulting limited statistical power (recently reviewed in 26). Prior experiences with this macaque model allowed us to consider potential group sizes and power to detect modest risk differences. We had previously demonstrated a relative risk of 2.5 (95% CI 1.1 – 5.6) for vaginal SHIV infection associated with pre-existing co-infection with STIs over STI-naïve animals, using a similar model of repeated vaginal SHIV exposures at sub-infectious virus doses21. Based on sample size calculations, we estimated the current study design allowed 80% power to detect a true odds ratio of approximately 2.5 with a 2-sided significance level of 0.05 if eight DMPA-treated animals readily became infected, and if the four real-time controls behaved like the seven historic controls. However, we did not observe early infections in all animals, as one animal did not become infected during DMPA treatment, and two additional animals had infections after 19 virus exposures. Thus, there was insufficient power to conclude statistically significant differences for an observed odds ratio <2.5.
The selection of a 1.5 mg/kg DMPA dose as similar to human Depo-Provera® use has previously been justified and discussed 18. It was based on a combination of factors such as effectiveness of ovulation suppression over two months, MPA levels, vaginal thinning, and vaginal pH changes in a group of three animals. We here obtained similar results for changes in some of these factors, in a larger group of animals, and over a longer period of time. Changes in pH were not measured throughout because it requires vaginal swabbing, thus, this was intentionally not undertaken in this trial so the vaginal mucosa was not disturbed manually before virus inoculations. It is possible and even likely that higher or lower DMPA doses might result in different infection outcomes. Of note, and consistent with our results, an even lower dose of 3 mg DMPA per animal did not decrease efficacy of Truvada for HIV prevention in a similar pigtail macaque model16,17. This suggests that the lower 3 mg DMPA dose also did not increase SHIV infection risk, although risk was not directly evaluated at this dose16,17.
Taken together with previous data from DMPA use in macaques, our results suggest a dose dependency of increased vaginal SIV/ SHIV infection risk in macaques. It remains unclear whether a similar DMPA/ HIV risk dose-dependency exists in women. Women receive a Depo-Provera® dose of 150 mg, regardless of their weight, age, or reproductive history. This dose peaks and then declines over the course of three months, when the next injection is due. MPA levels thus vary over the three months of contraceptive efficacy in each woman. There is therefore no one DMPA dose that perfectly models all possible human scenarios. Our results are somewhat reassuring regarding HIV infection risk, since we considered the dose as likely equivalent to an average human dose, with the caveats of animal model data as discussed above. At the same time, one could hypothesize that women with low body mass who receive the standard 150 mg MPA dose (e.g., small women with low body weight) could have a relatively higher HIV infection risk during Depo-Provera® use than heavier women. If confirmed by human data, this could be avoided by administering DMPA on a strict per-weight basis to women.
Although we could not demonstrate a statistically significant risk increase associated with 1.5 mg/kg DMPA, we still observed increases in putative HIV risk factors such as vaginal thinning and, in our previous work, pH increases that likely reflect changes in the vaginal microbiome18. We have previously demonstrated a relationship between vaginal epithelial thinning and SHIV infection risk in this model 24, although vaginal thinning occurs to a lesser degree in women, if at all 27. In humans, the link between several biological changes and HIV infection risk during DMPA use remains under active study and discussion (reviewed by 5). Furthermore, our data do not exclude the possibility that there is a moderate risk increase associated with a human-like DMPA dose in macaques. Other research efforts, such as the planned randomized clinical trial on DMPA use and HIV acquisition risk, as well as thorough investigations of potential alternatives to DMPA (e.g., the injectable contraceptive drug Net-en 6), will help identify the safest contraceptive choices for women with regards to HIV acquisition risk.
Supplementary Material
Acknowledgements
We thank Dr. Brianna Skinner and Dr. George Lathrop for serving as the attending veterinarians, James Mitchell, Leecresia Jenkins, Frank Deyounks, and Kristen Kelley for animal technical assistance, and Dr. David Garber for programmatic support. We also thank Dr. Norma Harris for helpful discussions. This work was funded by the CDC and by Interagency Agreement Y1-Al-0681-02 between the CDC and the NIH. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the CDC.
Footnotes
Meetings at which parts of the data were presented: Data in part submitted for presentation to the Annual Symposium for Nonhuman Primate Models for AIDS, Monterey, CA, October 2015
Conflicts of Interest: All authors declare no conflict of interest
References
- 1.Ralph LJ, McCoy SI, Shiu K, Padian NS. Hormonal contraceptive use and women's risk of HIV acquisition: a meta-analysis of observational studies. Lancet Infect Dis. 2015 Feb;15(2):181–189. doi: 10.1016/S1473-3099(14)71052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polis CB, Curtis KM. Use of hormonal contraceptives and HIV acquisition in women: a systematic review of the epidemiological evidence. Lancet Infect Dis. 2013 Jul 18; doi: 10.1016/S1473-3099(13)70155-5. [DOI] [PubMed] [Google Scholar]
- 3.Polis CB, Phillips SJ, Curtis KM, et al. Hormonal contraceptive methods and risk of HIV acquisition in women: a systematic review of epidemiological evidence. Contraception. 2014 Oct;90(4):360–390. doi: 10.1016/j.contraception.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Morrison CS, Chen PL, Kwok C, et al. Hormonal contraception and the risk of HIV acquisition: an individual participant data meta-analysis. PLoS medicine. 2015 Jan;12(1):e1001778. doi: 10.1371/journal.pmed.1001778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy K, Irvin SC, Herold BC. Research gaps in defining the biological link between HIV risk and hormonal contraception. Am J Reprod Immunol. 2014 Aug;72(2):228–235. doi: 10.1111/aji.12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noguchi LM, Richardson BA, Baeten JM, et al. Risk of HIV-1 acquisition among women who use different types of injectable progestin contraception in South Africa: a prospective cohort study. The lancet HIV. 2015 Jul 1;2(7):e279–e287. doi: 10.1016/S2352-3018(15)00058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morrison CS, Chen PL, Kwok C, et al. Hormonal contraception and HIV acquisition: reanalysis using marginal structural modeling. AIDS (London, England) 2010 Jul 17;24(11):1778–1781. doi: 10.1097/QAD.0b013e32833a2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grabowski MK, Gray RH, Makumbi F, et al. Use of injectable hormonal contraception and women's risk of herpes simplex virus type 2 acquisition: a prospective study of couples in Rakai, Uganda. The Lancet. Global health. 2015 Aug;3(8):e478–486. doi: 10.1016/S2214-109X(15)00086-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rees H, Consortium E. DMPA and HIV: why we need a trial. Contraception. 2014 Oct;90(4):354–356. doi: 10.1016/j.contraception.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Marx PA, Spira AI, Gettie A, et al. Progesterone implants enhance SIV vaginal transmission and early virus load. Nature medicine. 1996 Oct;2(10):1084–1089. doi: 10.1038/nm1096-1084. [DOI] [PubMed] [Google Scholar]
- 11.Hild-Petito S, Veazey RS, Larner JM, Reel JR, Blye RP. Effects of two progestin-only contraceptives, Depo- Provera and Norplant-II, on the vaginal epithelium of rhesus monkeys. AIDS research and human retroviruses. 1998 Apr;14(Suppl 1):S125–130. [PubMed] [Google Scholar]
- 12.Smith JM, Srinivasan P, Teller RS, et al. Tenofovir disoproxil fumarate intravaginal ring protects high-dose depot medroxyprogesterone acetate-treated macaques from multiple SHIV exposures. Journal of acquired immune deficiency syndromes (1999) 2015 Jan 1;68(1):1–5. doi: 10.1097/QAI.0000000000000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veazey RS, Shattock RJ, Klasse PJ, Moore JP. Animal models for microbicide studies. Curr HIV Res. 2012 Jan 1;10(1):79–87. doi: 10.2174/157016212799304715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fryar CD, Gu Q, Ogden CL. Anthropometric reference data for children and adults: United States, 2007-2010. Vital and health statistics. Series 11, Data from the national health survey. Oct;2012(252):1–48. [PubMed] [Google Scholar]
- 15.Mora G, Johansson ED. Plasma levels of medroxyprogesterone acetate (MPA), estradiol and progesterone in the rhesus monkey after intramuscular adminstration of Depo-Provera. Contraception. 1976 Sep;14(3):343–350. doi: 10.1016/0010-7824(76)90101-3. [DOI] [PubMed] [Google Scholar]
- 16.Radzio J, Hanley K, Mitchell J, et al. Depot-medroxyprogesterone acetate does not reduce the prophylactic efficacy of emtricitabine and tenofovir disoproxil fumarate in macaques. Journal of acquired immune deficiency syndromes (1999) 2014 Dec 1;67(4):365–369. doi: 10.1097/QAI.0000000000000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radzio J, Hanley K, Mitchell J, et al. Physiologic doses of depot-medroxyprogesterone acetate do not increase acute plasma simian HIV viremia or mucosal virus shedding in pigtail macaques. AIDS (London, England) 2014 Jun 19;28(10):1431–1439. doi: 10.1097/QAD.0000000000000294. [DOI] [PubMed] [Google Scholar]
- 18.Butler K, Ritter J, Ellis S, et al. Analysis of Putative Mucosal SHIV Susceptibility Factors during Repeated DMPA Treatments in Pigtail Macaques. Journal of medical primatology. 2015 doi: 10.1111/jmp.12188. in press. [DOI] [PubMed] [Google Scholar]
- 19.Kersh EN, Henning TC, Dobard C, Heneine W, McNicholl JM. Practical Experience with Analysis and Design of Repeat Low-Dose SHIVSF162P3 Exposure Studies in Female Pigtail Macaques with Varying Susceptibility during Menstrual Cycling. AIDS research and human retroviruses. 2015 Jul 13; doi: 10.1089/aid.2014.0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Research Council (U.S.). Committee for the Update of the Guide for the Care and Use of Laboratory Animals., Institute for Laboratory Animal Research (U.S.), National Academies Press (U.S.) Guide for the care and use of laboratory animals. 8th ed. National Academies Press; Washington, D.C.: 2011. [Google Scholar]
- 21.Henning TR, Butler K, Hanson D, et al. Increased susceptibility to vaginal simian/human immunodeficiency virus transmission in pig-tailed macaques coinfected with Chlamydia trachomatis and Trichomonas vaginalis. The Journal of infectious diseases. 2014 Oct 15;210(8):1239–1247. doi: 10.1093/infdis/jiu240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harouse JM, Gettie A, Eshetu T, et al. Mucosal transmission and induction of simian AIDS by CCR5- specific simian/human immunodeficiency virus SHIV(SF162P3). Journal of virology. 2001 Feb;75(4):1990–1995. doi: 10.1128/JVI.75.4.1990-1995.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livingston L, Sweeney E, Mitchell J, et al. Hormonal synchronization of the menstrual cycles of pigtail macaques to facilitate biomedical research including modeling HIV susceptibility. Journal of medical primatology. 2011 Jun;40(3):164–170. doi: 10.1111/j.1600-0684.2010.00465.x. [DOI] [PubMed] [Google Scholar]
- 24.Kersh EN, Ritter J, Butler K, et al. A Thinned Vaginal Stratum Corneum is a Susceptibility Factor for SHIV Acquisition. poster, CROI. 2015 [Google Scholar]
- 25.Nolen TL, Hudgens MG, Senb PK, Koch GG. Analysis of repeated low-dose challenge studies. Stat Med. 2015 May 30;34(12):1981–1992. doi: 10.1002/sim.6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henning TR, McNicholl JM, Vishwanathan SA, Kersh EN. Macaque models of enhanced susceptibility to HIV. Virology journal. 2015 Jun 14;12(1):90. doi: 10.1186/s12985-015-0320-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mauck CK, Callahan MM, Baker J, et al. The effect of one injection of Depo-Provera on the human vaginal epithelium and cervical ectopy. Contraception. 1999 Jul;60(1):15–24. doi: 10.1016/s0010-7824(99)00058-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.