Abstract
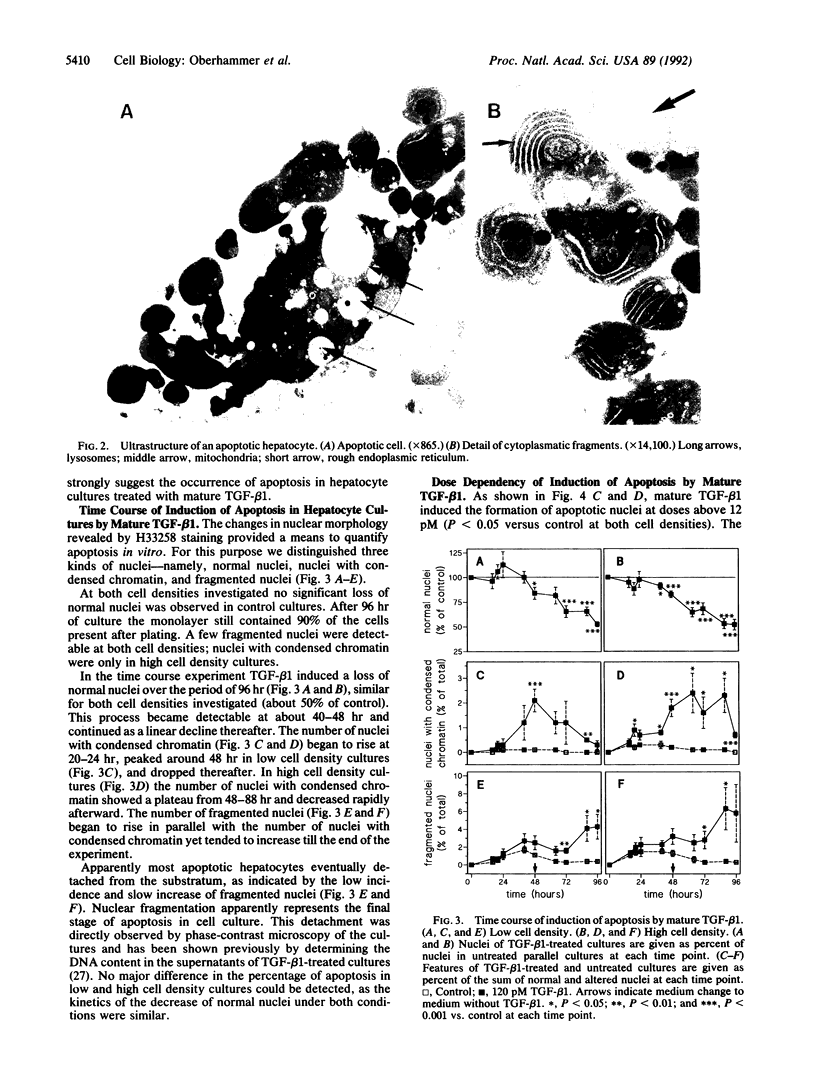
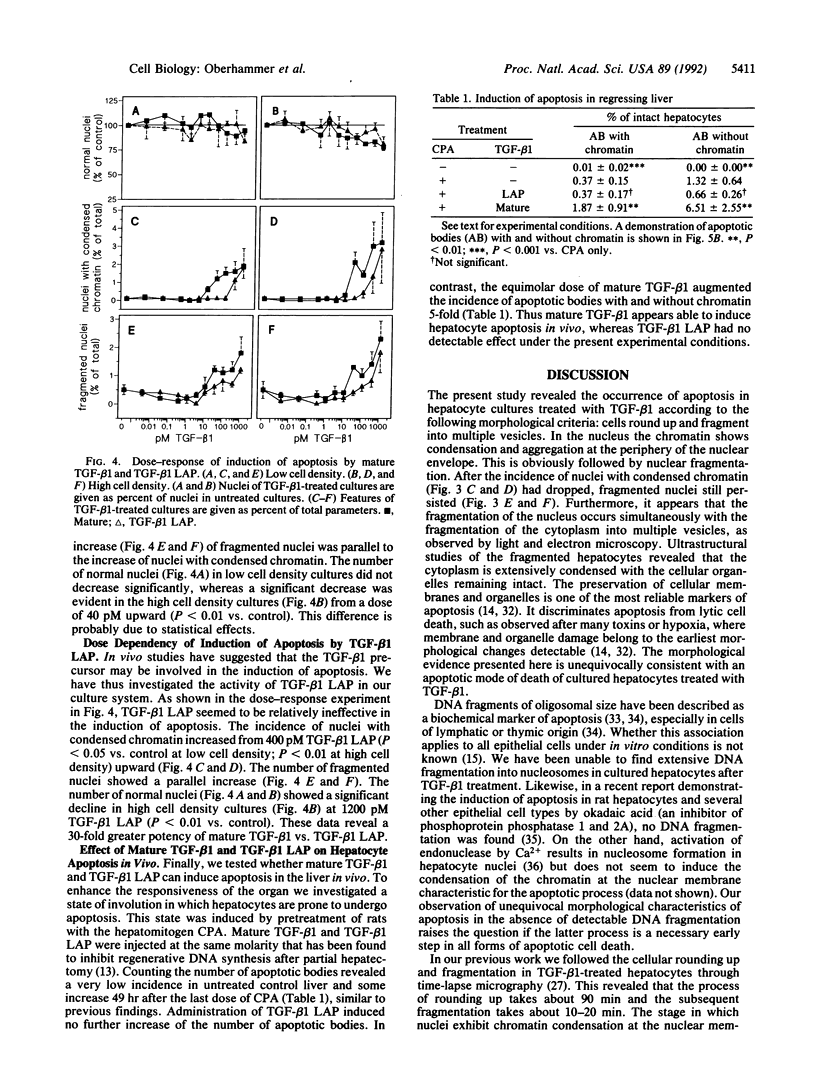
In previous studies hepatocytes undergoing cell death by apoptosis but not normal hepatocytes in rat liver showed immunostaining for transforming growth factor beta 1 (TGF-beta 1). Staining was much stronger with antibodies recognizing the pro-region of TGF-beta 1 than the mature peptide itself. Therefore we investigated the ability of both forms of TGF-beta 1 to induce apoptosis in primary cultures of rat hepatocytes. Mature TGF-beta 1 induced rounding up of the cells and fragmentation into multiple vesicles. As revealed by the DNA-specific stain H33258, the chromatin of these cells condensed and segregated into masses at the nuclear membrane; this was obviously followed by fragmentation of the nucleus. Ultrastructurally the cytoplasm was well preserved, as demonstrated by the presence of intact cell organelles. These features strongly suggest the occurrence of apoptosis. Quantification of nuclei with condensed chromatin revealed that mature TGF-beta 1 was 30-fold more effective than the TGF-beta 1 latency-associated protein complex. Finally, we administered TGF-beta 1 in vivo using an experimental model in which regression of rat liver was initiated by a short preceding treatment with the hepatomitogen cyproterone acetate. Two doses of TGF-beta 1, each 1 nM/kg, augmented the incidence of apoptotic hepatocytes 5-fold. Equimolar doses of TGF-beta 1 latency-associated protein complex were ineffective. These studies suggest that TGF-beta 1 is involved in the initiation of apoptosis in the liver and that the mature form of TGF-beta 1 is the active principle.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alison M. R. Regulation of hepatic growth. Physiol Rev. 1986 Jul;66(3):499–541. doi: 10.1152/physrev.1986.66.3.499. [DOI] [PubMed] [Google Scholar]
- Braun L., Mead J. E., Panzica M., Mikumo R., Bell G. I., Fausto N. Transforming growth factor beta mRNA increases during liver regeneration: a possible paracrine mechanism of growth regulation. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1539–1543. doi: 10.1073/pnas.85.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bursch W., Düsterberg B., Schulte-Hermann R. Growth, regression and cell death in rat liver as related to tissue levels of the hepatomitogen cyproterone acetate. Arch Toxicol. 1986 Dec;59(4):221–227. doi: 10.1007/BF00290542. [DOI] [PubMed] [Google Scholar]
- Bursch W., Lauer B., Timmermann-Trosiener I., Barthel G., Schuppler J., Schulte-Hermann R. Controlled death (apoptosis) of normal and putative preneoplastic cells in rat liver following withdrawal of tumor promoters. Carcinogenesis. 1984 Apr;5(4):453–458. doi: 10.1093/carcin/5.4.453. [DOI] [PubMed] [Google Scholar]
- Bursch W., Paffe S., Putz B., Barthel G., Schulte-Hermann R. Determination of the length of the histological stages of apoptosis in normal liver and in altered hepatic foci of rats. Carcinogenesis. 1990 May;11(5):847–853. doi: 10.1093/carcin/11.5.847. [DOI] [PubMed] [Google Scholar]
- Bøe R., Gjertsen B. T., Vintermyr O. K., Houge G., Lanotte M., Døskeland S. O. The protein phosphatase inhibitor okadaic acid induces morphological changes typical of apoptosis in mammalian cells. Exp Cell Res. 1991 Jul;195(1):237–246. doi: 10.1016/0014-4827(91)90523-w. [DOI] [PubMed] [Google Scholar]
- Carr B. I., Hayashi I., Branum E. L., Moses H. L. Inhibition of DNA synthesis in rat hepatocytes by platelet-derived type beta transforming growth factor. Cancer Res. 1986 May;46(5):2330–2334. [PubMed] [Google Scholar]
- Chambard M., Gabrion J., Mauchamp J. Influence of collagen gel on the orientation of epithelial cell polarity: follicle formation from isolated thyroid cells and from preformed monolayers. J Cell Biol. 1981 Oct;91(1):157–166. doi: 10.1083/jcb.91.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey R. J., Jr, Kost L. J., Lyons R. M., Moses H. L., LaRusso N. F. Hepatic processing of transforming growth factor beta in the rat. Uptake, metabolism, and biliary excretion. J Clin Invest. 1987 Sep;80(3):750–757. doi: 10.1172/JCI113130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruise J. L., Knechtle S. J., Bollinger R. R., Kuhn C., Michalopoulos G. Alpha 1-adrenergic effects and liver regeneration. Hepatology. 1987 Nov-Dec;7(6):1189–1194. doi: 10.1002/hep.1840070604. [DOI] [PubMed] [Google Scholar]
- Dennis P. A., Rifkin D. B. Cellular activation of latent transforming growth factor beta requires binding to the cation-independent mannose 6-phosphate/insulin-like growth factor type II receptor. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):580–584. doi: 10.1073/pnas.88.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry L. E., Lioubin M. N., Purchio A. F., Marquardt H. Molecular events in the processing of recombinant type 1 pre-pro-transforming growth factor beta to the mature polypeptide. Mol Cell Biol. 1988 Oct;8(10):4162–4168. doi: 10.1128/mcb.8.10.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry L. E., Webb N. R., Lim G. J., Brunner A. M., Ranchalis J. E., Twardzik D. R., Lioubin M. N., Marquardt H., Purchio A. F. Type 1 transforming growth factor beta: amplified expression and secretion of mature and precursor polypeptides in Chinese hamster ovary cells. Mol Cell Biol. 1987 Oct;7(10):3418–3427. doi: 10.1128/mcb.7.10.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houck K. A., Cruise J. L., Michalopoulos G. Norepinephrine modulates the growth-inhibitory effect of transforming growth factor-beta in primary rat hepatocyte cultures. J Cell Physiol. 1988 Jun;135(3):551–555. doi: 10.1002/jcp.1041350327. [DOI] [PubMed] [Google Scholar]
- Jakowlew S. B., Mead J. E., Danielpour D., Wu J., Roberts A. B., Fausto N. Transforming growth factor-beta (TGF-beta) isoforms in rat liver regeneration: messenger RNA expression and activation of latent TGF-beta. Cell Regul. 1991 Jul;2(7):535–548. doi: 10.1091/mbc.2.7.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirtle R. L., Carr B. I., Scott C. D. Modulation of insulin-like growth factor-II/mannose 6-phosphate receptors and transforming growth factor-beta 1 during liver regeneration. J Biol Chem. 1991 Nov 25;266(33):22444–22450. [PubMed] [Google Scholar]
- Jones D. P., McConkey D. J., Nicotera P., Orrenius S. Calcium-activated DNA fragmentation in rat liver nuclei. J Biol Chem. 1989 Apr 15;264(11):6398–6403. [PubMed] [Google Scholar]
- Kumatori A., Nakamura T., Ichihara A. Cell-density dependent expression of the c-myc gene in primary cultured rat hepatocytes. Biochem Biophys Res Commun. 1991 Jul 31;178(2):480–485. doi: 10.1016/0006-291x(91)90132-q. [DOI] [PubMed] [Google Scholar]
- Kyprianou N., English H. F., Davidson N. E., Isaacs J. T. Programmed cell death during regression of the MCF-7 human breast cancer following estrogen ablation. Cancer Res. 1991 Jan 1;51(1):162–166. [PubMed] [Google Scholar]
- Kyprianou N., Isaacs J. T. Expression of transforming growth factor-beta in the rat ventral prostate during castration-induced programmed cell death. Mol Endocrinol. 1989 Oct;3(10):1515–1522. doi: 10.1210/mend-3-10-1515. [DOI] [PubMed] [Google Scholar]
- Lin J. K., Chou C. K. In vitro apoptosis in the human hepatoma cell line induced by transforming growth factor beta 1. Cancer Res. 1992 Jan 15;52(2):385–388. [PubMed] [Google Scholar]
- Lockshin R. A., Zakeri Z. F. Programmed cell death: new thoughts and relevance to aging. J Gerontol. 1990 Sep;45(5):B135–B140. doi: 10.1093/geronj/45.5.b135. [DOI] [PubMed] [Google Scholar]
- Massagué J., Czech M. P. The subunit structures of two distinct receptors for insulin-like growth factors I and II and their relationship to the insulin receptor. J Biol Chem. 1982 May 10;257(9):5038–5045. [PubMed] [Google Scholar]
- McGowan J. A., Strain A. J., Bucher N. L. DNA synthesis in primary cultures of adult rat hepatocytes in a defined medium: effects of epidermal growth factor, insulin, glucagon, and cyclic-AMP. J Cell Physiol. 1981 Sep;108(3):353–363. doi: 10.1002/jcp.1041080309. [DOI] [PubMed] [Google Scholar]
- Mead J. E., Fausto N. Transforming growth factor alpha may be a physiological regulator of liver regeneration by means of an autocrine mechanism. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1558–1562. doi: 10.1073/pnas.86.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazono K., Heldin C. H. Role for carbohydrate structures in TGF-beta 1 latency. Nature. 1989 Mar 9;338(6211):158–160. doi: 10.1038/338158a0. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Arakaki R., Ichihara A. Interleukin-1 beta is a potent growth inhibitor of adult rat hepatocytes in primary culture. Exp Cell Res. 1988 Dec;179(2):488–497. doi: 10.1016/0014-4827(88)90286-8. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Nishizawa T., Hagiya M., Seki T., Shimonishi M., Sugimura A., Tashiro K., Shimizu S. Molecular cloning and expression of human hepatocyte growth factor. Nature. 1989 Nov 23;342(6248):440–443. doi: 10.1038/342440a0. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Tomita Y., Hirai R., Yamaoka K., Kaji K., Ichihara A. Inhibitory effect of transforming growth factor-beta on DNA synthesis of adult rat hepatocytes in primary culture. Biochem Biophys Res Commun. 1985 Dec 31;133(3):1042–1050. doi: 10.1016/0006-291x(85)91241-0. [DOI] [PubMed] [Google Scholar]
- Oberhammer F., Bursch W., Parzefall W., Breit P., Erber E., Stadler M., Schulte-Hermann R. Effect of transforming growth factor beta on cell death of cultured rat hepatocytes. Cancer Res. 1991 May 1;51(9):2478–2485. [PubMed] [Google Scholar]
- Orrenius S., McConkey D. J., Bellomo G., Nicotera P. Role of Ca2+ in toxic cell killing. Trends Pharmacol Sci. 1989 Jul;10(7):281–285. doi: 10.1016/0165-6147(89)90029-1. [DOI] [PubMed] [Google Scholar]
- Rotello R. J., Lieberman R. C., Purchio A. F., Gerschenson L. E. Coordinated regulation of apoptosis and cell proliferation by transforming growth factor beta 1 in cultured uterine epithelial cells. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3412–3415. doi: 10.1073/pnas.88.8.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell W. E., Coffey R. J., Jr, Ouellette A. J., Moses H. L. Type beta transforming growth factor reversibly inhibits the early proliferative response to partial hepatectomy in the rat. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5126–5130. doi: 10.1073/pnas.85.14.5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandford N. L., Searle J. W., Kerr J. F. Successive waves of apoptosis in the rat prostate after repeated withdrawal of testosterone stimulation. Pathology. 1984 Oct;16(4):406–410. doi: 10.3109/00313028409084731. [DOI] [PubMed] [Google Scholar]
- Schulte-Hermann R., Timmermann-Trosiener I., Barthel G., Bursch W. DNA synthesis, apoptosis, and phenotypic expression as determinants of growth of altered foci in rat liver during phenobarbital promotion. Cancer Res. 1990 Aug 15;50(16):5127–5135. [PubMed] [Google Scholar]
- Scott C. D., Baxter R. C. Insulin-like growth factor-II receptors in cultured rat hepatocytes: regulation by cell density. J Cell Physiol. 1987 Dec;133(3):532–538. doi: 10.1002/jcp.1041330314. [DOI] [PubMed] [Google Scholar]
- Searle J., Kerr J. F., Bishop C. J. Necrosis and apoptosis: distinct modes of cell death with fundamentally different significance. Pathol Annu. 1982;17(Pt 2):229–259. [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Silberstein G. B., Daniel C. W. Reversible inhibition of mammary gland growth by transforming growth factor-beta. Science. 1987 Jul 17;237(4812):291–293. doi: 10.1126/science.3474783. [DOI] [PubMed] [Google Scholar]
- Strain A. J., Frazer A., Hill D. J., Milner R. D. Transforming growth factor beta inhibits DNA synthesis in hepatocytes isolated from normal and regenerating rat liver. Biochem Biophys Res Commun. 1987 May 29;145(1):436–442. doi: 10.1016/0006-291x(87)91340-4. [DOI] [PubMed] [Google Scholar]
- Wakefield L. M., Winokur T. S., Hollands R. S., Christopherson K., Levinson A. D., Sporn M. B. Recombinant latent transforming growth factor beta 1 has a longer plasma half-life in rats than active transforming growth factor beta 1, and a different tissue distribution. J Clin Invest. 1990 Dec;86(6):1976–1984. doi: 10.1172/JCI114932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Macaskill I. A., Currie A. R. Adrenocortical cell deletion: the role of ACTH. J Pathol. 1973 Oct;111(2):85–94. doi: 10.1002/path.1711110203. [DOI] [PubMed] [Google Scholar]
- Zarnegar R., Michalopoulos G. Purification and biological characterization of human hepatopoietin A, a polypeptide growth factor for hepatocytes. Cancer Res. 1989 Jun 15;49(12):3314–3320. [PubMed] [Google Scholar]