Abstract
Functionalized tryptamines are targets of interest for development as small molecule therapeutics. The ring opening of aziridines with indoles is a powerful method for tryptamine synthesis if site selectivity can be controlled. 4-Nitrobenzyl carbamate (PNZ)-protected aziridines undergo regioselective ring opening to produce β-substituted tryptamines for a series of indoles. The PNZ-protected tryptamines can be further manipulated by PNZ removal under mild conditions.
Keywords: Aziridine, Indole, Tryptamine, Ring opening, Regioselective
1. Introduction
The demand for nitrogen-containing small molecules facilitates the search for new pathways to construct chiral amine building blocks.1 The ring opening of aziridines is a well-established method for incorporating nitrogen into organic molecules.2–4 Despite significant history in the development of aziridine opening, new advances are continuing to provide unique bond constructions and product selectivities.5–9 The inherent ring strain in aziridines is sufficient to initiate reactivity, but the nitrogen atom is often modified with an electron-withdrawing (protecting) group for increased activation.10 The resulting product retains this moiety as a nitrogen protecting group which must be removed prior to nitrogen functionalization. Synthetic design must account for the aziridine activation group to ensure its removal is compatible with ancillary functional groups in the final product.
Sulfonamides stand out as the most commonly utilized aziridine protecting groups for ring opening reactions.5,7,10 A majority of these methodologies incorporate the p-toluenesulfonamide (Ts) substrates, which are easy to generate from alkenes.11–13 However, the subsequent Ts deprotections can be challenging, and alternative sulfonamide-protected aziridines have been developed.14–17 Carbamates, such as benzyl (Cbz) and tert-butyl (Boc), can be removed under very mild conditions, but are less common as aziridine protecting groups since rearrangement byproducts are possible.18–21 The 4-nitrobenzyl carbamate (PNZ) had been sparingly used as an aziridine activating group until recently.22–31 The PNZ group is more activating than Cbz and more resistant to cleavage under acidic conditions.
Our group has been interested in the development of novel aziridine ring opening reactions, particularly for N-acylaziridines.32,33 The addition of indoles to aziridines produces tryptamine derivatives, which are known to possess extensive biological activity.34,35 An initial report of indoles adding to aziridinium ions36 was followed by methods for opening carbamate-protected aziridines.37,38 Extensive effort has been made to control the final tryptamine structure in high yield,21,39–41 where unsymmetrical aziridines, such as 1, can open at either backbone carbon (Scheme 1). For carbamate-protected aziridines with a C2 ester substituent (R1), addition of indole to the least hindered carbon produces the α-substituted tryptamine (2). Yields of 2 have steadily improved as new Lewis acid catalysts are developed and side reactions are minimized.21 The synthesis of a β-substituted tryptamine (3) from a mono-substituted aziridine has only been reported in low yields for carbamate-protected aziridines;21,42 however, limited examples with alternative nitrogen protecting groups are known.33,43 This report details our discovery that PNZ-protected, alkyl substituted aziridines (4) undergo selective ring opening by C2 attack with indoles to produce β-substituted tryptamines (5).7,44,45 The tryptamine products can be readily functionalized at nitrogen after PNZ removal under mild conditions.
Scheme 1.
Synthesis of tryptamines by indole addition to aziridines.
2. Results
Aziridine 6 was synthesized in racemic and enantiopure form and subjected to ring opening in the presence of 1-methylindole (Scheme 2). The choice of BF3 as the Lewis acid promoter was directed by previous reports25–27,30,43 and unpublished data from our laboratory. The reaction conditions were remarkably mild with complete consumption of 6 in 1 h at −78 °C. The PNZ-protected tryptamine (7) was isolated in 71% yield as a single enantiomer as confirmed by HPLC46 Excess indole was employed, based on previous data, to maximize tryptamine yield.33
Scheme 2.
Regioselective, enantiospecific ring opening of aziridine 6.
Optimized conditions for the BF3-promoted tryptamine synthesis were applied for a series of functionalized indoles (Table 1). The racemic aziridine 6 and a meso disubstituted PNZ-aziridine (8) were chosen for the investigation. Reactions were performed in duplicate on a 1 mmol scale to generate sufficient tryptamine for further functionalization. The yield for ring opening of rac-6 ranged from good to excellent with a variety of indole substitution patterns (entries 1–7). Tryptamines were produced in high regioselectivity favoring attack at the more substituted aziridine carbon. The 2, 5, and 6 positions of a 1H-indole were well-tolerated with functional groups including an ether (entry 4) and halogens (entries 5–6). Slightly lower yields were observed for aziridine 8, compared to rac-6, with similar indoles (entries 8–12). The reactions with 2-methylindole (entries 2 and 9) produced the highest yields, indicating unidentified byproducts from attack at the indole C2 may be reducing product yield in the remaining examples.
Table 1.
Substrate scope for the synthesis of β-substituted aziridinesa
 | ||||||
|---|---|---|---|---|---|---|
| Entry | R1 | R2 | R3 | R4 | Product | Yield (%)b,c |
| 1 | CH3 | H | H | H | 9 | 60 |
| 2 | CH3 | H | 2–CH3 | H | 10 | 90d |
| 3 | CH3 | H | 5–CH3 | H | 11 | 62 |
| 4 | CH3 | H | 5–OCH3 | H | 12 | 75 |
| 5 | CH3 | H | 5–CI | H | 13 | 63 |
| 6 | CH3 | H | 6–F | H | 14 | 57 |
| 7 | CH3 | H | H | CH3 | rac-7 | 68 |
| 8 | –CH2CH2CH2CH2– | H | H | 15 | 60 | |
| 9 | –CH2CH2CH2CH2– | 2–CH3 | H | 16 | 76 | |
| 10 | –CH2CH2CH2CH2– | 5–CH3 | H | 17 | 56 | |
| 11 | –CH2CH2CH2CH2– | 5–OCH3 | H | 18 | 62 | |
| 12 | –CH2CH2CH2CH2– | H | CH3 | 19 | 48 | |
Reactions were performed with aziridine rac-6 or 8 (1 mmol), indole (3 mmol), and BF3·OEt2 (1 mmol) in CH2CI2 (0.17 M) for 2 h at −78 °C.
Isolated yield from an average of two runs.
Product regioisomer ratio was >10:1 based on 1H NMR of entries 1 and 3–7. Entries 8–12 were formed as a racemic mixture.
Regioisomer ratio of 7:1.
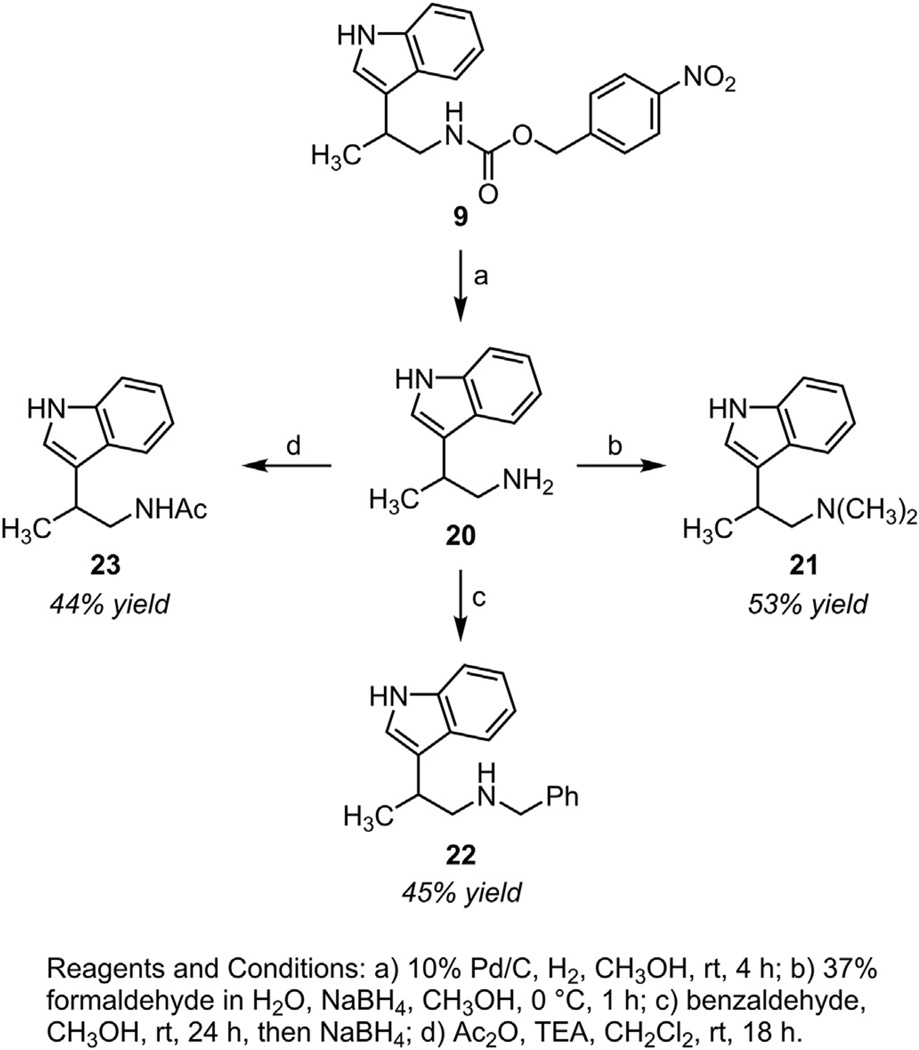
We envisioned access to PNZ-protected, β-substituted tryptamines would provide a single intermediate for the synthesis of biologically relevant tryptamine derivatives. To this end, the PNZ group of 9 was readily removed under standard hydrogenation conditions (Scheme 3). Tryptamine 20 was formed as a mixture with toluidine from PNZ reduction in quantitative yield. With this byproduct in mind, we employed literature methods to selectively functionalize the alkyl nitrogen of 20. Dimethylation47 (21) and benzylation48 (22) were accomplished by reductive amination in moderate yield. Site selective acylation led to the melatonin-like tryptamine 23 in modest yield.49 Studies are underway to functionalize each PNZ-tryptamine in Table 1 to generate a library of molecules for biological activity screening.
Scheme 3.
Deprotection and functionalization of PNZ-tryptamine 9.
A general method for the synthesis of β-substituted tryptamines by the addition of indoles to aziridines has been described. The PNZ protecting group functions to activate the aziridine for selective addition and is removed from the tryptamine product under mild conditions. Future experiments will be directed toward expansion of our β-substituted tryptamine library, and further development of ring opening reactions with underutilized PNZ-protected aziridines.
3. Experimental
3.1. General
1H NMR spectra were recorded on Bruker DRX (400 MHz) or Bruker Avance (600 MHz) spectrometer. Chemical shifts are reported in ppm from tetramethylsilane with the solvent resonance as the internal standard (CDCl3: 7.27 ppm). Data are reported as follows: chemical shift, multiplicity (s=singlet, d=doublet, t=triplet, q=quartet, br=broad, m=multiplet), coupling constants (Hz), and integration. 13C NMR spectra were recorded on a Bruker DRX 400 (100 MHz) or Bruker Avance 600 (151 MHz) spectrometer with complete proton decoupling. Chemical shifts are reported in ppm from tetramethylsilane with the solvent as the internal standard (CDCl3: 77.1 ppm). High resolution mass spectrometry was acquired with a Bruker MicrOTOF-Q II at the University of North Carolina Wilmington. Infrared (IR) spectra were obtained using a Nicolet iS5 FT-IR.
Liquid chromatography was performed using forced flow (flash chromatography) on silica gel (SiO2, 32–63 µm) purchased from Dynamic Absorbents Inc. Thin layer chromatography (TLC) was performed on EMD Chemicals 0.25 mm silica gel 60 plates. Visualization was achieved UV light (254 nm) or basic potassium permanganate in water followed by heating. High pressure liquid chromatography (HPLC) was performed on an HP instrument equipped with an autosampler and a UV detector.
All reactions were conducted in oven and flame dried glassware under an inert atmosphere of argon. All solvents were EMD Chemicals anhydrous solvents in Sure-Seal bottles sold by VWR International, unless otherwise noted. 2-Methylaziridine (90%) was purchased from Aldrich Chemical Company. 4-Nitrobenzyl chloroformate was purchased from the Acros Chemical Company. All reagents were used as received.
3.2. Representative procedure for synthesis of aziridine 6
A 250 mL round bottom flask with a Teflon®-coated magnetic stir bar was charged with 50 mL of THF and 20 mL of H2O. Solid sodium carbonate (6.36 g, 60 mmol) and 2-methylaziridine (1.57 mL, 20 mmol) were added to the stirred suspension at room temperature under argon. The flask was cooled to 0 °C where 4-nitrobenzyl chloroformate (4.67 g, 21 mmol) in 10 mL of THF was added dropwise. The mixture was stirred at 0 °C for 1 h and then diluted with 30 mL of H2O and 40 mL of ethyl acetate. The flask was warmed to room temperature and the organic layer was taken. The aqueous layer was extracted twice with 25 mL of ethyl acetate. The combined organics layer was then washed with 30 mL of brine and dried over MgSO4. The crude reaction was concentrated via rotary evaporation and further purified by silica gel chromatography (4:1 hexanes/acetone) to yield rac-6 as a white solid in 62% yield (3.23 g). An enantioenriched sample of 6 was synthesized from (S)-2-methylaziridine.50
3.2.1. 4-nitrobenzyl 2-methylaziridine-1-carboxylate
1H NMR (400 MHz, Chloroform-d) δ 8.23 (d, J=8.7 Hz, 2H), 7.54 (d, J=8.7 Hz, 2H), 5.27–5.18 (m, 2H), 2.61–2.53 (m, 1H), 2.38 (d, J=5.9 Hz, 1H), 2.02 (d, J=3.9 Hz, 1H), 1.32 (d, J=5.6 Hz, 3H). 13C NMR (151 MHz, Chloroform-d) δ 163.0, 147.9, 143.3, 128.5, 124.0, 66.6, 34.3, 32.9, 17.6. HRMS: calcd for : 259.0689 (M+Na+), found 259.0697 (M+Na+). IR (diamond ATR crystal): 1713, 1536, 2969, 2933, 3110, 3087.
3.3. Representative procedure for synthesis of aziridine 8
In a flame-dried round bottom flask equipped with a Teflon®-coated magnetic stir bar the lithium naphthalenide reagent was freshly prepared by vigorously stirring finely chopped lithium metal (1.0 equiv) and naphthalene (1.1 equiv) in dry THF (1 M) under argon for 24 h at room temperature.
A flame-dried round bottom flask equipped with a Teflon®-coated magnetic stir bar was charged with 3.77 g (15 mmol) of 7-tosyl-7-azabicyclo[4.1.0]heptane11 and 60 mL of THF. The solution was cooled to −78 °C in a dry ice and acetone bath. The lithium naphthalenide reagent was added dropwise until the dark green endpoint was observed (~40 mL of stock 1 M solution). The reaction was quenched with 20 mL of aqueous saturated sodium bicarbonate and slowly warmed to 0 °C. Solid 4-nitrobenzyl chloroformate (3.50 g, 16.2 mmol) was added all at once, and the suspension was stirred for a total of 2 h at 0 °C. After this time, the mixture was diluted with 20 mL of H2O and 30 mL of ethyl acetate. The flask was warmed to room temperature and the organic layer was taken. The aqueous layer was extracted twice with 20 mL of ethyl acetate. The combined organics layer was then washed with 30 mL of brine and dried over MgSO4. The crude reaction mixture was concentrated and further purified with silica gel chromatography (4:1 hexanes/ethyl acetate) to yield 8 as a white solid in 66% yield (2.74 g).
3.3.1. 4-nitrobenzyl 7-azobicyclo[4.1.0]heptane-7-carboxylate
1H NMR (600 MHz, Chloroform-d) δ 8.21 (d, J=8.7 Hz, 2H), 7.52 (d, J=8.7 Hz, 2H), 5.20 (s, 2H), 2.72–2.67 (m, 2H), 1.99–1.91 (m, 2H), 1.85–1.78 (m, 2H), 1.46–1.37 (m, 2H), 1.29–1.21 (m, 2H). 13C NMR (151 MHz, Chloroform-d) δ 163.5, 147.7, 143.5, 128.4, 123.8, 66.3, 37.4, 23.7, 19.8. HRMS: calcd for : 299.1002 (M+Na+), found 299.1014 (M+Na+). IR (diamond ATR crystal): 1348, 1534, 1709, 2854, 2934, 3316.
3.4. Enantiospecific synthesis of β-substituted tryptamine 7
A 1 dram vial equipped with a Teflon®-coated magnetic stir bar was purged with argon. The flask was charged with aziridine 6 (0.0236 g, 0.1 mmol) and 1-methylindole (0.0374 mL, 0.3 mmol). Dry DCM (0.5 mL, 0.20 M) was added, and the solution was cooled to −78 °C with a dry ice/acetone bath. A 1 M solution of BF3·OEt2 in DCM (0.1 mL, 0.1 mmol) was then added dropwise. The reaction was stirred for a total of 1 h at −78 °C and then quenched with 0.5 mL of aqueous saturated sodium bicarbonate. After slowly warming to room temperature, the mixture was extracted with 1 mL ethyl acetate three times and the combined organic layers were dried over MgSO4. The crude reaction mixture was then filtered and concentrated via rotary evaporation. The crude mixture was purified using silica gel chromatography (3:1 hexanes/ethyl acetate) afforded tryptamine 7 as a viscous yellow oil in 71% yield (0.0260 g).
3.4.1. 4-Nitrobenzyl (2-(1-methyl-1H-indol-3-yl)propyl) carbamate
1H NMR (400 MHz, Chloroform-d) δ 8.19 (d, J=8.5 Hz, 2H), 7.65 (d, J=7.9 Hz, 1H), 7.45 (d, J=8.5 Hz, 2H), 7.34 (d, J=8.1 Hz, 1H), 7.26 (t, J=7.2 Hz, 1H), 7.00 (t, J=7.5 Hz, 1H) 6.89 (s, 1H), 5.19 (d, J=13.4 Hz, 1H), 5.16 (d, J=13.4 Hz, 1H), 4.87 (br s, 1H), 3.77 (s, 3H), 3.59–3.52 (m, 1H), 3.52–3.44 (m, 1H), 3.35–3.26 (m, 1H), 1.40 (d, J=7.0 Hz, 3H). 13C NMR (151 MHz, CDCl3) δ 156.0, 147.6, 144.3, 137.3, 128.1, 127.1, 125.7, 123.8, 122.0, 119.3, 119.0, 117.0, 109.5, 65.1, 47.5, 32.8, 31.5, 18.9. HRMS: calcd for : 390.1424 (M+Na+), found 390.1433 (M+Na+). IR (diamond ATR crystal): 1514, 1716, 2875, 2929, 2961, 3411.
3.5. Representative for the synthesis of β-substituted tryptamines
A flame-dried round bottom flask equipped with a Teflon®-coated magnetic stir bar was purged with argon. The flask was charged with the required aziridine (1 mmol) and indole (3 mmol). Dry DCM (5 mL, 0.20 M) was added, and the solution was cooled to −78 °C with a dry ice/acetone bath. A 1 M solution of BF3·OEt2 in DCM (1 mL, 1 mmol) was then added dropwise. The reaction was stirred for a total of 2 h at −78 °C and then quenched with 5 mL of aqueous saturated sodium bicarbonate. After slowly warming to room temperature, the mixture was extracted with 10 mL ethyl acetate three times and the combined organic layers were dried over MgSO4. The crude reaction mixture was then filtered and concentrated via rotary evaporation. The crude mixture was purified using silica gel chromatography yielding the respective β-substituted tryptamine.
3.5.1. 4-Nitrobenzyl (2-(1H-indol-3-yl)propyl)carbamate (9)
Synthesized from 0.2362 g of rac-6 and 0.3514 g of 1H-indole by the standard procedure. Purification by silica gel chromatography (3:2 hexanes/ethyl acetate) afforded a yellow solid in 71% yield (0.2499 g). 1H NMR (600 MHz, Chloroform-d) δ 8.20 (d, J=8.4 Hz, 2H), 8.04 (br s, 1H), 7.66 (d, J=8.0 Hz, 1H), 7.45 (d, J=8.3 Hz, 2H), 7.40 (d, J=8.1 Hz, 1H), 7.23 (t, J=7.6 Hz, 1H), 7.13 (t, J=7.5 Hz, 1H), 7.05 (s, 1H), 5.19 (d, J=12.0 Hz, 1H), 5.15 (d, J=12.0 Hz, 1H), 4.82 (br s, 1H), 3.60–3.46 (m, 2H), 3.36–3.26 (m, 1H), 1.41 (d, J=7.0 Hz, 3H).13C NMR (151 MHz, Chloroform-d) δ 156.1, 147.6, 144.3, 136.7, 128.1, 126.7, 123.8, 122.4, 120.9, 119.6, 119.2, 118.6, 111.5, 65.1, 47.3, 31.6, 18.7. HRMS: calcd for : 376.1267 (M+Na+), found 376.1275 (M+Na+). IR (diamond ATR crystal): 1509, 1715, 3313, 3405.
3.5.2. 4-Nitrobenzyl (2-(2-methyl-1H-indol-3-yl)propyl) carbamate (10)
Synthesized from 0.2362 g of rac-6 and 0.3935 g of 2-methylindole by the standard procedure. Purification by silica gel chromatography (1% acetone in DCM) afforded a yellow solid in 90% yield (0.3327 g) 1H NMR (600 MHz, Chloroform-d) δ 8.18 (d, J=8.6 Hz, 2H), 7.89 (s, 1H), 7.59 (d, J=7.9 Hz, 1H), 7.42 (d, J=8.4 Hz, 2H), 7.30 (d, J=8.1 Hz, 1H), 7.14 (t, J=7.5 Hz, 1H), 7.07 (t, J=7.5 Hz, 1H), 5.15 (s, 2H), 4.77 (br s 1H), 3.74–3.67 (m, 1H), 3.48–3.41 (m, 1H), 3.28–3.19 (m, 1H), 2.36 (s, 3H), 1.44 (d, J=7.1 Hz, 3H). 13C NMR (151 MHz, Chloroform-d) δ 155.8, 147.5, 144.2, 135.5, 131.6, 128.0, 127.0, 123.7, 123.7, 121.1, 119.2, 118.9, 112.5, 110.6, 64.9, 46.4, 31.7, 18.3, 12.0. HRMS: calcd for : 390.1424 (M+Na+), found 390.1437 (M+Na+). IR (diamond ATR crystal): 1514, 1701, 2926, 2959, 3333, 3398.
3.5.3. 4-Nitrobenzyl (2-(5-methyl-1H-indol-3-yl)propyl) carbamate (11)
Synthesized from 0.2362 g of rac-6 and 0.3935 g of 5-methylindole by the standard procedure. Purification by silica gel chromatography (3:2 hexanes/ethyl acetate) afforded a yellow solid in 65% yield (0.2431 g). 1H NMR (600 MHz, Chloroform-d) δ 8.18 (d, J=8.7 Hz, 2H), 8.02 (br s, 1H), 7.44 (d, J=2.0 Hz, 2H), 7.43 (s, 1H), 7.28 (d, J=8.3 Hz, 1H), 7.06 (d, J=8.3 Hz, 1H), 6.99 (s, 1H), 5.17 (s, 2H), 4.85 (br s, 1H), 3.60–3.53 (m, 1H), 3.53–3.46 (m, 1H), 3.33–3.24 (m, 1H), 2.46 (s, 3H), 1.40 (d, J=7.0 Hz, 3H). 13C NMR (151 MHz, Chloroform-d) δ 156.0, 147.5, 144.2, 134.9, 128.7, 128.0, 126.8, 123.9, 123.7, 121.0, 118.8, 117.9, 111.0, 65.0, 47.1, 31.5, 21.6, 18.7. Calculated for : 390.1424 (M+Na+), found 390.1422 (M+Na+). IR (diamond ATR crystal): 1509, 1714, 2849, 2912, 2950, 3309, 3386.
3.5.4. 4-Nitrobenzyl (2-(5-methoxy-1H-indol-3-yl)propyl) carbamate (12)
Synthesized from 0.2362 g of rac-6 and 0.4415 g of 5-methoxylindole by the standard procedure. Purification by silica gel chromatography (1% THF in DCM) afforded an orange solid in 73% yield (0.2951 g). 1H NMR (600 MHz, Chloroform-d) δ 8.18 (d, J=8.9 Hz, 2H), 8.08 (br s, 1H), 7.43 (d, J=8.6 Hz, 2H), 7.28 (d, J=8.7 Hz, 1H), 7.10 (d, J=2.5 Hz, 1H), 7.01 (d, J=2.5 Hz, 1H), 6.89 (dd, J=8.8, 2.5 Hz, 1H), 5.18 (s, 2H), 4.90 (br s, 1H), 3.86 (s, 3H), 3.58–3.45 (m, 2H), 3.33–3.21 (m, 1H), 1.39 (d, J=7.0 Hz, 3H). 13C NMR (151 MHz, Chloroform-d) δ 156.1, 154.0, 147.6, 144.3, 131.8, 128.1, 127.1, 123.8, 121.6, 118.3, 112.4, 112.2, 101.2, 65.1, 56.1, 47.3, 31.5, 18.6. HRMS: calcd for : 406.1373 (M+Na+), found 406.1386 (M+Na+). IR (diamond ATR crystal): 1514, 1712, 2868, 2911, 2952, 3323, 3396.
3.5.5. 4-Nitrobenzyl (2-(5-chloro-1H-indol-3-yl)propyl) carbamate (13)
Synthesized from 0.2362 g of rac-6 and 0.4548 g of 5-chloroindole by the standard procedure. Purification by silica gel chromatography (3:2 hexanes/ethyl acetate) afforded a yellow solid in 65% yield (0.2551 g). 1H NMR (600 MHz, Chloroform-d) δ 8.20 (br s, 1H), 8.18 (d, J=8.3 Hz, 2H), 7.61 (s, 1H), 7.44 (d, J=8.3 Hz, 2H), 7.30 (d, J=8.6 Hz, 1H), 7.16 (d, J=8.6, 1H), 7.06 (s, 1H), 5.17 (s, 2H), 4.86 (br s, 1H), 3.59–3.48 (m, 1H), 3.48–3.38 (m, 1H), 3.29–3.21 (m, 1H), 1.38 (d, J=7.0 Hz, 3H). 13C NMR (151 MHz, Chloroform-d) δ 156.0, 147.5, 144.1, 134.8, 128.0, 127.8, 125.2, 123.7, 122.6, 122.1, 118.6, 118.4, 112.4, 65.1, 47.4, 31.4, 18.6. HRMS: calcd for : 410.0878 (M+Na+), found 410.0886 (M+Na+). IR (diamond ATR crystal): 1510, 1704, 2913, 3287, 3406.
3.5.6. 4-Nitrobenzyl (2-(6-fluoro-1H-indol-3-yl)propyl) carbamate (14)
Synthesized from 0.2362 g of rac-6 and 0.4053 g of 6-fluoroindole by the standard procedure. Purification by silica gel chromatography (3:2 hexanes/ethyl acetate) to afforded a yellow solid in 57% yield (0.2107 g). 1H NMR (600 MHz, Chloroform-d) δ 8.20 (d, J=8.4 Hz, 2H), 8.02 (br s, 1H), 7.56 (dd, J=8.8, 5.3 Hz, 1H), 7.46 (d, J=8.4 Hz, 2H), 7.07 (d, J=9.6, 1H), 7.01 (s, 1H), 6.89 (t, J=9.1 Hz, 1H), 5.17 (s, 2H), 4.83 (br s, 1H), 3.50 (t, J=6.4 Hz, 2H), 3.33–3.23 (m, 1H), 1.39 (d, J=7.0 Hz, 3H). 13C NMR (151 MHz, Chloroform-d) δ 160.0 (d, J=237.9 Hz), 156.1, 147.5, 144.2, 136.5 (d, J=12.4 Hz), 128.0, 123.7, 123.3, 121.1 (d, J=3.5 Hz), 119.8 (d, J=10.1 Hz), 118.6, 108.2 (d, J=24.5 Hz), 97.7 (d, J=25.8 Hz), 65.1, 47.3, 31.5, 18.5. HRMS: calcd for : 394.1173 (M+Na+), found 394.1183 (M+Na+). IR (diamond ATR crystal): 1708, 2954, 2914, 3284, 3397.
3.5.7. 4-Nitrobenzyl (2-(1H-indol-3-yl)cyclohexyl)carbamate (15)
Synthesized from 0.2763 g of 8 and 0.3514 g of 1H-indole by the standard procedure. Purification by silica gel chromatography (2:1 hexanes/ethyl acetate) afforded a yellow solid in 73% yield (0.2903 g) 1H NMR (400 MHz, Chloroform-d) δ 8.18 (br s, 1H), 8.05 (d, J=8.0 Hz, 2H), 7.64 (d, J=7.6 Hz, 1H), 7.39 (d, J=7.4 Hz, 1H), 7.22 (t, J=7.2 Hz, 1H), 7.13 (t, J=7.1 Hz, 2H), 7.09 (d, J=7.1 Hz, 1H), 7.00 (s, 1H), 5.03 (d, J=13.6 Hz, 1H), 4.90 (d, J=13.6 Hz, 1H), 4.77 (br s, 1H), 3.92–3.77 (m, 1H), 2.85–2.73 (m, 1H), 2.32–2.23 (m, 1H), 2.16–2.05 (m, 1H), 1.94–1.77 (m, 2H), 1.65–1.31 (m, 4H). 13C NMR (151 MHz, Chloroform-d) δ 155.4, 147.3, 144.5, 136.2, 127.4, 127.2, 123.6, 122.0, 120.8, 119.4, 118.7, 118.3, 111.4, 64.6, 55.2, 41.5, 34.7, 34.6, 26.3, 25.5. HRMS: calcd for : 416.1581 (M+Na+), found 416.1586 (M+Na+). IR (diamond ATR crystal): 1512, 1712, 2855, 2928, 3311, 3401.
3.5.8. 4-Nitrobenzyl (2-(2-methyl-1H-indol-3-yl)cyclohexyl) carbamate (16)
Synthesized from 0.2763 g of 8 and 0.3935 g of 2-methylindole by the standard procedure. Purification by silica gel chromatography (1% acetone in DCM) afforded a yellow solid in 78% yield (0.3112 g). 1H NMR (600 MHz, DMSO-d6) δ 10.65 (s, 1H), 8.07 (d, J=8.2 Hz, 2H), 7.54 (d, J=7.9 Hz, 1H), 7.21 (d, J=8.0 Hz, 2H), 7.18–7.01 (m, 2H), 7.01–6.79 (m, 2H), 4.99 (d, J=14.5 Hz, 1H), 4.87 (d, J=14.5 Hz, 1H), 3.90 (m, 1H), 2.74 (m, 1H), 2.33 (s, 3H), 2.05–1.65 (m, 4H), 1.54–1.20 (m, 4H). 13C NMR (151 MHz, DMSO-d6) δ 154.7, 146.6, 145.7, 135.2, 131.7, 127.2, 126.9, 123.3, 119.3, 118.8, 117.6, 112.0, 110.2, 63.1, 52.8, 40.5, 34.1, 32.5, 26.1, 25.3. HRMS: Calculated for : 430.1737 (M+Na+), found 430.1736 (M+Na+). IR (diamond ATR crystal): 1508, 1708, 2854, 2912, 3339, 3399.
3.5.9. 4-Nitrobenzyl (2-(5-methyl-1H-indol-3-yl) cyclohexyl) carbamate (17)
Synthesized from 0.2763 g of 8 and 0.3935 g of 5-methylindole by the standard procedure. Purification by silica gel chromatography (3:2 hexanes/ethyl acetate) afforded a yellow solid in 59% yield (0.2422 g). 1H NMR (600 MHz, Chloroform-d) δ 8.05 (d, J=8.1 Hz, 2H), 8.02 (br s, 1H), 7.40 (s, 1H), 7.27 (d, J=8.1 Hz, 1H), 7.11 (d, J=8.3 Hz, 2H), 7.04 (d, J=8.2 Hz, 1H), 6.97 (s, 1H), 5.02 (d, J=13.7 Hz, 1H), 4.91 (d, J=13.7 Hz, 1H), 4.74 (br s, 1H), 3.88–3.76 (m, 1H), 2.82–2.68 (m, 1H), 2.45 (s, 3H), 2.32–2.24 (m, 1H), 2.14–2.06 (m, 1H), 1.92–1.77 (m, 2H), 1.64–1.30 (m, 4H). 13C NMR (151 MHz, Chloroform-d) δ 155.4, 147.3, 144.5, 134.6, 128.6, 127.4, 123.6, 121.0, 118.3, 117.8, 111.1, 64.6, 55.1, 41.5, 34.8, 34.6, 26.3, 25.5, 21.7. Calculated for : 430.1737 (M+Na+), found 430.1735 (M+Na+). IR (diamond ATR crystal): 1520, 1700, 2865, 2917, 3338, 3390, 3407.
3.5.10. 4-Nitrobenzyl (2-(5-methoxy-1H-indol-3-yl) cyclohexyl) carbamate (18)
Synthesized from 0.2763 g of 8 and 0.4415 g of 5-methoxylindole by the standard procedure. Purification by silica gel chromatography (2% acetone in DCM) afforded a yellow solid in 63% yield (0.2626 g). 1H NMR (400 MHz, Chloroform-d) δ 8.08 (d, J=8.7 Hz, 2H), 7.87 (br s, 1H), 7.28 (d, J=8.7 Hz, 1H), 7.16 (d, J=8.7 Hz, 2H), 7.03 (s, 2H), 6.88 (dd, J=8.8, 2.4 Hz, 1H), 5.03 (d, J=13.8 Hz, 1H), 4.93 (d, J=13.8 Hz, 1H), 4.67 (br s, 1H), 3.86 (s, 3H), 3.87–3.73 (m, 1H), 2.78–2.65 (m, 1H), 2.31–2.21 (m, 1H), 2.16–2.06 (m, 1H), 1.92–1.76 (m, 2H), 1.61–1.29 (m, 4H). 13C NMR (151 MHz, Chloroform-d) δ 155.4, 154.0, 147.3, 144.5, 131.4, 127.7, 127.5, 123.7, 121.7, 118.0, 112.0, 111.9, 100.9, 64.6, 56.1, 55.2, 41.5, 34.7, 34.6, 26.3, 25.5. HRMS: calcd for : 446.1686 (M+Na+), found 446.1688 (M+Na+). IR (diamond ATR crystal): 1515, 1692, 2849, 2913, 2935, 3322, 3409.
3.5.11. 4-Nitrobenzyl (2-(1-methyl-1H-indol-3-yl) cyclohexyl) carbamate (19)
Synthesized from 0.2763 g of 8 and 0.375 mL of 1-methylindole by the standard procedure. Purification by silica gel chromatography (3:2 hexanes/ethyl acetate) afforded a yellow solid in 63% yield (0.2607 g). 1H NMR (600 MHz, Chloroform-d) δ 8.07 (d, J=8.3 Hz, 2H), 7.63 (d, J=7.8 Hz, 1H), 7.33 (d, J=8.2 Hz, 1H), 7.25 (d, J=7.8 Hz, 1H), 7.15 (d, J=8.3 Hz, 2H), 7.12 (t, J=7.5 Hz, 1H), 6.90 (s, 1H), 5.05 (d, J=13.9 Hz, 1H), 4.94 (d, J=13.9 Hz, 1H), 4.74 (br s, 1H), 3.86–3.76 (m, 1H), 3.71 (s, 3H), 2.84–2.72 (m, 1H), 2.35–2.26 (m, 1H), 2.16–2.05 (m, 1H), 1.92–1.78 (m, 2H), 1.65–1.28 (m, 4H). 13C NMR (151 MHz, Chloroform-d) δ 155.3, 147.3, 144.6, 137.0, 127.6, 127.5, 125.6, 123.6, 121.6, 118.9, 118.8, 116.9, 109.5, 64.6, 55.2, 41.4, 35.1, 34.6, 32.8, 26.3, 25.5. HRMS: calcd for : 430.1737 (M+Na+), found 430.1737 (M+Na+). IR (diamond ATR crystal): 1521, 1542, 1692, 2848, 2915, 3328.
3.6. Representative procedure for the tryptamine functionalization
An oven-dried 1-dram vial equipped with a Teflon®-coated magnetic stir bar was purged with argon. The vial was charged with 0.0706 g (0.2 mmol) of tryptamine 9 and 0.0400 g (0.02 mmol) of 10% palladium on carbon (activated, wet). Dry methanol (1.0 mL) was added under an argon atmosphere. Hydrogen gas was bubbled into solution for 5 min, then the hydrogen atmosphere was maintained under balloon pressure. The reaction was stirred for 4 h after which no starting material remained. The solution was filtered through a Celite© plug using excess methanol, and the crude filtrate was concentrated via rotary evaporation. Unpurified tryptamine 20 was used without purification for subsequent reactions.
3.6.1. 2-(1H-indol-3-yl)-N,N-dimethylpropan-1-amine (21)
Synthesized from tryptamine 20 (0.33 mmol) by a published procedure.47 Purification by silica gel chromatography (10% methanol and 1% NH4OH in DCM) afforded a white solid in 53% yield (0.0359 g). 1H NMR (400 MHz, Chloroform-d) δ 8.03 (br s, 1H), 7.66 (d, J=7.9 Hz, 1H), 7.35 (d, J=8.1, 1H), 7.22–7.16 (m, 1H), 7.15–7.09 (m, 1H), 7.00 (d, J=2.4 Hz, 1H), 3.33–3.20 (m, 1H), 2.61 (dd, J=12.0, 5.7 Hz, 1H), 2.49 (dd, J=12.0, 9.2 Hz, 1H), 2.30 (s, 6H), 1.41 (d, J=6.8 Hz, 3H). 13C NMR (151 MHz, CDCl3) δ 136.6, 126.9, 122.0, 121.0, 120.3, 119.3, 119.2, 111.4, 67.1, 46.1, 29.3, 19.7.
3.6.2. N-benzyl-2-(1H-indol-3-yl)propan-1-amine (22)
Synthesized from tryptamine 20 (0.2 mmol) by a published procedure.48 Purification by silica gel chromatography (5–7.5% methanol in DCM) afforded a yellow oil in 45% yield (0.0238 g). 1H NMR (400 MHz, Chloroform-d) δ 8.17 (br s, 1H), 7.68 (d, J=7.9 Hz, 1H), 7.38 (d, J=8.1 Hz, 1H), 7.35–7.19 (m, 6H), 7.13 (t, J=8.0 Hz, 1H), 7.00 (s, 1H), 3.86 (d, J=13.4, 1H), 3.81 (d, J=13.4, 1H), 3.42–3.30 (m, 1H), 3.04 (dd, J=11.4, 7.9 Hz, 1H), 2.91 (dd, J=11.4, 6.1 Hz, 1H), 2.17 (br s, 1H), 1.42 (d, J=6.9 Hz, 3H).13C NMR (151 MHz, CDCl3) δ 140.2, 136.7, 128.5, 128.2, 127.0, 126.7, 122.1, 120.9, 119.7, 119.5, 119.3, 111.4, 55.5, 53.8, 31.5, 19.8.
3.6.3. N-(2-(1H-indol-3-yl)propyl)acetamide (23)
Synthesized from tryptamine 20 (0.2 mmol) by a published procedure.49 Purification by silica gel chromatography (2:1 ethyl acetate/hexanes) afforded a white solid in 44% yield (0.0191 g). 1H NMR (400 MHz, Chloroform-d) δ 8.25 (br s, 1H), 7.67 (d, J=7.9 Hz, 1H), 7.40 (d, J=8.1 Hz, 1H), 7.22 (t, J=7.5, 1H), 7.14 (t, J=7.5, 1H), 7.05 (d, J=2.4 Hz, 1H), 5.46 (br s, 1H), 3.73–3.64 (m, 1H), 3.45 (ddd, J=13.2, 8.2, 5.1 Hz, 1H), 3.35–3.24 (m, 1H), 1.89 (s, 3H), 1.38 (d, J=7.0 Hz, 3H). 13C NMR (151 MHz, CDCl3) δ 170.3, 136.7, 126.8, 122.4, 120.9, 119.6, 119.2, 118.9, 111.5, 45.8, 31.3, 23.5, 19.0.
Supplementary Material
Acknowledgments
The authors are grateful to the National Institute of General Medical Sciences of the National Institutes of Health (R15GM114766) for financial support. The HRMS data was collected from a Bruker MicrOTOF-Q II instrument purchased with funds from the National Science Foundation (CHE-1039784) and University of North Carolina Wilmington. The 600 MHz NMR data was obtained using a Bruker instrument purchased with funds from the National Science Foundation (CHE-0821552).
Footnotes
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supplementary data (1H and 13C NMR spectra for compounds 6–19 and 21–23 and HPLC data for compound 6 and 7.) associated with this article can be found in the online version, at http://dx.doi.org/10.1016/j.tet.2016.03.031.
References and notes
- 1.Nugent TC, editor. Chiral Amine Synthesis. Weinheim: Wiley-VCH GmbH & Co. KGaA; 2010. [Google Scholar]
- 2.Tanner D. Angew. Chem. Int. Ed. Engl. 1994;33:599–619. [Google Scholar]
- 3.McCoull W, Davis FA. Synthesis. 2000:1347–1365. [Google Scholar]
- 4.Dahanukar VH, Zavialov IA. Curr. Opin. Drug Dis. Dev. 2002;5:918–927. [PubMed] [Google Scholar]
- 5.Hu XE. Tetrahedron. 2004;60:2701–2743. [Google Scholar]
- 6.Krake SH, Bergmeier SC. Tetrahedron. 2010;66:7337–7360. [Google Scholar]
- 7.Lu PF. Tetrahedron. 2010;66:2549–2560. [Google Scholar]
- 8.Callebaut G, Meiresonne T, De Kimpe N, Mangelinckx S. Chem. Rev. 2014;114:7954–8015. doi: 10.1021/cr400582d. [DOI] [PubMed] [Google Scholar]
- 9.Huang CY, Doyle AG. Chem. Rev. 2014;114:8153–8198. doi: 10.1021/cr500036t. [DOI] [PubMed] [Google Scholar]
- 10.Sweeney JB. Chem. Soc. Rev. 2002;31:247–258. doi: 10.1039/b006015l. [DOI] [PubMed] [Google Scholar]
- 11.Ando T, Kano D, Minakata S, Ryu I, Komatsu M. Tetrahedron. 1998;54:13485–13494. [Google Scholar]
- 12.Jeong JU, Tao B, Sagasser I, Henniges H, Sharpless KB. J. Am. Chem. Soc. 1998;120:6844–6845. [Google Scholar]
- 13.Müller P, Fruit C. Chem. Rev. 2003;103:2905–2919. doi: 10.1021/cr020043t. [DOI] [PubMed] [Google Scholar]
- 14.Maligres PE, See MM, Askin D, Reider P. J. Tetrahedron Lett. 1997;38:5253–5256. [Google Scholar]
- 15.Dauban P, Dodd RH. J. Org. Chem. 1999;64:5304–5307. doi: 10.1021/jo990356x. [DOI] [PubMed] [Google Scholar]
- 16.Gontcharov AV, Liu H, Sharpless KB. Org. Lett. 1999;1:783–786. doi: 10.1021/ol990761a. [DOI] [PubMed] [Google Scholar]
- 17. Bornholdt J, Felding J, Clausen RP, Kristensen JL. Chem.—Eur. J. 2010;16 doi: 10.1002/chem.201001026. 12474 where R12471 is typically a protected ester12480.
- 18.Tomasini C, Vecchione A. Org. Lett. 1999;1:2153–2156. [Google Scholar]
- 19.Cardillo G, Gentilucci L, Gianotti M, Tolomelli A. Synlett. 2000:1309–1311. [Google Scholar]
- 20.Lucarini S, Tomasini C. J. Org. Chem. 2001;66:727–732. doi: 10.1021/jo005583+. [DOI] [PubMed] [Google Scholar]
- 21.Tirotta I, Fifer NL, Eakins J, Hutton CA. Tetrahedron Lett. 2013;54:618–620. [Google Scholar]
- 22.Mente PG, Heine HW. J. Org. Chem. 1971;36:3076–3078. [Google Scholar]
- 23.Baldwin JE, Adlington RM, Robinson NG. J. Chem. Soc. Chem. Commun. 1987:153–155. [Google Scholar]
- 24.Mauger AB, Burke PJ, Somani HH, Friedlos F, Knox RJ. J. Med. Chem. 1994;37:3452–3458. doi: 10.1021/jm00047a002. [DOI] [PubMed] [Google Scholar]
- 25.McKeever B, Pattenden G. Tetrahedron. 2003;59:2701–2712. [Google Scholar]
- 26.Liu HQ, Pattabiraman VR, Vederas JC. Org. Lett. 2007;9:4211–4214. doi: 10.1021/ol701742x. [DOI] [PubMed] [Google Scholar]
- 27.Liu W, Chan ASH, Liu HQ, Cochrane SA, Vederas JC. J. Am. Chem. Soc. 2011;133:14216–14219. doi: 10.1021/ja206017p. [DOI] [PubMed] [Google Scholar]
- 28.O’Brien K, Kelleher F. Tetrahedron Lett. 2013;54:6627–6630. [Google Scholar]
- 29.O’Brien K, Proinsias KO, Kelleher F. Tetrahedron Lett. 2013;54:2395–2397. [Google Scholar]
- 30.Bleriot Y, Tran AT, Prencipe G, Jagadeesh Y, Auberger N, Zhu S, Gauthier C, Zhang YM, Desire J, Adachi I, Kato A, Sollogoub M. Org. Lett. 2014;16:5516–5519. doi: 10.1021/ol502929h. [DOI] [PubMed] [Google Scholar]
- 31.O’Brien K, Proinsias KO, Kelleher F. Tetrahedron. 2014;70:5082–5092. [Google Scholar]
- 32.Martin A, Casto K, Morris W, Morgan JB. Org. Lett. 2011;13:5444–5447. doi: 10.1021/ol202410v. [DOI] [PubMed] [Google Scholar]
- 33.Cockrell J, Wilhelmsen C, Rubin H, Martin A, Morgan JB. Angew. Chem. Int. Ed. 2012;51:9842–9845. doi: 10.1002/anie.201204224. [DOI] [PubMed] [Google Scholar]
- 34.Kochanowska-Karamyan AJ, Hamann MT. Chem. Rev. 2010;110:4489–4497. doi: 10.1021/cr900211p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greene SL. Novel Psychoactive Substances: Classification, Pharmacology, and Toxicology. Elsevier Inc; 2013. pp. 363–381. http://www.sciencedirect.com/science/book/9780124158160. [Google Scholar]
- 36.Pfeil E, Harder U. Angew. Chem. Int. Ed. 1967;6:178. [Google Scholar]
- 37.Sato K, Kozikowski AP. Tetrahedron Lett. 1989;30:4073–4076. [Google Scholar]
- 38.Shima I, Shimazaki N, Imai K, Hemmi K, Hashimoto M. Chem. Pharm. Bull. 1990;38:564–566. doi: 10.1248/cpb.35.3527. [DOI] [PubMed] [Google Scholar]
- 39.Bennani YL, Zhu GD, Freeman JC. Synlett. 1998:754–756. [Google Scholar]
- 40.Nishikawa T, Ishikawa M, Wada K, Isobe M. Synlett. 2001:945–947. [Google Scholar]
- 41.Nishikawa T, Kajii S, Wada K, Ishikawa M, Isobe M. Synthesis. 2002:1658–1662. [Google Scholar]
- 42.Harada H, Fujii A, Odai O, Kato S. Org. Process Res. Dev. 2004;8:238–245. [Google Scholar]
- 43.Farr RN, Alabaster RJ, Chung JYL, Craig B, Edwards JS, Gibson AW, Ho GJ, Humphrey GR, Johnson SA, Grabowski EJJ. Tetrahedron: Asymmetry. 2003;14:3503–3515. [Google Scholar]
- 44.Stankovic S, D’hooghe M, Catak S, Eum H, Waroquier M, Van Speybroeck V, De Kimpe N, Ha H. J. Chem. Soc. Rev. 2012;41:643–665. doi: 10.1039/c1cs15140a. [DOI] [PubMed] [Google Scholar]
- 45.Ghorai MK, Tiwari DP, Jain N. J. Org. Chem. 2013;78:7121–7130. doi: 10.1021/jo401028j. [DOI] [PubMed] [Google Scholar]
- 46.The absolute stereochemistry of 7 was not rigorously determined in this study. Inversion of stereochemistry is inferred from previous work with Nosylprotected aziridines (see Ref. 43)
- 47.Bosch J, Roca T, Armengol M, Fernandez-Forner D. Tetrahedron. 2001;57:1041–1048. [Google Scholar]
- 48.Martin DBC, Vanderwal CD. J. Am. Chem. Soc. 2009;131:3472–3473. doi: 10.1021/ja900640v. [DOI] [PubMed] [Google Scholar]
- 49.Schuck DC, Jordao AK, Nakabashi M, Cunha AC, Ferreira VF, Garcia CRS. Eur. J. Med. Chem. 2014;78:375–382. doi: 10.1016/j.ejmech.2014.03.055. [DOI] [PubMed] [Google Scholar]
- 50.Veitia MSI, Brun PL, Jorda P, Falguieres A, Ferroud C. Tetrahedron: Asymmetry. 2009;20:2077–2089. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.