Introduction
Cardiac arrhythmias are a major cause of morbidity and mortality in heart disease, and are the likely cause of more than a quarter of a million deaths annually in the U.S. alone. While our current approach to reducing suffering and death from arrhythmias relies largely on diagnosis and therapy aimed at early recognition and treatment, the benefits of prevention could be even more substantial. As we consider how the approach to arrhythmias may evolve in the coming decades, it is useful to consider the great breadth of presentations and scenarios that are encountered. Human arrhythmias occur in two scenarios. First, and particularly early in life, they arise as a consequence of inborn (congenital) constellations of anatomy and electrophysiology that change over time. There may be no structural cardiac abnormality (e.g., long QT syndrome), a minimal structural abnormality (e.g., an accessory pathway causing WPW) or a severe structural abnormality (e.g., endocardial cushion defect with heart block). Secondly, arrhythmias emerge later in life as a consequence of acquired disease (e.g., ventricular tachycardia late after myocardial infarction) or aging (atrial fibrillation). Genetic susceptibilities influence all aspects of the pathophysiology. As approaches to prevention and therapy evolve, genetic influences will receive increasing attention.
Diagnostic Strategies
Advances in Monitoring and Detection of Arrhythmias: Moving Care from the Office to the Home
Mobile applications and monitors are revolutionizing the way we interact with patients who have or are at risk for arrhythmias. Over the past decade, major advances have occurred in both external and implantable monitoring devices. External ambulatory monitors have decreased in size and complexity; while at the same time the storage capacity has increased. Self-contained wireless “patch” monitors that self-adhere to the patient’s chest are capable of recording and storing single lead ECG recordings for up to 30 days. Monitoring can be extended further, up to 3 years, with subcutaneously implanted loop recorders. These implanted devices have already been miniaturized to the size of less than half of a triple-A battery such that an injection-like technique can be used for implantation. These types of monitors will replace traditional Holter monitors and looping recording devices over the next decade. Ongoing clinical trials will be critical in establishing the efficacy and cost-effectiveness of new technologies relative to conventional methods of monitoring patients. Improvements in size, ease of use, functionality, accuracy, and longevity over the next decade will spur on the current movement to extend the use of these monitors beyond traditional diagnostic indications to their use as screening tools in targeted populations, as will be discussed later.
In addition to current FDA-approved medical devices, there has been an explosion of direct-to-consumer hand held or wearable monitors (i.e., watches), mobile phone adaptors, and smart phone applications that allow patients to monitor their own heart rhythm1, as well as other biologic parameters such as blood pressure, glucose, etc2. Recent developments include both multiple lead recording from smart phones and continuous recording from a “smartwatch.” The rapid development of these technologies has outpaced their real-world validation, and large-scale, pragmatic studies are needed to determine their accuracy and how they might be incorporated into medical care. Many other issues will need to be addressed before these monitors enter standard clinic practice. The security of the recorded data is an important consideration. Legal issues of responsibility for data accuracy and reliability will emerge, as when a dangerous arrhythmia is not recognized, or misinterpretation of artifact results in an expensive emergency room evaluation, and need to be solved. If the devices are used to improve health, will they be paid for; will the time that physicians spend evaluating these recordings be reimbursed? In the next decade, it is likely that we will work through these issues, and these technologies will be woven into practice, providing us with a way of easily monitoring our patients and transitioning a greater proportion of care to the home setting through electronic data transfer and communication.
The transition to more sophisticated care at home has already transformed the care of patients with implantable cardiac pacemakers and defibrillators. The automatic monitoring and reporting that this technology provides has reduced the frequency of office visits and has allowed more rapid diagnosis and treatment of arrhythmias and device malfunctions. Physicians are notified quickly regarding actionable events, rather than having to wait for patients to be seen at scheduled office visits, or to present urgently when the arrhythmia reaches the threshold to cause symptoms. In large scale studies, the use of remote monitoring has been associated with improvements in survival and a decrease in adverse outcomes, such as hospitalizations3, 4. Over the next decade, advances in this arena will include direct electronic messaging/transmission of remote monitoring data to patients and physicians through electronic health records, coupled with the ability to alter the programming of implantable devices through remote platforms. In order for the latter to become a reality, technological advances will be required to address potential security, safety, and privacy issues.
Implantable Devices and Lead Technology
In addition to the advances in remote monitoring described above, there have been significant advances in pacing and implantable defibrillator technology over the past decade. The relationships between ventricular activation sequence and cardiac mechanical performance are now better appreciated, and will become more completely understood. Even in the setting of infra-His block and bundle branch block5, ventricular activation over the Purkinje system can now be achieved in many patients with direct pacing of the His bundle, providing a more physiologic alternative to RV pacing that should avoid pacing induced ventricular dysfunction6, as well as provide an alternative to left ventricular pacing for CRT7. Advances in the lead technology will increase ease and use of this form of pacing. When activation of the ventricles from the His Purkinje system is not feasible, LV pacing for cardiac resynchronization therapy (CRT) will continue to be important for patients with depressed ventricular function associated with left bundle branch block. Surprisingly, CRT is often beneficial even though present implementation is limited to the few LV pacing sites accessible through the coronary venous system. The advent of pacing leads with multiple electrodes for placement in the coronary venous system is a notable advance, that incontrast to traditional bipolar leads, provides multiple LV pacing configurations from which to select the optimal site for LV pacing without compromising lead stability. This option also addresses problems of phrenic nerve stimulation and high pacing thresholds that often limit delievery of LV pacing. These leads will also allow performance of simultaneous pacing from multiple LV sites, which may improve mechanical performance in situations other than left bundle branch block6.
There will be continued advances in disruptive leadless technologies for pacing and defibrillation, which will change the way we deliver these therapies to an increasing proportion of patients. The intravascular that are the mainstay of current pacing and defibrillator systems are recognized as a weak link in the system, susceptible to long-term “wear and tear” due to mechanical stress, and providing a portal and nidus for intravascular infections that occur in up to 2% of patients.8 The vulnerability of transvenous leads was notably underscored by the recent accelerated failure rates of some of the newer generation high-voltage ICD leads, which resulted in widespread need for re-operations for lead revision/extraction along with adverse patient outcomes due to inappropriate shocks and failure to deliver therapy.9 For all of these reasons, safer and more durable alternatives to transvenous lead systems are highly anticipated major breakthroughs in device therapy.
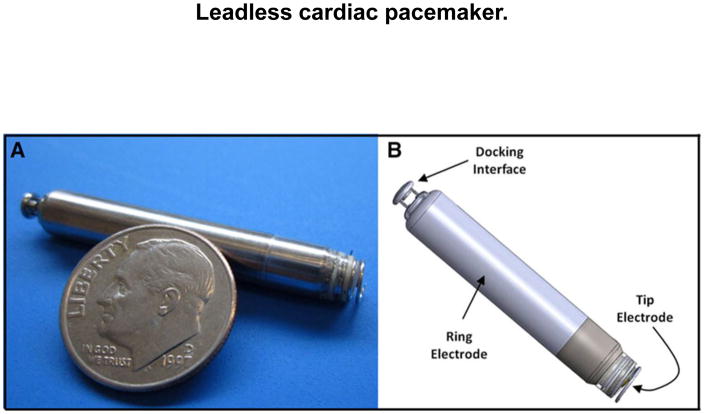
Current leadless pacing systems involve a self-contained leadless single chamber pacemaker delivered via catheter and attached to the right ventricle via a femoral vein, eliminating the need for the traditional surgical pocket. These 0.8–1 cm3 “bullet shaped” devices contain all of the pacemaker electronics, lithium battery, and electrodes (Figure 1)10. The longevity of the current battery is reported to be up to 15 years, but long-term studies are not yet available. Two recent, feasibility and safety studies involving 1251 patients demonstrated acceptable short-term performance of two versions of the leadless pacemakers with major complications rates (4.0% to 6.5%) reported to be similar to historical controls11, 12. The latter finding needs to be interpreted with caution. The comparator control population was not randomized and contained a high proportion of patients with dual chamber devices, where the complication rate is known to be higher. The current leadless pacing systems are limited to single chamber pacing. However, dual chamber and CRT leadless systems will be developed utilizing multiple self-contained pacing devices that will have the ability to communicate with one another. CRT systems will include both endocardial and epicardial LV pacing approaches. Endocardial LV devices that link via ultrasound to a subcutaneous transmitter have already been developed. With these devices, pacing sites for resynchronization will no longer be limited by coronary sinus venous anatomy.
Figure 1.
Leadless cardiac pacemaker. A, Picture of the leadless cardiac pacemaker with a US dime to indicate scale. B, Rendering of the device with pertinent components labeled10.
With respect to leadless defibrillator systems, the current version of the subcutaneous ICD (S-ICD) involves an extra- thoracic subcutaneous electrode tunneled subcutaneously along the sternum and connected to an S-ICD generator implanted subcutaneously in the mid-axillary line13, 14. Disadvantages of the present system include: absence of pacing for bradycardia, resynchronization or tachycardia termination, inadequate sensing in ~10% of patients, large size of the generator, undesirable cosmetics, and cost15. For these reasons, some centers restrict the device to select patients who have poor vascular status, are at high risk for intravascular infection, or young patients receiving primary prevention devices who are at particularly high risk of complications from intravascular of leads16; other centers are more liberal in their apporach17. These systems will evolve. The size of the generator will decrease, and leadless pacemakers will be incorporated to deliver bradycardia and anti-tachycardia pacing. Epicardial LV leadless pacemakers and/or sensors linked to the S-ICD could even permit CRT.
For these leadless technologies to be truly transformative over the next decade, the technology will need to advance the point where the efficacy, safety, durability, and costs are comparable to current transvenous systems. Long-term studies will need to be performed to determine whether these devices remain safe and effective over time and are as durable as transvenous devices. To date, limited data exist relating to infection and failure risk of chronically implanted leadless pacemakers, and it is unclear how these issues will be managed. Although many concerns will need to be addressed, these technologies will have a much stronger presence in the future.
Ablation Strategies
Tachyarrhythmias have either an anatomic source (automatic focus or reentry circuit) and/or initiating factors (triggering foci, autonomic influences) that can be targeted for ablation. Catheter ablation is an effective treatment for a variety of arrhythmias, including most supraventricular tachycardias, many ventricular tachycardias, and perhaps most impressively over the past decade, some forms of atrial fibrillation. These efforts have been greatly aided by better understanding of anatomy and the ability to incorporate imaging with cardiac MRI, CT and ultrasound into electrophysiologic mapping systems. Ablation failures are related to inadequate localization of the arrhythmia substrate, inability to permanently damage the substrate and, for some, inadequate understanding of the pathophysiologic mechanisms.
Localization of the arrhythmia source (mapping) has improved greatly with better tools for mapping and incorporation of electrophysiologic data into anatomic displays in electroanatomic mapping systems. However, the fundamental method of recording and processing cardiac electrical activity from catheters in clinical use has not changed substantially over two decades. More recently, an appreciation of the need to improve recording quality to achieve detection of very low amplitude signals, particularly to guide ablation of scar related arrhythmias and some idiopathic arrhythmias, has begun to receive appropriate attention.18, 19 Better mapping catheters and recording equipment with refined signal processing will facilitate definition of arrhythmias and further improve our understanding of pathophysiology and ability to target ablation.
The ability to derive detailed cardiac electrophysiologic information, well beyond that of the standard ECG, noninvasively from body surface recordings is already here.20–22 While initial efforts largely reflect activation at the epicardial surface, combining this information with anatomic imaging will further refine localization of arrhythmia substrate, both prior to the ablation procedure, and in real time in the electrophysiology laboratory.
Imaging technologies will continue to improve ablation efficacy. The ability to detect and define scar, which participates in the arrhythmia substrate for ventricular and atrial arrhythmias in heart disease and aging, has great potential to better define the arrhythmia substrate and guide ablation.23, 24 Pre-procedure cardiac MR imaging to define areas of fibrosis that are associated with reentry or focal arrhythmias is now in common use. Resolution is not quite sufficient to define microscopic arrhythmia sources or reentry channels, but progress is being made. The concept of directing ablation to anatomic substrate defined from noninvasive imaging has been demonstrated and will become a reality as imaging resolution improves.18, 24 CT imaging which has better spatial resolution but is perhaps more limited in distinguishing fibrous scar from myocytes, and ultrasound imaging, also presently limited in the ability to define the fine structure of scars, is none the less showing promise.25, 26 The use of nuclear pharmaceuticals to assess abnormal innervation in the arrhythmogenic area may also provide further insights into arrhythmogenesis and its prevention.27 Pre-procedure imaging can be combined with noninvasive body surface electrocardiogram recordings to refine characterization of the arrhythmia substrate.22
Incorporating real time imaging in the electrophysiology laboratory also offers the prospect of improving the creation of durable ablation lesions (see below), although substantial challenges need to be overcome.28 Greater resolution will need to be attained, likely without increasing MR field strength. What is learned from MR imaging will be applied to adapt modalities that are easier to use in the EP laboratory environment, notably ultrasound imaging, for the purpose of defining arrhythmia substrate targets. However, the trade off of better substrate definition with pre-acquired imaging versus use of real time imaging systems in the EP laboratory that will allow assessment of ablation lesions as they are created will likely remain for some time.
New technologies will also facilitate the creation of more effective and permanent ablation lesions. Presently ablation is achieved with a thermal injury, either heating or freezing. These methods require firm contact between the catheter applying the energy and the tissue and this contact can now be assessed with both imaging (intracardiac ultrasound) and force sensing catheters. The next step is real time assessment of lesion creation which can be potentially provided by methods to measure tissue temperature and assess necrosis.29–31 High resolution recording methods also have the potential to better define effective ablation lesions. While radiofrequency current will remain a simple, safe and easily applied energy source for ablation, a number of other energy sources for ablation will find applications for difficult arrhythmia substrates. The inadequate lesion depth for some intramural substrates will be addressed with bipolar ablation from electrodes bracketing the target area, intramural needle ablation, and alternative energy sources, such as microwave and ultrasound energy, which do not require tissue contact to produce deep tissue heating.31–34
Eventually advances in imaging, body surface recordings and radiation therapy will combine to allow noninvasive ablation of arrhythmogenic substrates using stereotactic application of external beam radiation or focused ultrasound.34, 35 Ablation with some forms of radiation will be fundamentally different from present methods not only in application, but also in the time course of the effect, which occurs later after the application, and which will therefore not be suitable for achieving urgent control of arrhythmias, but may be well suited for addressing many chronic arrhythmias, notably atrial fibrillation and recurrent sustained monomorphic VT.
Adverse effects of ablation by heating or freezing are largely due to unintended injury to adjacent structures (such as the esophagus for AF ablation) or excessive heating causing steam pops and perforation or charring of the endocardium creating a nidus for thrombus. These effects can potentially be avoided with the use of biologic therapies that create fibrous scar by inducing formation of fibroblasts or by direct injection into the target region.36
Pharmacologic Therapies
Antiarrhythmic drugs that target ion channels have had important but limited efficacy in treating arrhythmias. The limited differences between normal myocytes and those causing arrhythmias contributes to a narrow useful therapeutic window for these agents. Pharmacologic agents that modulate cell coupling will be evaluated further but may share similar issues. Diminishing or improving cell coupling will likely have off target effects, as cell coupling is important in all tissues. Selective drug delivery to the target area may address these concerns. Novel methods of guiding drugs to the desired target, as with the use of magnetic nanoparticles, could mitigate some of these problems. Modulation of conduction has the potential for both arrhythmia suppression and proarrhythmia. Pharmacologic therapies may have greater promise for prevention by influencing processes that promote arrhythmogenesis, such as by slowing age related apoptosis and fibrosis.
Autonomic Therapies
Cardiac arrhythmias are modulated by the effects of the autonomic nervous system. Therapies to modulate autonomic outflow to the heart, such as stimulation of the spinal cord, carotid body, and vagus nerve, and targeted ablation of autonomic fibers, such as the perivascular renal nerves, stellate ganglia and upper thoracic sympathetic ganglia, and cardiac ganglia are already available or in trials, but our understanding of the physiology involved is limited. 37 Refined methods for autonomic modulation have promise to reduce arrhythmias and also to favorably influence the underlying structural heart disease. As these therapies are refined, more targeted approaches will be developed. These will be important adjuvants to combined approaches to prevent arrhythmias in high risk patients.
Genomic Therapies and Approaches to Arrhythmias
Over the last decade, there have been major advances in our understanding of the genetic architecture of rare inherited arrhythmic syndromes and common arrhythmias such as atrial fibrillation and sudden cardiac death38, 39. In the next decade, advances in our ability to perform genome wide sequencing and high throughput functional assays of presumed pathogenic genetic variants in large patient populations will usher in personalized medicine approaches for the treatment and prevention of arrhythmias. Once these are readily available, large-scale clinical studies will be designed to test utilization of this information for optimizing therapeutic approaches to both common arrhythmias such as AF and VT and rare inherited arrhythmic diseases (IAS) such as LQTS, Brugada, etc. For example, genetic information may be used to both prognosticate adverse outcomes warranting more aggressive therapies and to identify subgroups that will or will not respond to particular therapeutic approaches, such as ablation and/or specific drugs. Data from ongoing large-scale genomic studies will also identify novel drug and/or genetic targets leading to the development of new pharmacologic and/or genetic approaches to the treatment and prevention of arrhythmias.
Gene therapy for arrhythmias will proceed beyond animal models and reach the clinical trial stage over the next decade. Interventional and/or other targeted delivery techniques (such as exosomes) will be developed that will allow us to test genetic modulation approaches for a variety of arrhythmias40. In addition to the potential for biologic pacing, genetic therapeutic approaches to modulation of AV nodal and other conduction tissue(s) have potential utility for rate control in atrial fibrillation and treatment of supraventricular arrhythmias. 41 Targeted modulation of ion channel components and associated proteins that affect cell-to cell coupling and intracellular matrix formation designed to reduce myocardial electrical heterogeneity and fibrosis may have utility as therapeutic and preventive strategies for atrial fibrillation, ventricular tachycardia, and other reentrant arrhythmias.42, 43 There are substantial hurdles. The means of identifying the areas to be treated and delivery systems may be available sooner than the required advances in myocyte biology. Proarrhythmia, will be a major challenge, and destructive methods of ablation may be needed to resculpt areas of new myocyte function.42
Sudden Death and Ventricular Arrhythmias
Prevention of Sudden Cardiac Death
In the western world, coronary heart disease (CHD) still underlies the majority of SCDs. Despite major advances over the past decade in treatment and prevention of CHD and landmark trials proving the efficacy of implantable cardioverter defibrillators (ICDs) in preventing SCD in patients with significant systolic dysfunction44, declines in SCD rates45 have not kept pace with declines in other causes of CHD death46–48. There also appears to be a growing fraction of SCD not due to CHD and/or ventricular arrhythmias, particularly among certain subsets of the population49. These latter findings have profound implications for our current SCD prevention strategies focused on CHD prevention and ICDs. To date, prior work in this arena has focused on stratifying risk among patients with low left ventricular ejection fractions (LVEF), who, although at increased risk, comprise a minority of the population who goes on to experience a sudden cardiac arrest50.. As such, the societal impact is limited51. In the future, SCD prevention will need to move beyond this narrow focus and encompass SCD prevention strategies in broader populations at varying levels of risk. This will not only involve the expansion of indications for the ICD through the use of combinations of novel markers to identify patient populations at a high risk for arrhythmic death, but also the development of novel strategies for preventing SCD in broader populations with more modest elevations in risk. Technologic advances in implantable loop recorders and/or external monitoring devices will have a major impact on how we approach SCD risk stratification and resuscitation. Not only will they be utilized for ongoing risk assessment, but with the development of localization and transmission capabilities, these monitors could be integrated into the emergency response system to allow rapid dispatch of emergency medical technicians at the time when life-threatening ventricular arrhythmias are detected. Technologic advances in the wearable external defibrillator, such as a decrease in size and ease of use, could allow this technology to be expanded to broader populations as a more permanent “destination therapy” rather than a temporary “bridge therapy” to the ICD52. Similarly, developments in the S-ICD could also expand the landscape of patients treated with defibrillator therapy. In all these areas, there will be increasing pressure to address cost-effectiveness as well as to refine risk stratification approaches.
There is also growing recognition that despite our best efforts to identify patients who may be at higher risk of SCD, there will always be a significant proportion of patients for whom SCD is their first encounter with the healthcare system. In these patients, SCD can only be prevented through population-based interventions and/or improvements in resuscitation of cardiac arrest. Several major advances in CPR and post resuscitation care53 have resulted in improved resuscitation rates from OHCA54, 55 There will continue to be several advances in the latter arena including population-based CPR and first responder initiatives coupled with better allocation of AEDs. There are already mobile applications that alert potential first responders to the location of a nearby cardiac arrest and AED to facilitate rapid CPR and defibrillation, and likely these type of technologies will continue to expand. There is also the potential for automated mobile AEDs that are dispatched to the site of cardiac arrest by drone transport. Even with these advances, in the absence of a transmitting implanted or external monitoring device that alerts responders, an arrest will need to be witnessed for resuscitation to take place. Given that the majority of arrests are unwitnessed54, in the future preventive measures applied to the population at large may have a great impact on SCD incidence. Data from epidemiologic studies support the notion that a significant fraction of SCD in the population could be prevented through the adoption of healthy lifestyle56, 57. AHA and other health agency and governmental public health initiatives to reduce weight, quit smoking, eat a healthy diet, and exercise moderately have gained momentum58, 59, and providers have begun to recognize the importance of prevention. In our future healthcare environment, this emphasis on prevention will continue to grow, as will public participation in preventative interventions, which should serve to lower the incidence of SCD.
Ventricular tachycardia in Structural Heart Disease
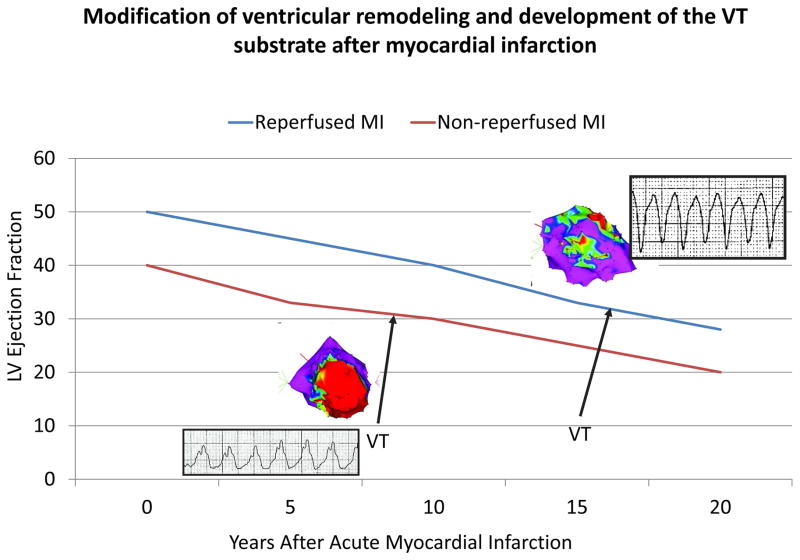
In patients with structural heart disease, sustained monomorphic VT is usually due to reentry involving regions of ventricular scar. In patients with myocardial infarction, early reperfusion therapy has reduced these arrhythmias and delayed their development (Figure 2). As the success in prevention and treatment of coronary disease continue, nonischemic cardiomyopathies and congenital and genetic heart diseases will comprise an increasing proportion of patients who require management for ventricular arrhythmias. The location and characteristics of the scar are important determinants of the risk of this arrhythmia. Advanced imaging methods combined with noninvasive electrocardiogram recordings will define high risk scars and delineate the potential reentry circuit channels they contain. 25, 60 The approach to VT and sudden death prevention will be tailored to the heart disease and individual patient. Patients at risk can receive implantable monitors for continuous monitoring of markers for increasing risk, and thresholds at which implementation of a therapeutic strategy for protection, as with an implanted arrhythmia treatment device, such as a defibrillator, will be defined. High risk individuals with anatomic substrates that can be targeted for ablation will receive this therapy to prevent VT. Such an approach is already starting to be realized for patients with repaired Tetralogy of Fallot and studies in animal models support its feasibility for infarct related VTs.61 However, the arrhythmia substrate continues to evolve with time and therapies that slow progression of the arrhythmia substrate will be critically important and employed in patients with and at risk for scar-related arrhythmias.
Figure 2.
The impact of acute reperfusion on the development of recurrent sustained VT in patients referred for catheter ablation late after surviving acute myocardial infarction. Compared to patients who did not have acute reperfusion at the time of infarction, those with successful reperfusion develop recurrent VT an average of 10 years later, have smaller, more mottled infarct scars (as indicated in the voltage maps of the LV shown in the figure) and generally have faster VTs.81
Atrial Fibrillation
Atrial fibrillation (AF), the most common arrhythmia requiring treatment, is a major and increasing health problem in adults.62,63–65 The prevalence in the U.S. and European Union of approximately 11 million people in 2010 is projected to increase to 24 to 30 million by 2050.63 Potential reasons for this precipitous rise in AF prevalence include a combination of the aging population, improved longevity from predisposing cardiovascular disease, improved monitoring and detection of AF, and rising rates of predisposing risk factors66. Rates of obesity have exploded, and a substantial proportion of AF in contemporary populations is attributable to obesity and hypertension66, 67.
Presently we are focused on treatment of clinically detected atrial fibrillation, primarily when it becomes highly symptomatic. The future will involve earlier identification and treatment of AF to prevent progression to more therapeutically resistent forms and to reduce AF-related adverse outcomes. AF promotes electrophysiologic and structural changes that further promote and sustain AF, and early initiation of strategies to maintain sinus rhythm might prevent or delay AF-related morbidity and mortality. Previously discussed advances in monitoring technologies will facilitate prompt recognition of AF, and the role of possible precursor arrhythmias that may indicate susceptibility, such as atrial tachycardia and premature atrial beats will be defined. Population-based screening for AF will become a reality, and we will subsequently need to evaluate the effectiveness of therapies and interventions within this population68. Early identification of AF and its precursors will allow for prompt implementation of therapies to prevent stroke, reduce progression of atrial fibrosis, and maintain sinus rhythm, all of which should meaningfully improve outcomes and delay or prevent emergence of symptomatic atrial fibrillation.
As ablation therapies continue to improve in efficacy and safety, early ablation will find a role in this strategy and continue to be used to treat patients with established AF. The arrhythmia substrate is complex and better definition of optimal ablation strategies and targets will be achieved as insights from basic and clinical studies of the arrhythmia are translated to the clinic. If ongoing randomized trials of AF ablation demonstrate a significant benefit on morbidity and mortality, then the population of patients undergoing AF ablation will expand. At the same time, there will be financial pressures placed on the electrophysiology community to better identify patients likely to respond to and/or benefit from AF ablation since multiple repeat procedures are unlikely to be reimbursed in our changing health care environment. There will also be increasing emphasis on the role of AF risk factor and lifestyle modification, particularly weight reduction and blood pressure control, as adjuncts to ablation and other traditional therapeutic approaches to prevent recurring AF episodes and to improve quality of life among patients with AF69, 70.
AF contributes to 25% of strokes in the US; thus, stroke prevention in AF will remain of primary importance.63,71 For many years, there was major doubt that there would ever be an alternative to warfarin. The last decade has changed all of that, and now we have FDA approved direct acting oral anticoagulants. With the advent of reversal agents, and the eventual lowering of costs, it is likely that these agents will become the dominant anticoagulant for AF patients. As anticoagulants continue to evolve and their risk benefit profiles improve, the indications for these agents in AF will expand. However, as was learned from the failure of dabigitran to protect patients with mechanical valves, agents that provide a targeted approach to therapeutic interruption of the coagulation cascade need to be carefully studied in diverse patient populations.72 Clinical trials will determine the efficacy of anticoagulantion implemented according to risk in new patient populations even before AF emerges. There will still remain a need for alternatives to anticoagulants in patients at high risk for bleeding. Further data will be accrued to clarify the role of therapies that exclude the left atrial appendage from the circulation. Trials in the high risk elderly will be particularly important as this is population where the need is great. Beyond stroke, attention will also be directed toward AF-related complications that currently comprise a higher proportion of AF-related mortality, in particular heart failure and sudden cardiac death73. AF patients may be a defined high risk population who might be candidates for long-term monitoring of ventricular arrhythmias and/or in whom to test preventive strategies for heart failure beyond maintenence of sinus rhythm.
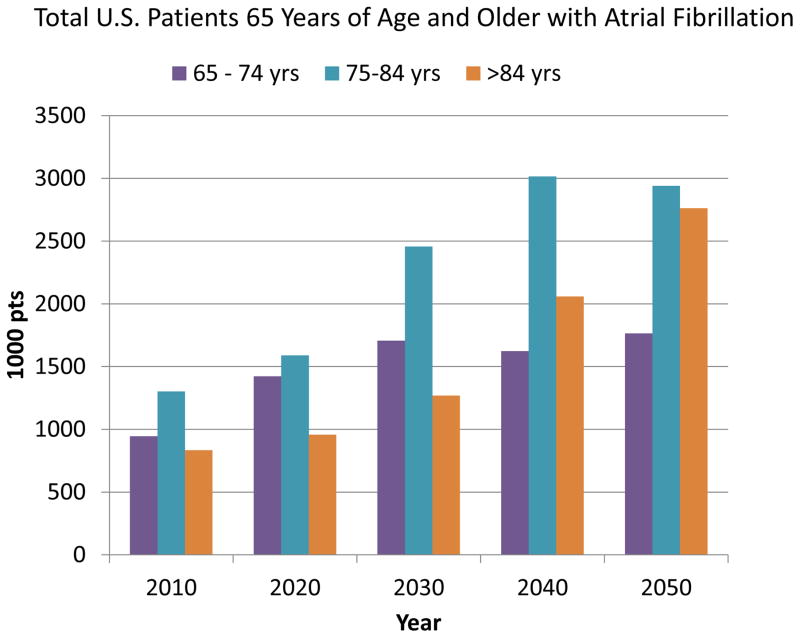
The greatest impact will likely be achieved by preventive strategies that prevent or delay initial emergence of AF. Over the last decade, the knowledge base regarding contemporary risk factors for AF in the population has expanded. AF will continue to be an important disease of the elderly, emerging progressively later in life, as discussed below (figure 3). However, there are other risk factors for AF that are modifiable and amenable to broad-based public interventions74. These easily measured risk factors have also been combined into AF risk scores75, 76 that could be utilized alone or in combination with advanced monitoring techniques to identify patient populations at high risk for AF. Such targeted populations could then allow potential preventive interventions to be tested in adequately powered cost-effective manner.
Figure 3.
Projection of the total numbers of patients age 65 years and older with atrial fibrillation over the next 40 years. The number of patients with AF will increase in all age groups with a disproportionate increase in the very elderly. Data extrapolated from US census projection and Medicare data and assumes no increase in AF risk for individual age groups.71, 79
Arrhythmias in Structurally Normal Hearts
Therapies of paroxysmal supraventricular tachycardia and idiopathic ventricular arrhythmias are already at an advanced level. As ablation therapies continue to evolve in the treatment of more difficult arrhythmias, the knowledge and technology accrued will also be applied to patients with more benign arrhythmias that are not associated with structural heart disease. Safer, more effective, curative out-patient procedures will be an increasing first line therapy for these arrhythmias and reduce the number of patients with arrhythmias that can not be adequately treated by ablation.77
General Trends
Aging
Much cardiac disease is a consequence of, or importantly exacerbated by aging. The development of atrial fibrosis that diminishes cell to cell coupling, facilitates emergence of automaticity and reentry leading to AF in some, bradyarrhythmias from sinus node dysfunction and AV block in others, and both in many elderly people. As effective treatments delay cardiac aging, these diseases will not disappear, but their emergence will be shifted later in life. Therapeutic considerations will have to evolve. A few years ago placing an ICD in an octagenerian was rare, it is now common place. From 1935 to 2010 the crude death rate in the US fell by 27%, but adjusting for the aging population, the age-adjusted death rate fell by 60%, with a 41% decrease between 1969 and 2010.78 By 2050, 20% of the US population will be 65 years of age or older and 4% (7.5 million) will be older than 85 years.79 By this date people older than 65 years will outnumber those younger than 15 years of age for most countries.80 Physicians will continue to be called on to address arrhythmias occurring in concert with co-morbid diseases. Even now, over half of patients 65 years of age and older with AF have at least 5 other chronic conditions, such as cancer, dementia, hypertension, renal disease, and heart failure.79 Utilization of technologies that have benefit for increasing survival in the last decade of life will require continued assessment for appropriate utilization. Developing rational strategies for the application of detection and treatment strategies in the face of competing risks from comorbidities, will remain an ongoing process as the population and therapies evolve.
Shifting heart disease etiologies
Although cardiovascular disease remains the leading cause of death in the US and accounts for 30% of global deaths, deaths from coronary heart disease in the US are declining, with a 37% reduction from 2002 to 2011.63 Better population health from addressing atherosclerotic heart disease risk factors and better treatment of heart disease are paying off. Coronary artery disease and its consequences, while still affecting millions of people, will continue to decline. In contrast, congential heart disease in adults is increasing. Approximately 40,000 infants are born with congenital heart disease in the US annually and mortality is declining.63 There are approximately 1.6 million people with congenital heart disease in the US and this population is anticipated to increase by 1 to 5% per year, with more than half being adults.63 There is no reason to suspect a decline in other nonischemic cardiomyopathies. Consequently, patients with nonischemic forms of heart disease will comprise an increasing portion of patients who require arrhythmia management.
Conclusions
The next decades will see substantial evolution of diagnostic and therapeutic approaches that will reduce suffering and death from arrhythmias. Some approaches that have great promise will fail, others will succeed. New insights from basic science could provide paradigm shifting approaches. Atrial fibrillation and scar related VTs from coronary disease will be encountered in an increasingly elderly patient population, and a greater proportion of younger patients with arrhythmias will have recognized genetic predispositions and etiologies. This latter heterogeneous population will benefit from strategies tailored to the disease process and patient characteristics. At the core of physician ministrations, will remain the human interaction between the patient and the health care provider in the continued quest to tailor and optimize preventive, diagnostic, and treatment strategies for the individual.
Footnotes
Disclosures
William Stevenson is co-holder of a patent for needle ablation that is consigned to Brigham and Women’s hospital and his spouse has received research support from St Jude Medical, inc.
Christine Albert is the Principal Investigator on research grants received from National Heart, Lung, and Blood Institute and St. Jude Medical Inc to identify biologic markers of sudden cardiac death.
Contributor Information
Christine M. Albert, Professor of Medicine Harvard Medical School, Director, Center for Arrhythmia Prevention, Division of Cardiovascular and Preventive Medicine, Brigham and Women’s Hospital.
William G. Stevenson, Professor of Medicine, Harvard Medical School, Director, Cardiac Arrhythmia Program, Division of Cardiovascular Medicine, Brigham and Women’s Hospital.
Bibliography
- 1.Walsh JA, Topol EJ, Steinhubl SR. Novel wireless devices for cardiac monitoring. Circulation. 2014;130:573–581. doi: 10.1161/CIRCULATIONAHA.114.009024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burke LE, Ma J, Azar KM, Bennett GG, Peterson ED, Zheng Y, Riley W, Stephens J, Shah SH, Suffoletto B, Turan TN, Spring B, Steinberger J, Quinn CC American Heart Association Publications Committee of the Council on E, Prevention BCCotCoCHCoC, Stroke Nursing CoFG, Translational Biology CoQoC, Outcomes R, Stroke C. Current science on consumer use of mobile health for cardiovascular disease prevention: A scientific statement from the american heart association. Circulation. 2015;132:1157–1213. doi: 10.1161/CIR.0000000000000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akar JG, Bao H, Jones P, Wang Y, Varosy P, Masoudi FA, Stein K, Saxon LA, Normand S-LT, Curtis JP. Use of remote monitoring is associated with lower risk of adverse outcomes among patients with implanted cardiac defibrillators. Circulation: Arrhythmia and Electrophysiology. 2015 doi: 10.1161/CIRCEP.114.003030. [DOI] [PubMed] [Google Scholar]
- 4.Varma N, Piccini JP, Snell J, Fischer A, Dalal N, Mittal S. The relationship between level of adherence to automatic wireless remote monitoring and survival in pacemaker and defibrillator patients. J Am Coll Cardiol. 2015;65:2601–2610. doi: 10.1016/j.jacc.2015.04.033. [DOI] [PubMed] [Google Scholar]
- 5.El-Sherif N, Amay YLF, Schonfield C, Scherlag BJ, Rosen K, Lazzara R, Wyndham C. Normalization of bundle branch block patterns by distal his bundle pacing. Clinical and experimental evidence of longitudinal dissociation in the pathologic his bundle. Circulation. 1978;57:473–483. doi: 10.1161/01.cir.57.3.473. [DOI] [PubMed] [Google Scholar]
- 6.Sharma PS, Dandamudi G, Naperkowski A, Oren JW, Storm RH, Ellenbogen KA, Vijayaraman P. Permanent his-bundle pacing is feasible, safe, and superior to right ventricular pacing in routine clinical practice. Heart Rhythm. 2015;12:305–312. doi: 10.1016/j.hrthm.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 7.Lustgarten DL, Crespo EM, Arkhipova-Jenkins I, Lobel R, Winget J, Koehler J, Liberman E, Sheldon T. His-bundle pacing versus biventricular pacing in cardiac resynchronization therapy patients: A crossover design comparison. Heart Rhythm. 2015;12:1548–1557. doi: 10.1016/j.hrthm.2015.03.048. [DOI] [PubMed] [Google Scholar]
- 8.Prutkin JM, Reynolds MR, Bao H, Curtis JP, Al-Khatib SM, Aggarwal S, Uslan DZ. Rates of and factors associated with infection in 200 909 medicare implantable cardioverter-defibrillator implants: Results from the national cardiovascular data registry. Circulation. 2014;130:1037–1043. doi: 10.1161/CIRCULATIONAHA.114.009081. [DOI] [PubMed] [Google Scholar]
- 9.Krahn AD, Morissette J, Lahm R, Haddad T, Baxter WW, McVenes R, Crystal E, Ayala-Paredes F, Cameron D, Verma A, Simpson CS, Exner DV, Birnie DH. Radiographic predictors of lead conductor fracture. Circ Arrhythm Electrophysiol. 2014;7:1070–1077. doi: 10.1161/CIRCEP.114.001612. [DOI] [PubMed] [Google Scholar]
- 10.Reddy VY, Knops RE, Sperzel J, Miller MA, Petru J, Simon J, Sediva L, de Groot JR, Tjong FV, Jacobson P, Ostrosff A, Dukkipati SR, Koruth JS, Wilde AA, Kautzner J, Neuzil P. Permanent leadless cardiac pacing: Results of the leadless trial. Circulation. 2014;129:1466–1471. doi: 10.1161/CIRCULATIONAHA.113.006987. [DOI] [PubMed] [Google Scholar]
- 11.Reddy VY, Exner DV, Cantillon DJ, Doshi R, Bunch TJ, Tomassoni GF, Friedman PA, Estes NA, 3rd, Ip J, Niazi I, Plunkitt K, Banker R, Porterfield J, Ip JE, Dukkipati SR Investigators LIS. Percutaneous implantation of an entirely intracardiac leadless pacemaker. N Engl J Med. 2015;373:1125–1135. doi: 10.1056/NEJMoa1507192. [DOI] [PubMed] [Google Scholar]
- 12.Reynolds D, Duray GZ, Omar R, Soejima K, Neuzil P, Zhang S, Narasimhan C, Steinwender C, Brugada J, Lloyd M, Roberts PR, Sagi V, Hummel J, Bongiorni MG, Knops RE, Ellis CR, Gornick CC, Bernabei MA, Laager V, Stromberg K, Williams ER, Hudnall JH, Ritter P Micra Transcatheter Pacing Study G. A leadless intracardiac transcatheter pacing system. N Engl J Med. 2015 [Google Scholar]
- 13.Bardy GH, Smith WM, Hood MA, Crozier IG, Melton IC, Jordaens L, Theuns D, Park RE, Wright DJ, Connelly DT, Fynn SP, Murgatroyd FD, Sperzel J, Neuzner J, Spitzer SG, Ardashev AV, Oduro A, Boersma L, Maass AH, Van Gelder IC, Wilde AA, van Dessel PF, Knops RE, Barr CS, Lupo P, Cappato R, Grace AA. An entirely subcutaneous implantable cardioverter-defibrillator. N Engl J Med. 2010;363:36–44. doi: 10.1056/NEJMoa0909545. [DOI] [PubMed] [Google Scholar]
- 14.Weiss R, Knight BP, Gold MR, Leon AR, Herre JM, Hood M, Rashtian M, Kremers M, Crozier I, Lee KL, Smith W, Burke MC. Safety and efficacy of a totally subcutaneous implantable-cardioverter defibrillator. Circulation. 2013;128:944–953. doi: 10.1161/CIRCULATIONAHA.113.003042. [DOI] [PubMed] [Google Scholar]
- 15.McLeod CJ, Boersma L, Okamura H, Friedman PA. The subcutaneous implantable cardioverter defibrillator: State-of-the-art review. Eur Heart J. 2015 doi: 10.1093/eurheartj/ehv507. [DOI] [PubMed] [Google Scholar]
- 16.Acha MR, Milan D. Who should receive the subcutaneous implanted defibrillator?: Timing is not right to replace the transvenous implantable cardioverter defibrillator. Circulation: Arrhythmia and Electrophysiology. 2013;6:1246–1251. doi: 10.1161/CIRCEP.113.000445. [DOI] [PubMed] [Google Scholar]
- 17.Poole JE, Gold MR. Who should receive the subcutaneous implanted defibrillator?: The subcutaneous implantable cardioverter defibrillator (icd) should be considered in all icd patients who do not require pacing. Circ Arrhythm Electrophysiol. 2013;6:1236–1244. doi: 10.1161/CIRCEP.113.000481. discussion 1244–1235. [DOI] [PubMed] [Google Scholar]
- 18.Thajudeen A, Jackman WM, Stewart B, Cokic I, Nakagawa H, Shehata M, Amorn AM, Kali A, Liu E, Harlev D, Bennett N, Dharmakumar R, Chugh SS, Wang X. Correlation of scar in cardiac mri and high-resolution contact mapping of left ventricle in a chronic infarct model. Pacing Clin Electrophysiol. 2015;38:663–674. doi: 10.1111/pace.12581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anter E, Tschabrunn CM, Josephson ME. High-resolution mapping of scar-related atrial arrhythmias using smaller electrodes with closer interelectrode spacing. Circ Arrhythm Electrophysiol. 2015 doi: 10.1161/CIRCEP.114.002737. [DOI] [PubMed] [Google Scholar]
- 20.Rudy Y, Lindsay BD. Electrocardiographic imaging of heart rhythm disorders: From bench to bedside. Card Electrophysiol Clin. 2015;7:17–35. doi: 10.1016/j.ccep.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sapp JL, Dawoud F, Clements JC, Horacek BM. Inverse solution mapping of epicardial potentials: Quantitative comparison with epicardial contact mapping. Circ Arrhythm Electrophysiol. 2012;5:1001–1009. doi: 10.1161/CIRCEP.111.970160. [DOI] [PubMed] [Google Scholar]
- 22.Haissaguerre M, Hocini M, Denis A, Shah AJ, Komatsu Y, Yamashita S, Daly M, Amraoui S, Zellerhoff S, Picat MQ, Quotb A, Jesel L, Lim H, Ploux S, Bordachar P, Attuel G, Meillet V, Ritter P, Derval N, Sacher F, Bernus O, Cochet H, Jais P, Dubois R. Driver domains in persistent atrial fibrillation. Circulation. 2014;130:530–538. doi: 10.1161/CIRCULATIONAHA.113.005421. [DOI] [PubMed] [Google Scholar]
- 23.Desjardins B, Yokokawa M, Good E, Crawford T, Latchamsetty R, Jongnarangsin K, Ghanbari H, Oral H, Pelosi F, Jr, Chugh A, Morady F, Bogun F. Characteristics of intramural scar in patients with nonischemic cardiomyopathy and relation to intramural ventricular arrhythmias. Circ Arrhythm Electrophysiol. 2013;6:891–897. doi: 10.1161/CIRCEP.113.000073. [DOI] [PubMed] [Google Scholar]
- 24.Dickfeld T, Tian J, Ahmad G, Jimenez A, Turgeman A, Kuk R, Peters M, Saliaris A, Saba M, Shorofsky S, Jeudy J. Mri-guided ventricular tachycardia ablation: Integration of late gadolinium-enhanced 3d scar in patients with implantable cardioverter-defibrillators. Circ Arrhythm Electrophysiol. 2011;4:172–184. doi: 10.1161/CIRCEP.110.958744. [DOI] [PubMed] [Google Scholar]
- 25.Arevalo H, Plank G, Helm P, Halperin H, Trayanova N. Tachycardia in post-infarction hearts: Insights from 3d image-based ventricular models. PLoS One. 2013;8:e68872. doi: 10.1371/journal.pone.0068872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bala R, Ren JF, Hutchinson MD, Desjardins B, Tschabrunn C, Gerstenfeld EP, Deo R, Dixit S, Garcia FC, Cooper J, Lin D, Riley MP, Tzou WS, Verdino R, Epstein AE, Callans DJ, Marchlinski FE. Assessing epicardial substrate using intracardiac echocardiography during vt ablation. Circ Arrhythm Electrophysiol. 2011;4:667–673. doi: 10.1161/CIRCEP.111.963553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klein T, Abdulghani M, Smith M, Huang R, Asoglu R, Remo BF, Turgeman A, Mesubi O, Sidhu S, Synowski S, Saliaris A, See V, Shorofsky S, Chen W, Dilsizian V, Dickfeld T. Three-dimensional 123i-meta-iodobenzylguanidine cardiac innervation maps to assess substrate and successful ablation sites for ventricular tachycardia: A feasibility study for a novel paradigm of innervation imaging. Circ Arrhythm Electrophysiol. 2015 doi: 10.1161/CIRCEP.114.002105. [DOI] [PubMed] [Google Scholar]
- 28.Grothoff M, Piorkowski C, Eitel C, Gaspar T, Lehmkuhl L, Lucke C, Hoffmann J, Hildebrand L, Wedan S, Lloyd T, Sunnarborg D, Schnackenburg B, Hindricks G, Sommer P, Gutberlet M. Mr imaging-guided electrophysiological ablation studies in humans with passive catheter tracking: Initial results. Radiology. 2014;271:695–702. doi: 10.1148/radiol.13122671. [DOI] [PubMed] [Google Scholar]
- 29.Kolandaivelu A, Zviman MM, Castro V, Lardo AC, Berger RD, Halperin HR. Noninvasive assessment of tissue heating during cardiac radiofrequency ablation using mri thermography. Circ Arrhythm Electrophysiol. 2010;3:521–529. doi: 10.1161/CIRCEP.110.942433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nazarian S, Bluemke DA, Halperin HR. Applications of cardiac magnetic resonance in electrophysiology. Circ Arrhythm Electrophysiol. 2009;2:63–71. doi: 10.1161/CIRCEP.108.811562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu Z, Gudur MS, Deng CX. Transmural ultrasound imaging of thermal lesion and action potential changes in perfused canine cardiac wedge preparations by high intensity focused ultrasound ablation. PLoS One. 2013;8:e82689. doi: 10.1371/journal.pone.0082689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sapp JL, Beeckler C, Pike R, Parkash R, Gray CJ, Zeppenfeld K, Kuriachan V, Stevenson WG. Initial human feasibility of infusion needle catheter ablation for refractory ventricular tachycardia. Circulation. 2013;128:2289–2295. doi: 10.1161/CIRCULATIONAHA.113.003423. [DOI] [PubMed] [Google Scholar]
- 33.Nazer B, Gerstenfeld EP, Hata A, Crum LA, Matula TJ. Cardiovascular applications of therapeutic ultrasound. J Interv Card Electrophysiol. 2014;39:287–294. doi: 10.1007/s10840-013-9845-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strickberger SA, Tokano T, Kluiwstra JU, Morady F, Cain C. Extracardiac ablation of the canine atrioventricular junction by use of high-intensity focused ultrasound. Circulation. 1999;100:203–208. doi: 10.1161/01.cir.100.2.203. [DOI] [PubMed] [Google Scholar]
- 35.Lehmann HI, Richter D, Prokesch H, Graeff C, Prall M, Simoniello P, Fournier C, Bauer J, Kaderka R, Weymann A, Szabo G, Sonnenberg K, Constantinescu AM, Johnson SB, Misiri J, Takami M, Miller RC, Herman MG, Asirvatham SJ, Brons S, Jakel O, Haberer T, Debus J, Durante M, Bert C, Packer DL. Atrioventricular node ablation in langendorff-perfused porcine hearts using carbon ion particle therapy: Methods and an in vivo feasibility investigation for catheter-free ablation of cardiac arrhythmias. Circ Arrhythm Electrophysiol. 2015;8:429–438. doi: 10.1161/CIRCEP.114.002436. [DOI] [PubMed] [Google Scholar]
- 36.Bunch TJ, Mahapatra S, Bruce GK, Johnson SB, Miller DV, Horne BD, Wang XL, Lee HC, Caplice NM, Packer DL. Impact of transforming growth factor-beta1 on atrioventricular node conduction modification by injected autologous fibroblasts in the canine heart. Circulation. 2006;113:2485–2494. doi: 10.1161/CIRCULATIONAHA.105.570796. [DOI] [PubMed] [Google Scholar]
- 37.Fukuda K, Kanazawa H, Aizawa Y, Ardell JL, Shivkumar K. Cardiac innervation and sudden cardiac death. Circ Res. 2015;116:2005–2019. doi: 10.1161/CIRCRESAHA.116.304679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bezzina CR, Lahrouchi N, Priori SG. Genetics of sudden cardiac death. Circ Res. 2015;116:1919–1936. doi: 10.1161/CIRCRESAHA.116.304030. [DOI] [PubMed] [Google Scholar]
- 39.Tucker NR, Ellinor PT. Emerging directions in the genetics of atrial fibrillation. Circ Res. 2014;114:1469–1482. doi: 10.1161/CIRCRESAHA.114.302225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bongianino R, Priori SG. Gene therapy to treat cardiac arrhythmias. Nat Rev Cardiol. 2015;12:531–546. doi: 10.1038/nrcardio.2015.61. [DOI] [PubMed] [Google Scholar]
- 41.Rosen MR. Gene therapy and biological pacing. N Engl J Med. 2014;371:1158–1159. doi: 10.1056/NEJMcibr1408897. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen TP, Qu Z, Weiss JN. Cardiac fibrosis and arrhythmogenesis: The road to repair is paved with perils. J Mol Cell Cardiol. 2014;70:83–91. doi: 10.1016/j.yjmcc.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boink GJ, Lu J, Driessen HE, Duan L, Sosunov EA, Anyukhovsky EP, Shlapakova IN, Lau DH, Rosen TS, Danilo P, Jia Z, Ozgen N, Bobkov Y, Guo Y, Brink PR, Kryukova Y, Robinson RB, Entcheva E, Cohen IS, Rosen MR. Effect of skeletal muscle na(+) channel delivered via a cell platform on cardiac conduction and arrhythmia induction. Circ Arrhythm Electrophysiol. 2012;5:831–840. doi: 10.1161/CIRCEP.111.969907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Josephson M, Wellens HJ. Implantable defibrillators and sudden cardiac death. Circulation. 2004;109:2685–2691. doi: 10.1161/01.CIR.0000129322.97266.F3. [DOI] [PubMed] [Google Scholar]
- 45.Niemeijer MN, van den Berg ME, Leening MJ, Hofman A, Franco OH, Deckers JW, Heeringa J, Rijnbeek PR, Stricker BH, Eijgelsheim M. Declining incidence of sudden cardiac death from 1990–2010 in a general middle-aged and elderly population: The rotterdam study. Heart Rhythm. 2015;12:123–129. doi: 10.1016/j.hrthm.2014.09.054. [DOI] [PubMed] [Google Scholar]
- 46.Fox CS, Evans JC, Larson MG, Kannel WB, Levy D. Temporal trends in coronary heart disease mortality and sudden cardiac death from 1950 to 1999: The framingham heart study. Circulation. 2004;110:522–527. doi: 10.1161/01.CIR.0000136993.34344.41. [DOI] [PubMed] [Google Scholar]
- 47.Dudas K, Lappas G, Stewart S, Rosengren A. Trends in out-of-hospital deaths due to coronary heart disease in sweden (1991 to 2006) Circulation. 2011;123:46–52. doi: 10.1161/CIRCULATIONAHA.110.964999. [DOI] [PubMed] [Google Scholar]
- 48.Stecker EC, Reinier K, Marijon E, Narayanan K, Teodorescu C, Uy-Evanado A, Gunson K, Jui J, Chugh SS. Public health burden of sudden cardiac death in the united states. Circ Arrhythm Electrophysiol. 2014;7:212–217. doi: 10.1161/CIRCEP.113.001034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hayashi M, Shimizu W, Albert CM. The spectrum of epidemiology underlying sudden cardiac death. Circ Res. 2015;116:1887–1906. doi: 10.1161/CIRCRESAHA.116.304521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Narayanan K, Reinier K, Uy-Evanado A, Teodorescu C, Chugh H, Marijon E, Gunson K, Jui J, Chugh SS. Frequency and determinants of implantable cardioverter defibrillator deployment among primary prevention candidates with subsequent sudden cardiac arrest in the community. Circulation. 2013;128:1733–1738. doi: 10.1161/CIRCULATIONAHA.113.002539. [DOI] [PubMed] [Google Scholar]
- 51.Albert CM, Stevenson WG. Implantable cardioverter-defibrillators for primary prevention of sudden cardiac death: Too little and too late? Circulation. 2013;128:1721–1723. doi: 10.1161/CIRCULATIONAHA.113.005832. [DOI] [PubMed] [Google Scholar]
- 52.Kutyifa V, Moss AJ, Klein H, Biton Y, McNitt S, MacKecknie B, Zareba W, Goldenberg I. Use of the wearable cardioverter defibrillator in high-risk cardiac patients: Data from the prospective registry of patients using the wearable cardioverter defibrillator (wearit-ii registry) Circulation. 2015;132:1613–1619. doi: 10.1161/CIRCULATIONAHA.115.015677. [DOI] [PubMed] [Google Scholar]
- 53.Field JM, Hazinski MF, Sayre MR, Chameides L, Schexnayder SM, Hemphill R, Samson RA, Kattwinkel J, Berg RA, Bhanji F, Cave DM, Jauch EC, Kudenchuk PJ, Neumar RW, Peberdy MA, Perlman JM, Sinz E, Travers AH, Berg MD, Billi JE, Eigel B, Hickey RW, Kleinman ME, Link MS, Morrison LJ, O’Connor RE, Shuster M, Callaway CW, Cucchiara B, Ferguson JD, Rea TD, Vanden Hoek TL. Part 1: Executive summary: 2010 american heart association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 122:S640–656. doi: 10.1161/CIRCULATIONAHA.110.970889. [DOI] [PubMed] [Google Scholar]
- 54.Chan PS, McNally B, Tang F, Kellermann A, Group CS. Recent trends in survival from out-of-hospital cardiac arrest in the united states. Circulation. 2014;130:1876–1882. doi: 10.1161/CIRCULATIONAHA.114.009711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wissenberg M, Lippert FK, Folke F, Weeke P, Hansen CM, Christensen EF, Jans H, Hansen PA, Lang-Jensen T, Olesen JB, Lindhardsen J, Fosbol EL, Nielsen SL, Gislason GH, Kober L, Torp-Pedersen C. Association of national initiatives to improve cardiac arrest management with rates of bystander intervention and patient survival after out-of-hospital cardiac arrest. JAMA. 2013;310:1377–1384. doi: 10.1001/jama.2013.278483. [DOI] [PubMed] [Google Scholar]
- 56.Deo R, Albert CM. Epidemiology and genetics of sudden cardiac death. Circulation. 2012;125:620–637. doi: 10.1161/CIRCULATIONAHA.111.023838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chiuve SE, Fung TT, Rexrode KM, Spiegelman D, Manson JE, Stampfer MJ, Albert CM. Adherence to a low-risk, healthy lifestyle and risk of sudden cardiac death among women. JAMA. 2011;306:62–69. doi: 10.1001/jama.2011.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hurt RD, Weston SA, Ebbert JO, McNallan SM, Croghan IT, Schroeder DR, Roger VL. Myocardial infarction and sudden cardiac death in olmsted county, minnesota, before and after smoke-free workplace laws. Arch Intern Med. 2012;172:1635–1641. doi: 10.1001/2013.jamainternmed.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD Force obotAHASPT, Committee S. Defining and setting national goals for cardiovascular health promotion and disease reduction: The american heart association’s strategic impact goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 60.Ashikaga H, Arevalo H, Vadakkumpadan F, Blake RC, 3rd, Bayer JD, Nazarian S, Muz Zviman M, Tandri H, Berger RD, Calkins H, Herzka DA, Trayanova NA, Halperin HR. Feasibility of image-based simulation to estimate ablation target in human ventricular arrhythmia. Heart Rhythm. 2013;10:1109–1116. doi: 10.1016/j.hrthm.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hsieh CH, Chia EM, Huang K, Lu J, Barry M, Pouliopoulos J, Ross DL, Thomas SP, Kovoor P. Primary radiofrequency ablation of ventricular tachycardia early after myocardial infarction: Evaluation in an ovine model. Circ Arrhythm Electrophysiol. 2013;6:1215–1221. doi: 10.1161/CIRCEP.113.000447. [DOI] [PubMed] [Google Scholar]
- 62.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 aha/acc/hrs guideline for the management of patients with atrial fibrillation: Executive summary: A report of the american college of cardiology/american heart association task force on practice guidelines and the heart rhythm society. Circulation. 2014;130:2071–2104. doi: 10.1161/CIR.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 63.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB. Heart disease and stroke statistics--2015 update: A report from the american heart association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 64.Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim Y-H, McAnulty JH, Zheng Z-J, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJL. Worldwide epidemiology of atrial fibrillation: A global burden of disease 2010 study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Patel NJ, Deshmukh A, Pant S, Singh V, Patel N, Arora S, Shah N, Chothani A, Savani GT, Mehta K, Parikh V, Rathod A, Badheka AO, Lafferty J, Kowalski M, Mehta JL, Mitrani RD, Viles-Gonzalez JF, Paydak H. Contemporary trends of hospitalization for atrial fibrillation in the united states, 2000 through 2010: Implications for healthcare planning. Circulation. 2014;129:2371–2379. doi: 10.1161/CIRCULATIONAHA.114.008201. [DOI] [PubMed] [Google Scholar]
- 66.Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, Newton-Cheh C, Lubitz SA, Magnani JW, Ellinor PT, Seshadri S, Wolf PA, Vasan RS, Benjamin EJ, Levy D. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the framingham heart study: A cohort study. Lancet. 2015;386:154–162. doi: 10.1016/S0140-6736(14)61774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Perez MV, Wang PJ, Larson JC, Soliman EZ, Limacher M, Rodriguez B, Klein L, Manson JE, Martin LW, Prineas R, Connelly S, Hlatky M, Wassertheil-Smoller S, Stefanick ML. Risk factors for atrial fibrillation and their population burden in postmenopausal women: The women’s health initiative observational study. Heart. 2013;99:1173–1178. doi: 10.1136/heartjnl-2013-303798. [DOI] [PubMed] [Google Scholar]
- 68.Ben Freedman S, Lowres N. Asymptomatic atrial fibrillation: The case for screening to prevent stroke. JAMA. 2015;314:1911–1912. doi: 10.1001/jama.2015.9846. [DOI] [PubMed] [Google Scholar]
- 69.Pathak RK, Middeldorp ME, Lau DH, Mehta AB, Mahajan R, Twomey D, Alasady M, Hanley L, Antic NA, McEvoy RD, Kalman JM, Abhayaratna WP, Sanders P. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: The arrest-af cohort study. J Am Coll Cardiol. 2014;64:2222–2231. doi: 10.1016/j.jacc.2014.09.028. [DOI] [PubMed] [Google Scholar]
- 70.Abed HS, Wittert GA, Leong DP, Shirazi MG, Bahrami B, Middeldorp ME, Lorimer MF, Lau DH, Antic NA, Brooks AG, Abhayaratna WP, Kalman JM, Sanders P. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: A randomized clinical trial. JAMA. 2013;310:2050–2060. doi: 10.1001/jama.2013.280521. [DOI] [PubMed] [Google Scholar]
- 71.Shroff GR, Solid CA, Herzog CA. Atrial fibrillation, stroke, and anticoagulation in medicare beneficiaries: Trends by age, sex, and race, 1992–2010. J Am Heart Assoc. 2014;3:e000756. doi: 10.1161/JAHA.113.000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ezekowitz MD, Kent AP. Novel anticoagulants eliminate the need for left atrial appendage exclusion devices. Circulation. 2014;130:1505–1514. doi: 10.1161/CIRCULATIONAHA.114.008139. [DOI] [PubMed] [Google Scholar]
- 73.Marijon E, Le Heuzey JY, Connolly S, Yang S, Pogue J, Brueckmann M, Eikelboom J, Themeles E, Ezekowitz M, Wallentin L, Yusuf S Investigators R-L. Causes of death and influencing factors in patients with atrial fibrillation: A competing-risk analysis from the randomized evaluation of long-term anticoagulant therapy study. Circulation. 2013;128:2192–2201. doi: 10.1161/CIRCULATIONAHA.112.000491. [DOI] [PubMed] [Google Scholar]
- 74.Huxley RR, Lopez FL, Folsom AR, Agarwal SK, Loehr LR, Soliman EZ, Maclehose R, Konety S, Alonso A. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: The atherosclerosis risk in communities (aric) study. Circulation. 2011;123:1501–1508. doi: 10.1161/CIRCULATIONAHA.110.009035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alonso A, Krijthe BP, Aspelund T, Stepas KA, Pencina MJ, Moser CB, Sinner MF, Sotoodehnia N, Fontes JD, Janssens AC, Kronmal RA, Magnani JW, Witteman JC, Chamberlain AM, Lubitz SA, Schnabel RB, Agarwal SK, McManus DD, Ellinor PT, Larson MG, Burke GL, Launer LJ, Hofman A, Levy D, Gottdiener JS, Kaab S, Couper D, Harris TB, Soliman EZ, Stricker BH, Gudnason V, Heckbert SR, Benjamin EJ. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: The charge-af consortium. J Am Heart Assoc. 2013;2:e000102. doi: 10.1161/JAHA.112.000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Everett BM, Cook NR, Conen D, Chasman DI, Ridker PM, Albert CM. Novel genetic markers improve measures of atrial fibrillation risk prediction. Eur Heart J. 2013 doi: 10.1093/eurheartj/eht033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nagashima K, Choi EK, Lin KY, Kumar S, Tedrow UB, Koplan BA, Michaud GF, John RM, Epstein LM, Tokuda M, Inada K, Couper GS, Stevenson WG. Ventricular arrhythmias near the distal great cardiac vein: Challenging arrhythmia for ablation. Circ Arrhythm Electrophysiol. 2014;7:906–912. doi: 10.1161/CIRCEP.114.001615. [DOI] [PubMed] [Google Scholar]
- 78.Hoyert DL. 75 years of mortality in the united states, 1935–2010. NCHS Data Brief. 2012;88 [PubMed] [Google Scholar]
- 79.Administration on aging. 2015 www.aoa.acl.gov/aging_Statistics.
- 80.Kochhar R. 10 projections for the global population in 2050. 2014. [Google Scholar]
- 81.Wijnmaalen AP, Schalij MJ, von der Thusen JH, Klautz RJ, Zeppenfeld K. Early reperfusion during acute myocardial infarction affects ventricular tachycardia characteristics and the chronic electroanatomic and histological substrate. Circulation. 2010;121:1887–1895. doi: 10.1161/CIRCULATIONAHA.109.891242. [DOI] [PubMed] [Google Scholar]