Abstract
Heart failure (HF) with preserved ejection fraction (EF) (HFpEF) accounts for 50% of HF cases and its prevalence relative to HF with reduced EF (HFrEF) continues to rise. In contrast to HFrEF, large trials testing neurohumoral inhibition in HFpEF failed to reach a positive outcome. This failure was recently attributed to distinct systemic and myocardial signaling in HFpEF and to diversity of HFpEF phenotypes. In this review, a HFpEF treatment strategy is proposed which addresses HFpEF-specific signaling and phenotypic diversity. In HFpEF, extracardiac comorbidities such as metabolic risk, arterial hypertension and renal insufficiency drive left ventricular (LV) remodeling and dysfunction through systemic inflammation and coronary microvascular endothelial dysfunction. The latter affects LV diastolic dysfunction through macrophage infiltration resulting in interstitial fibrosis and through altered paracrine signaling to cardiomyocytes, which become hypertrophied and stiff because of low nitric oxide (NO) and cyclic guanosine monophosphate (cGMP). Systemic inflammation also affects other organs such as lungs, skeletal muscle and kidneys leading respectively to pulmonary hypertension, muscle weakness and sodium retention. Individual steps of these signaling cascades can be targeted by specific interventions: metabolic risk by caloric restriction, systemic inflammation by statins, pulmonary hypertension by phosphodiesterase (PDE) 5 inhibitors, muscle weakness by exercise training, sodium retention by diuretics and monitoring devices, myocardial NO bioavailability by inorganic nitrate-nitrite, myocardial cGMP content by neprilysin or PDE 9 inhibition and myocardial fibrosis by spironolactone. Because of phenotypic diversity in HFpEF, personalized therapeutic strategies are proposed, which are configured in a matrix with HFpEF presentations in the abscissa and HFpEF predispositions in the ordinate.
Keywords: heart failure; diastole; diastolic heart failure; treatment; preserved left ventricular function, phenotypes
INTRODUCTION
Heart Failure (HF) with preserved Ejection Fraction (EF) (HFpEF) currently accounts for more than 50% of all heart failure cases and its prevalence relative to HF with reduced Ejection Fraction (HFrEF) continues to rise at an alarming rate of 1% per year1. In the past three decades, HFrEF evolved to a distinct therapeutic entity partly because large outcome trials demonstrated efficacy of neurohumoral inhibition. No similar evolution has occurred in HFpEF, where large trials testing neurohumoral inhibition consistently failed to reach a positive primary outcome either individually2 or on meta-analysis3. In trials testing angiotensin converting enzyme inhibitors (ACEIs), angiotensin II receptor blockers (ARBs) or mineralocorticoid receptor antagonists (MRA) a modest positive trend was sometimes observed but only for secondary outcomes4 or retrospectively defined subgroups5,6. The failure of neurohumoral inhibition in the large HFpEF outcome trials led some investigators to challenge HFpEF as a distinct HF phenotype7-9. More recent views attributed this failure to different systemic and myocardial signalling in HFpEF and HFrEF10 or to diverse phenotypes within the HFpEF patient population11-14. In line with these views, the current HFpEF treatment roadmap first addresses HFpEF specific systemic and myocardial signalling, subsequently configures HFpEF phenotypes in a matrix of predispositions and presentations and finally discusses therapeutic inroads that fit into the phenotypic framework.
SYSTEMIC AND MYOCARDIAL SIGNALLING
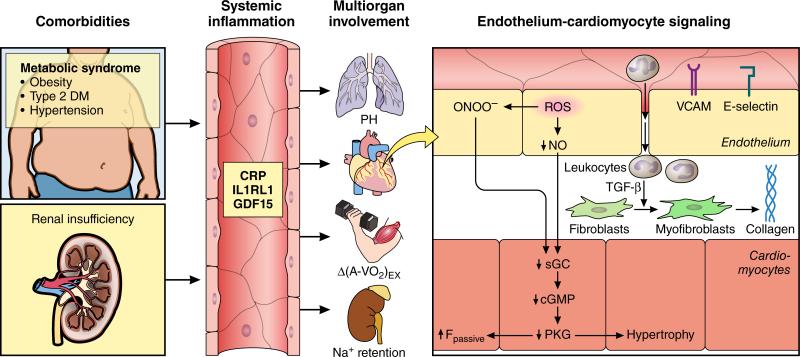
Large outcome trials and registries all revealed HFpEF patients to be of advanced age and predominantly women and to suffer from multiple comorbidities such as overweight/obesity (84%)15, arterial hypertension (60-80%)16, type 2 diabetes mellitus (20-45%)16, renal insufficiency and sleep apnea. Aging and the aforementioned comorbidities may initiate chronic systemic inflammation as manifest from biomarker profiles which revealed high plasma levels of soluble interleukin 1 receptor-like 1 (IL1RL1), C-reactive protein (CRP) and growth differentiation factor 15 (GDF15) in HFpEF17-20. Initial studies revealed plasma levels to be similarly elevated in HFpEF and HFrEF17 but recent studies observed them to be higher in HFpEF19 and therefore suggested a larger involvement of systemic inflammation in HFpEF. Systemic inflammation may affect myocardial remodeling and dysfunction in HFpEF through a signalling cascade, which begins with coronary microvascular endothelial dysfunction (Figure 1)10,21. It subsequently involves myocardial infiltration by activated macrophages, which induce reactive interstitial fibrosis22 and altered paracrine communication between endothelial cells and surrounding cardiomyocytes21. The latter deprives cardiomyocytes of nitric oxide (NO) and of cyclic guanosine monophosphate (cGMP), which renders them hypertrophied and stiff23. High cardiomyocyte stiffness is caused by diminished distensibility of the giant cytoskeletal protein titin, whose elastic properties are dynamically modulated by isoform shifts, phosphorylation and oxidation24,25. Strong support for an extramyocardial origin of HFpEF came from “parabiosis” experiments in which hearts of young animals acquired HFpEF-like features when exposed to blood from old animals and vice versa, as hearts of old animals reversed HFpEF-like features when exposed to blood of young animals26.
Figure 1. Systemic and myocardial signalling in HFPEF.
Comorbidities induce systemic inflammation, evident from elevated plasma levels of inflammatory biomarkers such as soluble interleukin 1 receptor-like 1 (IL1RL1), C-reactive protein (CRP) and growth differentiation factor 15 (GDF15). Chronic inflammation affects the lungs, myocardium, skeletal muscle and kidneys leading to diverse HFpEF phenotypes with variable involvement of pulmonary hypertension (PH), myocardial remodeling, deficient skeletal muscle oxygen extraction (ΔA-VO2) during exercise (Ex) and renal Na+ retention. Myocardial remodeling and dysfunction begins with coronary endothelial microvascular inflammation manifest from endothelial expression of adhesion molecules such as vascular cell adhesion molecule (VCAM) and E-Selectin. Expression of adhesion molecules attracts infiltrating leukocytes secreting transforming growth factor β (TGF- β), which converts fibroblasts to myofibroblasts with enhanced interstitial collagen deposition. Endothelial inflammation also results in presence of reactive oxygen species (ROS), reduced nitric oxide (NO) bioavailability and production of peroxynitrite (ONOO−). This reduces soluble guanylate cyclase (sGC) activity, cyclic guanosine monophosphate (cGMP) content and the favorable effects of protein kinase G (PKG) on cardiomyocyte stiffness and hypertrophy.
The extramyocardial origin of HFpEF differs from the intramyocardial origin of HFrEF, where remodeling is driven by cardiomyocyte cell death because of ischemia, infection or toxicity27. Distinct origins of HFpEF and HFrEF are mirrored by unequal LV structural and ultrastructural remodeling (Table). Biomarker profiles in HFpEF and HFrEF are consistent with the distinct origins of both HF phenotypes as they show lower markers of myocardial injury (high-sensitivity troponin T; hsTNT)) or of myocardial stress (N-terminal pro brain natriuretic peptide; NT-proBNP) in HFpEF17-20,28-30. Lower hsTNT is explained by less cardiomyocyte damage as a result of limited upregulation in HFpEF myocardium of nicotinamide adenine dinucleotide phosphate oxidase 2 (Nox2) evident in infiltrating macrophages or endothelial cells but not in cardiomyocytes21. Lower NT-proBNP is explained by concentric LV remodeling/hypertrophy in HFpEF in contrast to eccentric LV remodeling/hypertrophy in HFrEF31 and by visceral distribution of adipose tissue in the mostly overweight or obese HFpEF patients32, which is associated with decreased production and increased clearance of natriuretic peptides (NP).
Table.
Unequal structural, functional and ultrastructural LV characteristics in HFpEF and HFrEF
| HFpEF | HFrEF | |
|---|---|---|
| LV Structure/Function | ||
| End-DiastolicVolume | ↔ | ↑ |
| End-Systolic Volume | ↔ | ↑ |
| Wall Thickness | ↑ | ↔ |
| Mass | ↑ | ↑ |
| Mass/Volume Ratio | ↑ | ↓ |
| Remodeling | Concentric | Eccentric |
| Ejection Fraction | ↔ | ↓ |
| Stroke Work | ↔ | ↓ |
| End-Systolic Elastance | ↔ | ↓ |
| End-DiastolicStiffness | ↑ | ↓ |
| LV Ultrastructure | ||
| Myocyte Diameter | ↑ | ↔ |
| Myocyte Length | ↔ | ↑ |
| Myocyte Remodeling | Concentric | Eccentric |
| Fibrosis | Interstitial/Reactive | Focal/Replacement |
In HFpEF, chronic systemic inflammation affects not only the myocardium but also other organs such as lungs, skeletal muscles and kidneys (Figure 1). Although HFpEF patients may stop exercising because of a rapid and brisk rise in LV filling pressures33-38, in a substantial subset of patients effort tolerance is limited by inappropriate pulmonary vasoconstriction evident from pulmonary hypertension, or by inadequate peripheral skeletal muscle vasodilation, perfusion and oxygen utilization evident from absent widening of arterio-venous oxygen difference39-43. Systemic inflammation also affects the renal microcirculation and the ability of the kidneys to excrete a sodium load44. Inability to excrete a sodium load contributes to the progressive volume expansion observed during transition from chronic compensated to acute decompensated HFpEF45-46 and explains the efficacy of diuretics as they restore the pressure-natriuresis relationship.
PHENOTYPIC FRAMEWORK
HFpEF clinically presents as a diverse syndrome initiated by a variety of comorbidities and inflammatory mediators with extracardiac manifestations and cardiac abnormalities 13,47-49. Despite the diversity of the HFpEF syndrome, the treatment strategy thus far has focused on a “one-size-fits-all” approach that has worked relatively well for chronic HFrEF. However, virtually all clinical syndromes benefit from more tailored, personalized therapy, and this may also be true of HFpEF. Successfully addressing the diversity of HFpEF is an active area of investigation, and solutions for the problem range from simple (e.g., stratifying based on type of clinical presentation47) to sophisticated (e.g. machine learning techniques to perform data reduction in order to classify patients based on intrinsic patterns in dense phenotypic data49). While the field of machine learning is not new50, its application to clinical medicine is still relatively novel and these techniques will require iterative testing and application to clinical trials before they can be applied clinically on a routine basis.
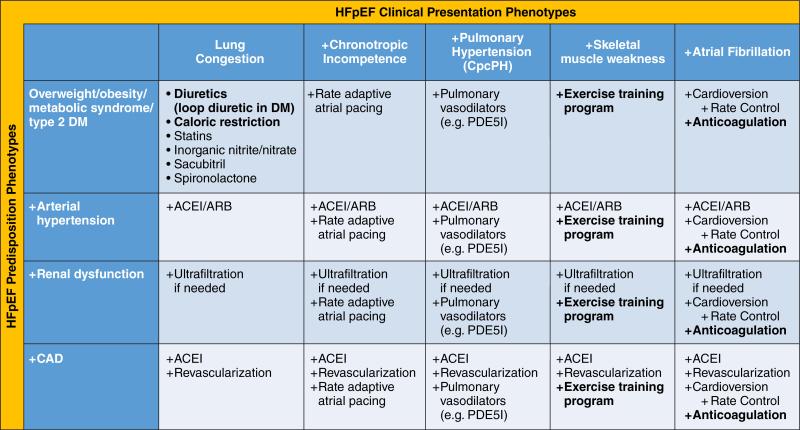
In the absence of compelling outcome data to support individual therapies, we propose a matrix configuration combining predisposition phenotypes with clinical presentation phenotypes as a starting point to guide current clinical care and future prospective research (Figure 2). Rare etiologies such as constrictive pericarditis, valvular heart disease, high output failure or infiltrative cardiomyopathies are presumed to be excluded beforehand. Figure 2 displays a stepwise approach that begins in the left hand upper corner of the matrix with general treatment recommendations, presumed to be beneficial to the vast majority of HFpEF patients as they address the presentation phenotype of lung congestion and the predisposition phenotype of overweight/obesity present in >80% of HFpEF patients15. Subsequently, supplementary recommendations are suggested for additional predisposition-related phenotypic features when moving downward in the matrix and for additional presentation-related phenotypic features when moving rightward in the matrix. Arterial hypertension, renal dysfunction and coronary artery disease are proposed as additional predisposition phenotypes (Figure 2). Additional clinical presentation phenotypes, in whom specific therapeutic interventions could be meaningful, are chronotropic incompetence, pulmonary hypertension (especially combined precapillary and postcapillary pulmonary hypertension; CpcPH), skeletal muscle weakness and atrial fibrillation. Apart from use of diuretics, caloric restriction diet, exercise training and anticoagulation in the presence of atrial fibrillation, all recommendations need to be confirmed by prospective outcome trials in the respective phenotypic subsets.
Figure 2. Phenotype-specific HFpEF treatment strategy using a matrix of predisposition phenotypes and clinical presentation phenotypes.
A stepwise approach is proposed that begins in the left hand upper corner of the matrix with general treatment recommendations, presumed to be beneficial to the vast majority of HFpEF patients as they address the presentation phenotype of lung congestion and the predisposition phenotype of overweight/obesity present in >80% of HFpEF patients. Subsequently, supplementary (+) recommendations are suggested for additional predisposition-related phenotypic features when moving downward in the matrix and for additional presentation-related phenotypic features when moving rightward in the matrix. Arterial hypertension, renal dysfunction and coronary artery disease are proposed as additional predisposition phenotypes. Additional clinical presentation phenotypes, in whom specific therapeutic interventions could be meaningful, include chronotropic incompetence, pulmonary hypertension (especially combined precapillary and postcapillary pulmonary hypertension; CpcPH), skeletal muscle weakness and atrial fibrillation. Only therapeutic measures indicated in bold are currently established. All other therapeutic measures require further testing in specific phenotypes.
PHENOTYPIC TREATMENT STRATEGY
Numerous steps of the HFpEF signalling cascade, which ranges from systemic inflammation to myocardial titin elasticity, are valid treatment targets either for the vast majority of the HFpEF population (i.e. the lung congestion/metabolic risk phenotype in the upper left hand corner of Figure 2) or for specific presentation/predisposition HFpEF phenotypes (Figure 2).
Lung congestion/metabolic risk phenotype
The lung congestion/metabolic risk phenotype is considered the “garden variety” of HFpEF because by definition, HF patients have evidence of lung congestion at rest or during exercise and because overweight/obesity (BMI > 25 kg/m2) is highly prevalent in HFpEF (>80%)15 and increasingly recognized to drive HFpEF development. The latter was evident from recent longitudinal non-invasive studies, which revealed close correlations over a 4 year time interval between diastolic LV stiffness and body mass index (BMI) and concluded that central adiposity predisposed to HFpEF51,52. Similar evidence was already provided by the ALLHAT trial, which enrolled patients with arterial hypertension and one additional cardiovascular risk factor, and observed a high BMI at enrolment to be the strongest predictor of HFpEF development53.
Diuretics
Lowering of LV filling pressures with diuretics is of paramount importance for HFpEF patients to achieve symptomatic benefit, to reduce pulmonary artery pressures and to improve RV loading54. Their efficacy relates to a restored pressure-natriuresis relationship in the presence of renal microvascular inflammation55. Administration of diuretics can be guided by use of implantable hemodynamic monitors that either directly and continuously measure diastolic LV pressures or provide surrogates of pressure45. Studies evaluating hemodynamic monitoring have demonstrated that even in HFpEF patients considered to be “compensated” by expert HF clinicians, diastolic LV pressures are elevated and these elevations have important prognostic implications56. When transition to decompensated HF occurs, diastolic LV pressures progressively increase over weeks. During this time interval hemodynamic monitoring allows for early uptitration of diuretics, which improves outcome as demonstrated in the CHAMPION trial. In this study, treatment guided by implantable hemodynamic monitoring significantly decreased cardiovascular death and HF hospitalizations in HFPEF patients57,58.
Caloric restriction
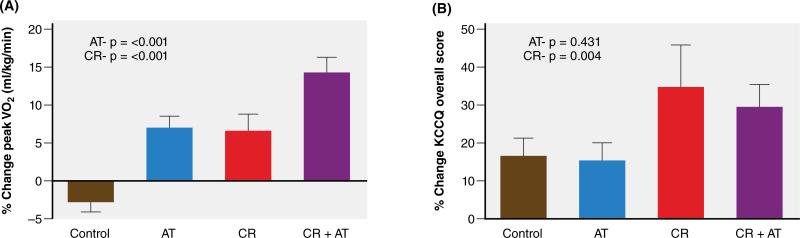
As increased body adiposity promotes inflammation and impairs cardiac, arterial, renal and skeletal muscle function, weight loss should be considered in a treatment strategy for the vast majority of HFpEF patients. Kitzman et al. recently reported that a 20-week caloric restriction diet was feasible and appeared safe in older, obese HFpEF patients, and significantly improved their symptoms, peak oxygen consumption (VO2), and quality of life (QOL) scores (Figure 3)59. The QOL improvement was significantly greater with diet than exercise. The combination of diet with endurance exercise training was additive and produced a large (2.5 mL/kg/min) increase in peak VO2 (Figure 3), similar or larger than what most drug or other treatments produced in HFrEF patients. The validity of the increase in peak VO2 was supported by significant increases in 5 other measures of physical performance that are independent of body mass: VO2 reserve, exercise time to exhaustion, workload, six-minute walk distance, and leg power. The increase in peak VO2 was strongly correlated with reduced body fat mass, increased percent lean body mass, higher thigh muscle/intermuscular fat ratio and lower biomarkers of inflammation supporting the hypothesis that overweight/obesity contributes to exercise intolerance in HFpEF through systemic inflammation59.
Figure 3. Effects of a 20 week caloric restriction diet on exercise capacity and quality of life in HFpEF.
The graph displays percent changes ± standard errors at the 20-week follow-up relative to baseline by randomized group for: peak VO2 (ml/kg/min, panel A), and KCCQ (Kansas City Cardiomyopathy Questionnaire) overall score (= Quality of Life Score) (panel B). AT=aerobic exercise training; CR=caloric restriction diet. P-values represent effects for AT and CR.
Statins
The presence of systemic inflammation supports the use of statins in HFpEF. Statins improve endothelial redox balance and restore NO bioavailability, independently of low-density lipoprotein lowering60-61. Analysis of endomyocardial biopsy material revealed statin-treated HFpEF patients to have less myocardial nitrotyrosine, higher myocardial PKG activity, less cardiomyocyte hypertrophy and lower cardiomyocyte resting tension10. In an observational study, statin-treated HFpEF patients were also less prone to develop atrial fibrillation62. These findings support the positive outcome of small phase 2 trials and HF registries that showed statin use to improve outcome of HFpEF patients63-65. It remains to be explored whether other novel approaches to treat systemic inflammation might be effective in HFpEF66.
Inorganic nitrite/nitrate
In the HFpEF signaling cascade cardiomyocytes are deprived of NO and cGMP because of altered paracrine communication between inflamed microvascular endothelial cells and cardiomyocytes (Figure 1). Organic NO donors were therefore suggested to be potentially useful in HFpEF as they could restore myocardial NO content and concomitantly correct the elevated arterial load. Recently however, Redfield et al. demonstrated in patients with HFpEF that the organic nitrate isosorbide mononitrate tended to reduce chronic activity levels measured by accelerometry, with no improvement in submaximal exercise capacity67. This result might be interpreted as disproving the NO hypothesis in HFpEF but there are some important caveats to consider. Organic nitrates may produce greater than expected hypotensive effects in people with HFpEF or potentially impair cardiac output owing to excessive preload reduction68. Organic nitrates tonically increase local NO levels and require bioactivation in the tissues. The latter can cause pharmacologic tolerance, while the former can chronically lower renal perfusion pressure, which, as alluded to before, is countered by renal sodium retention. This may override any beneficial reduction in filling pressures, a phenomenon known as pseudo-tolerance. Perhaps more importantly, organic nitrates such as isosorbide mononitrate have also been shown to cause endothelial dysfunction69,70 which plays a central role in the HFpEF signaling cascade.
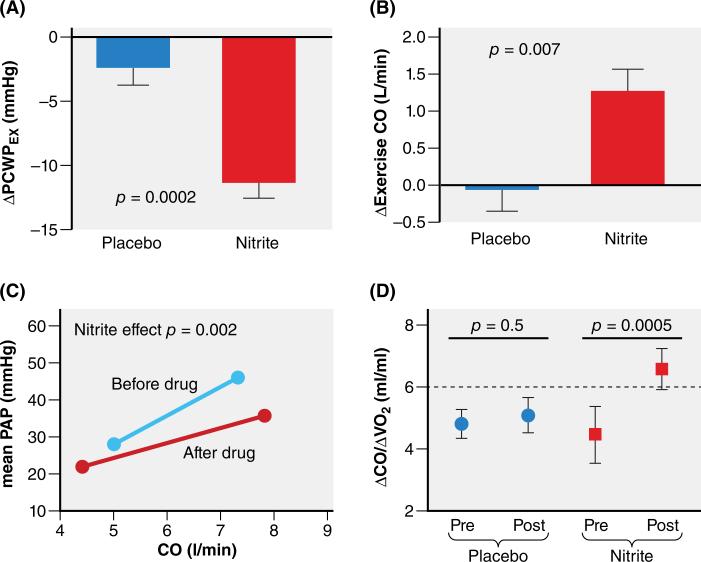
In contrast to organic nitrates, the inorganic nitrate-nitrite pathway represents an important alternative route to restore NO signaling in HFpEF71. Formerly considered as an inert byproduct of NO metabolism, nitrite is now known to function as an important in vivo NO reservoir. Importantly, nitrite is preferentially reduced to NO in the presence of hypoxia and acidosis, which occurs during physical exercise, thus delivering NO at the time and locations (i.e. skeletal and cardiac muscles) of greatest need. Nitrate-nitrite preparations have been shown to improve conduit artery stiffness in healthy volunteers and improve systemic vasodilation during exercise in patients with HFpEF72,73. More recently, acute infusion of sodium nitrite was shown in a placebo-controlled trial of patients with HFpEF to preferentially reduce diastolic LV pressures and pulmonary artery pressures during exercise while restoring cardiac output reserve toward normal (Figure 4)74. Part of this benefit was mediated by vasodilation, but evidence for a direct myocardial benefit such as increased stroke work was also observed. Another recent study found that inorganic nitrate, delivered as one week of once-daily beetroot juice consumption, improved submaximal exercise endurance75.
Figure 4. Effects of acute infusion of inorganic nitrite on exercise hemodynamics in HFpEF.
ΔPCWPEX : change in exercise induced pulmonary capillary wedge pressure; ΔExerciseCO : increase in exercise induced cardiac output; mean PAP: mean pulmonary artery pressure; ΔCO/ ΔVO2: ratio of exercise induced increase in cardiac output over exercise induced increase in oxygen consumption.
Sacubitril and other PKG stimulating drugs
A substantial number of HFpEF patients have pathological ventricular hypertrophy, with interstitial fibrosis and diastolic chamber stiffening. This has encouraged efforts to block key activators and to stimulate intrinsic suppressors of these changes. Among the attractive pathways representing the latter approach are those coupled to cyclic guanosine monophosphate (cGMP) and its cognate kinase – protein kinase G (PKG). PKG stimulation has potent anti-fibrotic and anti-hypertrophic effects in cultured myocytes and fibroblasts76-79, and has been protective in a wide array of experimental cardiac disease models including pressure-overload hypertrophy80-82. Moreover, there are multiple therapeutic approaches to stimulate PKG already in clinical use or under active investigation, which increases the potential translational relevance of this pathway.
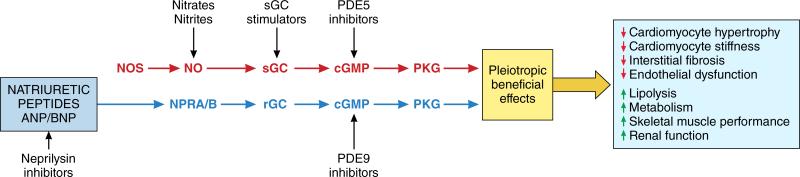
Stimulation of PKG requires cGMP which is either synthesized by soluble guanylate cyclase (sGC) activated by NO or by receptor guanylate cyclase (rGC) linked to the natriuretic peptide (NP) receptor83-85. This is in turn counterbalanced by hydrolysis of cGMP back to GMP by select members of the phosphodiesterase (PDE) superfamily, and their inhibition, which leads to increased cGMP, can also increase PKG activity (Figure 5). cGMP also controls cyclic adenosine monophosphate (cAMP) levels by feedback modulation of PDE2 and PDE3. At low levels of cGMP, pro-inotropic effects via cAMP have been observed while at higher levels and with cAMP co-stimulation, cGMP induces an anti-adrenergic effect. Four members of the PDE superfamily (PDE1, PDE2, PDE5, and PDE9) regulate cGMP in the heart of which both PDE5 and PDE9 are selective for cGMP. They are not redundant, but target different intracellular pools, with PDE5 largely impacting NO-sGC derived cGMP, while PDE9 regulates NP-rGC derived pools80. These local pools impact different intracellular compartments of PKG as detected by differences in net phosphokinomes and effects on transcriptional regulation80.
Figure 5. Myocardial cGMP signaling and pharmacological interventions in HFpEF.
Nitric Oxide (NO) produced by nitric oxide synthases (NOS) stimulates soluble guanylate cyclase (sGC) to produce cyclic guanosine monophosphate (cGMP), which activates protein kinase G (PKG). Inorganic nitrate/nitrite, sGC stimulators and phosphodiesterase (PDE) 5 inhibitors target this pathway. The natriuretic peptides ANP and BNP attach to the natriuretic peptide receptors A/B (NPRA/NPRB). This stimulates receptor guanylate cyclase (rGC) to produce cGMP, which again activates PKG. Neprilysin inhibitors such as sacubitril and PDE9 inhibitors act through this pathway.
Recent studies have defined multiple targets relevant to the lusitropic, anti-hypertrophic and anti-fibrotic impact of PKG. HFpEF cardiomyocytes display greater passive diastolic stiffness that has been linked to changes in titin phosphorylation at PEVK-region residues modulated by PKA and PKG23,86-88. The latter is a particularly potent regulator of titin stiffness, which in turn impacts cardiac muscle stiffness23. Anti-hypertrophic and anti-fibrotic mechanisms include PKG-suppression of transforming growth factor-β (TGF-β) signaling by phosphorylation of Smad proteins that blocks their nuclear translocation and signaling89.
Data regarding myocardial cGMP/PKG signaling in HFpEF remain fairly limited, but several studies have revealed likely critical features in this disease that could ultimately dictate how a successful therapy would need to work. Most pertinently, human LV biopsy analysis from HFpEF has reported very low levels of cGMP and associated PKG activity, particularly when compared to HFrEF and aortic stenosis patients23. This may help explain reduced titin phosphorylation and muscle stiffening, as well as contributory signaling to hypertrophy and fibrosis – i.e. the brake has been removed. It also raises questions regarding the initiating mechanism. Myocardial oxidative stress coupled with a pro-inflammatory microvascular environment has been proposed10 and was recently supported by comparative analysis of HFpEF, HFrEF and aortic stenosis samples, which revealed in HFpEF higher microvascular expression of adhesion molecules and Nox2 with higher hydrogen peroxide and lower nitrite/nitrate content21.
Administration of sGC activators or stimulators could provide downstream correction for the low myocardial NO bioavailability in HFpEF. Use of the sGC activator cinaciguat in HFrEF was hampered by hypotension90. The oral sGC stimulator riociguat improved exercise tolerance or quality of life in pulmonary arterial hypertension (PATENT)91, in chronic thromboembolic pulmonary hypertension (CHEST)92 and in pulmonary hypertension due to HFrEF (LEPHT)93. In these three studies, arterial blood pressure also fell by up to 9 mmHg and this is especially worrisome for HFpEF patients because of their limited ability to raise LV stroke volume68. The use of vericiguat, another sGC stimulator, was well tolerated in HFrEF but failed to lower NP except at the highest dose94. It is currently being tested in HFpEF in the SOCRATES-PRESERVED trial.
Because of concentric LV remodeling, NP stimulation is less marked in HFpEF than HFrEF, a finding that may limit counter stimulation via this pathway. NPs are degraded by circulating neprilysin. Inhibition of this peptidase could augment deficient NP-rGC signaling and therefore be beneficial in HFpEF as suggested by the fall in NP following administration of valsartan/sacubitril in the phase 2 PARAMOUNT study95. Use of valsartan/sacubitril is currently being tested in the multicenter PARAGON-HF trial.
Another approach is to block PDEs to increase cGMP levels and hence PKG activity. PDE5 upregulation in HFrEF was reported by multiple96,97 but not all98 laboratories. Data in HFpEF did not support a similar elevation23, and two PDE5-inhibitor trials in HFpEF yielded a neutral outcome99,100. An alternative may therefore be inhibiting PDE9. A recent study found marked upregulation of PDE9 protein in human left ventricular biopsies from HFpEF patients as well as HFrEF and aortic stenosis patients80. This suggests the low cGMP levels might be related to enhanced expression of PDE9, and if so, inhibiting this PDE should have beneficial effects. In mice subjected to sustained pressure-overload, blocking PDE9 by gene deletion or selective pharmacological inhibition suppressed hypertrophy, fibrosis, and chamber dysfunction80. PDE9 inhibition has been previously examined clinically for its potential to alter cognition101 but these new data may trigger interest in HFpEF and other forms of heart failure.
Spironolactone and E-matrix modification
The extracellular matrix is composed of fibrillary proteins (such as collagen, elastin), non-fibrillary proteins (such as aminoglycans, fibronectin, laminin) and bioactive proteins such as TGF-β, matrix metalloproteinases (MMPs), tissue inhibitors of matrix metalloproteinases (TIMPs) and matricellular proteins. The homeostatic control of collagen is especially important for abnormal diastolic function in HF102. Important differences in geometry, composition and homeostatic mechanisms are seen in HFpEF vs. HFrEF. HFpEF is more often associated with interstitial, reactive fibrosis and HFrEF with focal, replacement fibrosis (Table). The extent of collagen cross-linking tends to be higher in HFpEF103 and homeostasis in HFpEF is profibrotic while fibrinolytic in HFrEF. The latter is reflected in the respective plasma biomarker profiles104.
Resident myocardial fibroblasts control collagen homeostasis in normal hearts. Whether resident fibroblasts remain responsible for increased collagen production or whether recruitment of fibroblasts occurs from a different source such as bone-marrow or microvascular endothelium remains uncertain. In murine HFpEF models, resident fibroblasts and not bone-marrow derived cells or endothelial-mesenchymal transition were primarily responsible for myocardial collagen production following transverse aortic constriction105,106. Recruited cells could however still be involved through secretion of cytokines or matricellular proteins. Myofibroblasts have also been implicated in collagen deposition in HFpEF as they are closely associated with fibrotic collagen deposition and scar contracture.In HFpEF, fibroblasts are presumed to convert to myofibroblasts because of exposure to TGF-β as a result of monocyte/macrophage myocardial infiltration22.
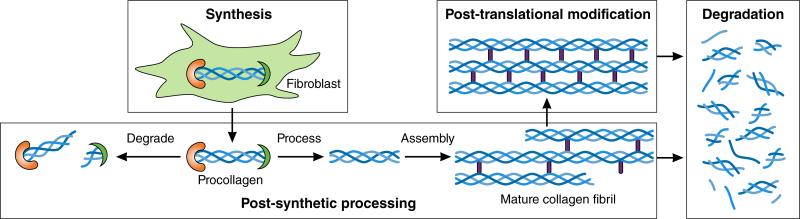
Collagen metabolism requires sequential, highly orchestrated and regulated steps: 1) procollagen synthesis and secretion; 2) procollagen post-synthetic processing; 3) collagen post-translational modification and 4) collagen degradation. Each of these steps is altered in HFpEF, contributes either individually or in aggregate to LV diastolic dysfunction, is mirrored in plasma biomarkers and serves as a unique treatment target (Figure 6). Procollagen I and III are synthesized in myocardial fibroblasts and secreted as a soluble molecule with NH2- (N) and COOH- (C) terminal propeptides attached. These are removed to create insoluble collagen107 and appear in plasma as procollagen I C-terminal peptide (PICP), procollagen III C-terminal peptide (PIIICP), procollagen I N-terminal peptide (PINP) and procollagen III N-terminal peptide (PIIINP), all of which reflect rate of collagen synthesis. Subsequent formation of insoluble collagen requires enzymatic formation of crosslinks by lysyl- or hydroxylysyloxidase. Non-enzymatic crosslinks can also be formed by advanced glycation endproducts (AGEs), which can activate profibrotic pathways through binding with the receptor for AGE (RAGE). Insoluble collagen formation is promoted by matricellular proteins (SPARC, thrombospondin, osteopontin). To maintain collagen homeostasis, insoluble collagen is continuously degraded by matrix metalloproteinases (MMPs), which are in turn regulated by tissue inhibitors of MMPs (TIMPs) 108. Collagen degradation results in formation of collagen telopeptides (CITP, CIIITP). MMPs, CITP and CIIITP can be measured in plasma and in combination with PICP, PINP, PIIICP and PIIINP allow for an integrated multi-biomarker assessment of collagen homeostasis103,104. In HFpEF such an assessment revealed collagen synthesis to be increased and collagen degradation to be decreased resulting in a net increase in collagen content109. Additional biomarkers that are useful estimates of myocardial collagen content are galectin-3 (Gal-3) and solubleST2 (sST2). The former is secreted by infiltrating macrophages and stimulates fibroblasts while the latter is a member of the interleukin 1 receptor family which is also profibrotic as it acts as a decoy for interleukin-33 (IL-33), which inhibits profibrotic signaling.
Figure 6. Sequential steps of collagen metabolism.
Collagen metabolism involves sequential steps consisting of procollagen synthesis, procollagen processing to collagen fibrils, post-translational modification of collagen fibrils and collagen degradation.
To date, three pharmaceutical agents that affect the extracellular matrix have been tested in HFpEF: spironolactone in TOPCAT, valsartan/sacubitril in PARAMOUNT and torasemide. In TOPCAT, spironolactone (a mineralocorticoid receptor antagonist, MRA) failed to reduce the composite primary endpoint in the overall trial population110 but not in patients with elevated BNP, which was a marker of enrollment in the Americas (p=0.003)111. The neutral outcome in the overall population may have been related to aberrant patient enrolment in Russia/Republic of Georgia rather than to inefficacy of spironolactone. In PARAMOUNT, salutary effects of valsartan/sacubitril were observed in HFpEF patients consisting of a significant decrease in NT-proBNP and left atrial volume95. These effects support fibrosis-specific therapy for HFpEF patients with advanced extracellular matrix modification112. The loop diuretic torasemide affects collagen crosslinking and its use has been shown to improve diastolic LV dysfunction in patients with hypertensive heart disease113. Finally, use of mesenchymal stem cells has been examined in Dahl salt-sensitive rats with promising results. In this model, a single intracoronary dose of allogeneic mesenchymal stem cells reduced myocardial collagen volume fraction and normalized diastolic LV function without effect on cardiomyocyte hypertrophy114.
Arterial Hypertension
Arterial hypertension is found in 80% or more of HFpEF patients. Treatment of arterial hypertension in older people without HF reduces incident HF53. In acutely decompensated HFpEF patients with elevated blood pressure, symptoms may improve markedly with blood pressure lowering alone even before diuresis is achieved. However, in chronic stable HFpEF patients there is uncertainty whether adding blood pressure lowering medications provides additional benefit. A discordance was indeed present between substantial blood pressure lowering and outcome in large trials testing neurohumoral inhibition in HFpEF2. This was even more surprising, since along with blood pressure lowering, there were numerous other mechanisms whereby neurohumoral inhibition was expected to benefit HFpEF, including improvements in myocardial hypertrophy, myocardial fibrosis and vascular stiffness. However, treating arterial hypertension for non-HF related macrovascular indications (e.g. stroke, myocardial infarction) remains an important goal also in HFpEF patients. In this regard, it is worth noting that large outcome trials confirmed ACEIs and ARBs to be safe and well-tolerated as antihypertensives2,4. Diuretics, spironolactone and ACEIs/ARBs are therefore reasonable first choices to control blood pressure based upon the currently available data. While it is true that previously tested ACEIs and ARBs did not reduce mortality, increasing the quality of life may be the better strategy in HFpEF patients because they are often elderly and debilitated and because some of the previously completed trials of ACEIs/ARBs had relevant symptomatic benefits such as reduced HF hospitalization2,4.
Arterial hypertension can affect myocardial remodeling and dysfunction in HFpEF through myocardial overload115 or systemic inflammation116. The importance of overload is unclear because in a concentrically remodeled left ventricle with normal EF, a favorable late-systolic Laplace relation protects LV myocardium from loading increments provoked by large reflected arterial pressure waves. However, in the presence of a minor LV shortening deficit, hypertensive HFpEF patients may develop late-peaking systolic LV wall stress. This may explain the favorable effects in HFpEF patients of nitrate-rich beetroot juice, which reduces magnitude of reflected arterial pressure waves75, or of the sodium-restricted DASH (Dietary Approaches to Stop Hypertension) diet, which improves ventricular-arterial coupling117.
Renal Dysfunction
HFpEF and renal dysfunction are mutually promoting (Figure 1)118. HFpEF promotes renal dysfunction by 1) an elevated central venous pressure, which results from pulmonary hypertension and right ventricular dysfunction; 2) inability to raise cardiac output following arterial vasodilation because of chronotropic incompetence and fixed LV stroke volume119; 3) systemic inflammation, endothelial dysfunction and low NO bioavailability, which reduces renal blood flow 55,120 and sodium excretion44. Renal dysfunction promotes HFpEF by worsening systemic inflammation, endothelial dysfunction and NO bioavailability partially because of renal-specific mediators such as high levels of fibroblast growth factor 23, phosphorus, parathyroid hormone or uremic toxins and low levels of vitamin D or erythropoietin118. Limited tolerability of systemic vasodilation and impaired sodium excretion are of therapeutic importance68. Impaired sodium excretion implies the arterial pressure-natriuresis relationship to be shifted to the right. Under these conditions, a fall in arterial pressure because of systemic vasodilation without cardiac output increase is especially deleterious as it leads to additional sodium retention and extracellular volume expansion, which wipes out any direct beneficial effect of vasodilation on LV filling pressures68. This mechanism could partially account for the neutral outcome of the RELAX trial99, where sildenafil lowered arterial pressure, raised plasma creatinine and urea levels and failed to improve exercise tolerance.
HFpEF in the presence of renal dysfunction recently emerged as a distinct phenotype with more LV hypertrophy, a larger LV systolic functional deficit, impaired left atrial mechanics, right ventricular dysfunction and poor prognosis121,122. The latter relates to exaggerated reactive pulmonary hypertension and right ventricular dysfunction. Because of right ventricular dysfunction, renal venous congestion importantly contributes to renal dysfunction in HFpEF. Vigorous diuresis (and ultrafiltration if necessary) are therefore important in HFpEF patients with renal dysfunction.
Coronary Artery Disease
The presence of coronary artery disease (CAD) also identifies a distinct HFpEF phenotype with a larger LV systolic functional deficit, poor prognosis123,124 and a high incidence of sudden death125. Use of ACEIs is recommended for prevention of new cardiovascular events. In HFpEF patients with CAD, observational data suggest that complete revascularization is associated with better preservation of LV systolic function and an improved prognosis, though prospective trial data are still lacking123.
Chronotropic Incompetence
Many patients with HFpEF display marked impairments in cardiac output reserve during exercise, despite normal resting values126. Impaired cardiac output reserve in HFpEF is related not only to decreased stroke volume augmentation but also to chronotropic incompetence127-129. One study actually indicated that chronotropic incompetence was the major contributor to reduced cardiac output reserve in HFpEF42. The importance of chronotropic incompetence is further supported by the worsened exercise capacity when heart rate was slowed by the If blocker ivabradine130. Chronotropic incompetence was previously shown to be related to endothelial dysfunction and systemic inflammation131 and therefore fits well into the multiorgan signaling cascade, which appears to drive HFpEF development. Because there is a direct relationship between heart rate response to activity and aerobic capacity128, a clinical trial is currently testing whether rate adaptive atrial pacing can improve exercise capacity in patients with HFpEF (NCT02145351).
Pulmonary Hypertension
Recent evidence stressed the importance in HFpEF of pathophysiologic targets beyond the heart (Figure 1)54. Pulmonary hypertension is frequently present at rest132 and patients can also develop an exaggerated pulmonary hypertensive response to exercise36,37,39. In HFpEF, pulmonary pressures can be augmented by increased left atrial pressure and/or by pulmonary vasoconstriction. When both mechanisms prevail, combined precapillary and postcapillary pulmonary hypertension (CpcPH) is present.
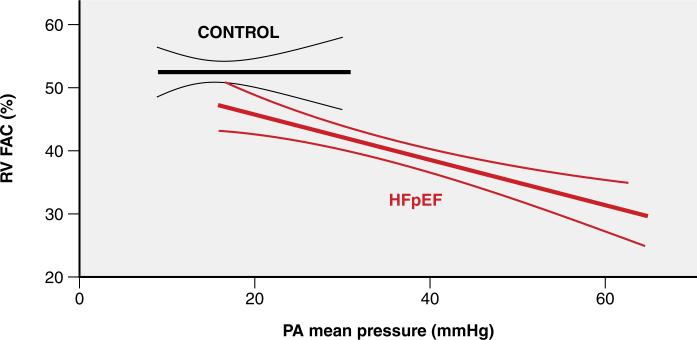
Because of pulmonary hypertension and shared predisposing mechanisms, right ventricular dysfunction is common in HFpEF and is associated with increased morbidity and mortality133-135. The right ventricle in HFpEF displays heightened afterload-sensitivity, suggesting favorable potential for benefit from reduction in pulmonary pressures (Figure 7)133. An early single-center trial recruiting mainly CpcPH patients indeed reported salutary effects on hemodynamics and right ventricular function following treatment with the phosphodiesterase 5 inhibitor (PDE5I) sildenafil136. However, two subsequent larger trials in patients with IpcPH (isolated postcapillary pulmonary hypertension) and CpcPH failed to corroborate this finding99,100. A recent trial reported significant improvement in pulmonary vascular function in response to dobutamine in HFpEF patients, greatly exceeding the pulmonary vasodilatory response seen in non-HF controls137. Improved right ventricular-pulmonary artery coupling in this study was achieved predominantly through reduction in afterload rather than enhanced right ventricular function, highlighting the importance of management of pulmonary hypertension in HFpEF. A number of trials have or are currently testing the effects of pulmonary vasodilators targeting cGMP138, endothelin139 and NO (NCT02713126; NCT02262078) in subjects with HFpEF.
Figure 7. Heightened afterload sensitivity of the right ventricle in HFpEF.
The relation between echocardiographic right ventricular (RV) fractional area change (FAC) and mean pulmonary artery (PA) pressure is flat in controls but steep in HFpEF patients.
In contrast to the pulmonary vasculature, changes in the lung parenchyma are less characterized in HFpEF but likely also play an important role. Impairments in pulmonary function predict incident development of HFpEF independent of cardiac function140. Patients with HFpEF display gas exchange abnormalities manifest by reduced alveolar capillary membrane conductance141,142. These impairments become more dramatic during exercise owing to high LV filling pressures during stress141. HFpEF patients with increased interstitial pulmonary edema display greater pulmonary vascular abnormalities and RV dysfunction, supporting aggressive therapies to reduce left heart filling pressures chronically in patients with HFpEF142.
Skeletal Muscle Weakness
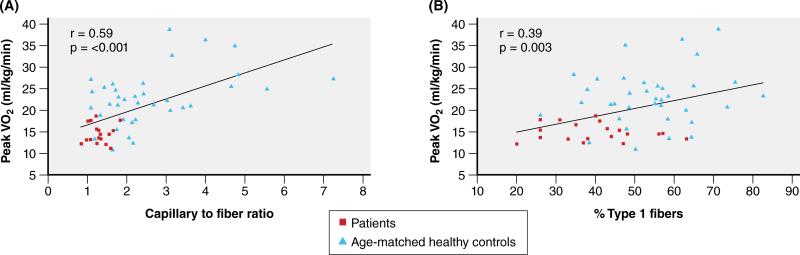
Exercise intolerance can be objectively measured as peak VO2. By the Fick equation, VO2 is the product of cardiac output and arteriovenous oxygen difference (ΔA-VO2). Multiple studies indicate that peak exercise ΔA-VO2 is significantly reduced in HFpEF and accounts for 50% or more of their severely reduced peak VO240,143. What are the causes of the reduced peak ΔA-VO2 in HFpEF patients? HFpEF patients have abnormalities in skeletal muscle mass, composition, capillary density, and oxidative metabolism. Haykowsky et al. showed that compared to age-matched healthy controls, older HFpEF patients have significantly reduced percent total lean body mass and percent leg lean mass43. When peak VO2 was indexed to total lean body mass or leg lean mass, it remained significantly reduced. Thus, HFpEF patients have abnormal O2 utilization that is independent of and in addition to their reduced muscle mass. HFpEF patients also have abnormal skeletal muscle composition with infiltration of adipose tissue, which is directly related to their reduced peak VO2144. Increased intramuscular fat reduces capillary density, thereby increasing the distance O2 must traverse from the capillaries to the muscle fibers. In HFpEF patients the reduced thigh muscle capillary density is associated with their reduced peak VO2 (Figure 8)145. Multiple studies indicate that HFpEF patients also have impaired skeletal muscle oxidative metabolism. Kitzman et al. showed that compared to healthy age-matched controls, HFpEF patients have a shift in skeletal muscle fiber type distribution from oxidative, slow type 1 fibers to glycolytic, fast type 2 fibers, which results in a lower type 1/type 2 fiber ratio. Similarly to capillary density, these alterations are also associated with their severely reduced peak exercise VO2 (Figure 8)145. A consequence of this fiber type shift is reduced skeletal muscle oxidative metabolism during exercise, which was evident after cessation of exercise from the delayed regeneration of quadriceps muscle phosphocreatine stores using phosphorus magnetic resonance spectroscopy146.
Figure 8. Peak VO2 and skeletal muscle histology in HFpEF.
Relationship of capillary to fiber ratio (Panel A) and percent type 1 muscle fibers (Panel B) with peak VO2 in older HFpEF patients (squares) and age-matched healthy controls (triangles).
What are the implications of these extensive skeletal muscle abnormalities in HFpEF? First, they confirm that HFpEF is a systemic disorder involving not only the heart but also other organ systems and that skeletal muscle and cardiac abnormalities are incited by common, circulating factors such as proinflammatory cytokines originating from multiple comorbidities147. Second, they suggest opportunities for novel interventions. Unlike the myocardium, which is terminally differentiated and has minimal capacity for regeneration, skeletal muscle has robust capacity for rapid repair, regeneration, and growth, which can be exploited by participation in an exercise training program148. Exercise training, shown in multiple studies to significantly improve peak VO2 in HFpEF148-151, achieves this primarily by improving skeletal muscle mitochondrial mass or function148. Most studies to date have used endurance training; high-intensity and strength training might produce even larger improvements but have not been examined systematically148.
Atrial Fibrillation
Prevalent atrial fibrillation in HFpEF goes along with a more advanced stage of cardiac remodeling evident from a larger left atrium and uniformly carries a worse prognosis62,152,153. Incident atrial fibrillation in HFpEF also accompanies worse LV diastolic dysfunction and was inversely related to statin use62. Prevalent atrial fibrillation was shown to be associated with incident HFpEF and prevalent HFpEF with incident atrial fibrillation154. These interactions suggest atrial fibrillation to beget HFpEF and vice versa and suggest efforts to restore sinus rhythm could be included in a HFpEF treatment strategy. Similar to HFpEF, atrial fibrillation reacts favorably to exercise training and weight loss155. To restore sinus rhythm, only cardioversion is recommended as catheter ablation of atrial fibrillation had limited long term success in HFpEF with single- and multiple-procedure drug-free success rates of respectively 27 and 45%156. If cardioversion is unsuccessful, rate control and permanent anticoagulation become mandatory.
CONCLUSIONS
HFpEF is the most common form of HF, is increasing out of proportion to HFrEF, and is associated with significant morbidity and mortality. Medication trials to date have been largely neutral on their primary outcomes, and so far only exercise training and weight loss appear to improve exercise intolerance and quality of life. Recent insights provide an understanding of the fundamental basis of left ventricular dysfunction in HFpEF, which involves systemic inflammation, coronary microcirculatory disturbances, cardiomyocyte stiffening and myocardial fibrosis. These insights also provide a more expanded view on HFpEF that includes involvement of the pulmonary circulation, right ventricular failure, skeletal muscle weakness and renal dysfunction. These new perspectives on HFpEF open an array of novel therapeutic targets either in the “garden variety” phenotype of lung congestion/metabolic risk or in specific phenotypes that may propel future advances in treatment and prevention of this important disorder.
Acknowledgments
SOURCES OF FUNDING
SJS is supported by grants from the National Institutes of Health (RO1 HL107577 and RO1 HL127028). BAB is supported by funding from the National Heart Lung and Blood Institute (NHLBI) Heart Failure Research Network (U10 HL110262-01) and research grants of Aires Pharmaceuticals, Medtronic, Teva and GSK. DWK is supported by National Institute of Health (NIH) grants R01AG18915 and P30AG21332 and by the Kermit Glenn Phillips II Chair in Cardiovascular Medicine. LvH is supported by grants from the European Commission (FP7-Health-2010; MEDIA-26140) and from CardioVasculair Onderzoek Nederland (CVON), Dutch Heart Foundation, The Netherlands (RECONNECT). MRZ receives research support from NHLBI, Veterans Administration, Bayer, CVRx, Medtronic, Novartis. DAK is supported by RO1 HL119012 and HL114910. WJP is supported by grants from the European Commission (FP7-Health-2010; MEDIA-26140) and from CVON, Dutch Heart Foundation, The Netherlands (ARENA, RECONNECT, EARLY-HFPEF).
Footnotes
DISCLOSURES
SJS has received consulting fees from AstraZeneca, Bayer, Merck and Novartis and grant funding from Actelion and Novartis. DWK serves as a consultant for Relypsa, Corvia Medical, Abbvie, Merck, Bayer, GSK, and Regeneron, has a trial grant from Novartis, and owns stock in Gilead Sciences and Relypsa. BAB received consulting fees from Amgen, Merck and Astra Zeneca. LvH: None. MRZ receives honorarium as a consultant for Abbott, Amgen, A-Z, Bayer, BMS, BMRC of Singapore-ATTRaCT, CVRx, European Union Research and Innovation, Capricor, Corvia, CVRx, DC Devices, Eli Lilly, Enopace, Idenex, Ironwood, Medtronic, Merck, MicroVide, Novartis, St. Jude Medical. DAK has received research funding from Pfizer Inc. and has provided consulting with Merck and Ironwood. WJP received consulting fees from Amgen and Servier and owns stock in Novartis and Pfizer.
REFERENCES
- 1.Borlaug BA. Heart Failure with Preserved Ejection Fraction. In: Baliga Ragavendra R., Haas Garrie J., editors. Management of Heart Failure. Springer Verlag; 2015. [Google Scholar]
- 2.Paulus WJ, van Ballegoij JJ. Treatment of heart failure with normal ejection fraction: an inconvenient truth! J Am Coll Cardiol. 2010;55:526–37. doi: 10.1016/j.jacc.2009.06.067. [DOI] [PubMed] [Google Scholar]
- 3.Holland DJ, Kumbhani DJ, Ahmed SH, Marwick TH. Effects of treatment on exercise tolerance, cardiac function, and mortality in heart failure with preserved ejection fraction. A meta-analysis. J Am Coll Cardiol. 2011;57:1676–86. doi: 10.1016/j.jacc.2010.10.057. [DOI] [PubMed] [Google Scholar]
- 4.Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJV, Michelson EL, Olofsson B, Östergren J, for the CHARM Investigators and Committees Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777–781. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 5.Anand IS, Rector TS, Cleland JG, Kuskowski M, McKelvie RS, Persson H, McMurray JJ, Zile MR, Komajda M, Massie BM, Carson PE. Prognostic value of baseline plasma amino-terminal pro-brain natriuretic peptide and its interactions with irbesartan treatment effects in patients with heart failure and preserved ejection fraction: findings from the I-PRESERVE trial. Circ Heart Fail. 2011;4:569–577. doi: 10.1161/CIRCHEARTFAILURE.111.962654. [DOI] [PubMed] [Google Scholar]
- 6.Solomon SD, Claggett B, Lewis EF, Desai A, Anand I, Sweitzer NK, O'Meara E, Shah SJ, McKinlay S, Fleg JL, Sopko G, Pitt B, Pfeffer MA, TOPCAT Investigators Influence of ejection fraction on outcomes and efficacy of spironolactone in patients with heart failure with preserved ejection fraction. Eur Heart J. 2016;37:455–462. doi: 10.1093/eurheartj/ehv464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanderson JE. Heart failure with a normal ejection fraction. Heart. 2007;93:155–8. doi: 10.1136/hrt.2005.074187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Keulenaer GW, Brutsaert DL. Systolic and diastolic heart failure are overlapping phenotypes within the heart failure spectrum. Circulation. 2011;123:1996–2004. doi: 10.1161/CIRCULATIONAHA.110.981431. [DOI] [PubMed] [Google Scholar]
- 9.Packer M. Can brain natriuretic peptide be used to guide the management of patients with heart failure and a preserved ejection fraction? The wrong way to identify new treatments for a nonexistent disease. Circ Heart Fail. 2011;4:538–40. doi: 10.1161/CIRCHEARTFAILURE.111.963710. [DOI] [PubMed] [Google Scholar]
- 10.Paulus WJ, Tschoepe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–71. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 11.Shah AM, Solomon SD. Phenotypic and pathophysiological heterogeneity in heart failure with preserved ejection fraction. Eur Heart J. 2012;33:1716–1717. doi: 10.1093/eurheartj/ehs124. [DOI] [PubMed] [Google Scholar]
- 12.Shah AM, Pfeffer MA. The many faces of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2012;9:555–556. doi: 10.1038/nrcardio.2012.123. [DOI] [PubMed] [Google Scholar]
- 13.Shah SJ, Katz DH, Deo RC. Phenotypic spectrum of heart failure with preserved ejection fraction. Heart Fail Clin. 2014;10:407–418. doi: 10.1016/j.hfc.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrari R, Böhm M, Cleland JG, Paulus WJ, Pieske B, Rapezzi C, Tavazzi L. Heart failure with preserved ejection fraction: uncertainties and dilemmas. Eur J Heart Fail. 2015;17:665–671. doi: 10.1002/ejhf.304. [DOI] [PubMed] [Google Scholar]
- 15.Haass M, Kitzman DW, Anand IS, Miller A, Zile MR, Massie BM, Carson PE. Body Mass Index and Adverse Cardiovascular Outcomes in Heart Failure Patients With Preserved Ejection Fraction / Clinical Perspective. Circ Heart Fail. 2011;4:324–331. doi: 10.1161/CIRCHEARTFAILURE.110.959890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhingra A, Garg A, Kaur S, Chopra S, Batra JS, Pandey A, Chaanine AH, Agarwal SK. Epidemiology of heart failure with preserved ejection fraction. Curr Heart Fail Rep. 2014;11:354–365. doi: 10.1007/s11897-014-0223-7. [DOI] [PubMed] [Google Scholar]
- 17.Santhanakrishnan R, Chong JP, Ng TP, Ling LH, Sim D, Leong KT, Yeo PS, Ong HY, Jaufeerally F, Wong R, Chai P, Low AF, Richards AM, Lam CS. Growth differentiation factor 15, ST2, high-sensitivity troponin T, and N-terminal pro brain natriuretic peptide in heart failure with preserved vs. reduced ejection fraction. Eur J Heart Fail. 2012;14:1338–1347. doi: 10.1093/eurjhf/hfs130. [DOI] [PubMed] [Google Scholar]
- 18.Cheng JM, Akkerhuis KM, Battes LC, van Vark LC, Hillege HL, Paulus WJ, Boersma E, Kardys I. Biomarkers of heart failure with normal ejection fraction: a systematic review. Eur J Heart Fail. 2013;15:1350–1362. doi: 10.1093/eurjhf/hft106. [DOI] [PubMed] [Google Scholar]
- 19.Sanders-van Wijk S, van Empel V, Davarzani N, Maeder MT, Handschin R, Pfisterer ME, Brunner-La Rocca HP, TIME-CHF investigators Circulating biomarkers of distinct pathophysiological pathways in heart failure with preserved vs. reduced left ventricular ejection fraction. Eur J Heart Fail. 2015;17:1006–1014. doi: 10.1002/ejhf.414. [DOI] [PubMed] [Google Scholar]
- 20.D'Elia E, Vaduganathan M, Gori M, Gavazzi A, Butler J, Senni M. Role of biomarkers in cardiac structure phenotyping in heart failure with preserved ejection fraction: critical appraisal and practical use. Eur J Heart Fail. 2015;17:1231–1239. doi: 10.1002/ejhf.430. [DOI] [PubMed] [Google Scholar]
- 21.Franssen C, Chen S, Unger A, Korkmaz HI, De Keulenaer GW, Tschöpe C, Leite-Moreira AF, Musters R, Niessen HWM, Linke WA, Paulus WJ, Hamdani N. Myocardial microvascular inflammatory endothelial activation in heart failure with preserved ejection fraction. JACC Heart Fail. 2016;4:312–324. doi: 10.1016/j.jchf.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 22.Westermann D, Lindner D, Kasner M, Zietsch C, Savvatis K, Escher F, von Schlippenbach J, Skurk C, Steendijk P, Riad A, Poller W, Schultheiss HP, Tschöpe C. Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ Heart Fail. 2011;4:44–52. doi: 10.1161/CIRCHEARTFAILURE.109.931451. [DOI] [PubMed] [Google Scholar]
- 23.Van Heerebeek L, Hamdani N, Falcão-Pires I, Leite-Moreira AF, Begieneman MPV, Bronzwaer JGF, van der Velden J, Stienen GJ, Laarman GJ, Somsen A, Verheugt FW, Niessen HW, Paulus WJ. Low Myocardial Protein Kinase G Activity in Heart Failure with Preserved Ejection Fraction. Circulation. 2012;126:830–839. doi: 10.1161/CIRCULATIONAHA.111.076075. [DOI] [PubMed] [Google Scholar]
- 24.LeWinter MM, Granzier HL. Cardiac titin and heart disease. J Cardiovasc Pharmacol. 2014;63:207–12. doi: 10.1097/FJC.0000000000000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linke WA, Hamdani N. Gigantic business: titin properties and function through thick and thin. Circ Res. 2014;114:1052–68. doi: 10.1161/CIRCRESAHA.114.301286. [DOI] [PubMed] [Google Scholar]
- 26.Loffredo FS, Steinhauser ML, Jay SM, Gannon J, Pancoast JR, Yalamanchi P, Sinha M, Dall'Osso C, Khong D, Shadrach JL, Miller CM, Singer BS, Stewart A, Psychogios N, Gerszten RE, Hartigan AJ, Kim MJ, Serwold T, Wagers AJ, Lee RT. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013;153:828–39. doi: 10.1016/j.cell.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.González A, Ravassa S, Beaumont J, López B, Díez J. New targets to treat the structural remodeling of the myocardium. J Am Coll Cardiol. 2011;58:1833–43. doi: 10.1016/j.jacc.2011.06.058. [DOI] [PubMed] [Google Scholar]
- 28.de Denus S, Lavoie J, Ducharme A, O'Meara E, Racine N, Sirois MG, Neagoe PE, Zhu L, Rouleau JL, White M. Differences in biomarkers in patients with heart failure with a reduced vs a preserved left ventricular ejection fraction. Can J Cardiol. 2012;28:62–68. doi: 10.1016/j.cjca.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 29.van Veldhuisen DJ, Linssen GC, Jaarsma T, van Gilst WH, Hoes AW, Tijssen JG, Paulus WJ, Voors AA, Hillege HL. B-type natriuretic peptide and prognosis in heart failure patients with preserved and reduced ejection fraction. J Am Coll Cardiol. 2013;61:1498–1506. doi: 10.1016/j.jacc.2012.12.044. [DOI] [PubMed] [Google Scholar]
- 30.Bishu K, Deswal A, Chen HH, LeWinter MM, Lewis GD, Semigran MJ, Borlaug BA, McNulty S, Hernandez AF, Braunwald E, Redfield MM. Biomarkers in acutely decompensated heart failure with preserved or reduced ejection fraction. Am Heart J. 2012;164:763–770. doi: 10.1016/j.ahj.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaasch WH, Zile MR. Left ventricular structural remodeling in health and disease: with special emphasis on volume, mass, and geometry. J Am Coll Cardiol. 2011;58:1733–1740. doi: 10.1016/j.jacc.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 32.Neeland IJ, Winders BR, Ayers CR, Das SR, Chang AY, Berry JD, Khera A, McGuire DK, Vega GL, de Lemos JA, Turer AT. Higher natriuretic peptide levels associate with a favorable adipose tissue distribution profile. J Am Coll Cardiol. 2013;62:752–760. doi: 10.1016/j.jacc.2013.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paulus WJ. Culprit Mechanism(s) for Exercise Intolerance in heart failure with normal ejection fraction. J Am Coll Cardiol. 2010;56:864–866. doi: 10.1016/j.jacc.2010.04.041. [DOI] [PubMed] [Google Scholar]
- 34.Borlaug BA, Olson TP, Lam CS, Flood KS, Lerman A, Johnson BD, Redfield MM. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56:845–854. doi: 10.1016/j.jacc.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maeder MT, Thompson BR, Brunner-La Rocca HP, Kaye DM. Hemodynamic basis of exercise limitation in patients with heart failure and normal ejection fraction. J Am Coll Cardiol. 2010;56:855–863. doi: 10.1016/j.jacc.2010.04.040. [DOI] [PubMed] [Google Scholar]
- 36.Andersen MJ, Olson TP, Melenovsky V, Kane GC, Borlaug BA. Differential hemodynamic effects of exercise and volume expansion in people with and without heart failure. Circ Heart Fail. 2015;8:41–48. doi: 10.1161/CIRCHEARTFAILURE.114.001731. [DOI] [PubMed] [Google Scholar]
- 37.Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:588–595. doi: 10.1161/CIRCHEARTFAILURE.109.930701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borlaug BA, Jaber WA, Ommen SR, Lam CS, Redfield MM, Nishimura RA. Diastolic relaxation and compliance reserve during dynamic exercise in heart failure with preserved ejection fraction. Heart. 2011;97:964–969. doi: 10.1136/hrt.2010.212787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santos M, Opotowsky AR, Shah AM, Tracy J, Waxman AB, Systrom DM. Central cardiac limit to aerobic capacity in patients with exertional pulmonary venous hypertension: implications for heart failure with preserved ejection fraction. Circ Heart Fail. 2015;8:278–285. doi: 10.1161/CIRCHEARTFAILURE.114.001551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dhakal BP, Malhotra R, Murphy RM, Pappagianopoulos PP, Baggish AL, Weiner RB, Houstis NE, Eisman AS, Hough SS, Lewis GD. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction: the role of abnormal peripheral oxygen extraction. Circ Heart Fail. 2015;8:286–294. doi: 10.1161/CIRCHEARTFAILURE.114.001825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Empel VP, Mariani J, Borlaug BA, Kaye DM. Impaired myocardial oxygen availability contributes to abnormal exercise hemodynamics in heart failure with preserved ejection fraction. J Am Heart Assoc. 2014;3:e001293. doi: 10.1161/JAHA.114.001293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM, Kitzman DW. Determinants of Exercise Intolerance in Elderly Heart Failure Patients With Preserved Ejection Fraction. J Am Coll Cardiol. 2011;58:265–274. doi: 10.1016/j.jacc.2011.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haykowsky M, Brubaker P, Morgan T, Kritchevsky S, Eggebeen J, Kitzman D. Impaired aerobic capacity and physical functional performance in older heart failure patients with preserved ejection fraction: role of lean body mass. J Gerontol A Biol Sci Med Sci. 2013;68:968–975. doi: 10.1093/gerona/glt011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cowley AW, Jr, Abe M, Mori T, O'Connor PM, Ohsaki Y, Zheleznova NN. Reactive oxygen species as important determinants of medullary flow, sodium excretion, and hypertension. Am J Physiol Renal Physiol. 2015;308:F179–197. doi: 10.1152/ajprenal.00455.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zile MR, Bennett TD, St John Sutton M, Cho YK, Adamson PB, Aaron MF, Aranda JM, Jr, Abraham WT, Smart FW, Stevenson LW, Kueffer FJ, Bourge RC. Transition from chronic compensated to acute decompensated heart failure: pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation. 2008;118:1433–1441. doi: 10.1161/CIRCULATIONAHA.108.783910. [DOI] [PubMed] [Google Scholar]
- 46.Maisel AS, Shah KS, Barnard D, Jaski B, Frivold G, Marais J, Azer M, Miyamoto MI, Lombardo D, Kelsay D, Iqbal N, Taub PR, Kupfer K, Lee E, Clopton P, Zile M, Greenberg B. How B-Type Natriuretic Peptide (BNP) and Body Weight Changes Vary in Heart Failure With Preserved Ejection Fraction Compared With Reduced Ejection Fraction: Secondary Results of the HABIT (HF Assessment With BNP in the Home) Trial. J Card Fail. 2016;22:283–293. doi: 10.1016/j.cardfail.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 47.Senni M, Paulus WJ, Gavazzi A, Fraser AG, Díez J, Solomon SD, Smiseth OA, Guazzi M, Lam CS, Maggioni AP, Tschöpe C, Metra M, Hummel SL, Edelmann F, Ambrosio G, Stewart Coats AJ, Filippatos GS, Gheorghiade M, Anker SD, Levy D, Pfeffer MA, Stough WG, Pieske BM. New strategies for heart failure with preserved ejection fraction: the importance of targeted therapies for heart failure phenotypes. Eur Heart J. 2014;35:2797–2815. doi: 10.1093/eurheartj/ehu204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharma K, Kass DA. Heart failure with preserved ejection fraction: mechanisms, clinical features, and therapies. Circ Res. 2014;115:79–96. doi: 10.1161/CIRCRESAHA.115.302922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shah SJ, Katz DH, Selvaraj S, Burke MA, Yancy CW, Gheorghiade M, Bonow RO, Huang CC, Deo RC. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation. 2015;131:269–279. doi: 10.1161/CIRCULATIONAHA.114.010637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deo RC. Machine Learning in Medicine. Circulation. 2015;132:1920–1930. doi: 10.1161/CIRCULATIONAHA.115.001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Borlaug BA, Redfield MM, Melenovsky V, Kane GC, Karon BL, Jacobsen SJ, Rodeheffer RJ. Longitudinal changes in left ventricular stiffness: a community-based study. Circ Heart Fail. 2013;6:944–952. doi: 10.1161/CIRCHEARTFAILURE.113.000383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wohlfahrt P, Redfield MM, Lopez-Jimenez F, Melenovsky V, Kane GC, Rodeheffer RJ, Borlaug BA. Impact of general and central adiposity on ventricular-arterial aging in women and men. JACC Heart Fail. 2014;2:489–499. doi: 10.1016/j.jchf.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davis BR, Kostis JB, Simpson LM, Black HR, Cushman WC, Einhorn PT, Farber MA, Ford CE, Levy D, Massie BM, Nawaz S, ALLHAT Collaborative Research Group Heart failure with preserved and reduced left ventricular ejection fraction in the antihypertensive and lipid-lowering treatment to prevent heart attack trial. Circulation. 2008;118:2259–2267. doi: 10.1161/CIRCULATIONAHA.107.762229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2014;11:507–515. doi: 10.1038/nrcardio.2014.83. [DOI] [PubMed] [Google Scholar]
- 55.van Dijk CG, Oosterhuis NR, Xu YJ, Brandt M, Paulus WJ, van Heerebeek L, Duncker DJ, Verhaar MC, Fontoura D, Lourenço AP, Leite-Moreira AF, Falcão-Pires I, Joles JA, Cheng C. Distinct Endothelial Cell Responses in the Heart and Kidney Microvasculature Characterize the Progression of Heart Failure With Preserved Ejection Fraction in the Obese ZSF1 Rat With Cardiorenal Metabolic Syndrome. Circ Heart Fail. 2016;9:e002760. doi: 10.1161/CIRCHEARTFAILURE.115.002760. [DOI] [PubMed] [Google Scholar]
- 56.Zile MR, Sharma V, Johnson JW, Warman EN, Baicu CF, Bennett TD. Prediction of All-Cause Mortality Based on the Direct Measurement of Intrathoracic Impedance. Circ Heart Fail. 2016;9:e002543. doi: 10.1161/CIRCHEARTFAILURE.115.002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adamson PB, Abraham WT, Bourge RC, Costanzo MR, Hasan A, Yadav C, Henderson J, Cowart P, Stevenson LW. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail. 2014;7:935–944. doi: 10.1161/CIRCHEARTFAILURE.113.001229. [DOI] [PubMed] [Google Scholar]
- 58.Abraham WT, Stevenson LW, Bourge RC, Lindenfeld JA, Bauman JG, Adamson PB, CHAMPION Trial Study Group Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: complete follow-up results from the CHAMPION randomised trial. Lancet. 2016;387:453–461. doi: 10.1016/S0140-6736(15)00723-0. [DOI] [PubMed] [Google Scholar]
- 59.Kitzman DW, Brubaker P, Morgan T, Haykowsky M, Hundley G, Kraus WE, Eggebeen J, Nicklas BJ. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2016;315:36–46. doi: 10.1001/jama.2015.17346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ramasubbu K, Estep J, White DL, Deswal A, Mann DL. Experimental and clinical basis for the use of statins in patients with ischemic and nonischemic cardiomyopathy. J Am Coll Cardiol. 2008;51:415–426. doi: 10.1016/j.jacc.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 61.Antoniades C, Bakogiannis C, Leeson P, Guzik TJ, Zhang MH, Tousoulis D, Antonopoulos AS, Demosthenous M, Marinou K, Hale A, Paschalis A, Psarros C, Triantafyllou C, Bendall J, Casadei B, Stefanadis C, Channon KM. Rapid, direct effects of statin treatment on arterial redox state and nitric oxide bioavailability in human atherosclerosis via tetrahydrobiopterin-mediated endothelial nitric oxide synthase coupling. Circulation. 2011;124:335–345. doi: 10.1161/CIRCULATIONAHA.110.985150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zakeri R, Chamberlain AM, Roger VL, Redfield MM. Temporal relationship and prognostic significance of atrial fibrillation in heart failure patients with preserved ejection fraction: a community-based study. Circulation. 2013;128:1085–1093. doi: 10.1161/CIRCULATIONAHA.113.001475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fukuta H, Sane DC, Brucks S, Little WC. Statin therapy may be associated with lower mortality in patients with diastolic heart failure: A preliminary report. Circulation. 2005;112:357–363. doi: 10.1161/CIRCULATIONAHA.104.519876. [DOI] [PubMed] [Google Scholar]
- 64.Nochioka K, Sakata Y, Miyata S, Miura M, Takada T, Tadaki S, Ushigome R, Yamauchi T, Takahashi J, Shimokawa H, CHART-2 Investigators Prognostic impact of statin use in patients with heart failure and preserved ejection fraction. Circ J. 2015;79:574–582. doi: 10.1253/circj.CJ-14-0865. [DOI] [PubMed] [Google Scholar]
- 65.Alehagen U, Benson L, Edner M, Dahlström U, Lund LH. Association Between Use of Statins and Mortality in Patients With Heart Failure and Ejection Fraction of ≥50. Circ Heart Fail. 2015;8:862–870. doi: 10.1161/CIRCHEARTFAILURE.115.002143. [DOI] [PubMed] [Google Scholar]
- 66.Gomberg-Maitland M, Shah SJ, Guazzi M. Inflammation in Heart Failure With Preserved Ejection Fraction: Time to Put Out the Fire. JACC Heart Fail. 2016;4:325–328. doi: 10.1016/j.jchf.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 67.Redfield MM, Anstrom KJ, Levine JA, Koepp GA, Borlaug BA, Chen HH, LeWinter MM, Joseph SM, Shah SJ, Semigran MJ, Felker GM, Cole RT, Reeves GR, Tedford RJ, Tang WH, McNulty SE, Velazquez EJ, Shah MR, Braunwald E. Isosorbide mononitrate in heart failure with preserved ejection fraction. N Engl J Med. 2015;373:2314–2324. doi: 10.1056/NEJMoa1510774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schwartzenberg S, Redfield MM, From AM, Sorajja P, Nishimura RA, Borlaug BA. Effects of vasodilation in heart failure with preserved or reduced ejection fraction implications of distinct pathophysiologies on response to therapy. J Am Coll Cardiol. 2012;59:442–51. doi: 10.1016/j.jacc.2011.09.062. [DOI] [PubMed] [Google Scholar]
- 69.Thomas GR, DiFabio JM, Gori T, Parker JD. Once daily therapy with isosorbide-5-mononitrate causes endothelial dysfunction in humans: Evidence of a free-radical-mediated mechanism. J Am Coll Cardiol. 2007;49:1289–1295. doi: 10.1016/j.jacc.2006.10.074. [DOI] [PubMed] [Google Scholar]
- 70.Oelze M, Knorr M, Kroller-Schon S, Kossmann S, Gottschlich A, Rummler R, Schuff A, Daub S, Doppler C, Kleinert H, Gori T, Daiber A, Munzel T. Chronic therapy with isosorbide-5-mononitrate causes endothelial dysfunction, oxidative stress, and a marked increase in vascular endothelin-1 expression. Eur Heart J. 2013;34:3206–3216. doi: 10.1093/eurheartj/ehs100. [DOI] [PubMed] [Google Scholar]
- 71.Vanderpool R, Gladwin MT. Harnessing the nitrate-nitrite-nitric oxide pathway for therapy of heart failure with preserved ejection fraction. Circulation. 2015;131:334–336. doi: 10.1161/CIRCULATIONAHA.114.014149. [DOI] [PubMed] [Google Scholar]
- 72.Omar SA, Fok H, Tilgner KD, Nair A, Hunt J, Jiang B, Taylor P, Chowienczyk P, Webb AJ. Paradoxical normoxia-dependent selective actions of inorganic nitrite in human muscular conduit arteries and related selective actions on central blood pressures. Circulation. 2015;131:381–389. doi: 10.1161/CIRCULATIONAHA.114.009554. [DOI] [PubMed] [Google Scholar]
- 73.Zamani P, Rawat D, Shiva-Kumar P, Geraci S, Bhuva R, Konda P, Doulias PT, Ischiropoulos H, Townsend RR, Margulies KB, Cappola TP, Poole DC, Chirinos JA. Effect of inorganic nitrate on exercise capacity in heart failure with preserved ejection fraction. Circulation. 2015;131:371–380. doi: 10.1161/CIRCULATIONAHA.114.012957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Borlaug BA, Koepp KE, Melenovsky V. Sodium nitrite improves exercise hemodynamics and ventricular performance in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2015;66:1672–1682. doi: 10.1016/j.jacc.2015.07.067. [DOI] [PubMed] [Google Scholar]
- 75.Eggebeen J, Kim-Shapiro DB, Haykowsky M, Morgan TM, Basu S, Brubaker P, Rejeski J, Kitzman DW. One Week of Daily Dosing With Beetroot Juice Improves Submaximal Endurance and Blood Pressure in Older Patients With Heart Failure and Preserved Ejection Fraction. JACC Heart Fail. 2016 doi: 10.1016/j.jchf.2015.12.013. doi:10.1016/j.jchf.2015.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Blanton RM, Takimoto E, Lane AM, Aronovitz M, Piotrowski R, Karas RH, Kass DA, Mendelsohn ME. Protein kinase Giα inhibits pressure overload-induced cardiac remodeling and is required for the cardioprotective effect of sildenafil in vivo. J Am Heart Assoc. 2012;1:e003731. doi: 10.1161/JAHA.112.003731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Koitabashi N, Aiba T, Hesketh GG, Rowell J, Zhang M, Takimoto E, Tomaselli GF, Kass DA. Cyclic GMP/PKG-dependent inhibition of TRPC6 channel activity and expression negatively regulates cardiomyocyte NFAT activation: novel mechanism of cardiac stress modulation by PDE5 inhibition. J Mol Cell Cardiol. 2010;48:713–724. doi: 10.1016/j.yjmcc.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Takimoto E, Koitabashi N, Hsu S, Ketner EA, Zhang M, Nagayama T, Bedja D, Gabrielson KL, Blanton R, Siderovski DP, Mendelsohn ME, Kass DA. Regulator of G protein signaling 2 mediates cardiac compensation to pressure overload and antihypertrophic effects of PDE5 inhibition in mice. J Clin Invest. 2009;119:408–420. doi: 10.1172/JCI35620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang M, Takimoto E, Hsu S, Lee DI, Nagayama T, Danner T, Koitabashi N, Barth AS, Bedja D, Gabrielson KL, Wang Y, Kass DA. Myocardial remodeling is controlled by myocyte-targeted gene regulation of phosphodiesterase type 5. J Am Coll Cardiol. 2010;56:2021–2030. doi: 10.1016/j.jacc.2010.08.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee DI, Zhu G, Sasaki T, Cho GS, Hamdani N, Holewinski R, Jo SH, Danner T, Zhang M, Rainer PP, Bedja D, Kirk JA, Ranek MJ, Dostmann WR, Kwon C, Margulies KB, Van Eyk JE, Paulus WJ, Takimoto E, Kass DA. Phosphodiesterase 9a controls nitric-oxide-independent cGMP and hypertrophic heart disease. Nature. 2015;519:472–476. doi: 10.1038/nature14332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguez ER, Bedja D, Gabrielson KL, Wang Y, Kass DA. Chronic inhibition of cyclic GMP phosphodiesterase 5a prevents and reverses cardiac hypertrophy. Nat.Med. 2005;11:214–222. doi: 10.1038/nm1175. [DOI] [PubMed] [Google Scholar]
- 82.Ranek MJ, Terpstra EJ, Li J, Kass DA, Wang X. Protein kinase G positively regulates proteasome-mediated degradation of misfolded proteins. Circulation. 2013;128:365–376. doi: 10.1161/CIRCULATIONAHA.113.001971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Omori K, Kotera J. Overview of PDEs and their regulation. Circ Res. 2007;100:309–327. doi: 10.1161/01.RES.0000256354.95791.f1. [DOI] [PubMed] [Google Scholar]
- 84.Lee DI, Kass DA. Phosphodiesterases and cyclic GMP regulation in heart muscle. Physiology (Bethesda) 2012;27:248–258. doi: 10.1152/physiol.00011.2012. [DOI] [PubMed] [Google Scholar]
- 85.Kirk JA, Holewinski RJ, Crowgey EL, Van Eyk JE. Protein kinase G signaling in cardiac pathophysiology: Impact of proteomics on clinical trials. Proteomics. 2016;16:894–905. doi: 10.1002/pmic.201500401. [DOI] [PubMed] [Google Scholar]
- 86.Kruger M, Kotter S, Grutzner A, Lang P, Andresen C, Redfield MM, Butt E, dos Remedios CG, Linke WA. Protein kinase g modulates human myocardial passive stiffness by phosphorylation of the titin springs. Circ.Res. 2009;104:87–94. doi: 10.1161/CIRCRESAHA.108.184408. [DOI] [PubMed] [Google Scholar]
- 87.Borbély A, Falcao-Pires I, van Heerebeek L, Hamdani N, Edes I, Gavina C, Leite-Moreira AF, Bronzwaer JG, Papp Z, van der Velden J, Stienen GJ, Paulus WJ. Hypophosphorylation of the Stiff N2B titin isoform raises cardiomyocyte resting tension in failing human myocardium. Circ Res. 2009;104:780–6. doi: 10.1161/CIRCRESAHA.108.193326. [DOI] [PubMed] [Google Scholar]
- 88.Hamdani N, Bishu KG, von Frieling-Salewsky M, Redfield MM, Linke WA. Deranged myofilament phosphorylation and function in experimental heart failure with preserved ejection fraction. Cardiovasc Res. 2013;97:464–471. doi: 10.1093/cvr/cvs353. [DOI] [PubMed] [Google Scholar]
- 89.Li P, Oparil S, Novak L, Cao X, Shi W, Lucas J, Chen YF. ANP signaling inhibits TGF-beta-induced smad2 and smad3 nuclear translocation and extracellular matrix expression in rat pulmonary arterial smooth muscle cells. J Appl.Physiol. 2007;102:390–398. doi: 10.1152/japplphysiol.00468.2006. [DOI] [PubMed] [Google Scholar]
- 90.Gheorghiade M, Greene SJ, Filippatos G, Erdmann E, Ferrari R, Levy PD, Maggioni A, Nowack C, Mebazaa A, COMPOSE Investigators and Coordinators Cinaciguat, a soluble guanylate cyclase activator: results from the randomized, controlled, phase IIb COMPOSE programme in acute heart failure syndromes. Eur J Heart Fail. 2012;14:1056–1066. doi: 10.1093/eurjhf/hfs093. [DOI] [PubMed] [Google Scholar]
- 91.Ghofrani HA, Galiè N, Grimminger F, Grünig E, Humbert M, Jing ZC, Keogh AM, Langleben D, Kilama MO, Fritsch A, Neuser D, Rubin LJ, PATENT-1 Study Group Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. 2013;369:330–340. doi: 10.1056/NEJMoa1209655. [DOI] [PubMed] [Google Scholar]
- 92.Ghofrani HA, D'Armini AM, Grimminger F, Hoeper MM, Jansa P, Kim NH, Mayer E, Simonneau G, Wilkins MR, Fritsch A, Neuser D, Weimann G, Wang C, CHEST-1 Study Group Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013;369:319–329. doi: 10.1056/NEJMoa1209657. [DOI] [PubMed] [Google Scholar]
- 93.Bonderman D, Ghio S, Felix SB, Ghofrani HA, Michelakis E, Mitrovic V, Oudiz RJ, Boateng F, Scalise AV, Roessig L, Semigran MJ, Left Ventricular Systolic Dysfunction Associated With Pulmonary Hypertension Riociguat Trial (LEPHT) Study Group Riociguat for patients with pulmonary hypertension caused by systolic left ventricular dysfunction: a phase IIb double-blind, randomized, placebo-controlled, dose-ranging hemodynamic study. Circulation. 2013;128:502–511. doi: 10.1161/CIRCULATIONAHA.113.001458. [DOI] [PubMed] [Google Scholar]
- 94.Gheorghiade M, Greene SJ, Butler J, Filippatos G, Lam CS, Maggioni AP, Ponikowski P, Shah SJ, Solomon SD, Kraigher-Krainer E, Samano ET, Müller K, Roessig L, Pieske B, SOCRATES-REDUCED Investigators and Coordinators Effect of Vericiguat, a Soluble Guanylate Cyclase Stimulator, on Natriuretic Peptide Levels in Patients With Worsening Chronic Heart Failure and Reduced Ejection Fraction: The SOCRATES-REDUCED Randomized Trial. JAMA. 2015;314:2251–2262. doi: 10.1001/jama.2015.15734. [DOI] [PubMed] [Google Scholar]
- 95.Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, Shi V, Bransford T, Takeuchi M, Gong J, Lefkowitz M, Packer M, McMurray JJ, Prospective comparison of ARNI with ARB on Management Of heart failUre with preserved ejectioN fracTion (PARAMOUNT) Investigators The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet. 2012;380:1387–1395. doi: 10.1016/S0140-6736(12)61227-6. [DOI] [PubMed] [Google Scholar]
- 96.Pokreisz P, Vandenwijngaert S, Bito V, Van den BA, Lenaerts I, Busch C, Marsboom G, Gheysens O, Vermeersch P, Biesmans L, Liu X, Gillijns H, Pellens M, Van Lommel A, Buys E, Schoonjans L, Vanhaecke J, Verbeken E, Sipido K, Herijgers P, Bloch KD, Janssens SP. Ventricular phosphodiesterase-5 expression is increased in patients with advanced heart failure and contributes to adverse ventricular remodeling after myocardial infarction in mice. Circulation. 2009;119:408–416. doi: 10.1161/CIRCULATIONAHA.108.822072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shan X, Quaile MP, Monk JK, French B, Cappola TP, Margulies KB. Differential expression of pde5 in failing and nonfailing human myocardium. Circ Heart Fail. 2012;5:79–86. doi: 10.1161/CIRCHEARTFAILURE.111.961706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Degen CV, Bishu K, Zakeri R, Ogut O, Redfield MM, Brozovich FV. The emperor's new clothes: PDE5 and the heart. PLoS One. 2015;10:e0118664. doi: 10.1371/journal.pone.0118664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, Lewinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O'Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: A randomized clinical trial. JAMA. 2013;309:1268–1277. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hoendermis ES, Liu LC, Hummel YM, van der Meer P, de Boer RA, Berger RM, van Veldhuisen DJ, Voors AA. Effects of sildenafil on invasive haemodynamics and exercise capacity in heart failure patients with preserved ejection fraction and pulmonary hypertension: A randomized controlled trial. Eur Heart J. 2015;36:2565–2573. doi: 10.1093/eurheartj/ehv336. [DOI] [PubMed] [Google Scholar]
- 101.Kroker KS, Mathis C, Marti A, Cassel JC, Rosenbrock H, Dorner-Ciossek C. PDE9a inhibition rescues amyloid beta-induced deficits in synaptic plasticity and cognition. Neurobiology of aging. 2014;35:2072–2078. doi: 10.1016/j.neurobiolaging.2014.03.023. [DOI] [PubMed] [Google Scholar]
- 102.Spinale FG, Zile MR. Integrating the Myocardial Matrix into Heart Failure Recognition and Management. Circ Res. 2013;113:725–738. doi: 10.1161/CIRCRESAHA.113.300309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.López B, Ravassa S, González A, Zubillaga E, Bonavila C, Bergés M, Echegaray K, Beaumont J, Moreno MU, San José G, Larman M, Querejeta R, Díez J. Myocardial Collagen Cross-Linking Is Associated With Heart Failure Hospitalization in Patients With Hypertensive Heart Failure. J Am Coll Cardiol. 2016;67:251–260. doi: 10.1016/j.jacc.2015.10.063. [DOI] [PubMed] [Google Scholar]
- 104.Chow SL, Maisel AS, Anand I, Bozkurt B, de Boer RA, Felker GM, Fonarow GC, Greenberg B, Januzzi JL, Kiernan MS, Liu P, Wang TJ, Yancy CW, Zile MR. The Role of Biomarkers for the Prevention, Assessment, and Management of Heart Failure. A Consensus Statement for Healthcare Professions from the American Heart Association. Circulation. 2016 doi: 10.1161/CIR.0000000000000490. in press. [DOI] [PubMed] [Google Scholar]
- 105.Moore-Morris T, Cattaneo P, Puceat M, Evans SM. Origins of cardiac fibroblasts. J Mol Cell Cardiol. 2015;91:1–5. doi: 10.1016/j.yjmcc.2015.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Moore-Morris T, Guimarães-Camboa N, Banerjee I, Zambon AC, Kisseleva T, Velayoudon A, Stallcup WB, Gu Y, Dalton ND, Cedenilla M, Gomez-Amaro R, Zhou B, Brenner DA, Peterson KL, Chen J, Evans SM. Resident fibroblast lineages mediate pressure overload-induced cardiac fibrosis. J Clin Invest. 2014;124:2921–2934. doi: 10.1172/JCI74783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Baicu CF, Zhang Y, Van Laer AO, Renaud L, Zile MR, Bradshaw AD. Effects of the absence of Procollagen C-Endopeptidase Enhancer-2 (PCOLCE-2) on myocardial collagen accumulation in chronic pressure-overload. Am J Physiol Heart Circ Physiol. 2012;303:H234–240. doi: 10.1152/ajpheart.00227.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Spinale FG, Janicki JS, Zile MR. Membrane Associated Matrix Proteolysis and Heart Failure. Circ Res. 2013;112:195–208. doi: 10.1161/CIRCRESAHA.112.266882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zile MR, Baicu CF. Biomarkers of Diastolic Dysfunction and Myocardial Fibrosis: Application to Heart Failure with Preserved Ejection Fraction. J Cardiovasc Transl Res. 2013;6:501–15. doi: 10.1007/s12265-013-9472-1. [DOI] [PubMed] [Google Scholar]
- 110.Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O'Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM, TOPCAT Investigators Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–92. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 111.Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Heitner JF, Lewis EF, O'Meara E, Rouleau JL, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, McKinlay SM, Pitt B. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation. 2015;131:34–42. doi: 10.1161/CIRCULATIONAHA.114.013255. [DOI] [PubMed] [Google Scholar]
- 112.Zile MR, Jhund PS, Baicu CF, Claggett BL, Pieske B, Voors AA, Prescott MF, Shi V, Lefkowitz M, McMurray JJ, Solomon SD. Prospective Comparison of ARNI With ARB on Management of Heart Failure With Preserved Ejection Fraction (PARAMOUNT) Investigators. Plasma Biomarkers Reflecting Profibrotic Processes in Heart Failure With a Preserved Ejection Fraction: Data From the Prospective Comparison of ARNI With ARB on Management of Heart Failure With Preserved Ejection Fraction Study. Circ Heart Fail. 2016;9:e002551. doi: 10.1161/CIRCHEARTFAILURE.115.002551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.López B, Querejeta R, González A, Beaumont J, Larman M, Díez J. Impact of treatment on myocardial lysyl oxidase expression and collagen cross-linking in patients with heart failure. Hypertension. 2009;53:236–42. doi: 10.1161/HYPERTENSIONAHA.108.125278. [DOI] [PubMed] [Google Scholar]
- 114.Gallet R, de Couto G, Simsolo E, Valle J, Sun B, Liu W, Tseliou E, Zile MR, Marbán E. Cardiosphere-derived cells reverse heart failure with preserved ejection fraction (HFpEF) in rats by decreasing fibrosis and inflammation. JACC Basic Transl Sci. 2016;1:14–28. doi: 10.1016/j.jacbts.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Borlaug BA, Lewis GD, McNulty SE, Semigran MJ, LeWinter M, Chen H, Lin G, Deswal A, Margulies KB, Redfield MM. Effects of sildenafil on ventricular and vascular function in heart failure with preserved ejection fraction. Circ Heart Fail. 2015;8:533–541. doi: 10.1161/CIRCHEARTFAILURE.114.001915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rodríguez-Iturbe B, Pons H, Quiroz Y, Johnson RJ. The immunological basis of hypertension. Am. J. Hypertens. 2014;27:1327–1337. doi: 10.1093/ajh/hpu142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hummel SL, Seymour EM, Brook RD, Sheth SS, Ghosh E, Zhu S, Weder AB, Kovács SJ, Kolias TJ. Low-sodium DASH diet improves diastolic function and ventricular-arterial coupling in hypertensive heart failure with preserved ejection fraction. Circ Heart Fail. 2013;6:1165–1171. doi: 10.1161/CIRCHEARTFAILURE.113.000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.ter Maaten JM, Damman K, Verhaar MC, Paulus WJ, Duncker DJ, Cheng C, van Heerebeek L, Hillege HL, Lam CS, Navis G, Voors AA. Connecting heart failure with preserved ejection fraction and renal dysfunction – the role of endothelial dysfunction and inflammation. Eur J Heart Fail. 2016 doi: 10.1002/ejhf.497. doi: 10.1002/ejhf.497. [DOI] [PubMed] [Google Scholar]
- 119.Klein DA, Katz DH, Beussink-Nelson L, Sanchez CL, Strzelczyk TA, Shah SJ. Association of Chronic Kidney Disease With Chronotropic Incompetence in Heart Failure With Preserved Ejection Fraction. Am J Cardiol. 2015;116:1093–1100. doi: 10.1016/j.amjcard.2015.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Damkjaer M, Vafaee M, Moller ML, Braad PE, Petersen H, Hoilund-Carlsen PF, Bie P. Renal cortical and medullary blood flow responses to altered NO availability in humans. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1449–1455. doi: 10.1152/ajpregu.00440.2010. [DOI] [PubMed] [Google Scholar]
- 121.Gori M, Senni M, Gupta DK, Charytan DM, Kraigher-Krainer E, Pieske B, Claggett B, Shah AM, Santos AB, Zile MR, Voors AA, McMurray JJ, Packer M, Bransford T, Lefkowitz M, Solomon SD, PARAMOUNT Investigators Association between renal function and cardiovascular structure and function in heart failure with preserved ejection fraction. Eur Heart J. 2014;35:3442–3451. doi: 10.1093/eurheartj/ehu254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Unger ED, Dubin RF, Deo R, Daruwalla V, Friedman JL, Medina C, Beussink L, Freed BH, Shah SJ. Association of chronic kidney disease with abnormal cardiac mechanics and adverse outcomes in patients with heart failure and preserved ejection fraction. Eur J Heart Fail. 2016;18:103–12. doi: 10.1002/ejhf.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hwang SJ, Melenovsky V, Borlaug BA. Implications of coronary artery disease in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63:2817–2827. doi: 10.1016/j.jacc.2014.03.034. [DOI] [PubMed] [Google Scholar]
- 124.Greenberg B. Heart failure preserved ejection fraction with coronary artery disease: time for a new classification? J Am Coll Cardiol. 2014;63:2828–2830. doi: 10.1016/j.jacc.2014.03.033. [DOI] [PubMed] [Google Scholar]
- 125.Rusinaru D, Houpe D, Szymanski C, Lévy F, Maréchaux S, Tribouilloy C. Coronary artery disease and 10-year outcome after hospital admission for heart failure with preserved and with reduced ejection fraction. Eur J Heart Fail. 2014;16:967–976. doi: 10.1002/ejhf.142. [DOI] [PubMed] [Google Scholar]
- 126.Abudiab MM, Redfield MM, Melenovsky V, Olson TP, Kass DA, Johnson BD, Borlaug BA. Cardiac output response to exercise in relation to metabolic demand in heart failure with preserved ejection fraction. Eur J Heart Fail. 2013;15:776–785. doi: 10.1093/eurjhf/hft026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Borlaug BA, Melenovsky V, Russell SD, Kessler K, Pacak K, Becker LC, Kass DA. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation. 2006;114:2138–2147. doi: 10.1161/CIRCULATIONAHA.106.632745. [DOI] [PubMed] [Google Scholar]
- 128.Brubaker PH, Joo KC, Stewart KP, Fray B, Moore B, Kitzman DW. Chronotropic incompetence and its contribution to exercise intolerance in older heart failure patients. J Cardiopulm Rehabil. 2006;26:86–89. doi: 10.1097/00008483-200603000-00007. [DOI] [PubMed] [Google Scholar]
- 129.Phan TT, Nallur Shivu G, Abozguia K, Davies C, Nassimizadeh M, Jimenez D, Weaver R, Ahmed I, Frenneaux M. Impaired heart rate recovery and chronotropic incompetence in patients with heart failure with preserved ejection fraction. Circ Heart Fail. 2009;3:29–34. doi: 10.1161/CIRCHEARTFAILURE.109.877720. [DOI] [PubMed] [Google Scholar]
- 130.Pal N, Sivaswamy N, Mahmod M, Yavari A, Rudd A, Singh S, Dawson DK, Francis JM, Dwight JS, Watkins H, Neubauer S, Frenneaux M, Ashrafian H. Effect of Selective Heart Rate Slowing in Heart Failure With Preserved Ejection Fraction. Circulation. 2015;132:1719–1725. doi: 10.1161/CIRCULATIONAHA.115.017119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Huang PH, Leu HB, Chen JW, Wu TC, Lu TM, Ding YA, Lin SJ. Comparison of endothelial vasodilator function, inflammatory markers, and N-terminal pro-brain natriuretic peptide in patients with or without chronotropic incompetence to exercise test. Heart. 2006;92:609–614. doi: 10.1136/hrt.2005.064147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: A community-based study. J Am Coll Cardiol. 2009;53:1119–1126. doi: 10.1016/j.jacc.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Melenovsky V, Hwang SJ, Lin G, Redfield MM, Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J. 2014;35:3452–3462. doi: 10.1093/eurheartj/ehu193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mohammed SF, Hussain I, Abou Ezzeddine OF, Takahama H, Kwon SH, Forfia P, Roger VL, Redfield MM. Right ventricular function in heart failure with preserved ejection fraction: A community-based study. Circulation. 2014;130:2310–2320. doi: 10.1161/CIRCULATIONAHA.113.008461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Burke MA, Katz DH, Beussink L, Selvaraj S, Gupta DK, Fox J, Chakrabarti S, Sauer AJ, Rich JD, Freed BH, Shah SJ. Prognostic importance of pathophysiologic markers in patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2014;7:288–299. doi: 10.1161/CIRCHEARTFAILURE.113.000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Guazzi M, Vicenzi M, Arena R, Guazzi MD. Pulmonary hypertension in heart failure with preserved ejection fraction: A target of phosphodiesterase-5 inhibition in a 1-year study. Circulation. 2011;124:164–174. doi: 10.1161/CIRCULATIONAHA.110.983866. [DOI] [PubMed] [Google Scholar]
- 137.Andersen MJ, Hwang SJ, Kane GC, Melenovsky V, Olson TP, Fetterly K, Borlaug BA. Enhanced pulmonary vasodilator reserve and abnormal right ventricular: Pulmonary artery coupling in heart failure with preserved ejection fraction. Circ Heart Fail. 2015;8:542–550. doi: 10.1161/CIRCHEARTFAILURE.114.002114. [DOI] [PubMed] [Google Scholar]
- 138.Bonderman D, Pretsch I, Steringer-Mascherbauer R, Jansa P, Rosenkranz S, Tufaro C, Bojic A, Lam CS, Frey R, Ochan Kilama M, Unger S, Roessig L, Lang IM. Acute hemodynamic effects of riociguat in patients with pulmonary hypertension associated with diastolic heart failure (DILATE-1): a randomized, double-blind, placebo-controlled, single-dose study. Chest. 2014;146:1274–1285. doi: 10.1378/chest.14-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zile MR, Bourge RC, Redfield MM, Zhou D, Baicu CF, Little WC. Randomized, double-blind, placebo-controlled study of sitaxsentan to improve impaired exercise tolerance in patients with heart failure and a preserved ejection fraction. JACC Heart Fail. 2014;2:123–130. doi: 10.1016/j.jchf.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 140.Lam CS, Lyass A, Kraigher-Krainer E, Massaro JM, Lee DS, Ho JE, Levy D, Redfield MM, Pieske BM, Benjamin EJ, Vasan RS. Cardiac dysfunction and noncardiac dysfunction as precursors of heart failure with reduced and preserved ejection fraction in the community. Circulation. 2011;124:24–30. doi: 10.1161/CIRCULATIONAHA.110.979203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Melenovsky V, Andersen MJ, Andress K, Reddy YN, Borlaug BA. Lung congestion in chronic heart failure: haemodynamic, clinical, and prognostic implications. Eur J Heart Fail. 2015;17:1161–1171. doi: 10.1002/ejhf.417. [DOI] [PubMed] [Google Scholar]
- 142.Olson TP, Johnson BD, Borlaug BA. Impaired pulmonary diffusion in heart failure with preserved ejection fraction. JACC Heart Fail. 2016 doi: 10.1016/j.jchf.2016.03.001. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Haykowsky M, Kouba EJ, Brubaker PH, Nicklas BJ, Eggebeen J, Kitzman DW. Skeletal muscle composition and its relation to exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Cardiol. 2014;113:1211–1216. doi: 10.1016/j.amjcard.2013.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Heinonen I, Bucci M, Kemppainen J, Knuuti J, Nuutila P, Boushel R, Kalliokoski KK. Regulation of subcutaneous adipose tissue blood flow during exercise in humans. J Appl Physiol. 2012;112:1059–1063. doi: 10.1152/japplphysiol.00732.2011. [DOI] [PubMed] [Google Scholar]
- 145.Kitzman DW, Nicklas B, Kraus WE, Lyles MF, Eggebeen J, Morgan TM, Haykowsky M. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol. 2014;306:H1364–1370. doi: 10.1152/ajpheart.00004.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bhella PS, Prasad A, Heinicke K, Hastings JL, Arbab-Zadeh A, Adams-Huet B, Pacini EL, Shibata S, Palmer MD, Newcomer BR, Levine BD. Abnormal haemodynamic response to exercise in heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:1296–1304. doi: 10.1093/eurjhf/hfr133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ather S, Chan W, Bozkurt B, Aguilar D, Ramasubbu K, Zachariah AA, Wehrens XH, Deswal A. Impact of Noncardiac Comorbidities on Morbidity and Mortality in a Predominantly Male Population With Heart Failure and Preserved Versus Reduced Ejection Fraction. J Am Coll Cardiol. 2012;59:998–1005. doi: 10.1016/j.jacc.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kitzman DW, Haykowsky MJ. Mechanisms of exercise training in heart failure with preserved ejection fraction: central disappointment and peripheral promise. Am Heart J. 2012;164:807–809. doi: 10.1016/j.ahj.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 149.Kitzman D, Brubaker P, Morgan T, Stewart K, Little W. Exercise training in older patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2010;3:659–667. doi: 10.1161/CIRCHEARTFAILURE.110.958785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Edelmann F, Gelbrich G, Düngen HD, Fröhling S, Wachter R, Stahrenberg R, Binder L, Töpper A, Lashki DJ, Schwarz S, Herrmann-Lingen C, Löffler M, Hasenfuss G, Halle M, Pieske B. Exercise Training Improves Exercise Capacity and Diastolic Function in Patients With Heart Failure With Preserved Ejection Fraction: Results of the Ex-DHF (Exercise training in Diastolic Heart Failure) Pilot Study. J Am Coll Cardiol. 2011;58:1780–1791. doi: 10.1016/j.jacc.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 151.Fujimoto N, Prasad A, Hastings JL, Bhella PS, Shibata S, Palmer D, Levine BD. Cardiovascular effects of 1 year of progressive endurance exercise training in patients with heart failure with preserved ejection fraction. Am Heart J. 2012;164:869–877. doi: 10.1016/j.ahj.2012.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kao DP, Lewsey JD, Anand IS, Massie BM, Zile MR, Carson PE, McKelvie RS, Komajda M, McMurray JJ, Lindenfeld J. Characterization of subgroups of heart failure patients with preserved ejection fraction with possible implications for prognosis and treatment response. Eur J Heart Fail. 2015;17:925–935. doi: 10.1002/ejhf.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Melenovsky V, Hwang SJ, Redfield MM, Zakeri R, Lin G, Borlaug BA. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ Heart Fail. 2015;8:295–303. doi: 10.1161/CIRCHEARTFAILURE.114.001667. [DOI] [PubMed] [Google Scholar]
- 154.Santhanakrishnan R, Wang N, Larson MG, Magnani JW, McManus DD, Lubitz SA, Ellinor PT, Cheng S, Vasan RS, Lee DS, Wang TJ, Levy D, Benjamin EJ, Ho JE. Atrial Fibrillation Begets Heart Failure and Vice Versa: Temporal Associations and Differences in Preserved vs. Reduced Ejection Fraction. Circulation. 2016;133:484–492. doi: 10.1161/CIRCULATIONAHA.115.018614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Elliott AD, Mahajan R, Pathak RK, Lau DH, Sanders P. Exercise Training and Atrial Fibrillation: Further Evidence for the Importance of Lifestyle Change. Circulation. 2016;133:457–459. doi: 10.1161/CIRCULATIONAHA.115.020800. [DOI] [PubMed] [Google Scholar]
- 156.Machino-Ohtsuka T, Seo Y, Ishizu T, Sugano A, Atsumi A, Yamamoto M, Kawamura R, Machino T, Kuroki K, Yamasaki H, Igarashi M, Sekiguchi Y, Aonuma K. Efficacy, safety, and outcomes of catheter ablation of atrial fibrillation in patients with heart failure with preserved ejection fraction. J Am Coll Cardiol. 2013;62:1857–1865. doi: 10.1016/j.jacc.2013.07.020. [DOI] [PubMed] [Google Scholar]