Abstract
Mechanisms underlying the profound parental effects on cognitive, emotional and social development in humans remain poorly understood. Studies with nonhuman models suggest variations in parental care affect the limbic system, influential to learning, autobiography and emotional regulation. In some research, nonoptimal care relates to decreases in neurogenesis, although other work suggests early-postnatal social adversity accelerates the maturation of limbic structures associated with emotional learning. We explored whether maternal sensitivity predicts human limbic system development and functional connectivity patterns in a small sample of human infants. When infants were 6 months of age, 20 mother–infant dyads attended a laboratory-based observational session and the infants underwent neuroimaging at the same age. After considering age at imaging, household income and postnatal maternal anxiety, regression analyses demonstrated significant indirect associations between maternal sensitivity and bilateral hippocampal volume at six months, with the majority of associations between sensitivity and the amygdala demonstrating similar indirect, but not significant results. Moreover, functional analyses revealed direct associations between maternal sensitivity and connectivity between the hippocampus and areas important for emotional regulation and socio-emotional functioning. Sensitivity additionally predicted indirect associations between limbic structures and regions related to autobiographical memory. Our volumetric results are consistent with research indicating accelerated limbic development in response to early social adversity, and in combination with our functional results, if replicated in a larger sample, may suggest that subtle, but important, variations in maternal care influence neuroanatomical trajectories important to future cognitive and emotional functioning.
Introduction
The quality of parental care influences socio-emotional and cognitive development,1, 2, 3, 4 as well as mental health,5 although the degree to which normal variation in parental care influences neural development is less well understood. In contrast, there are well-documented effects of more extreme forms of childhood adversity, such as abuse and neglect, upon brain development and function.6, 7 In addition, the influence of variations in parental care that lie within the normal range upon neuronal development and behavior has been extensively studied in rodents,8 with similar neurodevelopmental findings in nonhuman primates.9, 10, 11 Nevertheless, studies of human child development do reveal the particular importance of variation in ‘maternal sensitivity' on developmental outcomes.12, 13 Maternal sensitivity refers to timely and accurate responsivity to situationally dependent infant signals, and is critical for the management of infant distress and the facilitation of exploration and autonomy.14, 15 Meta-analytic analyses confirm that maternal sensitivity predicts infant behavioral responses to potentially stressful situations,16 which are themselves used to qualitatively describe the mother–child attachment relationship17—a highly documented predictor of subsequent socio-emotional development and mental health.18 Likewise, sensitivity and/or secure mother–child attachment predict positive social relationships,19, 20, 21 enhanced cognitive abilities,22, 23, 24, 25 and inversely relate to internalizing difficulties26 including anxiety,27 and externalizing problems.26 Furthermore, enhancing sensitivity has a positive effect on child outcomes.28
Despite the compelling evidence for the importance of maternal sensitivity for child development, there are no magnetic resonance imaging (MRI) studies that directly examine effects on early-postnatal brain structure and function. Findings from three studies using the Parental Bonding Instrument29 to retrospectively assess the potential influence of variations in the quality of parental care suggest that poorer parental care during childhood and adolescence may directly and/or indirectly negatively impact hippocampal volume during adulthood and the elderly years.30, 31, 32 These findings are consistent with those from rodent studies, suggesting that normal variations in maternal care alter limbic system development and function.33, 34 However, the results of these human studies are compromised by both the use of self-reported retrospective assessment of parental care in adulthood and measures of neural structure in later life. Self-reported Parental Bonding Instrument scores may be biased, as the degree to which they correlate with likely experience assessed by an objective observer is affected by the degree to which participants idealize parents and dismiss the importance of attachment relationships.35 Moreover, retrospective accounts do not report specifically on the quality of mother–infant, as opposed to child, experience, nor on the timing of the parental influences. Furthermore, neuroanatomical assessment later in development may reflect potential confounds such as subsequent social and emotional experience.
Consistent with research examining childhood maltreatment,36 prospective studies with observer-rated variations in maternal care do suggest effects on amygdala and hippocampal volume in childhood, early adolescence and adulthood.37, 38, 39 That is, Moutsiana et al.39 have recently reported that secure infant–mother attachment relationships observed at 18 months predicted smaller amygdala volume in early adulthood, and Whittle et al.40 observed positive maternal behavior during early adolescence predicted subsequent attenuation in amygdala growth. With regard to the hippocampus, Luby et al.38 found maternal support during early childhood directly predictive of hippocampal volume among non-depressed school-age children, whereas Rao et al.37 found increased nurturance as assessed by the Home Observation for Measurement of the Environment (HOME) Inventory scale indirectly associated with hippocampal volume among young adolescents who had been prenatally exposed to cocaine. Unfortunately, it is difficult to determine why the direction of effects on the hippocampus differs as, in addition to differences in the participant pools, the studies also differed with regard to the specific forms of caregiving experience assessed, the timing of exposure and the timing of MRI assessment, with all of these factors likely to influence relations between experience and brain development.36
Moreover, the timing of MRI assessment is not just an important reflection of the duration of time, and presumably physiological change, since exposure. MRI provides a ‘snapshot' of the brain's structure at a specific stage of development, with recent work suggesting that human development may not be uniform, and is influenced by experience with adversity41, 42, 43, 44 and positive parenting.40 Studying the effects on limbic structures during infancy is important, as this period is characterized by rapid hippocampal and amygdala growth.45 For example, MRI studies indicate that the hippocampus rapidly changes within the first one46 to two years.46, 47 The hippocampus substantially increases in volume (that is, by 15–19%) from the first to second year,46 suggesting that alterations occurring within the first year may also impact the course of development for future growth.
Thus, although an effect of maternal sensitivity upon infant hippocampal and amygdala volume is expectable, the direction of the hypothesized association remains unclear. Past literature suggests that brain regions may be especially sensitive to insults during periods of rapid growth,48 and that adversity or stress hormone exposure may lead to decreased hippocampal volume,36, 49 with mixed findings concerning relations between adversity, glucocorticoids and amygdala volume;50, 51, 52 following this line of reasoning, increased sensitivity should directly relate to hippocampal volume, but may directly or indirectly relate to amygdala volume. In contrast, as both the hippocampus and amygdala show normative volumetric increases over early infancy,45 ideas concerning adversity and acceleration in development42, 43, 44, 53, 54 suggest that increased sensitivity should indirectly relate to both hippocampal and amygdala volumes.
To our knowledge, no studies have yet examined the potential effects of maternal behavior on hippocampal and amygdala structure over this early-postnatal period. In this report, we provide a novel determination of the association between variation in observed maternal sensitivity and infant hippocampal and amygdala volume assessed using structural MRI. Then, we explore the relation specifically between maternal sensitivity and, separately, hippocampal and amygdala functional connectivity assessed using resting-state functional MRI (fMRI) at 6 months of age. Resting-state fMRI enables a summary of complex patterns of brain functional organization, which can be examined in relation to maternal sensitivity in the level of hippocampal and amygdala functional networks.
Materials and methods
Participants
Participants were part of a larger prospective birth cohort study, Growing Up in Singapore Towards Healthy Outcomes (GUSTO).55 The GUSTO cohort consisted of pregnant Asian women attending the first trimester antenatal ultrasound scan clinic at the National University Hospital and KK Women's and Children's Hospital in Singapore. The parents were Singapore citizens or permanent residents of Chinese, Malay or Indian ethnic background. Birth outcome and pregnancy measures were obtained from hospital records. Socioeconomic status (household income) was extracted from survey questionnaires conducted as a part of a scheduled appointment during pregnancy. The GUSTO study was approved by the National Healthcare Group Domain Specific Review Board and the Sing Health Centralized Institutional Review Board, and all participating mothers provided informed consent.
Inclusion criteria for infants in the current research included an Apgar score of ⩾9, gestational age ⩾37 and <42 weeks, birth weight ⩾2500 and <4000 g, singleton birth and born to mothers with no pregnancy complications such as hypoglycemia, hypertension, preeclampsia, reported intrauterine growth retardation or gestational diabetes. In addition, included infants had usable data from: a 6-month structural and/or functional MRI session; a behavioral observation session occurring within ±2 weeks of their 6-month birthday; and a neonatal structural MRI scan. Four-hundred and thirty-four mother–infant dyads provided useable data from the 6-month behavioral assessment. One hundred and eighty-nine infants were recruited for the neuroimaging study shortly after birth. A subset of these infants was invited back for the 6-month imaging visit. Among those invited for the 6-month visit, 42 infants came back for the second MRI scan at 6 months of age, although at 6 months only 32 of these participants provided artifact-free structural MRI data, and only 24 of these participants provided artifact-free functional MRI data.
Maternal sensitivity
A 15-min mother–child interaction was recorded as part of a 3-h laboratory visit when infants were 6 months of age (±2 weeks). The mother was asked to ‘interact or play' with her 6-month-old infant ‘as she normally would at home.' The room was equipped with a foldable chair, highchair and a mat, but no toys for the first 5 min. After 5 min, a standard set of attractive toys and books was brought into the room. Maternal sensitivity was assessed using the Revised Mini-A short form of the Maternal Behavioral Q-Sort-V (Mini-MBQS-V).56, 57, 58 The Mini-MBQS-V consists of 25 items, each representing different possible aspects of sensitive, and inversely, insensitive, maternal behavior during interaction with an infant. Coders sort the 25 items into piles of 5, ranging from 1 being ‘least like the mother' to 5 being ‘most like the mother.' Ratings are then correlated with that of a theoretically constructed prototypical sensitive mother to derive the global sensitivity score, ranging from −1 (very much unlike a prototypical sensitive mother) to 1 (very much similar to a prototypical sensitive mother). For example, if the mother's behavior is very similar to a ‘prototypically sensitive mother', coders might assign values of ‘5' to cards such as: ‘mother builds on the focus of the baby's attention' and ‘mother responds to the baby's distress and non-distress signals even when engaged in some other activity.' Likewise, when viewing a mother who is very similar to a prototypically sensitive mother, coders might assign values of ‘1' to cards describing insensitive behavior such as, ‘mother tends to tune out and not notice the infant's bids for attention' and ‘the content and pace of the interaction is set by the mother rather than the baby's response.' The two southeast Asian coders who scored the majority of the current study's cases were directly trained by the developers of the Mini-MBQS-V coding system (D Pederson and S Bento). Together the local coders were fluent in both English and the predominant mother-tongue languages of Singapore, with one coder fluent in both English and Tamil, whereas the other is fluent in English, Bahasa Melayu and Mandarin. Training included the scoring of western and Singaporean tapes. To ensure reliability within the current sample, coders double coded all recordings where mothers predominantly spoke in English (that is, 70% of the current sample) and achieved an intra-class correlation of r=0.937. In addition, at the time of writing, the two local coders had achieved an high interclass correlation (r=0.923) on roughly 15% of useable cases comprising these and other cases within the larger GUSTO sample (n=434).
MRI acquisition
During the acquisition sessions, infants slept in a 1.5-Tesla GE scanner (GE Healthcare, Milwaukee, WI, USA) at the Department of Diagnostic and Interventional Imaging of the KK Hospital. No sedation was used, and precautions were taken to reduce exposure to MRI scanner noise. A neonatologist was present during each scan. A pulse oximeter was used to monitor heart rate and oxygen saturation through the entirety of the scans.
The imaging protocols were (i) fast spin-echo T2-weighted MRI (axial acquisition; TR=3500 ms; TE=110 ms; FOV=256 × 256 mm; matrix size=256 × 256; 50 axial slices with 2.0 mm thickness); (ii) fast spin-echo T2-weighted MRI (coronal acquisition; TR=3500 ms; TE=110 ms; FOV=256 × 256 mm; matrix size=256 × 256; 50 axial slices with 2.0 mm thickness); (iii) echo planar resting-state fMRI (axial acquisition; TR=2500 ms; TE=40 ms; FOV=192 × 192 mm; matrix size=64 × 64; 40 axial slices with 3.0 mm thickness, 120 volumes). The coronal T2-weighted MRI data were acquired parallel to the anterior–posterior axis of the hippocampus and only covered the temporal lobe. Each subject obtained two acquisitions of the axial T2-weighted MRI and one acquisition of the coronal T2-weighted MRI at baseline and follow-up. All brain scans were reviewed by a neuroradiologist (MVF). Images were analyzed blind to sensitivity ratings.
Hippocampus and amygdala delineation
Within individual subjects two T2-weighted MRI acquisitions were first rigidly aligned and averaged to increase signal-to-noise ratio. In cases where only one scan was acquired, data from one scan were used in lieu of the average axial image. The skull of the averaged axial image was removed using the Brain Extraction Tool59 and used manual and automated segmentation of the hippocampus and amygdala. The delineation protocol for the neonatal and 6-month-old infant's brain is detailed elsewhere.60 Intra-class correlation coefficients for the manual segmentation were, respectively, 0.79 and 0.82 for the hippocampus of neonates and 6-month-old infants, and 0.77 and 0.80 for the amygdala of neonates and 6-month-old infants.
Limbic system functional connectivity analysis
Preprocessing
The resting-state fMRI data were first processed with slice timing, motion correction, skull stripping, band-pass filtering (0.01–0.1 Hz) and grand mean scaling of the data. We only included subjects with framewise displacement (head motion characteristics, ranging from 0.05 to 0.47 in our sample) <0.5 as suggested by Power et al.61 However, three subjects showed sudden ‘jerk-like' head movements at the first three volumes. We manually removed these three volumes to create the new fMRI data sets for these three subjects that satisfied the head motion criteria. Within each subject, the six parameters of the head motion and cerebrospinal fluid and white matter signals were regressed out from the fMRI images. Finally, the fMRI images were aligned to the T2-weighted image.
Functional connectivity
To analyze the hippocampus and amygdala functional networks, we first computed the mean signals within the hippocampus/amgdala marks delineated from the T2-weighted image. We then calculated Pearson's correlation coefficients of the hippocampal/amygdala fMRI signal with the fMRI signals of the rest of the brain and converted them as z-score using Fisher's z-transformation. Finally, the z-score images were aligned to the 6-month-old infant atlas based on the nonlinear transformation obtained from large deformation diffeomorphic metric mapping.62 These images in the atlas space were used for statistical analysis.
Statistical analysis
Sensitivity and limbic structure volumes
The relation between maternal sensitivity and 6-month right and left hippocampal/amygdala volumes were determined via separate regressions. In these regressions, postmenstrual age at the time of MRI was entered in the first block, whereas maternal sensitivity was entered in the second block. Six-month left/right hippocampal/amygdala volume served as the outcome.
In addition, to better determine the specificity of effects, we also examined relations between maternal sensitivity and potential confounders incuding: gestational age, birth weight, birth length, pre- and postnatal maternal state and trait anxiety, pre- and postnatal maternal depression, household income, maternal education, maternal age, ethnicity and infant gender. The examinations of sensitivity and potential confounds were conducted within the larger sample of participants who had similar inclusion/exclusion criteria. No significant associations were observed for the majority of potential confounders. However, household income significantly related to sensitivity (r=0.213, P=0.001, n=261) and we observed a similar marginal association between sensitivity and maternal education (r=0.115, P=0.058, n=271). Likewise, the relation between levels of maternal state anxiety when infants were 3 months of age and maternal sensitivity at 6 months approached marginal significance (r=−0.105, P=0.116, n=227). In addition, we examined the relation between maternal sensitivity and neonatal hippocampal/amygdala volume among cases with similar inclusion/exclusion criteria who attended the neonatal scan, as sensitivity is known to relate to mental states16 associated with adult physiology,63 which could influence fetal development.64 Using partial correlations that controlled for age at the neonatal scan, and examining 80 dyads, maternal sensitivity did not significantly relate to left (r=−0.173, P=0.128) nor right (r=−0.097, P=0.397) neonatal hippocampal volume, nor left (r=−0.006, P=0.956) nor right (r=0.071, P=0.536) neonatal amygdala volume.
On the basis of the results of our covariate analyses, we repeated our regression analyses as above, but (a) first entered both postmenstrual age at the time of MRI and household income, before entering maternal sensitivity and (b) in a subsample for whom postnatal state anxiety information was available, first entered both postmenstrual age at the time of MRI and postnatal anxiety, before entering maternal sensitivity. All volumetric tests report two-sided P-values.
Sensitivity and limbic system functional connectivity
Voxel-based analysis was examined to first (separately) determine the hippocampal/amygdala functional connectivity networks and then investigate the potential influence of maternal sensitivity on the hippocampal/amygdala functional connectivity using SPM8 (Wellcome Trust Center for Neuroimaging, University College London, UK). The fMRI z-score images were smoothed with a Gaussian kernel with full width at half maximum of 6 mm. Regression analysis was first applied to the smoothed z-score images to identify the hippocampus/amygdala functional networks, with age at the 6-month MRI session as a covariate. Regression analysis was then used to examine the associations between maternal sensitivity and hippocampus/amygdala functional networks, with age at the 6-month MRI session and family income as covariates. Given the small sample size and concerns regarding power, as well as the weaker correlation between postnatal anxiety and sensitivity, postnatal anxiety was not entered as a covariate in these exploratory functional analyses. Consistent with similar research,65 all statistical results at each voxel were thresholded at the level of significance (P<0.01), the size of each cluster was greater than 100 voxels, uncorrected for multiple comparisons.
Results
Of the 42 infants who participated in the 6-month MRI session, 32 participants had acceptable 6-month structural MRI data. Of these, 8 (25%) were excluded due to perinatal or pregnancy risks (for example, hypertensive pregnancy, prematurity, gestational diabetes and so on). Of the remaining 24 infants with usable 6-month volumetric data, in two cases the behavioral recording was corrupted—one was sick on the day of the behavioral assessment and one took multiple breaks during the testing procedure. Thus analyses examining the relation between maternal sensitivity and limbic structure volume included data from 20 infants who had income data, both neonatal and 6-month structural MRI data, sensitivity data and satisfied the above subject selection criteria.
Twenty-four infants had usable 6-month functional MRI data. Of these, four (17%) were excluded due to perinatal or pregnancy risks. Of the remaining 20 infants with usable 6-month functional data, two had corrupted behavioral data recordings, and one took multiple breaks during the testing procedure. Therefore, the analyses regarding the relation between maternal sensitivity and limbic structure functional connectivity included 17 subjects who had income data, 6-month sensitivity and resting-state MRI data, and satisfied the above subject selection criteria. Thus, these participants represent a smaller subsample than those included in the above structural analyses, although there is considerable overlap between the structural and functional samples.
To ensure the representative nature of the sample, we compared the maternal sensitivity scores of the mothers of the 20 infants included in the structural MRI analyses (M=0.29, s.d.=0.45) with the 258 mothers who did not have infants taking part in MRI (M=0.20, s.d.=0.42), but who otherwise fulfilled the perinatal/birth outcome criteria used in this study. No significant differences in sensitivity scores were observed, t(1,276)=0.864, P=0.388.
Limbic structure volumes
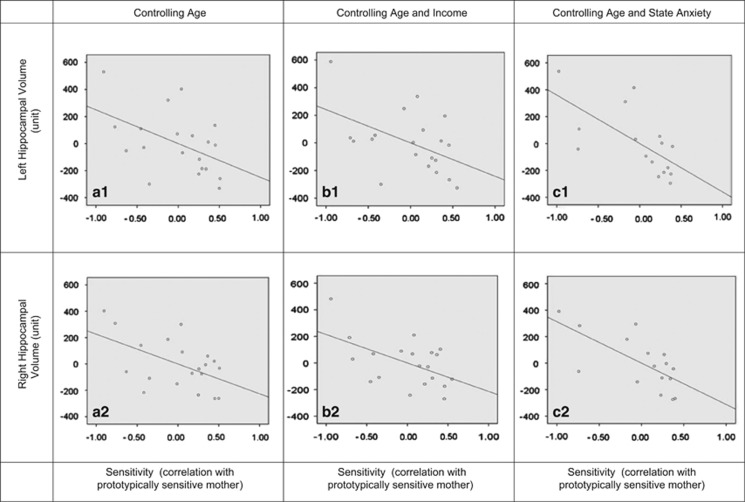
The findings are summarized in Figures 1 and 2. After controlling for age, maternal sensitivity significantly predicted left (B=−0.490, P=0.037; B=−249.88, 95% confidence interval (CI)=−483.078 to −16.682) and right (B=−0.506, P=0.022; B=−226.045; 95% CI=−416.019 to −36.07) hippocampal volume at 6 months (see Figure 1). The associations were unlikely due to income as, after controlling for both age and family income, maternal sensitivity significantly predicted left (B=−0.474, P=0.045; B=−241.518, 95% CI=−477.348 to −5.688) and right (B=−0.479, P=0.023; B=−214.074, 95% CI=−394.702 to −33.446) hippocampal volume. Likewise, the associations were unlikely due to postnatal anxiety, as after controlling for age and maternal levels of state anxiety among the 16 cases for whom anxiety information was available, sensitivity significantly predicted left (B=−0.657, P=0.011; B=−360.011, 95% CI=−620.129 to −99.892) and right (B=−0.638, P=0.008; B=−312.220, 95% CI=−524.856 to −99.584) hippocampal volume. Post hoc-observed power analyses66, 67 indicated lower power for analyses involving the left hippocampus (that is, 0.51–0.71) than the right hippocampus (0.75–0.86).
Figure 1.
Partial regression plots of the association between maternal sensitivity and hippocampal volume. (a1) The association between maternal sensitivity and left hippocampal volume, controlling for age at MRI. (a2) The association between maternal sensitivity and right hippocampal volume, controlling for age at MRI. (b1) The association between maternal sensitivity and left hippocampal volume, controlling for age at MRI and household income. (b2) The association between maternal sensitivity and right hippocampal volume, controlling for age at MRI and household income. (c1) The association between maternal sensitivity and left hippocampal volume, controlling for age at MRI and maternal postnatal state anxiety at 3 months. (c2) The association between maternal sensitivity and right hippocampal volume, controlling for age at MRI and maternal postnatal state anxiety at 3 months. MRI, magnetic resonance imaging.
Figure 2.
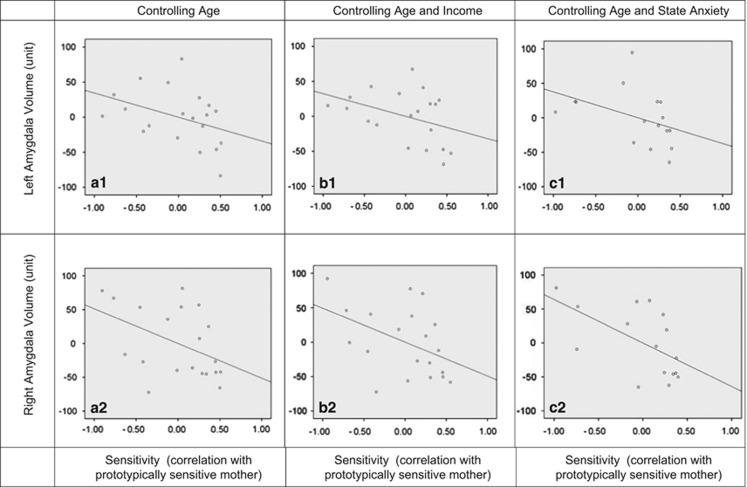
Partial regression plots of the association between maternal sensitivity and amygdala volume. (a1) The association between maternal sensitivity and left amygdala volume, controlling for age at MRI. (a2) The association between maternal sensitivity and right amygdala volume, controlling for age at MRI. (b1) The association between maternal sensitivity and left amygdala volume, controlling for age at MRI and household income. (b2) The association between maternal sensitivity and right amygdala volume, controlling for age at MRI and household income. (c1) The association between maternal sensitivity and left amygdala volume, controlling for age at MRI and maternal postnatal state anxiety at 3 months. (c2) The association between maternal sensitivity and right amygdala volume, controlling for age at MRI and maternal postnatal state anxiety at 3 months. MRI, magnetic resonance imaging.
The associations between sensitivity and hippocampal volume were unlikely due to outlying values, as in all cases, all data points fell within 3 s.d. of the regression slope, and all points fell within 3 s.d. from the mean for hippocampal volumes, sensitivity scores, age, income and postnatal anxiety. One case did have a Cook's distance exceeding the suggested 0.20 for this sample size both when income was not considered and when it was taken into account; when this case was removed from analyses, the results, although remaining consistent in their direction, no longer reached significance. Nevertheless, it is important to note that despite its Cook's distance, this case fell within 2 s.d. for the regression slope, which suggests that the large Cook's distance value may have been due to leverage, or the distance between this case and other cases in our small sample. Thus, given the case's biological feasibility and our small sample size, we believe it is best to retain this case in analyses. In addition, one (different) case had a Cook's distance exceeding the suggested 0.25 for the 16-dyad-smaller sample wherein postnatal anxiety was also considered; when this case was removed from analyses, the results controlling for age and anxiety and examining maternal sensitivity and hippocampal volume remained significant (left: B=−0.777, P=0.002; B=−475.927, 95% CI=−738.911 to −212.943; right: B=−0.780, P=0.001; B=−422.673, 95% CI=621.939 to −223.407).
The pattern of results was similar when we investigated the relation between maternal sensitivity and amygdala volume (see Figure 2), although these results generally failed to pass conventional significance levels. That is, maternal sensitivity marginally related to left (B=−0.389, P=0.102; B=−34.292, 95% CI=−76.104 to 7.52) and right (B=−0.451, P=0.057; B=−51.265, 95% CI=−104.288 to 1.759) amygdala volume. The results remained similar when controlling for income (left: B=−0.365, P=0.116; B=−32.166, 95% CI=−73.244 to 8.913; right: B=−0.433, P=0.068; B=−49.19, 95% CI=−102.526 to 4.147). Furthermore, when controlling for maternal postnatal state anxiety levels, the relation to the left amygdala remained nonsignificant (B=−0.386, P=0.144; B=−37.363, 95% CI=−89.409 to −14.684), whereas the relation to the right amygdala was significant (B=−0.552, P=0.034; B=−64.174, 95% CI=−122.572 to −5.777). Analyses were underpowered with post hoc-observed power analyses,66, 67 indicating power levels between 0.38–0.49 (left amygdala) and 0.44–0.57 (right amygdala). All values were within 3 s.d. of the regression slope, amygdala volumes, sensitivity, age, income and anxiety. For the most part, no points exceeded the suggested Cook's distance values; however, when anxiety was also considered one value exceeded the suggested Cook's distance for regressions involving the left amygdala, and two values exceeded the suggested value for regressions concerning the right amygdala. Nevertheless, when these values were respectively omitted from further analyses, results remained similar to the observed association between maternal sensitivity, controlling for age and postnatal anxiety on the left amygdala, failing to reach significance (B=−0.323, P=0.262; B=−27.256, 95% CI=−78.049 to −23.538), whereas the relation to the right amygdala remained significant (B=−0.692, P=0.005; B=−87.856, 95% CI=−142.348 to −33.364).
Functional connectivity
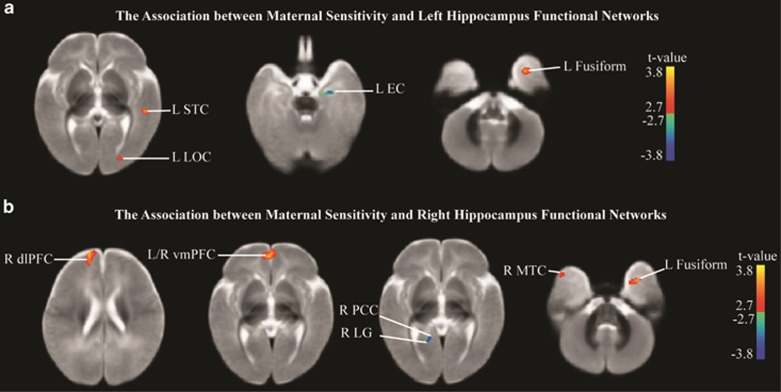
After controlling for age and family income, maternal sensitivity significantly (that is, P<0.01, with clusters of over 100 voxels, uncorrected) positively predicted functional connectivity between the right hippocampus and regions associated with emotion regulation (that is, bilateral ventromedial prefrontal cortex (vmPFC)68), cognitive flexibility (that is, right dorsolateral prefrontal cortex (dlPFC)69) and social communication (that is, left fusiform70 and right middle temporal cortex71). Likewise, maternal sensitivity positively predicted left hippocampal connectivity to regions important to social communication (that is, the left fusiform70 and left superior temporal cortex72), as well as the left lateral occipital cortex. Maternal sensitivity negatively predicted connectivity with regions potentially important to autobiographical memory, such as the right lingual gyrus73, 74 and right posterior cingulate,75 as well as left hippocampal connectivity, with the left entorhinal cortex.76 Results are listed in Table 1 and displayed in Figures 3 and 4.
Table 1. The association between maternal sensitivity and limbic structure functional connectivity.
|
Left hippocampus |
Right hippocampus |
||
|---|---|---|---|
| Positive associations | Negative associations | Positive associations | Negative associations |
| L superior temporal cortex | L entorhinal cortex | R dorsolateral prefrontal cortex | R lingual gyrus |
| L fusiform | L/R ventromedial prefrontal cortex | R posterior cingulate cortex | |
| L lateral occipital cortex | R middle temporal cortex | ||
| L fusiform | |||
|
Left amygdala |
Right amygdala |
||
|---|---|---|---|
| Positive associations | Negative associations | Positive associations | Negative associations |
| NA | L entorhinal cortex | NA | L inferior temporal cortex |
| L middle temporal cortex | |||
Abbreviations: L, left; NA, not applicable; R, right.
Figure 3.
The association between maternal sensitivity and hippocampus functional connectivity. (a) The association between maternal sensitivity and left hippocampus functional connectivity. (b) The association between maternal sensitivity and right hippocampus functional connectivity. dlPFC, dorsolateral prefrontal cortex; EC, entorhinal cortex; L, left; LG, lingual gyrus; LOC, lateral occipital cortex; MTC, middle temporal cortex; PCC, posterior cingulate cortex; R, right; STC, superior temporal cortex; vmPFC, ventromedial prefrontal cortex.
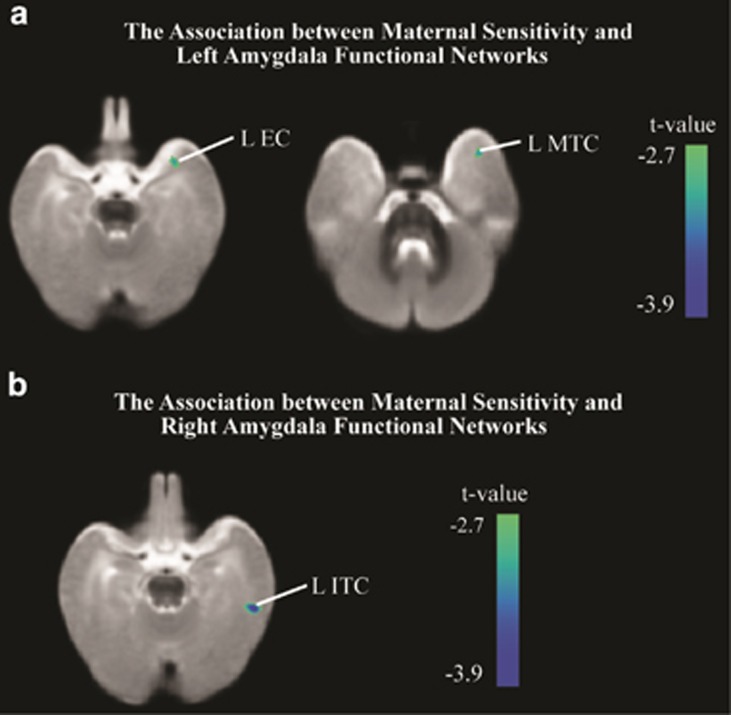
Figure 4.
The association between maternal sensitivity and amygdala functional connectivity. (a) The association between maternal sensitivity and left amygdala functional connectivity. (b) The association between maternal sensitivity and right amygdala functional connectivity. EC, entorhinal cortex; ITC, inferior temporal cortex; L, left; MTC, middle temporal cortex.
After controlling for age and family income, maternal sensitivity significantly (that is, P<0.01, with clusters of over 100 voxels, uncorrected) negatively predicted functional connectivity between the right amygdala and a region important to processing visual emotional stimuli (that is, the left inferior temporal cortex).77 In addition, sensitivity negatively predicted connectivity between the left amygdala and a region potentially important to autobiographical memory (that is, the left entorhinal cortex76), as well as the left middle temporal cortex).
Discussion
Here, in our exploratory study, we focused on the impact of maternal care during infancy, a time when the hippocampus and amygdala undergo rapid development.45, 46, 47 Although our results cannot be considered representative of the larger population, and require replication in a larger sample where more rigorous statistical approaches may be applied, we nevertheless observed maternal sensitivity significantly (hippocampus) and marginally (amygdala) predictive of limbic structure volume in human infants. We also found preliminary evidence that maternal sensitivity was related to functional connectivity between the hippocampus and regions important to emotion regulation (that is, vmPFC68), cognitive flexibility (that is, dlPFC69), social communication (that is, fusiform,70 superior temporal cortex72 and middle temporal cortex71) and memory (that is, entorhinal cortex,76 lingual gyrus,73, 74 and posterior cingulate cortex75). Emotion regulation,78, 79 cognitive flexibility,80, 81 social behavior19, 82 and reported autobiographical memory83, 84, 85, 86 are all functions that vary with early mother–child relationships.
In contrast to findings concerning parenting and adolescent or adult hippocampal volume,30, 32, 38 our findings tentatively suggest that reduced maternal sensitivity associated with larger hippocampal volume during the infancy period. Although the direction of our volumetric finding may appear counterintuitive, this finding is consistent with the results of one study examining young adolescents.37 Moreover, although two meta-analyses of maltreatment-related post-traumatic stress disorder (PTSD) and pediatric hippocampal volume, as well as a recent meta-analysis examining maltreatment,36 suggest overall nil effects of childhood maltreatment on pediatric brain volumes, Tupler and De Bellis87 reported that among children with PTSD, childhood maltreatment was associated with a larger hippocampal volume, which in turn was associated with total level of risk for psychopathology on the Child Behavior Checklist. Likewise, Qiu et al.60 previously reported a positive association (B=0.992) specifically between right hippocampal growth within the first 6 months and postnatal maternal anxiety, which is linked to forms of care that may be nonoptimal for infant development.88 Indeed, these findings and those of the current study are consistent with an emerging view that social adversity in early life may increase the maturational rate of limbic structures, which mediate the activation of stress responses and emotional learning. Although mother–infant interactions in rodents, such as pup licking/grooming, dampen hypothalamic–pituitary–adrenal activity,89 stress accelerates the development of amygdala-dependent emotional learning in the rat, an effect that is mediated by stress-induced increases in glucocorticoids.53, 54, 90 Likewise, a remarkable translational study44 showed that early institutionalization and the associated absence of parental care was associated with accelerated maturation of fronto-amygdala connectivity. This effect was statistically mediated by cortisol levels, which were elevated in the previously institutionalized children (also see Gunnar et al.91), suggesting a parallel to the rodent models.
Thus, despite literature suggesting a negative influence of early-life stress on hippocampal volume,92, 93 the association between sensitive parenting and decreased limbic structure volume has precedence in both animal and human research. Indeed, there are many possible explanations for the discrepancies including the moderating influences of gender30, 94 and genotype,95 as well as the time course of limbic system development. The hippocampus develops rapidly in the first 2 years of life before its growth rate begins to plateau.46, 47 In later childhood to early adulthood (that is, 8–30 years), the hippocampus continues to grow,96 and its relation with age is influenced by pubertal status.97 Moreover, the relation with age is nonlinear, growing faster at earlier time points96 and following an inverted U-shaped curve, peaking at around 17 years of age.98 Thus, if stress exposure influences accelerated development, the timing of MRI acquisition may greatly influence whether relations between stress exposure and volume are direct, indirect or nil.
In addition to our structural findings, our results, although preliminary, also suggest environmental effects on functional connectivity apparent as early as 6 months of life. Although somewhat speculative, our small-sample findings suggest the possibility that increased maternal sensitivity associates with increased connectivity of pathways that dampen stress reactivity. The hippocampus is implicated in the regulation of stress responses,99 and both fMRI and positron emission tomography studies show that acute social stress leads to deactivation of the hippocampus and prefrontal regions.100 Furthermore, an fMRI experiment100 showed that the cortisol response to acute social stress was predicted by bilateral hippocampal deactivation. Although the hippocampus may inhibit cortisol responses to stress, other regions may be essential to assessing the nature and valence of the experience, to determine whether it is indeed stressful. The medial PFC is important in the interpretation of the personal relevance of concurrent and subsequent challenging situations. The left medial PFC exhibits enhanced fMRI activity when adults are asked to make self-referential judgments as well when these judgments are later remembered.75 fMRI studies demonstrate that the vmPFC, in conjunction with the hippocampus, is also important to context-based fear extinction.68 Milad et al.68 suggest that the vmPFC is important for associations concerning fear, but that the co-activation of the hippocampus is necessary to learn the conditions under which cues are no longer a valid signal of danger (that is, safety signals). Jin et al.101 found decreased positive connectivity between prefrontal and hippocampal regions among adults with PTSD, a disorder in which past trauma is undifferentiated from current context. In the current work, we found that maternal sensitivity was positively related to functional connectivity in infants between the hippocampus and bilateral vmPFC. Whether such variation in hippocampal–vmPFC connectivity mediates relations between maternal sensitivity and infants' perception of challenging situations and their accompanying stress responses102, 103, 104 may therefore be an interesting question for future research. Hippocampal deactivation and accompanying hypothalamic-pituitary-adrenal activity may influence additional prefrontal areas, including the dlPFC, important to executive functioning and attention.69 Here, our results suggest that maternal sensitivity was positively correlated with functional connectivity between the right hippocampus and right dlPFC. Behavioral and fMRI research in humans indicates that stress induction negatively impacts working memory, conceptualized via accuracy and reaction time during the 2-back (working memory) versus 0-back condition of an n-back test, and that stress induction decreases dlPFC activity during this test.69 Additional fMRI work with adults during an n-back test demonstrates the importance of dorsolateral–hippocampal coupling, such that decreased coupling relates to faster 2-back processing.105 In addition, Bernal-Casis et al.106 find consistent right dlPFC and left hippocampal decoupling during an n-back test across three study sites. Interestingly, recent findings demonstrating that sensitivity80, 81 and the closely related construct of secure mother–infant attachment81 predict enhanced childhood executive functioning, which requires flexibility in attention. Our sensitivity findings within this exploratory study, in conjunction with the aforementioned work concerning stress, and dlPFC–hippocampal connectivity, may begin to suggest a biological pathway through which early-life care affects later cognitive control. Variation in the flexibility of attention has additionally been considered important to the quality of attachment strategies,107 a developmental correlate of experience with sensitive care.16 Attachment strategies can be characterized as differential displays of attentional flexibility, with children previously experiencing sensitive care able to shift attentional demands based on environmental input, whereas those who have experienced less sensitive care may be constrained to following rigid strategies regardless of external experience.107
Beyond the associations between sensitivity and right hippocampal–prefrontal connectivity, we also observed preliminary evidence for positive relations between the hippocampus and regions less directly implicated in stress physiology and the management of emotion, but of potential importance to social behavior and communication. As noted, maternal sensitivity is a building block for mother–infant attachment relationships,16 which form a blueprint for social relationships throughout development.83, 84, 85 These blueprints have been repeatedly linked to thoughts and emotions important to social behavior, including friendship108 and intimacy.109 In adulthood, attachment representations, which are theoretically and empirically linked to early experiences with sensitive (versus insensitive) care,83, 84, 85, 86 correlate with electrophysiological correlates of face processing,110 and so may involve the superior temporal sulcus111 and/or the fusiform gyrus.112 Here, we observed sensitivity positively related to functional connectivity between the bilateral hippocampus and the left fusiform gyrus. Although limited work113 has examined the functional significance of such connectivity, a recent diffusion tensor imaging study revealed parallel bilateral pathways between the hippocampus and fusiform, with greater left laterality and potentially more myelination in human adults.114 Using functional connectivity methods, Miller and D'Espisito113 demonstrated right fusiform activity proceeding bilateral hippocampal activation during both encoding and retrieval phases of a facial memory task. These connectivity findings are consistent with accounts of hippocampal and fusiform co-activation during fMRI experiments examining encoding115 and response to novelty.116 Furthermore, in keeping with the notion that early experience affects adult social behavior, individual differences in emotionality,117 maltreatment118, 119 and trauma120 are all also predictive of hippocampal and fusiform co-activation during face-processing tasks. Likewise, in our exploratory study, maternal sensitivity predicted greater connectivity between the left hippocampus and the left middle temporal gyrus, a region also associated with experience with maltreatment,121 and, which, in coordination with the hippocampus, may support information processing relevant to social cognition.122 When viewing pictures of faces, both the left middle temporal gyrus and the left hippocampus show greater activation when biographical information is simultaneously retrieved than when it is not.122 In addition, both regions are also more responsive to ‘happy' as compared with neutral paired faces and voices.123 In addition, we also noted a positive association between maternal sensitivity and connectivity between the hippocampus and left superior temporal gyrus. Alterations in the activity levels of both the left superior temporal gyrus and hippocampus during a facial expression discrimination task have been observed in patients with Fragile X syndrome, which is partially characterized by social difficulties.124 Finally, we also observed a negative association between the right amygdala and the left inferior temporal cortex. Co-activation of the amygdala and inferior temporal cortex has been observed during the initial processing of emotional stimuli and is expected for environmentally salient visual stimuli, although inferior temporal activity continues even after the amygdala has habituated.125
In addition, we also observed associations between higher sensitivity and less connectivity between the limbic structures and structures involved in memory formation. Children who have experienced more sensitive maternal behaviors, and accordingly less insensitive behavior, in infancy are more likely than their counterparts to evidence rich autobiographical memories for childhood relationships in young adulthood. In specific, depending on the form of insensitive caregiving experienced, children and adults judged to have received high amounts of maternal insensitivity may be more likely to evidence little memory for childhood or excessive detail for early experiences.83, 84, 85, 86, 126 Here, in our exploratory study, we observed maternal sensitivity related to less connectivity between the hippocampus and regions important to memory. Namely, sensitivity negatively associated with functional connectivity between the left hippocampus and left entorhinal cortex, as well as the right hippocampus and the right lingual gyrus and right posterior cortex. Likewise, sensitivity also predicted less connectivity between the left amygdala and left entorhinal cortex. The entorhinal cortex may be important to autobiography, working as an interface between the hippocampus and frontal cortex to affect memory storage and retrieval.76 As one example, the entorhinal cortex co-activates with the hippocampus, during a delay period following the viewing of familiar faces that are subsequently accurately and confidently judged to have just been seen.127 The lingual gyrus shows enhanced activity during spatial73 and visual working memory,74 and altered resting-state lingual function differs in those likely to have experienced chronic perceived stress128 and may be associated with resilience to childhood maltreatment.129 The posterior cingulate cortex is also involved in memory, specifically showing increased activity in response to self-referential versus semantic judgments, with co-activation between the left hippocampus and posterior cingulate occurring during the encoding of referential material that is later remembered.75 Interestingly, Bluhm et al.130 find the functional connectivity between the right hippocampus and posterior cingulate is disrupted in women with PTSD, and suggest this alteration may explain related difficulties in distinguishing past trauma from the current environment. Moreover, Zhou et al.131 find that connectivity between the posterior cingulate cortex and right hippocampus/amygdala within days of trauma exposure predicts PTSD symptom severity. These results, in association with links between early attachment status and risk for dissociation in adolescence132 as well as adult disorganization in the face of loss or trauma,84 may imply that prior, early occurring environmental risk affects neuronal connectivity important to the integration of stressful or traumatic experience into adulthood. In sum, although speculative, the current results suggest that early experiences may shape connectivity patterns between neuroanatomical regions relevant for attachment and parenting-related memories later in life.
Understanding the influence of maternal sensitivity upon developmental trajectories and the influence of early variation in brain volume and function upon later memory formation, emotion and stress regulation will be an important avenue for future research using much larger samples. Of note, within the current analyses, one of the dyads, which, while still in the normal range, scored lowest on maternal sensitivity, may have had undue influence on the majority of hippocampal results, and in fact, relevant results did not remain significant when this case was removed. Thus, it is essential the current findings are replicated in larger-scale research, not only to ensure that they are not spurious, but also in order to better understand the nature of any association. That is, although the current small-sample research suggests a linear association, larger-scale research may be able to determine whether any effects of sensitivity on limbic development are more categorical in nature, with a greater influence being seen in cases at the lower end of the sensitivity spectrum. In low-risk samples, less than 40% of cases may be expected to exhibit a low degree of sensitive behavior.133 Thus, larger samples may be especially important to elucidate the potential influence of relatively extreme low sensitivity scores in a nonclinical group. Likewise, it will be essential to follow individuals over time to better understand whether the direction of the relation between caregiving adversity and hippocampal volume is dependent upon developmental stage, and whether this predicts subsequent risk. Considering both the rapid pace of hippocampal growth within the first 2 years of life,46, 47 and the results from this exploratory study suggesting that the infant hippocampus responds to relatively subtle forms of caregiving adversity with accelerated development, it is quite possible we would not have uncovered any relation between maternal behavior and hippocampal volume in older, low-risk children, although a lasting impact on functional connectivity may have still been detected. Indeed, in their recent work examining relations between infant attachment and adult brain volume, effects were observed on functional activity during an emotional task,78 and amygdala, but not, hippocampal volume.39 Despite this study's small sample size, a large effect on everyday variation in maternal care upon the 6-month-old hippocampii were identified, with similar, but, for the most part, only marginally significant effects on amygdala volume. Moreover, although our functional analyses with this sample did not pass multiple comparisons, here we found sensitivity related to the connectivity strength between the hippocampus and regions important to emotional and cognitive control, social functioning and memory. If replicated in a larger group, this work will suggest a clear need for widespread early parenting intervention programs. Although by definition maternal sensitive behavior can only be observed in the postnatal period, variation in sensitivity is known to associate with mental states134 that may impact hypothalamic-pituitary-adrenal activity63 and accordingly the uterine environment. However, in our analyses of potential confounding variables within the larger sample, maternal sensitivity did not significantly relate to infants' hippocampal volumes within the first 2 weeks of birth. Thus, a primary role of antenatal or solely genetic factors, which if fully explanatory, would likely have also affected the neonatal brain, is unlikely. Rather, our results tentatively suggest that the variation in species-relevant maternal care influences biological mechanisms to shape hippocampal development and functional connectivity. Given that roughly 30–50% of low-risk mothers are unlikely to consistently score high on measures of maternal sensitivity,133, 135 the current findings, if replicated in larger research, may help to explain a great deal of the variance in children's stress and emotional regulation within nonclinical groups. In sum, similar to the animal work, the current study suggests that species-specific subtle variation in parenting cues may impact the development of the infant brain in regions known to impact endocrine, cognitive and emotional functioning.
Acknowledgments
This study was supported by National Medical Research Council (NMRC; NMRC/TCR/004-NUS/2008 and NMRC/CBRG/0039/2013), the Young Investigator Award at the National University of Singapore (NUSYIA FY10 P07), the Singapore Ministry of Education Academic Research Fund Tier 2 (MOE2012-T2-2-130) and the Young Investigator Award at the Singapore Institute for Clinical Sciences (SICS/YIG/2013/002). We are extremely thankful to Sandi Bento at the University of Western Ontario for her mentorship in the scoring of sensitivity, as well as Greg Moran at the University of Western Ontario for his associated input. We further thank the GUSTO study group and all clinical and home visit staff involved. The voluntary participation of all participants is greatly appreciated. The GUSTO study group includes Pratibha Agarwal, Arijit Biswas, Choon Looi Bong, Shirong Cai, Jerry Kok Yen Chan, Yiong Huak Chan, Cornelia Yin Ing Chee, Yin Bun Cheung, Audrey Chia, Amutha Chinnadurai, Chai Kiat Chng, Mary Foong-Fong Chong, Shang Chee Chong, Mei Chien Chua, Chun Ming Ding, Eric Andrew Finkelstein, Doris Fok, Keith M Godfrey, Anne Eng Neo Goh, Yam Thiam Daniel Goh, Joshua J Gooley, Wee Meng Han, Mark Hanson, Christiani Jeyakumar Henry, Joanna D Holbrook, Chin-Ying Hsu, Hazel Inskip, Jeevesh Kapur, Ivy Yee-Man Lau, Bee Wah Lee, Yung Seng Lee, Ngee Lek, Sok Bee Lim, Yen-Ling Low, Iliana Magiati, Lourdes Mary Daniel, Cheryl Ngo, Krishnamoorthy Naiduvaje, Wei Wei Pang, Boon Long Quah, Victor Samuel Rajadurai, Mary Rauff, Salome A Rebello, Jenny L Richmond, Lynette Pei-Chi Shek, Allan Sheppard, Borys Shuter, Leher Singh, Shu-E Soh, Walter Stunkel, Lin Lin Su, Kok Hian Tan, Oon Hoe Teoh, Mya Thway Tint, Hugo P S van Bever, Rob M van Dam, Inez Bik Yun Wong, PC Wong, Fabian Yap, George Seow Heong Yeo.
The authors declare no conflict of interest.
References
- Erath SA, El-Sheikh M, Mark Cummings E. Harsh parenting and child externalizing behavior: skin conductance level reactivity as a moderator. Child Dev 2009; 80: 578–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooklin AR, Giallo R, D'Esposito F, Crawford S, Nicholson JM. Postpartum maternal separation anxiety, overprotective parenting, and children's social-emotional well-being: longitudinal evidence from an Australian cohort. J Fam Psychol 2013; 27: 618–628. [DOI] [PubMed] [Google Scholar]
- Wang Y, Dix T. Patterns of depressive parenting: why they occur and their role in early developmental risk. J Fam Psychol 2013; 27: 884–895. [DOI] [PubMed] [Google Scholar]
- Zaslow MJ, Weinfield NS, Gallagher M, Hair EC, Ogawa JR, Egeland B et al. Longitudinal prediction of child outcomes from differing measures of parenting in a low-income sample. Dev Psychol 2006; 42: 27–37. [DOI] [PubMed] [Google Scholar]
- Gonzales NA, Coxe S, Roosa MW, White RM, Knight GP, Zeiders KH et al. Economic hardship, neighborhood context, and parenting: prospective effects on Mexican-American adolescent's mental health. Am J Community Psychol 2011; 47: 98–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart H, Rubia K. Neuroimaging of child abuse: a critical review. Front Hum Neurosci 2012; 6: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woon FL, Hedges DW. Hippocampal and amygdala volumes in children and adults with childhood maltreatment-related posttraumatic stress disorder: a meta-analysis. Hippocampus 2008; 18: 729–736. [DOI] [PubMed] [Google Scholar]
- Kaffman A, Meaney MJ. Neurodevelopmental sequelae of postnatal maternal care in rodents: clinical and research implications of molecular insights. J Child Psychol Psychiatry 2007; 48: 224–244. [DOI] [PubMed] [Google Scholar]
- Bardi M, Huffman MA. Maternal behavior and maternal stress are associated with infant behavioral development in macaques. Dev Psychobiol 2006; 48: 1–9. [DOI] [PubMed] [Google Scholar]
- Coplan JD, Abdallah CG, Tang CY, Mathew SJ, Martinez J, Hof PR et al. The role of early life stress in development of the anterior limb of the internal capsule in nonhuman primates. Neurosci Lett 2010; 480: 93–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestripieri D, Hoffman CL, Anderson GM, Carter CS, Higley JD. Mother-infant interactions in free-ranging rhesus macaques: relationships between physiological and behavioral variables. Physiol Behav 2009; 96: 613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth MDS, Bell SM, Stayton DJ. Individual differences in strange situation behavior of one year olds. In: Schaffer HR (ed). The Origins of Human Social Relationships. Academic Press: : London, UK, 1971, pp 17–57. [Google Scholar]
- Ainsworth MS, Blehar MC, Waters E, Wall S. Patterns of Attachment: a Psychological Study of the Strange Situation. Lawrence Erlbaum: : Oxford, England, 1978. [Google Scholar]
- Pederson DR, Bailey HN, Tarabulsy GM, Bento S, Moran G. Understanding sensitivity: lessons learned from the legacy of Mary Ainsworth. Attachment Hum Dev 2014; 16: 261–270. [DOI] [PubMed] [Google Scholar]
- Ainsworth MD. Infancy in Uganda: Infant Care and the Growth of Love. Johns Hopkins Press: Baltimore, MD, USA, 1967. [Google Scholar]
- De Wolff MS, van Ijzendoorn MH. Sensitivity and attachment: a meta-analysis on parental antecedents of infant attachment. Child Dev 1997; 68: 571–591. [PubMed] [Google Scholar]
- Ainsworth MD. Patterns of attachment. Clin Psychol 1985; 38: 27–29. [Google Scholar]
- Fearon RP, Bakermans-Kranenburg MJ, van Ijzendoorn MH, Lapsley AM, Roisman GI. The significance of insecure attachment and disorganization in the development of children's externalizing behavior: a meta-analytic study. Child Dev 2010; 81: 435–456. [DOI] [PubMed] [Google Scholar]
- Suess GJ, Grossmann KE, Sroufe LA. Effects of attachment to mother and father on quality of adaptation in preschool: from dyadic to individual organization of self. Int J Behav Dev 1992; 15: 43–65. [Google Scholar]
- Schneider BH, Atkinson L, Tardif C. Child-parent attachment and children's peer relations: a quantitative review. Dev Psychol 2001; 37: 86–100. [PubMed] [Google Scholar]
- Pallini S, Baiocco R, Schneider BH, Madigan S, Atkinson L. Early child-parent attachment and peer relations: a meta-analysis of recent research. J Fam Psychol 2014; 28: 118–123. [DOI] [PubMed] [Google Scholar]
- Bernier A, Carlson SM, Whipple N. From external regulation to self-regulation: early parenting precursors of young children's executive functioning. Child Dev 2010; 81: 326–339. [DOI] [PubMed] [Google Scholar]
- Bergman K, Sarkar P, Glover V, O'Connor TG. Maternal prenatal cortisol and infant cognitive development: moderation by infant-mother attachment. Biol Psychiatry 2010; 67: 1026–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen T, Huss M, Fendrich M, Kruesi MJ, Ziegenhain U. Children's ability to delay gratification: longitudinal relations to mother-child attachment. J Genet Psychol 1997; 158: 411–426. [DOI] [PubMed] [Google Scholar]
- Fearon RM, Belsky J. Attachment and attention: protection in relation to gender and cumulative social-contextual adversity. Child Dev 2004; 75: 1677–1693. [DOI] [PubMed] [Google Scholar]
- Garai EP, Forehand RL, Colletti CJ, Reeslund K, Potts J, Compas B. The relation of maternal sensitivity to children's internalizing and externalizing problems within the context of maternal depressive symptoms. Behav Modif 2009; 33: 559–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount KS, Crockenberg SC, Jo PS, Wagar JL. Maternal and child correlates of anxiety in 2(1/2)-year-old children. Infant Behav Dev 2010; 33: 567–578. [DOI] [PubMed] [Google Scholar]
- Moss E, Dubois-Comtois K, Cyr C, Tarabulsy GM, St-Laurent D, Bernier A. Efficacy of a home-visiting intervention aimed at improving maternal sensitivity, child attachment, and behavioral outcomes for maltreated children: a randomized control trial. Dev Psychopathol 2011; 23: 195–210. [DOI] [PubMed] [Google Scholar]
- Parker G. The Parental Bonding Instrument. A decade of research. Soc Psychiatry Psychiatr Epidemiol 1990; 25: 281–282. [DOI] [PubMed] [Google Scholar]
- Buss C, Lord C, Wadiwalla M, Hellhammer DH, Lupien SJ, Meaney MJ et al. Maternal care modulates the relationship between prenatal risk and hippocampal volume in women but not in men. J Neurosci 2007; 27: 2592–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita K, Fujihara K, Takei Y, Suda M, Aoyama Y, Uehara T et al. Associations among parenting experiences during childhood and adolescence, hypothalamus-pituitary-adrenal axis hypoactivity, and hippocampal gray matter volume reduction in young adults. Hum Brain Mapp 2012; 33: 2211–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engert V, Buss C, Khalili-Mahani N, Wadiwalla M, Dedovic K, Pruessner JC. Investigating the association between early life parental care and stress responsivity in adulthood. Dev Neuropsychol 2010; 35: 570–581. [DOI] [PubMed] [Google Scholar]
- Bagot RC, van Hasselt FN, Champagne DL, Meaney MJ, Krugers HJ, Joels M. Maternal care determines rapid effects of stress mediators on synaptic plasticity in adult rat hippocampal dentate gyrus. Neurobiol Learn Mem 2009; 92: 292–300. [DOI] [PubMed] [Google Scholar]
- Champagne DL, Bagot RC, van Hasselt F, Ramakers G, Meaney MJ, de Kloet ER et al. Maternal care and hippocampal plasticity: evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. J Neurosci 2008; 28: 6037–6045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manassis K, Owens M, Adam KS, West M, Sheldon-Keller AE. Assessing attachment: convergent validity of the adult attachment interview and the parental bonding instrument. Aust N Z J psychiatry 1999; 33: 559–567. [DOI] [PubMed] [Google Scholar]
- Riem MM, Alink LR, Out D, Van Ijzendoorn MH, Bakermans-Kranenburg MJ. Beating the brain about abuse: empirical and meta-analytic studies of the association between maltreatment and hippocampal volume across childhood and adolescence. Dev Psychopathol 2015; 27: 507–520. [DOI] [PubMed] [Google Scholar]
- Rao H, Betancourt L, Giannetta JM, Brodsky NL, Korczykowski M, Avants BB et al. Early parental care is important for hippocampal maturation: evidence from brain morphology in humans. NeuroImage 2010; 49: 1144–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby JL, Barch DM, Belden A, Gaffrey MS, Tillman R, Babb C et al. Maternal support in early childhood predicts larger hippocampal volumes at school age. Proc Natl Acad Sci USA 2012; 109: 2854–2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutsiana C, Johnstone T, Murray L, Fearon P, Cooper PJ, Pliatsikas C et al. Insecure attachment during infancy predicts greater amygdala volumes in early adulthood. J Child Psychol Psychiatry 2015; 56: 540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S, Simmons JG, Dennison M, Vijayakumar N, Schwartz O, Yap MB et al. Positive parenting predicts the development of adolescent brain structure: a longitudinal study. Dev Cogn Neurosci 2014; 8: 7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Front Hum Neurosci 2009; 3: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxbe DE, Negriff S, Susman EJ, Trickett PK. Attenuated hypothalamic-pituitary-adrenal axis functioning predicts accelerated pubertal development in girls 1 year later. Dev Psychopathol 2015; 27: 819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Ruttle PL, Boyce WT, Armstrong JM, Essex MJ. Early adversity, elevated stress physiology, accelerated sexual maturation, and poor health in females. Dev psychol 2015; 51: 816–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH et al. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci USA 2013; 110: 15638–15643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu A, Matsui M, Tanaka C, Takahashi T, Noguchi K, Suzuki M et al. Developmental trajectories of amygdala and hippocampus from infancy to early adulthood in healthy individuals. PloS One 2012; 7: e46970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore JH, Shi F, Woolson SL, Knickmeyer RC, Short SJ, Lin W et al. Longitudinal development of cortical and subcortical gray matter from birth to 2 years. Cereb Cortex 2012; 22: 2478–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsunomiya H, Takano K, Okazaki M, Mitsudome A. Development of the temporal lobe in infants and children: analysis by MR-based volumetry. AJNR Am J Neuroradiol 1999; 20: 717–723. [PMC free article] [PubMed] [Google Scholar]
- Georgieff M. Nutrition and the developing brain: nutrient priorities and measurement. Am J Clin Nutr 2007; 85: 614S–620S. [DOI] [PubMed] [Google Scholar]
- Uno H, Eisele S, Sakai A, Shelton S, Baker E, DeJesus O et al. Neurotoxicity of glucocorticoids in the primate brain. Horm Behav 1994; 28: 336–348. [DOI] [PubMed] [Google Scholar]
- Buss C, Davis EP, Shahbaba B, Pruessner JC, Head K, Sandman CA. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc Natl Acad Sci USA 2012; 109: E1312–E1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ES, Woolston DJ, Frol AB. Amygdala volume in patients receiving chronic corticosteroid therapy. Biol Psychiatry 2008; 63: 705–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliaccio D, Luby JL, Bogdan R, Agrawal A, Gaffrey MS, Belden AC et al. Stress-system genes and life stress predict cortisol levels and amygdala and hippocampal volumes in children. Neuropsychopharmacology 2014; 39: 1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Shionoya K, Jakubs K, Sullivan RM. Early-life stress disrupts attachment learning: the role of amygdala corticosterone, locus ceruleus corticotropin releasing hormone, and olfactory bulb norepinephrine. J Neurosci 2009; 29: 15745–15755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Holman PJ. Transitions in sensitive period attachment learning in infancy: the role of corticosterone. Neurosci Biobehav Rev 2010; 34: 835–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soh SE, Tint MT, Gluckman PD, Godfrey KM, Rifkin-Graboi A, Chan YH et al. Cohort profile: Growing Up in Singapore Towards healthy Outcomes (GUSTO) birth cohort study. Int J Epidemiol 2013; 43: 1401–1409. [DOI] [PubMed] [Google Scholar]
- Moran G, Pederson DR, Bento S (2009). “Maternal Behavior Q-Sort (MBQS) - Overview, Available Materials and Support” The SelectedWorks of Greg Moran. Available at: http://works.bepress.com/gregmoran/48.
- Moran G (2009). “Mini-MBQS-V Revised Mini-MBQS 25 Item for Video Coding” The SelectedWorks of Greg Moran. Available at: http://works.bepress.com/gregmoran/49.
- Tarabulsy GM, Provost MA, Bordeleau S, Trudel-Fitzgerald C, Moran G, Pederson DR et al. Validation of a short version of the maternal behavior Q-set applied to a brief video record of mother-infant interaction. Infant Behav Dev 2009; 32: 132–136. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp 2002; 17: 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu A, Rifkin-Graboi A, Chen H, Chong YS, Kwek K, Gluckman PD et al. Maternal anxiety and infants hippocampal development: timing matters. Nat Transl Psychiatry 2013; 3: e306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 2012; 59: 2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MI, Qiu A. The emerging discipline of Computational Functional Anatomy. NeuroImage 2009; 45: S16–S39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin-Graboi A. Attachment status and salivary cortisol in a normal day and during simulated interpersonal stress in young men. Stress 2008; 11: 210–224. [DOI] [PubMed] [Google Scholar]
- Van den Bergh BR, Mulder EJ, Mennes M, Glover V. Antenatal maternal anxiety and stress and the neurobehavioural development of the fetus and child: links and possible mechanisms. A review. Neurosci Biobehav Rev 2005; 29: 237–258. [DOI] [PubMed] [Google Scholar]
- Buss C, Davis EP, Muftuler LT, Head K, Sandman CA. High pregnancy anxiety during mid-gestation is associated with decreased gray matter density in 6–9-year-old children. Psychoneuroendocrinology 2010; 35: 141–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soper DS (2015). Post-hoc statistical power calculator for multiple regression (software). http://www.danielsoper.com/statcalc3/calc.aspx?id=9.
- Cohen J, Cohen P, West SG, Aiken LS. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. 3rd edn, Lawrence Earlbaum Associates: : Mahwah, NJ, USA, 2003. [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry 2007; 62: 446–454. [DOI] [PubMed] [Google Scholar]
- Qin S, Hermans EJ, van Marle HJ, Luo J, Fernandez G. Acute psychological stress reduces working memory-related activity in the dorsolateral prefrontal cortex. Biol Psychiatry 2009; 66: 25–32. [DOI] [PubMed] [Google Scholar]
- Miki K, Kakigi R. Magnetoencephalographic study on facial movements. Front Hum Neurosci 2014; 8: 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holler J, Kokal I, Toni I, Hagoort P, Kelly SD, Ozyurek A. Eye'm talking to you: speakers' gaze direction modulates co-speech gesture processing in the right MTG. Soc Cogn Affect Neurosci 2014; 10: 255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigler ED, Mortensen S, Neeley ES, Ozonoff S, Krasny L, Johnson M et al. Superior temporal gyrus, language function, and autism. Dev Neuropsychol 2007; 31: 217–238. [DOI] [PubMed] [Google Scholar]
- de Rover M, Petersson KM, van der Werf SP, Cools AR, Berger HJ, Fernandez G. Neural correlates of strategic memory retrieval: differentiating between spatial-associative and temporal-associative strategies. Hum Brain Mapp 2008; 29: 1068–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migo EM, Mitterschiffthaler M, O'Daly O, Dawson GR, Dourish CT, Craig KJ et al. Alterations in working memory networks in amnestic mild cognitive impairment. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 2014; 22: 106–127. [DOI] [PubMed] [Google Scholar]
- Morel N, Villain N, Rauchs G, Gaubert M, Piolino P, Landeau B et al. Brain activity and functional coupling changes associated with self-reference effect during both encoding and retrieval. PloS One 2014; 9: e90488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehara-Nishiuchi K. Entorhinal cortex and consolidated memory. Neurosci Res 2014; 84: 27–33. [DOI] [PubMed] [Google Scholar]
- Wendt J, Weike AI, Lotze M, Hamm AO. The functional connectivity between amygdala and extrastriate visual cortex activity during emotional picture processing depends on stimulus novelty. Biol Psychol 2011; 86: 203–209. [DOI] [PubMed] [Google Scholar]
- Moutsiana C, Fearon P, Murray L, Cooper P, Goodyer I, Johnstone T et al. Making an effort to feel positive: insecure attachment in infancy predicts the neural underpinnings of emotion regulation in adulthood. J Child Psychol Psychiatry 2014; 55: 999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arend R, Gove FL, Sroufe LA. Continuity of individual adaptation from infancy to kindergarten: a predictive study of ego-resiliency and curiosity in preschoolers. Child Dev 1979; 50: 950–959. [PubMed] [Google Scholar]
- Kok R, Lucassen N, Bakermans-Kranenburg MJ, van Ijzendoorn MH, Ghassabian A, Roza SJ et al. Parenting, corpus callosum, and executive function in preschool children. Child Neuropsychol 2013; 20: 583–606. [DOI] [PubMed] [Google Scholar]
- Bernier A, Carlson SM, Deschenes M, Matte-Gagne C. Social factors in the development of early executive functioning: a closer look at the caregiving environment. Dev Sci 2012; 15: 12–24. [DOI] [PubMed] [Google Scholar]
- Simpson JA, Collins WA, Tran S, Haydon KC. Attachment and the experience and expression of emotions in romantic relationships: a developmental perspective. J Pers Soc Psychol 2007; 92: 355–367. [DOI] [PubMed] [Google Scholar]
- Waters E, Merrick S, Treboux D, Crowell J, Albersheim L. Attachment security in infancy and early adulthood: a twenty-year longitudinal study. Child Dev 2000; 71: 684–689. [DOI] [PubMed] [Google Scholar]
- Main M, Hesse E, Kaplan N Predictability of attachment behavior and representational processes at 1, 6, and 19 years of age: The Berkeley longitudinal study. In: Grossmann KE, Grossmann K, Waters E (eds). Attachment from Infancy to Adulthood: the Major Longitudinal Studies. Guilford Publications: New York, NY, USA, 2005, pp 245-304.
- Hamilton CE. Continuity and discontinuity of attachment from infancy through adolescence. Child Dev 2000; 71: 690–694. [DOI] [PubMed] [Google Scholar]
- Beckwith L, Cohen SE, Hamilton CE. Maternal sensitivity during infancy and subsequent life events relate to attachment representation at early adulthood. Dev Psychol 1999; 35: 693–700. [DOI] [PubMed] [Google Scholar]
- Tupler LA, De Bellis MD. Segmented hippocampal volume in children and adolescents with posttraumatic stress disorder. Biol Psychiatry 2006; 59: 523–529. [DOI] [PubMed] [Google Scholar]
- Nicol-Harper R, Harvey AG, Stein A. Interactions between mothers and infants: impact of maternal anxiety. Infant Behav Dev 2007; 30: 161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S. The ontogeny of the hypothalamic-pituitary-adrenal axis. The influence of maternal factors. Ann N Y Acad Sci 1994; 746: 275–288, discussion 289–293. [DOI] [PubMed] [Google Scholar]
- Callaghan BL, Graham BM, Li S, Richardson R. From resilience to vulnerability: mechanistic insights into the effects of stress on transitions in critical period plasticity. Front Psychiatry 2013; 4: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Morison SJ, Chisholm K, Schuder M. Salivary cortisol levels in children adopted from romanian orphanages. Dev Psychopathol 2001; 13: 611–628. [DOI] [PubMed] [Google Scholar]
- Hanson JL, Nacewicz BM, Sutterer MJ, Cayo AA, Schaefer SM, Rudolph KD et al. Behavioral problems after early life stress: contributions of the hippocampus and amygdala. Biol Psychiatry 2014; 77: 314–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Anderson CM, Polcari A. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc Natl Acad Sci USA 2012; 109: E563–E572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samplin E, Ikuta T, Malhotra AK, Szeszko PR, Derosse P. Sex differences in resilience to childhood maltreatment: effects of trauma history on hippocampal volume, general cognition and subclinical psychosis in healthy adults. J Psychiatr Res 2013; 47: 1174–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aas M, Haukvik UK, Djurovic S, Bergmann O, Athanasiu L, Tesli MS et al. BDNF val66met modulates the association between childhood trauma, cognitive and brain abnormalities in psychoses. Prog Neuropsychopharmacol Biol Psychiatry 2013; 46: 181–188. [DOI] [PubMed] [Google Scholar]
- Ostby Y, Tamnes CK, Fjell AM, Westlye LT, Due-Tonnessen P, Walhovd KB. Heterogeneity in subcortical brain development: a structural magnetic resonance imaging study of brain maturation from 8 to 30 years. J Neurosci 2009; 29: 11772–11782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddings AL, Mills KL, Clasen LS, Giedd JN, Viner RM, Blakemore SJ. The influence of puberty on subcortical brain development. NeuroImage 2014; 88: 242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga L, Langen M, Ambrosino S, van Dijk S, Oranje B, Durston S. Typical development of basal ganglia, hippocampus, amygdala and cerebellum from age 7 to 24. NeuroImage 2014; 96: 67–72. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Pruessner M, Lord C, Buss C, Collins L et al. Stress regulation in the central nervous system: evidence from structural and functional neuroimaging studies in human populations - 2008 Curt Richter Award Winner. Psychoneuroendocrinology 2010; 35: 179–191. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Khalili-Mahani N, Engert V, Pruessner M, Buss C et al. Deactivation of the limbic system during acute psychosocial stress: evidence from positron emission tomography and functional magnetic resonance imaging studies. Biol Psychiatry 2008; 63: 234–240. [DOI] [PubMed] [Google Scholar]
- Jin C, Qi R, Yin Y, Hu X, Duan L, Xu Q et al. Abnormalities in whole-brain functional connectivity observed in treatment-naive post-traumatic stress disorder patients following an earthquake. Psychol Med 2014; 44: 1927–1936. [DOI] [PubMed] [Google Scholar]
- Atkinson L, Gonzalez A, Kashy DA, Santo Basile V, Masellis M, Pereira J et al. Maternal sensitivity and infant and mother adrenocortical function across challenges. Psychoneuroendocrinology 2013; 38: 2943–2951. [DOI] [PubMed] [Google Scholar]
- Bosquet Enlow M, King L, Schreier HM, Howard JM, Rosenfield D, Ritz T et al. Maternal sensitivity and infant autonomic and endocrine stress responses. Early Hum Dev 2014; 90: 377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Granger D, Willoughby M, Kivlighan K. Maternal sensitivity is related to hypothalamic-pituitary-adrenal axis stress reactivity and regulation in response to emotion challenge in 6-month-old infants. Ann N Y Acad Sci 2006; 1094: 263–267. [DOI] [PubMed] [Google Scholar]
- Bilek E, Schafer A, Ochs E, Esslinger C, Zangl M, Plichta MM et al. Application of high-frequency repetitive transcranial magnetic stimulation to the DLPFC alters human prefrontal-hippocampal functional interaction. J Neurosci 2013; 33: 7050–7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal-Casas D, Balaguer-Ballester E, Gerchen MF, Iglesias S, Walter H, Heinz A et al. Multi-site reproducibility of prefrontal-hippocampal connectivity estimates by stochastic DCM. Neuroimage 2013; 82: 555–563. [DOI] [PubMed] [Google Scholar]
- Main M. The organized categories of infant, child, and adult attachment: flexible vs. inflexible attention under attachment-related stress. J Am Psychoanal Assoc 2000; 48: 1055–1096. [DOI] [PubMed] [Google Scholar]
- Zimmermann P. Attachment representations and characteristics of friendship relations during adolescence. J Exp Child Psychol 2004; 88: 83–101. [DOI] [PubMed] [Google Scholar]
- Scharf M, Mayseless O, Kivenson-Baron I. Adolescents' attachment representations and developmental tasks in emerging adulthood. Dev Psychol 2004; 40: 430–444. [DOI] [PubMed] [Google Scholar]
- Fraedrich EM, Lakatos K, Spangler G. Brain activity during emotion perception: the role of attachment representation. Attach Hum Dev 2010; 12: 231–248. [DOI] [PubMed] [Google Scholar]
- Nguyen VT, Cunnington R. The superior temporal sulcus and the N170 during face processing: single trial analysis of concurrent EEG-fMRI. Neuroimage 2014; 86: 492–502. [DOI] [PubMed] [Google Scholar]
- Sadeh B, Podlipsky I, Zhdanov A, Yovel G. Event-related potential and functional MRI measures of face-selectivity are highly correlated: a simultaneous ERP-fMRI investigation. Hum Brain Mapp 2010; 31: 1490–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BT, D'Esposito M. Spatial and temporal dynamics of cortical networks engaged in memory encoding and retrieval. Front Hum Neurosci 2012; 6: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CD, Lori NF, Akbudak E, Sorar E, Gultepe E, Shimony JS et al. MRI diffusion tensor tracking of a new amygdalo-fusiform and hippocampo-fusiform pathway system in humans. J Magn Reson Imaging 2009; 29: 1248–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson-Long M, Eslinger PJ, Wang J, Meadowcroft M, Yang QX. Functional MRI evidence for distinctive binding and consolidation pathways for face-name associations: analysis of activation maps and BOLD response amplitudes. Top Magn Reson Imaging 2009; 20: 271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan R, Horner AJ, Bandettini PA, Doeller CF, Burgess N. Human hippocampal processing of environmental novelty during spatial navigation. Hippocampus 2014; 24: 740–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kret ME, Denollet J, Grezes J, de Gelder B. The role of negative affectivity and social inhibition in perceiving social threat: an fMRI study. Neuropsychologia 2011; 49: 1187–1193. [DOI] [PubMed] [Google Scholar]
- Edmiston EK, Blackford JU. Childhood maltreatment and response to novel face stimuli presented during functional magnetic resonance imaging in adults. Psychiatry Res 2013; 212: 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozier JC, Wang L, Huettel SA, De Bellis MD. Neural correlates of cognitive and affective processing in maltreated youth with posttraumatic stress symptoms: does gender matter? Dev Psychopathol 2014; 26: 491–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig-Fast K, Werner NS, Lermer R, Latscha K, Meister F, Reiser M et al. After facing traumatic stress: brain activation, cognition and stress coping in policemen. J Psychiatr Res 2009; 43: 1146–1155. [DOI] [PubMed] [Google Scholar]
- De Brito SA, Viding E, Sebastian CL, Kelly PA, Mechelli A, Maris H et al. Reduced orbitofrontal and temporal grey matter in a community sample of maltreated children. J Child Psychol Psychiatry 2013; 54: 105–112. [DOI] [PubMed] [Google Scholar]
- Paller KA, Ranganath C, Gonsalves B, LaBar KS, Parrish TB, Gitelman DR et al. Neural correlates of person recognition. Learn Mem 2003; 10: 253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JY, Gu BM, Kang DH, Shin YW, Choi CH, Lee JM et al. Integration of cross-modal emotional information in the human brain: an fMRI study. Cortex 2010; 46: 161–169. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Holsen L, Abbeduto L, Davidson RJ. Brain function and gaze fixation during facial-emotion processing in fragile X and autism. Autism Res 2008; 1: 231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CI, Martis B, Schwartz CE, Shin LM, Fischer HH, McMullin K et al. Novelty responses and differential effects of order in the amygdala, substantia innominata, and inferior temporal cortex. Neuroimage 2003; 18: 660–669. [DOI] [PubMed] [Google Scholar]
- Main M, Kaplan N, Cassidy J. Monographs of the Society for Research in Child Development. Wiley, 1985; 50: pp 66–104. [Google Scholar]
- Olsen RK, Nichols EA, Chen J, Hunt JF, Glover GH, Gabrieli JD et al. Performance-related sustained and anticipatory activity in human medial temporal lobe during delayed match-to-sample. J Neurosci 2009; 29: 11880–11890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veer IM, Beckmann CF, van Tol MJ, Ferrarini L, Milles J, Veltman DJ et al. Whole brain resting-state analysis reveals decreased functional connectivity in major depression. Front Syst Neurosci 2010; 4: pii:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Werff SJ, Pannekoek JN, Veer IM, van Tol MJ, Aleman A, Veltman DJ et al. Resilience to childhood maltreatment is associated with increased resting-state functional connectivity of the salience network with the lingual gyrus. Child Abuse Negl 2013; 37: 1021–1029. [DOI] [PubMed] [Google Scholar]
- Bluhm RL, Williamson PC, Osuch EA, Frewen PA, Stevens TK, Boksman K et al. Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. J Psychiatry Neurosci 2009; 34: 187–194. [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Wang Z, Qin LD, Wan JQ, Sun YW, Su SS et al. Early altered resting-state functional connectivity predicts the severity of post-traumatic stress disorder symptoms in acutely traumatized subjects. PloS One 2012; 7: e46833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson EA. A prospective longitudinal study of attachment disorganization/disorientation. Child Dev 1998; 69: 1107–1128. [PubMed] [Google Scholar]
- van IJzendoorn MH, Kroonenberg PM. Cross cultural patterns of attachment: a meta analysis of the strange situation. Child Dev 1988; 59: 147–156. [Google Scholar]
- van IJzendoorn MH. Adult attachment representations, parental responsiveness, and infant attachment: a meta-analysis on the predictive validity of the Adult Attachment Interview. Psychol Bull 1995; 117: 387–403. [DOI] [PubMed] [Google Scholar]
- van Ijzendoorn MH, Schuengel C, Bakermans-Kranenburg MJ. Disorganized attachment in early childhood: meta-analysis of precursors, concomitants, and sequelae. Dev Psychopathol 1999; 11: 225–249. [DOI] [PubMed] [Google Scholar]