Abstract
Background
Despite modern antimicrobials and supportive therapy bacterial and fungal infections are still major complications in people with prolonged disease‐related or treatment‐related neutropenia. Transfusions of granulocytes have a long history of usage in clinical practice to support and treat severe infection in high‐risk groups of patients with neutropenia or neutrophil dysfunction. However, there is considerable current variability in therapeutic granulocyte transfusion practice, and uncertainty about the beneficial effect of transfusions given as an adjunct to antibiotics on mortality. This is an update of a Cochrane review first published in 2005.
Objectives
To determine the effectiveness and safety of granulocyte transfusions compared to no granulocyte transfusions as adjuncts to antimicrobials for treating infections in people with neutropenia or disorders of neutrophil function aimed at reducing mortality and other adverse outcomes related to infection.
Search methods
We searched for randomised controlled trials (RCTs) in the Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library 2016, Issue 2). MEDLINE (from 1946), Embase (from 1974), CINAHL (from 1937), the Transfusion Evidence Library (from 1980) and ongoing trial databases to 11 February 2016.
Selection criteria
RCTs comparing people with neutropenia or disorders of neutrophil dysfunction receiving granulocyte transfusions to treat infection with a control group receiving no granulocyte transfusions. Neonates are the subject of another Cochrane review and were excluded from this review. There was no restriction by outcomes examined, language or publication status.
Data collection and analysis
We used standard methodological procedures expected by the Cochrane Collaboration.
Main results
We identified 10 trials that met the inclusion criteria with a total of 587 participants. We also identified another ongoing trial. These trials were conducted between 1975 and 2015. None of the studies included people with neutrophil dysfunction. The studies differed in the type of infections they included. Six studies included both children and adults, however data were not reported separately for children and adults. The two newest studies gave granulocyte colony stimulating factor (G‐CSF) to donors; both were stopped early due to lack of recruitment. Three studies re‐randomised participants and therefore quantitative analysis was unable to be performed.
Overall the quality of the evidence was very low to low across different outcomes according to GRADE methodology. This was due to many of the studies being at high risk of bias, and many of the outcomes being imprecise.
There may be no difference in all‐cause mortality over 30 days between participants receiving therapeutic granulocyte transfusions and those that did not (six studies; 321 participants; RR 0.75, 95% CI 0.54 to 1.04; very low‐quality evidence). There were no differences between the granulocyte dose subgroups (< 1 x 1010 per day versus ≥ 1 x 1010 per day) (test for subgroup differences P = 0.39). There was a difference in all‐cause mortality between the studies based on the age of the study (published before 2000 versus published 2000 or later) (test for subgroup differences P = 0.03). There was no difference in all‐cause mortality between participants receiving granulocyte transfusions and those that did not in the newest study (one study; 111 participants; RR 1.10, 95% CI 0.70 to 1.73, low‐quality evidence). There may be a reduction in all‐cause mortality in participants receiving granulocyte transfusions compared to those that did not in studies published before the year 2000 (five studies; 210 participants; RR 0.53, 95% CI 0.33 to 0.85; low‐quality evidence).
There may be no difference in clinical reversal of concurrent infection between participants receiving therapeutic granulocyte transfusions and those that did not (five studies; 286 participants; RR 0.98, 95% CI 0.81 to 1.19; low‐quality evidence).
There is insufficient evidence to determine whether there is a difference in pulmonary serious adverse events (1 study; 24 participants; RR 0.85, 95% CI 0.38 to 1.88; very low‐quality evidence).
None of the studies reported number of days on therapeutic antibiotics, number of adverse events requiring discontinuation of treatment, or quality of life.
Six studies reported their funding sources and all were funded by governments or charities.
Authors' conclusions
In people who are neutropenic due to myelosuppressive chemotherapy or a haematopoietic stem cell transplant, there is insufficient evidence to determine whether granulocyte transfusions affect all‐cause mortality. To be able to detect a decrease in all‐cause mortality from 35% to 30% would require a study containing at least 2748 participants (80% power, 5% significance). There is low‐grade evidence that therapeutic granulocyte transfusions may not increase the number of participants with clinical resolution of an infection.
Plain language summary
Transfusions of white blood cells to treat infections in people with low white blood cell counts or white blood cells that do not function properly
Review question
We evaluated the evidence about whether white blood cell transfusions (also called granulocyte transfusions) given to treat infections are safe and reduce the risk of death or severe outcomes due to infection. Our target population was people with a very low white count (neutropenia) or white cells that did not function properly (neutrophil dysfunction).
Background
Functioning white blood cells are important for fighting life‐threatening bacterial and fungal infections. For many years some hospital physicians have given white blood cell transfusions to people with infections who have a low white blood count. The demand for white blood cells for transfusion has shown a steady increase since the 1990s mainly as a result of the introduction of a drug called granulocyte colony stimulating factor (G‐CSF), which if given to donors, leads to increased white blood cell numbers in the donor's blood and the collection of a larger dose of white blood cells than was previously possible.
Study Characteristics
The evidence is current to February 2016. In this update we identified 10 completed trials that compared giving white blood cell transfusions to treat infection compared to not giving white blood cells to treat infection. One additional trial has not yet been completed. The 10 trials containing a total of 587 participants were conducted between 1975 and 2015. The studies differed in the type of infections they included. Data from three trials were not included in the analyses because participants were included within the trial more than once. Six trials included both children and adults, but results were not reported separately for children and adults. The two newest trials gave G‐CSF to donors, both were stopped early due to lack of recruitment. Six studies reported their funding sources and all were funded by governments or charities.
Key results
Giving white blood cell transfusions to treat infection may not affect the risk of death or the number of people who recover from an infection.
It is unknown whether white blood cell transfusions increase the risk of having a serious adverse event.
None of the studies reported whether white blood cell transfusions reduced the number of days participants were on therapeutic antibiotics, or whether white blood cell transfusions had an effect on participants' quality of life.
Quality of the Evidence
The evidence for most of the findings are of low or very low quality. This was because the total number of participants included in this review was too small to detect a difference in this review's primary outcome. We calculated that a study would need at least 2748 participants to be able to detect a decrease in the risk of death from 35 people out of 100 to 30 people out of 100 (five additional lives saved per 100 people treated). Also participants and their doctors were likely to know which study arm they had been allocated to in all of the studies.
Summary of findings
for the main comparison.
| Therapeutic granulocytes compared with no granulocyte transfusions for treating infection in people with neutropenia or neutrophil dysfunction | ||||||
| Patient or population: treating infections in people with neutropenia or neutrophil dysfunction Setting: Hospitals Intervention: Therapeutic granulocyte transfusions Comparison: No therapeutic granulocyte transfusions | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no therapeutic granulocyte transfusions | Risk with Therapeutic granulocyte transfusions | |||||
| Overall mortality follow‐up: 30 days | Study population | RR 0.75 (0.54 to 1.04) | 321 (6 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 | ||
| 346 per 1000 | 259 per 1000 (187 to 360) | |||||
| Moderate | ||||||
| 350 per 1000 | 262 per 1000 (189 to 364) | |||||
| Clinical response to infection | Study population | RR 0.98 (0.81 to 1.19) | 286 (5 RCTs) | ⊕⊕⊝⊝ LOW 3 4 | ||
| 590 per 1000 | 578 per 1000 (478 to 702) | |||||
| Moderate | ||||||
| 430 per 1000 | 421 per 1000 (348 to 512) | |||||
| Length of time with fever ‐ not reported | see comment | see comment | not estimable | ( studies) | ‐ | |
| Number of days on therapeutic antibiotics ‐ not reported | see comment | see comment | not estimable | ( studies) | ‐ | |
| Number of serious adverse events ‐ pulmonary | Study population | RR 0.85 (0.38 to 1.88) |
24 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 2 3 | ||
| 545 per 1000 | 464 per 1000 (207 to 1000) |
|||||
| Number of adverse events requiring discontinuation of treatment ‐ not reported | see comment | see comment | not estimable | ( studies) | ‐ | |
| Quality of life ‐ not reported | see comment | see comment | not estimable | ( studies) | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 We downgraded the evidence by one because there was a difference in the effect seen between the newer study (Price 2015) and the older studies.
2 We downgraded the evidence by two due to very serious imprecision in the estimate.
3 Owing to the nature of the intervention (granulocyte transfusion) and difficulty blinding participants and physicians studies were likely to be at high risk of performance bias. We downgraded the evidence by 1 for risk of bias.
4 We downgraded the evidence by one due to imprecision of the estimate.
Background
Description of the condition
Functioning white blood cells (WBCs) are a vital component of the defence system against infection in humans. There are a variety of different WBCs that work together and perform complementary roles. Granulocytes are WBCs that contain granules that are visible when viewed through a light microscope. Neutrophils, a subtype of granulocytes are the most numerous circulating WBCs in healthy adults. Granulocytes in general, and neutrophils in particular, are crucial in protecting against bacterial and fungal infection. A persisting reduction in neutrophil numbers is called neutropenia, the severity of which has been classified by the World Health Organization (WHO 1992): when the peripheral blood count is below a level of 0.5 x 109/litre there is an increased risk of severe infection (the normal neutrophil count ranges from 2 to 7.5 x 109/litre in adults). Neutropenia usually occurs as a result of impaired production of neutrophils and other blood cells in the bone marrow. Diseases infiltrating the bone marrow such as leukaemias, or drugs that are toxic to the bone marrow such as chemotherapy, are typical reversible causes of neutropenia. Even if their number of neutrophils is normal, people may suffer from a similar inability to fight infections adequately if there is an impairment in the function of their neutrophils. Some people are born with such disorders, which may either be suspected from their family history or demonstrated by laboratory testing (Kuijpers 1999) e.g. chronic granulomatous disease.
Description of the intervention
Despite the use of specific and appropriate antibiotic and antifungal drugs, infection in people with neutropenia is associated with hospital admission, organ damage, and a significant number of deaths (Klastersky 2001; Legrand 2012). The infusion of granulocytes to prevent or treat infection in high‐risk patients has been part of clinical practice for over 40 years.
Granulocyte transfusion therapy has undergone several paradigm shifts. There was some preliminary evidence that granulocyte transfusions were effective in the context of clinical studies conducted with the prevailing standards of clinical care 30 to 40 years ago, including the trials analysed in this review. However, following this initial enthusiasm, concerns were raised about efficacy at the doses collected. More recent studies have suggested that the efficacy of granulocyte transfusions in neutropenic patients may be proportional to the dose of granulocytes transfused.
Uncertainty arising from the age of this clinical research is also compounded by problems of clinical diversity, methodological weaknesses and heterogeneity, all described in this review (see below). In addition, none of these earlier studies reported on quality control measures operating for blood components, including granulocytes, and again, it is expected that current blood products would be manufactured to a higher and more consistent standard.
In 1961, granulocytes were selectively collected from the blood of people with chronic myeloid leukaemia who had raised levels of leukaemic WBCs, including neutrophils (Freireich 1964). Apheresis (from the Greek 'to take away') was later developed and used for increased efficiency; this technique removes specific blood cells or fluid from the donor or patient whilst the cells or fluid that do not need to be removed are returned to the donor. Although apheresis involves equipment, it does allow selective collection of a larger dose of granulocytes than would be found in whole blood, with the added advantage for the donor of minimal red cell loss. In the early 1960s, granulocytes collected in this way were transfused into people with severe neutropenia that was not responsive to antibiotics (Freireich 1964). There are a number of different methods for collecting granulocytes for transfusion in humans.
Unstimulated apheresis collection of granulocytes
There are a number of technical problems that make it difficult to collect consistently adequate granulocyte doses for transfusion. Granulocytes are difficult to separate from other blood cells, even if this has been facilitated by commercially available long‐chain starch solutions (sedimenting agents) such as hetastarch and pentastarch. Also, normal donors do not have very high levels of circulating granulocytes in the peripheral blood, and as a result are able only to donate sufficient doses of granulocytes for very small children. Doses of less than 1 x 1010 granulocytes per m2 of body surface area are not associated with either a significant rise in the recipient's neutrophil count or a clinical response to established infection (Engelfriet 2000; Vamvakas 1996).
Stimulated apheresis collection of granulocytes
More recently, there has been a further resurgence of interest in granulocyte transfusions, reflecting recognition that higher doses of granulocytes could be collected for transfusion, by priming donors with steroids or granulocyte colony stimulating factor (G‐CSF), or both, to increase the circulating white cell count prior to apheresis (Dale 2000). In the early 1990s growth factors that stimulate the bone marrow to produce more WBCs (particularly granulocytes) became available for therapeutic use (Dale 2000; Engelfriet 2000; Hubel 2001; Robinson 2004; Strauss 1995). These drugs allowed high peripheral blood white cell counts to be achieved in healthy donors. The most commonly used growth factor is G‐CSF. Steroids can also increase the white cell count, by both increasing marrow release of granulocytes and decreasing efflux from peripheral blood, but steroids alone are not as effective as G‐CSF. The use of a single injection of G‐CSF alone or combined with a single oral dose of steroids has enabled the collection of significantly greater yields of granulocytes by apheresis. Using this method, adequate doses of granulocytes can be produced for larger children and adults. However, the general clinical utility of transfusion therapy has also been compromised logistically by the inability to store granulocyte products in a manner which preserves much of their function (Strauss 2003). These developments have occurred in conjunction with advances in the overall standards of supportive care given to people undergoing treatment for haematological malignancies and stem cell transplantation, including the diagnostic strategies for infection and the therapeutic armamentarium of anti‐microbial drugs (Dellinger 2013; NICE 2012).
The exposure of a healthy volunteer donor to any form of medication with potential side effects does, however, present ethical and safety issues. Most side effects related to G‐CSF are short term (Bux 2003; de la Rubia 2008; Hölig 2013). Repeated doses of G‐CSF have been reported to cause thrombosis, possibly as a result of the increased level of white cells in the blood (Hölig 2013). Cases of splenic rupture following repeated doses of G‐CSF have been reported among the more severe adverse events (Gutierrez 2001; Hölig 2013). Any drug can also cause allergic type reactions, including anaphylactoid reactions (Gutierrez 2001). The bone marrow expansion that occurs as a result of G‐CSF stimulation commonly leads to bone pain and flu‐like symptoms (Hölig 2013). Theoretical concerns regarding the long‐term effects of G‐CSF on the donor's bone marrow cells remain, although there is increasing evidence that prolonged repeated administration of G‐CSF to children and adults as therapy does not increase their risk of bone marrow disorders (de la Rubia 2008; Hölig 2013).
Due to the potential risks of G‐CSF, in England G‐CSF cannot be administered to healthy donors who are not giving a directed granulocyte donation. A small number of donations are also collected from relatives and friends of patients following the administration of G‐CSF and the steroid dexamethasone (Hubel 2002).
Pooled granulocytes
Granulocytes derived from whole blood donations (pooled buffy coat granulocytes) (Bashir 2008; Massey 2012) are provided in England. These provide granulocyte doses at least comparable to the higher doses recorded in the randomised trials identified in this review. The risks of clinical sequelae due to alloimmunisation after multiple‐donor rather than single‐donor granulocyte transfusions need to be assessed fully. In one small study alloimmunisation occurred in 10% (3/30) of participants receiving granulocytes in additive solution and plasma (GASP) (Massey 2012).
How the intervention might work
Clinical experience and data from animal studies suggest that control of infection in neutropenic patients requires recovery of bone marrow neutrophil production (Dale 1976). The first documented attempt to reverse neutropenia using granulocyte transfusions was during the 1930s (Strumia 1934). Twenty years later, Brecher and colleagues gave granulocyte transfusions to neutropenic dogs, in which they showed that the transfused cells migrated to the areas of infection (Brecher 1953). There is an obvious rationale for this intervention, in that the major risk factor for severe infection in such patients is neutropenia: transfusions of granulocytes is then a logical way of correcting the deficit of granulocytes.
Adverse events such as febrile reactions, occasional severe pulmonary complications with hypoxia and hypotension, and HLA (human leucocyte antigen) alloimmunisation (immune‐mediated mechanisms potentially complicating other transfusions) are well recognised complications of granulocyte transfusions.
Although there is limited evidence from laboratory testing that donated granulocytes are functional (Bashir 2003; Bashir 2008), published controlled trials have reported very conflicting results of clinical effect. A number of these issues have been raised in previous systematic reviews (Kadri 2015; Stanworth 2005; Vamvakas 1996; Vamvakas 1997).
A final consideration for any new trials of granulocyte transfusion must be a better understanding of methods to preserve functional activity of granulocytes after collection, since the current utility of granulocyte transfusion therapy is also limited by concerns about storage and loss of function (Bashir 2003; Strauss 2003).
Why it is important to do this review
This systematic review aimed to assess the effectiveness and safety of therapeutic granulocyte transfusions in people with neutropenia or neutrophil dysfunction. We are uncertain whether granulocyte transfusions as part of a strategy to treat infection in neutropenic people are more effective at treating infections than antibiotic or antifungal agents alone. If effective, we are uncertain of the harms associated with granulocyte transfusions. Previous Cochrane reviews have been performed with specific reference to neonatal practice and prophylactic granulocyte transfusions (Estcourt 2015; Pammi 2011). This systematic review would therefore complement the previous Cochrane reviews. This is an update of a previous Cochrane review (Stanworth 2005).
Objectives
To determine the effectiveness and safety of granulocyte transfusions compared to no granulocyte transfusions as adjuncts to antimicrobials for treating infections in patients with neutropenia or disorders of neutrophil function aimed at reducing mortality and other adverse outcomes related to infection.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs). There were no restrictions on language or publication status.
This review has not included studies whose method of allocation was undertaken on the basis of donor availability, since the control group in these studies would be expected to contain older individuals without siblings or who were already alloimmunised ‐ i.e. baseline equivalence between the two groups would not be anticipated. It was also considered that the inclusion of these reports would not have affected the main clinical conclusions drawn from the analysis of the strictly randomised trials.
Types of participants
Patients with neutropenia (whether due to treatment or disease, or whether reversible or irreversible, were considered) and infection. We also considered patients with inherited disorders of neutrophil dysfunction and infection.
We excluded granulocyte transfusion studies of neonates with sepsis, and granulocyte transfusions to prevent severe infections because these are the focus of separate Cochrane reviews (Estcourt 2015; Pammi 2011).
Types of interventions
Intervention
Granulocyte transfusions
We included all sources of granulocytes by different methods of collection. Granulocyte transfusions would be given for therapeutic indications to treat severe infection refractory to antimicrobial therapy, and not as (secondary) prophylaxis to prevent recurrence of previous severe infections. It is expected that granulocyte transfusions would always be given as an adjunct to antibiotics and antimicrobials, and not as a separate intervention on its own.
Control
No granulocyte transfusions
Types of outcome measures
Primary outcomes
Death (from all causes) up to 30 days from the start of the study.
Secondary outcomes
Clinical reversal of concurrent infection (whether systemic or at specific loci) (as defined by the individual studies and includes both complete resolution or partial resolution of infection)
Length of time with fever
Days on antimicrobials (at treatment doses)
Increment of neutrophil count and duration of neutropenia reversal after transfusion (neutropenia defined as count below 0.5 x 109/litre)
Adverse events
Serious adverse events: resulting in death or life‐threatening illness, requiring or prolonging hospitalisation, or resulting in persistent or significant disability/incapacity
Adverse events requiring discontinuation of treatment
Other adverse events e.g. flu‐like symptoms, bone pain (see Background)
It is acknowledged that the frequency of potentially important long‐term side effects may not be adequately captured by information in (small) RCTs.
Search methods for identification of studies
The Systematic Review Initiative Information Specialist (CD) formulated updated search strategies in collaboration with the Cochrane Infectious Diseases Group based on those used in the previous version of this review (Stanworth 2005).
Electronic searches
We searched for randomised controlled trials in the following databases:
CENTRAL (the Cochrane Library 2016, Issue 2) (Appendix 1)
MEDLINE (Ovid, 1946 to 11 February 2016) (Appendix 2)
EMBASE (Ovid, 1974 to 11 February 2016) (Appendix 3)
CINAHL (EBSCOhost, 1937 to 11 February 2016) (Appendix 4)
LILACS (BIREME/PAHO/WHO, 1982 to 11 February 2016) (Appendix 5)
KoreaMed (KAMJE, 1997 to 11 February 2016) (Appendix 6)
PakMediNet (2001 to 11 February 2016) (Appendix 6)
IndMed (ICMR‐NIC, 1986 to 11 February 2016) (Appendix 7)
Transfusion Evidence Library (www.transfusionevidencelibrary.com) (1980 to 11 February 2016) (Appendix 8)
We updated the searches performed in 2003 and October 2008 (Stanworth 2005). Searches in MEDLINE, EMBASE and CINAHL were combined with adaptations of the Cochrane RCT search filters, as detailed in the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011).
Databases of ongoing trials
We also searched ClinicalTrials.gov (http://clinicaltrials.gov/ct2/search) (Appendix 9), the WHO International Clinical Trials Registry (ICTRP) (http://apps.who.int/trialsearch/) (Appendix 9), and the ISRCTN Register (http://www.controlled‐trials.com/isrctn/) (Appendix 10), in order to identify ongoing trials on 11 February 2016.
All new search strategies are presented as indicated in Appendices 1‐10. Search strategies for the original (2003 and 2008) searches are presented in Appendix 11.
Searching other resources
We augmented database searching with the following:
Handsearching of reference lists
We checked references of all included trials, relevant review articles and current treatment guidelines for further literature. Theses searches were limited to the 'first generation' reference lists.
Personal contacts
We contacted authors of relevant studies, study groups and experts worldwide known to be active in the field for unpublished material or further information on ongoing studies.
Data collection and analysis
Selection of studies
We updated the selection of studies from that performed for the previous version of this review (Stanworth 2005).
One review author (CD) excluded all duplicates and studies that were clearly irrelevant (e.g. non‐human) that had been identified by the review search strategy. Two review authors (LE, SS) then independently screened all remaining electronically‐derived citations and abstracts of papers identified by the review search strategy for relevance. We excluded studies that were clearly irrelevant at this stage based on a review of the abstract. Two review authors (LE, SS) independently formally assessed the full texts of all potentially‐relevant trials for eligibility against the criteria outlined above. All disagreements were resolved by discussion without the need for a third review author (SS). We sought further information from study authors if the article contained insufficient data to make a decision about eligibility. A study eligibility form was designed for trials of granulocyte transfusion to help in the assessment of relevance, which included ascertaining whether the participants were neonates, and whether the two groups could be defined in the trial on the basis of a therapeutic‐only versus prophylactic granulocyte transfusion strategy. We recorded the reasons why potentially‐relevant studies failed to meet the eligibility criteria.
Data extraction and management
We updated the data extraction from that performed for the previous version of this review (Stanworth 2005). This included data extraction for all new studies that have been included since the previous review and an updated 'Risk of bias' assessment for all included studies.
Two review authors (LE, SS) conducted the data extraction according to the guidelines proposed by the Cochrane Collaboration (Higgins 2011a). We resolved potential disagreements between the review authors by consensus. The review authors were not blinded to names of authors, institutions, journals, or the outcomes of the trials. Due to minor changes in the format the data extraction forms were piloted on a further study, thereafter the two authors (LE, SS) extracted data independently for all the studies. The following data were extracted.
General information
Review author's name, date of data extraction, study ID, first author of study, author's contact address (if available), citation of paper, objectives of the trial.
Trial details
Trial design, location, setting, sample size, power calculation, treatment allocation, inclusion and exclusion criteria, reasons for exclusion, comparability of groups, length of follow‐up, stratification, stopping rules described, statistical analysis, results, conclusion, and funding.
Characteristics of participants
Age, gender, ethnicity, total number recruited, total number randomised, total number analysed, types of underlying disease, lost to follow‐up numbers, dropouts (percentage in each arm) with reasons, protocol violations, previous treatments, current treatment, prognostic factors.
Interventions
Experimental and control interventions, method of preparation and source of granulocytes for transfusion, timing of intervention, dosage of granulocyte given, compliance to interventions, any differences between interventions, the use of colony‐stimulating factors in recipients, particularly G‐CSF, and the use of therapeutic antibiotics and antifungals.
Assessment of bias
Sequence generation, allocation concealment, blinding (participants, personnel, and outcome assessors), incomplete outcome data, selective outcome reporting, other sources of bias.
Outcomes measured
Death (from all causes), clinical reversal of concurrent infections (whether systemic or at specific loci), length of time with fever, days on antimicrobials (at treatment doses), increment of neutrophil count and duration of neutropenia reversal after transfusion (neutropenia defined as count below 0.5 x 109/litre). Adverse events including: serious adverse events resulting in death or life‐threatening illness, requiring or prolonging hospitalisation, or resulting in persistent or significant disability/incapacity; adverse events requiring discontinuation of treatment; other adverse events e.g. flu‐like symptoms, bone pain (see Background).
Both full‐text versions and abstracts were used to retrieve the data. Publications reporting on more than one trial were extracted using one data extraction form for each trial. Trials reported in more than one publication were extracted on one form only. Where these sources did not provide sufficient information, we contacted authors and study groups for additional details.
Data entry into the software Review Manager 5.3 was done by one review author (LE) and checked for accuracy by a second review author (SS).
Assessment of risk of bias in included studies
We updated the 'Risk of bias' assessment from that performed for the previous version of this review (Stanworth 2005).
Two review authors (LE, SS) assessed all included studies for possible risk of bias (as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c). The assessment included information about the design, conduct and analysis of the trial. Each criterion was evaluated on a three‐point scale: low risk of bias, high risk of bias, or unclear. The 'Risk of bias' assessment tool includes the following domains.
Selection bias: random sequence generation and allocation concealment.
Performance bias: blinding of participants and personnel.
Detection bias: blinding of outcome assessment.
Attrition bias: incomplete outcome data.
Reporting bias: selective reporting.
Other bias.
Measures of treatment effect
For dichotomous outcomes, we recorded the number of events and the total number of participants in both the treatment and control groups and we estimated the treatment effect measures across individual studies as the relative effect measures (risk ratio (RR) with 95% confidence intervals (CIs)). For dichotomous outcomes we reported the pooled RR with 95% CIs. Where the number of observed events was small (< 5% of sample per group), and where trials had balanced treatment groups, we planned to report the Peto’s Odds Ratio (OR) with 95% CI (Deeks 2011).
For continuous outcomes we planned to record the mean and standard deviations, and total number of participants in both the treatment and control groups. For continuous outcomes measured using the same scale, we planned to report the effect measure mean difference (MD) with 95% CIs, or the standardised mean difference (SMD) for outcomes measured using different scales.
For time‐to‐event outcomes we planned to extract the hazard ratio (HR) from published data according to Parmar 1998 and Tierney 2007. However, no time‐to‐event data were reported.
If data allowed, we undertook quantitative assessments using Review Manager 5 (Review Manager 5.3).
If the data available could not be reported in any of the formats described above a narrative report was performed.
Unit of analysis issues
We did not pre‐specify in the protocol how we would deal with unit of analysis issues. There were several unit of analysis issues within the included trials. Three trials re‐randomised participants or analysed the number of febrile episodes rather than the participants and were not included in the quantitative analysis (Alavi 1977; Herzig 1977; Seidel 2008a).
In Alavi 1977, participants had more than one febrile episode included in the analysis. Thirty‐two people were randomised; data from 31 participants were analysed for a total of 62 febrile episodes.
Participants were re‐randomised in one study (Herzig 1977); 27 participants were randomised to the study. Three participants were re‐randomised three, eight and 12 months after the first episode (all the re‐randomisations were to the granulocyte transfusion group, two participants had previously been in the control group).
In Seidel 2008a, 74 participants were randomised within 79 infectious episodes.
Dealing with missing data
We dealt with missing data according to the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). We contacted authors in order to obtain information that was missing or unclear in the published report. We contacted the authors of the Seidel 2008a study who have agreed to provide additional data, but this will require further statistical analysis.
Within an outcome, the preferred analysis was an intention‐to‐treat analysis (ITT). Where data were missing, the number of patients lost to follow‐up was recorded for each trial.
Assessment of heterogeneity
If studies were considered sufficiently homogenous in their study design, we conducted a meta‐analysis and assessed the statistical heterogeneity (Deeks 2011). We assessed statistical heterogeneity of treatment effects between trials using a Chi2 test with a significance level at P < 0.1. We used the I2 statistic to quantify possible heterogeneity (I2 > 50% moderate heterogeneity, I2 > 80% considerable heterogeneity). We explored potential causes of heterogeneity by sensitivity and subgroup analyses if possible.
Assessment of reporting biases
We did not perform a formal assessment of potential publication bias (small‐trial bias) by generating a funnel plot and statistically by using a linear regression test (Sterne 2011) as no meta‐analysis contained 10 or more studies.
Data synthesis
We performed analyses according to the recommendations of the Cochrane Collaboration (Deeks 2011). We used aggregated data for analysis. For statistical analysis, we entered data into Review Manager 5.3.
Where meta‐analysis was feasible, we used the fixed‐effect model for pooling the data. We used the Mantel‐Haenszel method for dichotomous outcomes. We planned to use the inverse variance method for continuous outcomes. Even in the absence of statistical heterogeneity, we explored the robustness of any summary measures, particularly with respect to study methodological quality.
We planned to use the random‐effects model for sensitivity analyses as part of the exploration of heterogeneity. However, none of the analyses performed reported heterogeneity, as expressed by an I2 above 50%, therefore only the fixed‐effect model was reported.
Summary of Findings
We used GRADE to build a 'Summary of findings' table as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011) including the following domains.
All‐cause mortality
Clinical reversal of concurrent infection
Length of time with fever
Number of days on therapeutic antibiotics
Number of serious adverse events
Number of adverse events requiring discontinuation of treatment
Quality of life
A GRADE assessment had not been pre‐specified in the protocol.
Subgroup analysis and investigation of heterogeneity
It was intended that the granulocyte dose, other donor‐related factors (e.g. type or source of granulocyte preparation such as whether community or related), and HLA incompatibility would be examined as a subgroup effect. In the event, there was insufficient information to make this very meaningful, reflecting the limitations of study numbers and their quality (see Results).
Three subgroup analyses were pre‐specified in the previous version of this review (Stanworth 2005). These were:
granulocyte dose;
other donor‐related factors (e.g. type or source of granulocyte preparation such as whether community or related);
HLA incompatibility.
We performed a subgroup analysis on granulocyte dose, classifying studies in to low dose (mean granulocyte dose less than 1 x 1010 per day for an adult patient) and standard dose (mean granulocyte dose at least 1 x 1010 4 x 1010 per day for an adult patient).
Differences between subgroups were commented on narratIvely.
We were unable to perform subgroup analyses for other donor‐related factors or HLA incompatibility due to lack of data.
Investigation of heterogeneity between studies also included, if appropriate, age of the study (as the treatment of neutropenic patients has changed over the last 40 years).
Sensitivity analysis
We intend to assess the robustness of our findings by the following two sensitivity analyses:
Including only those trials at low risk of bias
Including only those trials in which 20% participants or less were lost to follow‐up.
A sensitivity analysis including only those studies at low risk of bias was not performed because none of the studies were at low risk of bias. A sensitivity analysis including only those in which less than 20% participants were lost to follow‐up was not performed because all studies had less than 20% participants lost to follow‐up.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; and Characteristics of ongoing studies.
Results of the search
See PRISMA Flow Diagram Figure 1.
1.
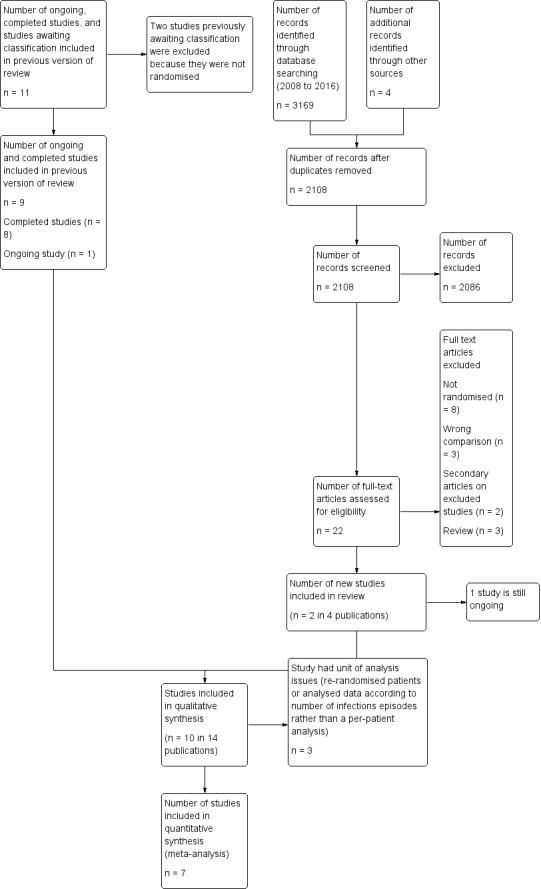
Study flow diagram.
The original systematic review search (conducted May 2003) identified 59 studies which appeared potentially relevant on the basis of the their full text or abstract. One review author performed the initial screening of abstracts. Two reviewers (EM, SS) independently assessed the 59 full‐text articles for inclusion or exclusion on the basis of the full text of abstract or paper using the stated criteria.
The updated search (conducted 11 February 2016) identified a total of 3173 potentially relevant records. There were 2108 records after duplicates were removed. Any two out of four reviewers (LE, CD, PB, SS) excluded 2086 records on the basis of the abstract.
The previous systematic review (Stanworth 2005) identified 11 potentially relevant trials that compared therapeutic granulocyte transfusions to no granulocyte transfusions, eight completed trials (Alavi 1977; Bow 1984; Herzig 1977; Higby 1975; Klastersky 1983; Scali 1978; Vogler 1977; Winston 1982a) one ongoing trial (Seidel 2008a), which is now included, and two studies awaiting classification (Adkins 1999; Blum 2001), which are now excluded.
This updated search identified two additional studies (Price 2015; DRKS00000218).
In total 11 studies were assessed and deemed eligible for inclusion (Alavi 1977; Bow 1984; DRKS00000218; Herzig 1977; Higby 1975; Klastersky 1983; Price 2015; Scali 1978; Seidel 2008a; Vogler 1977; Winston 1982a), however the DRKS00000218 study is still ongoing.
Included studies
See Characteristics of included studies and Characteristics of ongoing studies for full details of each study. Ten completed studies were eligible for inclusion in this review (Alavi 1977; Bow 1984; Herzig 1977; Higby 1975; Klastersky 1983; Price 2015; Scali 1978; Seidel 2008a; Vogler 1977; Winston 1982a).
Studies contributing to the main outcome
See Characteristics of included studies for full details of each study.
Study Design
There was one single‐centre parallel RCT (Winston 1982a), four multi‐centre parallel RCTs (Klastersky 1983; Price 2015; Seidel 2008a; Vogler 1977) and five parallel RCTs where the numbers of centres were unclear (Alavi 1977; Bow 1984; Herzig 1977; Higby 1975; Scali 1978).
Study Size
The number of participants enrolled in all the studies was small, ranging between 24 and 97 participants analysed. Only three studies recruited more than 50 participants (Price 2015; Seidel 2008a; Winston 1982a). These three studies presented information on sample sizes required to power the trial around a main outcome (Price 2015; Seidel 2008a; Winston 1982a). Two of the three studies performed prospective sample size calculations (Price 2015; Seidel 2008a); both studies were stopped early due to lack of recruitment. In the other study it was unclear when the sample size calculation was performed (Winston 1982a).
Setting
The 10 RCTs were published between 1975 and 2015. Six were conducted in the USA (Alavi 1977; Herzig 1977; Higby 1975; Price 2015; Vogler 1977; Winston 1982a), one in Canada (Bow 1984), one in Switzerland (Scali 1978, paper translated from German), one in Germany (Seidel 2008a) and one European multi‐centred study (Klastersky 1983). All studies were parallel RCTs and compared two groups of participants, one of which received granulocyte transfusions, the other no granulocyte transfusions.
Participants
In total, 587 participants were randomised, of these 471 were included in the analyses.
The study populations varied between the 10 trials. No trials enrolled patients with congenital disorders of neutrophil function or production. In all but one study (Herzig 1977), the majority of enrolled participants had acute myeloid leukaemia. The enrolled population in Herzig 1977 was predominantly acute lymphoblastic leukaemia. Six studies included children (Alavi 1977; Herzig 1977; Price 2015; Seidel 2008a; Vogler 1977; Winston 1982a). However, the number of children included in these studies was small because only one study had a median or mean age of study participants below 30 (Herzig 1977 median 15 years granulocyte transfusion group; 18 years control group). Only one study reported whether the participant's haematological disease was relapsed or refractory (Winston 1982a 13/48 participants granulocyte transfusion group; 11/47 participants control group). Four studies reported the type of treatment the participants were receiving for their haematological malignancy (Higby 1975; Price 2015; Scali 1978; Seidel 2008a). Two studies included participants receiving chemotherapy (Higby 1975; Scali 1978), two studies included participants receiving haematopoietic stem cell transplants (HSCT) or chemotherapy (Price 2015; Seidel 2008a). In Price 2015, the majority of participants (75%) were receiving chemotherapy and in Seidel 2008a, the majority of participants (53%) were receiving HSCT.
Intervention
Average dose and range
The mean (or median) dose of granulocytes transfused varied by a factor of 10, or one order of magnitude between studies (Table 2). The doses in the studies were, in ascending order, 0.5 x 1010 (median, Winston 1982a), 0.87 x 1010 (mean, Bow 1984), 0.4 or 1.7 x 1010/m2 (medians for two methods of collection, Herzig 1977), to exceed 1 x 1010/m2 (Klastersky 1983), 2.7 x 1010 (average, Vogler 1977), 2.9 x 1010 (average, Scali 1978), 3.7 x 1010 (average, Higby 1975), 4.6 x 1010 (median Seidel 2008a), 5 x 1010 (average, Alavi 1977), and 5.5 x 1010 (median Price 2015). Average doses of less than 1.0 x 1010 as reported in three studies would be considered low by contemporary standards (Bow 1984; Herzig 1977; Winston 1982a).
1. Characteristics of Studies.
| Study | Number of participants | Method of granulocyte procurement |
Dose of granulocyte transfusions Median (Range) |
Frequency of granulocyte transfusions | Granulocytes Irradiated | Period of observation post onset of fever | Donor pre‐medication | Donor selection |
| Alavi 1977 | 31 | Filtration leukapheresis | 3.3 x 1010/m2 children (Not reported) 3.2 x 1010/m2 adults (Not reported) |
Daily | Not reported | 1 to 24 hours | Hydrocortisone | Red cell compatibility alone |
| Bow 1984 | 24 | Discontinous flow centrifugation | Mean 0.49 x 1010/m2 SD 0.20a |
Not reported | Not reported | 72 hours | Dexamethasone | HLA compatibility WBC compatibility/crossmatch |
| Herzig 1977 | 27 | Continous flow centrifugation | 0.4 x 1010/m2 (0.2 to 0.8) |
Daily | Yes 1500 to 2500 rads |
variableb | None | WBC compatibility/crossmatch |
| Filtration leukapheresis | 1.7 x 1010/m2 (0.6 to 4.6) |
|||||||
| Higby 1975 | 36 | Filtration leukapheresis | 2.2 x 1010/m2 (1.1 to 5.0) |
Daily | Not reported | 48 hours | Dexamethasone | WBC compatibility/crossmatch |
| Klastersky 1983 | 39 | Not reported | > 1.0 x 1010/m2 (Not reported) |
Daily | Not reported | 24 hours | Not reported | Not reported |
| Price 2015 | 97 | Continuous flow centrifugation | 5.5 x 1010 (IQR 2.6 to 7.3) |
Daily | Yes 1500 to 2500 cGy |
variablec | G‐CSF and Dexamethasone | Red cell compatibility alone |
| Scali 1978 | 25 | Continous flow centrifugation | 2.9 x 1010 | Daily | Not reported | 24 hours | Not reported | Red cell compatibility alone |
| Seidel 2008a | 74 | Continous flow centrifugation | 4.6 x 1010 (0.84 to 11.2)d |
Alternate days | Yes 30Gy |
Not reported | G‐CSF | Not reported |
| Vogler 1977 | 30 | Continous flow centrifugation | 2.7 x 1010 (Not reported) |
At least 4 transfusions in an 8 day period | Not reported | variablee | None, hydrocortisone, or dexamethasone | HLA compatibility WBC compatibility/crossmatch |
| Winston 1982a | 95 | Discontinous flow centrifugation | 0.5 x 1010 (0.1 to 2.7) |
Daily | No | variablef | None | Red cell compatibility alone |
aConverted to per m2 by dividing by the estimated adult body surface area (1.79) in adult patients according to Sacco 2010.
bWithin 24 hours of a positive blood culture result.
cEligibility criteria changed during the trial. Initially treatment was within 24 hours of a proven or probable bacterial fungal infection. After 31 months this was liberalised within one week. Time from eligibility to first transfusion was 2.3 ± 1.2 days.
dAssuming weight is 70kg.
eWhen patient had received at least 72 hours of appropriate antibiotics (bactericidal in vitro) to treat organism identified on culture. Five participants had an infection for more than 10 days (2 control, 3 granulocyte transfusions); 13 participants had an infection for 5 to 10 days (5 control, 8 granulocyte transfusions); and 12 participants had an infection for 3 to 5 days (6 control, 6 granulocyte transfusions).
fWithin 24 hours of a positive blood culture result, the appearance of an infiltrate on chest X‐ray, or the initial development of a cellulitis or abscess.
Schedule for transfusion
Different policies applied to the starting criteria for granulocyte transfusions (Table 2). In five studies granulocyte transfusions were administered after a period of observation post the onset of fever (ranging from 24 to 72 hours), in order to assess clinical response to antibiotics (Alavi 1977; Bow 1984; Higby 1975; Klastersky 1983; Scali 1978). In four studies granulocyte transfusions were administered within 24 to 72 hours from a positive culture result (Herzig 1977; Price 2015; Vogler 1977; Winston 1982a). One study did not report the time‐frame (Seidel 2008a).
Seven studies planned to give daily granulocyte transfusions (Alavi 1977; Herzig 1977; Higby 1975; Klastersky 1983; Price 2015; Scali 1978; Winston 1982a). Two studies planned to give granulocyte transfusions at least every other day (Seidel 2008a; Vogler 1977), and one study did not report the frequency (Bow 1984).
Three studies had fixed time‐frames over which granulocyte transfusions were to be given (Higby 1975; Klastersky 1983; Vogler 1977), two studies stopped after four daily transfusions (Higby 1975; Klastersky 1983), and one study stopped after participants were given four or more transfusions within eight days (Vogler 1977).
Five studies had variable planned durations of administering granulocyte transfusion (Alavi 1977; Herzig 1977; Price 2015; Seidel 2008a; Winston 1982a). Three studies planned to stop administering granulocyte transfusions after the infection had resolved or there was evidence of neutrophil recovery (Herzig 1977; Price 2015; Winston 1982a), one study planned to stop when the infection had resolved (Alavi 1977), and one study planned to stop when there was evidence of neutrophil recovery (Seidel 2008a).
Two studies did not report their plan for stopping granulocyte transfusions (Bow 1984; Scali 1978), the average duration of granulocyte transfusions were 2.7 days in Scali 1978 and 6.4 days in Bow 1984. Overall, some studies described short durations of infusions, which currently would not be considered adequate.
Method of collection of granulocytes
The method of procurement of granulocytes varied between trials (Table 2). Granulocytes were collected by filtration leukapheresis in three studies ( Alavi 1977; Herzig 1977; Higby 1975), by discontinuous flow centrifugation in two studies (Bow 1984; Winston 1982a), and by continuous flow centrifugation in five studies (Herzig 1977; Price 2015; Scali 1978; Seidel 2008a; Vogler 1977). The method was not defined in one study (Klastersky 1983). Filtration leukapheresis is rarely used now, as despite high yields there are concerns about toxicity to both the donor and recipient, in addition to evidence of poor increments and functionality of the transfused granulocytes (Strauss 2003).
Pre‐medication of donors
Steroid pre‐medication of donors (dexamethasone or hydrocortisone) was reported in four studies (Alavi 1977; Bow 1984; Higby 1975; Price 2015). Only two studies administered steroids more than six hours prior to donation (Bow 1984; Price 2015), a shorter duration would not be expected to enhance granulocyte yield. Granulocyte colony stimulating factor (G‐CSF) pre‐medication was reported in two studies (Price 2015; Seidel 2008a), this was given between eight and 17 hours prior to donation. Eight studies were conducted prior to licensing of G‐CSF by the Food and Drug Administration (FDA) in 1991 and therefore it is very unlikely that donors in these studies received G‐CSF (Alavi 1977; Bow 1984; Herzig 1977; Higby 1975; Klastersky 1983; Scali 1978; Vogler 1977; Winston 1982a).
Donor selection
Donors were selected in part on the basis of human leucocyte antigen (HLA) compatibility in two studies (Bow 1984; Vogler 1977) and on the basis of white cell compatibility/cross match in four studies (Bow 1984; Herzig 1977; Higby 1975; Vogler 1977). Donors were selected only on the basis of red cell compatibility in four studies (Alavi 1977; Price 2015; Scali 1978; Winston 1982a). Two studies did not report their method of selecting donors (Klastersky 1983; Seidel 2008a).
Co‐interventions and/or alternative interventions
Differences between the studies were also identified in the co‐interventions provided to patients. This would include the diagnostic and therapeutic options then available for anti‐microbial practice.
Six studies defined the initial antibiotic therapy to be given (Alavi 1977; Bow 1984; Herzig 1977; Klastersky 1983; Vogler 1977; Winston 1982a). Four studies did not define the antibiotics to be given (Higby 1975; Price 2015; Scali 1978; Seidel 2008a).
Regimens for anti‐fungal diagnosis and therapy were only stated in two trials (Price 2015; Winston 1982a). Although standard therapy from 1975 to 1984 was amphotericin B.
One study recommended central line removal for participants with two or more positive blood cultures of Candida or Fusarium (Price 2015).
Funding sources
Six studies reported their funding sources and all were funded by governments or charities (Alavi 1977; Higby 1975; Klastersky 1983; Price 2015; Vogler 1977; Winston 1982a).
Definition of neutropenia
All studies used different inclusion definitions for neutropenia in patients, which varied from 0.1 x 109/L (Bow 1984) to 1 x 109/L (Herzig 1977). Marrow recovery or regeneration of counts was defined by two studies as a granulocyte count greater than 0.5 x 109/L (Alavi 1977; Winston 1982a), and by three studies as a granulocyte count greater than 1 x 109/L (Herzig 1977; Price 2015; Seidel 2008a). The other five studies did not report their definition of marrow recovery (Bow 1984; Higby 1975; Klastersky 1983; Scali 1978; Vogler 1977).
Definition of infection
Studies also applied different criteria for definition of infection. The inclusion criteria for granulocyte transfusions in two studies required positive microbiological cultures, including blood (Herzig 1977; Vogler 1977). In one study, positive cultures were required except in specific defined cases of localised infection with objective signs (Winston 1982a). Seven studies permitted a clinical diagnosis or 'possibility' of infection (Alavi 1977; Bow 1984; Higby 1975; Klastersky 1983; Price 2015; Scali 1978; Seidel 2008a), the exact definition of which varied between the studies. In Bow 1984, people with a definite infection were excluded from the randomised part of the study (Table 3).
2. Types of infection.
| Study | Number of participants analysed | Possible or probable infection | Localised infections (excluding oral candida) | Systemic infections (Bacteraemia or fungaemia | |||
| Therapeutic granulocytes | Control | Therapeutic granulocytes | Control | Therapeutic granulocytes | Control | ||
| Alavi 1977 | 31 (62 febrile episodes) |
2 participants | 3 participants | 2 participants 2 Abscess or cellulitis 0 Urinary tract infection |
2 participants 1 Abscess or cellulitis 1 Urinary tract infection |
10 participants 1 E. coli 4 Klebsiella 0 Pseudomonas 2 Gram negative (other) 2 Gram‐positive 1 Candidaemia |
14 participants 4 Pseudomonas 4 Klebsiella 2 E. coli 1 Gram‐negative (other) 3 Gram‐positive 0 Candidaemia |
| Bow 1984 | 24 | 13 | 11 | 0 | 0 | 0 | 0 |
| Herzig 1977 | 27 (30 septic episodes) |
0 | 0 | 0 | 0 | 9 E. coli 2 Klebsiella 2 Pseudomonas 0 Proteus 0 Neisseria 3 Mixed gram‐negative |
4 E. coli 4 Klebsiella 1 Pseudomonas 1 Proteus 1 Neisseria 3 Mixed gram‐negative |
| Higby 1975 | 27 (aged < 45 years) |
4 | 4 | 3 Pneumonia 4 Abscess (other) 4 Perirectal abscess 1Urinary tract infection 0 Other |
4 Pneumonia 1 Abscess (other) 3 Perirectal abscess 3 Urinary tract infection 1 Other |
5 Bacteraemia | 6 Bacteraemia |
| Price 2015 | 97 | 11 Probable pulmonary fungal | 9 Probable pulmonary fungal | 2 Pulmonary fungal 3 Sinus fungal 1 Skin/soft tissue fungal 2 Other fungal 5 Typhilitis 8 Invasive bacterial |
2 Pulmonary fungal 0 Sinus fungal 2 Skin/soft tissue fungal 4 Other fungal 2 Typhilitis 13 Invasive bacterial |
14 Bacteraemia 3 Candidaemia 0 Fungaemia (other) |
11 Bacteraemia 6 Candidaemia 2 Fungaemia (other) |
| Scali 1978 | 25 | 2 | 3 | 3 Pneumonia 5 Abscess 0 Other |
1 Pneumonia 0 Abscess 3 Other |
3 Bacteraemia | 5 Bacteraemia |
| Seidel 2008a | 67 (72 episodes) |
Probable fungal infection. Aspergillus 32, Aspergillus & Candida 1, Other 3 Unidentified infection 12 |
Detected fungal infection. Aspergillus 15, Candida 3, Both 1 Detected bacterial. Pseudomonas 2, Staph. aureus 1, Other (not defined) 2 |
||||
| Vogler 1977 | 30 | 0 | 0 | 7 (perirectal, skin, pulmonary, renal tract) |
4 Pseudomonas 8 Other |
3 Pseudomonas 8 Other |
|
| Winston 1982a | 95 | 0 | 0 | 4 Pneumonia 7 Celluluitis/abscess |
5 Pneumonia 3 Celluluitis/abscess |
12 E. coli 11 Klebsiella 4 Pseudomonas 0 Proteus 1 Gram negative (other) 4 Mixed gram‐negative 5 Gram‐positive |
10 E. coli 5 Klebsiella 12 Pseudomonas 1 Proteus 3 Gram negative (other) 5 Mixed gram‐negative 3 Gram‐positive |
Outcomes
Three trials re‐randomised participants or analysed the number of febrile episodes rather than the participants and were not included in the quantitative analysis (Alavi 1977; Herzig 1977; Seidel 2008a).
None of the studies examined exactly the same range of outcomes, however most trials reported mortality and information on type of infection (See Characteristics of included studies).
Excluded studies
We excluded 36 studies within 38 citations from the review because they failed to meet the stated eligibility criteria (Characteristics of excluded studies).
Nineteen studies were not randomised (Altrichter 2011; Atay 2011; Bhatia 1994; Blum 2001; Curtis 1977; Curtis 1982; Diaz 2014; Fortuny 1975; Granena 1978; Graw 1972; Graw 1977; Hershko 1978; Ikemoto 2012; Illerhaus 2002; Matsue 1984; NCT01932710; Oymak 2015; Stout 2015; Witt 2015).
Twelve studies were excluded because they assessed the prophylactic use of granulocyte transfusions (Adkins 1999; Clift 1978; Ford 1982; Gomez‐Villagran 1984; Mannoni 1979; NCT01204788; Oza 2006; Schiffer 1979; Strauss 1981; Sutton 1982; UMIN000014777; Winston 1982b).
Two studies compared two different types of granulocyte transfusions (Ambinder 1981; Freireich 2013).
Three publications were reviews (Pammi 2011; Strauss 2015; Yoshihara 2016).
Ongoing Studies
This updated review identified one ongoing study that was eligible for inclusion (DRKS00000218). This study plans to recruit 100 participants from three centres in Germany but has not yet opened to recruitment.
The previous systematic review (Stanworth 2005) identified three potentially relevant studies, one ongoing study that has now been completed and is included in the review (Seidel 2008a), and two studies that are now excluded, one non‐randomised study (Blum 2001), and one that compared the wrong intervention (Adkins 1999).
Risk of bias in included studies
See Figure 2 and Figure 3 for visual representations of the assessment of risk of bias across all studies and for each item in the included studies. See the Characteristics of included studies section 'Risk of bias tables' for further information about the bias identified within the individual studies.
2.
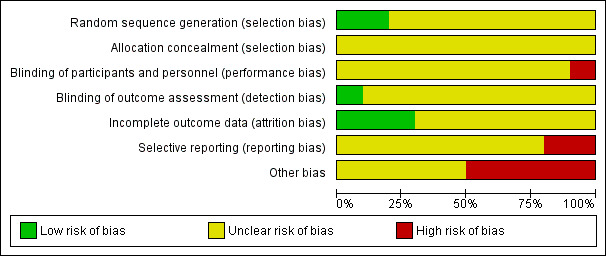
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
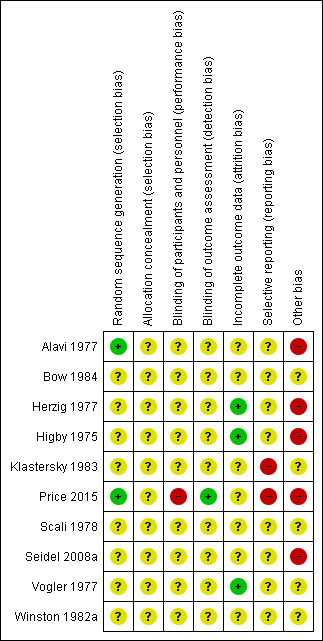
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All studies were at unclear risk of selection bias. Two studies were at low risk of bias due to random sequence generation (Alavi 1977; Price 2015). All other studies did not provide sufficient information to make an assessment and were considered at an unclear risk of bias. No studies explicitly reported allocation concealment, and therefore all 10 studies were at unclear risk of bias due to allocation concealment.
Blinding
One study was an open‐label study and was considered at high risk of performance bias (Price 2015). Nine studies did not provide any details on whether participants or investigators were blinded to the intervention, and therefore were considered at unclear risk of performance bias. However, owing to the nature of the intervention (granulocyte transfusion) and difficulty blinding participants and physicians it is highly likely that all studies were at high risk of performance bias.
One study had a blinded outcome assessment committee and was considered at low risk of detection bias (Price 2015). The other nine studies did not provide any details on whether outcome assessors were blinded to the intervention, and therefore were considered at unclear risk of detection bias.
Incomplete outcome data
There was also potential for bias in trial analysis, as not all the trials clearly reported reasons for withdrawals within the final analysis (Alavi 1977; Klastersky 1983; Vogler 1977). Details on these potential problems of attrition bias are stated in the summary sections for each trial, in the Characteristics of included studies. Moreover, in one trial it appears that the authors were confusing numbers of patients with numbers of infective/febrile episodes for some of the results data (Herzig 1977).
Selective reporting
Two studies were at high risk of reporting bias because protocols were available and not all outcomes were reported (Klastersky 1983; Price 2015). The other eight studies were at unclear risk of reporting bias because no protocols were available.
Other potential sources of bias
Due to the small numbers of participants in all the studies there is likely to be baseline imbalance between the study arms.
Effects of interventions
See: Table 1
Three studies either reported the data per infection episode (Alavi 1977) rather than per patient or re‐randomised patients (Herzig 1977; Seidel 2008a) and were excluded from the quantitative analysis.
All‐cause mortality up to 30 days (six studies, 321 participants)
Of the seven studies eligible for quantitative analysis, six studies reported information on overall mortality/survival (Bow 1984; Higby 1975; Scali 1978; Vogler 1977; Winston 1982a) or data could be extracted from Kaplan Mieier curves (Price 2015). Five studies reported mortality at 20 to 22 days (Bow 1984; Higby 1975; Price 2015; Scali 1978; Vogler 1977). The data in Winston 1982a were presented as survival/mortality at five days and undefined overall time points, and not at an equivalent time point between 20 to 22 days after randomisation.
A meta‐analysis showed no sub‐group differences between the data at five days and 20 to 22 days (test for subgroup differences: Chi² = 0.23, df = 1 (P = 0.63), I² = 0%). The overall result for all six studies showed no difference in all‐cause mortality up to 30 days in the participants receiving granulocyte transfusions and those that did not (risk ratio (RR) 0.75, 95% confidence interval (CI) 0.54 to 1.04, 321 participants) (Analysis 1.1).
1.1. Analysis.
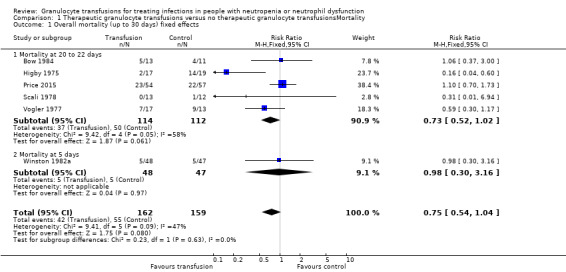
Comparison 1 Therapeutic granulocyte transfusions versus no therapeutic granulocyte transfusionsMortality, Outcome 1 Overall mortality (up to 30 days) fixed effects.
It should be noted that mortality rates for the control group varied considerably between studies. In the Higby 1975 trial, 20‐day mortality figures (after randomisation) in the control group were reported as 74% (14/19) whereas in Scali 1978 they were 8% (1/12). Part of the reason for this significant variation will be because of the small numbers involved in the included studies and the different inclusion and exclusion criteria.
Overall, in all six trials the mortality was 35%. We calculated that 2748 participants are required to have a 80% chance of detecting, as significant at the 5% level, a decrease in all‐cause mortality from 35% in the control group to 30% in the experimental group. We considered preventing five deaths per 100 people treated with granulocyte transfusions was a clinically significant difference. There are insufficient participants within the included trials (324 participants) to detect a difference in all‐cause mortality.
Clinical reversal of concurrent infection (five studies, 286 participants)
Of the seven studies eligible for quantitative analysis, five studies reported information on clinical reversal of concurrent infections (Bow 1984; Klastersky 1983; Price 2015; Scali 1978; Vogler 1977). The definitions of what constituted a clinical reversal of concurrent infection differed between studies (Table 4). Three studies included both partial or temporary resolution of infection (Klastersky 1983; Price 2015; Vogler 1977), one study included only complete resolution of infection (Winston 1982a), and one study did not define clinical reversal of concurrent infection further (Scali 1978). A meta‐analysis showed no difference between participants receiving therapeutic granulocyte transfusions and those that did not (RR 0.98, 95% CI 0.81 to 1.19) (Analysis 1.2).
3. Study definitions of resolution of infection.
| Study | Study definitions of resolution of infection | |
| Klastersky 1983 | Improved ‐ Lasting return of temperature, signs and symptoms to normal or to pre‐infectious state. | Temporarily improved ‐ As for "Improved" but with relapse in 3‐6 days despite continuing antibiotic therapy. |
| Price 2015 | Participant had to meet two criteria: 1. survival for 42 days after randomisation 2. clinical response of the study‐qualifying infection assessed at 42 days.
|
|
| Scali 1978 | Not further defined | |
| Vogler 1977 | Complete resolution of infectious episode: all evidence of infection must clear within the 21‐day period. Measurable parameters included resolution of fever, conversion of cultures to negative and healing of involved sites. | Partial resolution: less than complete clearing but definite clinical improvement in more than one measurable parameter for the 21‐day period. |
| Winston 1982a |
Resolution of septicaemia ‐ afebrile, antimicrobials have been stopped, and follow‐up blood cultures were negative Resolution of pneumonia ‐ afebrile, clinical and radiological improvement, and antimicrobials have been stopped Resolution of cellulitis or an abscess ‐ afebrile, no remaining localising signs of an infection, and antimicrobials have been stopped |
|
1.2. Analysis.
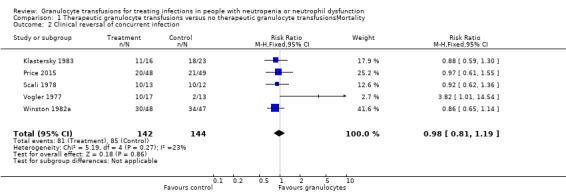
Comparison 1 Therapeutic granulocyte transfusions versus no therapeutic granulocyte transfusionsMortality, Outcome 2 Clinical reversal of concurrent infection.
In Price 2015, the response to infection with antimicrobials alone was 43% (21/49). We calculated that 642 patients are required to have a 80% chance of detecting, as significant at the 5% level, an increase in recovery from the infection from 43% in the control group to 54% in the experimental group (25% increase in recovery from infection). There are insufficient participants within the included trials (286 participants) to detect a difference in reversal of concurrent infections. We chose a 25% increase in recovery from infection because we felt this was a clinically significant difference that would lead to a change in practice if there were no significant side effects.
Length of time with fever (three studies, 144 participants)
Three studies reported the length of time with fever (Table 5). The format of the data for this outcome differed between the three studies, and was not combined. Two of the three trials reported a lower mean number of days with fever in the transfused compared to the control group.
4. Length of time with fever.
| Study | Definition of febrile day | Number of participants | Number of febrile days (Granulocyte transfusions) |
Number of febrile days (Control) |
| Bow 1984 | Not reported | 24 | Mean 9.1 SD ± 3.0 |
Mean 12.9 SD ± 7.0 |
| Higby 1975 | Not reported | 36 | All 16 patients who survived to day 10 were afebrile | All 11 patients who survived to day 10 were febrile. All 5 patients who survived to day 20 were febrile |
| Scali 1978 | Fever over 38 ° C in minimal 3 of 4 measurements within 24 hours | 25 | Mean 4.9 SD 5 |
Mean 8.3 SD 4 |
| Winston 1982a | Not reported | 97 | Median 8 (range 1 to 36 days) |
Median 5 (range 1 to 34 days) |
SD: standard deviation
Days on antimicrobials (no studies)
No studies reported numbers of days on antimicrobials.
Increment of neutrophil count and duration of neutropenia reversal after transfusion
Increment of neutrophil count (five studies)
Data on corrected one‐hour granulocyte increments were presented in five trials ( Alavi 1977; Herzig 1977; Higby 1975; Vogler 1977; Winston 1982a) (Table 6). These results need to be interpreted in the light of the method of granulocyte procurement.
5. Increment of neutrophil count.
| Study | Number of participants | Total number of transfusions (Number of transfusions increment reported if differed) | Type of collection | Corrected Count Increment (Range) |
Absolute neutrophil Increment (1 hour) Mean (SD) |
| Alavi 1977 | 31 | 176 (48) |
Filtration leukapheresis | mean 0.13 x 109/L (0 to 0.67) |
Not reported |
| Herzig 1977 | 27 | 197 | 129 ‐ leukapheresis 68 ‐ centrifugation |
leukapheresis 0 x 109/L (0 to 0.40) centrifugation 0.13 x 109/L (0 to 0.90) |
Not reported |
| Higby 1975 | 36 | 68 | Filtration leukapheresis | 0.26 x 109/L (0.05 to 3.00) |
Not reported |
| Price 2015 | 97 | 316 (209) |
Continuous flow centrifugation | Not reporteda | Not reported* |
| Scali 1978 | 25 | 32 | Continous flow centrifugation | Not reported | 0.68 ± 0.33 x 109/L |
| Seidel 2008a | 67 | Not reported | Continous flow centrifugation | Not reported | 0.48 x 109/L |
| Vogler 1977 | 30 | 62b | Continuous flow centrifugation | Not reportedc | Not reportedc |
| Winston 1982a | 95 | Not reported | Discontinuous flow centrifugation | 89 (0 to 841) |
Not reported |
aIn a generalised linear model for post‐transfusion absolute neutrophil count increment performed by the study authors, including both granulocyte dose/kg and time from product collection to administration, each additional 109 cells/kg administered was associated with an additional 1.75 x 109/L neutrophil count increment (P < 0.001) and each additional hour from collection to transfusion was associated with a 0.08 x 109/L lower increment (P = 0.056).
bDerived from figure 3 in the study publication (Vogler 1977).
cA scattergram showed a positive correlation between the number of granulocytes transfused and 1 hour increments but the corrected count increment was not reported.
Duration of neutropenia reversal (neutropenia defined as count below 0.5 x 109/litre) (no studies)
No studies reported duration of neutropenia reversal.
Adverse events
Only one study reported adverse events in both study arms (Bow 1984). There was no evidence of a difference in the number of participants who developed pulmonary complications (RR 0.85, 95% CI 0.38 to 1.88, 24 participants) (Analysis 1.3).
1.3. Analysis.
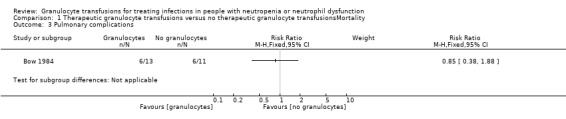
Comparison 1 Therapeutic granulocyte transfusions versus no therapeutic granulocyte transfusionsMortality, Outcome 3 Pulmonary complications.
Adverse events following granulocyte transfusions were reported in six studies (Alavi 1977; Herzig 1977; Higby 1975; Price 2015; Vogler 1977; Winston 1982a).
Serious adverse events
There was no difference on the number of pulmonary adverse events between study arms
Three studies reported serious adverse events in the granulocyte transfusion recipient (Alavi 1977; Higby 1975; Price 2015) (Table 7). One study reported no episodes (Higby 1975) and two studies each reported one episode (Alavi 1977; Price 2015).
6. Serious adverse events related to donor or recipients.
| Study | Number of participants receiving granulocyte transfusions | Number of granulocyte transfusions | Donor events | Recipient events |
| Alavi 1977 | 12 | 176 | Not reported | 1 Laryngospasm |
| Higby 1975 | 17 | 68 | 0 | 0 |
| Price 2015 | 56 | 316 | Not reported | 1 Hypoxia requiring ventilatory support |
One study reported no serious adverse events in granulocyte donors (Higby 1975) (Table 7).
Adverse events requiring discontinuation of treatment.
No studies reported adverse events that required discontinuation of treatment.
Other adverse events e.g. flu‐like symptoms, bone pain
Six studies reported adverse events in recipients associated with the granulocyte transfusions (Alavi 1977; Herzig 1977; Higby 1975; Price 2015; Vogler 1977; Winston 1982a) (Table 8).
7. Other adverse events.
| Study | Number of participants receiving granulocyte transfusions | Donor events | Recipient events |
| Alavi 1977 | 12 | Not reported | 2 participants developed urticaria 28/176 (16%) transfusions associated with fever 12/176 (7%) of transfusions associated with chills but no fever |
| Herzig 1977 | 15 | Not reported | < 10/68 (15%) obtained by continuous flow centrifugation > 96/129 (75%) obtained by leukapheresis were associated with transfusion reactions (fevers or chills) |
| Higby 1975 | 17 | 14/67 faintness 1/67 chills 4/67 nausea 2/67 fainted 1/67 arm pain |
40/68 transfusions associated with fever |
| Price 2015 | 56 | Not reported | 41% of participants had mild to moderate reactions (grade 1 to 2) ‐ fever, chills or changes in blood pressure 9 more severe reactions (grade 3 to 4), 6 hypoxia, 1 tachycardia, 1 hypotension, 1 allergic reactions |
| Vogler 1977 | 32 | 2 chills 3 periorbital tingling 3 faintness 2 headache |
No dyspnoea No febrile reactions |
| Winston 1982a | 48 | Not reported | 19 participants, 12 had fever and chills; 2 had fever, chills and a rash; 1 rash only; 4 dyspnoea |
Two studies reported adverse events in granulocyte donors (Higby 1975; Vogler 1977) (Table 8).
Of the three collecting granulocytes by filtration leukapheresis (Alavi 1977; Herzig 1977; Higby 1975), adverse events occurred in 23% of participants (mostly fever and chills, Alavi 1977) and up to 75% of transfusions ('untoward', Herzig 1977). In the study collecting granulocytes by discontinuous flow centrifugation (Winston 1982a), adverse events occurred in 40% of participants (mostly febrile reactions). Of the three studies collecting granulocytes by continuous flow centrifugation (Herzig 1977; Price 2015; Vogler 1977), mild to moderate adverse events occurred in up to 41% of participants (Price 2015) and more severe events occurred in nine participants (Price 2015) (Table 8).
Further subgroup analyses
In addition to the subgroup analysis of studies that collected and transfused average numbers of granulocytes that were greater than 1 x 1010 (shown above), it was also planned to undertake sensitivity analyses, as defined a priori in the methods, for the following subgroups:
those three studies where granulocytes were collected by filtration leukapheresis (Alavi 1977; Herzig 1977; Higby 1975);
those three studies where collections for transfusion were undertaken without any prior assessment of leucocyte compatibility (Alavi 1977; Price 2015; Winston 1982a).
As the mortality data in the Alavi study was recorded by febrile episodes, it was considered that the results of these sensitivity analyses would not be meaningful in view of the lack of relevant data.
Subgroup analyses
Average dose of granulocytes transfused
All‐cause mortality
When we excluded data from the two trials that collected and transfused average numbers of granulocytes below 1 x 1010 (Bow 1984; Winston 1982a), the four remaining trials (202 participants) (Higby 1975; Price 2015; Scali 1978; Vogler 1977), showed that participants receiving granulocyte transfusions may have a lower mortality than those not receiving granulocyte transfusions (RR 0.70; 95% CI 0.49 to 0.99) (Analysis 2.1; Figure 4). There was no evidence of a difference between the granulocyte dose subgroups (test for subgroup differences: Chi² = 0.73, df = 1 (P = 0.39), I² = 0%).
2.1. Analysis.
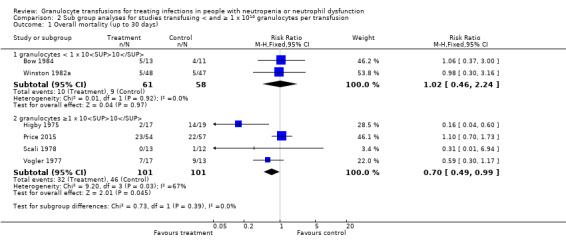
Comparison 2 Sub group analyses for studies transfusing < and ≥ 1 x 1010 granulocytes per transfusion, Outcome 1 Overall mortality (up to 30 days).
4.
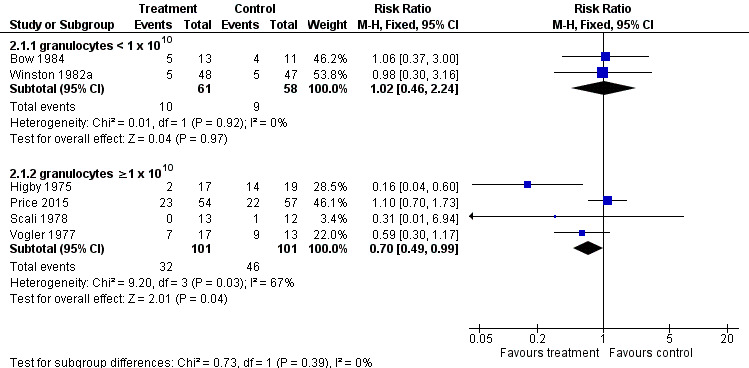
Forest plot of comparison: 2 Sub group analyses for studies transfusing < and ≥ 1 x 1010 granulocytes per transfusion, outcome: 2.1 Overall mortality (up to 30 days).
Clinical reversal of concurrent infections
When we excluded data from the one trial that collected and transfused average numbers of granulocytes below 1 x 1010 (Winston 1982a), the four remaining trials (191 participants) (Klastersky 1983; Price 2015; Scali 1978; Vogler 1977), showed that there may be no difference in clinical reversal of concurrent infections in those recipients receiving granulocyte transfusions compared to those not receiving granulocyte transfusions (RR 1.07; 95% CI 0.82 to 1.39) (Analysis 2.2). There was no evidence of a difference between the granulocyte dose subgroups (test for subgroup differences: Chi² = 1.15, df = 1 (P = 0.28), I² = 13.3%).
2.2. Analysis.
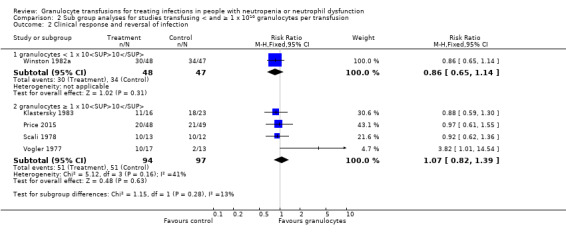
Comparison 2 Sub group analyses for studies transfusing < and ≥ 1 x 1010 granulocytes per transfusion, Outcome 2 Clinical response and reversal of infection.
Year of publication
All‐cause mortality
Only one trial published after 2000 (Price 2015) had mortality data available. This study showed no difference in overall mortality between those participants who received granulocyte transfusions and those that did not (RR 1.10; 95% CI 0.70 to 1.73, 111 participants) (Analysis 3.1). The five trials published before 2000 showed that there may be a reduction in overall mortality between those participants that received granulocyte transfusions and those that did not (RR 0.53; 95% CI 0.33 to 0.85) (Analysis 3.1). There was evidence that there may be a subgroup difference between newer and older studies (test for subgroup differences: Chi² = 4.84, df = 1 (P = 0.03), I² = 79.4%).
3.1. Analysis.
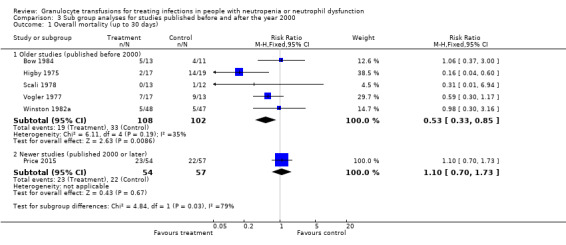
Comparison 3 Sub group analyses for studies published before and after the year 2000, Outcome 1 Overall mortality (up to 30 days).
Clinical response to infections
Only one trial published after 2000 (Price 2015) had data available. This study showed no difference in clinical response to infection between those participants who received granulocyte transfusions and those that did not (RR 0.97; 95% CI 0.61 to 1.55) (Analysis 3.2). The four trials published before 2000 also showed no difference in clinical response to infection between those participants who received granulocyte transfusions and those that did not (RR 0.99; 95% CI 0.80 to 1.21) (Analysis 3.2). There was no evidence of a subgroup difference between newer and older studies (test for subgroup differences: Chi² = 0.00, df = 1 (P = 0.96), I² = 0%).
3.2. Analysis.
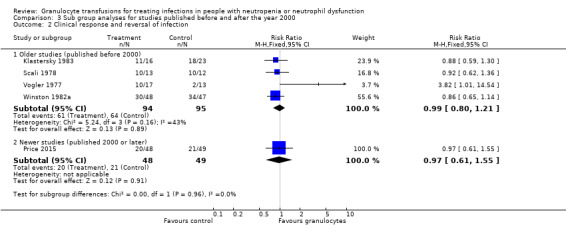
Comparison 3 Sub group analyses for studies published before and after the year 2000, Outcome 2 Clinical response and reversal of infection.
Discussion
Summary of main results
This Cochrane review aimed to evaluate the literature on the effectiveness and safety of therapeutic granulocyte transfusions. This review should be read in conjunction with another review undertaken to evaluate the randomised trial evidence for the use of prophylactic granulocyte transfusions (Estcourt 2015).
We identified 10 completed randomised controlled trials (RCTs) that met our inclusion criteria and one additional ongoing study (DRKS00000218). Ten trials were included in this review containing a total of 587 participants. None of the trials enrolled people with neutrophil dysfunction. Three studies re‐randomised participants and therefore quantitative analysis was unable to be performed.
There was insufficient evidence to detect a difference in all‐cause mortality between participants receiving therapeutic granulocyte transfusions and those that did not (six studies; 321 participants; RR 0.75, 95% CI 0.54 to 1.04).
Unlike the previous version of this review, we found that there were no differences between the granulocyte dose subgroups (< 1 x 1010 per day versus ≥ 1 x 1010 per day) (test for subgroup differences P = 0.39). We did find that there was a difference between the studies based on the age of the study (published before 2000 versus published 2000 or later) (test for subgroup differences P = 0.03). We found that there was no difference in all‐cause mortality between participants receiving granulocyte transfusions and those that did not in the newest study (one study; 111 participants; RR 1.10, 95% CI 0.70 to 1.73). The newest study was also the only study in the meta‐analysis that gave granulocyte colony stimulating factor (G‐CSF) to donors. We noted that there may be a reduction in all‐cause mortality in participants receiving granulocyte transfusions compared to those that did not in studies published before the year 2000 (five studies; 210 participants; RR 0.53, 95% CI 0.33 to 0.85; low‐quality evidence).
We found that there may be no difference in clinical reversal of concurrent infections between participants receiving therapeutic granulocyte transfusions and those that did not (five studies; 286 participants; RR 0.98, 95% CI 0.81 to 1.19; low‐quality evidence).
There is insufficient evidence to determine whether there is a difference in pulmonary serious adverse events between participants receiving therapeutic granulocyte transfusions and those that did not (one study; 24 participants; RR 0.85, 95% CI 0.38 to 1.88).
None of the studies reported number of days on therapeutic antibiotics, number of adverse events requiring discontinuation of treatment, or quality of life.
Overall completeness and applicability of evidence
This review provides the most up to date assessment of the effectiveness and safety of a therapeutic granulocyte transfusion policy compared with not administering granulocyte transfusions. This updated review identified two additional completed trials (Price 2015; Seidel 2008a) and one ongoing trial (DRKS00000218).
The results of this review should not be interpreted without taking into consideration the impact of the following factors.
None of the trials in this review specifically evaluated patients with congenital disorders of neutrophil function or production, although many clinicians might consider this an accepted practice to manage severe or refractory infections in this group of patients.
The one ongoing study (expected recruitment 100 participants) will be too small to provide sufficient additional data for this review’s primary outcome. For example, if we assumed that the risk of death was 35 out of 100 people with neutropenia who had a serious infection treated with antibiotics alone and that the risk of death decreased to 30 out of 100 people when they received therapeutic granulocyte transfusions as well as antibiotics, we would need to design a study with at least 2748 participants to detect this difference with 80% power and 5% significance (3680 participants required to detect a difference with 90% power) (calculated using a power calculator at http://www.sealedenvelope.com/power/binary‐superiority/).
None of the studies assessed the use of granulocytes derived from whole blood donations (Bashir 2008). This component has been assessed in a small safety study (Massey 2012). The process of obtaining granulocyte collections from directed G‐CSF and/ or steroid‐stimulated donors who are ’family and friends’ of patients or unrelated donors involves multiple steps. It is important that family and ’friends’ of patients are given time and an adequate explanation of the small risks to which they are exposed by both taking specific drugs (steroids or G‐CSF, or both) to mobilise granulocytes into the peripheral blood and by undergoing an apheresis procedure. To date, most of these risks have been theoretical or weak associations only but posterior capsular cataracts, splenic rupture and venous thrombosis have been described (Bennett 2006; Ghodsi 2001; Goldman 2006; Gutierrez 2001). There are also a number of potentially important constraints that can limit provision of apheresis products on a regular and timely basis; e.g. hospitals in Europe managing granulocyte collections by apheresis now have a requirement for meeting ’blood establishment status’ according to EU legislation.
Only two of the studies gave G‐CSF to the granulocyte donors (Price 2015; Seidel 2008a), both studies were stopped early due to lack of recruitment.
The effect of G‐CSF on donors was not reported and other side effects for granulocyte donors was only reported for two studies (Table 7; Table 8).
The studies included in this review range over a 40‐year period (1975 to 2015) during which chemotherapy protocols, predicted survival rates, supportive care, including antibiotics and anti‐fungal medication, have changed substantially. Newer less toxic anti‐fungal drug options are now available.
Data from three of the included studies could not be included in the meta‐analysis due to re‐randomisation of participants (Alavi 1977; Herzig 1977; Seidel 2008a).
The types of infection included within the trials varied (Table 3). In two studies participants had to have positive microbiological cultures (Herzig 1977; Vogler 1977). Seven studies permitted a 'possibility' of infection (Alavi 1977; Bow 1984; Higby 1975; Klastersky 1983; Price 2015; Scali 1978; Seidel 2008a), the exact definition of which varied between the studies. In Bow 1984 people with a definite infection were excluded from the randomised part of the study.
Quality of the evidence
Overall, the quality of the evidence was rated as very low to low across different outcomes according to GRADE methodology (Table 1). This was due to many of the outcome estimates being imprecise and many of the studies being at high risk of bias. Although most studies were at an unclear risk of bias due to blinding due to lack of information it is likely that these were all unblinded studies due to the difficultly in blinding participants and physicians to the intervention.
One outcome was graded low‐quality evidence because of a serious risk of imprecision and a serious risk of bias.
Clinical reversal of concurrent infections
Two outcomes were considered very low‐quality evidence because of a very serious risk of imprecision, a serious risk of bias, and a serious risk of inconsistency.
All‐cause mortality
Number of serious adverse events
The reason for the imprecision is because of the small number of participants within the trials and the low number of events. The reason for inconsistency for all‐cause mortality is because the effect of granulocyte transfusions appears to differ between newer and older studies.
See Figure 2 and Figure 3 for visual representations of the assessments of risk of bias across all studies and for each item within the individual studies.
Potential biases in the review process
To our knowledge, our review process is free from bias. We conducted a comprehensive search, searching data sources (including multiple databases, and clinical trial registries) to ensure that all relevant trials would be captured. The relevance of each paper identified was carefully assessed and all screening and data extractions were performed in duplicate. There were no restrictions on the language in which the paper was originally published. We prespecified all outcomes and subgroups prior to analysis. There were insufficient numbers of included studies within the meta‐analyses for us to use a funnel plot to examine the risk of publication bias.
Agreements and disagreements with other studies or reviews
We know of no other recent systematic reviews on the use of therapeutic granulocyte transfusions in people with neutropenia or neutrophil dysfunction. The last review on this subject was the previous version of this review (Stanworth 2005). This review no longer finds an overall benefit of therapeutic granulocyte transfusions on all‐cause mortality; this differs from the previous version of this review.
Authors' conclusions
Implications for practice.
There is insufficient evidence from randomised controlled trials (RCTs) to support or refute the use of granulocyte transfusion therapy in patients with neutropenia and severe infection to reduce mortality. None of the trials in this review specifically evaluated patients with congenital disorders of neutrophil function or production, although many clinicians might consider this as accepted practice to manage severe infection. None of the studies in this review assessed the use of granulocytes derived from whole blood donations. In keeping with the conclusions from the systematic review of the use of prophylactic granulocyte transfusions, the use of granulocyte transfusions should still be regarded as investigational and should ideally be conducted in the context of ongoing prospective trials designed to answer the question of effectiveness.
Implications for research.
Contemporary well‐designed prospective trials of sufficient power are required to evaluate the efficacy of granulocyte transfusions, in order to establish definitively whether it has clinical benefit or not with regards to reduction in mortality. The one ongoing trial is too small (100 participants) to be able to answer this review's primary outcome.
Side effects need to be evaluated not only for the transfused recipient but also with respect to donors.
What's new
| Date | Event | Description |
|---|---|---|
| 17 July 2018 | Review declared as stable | One ongoing study (DRKS00000218) was identified previously but stopped before recruitment started. No other new studies expected on this topic. |
History
Protocol first published: Issue 1, 2004 Review first published: Issue 3, 2005
| Date | Event | Description |
|---|---|---|
| 11 February 2016 | New citation required and conclusions have changed | There is no longer a difference in overall mortality between participants who received granulocyte transfusions compared to those that did not with the additional data from Price 2015. 'Risk of bias' assessment of all included studies updated. 'Summary of findings' table added. PRISMA flow diagram added (Stovold 2014). |
| 11 February 2016 | New search has been performed | New search, three new included trials were added, two completed studies were identified in the updated search (Price 2015; Seidel 2008a), and one ongoing study was identified (DRKS00000218) |
| 8 June 2010 | New search has been performed | Searches run in May 2009. One small Phase III RCT identified with poor recruitment (yet to be classified). A much larger on‐going trial also identified. The review will be fully updated when data from this large multi‐centre trial is available. |
| 14 October 2008 | Amended | Converted to new review format. |
| 20 May 2005 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
P Blanco who assisted with the selection of studies.
M Murphy, D Pamphilon, D Roberts, T Wallington, L Williamson for comments on the protocol and review.
S Brunskill, C Hyde, C. Navarrete, G Lucas, D Marks, U Paulus who were authors on the previous version of this review.
The authors and the Cochrane Gynaecological Neuro‐oncolgy and Orphan Cancer Group would like to thank and acknowledge the assistance of the Cochrane Infectious Diseases Group, with whom this title and protocol were originally registered and published, supported by a grant from the Department for International Development, UK. The review was transferred in March 2005.
We thank the National Institute of Health Research (NIHR). This review is part of a series of reviews that have been funded by the NIHR Cochrane Programme Grant ‐ Safe and Appropriate Use of Blood Components. This research was also supported by the NIHR Oxford Biomedical Research Centre Programme.
This project was also supported by the NIHR, via Cochrane Infrastructure funding to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group.
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. CENTRAL search strategy (2008 to 2016)
#1 MeSH descriptor: [Granulocytes] explode all trees #2 transfus* #3 #1 and #2 #4 MeSH descriptor: [Granulocytes] explode all trees and with qualifier(s): [Transplantation ‐ TR] #5 MeSH descriptor: [Leukocyte Transfusion] this term only #6 (buffy coat* or leukocyt* or leucocyt* or neutrophil* or white blood cell* or white cell* or eosinophil* or basophil*) near/5 (transfus* or infus*) #7 (granulocyte* near/5 (transfus* or infus* or concentrate*)) #8 #3 or #4 or #5 or #6 or #7
Appendix 2. MEDLINE (Ovid) search strategy (2008 to 2016)
1. exp Granulocytes/ and transfus*.mp. 2. (granulocyte* or buffy coat* or leukocyt* or leucocyt* or white blood cell* or white cell* or neutrophil* or eosinophil* or basophil*) adj concentrate*)).tw. 3. ((granulocyte* or buffy coat* or leukocyt* or leucocyt* or white blood cell* or white cell* or neutrophil* or eosinophil* or basophil*) adj5 (transfus* or infus*)).tw. 4. exp Granulocytes/tr 5. Leukocyte Transfusion/ 6. 1 or 2 or 3 or 4 or 5
Appendix 3. Embase (Ovid) search strategy (2008 to 2016)
1. (granulocyte* or buffy coat* or leukocyt* or leucocyt* or white blood cell* or white cell* or neutrophil* or eosinophil* or basophil*) adj concentrate*)).tw. 2. ((granulocyte* or buffy coat* or leukocyt* or leucocyt* or white blood cell* or white cell* or neutrophil* or eosinophil* or basophil*) adj5 (transfus* or infus*)).tw. 3. Leukocyte Transfusion/ 4. exp Granulocyte/ and transfus*.mp. 5. Granulocyte Transfusion/ 6. or/1‐5
Appendix 4. CINAHL (NHS Evidence) search strategy (2008 to 2016)
S1 (MH "Granulocytes+") S2 TX transfus* S3 S1 AND S2 S4 TI (((granulocyte* or buffy coat* or leukocyt* or leucocyt* or white blood cell* or white cell* or neutrophil* or eosinophil* or basophil*) N1 concentrate*)) OR AB (((granulocyte* or buffy coat* or leukocyt* or leucocyt* or white blood cell* or white cell* or neutrophil* or eosinophil* or basophil*) N1 concentrate*)) S5 TI (((granulocyte* or buffy coat* or leukocyt* or leucocyt* or white blood cell* or white cell* or neutrophil* or eosinophil* or basophil*) N5 (transfus* or infus*))) OR AB ( ((granulocyte* or buffy coat* or leukocyt* or leucocyt* or white blood cell* or white cell* or neutrophil* or eosinophil* or basophil*) N5 (transfus* or infus*))) S6 (MH "Granulocytes+/TR") S7 S3 OR S4 OR S5 OR S6
Appendix 5. LILACS search strategy (2008 to 2016)
tw:(granulocyte OR granulocytes OR basophil OR basophils OR eosinophil OR eosinophils OR white cell transfusion OR white blood cell transfusion) AND ( db:("LILACS") AND type_of_study:("clinical_trials"))
Appendix 6. KoreaMed and PakMediNet search strategy (2008 to 2016)
granulocyte* [TI] AND transfus* [ALL] OR white cell*[TI] AND transfus*[ALL] OR white blood cell*[TI] AND transfus*[ALL]
Appendix 7. IndMed search strategy (2008 to 2016)
(granulocyte OR granulocytes OR white cell OR white cells OR white blood cell OR white blood cells OR leucocyte OR leucocytes OR leukocyte OR leukocytes) AND (transfusion OR transfused OR transfusions OR transfusing) AND (randomised OR randomized OR randomly OR blind OR blinded OR trial OR allocation OR allocated OR assigned OR control group OR controlled study OR intervention)
Appendix 8. Transfusion Evidence Library search strategy (2008 to 2016)
title:granulocyte OR title:granulocytes OR keywords:granulocyte OR keywords:granulocytes OR keywords:"white cell transfusion" OR keywords:"white blood cell transfusion" OR keywords:"leukocyte transfusion"
Appendix 9. ClinicalTrials.gov & ICTRP Search Strategy (2008 to 2016)
Search Terms: granulocyte transfusion OR Intervention: granulocyte transfusion OR white cell transfusion OR leukocyte transfusion OR granulocytes OR white cells OR leukocytes
Appendix 10. ISRCTN search strategy (2008 to 2016)
(leukocyte% OR leucocyte% OR granulocyte% OR white blood cell% OR white cell% OR basophil% OR eosinophil%) AND transfus% OR granulocyte transfusion% OR white cell transfusion% OR white blood cell transfusion% OR leucocyte transfusion%
Appendix 11. Original search strategies (Stanworth 2005)
CENTRAL Search Strategy
#1 LEUKOCYTE TRANSFUSION explode all trees (MeSH) #2 GRANULOCYTES explode all trees (MeSH) #3 BLOOD TRANSFUSION explode all trees (MeSH) #4 #2 AND #3 #5 (granulocyt* OR buffy coat OR leukocyt* OR leucocyt* OR neutrophil OR white blood cell*) NEAR (transfus* OR infus*) #6 #1 OR #4 OR #5 #7neutrop* OR leukop* OR leukocyto* OR leucop* OR granulop* OR agranulocyto* OR granulocyto* OR prophyla* OR prevent* OR infect* #8 #6 AND #7
MEDLINE Search Strategy
1. LEUKOCYTE‐TRANSFUSION#.DE. 2. GRANULOCYTES#.DE. AND BLOOD‐TRANSFUSION#.DE. 3. GRANULOCYT$2 NEAR (TRANSFUS$4 OR INFUS$4) 4. BUFFY ADJ COAT NEAR (TRANSFUS$4 OR INFUS$4) 5. LEUKOCYT$2 NEAR (TRANSFUS$4 OR INFUS$4) 6. LEUCOCYT$2 NEAR (TRANSFUS$4 OR INFUS$4) 7. NEUTROPHIL$2 NEAR (TRANSFUS$4 OR INFUS$4) 8. WHITE ADJ BLOOD ADJ CELL$1 NEAR (TRANSFUS$4 OR INFUS$4) 9. 1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 10. NEUTROP$5 OR GRANULOCYTOP$5 OR GRANULOP$5 OR AGRANULOCYTOP$5 OR PROPHYLA$8 OR INFECT$4 OR PREVENT$4 11. 9 AND 10
Embase Search Strategy
1. GRANULOCYTE#.MJ. AND BLOOD‐TRANSFUSION#.MJ. 2. GRANULOCYTE‐TRANSFUSION.DE. 3. LEUKOCYTE‐TRANSFUSION.DE. 4. GRANULOCYT$2 NEAR TRANSFUS$4 5. BUFFY ADJ COAT NEAR TRANSFUS$4 6. LEUKOCYT$2 NEAR TRANSFUS$4 7. LEUCOCYT$2 NEAR TRANSFUS$4 8. NEUTROPHIL$2 NEAR TRANSFUS$4 9. WHITE ADJ BLOOD ADJ CELL$1 NEAR TRANSFUS$4 10. 1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 11. NEUTROP$5 OR GRANULOCYTONEUTROP$5 OR GRANULOCYTOP$5 OR GRANULOP$5 OR AGRANULOCYTOP$5 OR PROPHYLA$8 OR INFECT$4 OR PREVENT$4 12. 10 AND 11
Data and analyses
Comparison 1. Therapeutic granulocyte transfusions versus no therapeutic granulocyte transfusionsMortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall mortality (up to 30 days) fixed effects | 6 | 321 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.54, 1.04] |
| 1.1 Mortality at 20 to 22 days | 5 | 226 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.52, 1.02] |
| 1.2 Mortality at 5 days | 1 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.30, 3.16] |
| 2 Clinical reversal of concurrent infection | 5 | 286 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.81, 1.19] |
| 3 Pulmonary complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 2. Sub group analyses for studies transfusing < and ≥ 1 x 1010 granulocytes per transfusion.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall mortality (up to 30 days) | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 granulocytes < 1 x 1010 | 2 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.46, 2.24] |
| 1.2 granulocytes ≥1 x 1010 | 4 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.49, 0.99] |
| 2 Clinical response and reversal of infection | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 granulocytes < 1 x 1010 | 1 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.65, 1.14] |
| 2.2 granulocytes ≥ 1 x 1010 | 4 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.82, 1.39] |
Comparison 3. Sub group analyses for studies published before and after the year 2000.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall mortality (up to 30 days) | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Older studies (published before 2000) | 5 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.33, 0.85] |
| 1.2 Newer studies (published 2000 or later) | 1 | 111 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.70, 1.73] |
| 2 Clinical response and reversal of infection | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Older studies (published before 2000) | 4 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.80, 1.21] |
| 2.2 Newer studies (published 2000 or later) | 1 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.61, 1.55] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alavi 1977.
| Methods | Parallel RCT (period of enrolment not reported) (number of centres not reported) USA | |
| Participants |
Inclusion criteria: Patients with acute leukaemia or blastic phase of chronic myeloid leukaemia if the neutrophil count was < 0.25 x 109/L, and if infection was documented or strongly suspected. The eligibility criteria for children also required culture‐positive microbiology results. Exclusion criteria: No patient was eligible unless he or she was under treatment for acute leukaemia N = 32 patients, 31 included in the analysis Age range 8 to 75 years (Average 38 years) Arm 1 (Granulocyte transfusions): N = 13, 12 included in the analysis, Acute myeloid leukaemia = 8, Acute lymphocytic leukaemia = 3, Other = 1. Arm 2 (Control): N = 19, Acute myeloid leukaemia = 14, Acute lymphocytic leukaemia = 4, Other = 1 Type of treatment participants received for the acute leukaemia was not reported |
|
| Interventions |
Granulocyte dose: 5 x 1010/day. Average 3.3 x 1010/m2 children; Average 3.2 x 1010/m2 adults Granulocyte method of collection: Filtration leukapheresis Selection of donors: HLA typing results were not used to select prospective donors. Pre‐medication of donors: Hydrocortisone Initiation of granulocyte transfusions: Fever > 38.80C for 1 hour or persistent fever 38.00C for 24 hours Frequency of granulocyte transfusions: Daily Termination of granulocyte transfusions: Until antibiotics discontinued or until the patient was afebrile for 72 hours with negative cultures |
|
| Outcomes |
Primary Outcome(s): Not reported Secondary Outcome(s): Survival at day 21 Adverse events |
|
| Definition(s) of infection |
Proven infection: Cultures were positive Probable infection: If there was a clinical source but cultures were negative. |
|
| Definition of neutropenia | Neutrophil count <0.25 x 109/L was an inclusion criterion | |
| Co‐interventions |
Therapeutic antibiotics: All patients received broad‐spectrum antibiotics, including gentamicin and cephalothin, or a penicillinase‐resistant, semisynthetic penicillin, and most patients also received carbenicillin. Clindamycin was added if a gastrointestinal source of infection seemed likely. G‐CSF: G‐CSF not licensed by the Food and Drug Administration (FDA) until 1991. Therapeutic antifungals: Not reported |
|
| Notes |
Randomised: Patients were not randomised until antibiotics were started for presumed or proven infection Trial registration: none identified Sources of funding: Supported by a research grant (CA11630) and research career development awards (A100143 to RKR and AM38345 to RAC) from the National Institutes of Health and by a grant from the Radiation Management Corporation. Dr Schreiber is a Leukemia Scholar of the Leukemia Society of America Conflicts of Interest: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to control or transfusion groups by means of consecutively numbered cards, randomised by computer. The method of randomisation by the computer was not stated. |
| Allocation concealment (selection bias) | Unclear risk | The consecutively numbered cards were sealed in an envelope, but it did not state whether the envelopes were opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The report did not state whether participants were blinded to the intervention. The report did not state whether the clinical personnel or investigators were blinded to the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The report did not state whether the outcome assessors were blinded to the participants allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Two febrile episodes were excluded from analysis because of early death before the effects of the transfusions could be analysed: one patient in the transfusion group was excluded for this reason, but the control patient had also contributed to a prior febrile episode. In the transfusion group, one febrile episode was subsequently considered to be due to an allergic reaction and another due to viral infection, and these data were also excluded from the analysis of outcomes, including survival. |
| Selective reporting (reporting bias) | Unclear risk | The protocol was not available to assess whether any of the pre‐specified outcomes were not reported, or whether additional outcomes that had not been pre‐specified were reported |
| Other bias | High risk | There was an imbalance between the two arms in the number of participants allocated to each arm (19 vs. 12). There were unit‐of‐analysis issues as patients had more than one episode of fever. In the granulocyte arm there were 22 febrile episodes in 12 patients and in the control arm there were 40 febrile episodes within 19 patients. |
Bow 1984.
| Methods | Parallel RCT (period of enrolment not reported) (number of centres not reported) Canada | |
| Participants |
Inclusion criteria: Patients with: granulocytopenia (< 0.1 x 109 cells/L); fever (temperature > 38oC); unresponsive to broad spectrum antibiotics for at least 72 hours Exclusion criteria: Patients with a documented infection either clinically or microbiologically N = 24 patients The mean age of patients undergoing randomisation was not reported separately from patients in the concurrent prospective observational study of all patients receiving granulocytes but was between 52.6 years (SD 12.4) and 45.1 years (SD 20) (Table 2 (Bow 1984)). Arm 1 (Granulocyte transfusions): N = 13 Arm 2 (Control): N = 11 Predominantly acute myeloid leukaemia, but not reported separately from the 8 patients who only had a definite infection and were treated with granulocyte transfusions (Acute myeloid leukaemia = 22; Acute lymphoblastic leukaemia = 5; Chronic lymphocytic leukaemia = 2; Non‐Hodgkin's lymphoma = 2; carcinoma = 2). Type of treatment participants received for underlying disorder not reported |
|
| Interventions |
Granulocyte dose: 0.87 (+/‐ 0.35) x 1010 granulocytes given per transfusion (this relates to both the randomised study and the concurrent observational study) Granulocyte method of collection: Discontinuous flow centrifugation Selction of donors: ABO‐compatible, matched to at least 2 HLA antigens. If matching not possible cross‐match compatible Pre‐medication of donors: Dexamethasone 6 mg po (6 to 12 hours prior to collection) Initiation of granulocyte transfusions: Patients with: possible infection; granulocytopenia (< 0.1 x 109 cells/L); fever (temperature > 38oC); unresponsive to broad spectrum antibiotics for at least 72 hours Frequency of granulocyte transfusions: Not reported Termination of granulocyte transfusions: Not reported |
|
| Outcomes |
Primary Outcome(s): Not reported Secondary Outcome(s): Survival at day 21 Time to abatement of fever Pulmonary complications |
|
| Definition(s) of infection |
Proven infection: microbiologically documented if a pathogen was identified either in culture or in a histopathological preparation from an infected focus, as clinically documented if no pathogen was recovered Probable infection: febrile illness was compatible with infection but had no identifiable focus |
|
| Definition of neutropenia | Neutrophils < 1 x 109 cells/L; severe neutropenia < 0.1 x 109 cells/L | |
| Co‐interventions |
Therapeutic antibiotics: patients were treated empirically with an aminoglycoside and ticarcillin (or cefazolin if the patient was allergic to penicillin) G‐CSF: G‐CSF not licensed by the Food and Drug Administration (FDA) until 1991. Therapeutic antifungals: Not reported |
|
| Notes |
Randomised: Not reported Trial registration: none identified Sources of funding: Not reported in full. TJL was a recipient of a career development award from the Canadian Life and Health Insurance Association Conflicts of Interest: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It was unclear whether participants or clinical personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was unclear whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up was not reported. 21 day survival was 8/13 versus 7/11. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Unclear risk | The conduct of the study was poorly reported, and it was therefore unclear whether any other significant source of bias was present. |
Herzig 1977.
| Methods | Parallel RCT (period of enrolment not reported) (number of centres not reported) USA | |
| Participants |
Inclusion criteria: Patients with haematological malignancy, aplastic anaemia or solid tumours who had granulocytopenia (< 1 x 109/L) and had blood culture proven gram‐negative septicaemia Exclusion criteria: Equipment or personnel not available to perform a leukapheresis N =27 patients (30 episodes of sepsis). Three patients were re‐randomised 3, 8 & 12 months after the first episode (all the re‐randomisations were to the granulocyte transfusion group, 2 participants had previously been in the control group) Age range 2 to 57 years (Median 17 years) Arm 1 (Granulocyte transfusions): N = 16, 3 participants had been re‐randomised to this group, Acute myeloid leukaemia = 4, Acute lymphocytic leukaemia = 7, Aplastic anaemia = 1; Solid tumours = 2; Other = 2 Arm 2 (Control): N = 14, Acute myeloid leukaemia = 4, Acute lymphocytic leukaemia = 6, Aplastic anaemia = 1; Solid tumours = 0; Other = 3 Type of treatment participants received for underlying disorder not reported |
|
| Interventions |
Granulocyte dose: Median 1.7x1010/m2 filtration; Median 0.4x1010/m2 centrifugation Granulocyte method of collection: Filtration leukapheresis or continuous flow centrifugation Selection of donors: HLA typing results were not used to select prospective donors. Pre‐medication of donors: None Initiation of granulocyte transfusions: Granulocyte transfusions were started within 24 hours of the positive blood culture result Frequency of granulocyte transfusions: Daily Termination of granulocyte transfusions: Until 5 days with negative blood cultures , or 3 days without fever (defined as < 38oC); or neutrophil count >1 x109/L |
|
| Outcomes |
Primary Outcome(s): Not reported Secondary Outcome(s): Not clearly reported Median time to bone marrow recovery Survival at day 20/21 Adverse events |
|
| Definition(s) of infection | Septicaemia: Positive blood cultures ‐ gram negative organism | |
| Definition of neutropenia | Neutrophils < 1 x 109/L | |
| Co‐interventions |
Therapeutic antibiotics: Standard triple antibiotic regimen (cephalothin, gentamicin and carbenicillin) G‐CSF: G‐CSF not licensed by the Food and Drug Administration (FDA) until 1991. Therapeutic antifungals: Not reported |
|
| Notes |
Randomised: Not reported Trial registration: none identified Sources of funding: Not reported Conflicts of Interest: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | A deck of pre‐arranged sealed envelopes had been created, but how this prearrangement had been created was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Investigators drew a sealed envelope from a pre‐arranged deck. It was not reported whether the envelopes were opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The report did not state whether participants or clinical personnel were blinded to the outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The report did not state whether outcome assessors were blinded to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients who survived were followed up for at least 1 month after the end of the study (Figure 1 in the study report). The median time in the study was 21 days (9 to 56) in the control arm and 17 days (6 to 57) in the granulocyte arm. |
| Selective reporting (reporting bias) | Unclear risk | The protocol was not available to assess whether any outcomes had not been reported |
| Other bias | High risk | There was a unit of analysis issue in this study. 27 patients were randomised to receive or not receive granulocyte transfusions. 3 of these patients were re‐randomised. One patient in the granulocyte arm was re‐randomised to the same arm, and two of the five surviving patients in the control arm were re‐randomised to the granulocyte arm. |
Higby 1975.
| Methods | Parallel RCT (period of enrolment not reported) (number of centres not reported) USA | |
| Participants |
Inclusion criteria: Patients with haematological malignancies, neutrophil count < 0.5 x 109/L, clinical evidence of infection and fever > 38oC. Exclusion criteria: Not reported N = 36 9 patients aged > 45 years. Minimum age 15 years Arm 1 (Granulocyte transfusions): N = 17 (Acute myeloid leukaemia = 11). 1/17 patients aged > 45 years Arm 2 (Control): N = 19, (Acute myeloid leukaemia =12). 8/19 patients aged > 45 years Patients were receiving chemotherapy for acute leukaemia. 6 patients in each arm were being treated with remission induction chemotherapy. |
|
| Interventions |
Granulocyte dose: 2.2 x 1010/m2 (range 1.11 to 5) Granulocyte method of collection: Filtration leukapheresis Selection of donors: HLA typing results were not used to select prospective donors. Pre‐medication of donors: Dexamethasone immediately prior to collection (i.e. not priming) Initiation of granulocyte transfusions: Neutropenia < 0.5x109/L + fever >380C + antibiotics more than 2 days (i.e. 48‐hour period to assess response to antibiotics) + clinical evidence of infection (organism or focus) Frequency of granulocyte transfusions: Daily over 4 consecutive days Termination of granulocyte transfusions: After 4 consecutive days |
|
| Outcomes |
Primary Outcome(s): Not reported Secondary Outcome(s): Survival at day 20 Remission rates Adverse events |
|
| Definition(s) of infection | Clinical evidence of infection was not defined, but culture‐positive results were not required as mandatory prior to randomisation (although positive cultures were documented subsequently in 32 patients). | |
| Definition of neutropenia | neutrophil count < 0.5 x 109/L | |
| Co‐interventions |
Therapeutic antibiotics: type of antibiotics not specified G‐CSF: G‐CSF not licensed by the Food and Drug Administration (FDA) until 1991. Therapeutic antifungals: Not reported |
|
| Notes |
Randomised: Patients were not randomised until antibiotics had been given for two or more days and were judged clinically ineffective as evidenced by persistent fever and clinical deterioration Trial registration: none identified Sources of funding: Supported by research grants (CA‐5834 and CA‐10044) from the National Cancer Institute Conflicts of Interest: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients qualifying for this study were placed in the study group or the control group by drawing an assignment card from a prearranged deck. Method of sequence generation for the prearranged deck were not reported. |
| Allocation concealment (selection bias) | Unclear risk | Cards in prearranged deck were enclosed in sealed envelopes. It was not reported whether the envelopes were opaque |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It was not reported whether participants or clinical personnel were blinded to the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was not reported whether outcome assessors were blinded to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no loss to follow up at day 20. |
| Selective reporting (reporting bias) | Unclear risk | The protocol was not available to assess whether all pre‐specified outcomes were reported |
| Other bias | High risk | There was imbalance between the two groups in the baseline characteristics of the patients. 16/17 patients were aged under 45 years of age in the granulocyte transfusion arm, whereas 11/19 patients were aged under 45 years of age in the control arm. |
Klastersky 1983.
| Methods | Parallel RCT (period of enrolment ) (20 centres, majority of patients were from 5 centres) Belgium, Germany, Switzerland and UK | |
| Participants |
Inclusion criteria: People with at least 3 out of 4 characteristics predictive of gram negative septicaemia: neutropenia (< 0.1 x 109/L); fever (> 39.0°C); platelet count < 50x109/L; creatinine level > 1.0 mg AND hypocellular bone marrow (bone marrow aspirate) Exclusion criteria: Patients without a white blood cell donor Age range not reported N = 46 randomised, 39 included in analysis. Participants were excluded because of protocol violations or non‐documentation of fever Arm 1 (Granulocyte transfusions): N = 18, 16 included in the analysis, majority had acute myeloid leukaemia. Arm 2 (Control): N = 28 , 23 included in the analysis, majority had acute myeloid leukaemia Type of treatment participants received was not reported |
|
| Interventions |
Granulocyte dose: To exceed 1 x 1010/m2/day Granulocyte method of collection: Not specified, but the method of collection was the same for each recipient Selection of donors: Not reported Pre‐medication of donors: Not reported Initiation of granulocyte transfusions: Within 24 hours of onset of antibiotics Frequency of granulocyte transfusions: Daily for 4 days Termination of granulocyte transfusions: After 96 hours of antibiotic therapy those randomised not to receive granulocytes could be transfused and these patients were counted as a failure of the assigned therapy |
|
| Outcomes |
Primary Outcome(s): Not reported Secondary Outcome(s): Not reported Aims of the trial included: In poor‐risk patients what is the value of early administered granulocyte transfusions? Do differently collected granulocytes affect the outcome of severe infection in neutropenic patients in a different way? What are the side effects of transfusions of granulocytes and can they be related to the mode of collection of the granulocytes and to other factors such as pre‐medication of the donor and histocompatibility? |
|
| Definition(s) of infection |
Microbiologically documented: Signs and symptoms of infection present (i.e. primary site of infection recognised) and positive bacteriological cultures obtained from the suspected site, blood cultures, or histological sections.*
Clinically documented: Site of infection identified and progress consistent with infection. Negative cultures from primary site and blood.*
Possible infection: Signs, symptoms and progress are consistent with infection. Negative cultures and no site found despite complete re‐examinations, history, X‐rays and cultures at least every 3 days.
Infection doubted: Infection improbable on review of clinical signs and progress. *e.g., a pulmonary infiltrate is a pneumonia if consistent with proper signs and symptoms of infection‐ clinically documented ‐ but is microbiologically documented if (1) blood cultures are positive, (2) sputum culture is positive with a clear preponderance of one organism, the specimen is of good quality (i.e., not saliva), etc., or (3), positive results are obtained from transtracheal puncture, biopsy, etc . . . . Pneumonia remains clinically documented if blood cultures are negative or no blood cultures were taken and sputum sample was of poor quality or had mixed flora. |
|
| Definition of neutropenia | Neutrophil count < 1.0 x 109/L | |
| Co‐interventions |
Therapeutic antibiotics: treated with carbenicillin (or ticarcillin) and amikacin G‐CSF: G‐CSF not licensed by the Food and Drug Administration (FDA) until 1991. Therapeutic antifungals: Not reported |
|
| Notes |
Randomised: Patients were allocated to 'high risk' or 'poor prognosis', hospitalised at participating centre and donor available Trial registration: none identified Sources of funding: This was a European multicentre study under the auspices of the EORTC International Antimicrobial Therapy Project Group Conflicts of Interest: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Exact method used to randomise patients was not clear. However, the participating centres drew the next card from the stock supplied to them by the statistical centre. Each centre required details to be entered on to the postcard provided and this was sent to the Statistical Centre even if the patient was subsequently excluded from the trial. |
| Allocation concealment (selection bias) | Unclear risk | It was unclear whether the randomisation allocation was on the cards provided to the centres or they received the allocation once the card had been returned to the statistical centre. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study did not report whether participants or clinical personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The study did not report whether outcome assessors were blinded to the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There was an imbalance between the number of patients excluded from the 2 arms due to protocol violations or non‐documentation of fever. 46 patients were randomised, 18 patients in the granulocyte arm and 28 patients in the control arm. 2 patients were excluded from the analysis in the granulocyte arm (11%) and 5 patients were excluded in the control arm (18%) |
| Selective reporting (reporting bias) | High risk | A published protocol was available but did not state any primary or secondary outcomes. The objectives of the trial were to find out whether: In poor‐risk patients what is the value of early administered granulocyte transfusions? Do differently collected granulocytes affect the outcome of severe infection in neutropenic patients in a different way? What are the side effects of transfusions of granulocytes and can they be related to the mode of collection of the granulocytes and to other factors such as pre‐medication of the donor and histocompatibility Only the first objective was reported in the final report |
| Other bias | Unclear risk | There was an imbalance between study arms, 18 patients were randomised to the granulocyte arm and 28 patients were randomised to the control arm |
Price 2015.
| Methods | Parallel open‐label RCT (period of enrolment April 2008 to May 2013) (14 centres) USA | |
| Participants |
Inclusion criteria at study initiation Patients were those of any age with: 1) neutropenia, defined as absolute neutrophil count (ANC) < 0.5 x 109/L, due to aggressive chemotherapy or HSCT and 2) proven or probable bacterial or fungal infection. Participants were to be randomised within 24 hours of eligibility Inclusion criteria liberalised after 31 months of enrolment The eligibility criteria were liberalised to include: Patients with presumed infection (identical host and clinical criteria, but identification of organism not necessary), and to include patients with neutropenia due to underlying marrow disease (e.g. aplastic anaemia) The allowed time to randomisation was extended to one week from first meeting eligibility criteria Exclusion criteria Participants were excluded from the study if they were unlikely to survive five days, if there was evidence that the patient was unlikely to be neutropenic for at least five days, or if they had previously enrolled in this study. N = 114 participants, 97 included in the analysis (9 withdrew early, 8 primary endpoint could not be determined) 27 patients aged > 65 years 10 patients aged < 18 years. Mean age in granulocyte transfusion group (54.9 years ± 17.1) higher than in control group (46.9 years ± SD 20.2). Arm 1 (Granulocyte transfusions): N = 56, 48 included in the analysis, Acute myeloid leukaemia = 32, Acute lymphocytic leukaemia = 6, Non‐Hodgkin's lymphoma = 4, Other = 6. Chemotherapy = 37, HSCT = 8, Other = 3 Arm 2 (Control): N = 58, 49 included in the analysis. Acute myeloid leukaemia = 31, Acute lymphocytic leukaemia = 6, Non‐Hodgkin's lymphoma = 3, Other = 9. Chemotherapy = 36, HSCT = 8, Other = 5 |
|
| Interventions |
Granulocyte dose: Median 5.5 x 1010 (IQR 2.6 to 7.3). 15/49 (31%) participants in whom the dose was known received less than the planned dose of 0.6 x 109 cells/kg. Only 51 of the 56 participants randomised to the granulocyte transfusion arm received at least one granulocyte transfusion. Granulocyte method of collection: Continous flow centrifugation Selection of donors: Donors were not selected on the basis of HLA or granulocyte compatibility Pre‐medication of donors: G‐CSF and dexamethasone Initiation of granulocyte transfusions: Within 24 hours of diagnosis of a proven or probable bacterial or fungal infection (first 31 months). After this the allowed time to randomisation was extended to one week from first meeting eligibility criteria. Frequency of granulocyte transfusions: Daily Termination of granulocyte transfusions: Until neutrophil recovery, resolution or improvement of the underlying infection (at the discretion of the participant's physician) provided the participant received at least 5 granulocyte transfusions over at least a 7‐day period, life‐threatening toxicity, or patient had spent 42 days on the study. |
|
| Outcomes |
Primary Outcome
To be considered a success, the participant had to meet two criteria: 1) survival for 42 days after randomisation 2) evaluation of the clinical response of the study‐qualifying infection at 42 days Response for bloodstream infections was defined as a negative blood culture. For invasive bacterial or fungal infections, response was defined as resolution or improvement of clinical evidence of infection. A stable infection was considered to be a failure. Secondary Outcomes
|
|
| Definition(s) of infection | Criteria for the categorisation of proven or probable fungal infections were that of the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institutes of Allergy and Infectious Diseases Mycosis Study Group (EORTC/MSG) Consensus Group. Criteria for categorisation of proven or probable bacterial infection included: 1) Bacteraemia, the organism must be indicative of serious infection, for example gram negative bacteraemia or S. aureus bacteraemia. Coagulase‐negative staphylococcal bacteraemia was excluded AND
2) Typhlitis (neutropenic enterocolitis) demonstrated by clinical signs and symptoms compatible with disease AND evidence of disease by imaging techniques 3) Invasive tissue infection (infection of chest or sinuses) demonstrated by clinical signs and symptoms compatible with disease AND evidence of disease by imaging techniques 4) Invasive tissue infection (other than types listed above) demonstrated by clinical signs and symptoms compatible with disease e.g. intra‐abdominal abscess, perirectal cellulitis/ecthyma/abscess, lesions with crepitation or blebs, multiple skin lesions with bacteraemia |
|
| Definition of neutropenia | Neutrophil count < 0.5 x 109/L | |
| Co‐interventions |
Therapeutic antibiotics: Type of antibiotics not specified G‐CSF: Growth factors may be administered at the discretion of the attending physician, but should be discontinued if the neutrophil count exceeds 2.5 x 109/L Therapeutic antifungals: Invasive mould infections due to:
Candidemia or deep tissue invasive candidiasis: echinocandin (caspofungin, micafungin or anidulafungin), conventional amphotericin B (> 0.6 mg/kg/day); lipid formation of amphotericin B (3 to 5 mg/kg/day). Removal of central venous catheter is advised. |
|
| Notes |
Randomised: Patients were not randomised until they had a proven or probable fungal infection (first 31 months of study) this was liberalised to include presumed infection (after 31 months). Trial registration: registered prospectively on ClinicalTrials.gov NCT00627393 on 28 February 2008 Sources of funding: National Heart, Lung, and Blood Institute (NHLBI) Conflicts of Interest: P Ness is a consultant to TerumoBCT, Lakewood, CO. J McFarland is a member of the Scientific Advisory Board of Fenwal division of Fresenius Kabi and receives consulting fees for this service. The other authors declared no competing financial interests. Other: The planned target sample size of 118 participants per arm would have provided 80% power to detect a treatment difference if the true success rate with antimicrobial therapy alone was 50%, and the true success rate with the granulocyte treatment arm was 70%. The enrolment achieved was only 114, resulting in approximately 47% power to detect that difference.The study was stopped early due to a lower than expected accrual rate. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using randomly permuted blocks within strata. Participants were stratified according to risk status (high risk = stem cell transplant or relapsed leukaemia; low risk = other) and type of infection (invasive mould versus other), and allocation was also balanced within each clinical site using dynamic balancing. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This was an open‐label trial, therefore all clinical outcomes apart from all cause mortality are at risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Evaluation of response was performed by an independent adjudication panel blinded to the subject’s treatment arm. For all subjects, the panel confirmed the subject’s eligibility for the study and evaluated the appropriateness of the antimicrobial therapy. For subjects alive at Day 42, the panel also determined whether or not the subject’s infection resolved or improved. These decisions were based on clinical summaries, laboratory results, cultures, reports of imaging studies, and data from the standard case report forms. The panel was comprised of three infectious disease specialists and one radiologist, none of whom was affiliated with any of the participating clinical sites." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There was an imbalance in the number of participants who withdrew from the trial. 15/56 (27%) withdrew from the study in the granulocyte transfusion arm and 8/58 (14%) withdrew from the study in the control arm. |
| Selective reporting (reporting bias) | High risk | Several planned secondary outcomes were not reported:
|
| Other bias | High risk | Whether or not participants received the granulocyte dose specified in the protocol was not a random occurrence but was largely site specific. "These differences were not due to differences in the dose or timing of G‐CSF or dexamethasone, the timing of the leukapheresis, the donor’s neutrophil count at the time of collection, or the amount of blood processed during the collection (data not shown)". Approximately 30% did not receive the mean target dose of at least 0.6 x 109 granulocytes/kg per transfusion. The planned target sample size of 118 participants per arm would have provided 80% power to detect a treatment difference if the true success rate with antimicrobial therapy alone was 50%, and the true success rate with the granulocyte treatment arm was 70%. The enrolment achieved was only 114, resulting in approximately 47% power to detect that difference.The study was stopped early due to a lower than expected accrual rate. Study inclusion criteria were liberalised part way through the study (after 31 months) to increase the accrual rate. It was not reported how many participants had been recruited before and after the liberalisation of the inclusion criteria |
Scali 1978.
| Methods | Parallel RCT (Between 1973 and 1977) (number of centres not reported) Switzerland | |
| Participants | Acute leukaemias and predominantly acute myeloid leukaemia (as first treatment i.e. not refractory and not relapsed) Inclusion criteria: Patients with acute leukaemia or blastic phase of chronic myeloid leukaemia if the neutrophil count was < 0.25 x 109/L, and if infection was documented or strongly suspected. The eligibility criteria for children also required culture‐positive microbiology results. Exclusion criteria: No patient was eligible unless he was under treatment for acute leukaemia Age range not reported N = 25 patients included in the analysis, Acute myeloid leukaemia = 23, Acute lymphocytic leukaemia = 2. All participants were receiving induction chemotherapy Arm 1 (Granulocyte transfusions): N = 13 Arm 2 (Control): N = 12 |
|
| Interventions |
Granulocyte dose: average 2.9 x 1010 granulocytes given/day to each patient. Total dose received by patients from all transfusions 6.6 x 1010, range 1.7 to 14.7 x 1010 Granulocyte method of collection: continuous flow (cell separator) centrifugation Selection of donors: ABO‐compatible Pre‐medication of donors: not reported Initiation of granulocyte transfusions: neutropenia < 0.5 x 109/L & fever > 380C (at least 3 out of 4 measurements in 24 hours) & antibiotics given for more than 24hrs without response or recurrence after initial improvement & hypocellular bone marrow aspirate Frequency of granulocyte transfusions: Daily Termination of granulocyte transfusions: Not reported. Granulocytes were given for a total mean number of 2.7 days (range 1 to 5 days). |
|
| Outcomes |
Primary Outcome(s): Not reported Secondary Outcome(s): Not reported |
|
| Definition(s) of infection | Infections were retrospectively divided into: clinically and microbiologically documented; clinically definite source of infection (e.g. severe soft tissue infection even if no fever), and fever of unknown origin. | |
| Definition of neutropenia | Neutrophil count < 0.5 x 109/L | |
| Co‐interventions |
Therapeutic antibiotics: All patients received gentamicin: in 21 cases this was combined with cephalothin or carbenicillin, or both; in 4 cases it was combined with other antibiotics. G‐CSF: G‐CSF not licensed by the Food and Drug Administration (FDA) until 1991. Therapeutic antifungals: Not reported |
|
| Notes |
Randomised: Patients were not randomised until antibiotics were started for presumed or proven infection and a bone marrow aspirate had been performed that showed a pronounced hypocellularity or a complete aplasia with lack of myelopoiesis. Trial registration: none identified Sources of funding: Not reported Conflicts of Interest: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear whether there was loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available |
| Other bias | Unclear risk | Study is poorly reported, therefore the study may be at significant risk of bias. The more serious infections extending sepsis, pneumonia and soft tissue abscesses were unequally distributed between the two groups and overall were more common in the granulocyte transfusion group |
Seidel 2008a.
| Methods | Parallel RCT (1999 to 2005) (multi‐centre, 10 centres, only 5 recruited patients) | |
| Participants |
Inclusion criteria: Haematological malignancy, acquired marrow aplasia or solid tumour; febrile neutropenia (neutrophil count < 0.5 x 109/L and anticipated duration of aplasia > 5 days) after conventional chemotherapy or HSCT; pulmonary infiltrates (except exclusive bronchoalveolar lavage‐confirmed detection of virus and interstitial pneumonia) or soft tissue infiltration (> 5 cm diameter) or history of proven invasive fungal infection according to European Organisation for the Research and Treatment of Cancer and the Mycosis Study Group (EORTC/MSG)‐criteria and anticipated duration of neutropenia > 10 days. After an interim review of the study the inclusion criteria was broadened so that patients could be included before their neutrophil count fell below 0.5 x 109/L. Only 45 of the 72 infection episodes with complete data sets were randomised when the neutrophil count was < 0.5 x 109/L. Exclusion criteria: Adult respiratory distress syndrome; septic shock; or participation in another investigational drug study. N = 74 participants (79 infection episodes), 67 participants (72 infection episodes) included in the analysis. 4 participants were lost to follow‐up Mean age 47. Age range 14 to 75 years Arm 1 (Granulocyte transfusions): N = 39 participants. 40 randomised episodes (1 participant re‐randomised). Acute myeloid leukaemia = 27/40, Other leukaemias = 8/40, Other = 5/40. Chemotherapy 19/40, Allogeneic HSCT 18/40, Autologous HSCT = 1/40, Not reported = 2/40 Arm 2 (Control): N = 35 participants. 39 randomised episodes (4 participants re‐randomised). Acute myeloid leukaemia = 20/39, Other leukaemias = 11/39, Other = 8/39. Chemotherapy 13/39, Allogeneic HSCT 21/39, Autologous HSCT = 2/39, Not reported = 3/39 |
|
| Interventions |
Granulocyte dose: minimum recommended was 0.3 x 109/kg body weight (approximately 2 x 1010 for a 70 kg person) Granulocyte method of collection: continuous flow (cell separator) centrifugation Selection of donors: HIV1/2, HCV, CMV, HbsAg‐negative, no other selection criteria reported Pre‐medication of donors: G‐CSF Initiation of granulocyte transfusions: timing unclear. Median time from randomisation to initiation of granulocyte transfusions was 4 days (range 1 to 14 days) Frequency of granulocyte transfusions: Every other day, in many centres granulocyte transfusions were not given at weekends for logistical reasons Termination of granulocyte transfusions: administration of granulocyte transfusions was discontinued after white cell count had risen to above 1.0 x 109/L 48 hours after the previous granulocyte transfusion |
|
| Outcomes |
Primary Outcome(s): Survival on day 28 after randomisation Secondary Outcome(s): successful treatment of the infection neutrophil increment adverse effects incidence of acute and chronic GVHD |
|
| Definition(s) of infection | No definitions of infection provided | |
| Definition of neutropenia | Neutrophil count < 0.5 x 109/L | |
| Co‐interventions |
Therapeutic antibiotics: broad spectrum antibiotics according to local policies G‐CSF: daily 30 to 48 MIU continuously during neutropenic period and concurrently with granulocyte transfusions Therapeutic antifungals: not reported |
|
| Notes |
Randomised: not reported. 27 of the randomised infection episodes occurred when the neutrophil count was > 0.5 x 109/L. Trial registration: none identified Sources of funding: not reported. Amgen, Schering and Chugai Pharma provided travel grants Conflicts of Interest: not reported Other: The study was closed prematurely due to a dramatic decrease in recruitment rate from 15 on 2001 to 2 in 2005. The predicted sample size based on survival to 28 days was 160 participants (80 in each study arm) taking into consideration a 10% drop‐out proportion). This ws based on the assumption that the estimated survival to 28 days was 60% in the control arm and 80% in the granulocyte transfusion arm. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It was not reported whether participants or clinical personnel were blinded to the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was not reported whether outcome assessors were blinded to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The full data set was available for 67 out of 74 patients, and for many of the study calculations, the authors only looked at those who had a neutrophil count < 0.5 x 109/L at randomisation (n = 45) [unpublished data from author]. |
| Selective reporting (reporting bias) | Unclear risk | The protocol was not available to assess whether all pre‐specified outcomes were reported |
| Other bias | High risk | The study was closed prematurely due to a dramatic decrease in recruitment rate from 15 on 2001 to 2 in 2005. The predicted sample size based on survival to 28 days was 160 participants (80 in each study arm) taking into consideration a 10% drop‐out proportion). There was a unit of analysis issue in this study. 74 participants were randomised to receive or not receive granulocyte transfusions. 5 of these participants were re‐randomised. 4 of the 5 were re‐randomised to the control arm, one of these patients received a course of granulocyte transfusions despite being randomised to the control arm [unpublished data from author]. An interim review of the study changed the inclusion criteria to include people who were not neutropenic but expected to become neutropenic, 27 of the randomised infection episodes in which a full data set was available occurred when the neutrophil count was > 0.5 x 109/L. There was an imbalance between the number of episodes in which the neutrophil count was > 0.5 x 109/L at randomisation (27 randomised granulocyte transfusion episodes, 18 randomised control episodes). 40 infection episodes were randomised to receive granulocyte transfusions, only 33 definitely received granulocyte transfusions, 6 received none and 1 received an unknown number of granulocyte transfusions. 39 infection episodes were randomised to receive no granulocyte transfusions, however 6 episodes (3 participants) received granulocyte transfusions due to their deteriorating clinical state. 17 of the 39 episodes (44%) in which granulocyte transfusions were given (33 episodes in which participant was randomised to receive granulocytes) only 1 to 2 granulocyte transfusions were given before neutrophil recovery, suggesting that the potential effect of these granulocyte episodes would be minimal. The risk of death during a serious infectious episode decreased during the study period due to the introduction of new antimicrobial agents. 16% of granulocyte transfusions contained less than the minimum recommended dose (0.3 x 109/kg equivalent to 2 x 1010/L for a 70kg person) |
Vogler 1977.
| Methods | Parallel RCT (December 1973 to April 1976) (multi‐centre, 4 centres) | |
| Participants |
Inclusion criteria: had a suitable donor, culture proven infection, absolute neutrophil count < 0.3 x 109/L, failure to improve after at least 72 hours of appropriate antibiotic therapy, which was defined as organisms sensitive in vitro to antibiotics being administered Exclusion criteria: Patients with refractory underlying disease, the attending physician believed that further antileukaemic therapy would be of no benefit and that granulocyte transfusions would not be indicated N = 32 patients, 30 included in the analysis (2 patients died on day of randomisation not included in analysis) Age range 2 to 78 years. Average 35 years in granulocyte transfusion group and 45 years in control group. There were 3 children (< 15 years) in the granulocyte transfusion group and 2 children in the control group. Arm 1 (Granulocyte transfusions): N = 17 included in the analysis, Acute myeloid leukaemia = 12, Acute lymphocytic leukaemia = 2, Other = 3 Arm 2 (Control): N = 13, Acute myeloid leukaemia = 10, Acute lymphocytic leukaemia = 2, Other = 1 Type of treatment participants received was not reported. |
|
| Interventions |
Granulocyte dose: 2.7 x 1010 granulocytes given/day to each patient per dose Granulocyte method of collection: Continuous flow centrifugation Selection of donors: On the basis of ABO compatibility, best HLA match and health of the donor Pre‐medication of donors: initially donors did not receive pre‐medication, subsequently donors were given either hydrocortisone or dexamethasone as pre‐medication 2 hours before the procedure Initiation of granulocyte transfusions: Antibiotics more than 72 hours without response + culture positive infection Frequency of granulocyte transfusions: At least 4 transfusions in an 8‐day period. Daily if possible, but usually omitted at weekends. Termination of granulocyte transfusions: After 8 days of treatment. |
|
| Outcomes |
Primary Outcome(s): Not reported Secondary Outcome(s): Response to infection at 21 days Adverse events |
|
| Definition(s) of infection |
Complete resolution of infectious episode: all evidence of infection must clear within the 21‐day period. Measurable parameters included resolution of infection, conversion of cultures to negative, and healing of involved sites Partial resolution: less than complete clearing but definite clinical improvement in more than one measurable parameter for the 21‐day period No change: no measurable effect of granulocyte transfusions, or antibiotics, or both in patients surviving 21 days Worse: obvious progression of infection or death attributable to infection during the study period |
|
| Definition of neutropenia | Neutrophil count < 0.3 x 109/L | |
| Co‐interventions |
Therapeutic antibiotics: All patients were given combination antibiotics, most were given carbenicillin, cephalothin and gentamicin G‐CSF: G‐CSF not licensed by the Food and Drug Administration (FDA) until 1991. Therapeutic antifungals: Not reported |
|
| Notes |
Randomised: when patients had failed to improve after at least 72 hours of appropriate antibiotic therapy. Patients were stratified according to duration of infection (less than 5 days, 5 to 10 days, and more than 10 days) and type of infection (Pseudomonas or non‐Pseudomonas) Trial registration: none identified Sources of funding: Grant No. CA 14864 and Grant No. CA 16255 from the National Institutes of Health. Conflicts of Interest: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Patients were randomly assigned by sealed cards. It was not reported whether envelopes were opaque |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It was not reported whether participants or clinical personnel were blinded to the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was not reported whether outcome assessors were blinded to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Patients were followed up for at least 22 days. The median survival was 7.7 days in the control group and 22.5 days in the granulocyte transfusion group. |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available to assess whether any pre‐specified outcomes were not reported |
| Other bias | Unclear risk | There was an imbalance in the number of participants in each study arm (13 in the control arm versus 17 in the intervention arm).
The average age was younger in the granulocyte transfusion arm (35 years, range 2 to 75 versus 45 years, range 7 to 78). Pre‐medication to donors (steroids) was started part way through the study. |
Winston 1982a.
| Methods | Parallel RCT (period of enrolment not reported) (Single centre ) USA | |
| Participants |
Inclusion criteria: Patients with neutrophil count was < 0.5 x109/L, and a documented infection. Exclusion criteria: Patients with only fever and no documented infections, or patients with fungal or viral infections N = 95 patients randomised and analysed Age range 4 to 81 years. Median 49 years granulocyte transfusion group, 44 years control group. Arm 1 (Granulocyte transfusions): N = 48 included in the analysis, Acute myeloid leukaemia = 30 , Acute lymphocytic leukaemia = 1 , Other = 17. Refractory disease = 13. Arm 2 (Control): N = 47 , Acute myeloid leukaemia = 22 , Acute lymphocytic leukaemia = 10 , Other = 15. Refractory disease = 11. Type of treatment participants received was not reported. |
|
| Interventions |
Granulocyte dose: 0.5x1010/day (range 0.1 to 2.7 x 1010/day) Granulocyte method of collection: Discontinuous flow centrifugation Selection of donors: HLA typing results were not used to select prospective donors, selected on basis of ABO compatibility (related and unrelated donors) Pre‐medication of donors: None Initiation of granulocyte transfusions: Given within 24 hours of a positive blood culture, the appearance of an infiltrate on chest X‐ray, or the initial development of a cellulitis or abscess Frequency of granulocyte transfusions: Daily Termination of granulocyte transfusions:
|
|
| Outcomes |
Primary Outcome(s): Not reported Secondary Outcome(s): Overall survival (and at day 5) Clinical response rates Duration of time febrile Adverse events |
|
| Definition(s) of infection |
Documented infection: Either blood culture positive for a gram‐positive or gram‐negative organism with clinical features compatible with septicaemia, a lobar or segmental infiltrate on chest X‐ray consistent with bacterial pneumonia, or a localised area of cellulitis or abscess formation involving the skin or other soft tissues. Septic shock: hypotension, tachypnoea, decreased urine output, or altered mental state |
|
| Definition of neutropenia | Neutrophil count < 0.5 x109/L | |
| Co‐interventions |
Therapeutic antibiotics: Initial antimicrobial therapy in all patients consisted of an aminoglycoside (amikacin or netilmicin) plus an antipseudomonal penicillin (carbenicillin or piperacillin). For patients allergic to penicillin, cefazolin was substituted for penicillin. If a patient had a documented or strongly suspected staphylococcal infection an antistaphylococcal agent was incorporated into the patient's therapy. G‐CSF: G‐CSF not licensed by the Food and Drug Administration (FDA) until 1991. Therapeutic antifungals: Patients who remained febrile for 7 days or had surveillance cultures positive for fungi were eligible to receive amphotericin B. 19 patients in the granulocyte transfusion arm and 14 patients in the control arm received amphotericin B. |
|
| Notes |
Randomised: Patients were not randomised until antibiotics were started for presumed or proven infection Trial registration: none identified Sources of funding: Grants HB‐62971 from the national Heart, Lung and Blood Institute. CA‐23175 and CA‐15688 from the National Cancer Institute of Allergy and Insfections Diseases. Dr. Gale is a Scholar of the Leukaemia Sociaty of America. Conflicts of Interest: Not reported Other: "The probability of a type 2 error (not obtaining a statistically significant result when there actually is a difference) was computed for the alternative hypothesis using an assumed true odds ratio of 5.57. This odds ratio was based on previously reported data suggesting that infected patients receiving granulocyte transfusions have a response rate of 75% and that patients treated with only antimicrobial therapy have a response rate of 35%. The probability of type 2 error also considered the total number of favourable responses seen in both the transfused and control patients in the present study" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It was not reported whether participants or clinical personnel were blinded to the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was not reported whether outcome assessors were blinded to the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It was unclear how long patients were followed up for and whether any patients were lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available |
| Other bias | Unclear risk | Other sources of bias may have been present but this study was not reported in sufficient detail to assess the risk |
CMV: cytomegalovirus G‐CSF: granulocyte colony stimulating factor HCV: hepatitis C virus HLA: human leucocyte antigen HSCT: haematopoietic stem cell transplant MIU: million international units po: by mouth RCT: randomised controlled trial SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adkins 1999 | Prophylaxis |
| Altrichter 2011 | Non‐randomised study |
| Ambinder 1981 | Compared two types of leukapheresis product |
| Atay 2011 | Non‐randomised study |
| Bhatia 1994 | Non‐randomised study |
| Blum 2001 | Non‐randomised study |
| Clift 1978 | Prophylaxis |
| Curtis 1977 | Non‐randomised study |
| Curtis 1982 | Non‐randomised study |
| Diaz 2014 | Non‐randomised study |
| Ford 1982 | Prophylaxis |
| Fortuny 1975 | Non‐randomised study |
| Freireich 2013 | Comparing irradiated versus non‐irradiated granulocytes |
| Gomez‐Villagran 1984 | Prophylaxis |
| Granena 1978 | Non‐randomised study |
| Graw 1972 | Non‐randomised study |
| Graw 1977 | Non‐randomised study |
| Hershko 1978 | Non‐randomised study |
| Ikemoto 2012 | Assessing granulocyte yield in donors |
| Illerhaus 2002 | Non‐randomised study |
| Mannoni 1979 | Prophylaxis |
| Matsue 1984 | Non‐randomised study |
| NCT01204788 | Prophylaxis |
| NCT01932710 | Non‐randomised study |
| Oymak 2015 | Non‐randomised study |
| Oza 2006 | Prophylaxis |
| Pammi 2011 | Systematic review |
| Schiffer 1979 | Prophylaxis |
| Stout 2015 | Non‐randomised study |
| Strauss 1981 | Prophylaxis |
| Strauss 2015 | Review |
| Sutton 1982 | Prophylaxis |
| UMIN000014777 | Non‐randomised study |
| Winston 1982b | Prophylaxis |
| Witt 2015 | Non‐randomised study |
| Yoshihara 2016 | Review |
Characteristics of ongoing studies [ordered by study ID]
DRKS00000218.
| Trial name or title | GRANITE Transfusion of granulocytes for patients with febrile neutropenia |
| Methods | Parallel multi‐centre randomised controlled trial in Germany Planned recruitment 100 participants |
| Participants |
Inclusion Criteria People aged between 1 and 75 years of age With one of the following diseases:
Who have:
Exclusion Criteria
|
| Interventions | Arm 1: Intervention‐group: Transfusion of standardised apheresis‐products of granulocytes on every other day/alternating days + standard‐therapy (antibiotics/antimycotics) Arm 2: Control‐group: standard‐therapy without transfusion of granulocytes |
| Outcomes |
Primary outcome Measurement of temperature (auricularly or orally); Intervention group: 1h before, at starting time and ending of the transfusion of granulocytes, 1h, 12h and 24h after the transfusion. Control group: 0h (equates to 1h before the transfusion in the intervention group), 12h and 24h Endpoint: the normalisation of the temperature (measurement intraauricular or oral; <38°C) for 72h Secondary outcomes End of neutropenia (not as a consequence of transfusions); Value of neutrophil granulocytes in a blood sample > 500/μL on two following days in an upward trend |
| Starting date | Study has not yet opened to recruitment |
| Contact information |
Contact for Public or Scientific Queries Mr. Prof. Dr. med. Kai Hübel Klinik I für Innere Medizin Universitätsklinikum Köln Kerpener Str. 62 50937 Köln Germany e‐mail: kai.huebel at uni‐koeln.de Mr. Dr. med. Maximilian Fresen Klinik I für Innere Medizin Universitätsklinikum Köln Kerpener Str. 62 50937 Köln Germany e‐mail: Maximilian.Fresen at uk‐koeln.de |
| Notes |
Recruitment locations University Medical Center Klinik I für Innere Medizin, Köln University Medical Center Klinik für Innere Medizin, Hannover University Medical Center Klinik für Pädiatrie, Hannover Funding Public funding institutions financed by tax money/Government funding body (German Research Foundation (DFG), Federal Ministry of Education and Research (BMBF), etc.) Sponsor Universität zu Köln Albertus‐Magnus‐Platz 50923 Köln Germany Uni‐Köln‐478 (Prüfplancode) Ethics Committee 10‐103 , Ethik‐Kommission der Medizinischen Fakultät der Universität zu Köln Trial registrations DRKS‐ID: DRKS00000218 Universal Trial Number (UTN): U1111‐1111‐9560 EudraCT‐No. (for studies acc. to Drug Law): 2009‐010700‐28 |
NYHA: New York Heart Association WHO: World Health Organization
Differences between protocol and review
For continuous outcomes we planned to record the mean and standard deviations, and total number of participants in both the treatment and control groups. For continuous outcomes measured using the same scale, we planned to report the effect measure mean difference (MD) with 95% CIs, or the standardised mean difference (SMD) for outcomes measured using different scales. No continuous outcomes were reported. For time‐to‐event outcomes we planned to extract the hazard ratio (HR) from published data according to Parmar 1998 and Tierney 2007. However, no time‐to‐event data were reported.
We did not perform a formal assessment of potential publication bias (small‐trial bias) by generating a funnel plot and statistically using a linear regression test (Sterne 2011) as no meta‐analysis contained 10 or more studies.
We did not pre‐specify in the protocol how we would deal with unit of analysis issues. There were several unit of analysis issues within the included trials. Three trials re‐randomised patients or analysed the number of febrile episodes rather than the patient and these trials were not included in the quantitative analysis (Alavi 1977; Herzig 1977; Seidel 2008a).
We clarified that mortality would be reported up to 30 days.
We clarified that reversal of concurrent infection included both resolution of infection and improvement of clinical infection.
We used GRADE to build a 'Summary of findings' table as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011). This included the following domains.
Death from all causes up to 30 days from the start of the study
Clinical reversal of concurrent infections
Length of time with fever
Number of days on therapeutic antibiotics
Number of serious adverse events
Number of adverse events requiring discontinuation of treatment
Quality of life
A GRADE assessment had not been pre‐specified in the protocol.
Differences between the protocol and the review due to lack of data
We intended to assess the robustness of our findings by the following two sensitivity analyses.
Including only those trials at low risk of bias
Including only those trials in which 20% participants or less were lost to follow‐up.
A sensitivity analysis including only those studies at low risk of bias was not performed because none of the studies were at low risk of bias.
Contributions of authors
Lise Estcourt: searching, selection of studies, eligibility and methodological quality assessment, data extraction and analysis, and content expert. Simon Stanworth: protocol development, searching, selection of studies, eligibility and methodological quality assessment, data extraction and analysis, and content expert. Carolyn Doree who developed the search strategy and assisted with the selection of studies. Sally Hopewell: methodological expert. Marialena Trivella: methodological expert. Edwin Massey: protocol development, methodological quality assessment, data extraction and analysis and content expert.
Sources of support
Internal sources
National Blood Service, Research and Development, UK.
External sources
Department for International Development (DFID), UK.
-
National Institute for Health Research (NIHR) Cochrane Programme Grant, UK.
To provide funding for systematic reviewers and methodological support from the Centre for Statistics in Medicine, Oxford
Declarations of interest
Lise Estcourt is partly funded by an NIHR Cochrane Programme Grant. Simon Stanworth is involved in the design of clinical trials of granulocytes for transfusion. Carolyn Doree: none known. Sally Hopewell is partly funded by an NIHR Cochrane Programme Grant. Marialena Trivella is partly funded by an NIHR Cochrane Programme Grant. Edwin Massey is involved in the design of clinical trials of granulocytes for transfusion.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Alavi 1977 {published data only}
- Alavi JB, Roor RK, Djerassi I, Evans AE, Gluckman SJ, MacGregor RR, et al. A randomised clinical trial of granulocyte transfusions for infection in acute leukemia. New England Journal of Medicine 1977;296(13):706‐11. [DOI] [PubMed] [Google Scholar]
Bow 1984 {published data only}
- Bow EJ, Schroeder ML, Louie TJ. Pulmonary complications in patients receiving granulocyte transfusions and amphotericin B. Canadian Medical Association Journal 1984;130:593‐5. [PMC free article] [PubMed] [Google Scholar]
Herzig 1977 {published data only}
- Herzig R, Herzig GP, Graw RG, Bull MJ, Ray KK. Successful granulocyte transfusion therapy for gram‐negative septicemia. New England Journal of Medicine 1977;296(13):701‐5. [DOI] [PubMed] [Google Scholar]
Higby 1975 {published data only}
- Higby DJ, Yates JW, Henderson ES, Holland JF. Filtration leukapheresis for granulocyte transfusion therapy. New England Journal of Medicine 1975;292(15):762‐6. [DOI] [PubMed] [Google Scholar]
Klastersky 1983 {published data only}
- EORTC International Antimicrobial Therapy Project Group. Early granulocyte transfusions in high risk febrile neutropenic patients. Schweizerische Medizinische Wochenschrift 1983;113(Suppl. 14):46‐8. [PubMed] [Google Scholar]
- EORTC International Antimicrobial Therapy Project Group. Protocol for a cooperative trial of empirical antibiotic treatment and early granulocyte transfusions in febrile neutropenic patients. European Journal of Cancer 1977;13:617‐21. [DOI] [PubMed] [Google Scholar]
Price 2015 {published data only}
- NCT00627393. Safety and effectiveness of granulocyte transfusions in resolving infection in people With neutropenia (The RING Study). www.Clinical Trials.gov 2008 [Accessed 17th February 2015].
- Price TH, Boeckh M, Harrison RW, McCullough J, Ness PM, Strauss RG, et al. Efficacy of transfusion with granulocytes from G‐CSF/dexamethasone treated donors in neutropenic patients with infection. Blood 2015;126(18):2153‐61. [DOI: 10.1182/blood-2015-05-645986] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TH, McCullough J, Ness P, Strauss RG, Pulkrabek SM, Harrison R, et al. A randomized controlled trial on the efficacy of high‐dose granulocyte transfusion therapy in neutropenic patients with infection. Blood 2014;124(21):SCI‐16. [Google Scholar]
Scali 1978 {published data only}
- Scali G, Felten A, Fehr J, Sasuter Chr, Gmòr J. Granulocyte substitution in febrile leukemia patients with bone marrow aplasia. 1. Results of a prospective study [Granulozytensubstitution bei febrilen leukämiepatienten in knochenmarkaplasie]. Schweizerische Medizinische Wochenschrift 1978;108(41):1583‐5. [PubMed] [Google Scholar]
Seidel 2008a {published data only}
- Peters C, Seidel MG, Northoff H, Moog R, Boehme A, Silling G, et al. Granulocyte transfusions for treatment or prophylaxis of severe infections in immunocompromized neutropenic patients ‐ a randomized clinical trial. ASH Annual Meeting Abstracts 2006;108(11):2934. [Google Scholar]
- Seidel MG, Peters C, Wacker A, Northoff H, Moog R, Boehme A, et al. Randomized phase III study of granulocyte transfusions in neutropenic patients. Bone Marrow Transplantation 2008;42(10):679‐84. [DOI] [PubMed] [Google Scholar]
Vogler 1977 {published data only}
- Vogler WR, Winton EF. A controlled study of the efficacy of granulocyte transfusions in patients with neutropenia. American Journal of Medicine 1977;63:548‐55. [DOI] [PubMed] [Google Scholar]
Winston 1982a {published data only}
- Winston DJ, Winston G, Gale RP. Therapeutic granulocyte transfusions for documented infections ‐ A controlled trial in ninety‐five infectious granulocytopenic episodes. Annals of Internal Medicine 1982;97:509‐15. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adkins 1999 {published data only}
- Adkins D, Goodnough LT, Moellering J. Reduction in antibiotic utilization and in febrile days by transfusion of G‐CSF mobilised prophylactic granulocyte components: a randomised study. Blood 1999;94:590a. [Google Scholar]
Altrichter 2011 {published data only}
- Altrichter J, Sauer M, Kaftan K, Birken T, Gloger D, Gloger M, et al. Extracorporeal cell therapy of septic shock patients with donor granulocytes: a pilot study. Critical Care 2011;15(2):R82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ambinder 1981 {published data only}
- Ambinder EP, Button GR, Cheung T, Goldberg JD, Holland JF. Filtration versus gravity leukapheresis in febrile granulocytopenic patients: a randomized prospective trial. Blood 1981;57:836‐41. [PubMed] [Google Scholar]
Atay 2011 {published data only}
- Atay D, Ozturk G, Akcay A, Yanasik M, Anak S, Devecioglu O. Effect and safety of granulocyte transfusions in pediatric patients with febrile neutropenia or defective granulocyte functions. Journal of Pediatric Hematology/Oncology : Official Journal of the American Society of Pediatric Hematology/Oncology 2011;33(6):e220‐5. [DOI] [PubMed] [Google Scholar]
Bhatia 1994 {published data only}
- Bhatia S, McCullough J, Perry EH, Clay M, Ramsay MKC, Neglia JP. Granulocyte transfusions: efficacy in treating fungal infections in neutropenic patients following bone marrow transplantation. Transfusion 1994;34(3):226‐32. [DOI] [PubMed] [Google Scholar]
Blum 2001 {published data only}
- Blum W, Hallem C, Vij R. Improved survival in allogeneic peripheral blood stem cell transplant patients who received prophylactic granulocyte transfusions from HLA‐matched donors: long term follow up. Blood 2001;98:58a. [Google Scholar]
Clift 1978 {published data only}
- Clift RA, Sanders JE, Thomas ED, Williams B, Buckner CD. Granulocyte transfusions for the prevention of infection in patients receiving bone marrow transplants. New England Journal of Medicine 1978;298:1052‐7. [DOI] [PubMed] [Google Scholar]
Curtis 1977 {published data only}
- Curtis JE, Hasselback R, Bergsagel DE. Leukocyte transfusions for the prophylaxis and treatment of infections associated with granulocytopenia. Canadian Medical Association Journal 1977;117(4):341‐5. [PMC free article] [PubMed] [Google Scholar]
Curtis 1982 {published data only}
- Curtis J E. Efficacy of granulocyte transfusions collected from non‐stimulated donors. Progress in Clinical and Biological Research 1982;88:61‐73. [PubMed] [Google Scholar]
Diaz 2014 {published data only}
- Diaz, R, Soundar E, Hartman SK, Dreyer Z, Teruya J, Hui SK. Granulocyte transfusions for children with infection and neutropenia or granulocyte dysfunction. Pediatric Hematology & Oncology 2014;31(5):425‐34. [DOI] [PubMed] [Google Scholar]
Ford 1982 {published data only}
- Ford JM, Cullen MH, Roberts MM, Brown LM, Oliver RTD, Lister TA. Prophylactic granulocyte transfusions: Results of a randomised controlled trial in patients with acute myelogenous leukemia. Transfusion 1982;22:311‐6. [DOI] [PubMed] [Google Scholar]
Fortuny 1975 {published data only}
- Fortuny IE, Bloomfield CD, Hadlock DC, Goldman A, Kennedy BJ, McCullough JJ. Granulocyte transfusion: a controlled study in patients with acute nonlymphocytic leukemia. Transfusion 1975;15(6):548‐57. [DOI] [PubMed] [Google Scholar]
Freireich 2013 {published data only}
- Freireich EJ, Lichtiger B, Mattiuzzi G, Martinez F, Reddy V, Kyle WJ. A prospective, randomized, double‐blind study, comparing unirradiated to irradiated white blood cell transfusions in acute leukemia patients. Leukemia 2013;27(4):861‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00968838. A study for leukemia patients with life‐threatening infections. www.clinicaltrials.gov 2008 [Accessed 17th February 2015].
Gomez‐Villagran 1984 {published data only}
- Gomez‐Villagran JL, Torres‐Gomez A, Gomez‐Garcia P, Martinez‐Guibelalde F, Velasco‐Jimena F. A controlled trial of prophylactic granulocyte transfusions during induction chemotherapy for acute nonlymphoblastic leukemia. Cancer 1984;54:734‐8. [DOI] [PubMed] [Google Scholar]
Granena 1978 {published data only}
- Grañena A, Aranalde IM, Feliu E, Muncunill J, Montserrat E, Nieto H, Rozman C. The effectiveness of granulocyte transfusions in the treatment of sever injections in granulocytopenic patients. A controlled trial (author's transl) [Eficacia de las transfusiones de granulocitos en el tratemiento de infecciones graves de pacientes granulocitopénicos]. Sangre 1978;23(3):291‐7. [PubMed] [Google Scholar]
Graw 1972 {published data only}
- Graw RG, Herzig G, Perry S, Henderson ES. Normal granulocyte transfusion therapy ‐ treatment of septicemia due to gram‐negative bacteria. New England Journal of Medicine 1972;287(8):367‐71. [DOI] [PubMed] [Google Scholar]
Graw 1977 {published data only}
- Graw RG, Stout FG, Herzig RH, Herzig GP. Granulocyte transfusion therapy for life‐threatening bacteremia: results of transfusion trials at the National Cancer Institute. Progress in Clinical and Biological Research 1977;13:267‐80. [PubMed] [Google Scholar]
Hershko 1978 {published data only}
- Hershko C, Naparstek E, Eldor A, Izak G. Granulocyte transfusion therapy ‐ a clinical trial in patients with acute leukemia and sepsis. Vox Sanguinis 1978;34:129‐35. [DOI] [PubMed] [Google Scholar]
Ikemoto 2012 {published data only}
- Ikemoto J, Yoshihara S, Fujioka T, Ohtsuka Y, Fujita N, Kokubunji A, et al. Impact of the mobilization regimen and the harvesting technique on the granulocyte yield in healthy donors for granulocyte transfusion therapy. Transfusion 2012;52(12):2646‐52. [DOI] [PubMed] [Google Scholar]
Illerhaus 2002 {published data only}
- Illerhaus G, Wirth K, Dwenger A, Waller CF, Garbe A, Brass V, et al. Treatment and prophylaxis of severe infections in neutropenic patients by granulocyte transfusions. Annals of Hematology 2002;81:273‐81. [DOI] [PubMed] [Google Scholar]
Mannoni 1979 {published data only}
- Mannoni P, Rodet M, Vernant JP, Brun B, Coquin‐Radeau EI, Bracq C, et al. Efficiency of prophylactic granulocyte transfusions in preventing infections in acute leukemia. Revue Francaise de Transfusion et Immuno‐Hematologie 1979;22(5):503‐18. [PubMed] [Google Scholar]
Matsue 1984 {published data only}
- Matsue K, Harada M, Nakao S, Ueda M, Kondon K, Odaka K, et al. Controlled study of therapeutic granulocyte transfusion in granulocytopenic patients with severe infections. Japanese Journal of Clinical Oncology 1984;14(1):21‐30. [PubMed] [Google Scholar]
NCT01204788 {published data only}
- NCT01204788. Prophylactic white cell transfusions versus therapeutic white cell transfusions in patients with leukemia. www.Clinicaltrials.gov 2010 [Accessed 17 February 2015].
NCT01932710 {published data only}
- NCT01932710. A feasibility study of prophylactic white blood cell transfusions. Clinical.Trials.gov 2013:[Accessed 21st April 2015].
Oymak 2015 {published data only}
- Oymak Y, Ayhan Y, Karapinar TH, Devrim I, Ay Y, Sarihan H, et al. Granulocyte transfusion experience in pediatric neutropenic fever: Splitted product can be an alternative?. Transfusion & Apheresis Science 2015;53(3):348‐52. [DOI] [PubMed] [Google Scholar]
Oza 2006 {published data only}
- Oza A, Hallemeier C, Goodnough L, Khoury H, Shenoy S, Devine S, et al. Granulocyte‐colony‐stimulating factor‐mobilized prophylactic granulocyte transfusions given after allogeneic peripheral blood progenitor cell transplantation result in a modest reduction of febrile days and intravenous antibiotic usage. Transfusion 2006;46(1):14‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pammi 2011 {published data only}
- Pammi M, Brocklehurst P. Granulocyte transfusions for neonates with confirmed or suspected sepsis and neutropenia. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD003956.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schiffer 1979 {published data only}
- Schiffer CA, Aisner J, Daly PA, Schimpff SC, Wiernick PH. Alloimmunisation following prophylactic granulocyte transfusion. Blood 1979;54:766‐74. [PubMed] [Google Scholar]
Stout 2015 {published data only}
- Stout IP, Reeves HM, Maitta RW. Analysis of the effectiveness of granulocyte transfusions in neutropenic patients at a large tertiary academic medical center. Transfusion 2015;55:164A. [Google Scholar]
Strauss 1981 {published data only}
- NCT00000581. Granulocyte Transfusion Study. Clinical.Trials.gov 1999:[Accessed 21st April 2015].
- Strauss RG, Connett JE, Gale RP, Bloomfield CD, Herzig GP, McCullough J, et al. A controlled trial of prophylactic granulocyte transfusions during initial induction chemotherapy for acute myelogenous leukemia. New England Journal of Medicine 1981;305:597‐603. [DOI] [PubMed] [Google Scholar]
Strauss 2015 {published data only}
- Strauss RG. Neutrophil/granulocyte transfusions collected from G‐CSF + dexamethasone‐stimulated donors. Current Opinion in Hematology 2015;22(6):565‐7. [DOI] [PubMed] [Google Scholar]
Sutton 1982 {published data only}
- Sutton DMC, Shumak KH, Baker MA. Prophylactic granulocyte transfusions in acute leukemia. Plasma Therapy and Transfusion Technology 1982;3:45‐50. [Google Scholar]
UMIN000014777 {published data only}
- UMIN000014777. Study on safety and efficacy of granulocyte transfusion mobilized with G‐CSF in related donor. UMIN Clinical Trials Registry 2014:[Accessed 24th April 2015].
Winston 1982b {published data only}
- Winston DJ, Ho WG, Young LS, Gale RP. Prophylactic granulocyte transfusions during human bone marrow transplantation. American Journal of Medicine 1982;68:893‐7. [DOI] [PubMed] [Google Scholar]
Witt 2015 {published data only}
- Witt V, Pichler H. Survey of patients receiving Leukocyte transfusions from 2012 until 2014, a one center retrospective analysis. Transfusion Medicine and Hemotherapy 2015;48(42):9. [Google Scholar]
Yoshihara 2016 {published data only}
- Yoshihara S, Ikemoto J, Fujimori Y. Update on granulocyte transfusions: accumulation of promising data, but still lack of decisive evidence. Current Opinion in Hematology 2016;23(1):55‐60. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
DRKS00000218 {published data only}
- Transfusion of granulocytes for patients with febrile neutropenia. German Clinical Trials Register (DRKS) 2011 (Date Accessed 21 April 2015).
Additional references
Bashir 2003
- Bashir S, Cardigan R. Granulocyte concentrates: how can we assess their quality?. Transfusion Medicine 2003;13:245. [DOI] [PubMed] [Google Scholar]
Bashir 2008
- Bashir S, Stanworth S, Massey E, Goddard F, Cardigan R. Neutrophil function is preserved in a pooled granulocyte component prepared from whole blood donations. British Journal of Haematology 2008;140:701‐11. [DOI] [PubMed] [Google Scholar]
Bennett 2006
- Bennett CL, Evens AM, Andritsos LA. Haematological malignancies developing in previously healthy individuals who received haematopoietic growth factors: report from the Research on Adverse Drug Events and Reports (RADAR) project. British Journal of Haematology 2006;135(5):642‐50. [DOI] [PubMed] [Google Scholar]
Brecher 1953
- Brecher G, Wilbur KM, Cronkite EP. Transfusions of separated leukocytes into irradiated dogs with aplastic marrows. Proceedings of the Society for Experimental Biology and Medicine 1953;84:54‐6. [DOI] [PubMed] [Google Scholar]
Bux 2003
- Bux J, Cassens U, Dielschnider T, Duchscherer M, Edel E, Eichler H, et al. Tolerance of granulocyte donors toward granulocyte colony‐stimulating factor stimulation and of patients towards granulocyte transfusions: results of a multicentre study. Vox Sanguinis 2003;85:322‐5. [DOI] [PubMed] [Google Scholar]
Dale 1976
- Dale DC, Reynolds HY, Pennington JE, Elin RJ, Herzig GP. Experimental Pseudomonas pneumonia in leukopenic dogs: comparison of therapy with antibiotics and granulocyte transfusions. Blood 1976;47:869‐76. [PubMed] [Google Scholar]
Dale 2000
- Dale DC, Liles WC. Return of granulocyte transfusions. Current Opinion in Pediatrics 2000;12:18‐22. [DOI] [PubMed] [Google Scholar]
de la Rubia 2008
- Rubia J, Arriba F, Arbona C, Pascual MJ, Zamora C, Insunza A, et al. Follow‐up of healthy donors receiving granulocyte colony‐stimulating factor for peripheral blood progenitor cell mobilization and collection. Results of the Spanish Donor Registry. Haematologica 2008;93(5):735‐40. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Dellinger 2013
- Dellinger R, Levy M, Rhodes A, Annane D, Gerlach H, Opal S, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Medicine 2013;39:165‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
Engelfriet 2000
- Engelfriet CO, Reesink HW. Granulocyte transfusions. Vox Sanguinis 2000;79:59‐96. [PubMed] [Google Scholar]
Estcourt 2015
- Estcourt LJ, Stanworth S, Doree C, Blanco P, Hopewell S, Trivella M, et al. Granulocyte transfusions for preventing infections in people with neutropenia or neutrophil dysfunction. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD005341.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Freireich 1964
- Freireich EJ, Levin RH, Whang J, Carbone PP, Bronson W, Morse EE. The function and fate of transfused leukocytes from donors with chronic myelocytic leukemia in leukopenic recipients. Annals of the New York Academy of Sciences 1964;113:1081‐90. [DOI] [PubMed] [Google Scholar]
Ghodsi 2001
- Ghodsi Z, Strauss RG. Cataracts in neutrophil donors stimulated with adrenal corticosteroids. Transfusion 2001;41:1464‐8. [DOI] [PubMed] [Google Scholar]
Goldman 2006
- Goldman JM, Madrigal JA, Pamphilon D. Possible harmful effects of short course granulocyte colony‐stimulating factor in normal donors. British Journal of Haematology 2006 December;8:155‐60. [DOI] [PubMed] [Google Scholar]
Gutierrez 2001
- Gutierrez‐Delgado F, Bensigner W. Safety of granulocyte colony‐stimulating factor in normal donors. Current Opinion in Haematology 2001;8:155‐60. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011c
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hubel 2001
- Hubel K, Dale DC, Engert A, Liles WC. Current status of granulocyte (neutrophil) transfusion therapy for infectious diseases. Journal of Infectious Diseases 2001;183:321‐8. [DOI] [PubMed] [Google Scholar]
Hubel 2002
- Hubel K, Carter R, Liles WC, dale DC, Price TH, Bowden RA, et al. Granulocyte transfusion therapy for infections in candidates and recipients of hematopoietic stem cell transplant: a comparative analysis of feasibility and outcome of community donors versus related donors. Transfusion 2002;42:1414‐21. [DOI] [PubMed] [Google Scholar]
Hölig 2013
- Hölig K. G‐CSF in healthy allogeneic stem cell donors. Transfusion Medicine and Hemotherapy 2013;40(4):225‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kadri 2015
- Kadri SS, Remy KE, Strich JR, Gea‐Banacloche J, Leitman, SF. Role of granulocyte transfusions in invasive fusariosis: systematic review and single‐center experience. Transfusion 2015; Vol. On‐line ahead of print. [DOI] [PMC free article] [PubMed]
Klastersky 2001
- Klastersky J. Empirical treatment of sepsis in neutropenic patients. Hospital Medicine (London) 2001;62(2):101‐3. [DOI] [PubMed] [Google Scholar]
Kuijpers 1999
- Kuijpers TW, Weening RS, Roos D. Clinical and laboratory work‐up of patients with neutrophil shortage or dysfunction. Journal of Immunological Methods. 1999;232:211‐29. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Legrand 2012
- Legrand M, Max A, Peigne V, Mariotte E, Canet E, Debrumetz A, et al. Survival in neutropenic patients with severe sepsis or septic shock. Critical Care Medicine 2012;40(1):43‐9. [DOI] [PubMed] [Google Scholar]
Massey 2012
- Massey E, Harding K, Kahan BC, Llewelyn C, Wynn R, Moppett J, et al. The granulocytes in neutropenia 1 (GIN 1) study: a safety study of granulocytes collected from whole blood and stored in additive solution and plasma. Transfusion Medicine 2012;22(4):277‐84. [DOI] [PubMed] [Google Scholar]
NICE 2012
- National Institute for Health and Clinical Excellence (NICE). Neutropenic sepsis: prevention and management of neutropenic sepsis in cancer patients. NICE Guidelines 2012; Vol. CG151. [PubMed]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Review Manager 5.3 [Computer program]
- The Nordic Cochrane Centre. Review Manager (RevMan). Version Version 5.3. Copenhagen: The Cochrane Collaboration, 2014.
Robinson 2004
- Robinson SP, Marks DI. Granulocyte transfusions in the G‐CSF era. Where do we stand?. Bone Marrow Transplantation 2004;34(10):839‐46. [DOI] [PubMed] [Google Scholar]
Sacco 2010
- Sacco JJ, Botten J, Macbeth F, Bagust A, Clark P. The average body surface area of adult cancer patients in the UK: a multicentre retrospective study. PLoS One 2010;5(1):e8933. [DOI: 10.1371/journal.pone.0008933] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Sterne 2011
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Stovold 2014
- Stovold E, Beecher D, Foxlee R, Noel‐Storr A. Study flow diagrams in Cochrane systematic review updates: an adapted PRISMA flow diagram. Systematic Reviews 2014;3(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Strauss 1995
- Strauss RG. Clinical perspectives of granulocyte transfusions: efficacy to date. Journal of Clinical Apheresis 1995;10:114‐8. [DOI] [PubMed] [Google Scholar]
Strauss 2003
- Strauss RG. Granulocyte (neutrophil) transfusion. Apheresis: Principles and Practice. Bethesda: AABB Press, 2003:237‐52. [Google Scholar]
Strumia 1934
- Strumia MM. The effect of leukocytic cream injections in the treatment of the neutropenias. American Journal of Medicine 1934;187:527‐44. [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007; Vol. 8, issue 16. [DOI: 10.1186/1745-6215-8-16] [DOI] [PMC free article] [PubMed]
Vamvakas 1996
- Vamvakas EC, Pineda AA. Meta‐analysis of clinical studies of the efficacy of granulocyte transfusions in the treatment of bacterial sepsis. Journal of Clinical Apheresis 1996;11(1):1‐9. [DOI] [PubMed] [Google Scholar]
Vamvakas 1997
- Vamvakas EC, Pineda AA. Determinants of the efficacy of prophylactic granulocyte transfusions: a meta‐analysis. Journal of Clinical Apheresis 1997;12(2):74‐81. [DOI] [PubMed] [Google Scholar]
WHO 1992
- World Health Organization. International statistical classification of diseases and related health problems. 10th Edition. Vol. 1, Geneva: World Health Organization, 1992. [Google Scholar]
References to other published versions of this review
Stanworth 2005
- Stanworth S, Massey E, Hyde C, Brunskill SJ, Navarette C, Lucas G, et al. Granulocyte transfusions for treating infections in patients with neutropenia or neutrophil dysfunction. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD005339] [DOI] [PubMed] [Google Scholar]


