Abstract
In this issue of Immunity, Haghikia and colleagues (2015) demonstrate that dietary fatty acids, by modulating gut microbes and their metabolism, regulate mucosal immune cells to impact systemic immunity. Using this mechanism, dietary and bacteria-derived medium-chain and long-chain fatty acids exacerbate, whereas short-chain fatty acids ameliorate, autoimmunity in the brain.
Our understanding of the relationship between us as the host and gut microbes as the guests has been, and still is, undergoing continuous evolution. For a long time, we thought of this relationship as “commensal,” meaning that it is a one-way street with only the microbes as the beneficiaries of this living-together arrangement. Nonetheless, why our immune system does not view these microbes as aliens and tolerates their existence in our gut without mounting an overt immune response is still puzzling. Studies addressing this intriguing question have led to the discovery that gut microbes and their metabolites play an active role in modulating the mucosal immune system to establish a “tolerant” phenotype, facilitating the continuation of the co-existence (Ganapathy et al., 2013; Kim et al., 2014). Bacterial fermentation products, principally short-chain fatty acids (SCFAs), mediate the effects of gut microbes on the host immune system, regulating the differentiation and function of almost every cell type in the immune cell repertoire of the gut. The molecular mechanisms underlying this process involve not only cell-surface receptors such as GPR109A, GPR41, and GPR43 (Ganapathy et al., 2013; Kim et al., 2014) but also intracellular targets such as histone deacetylases (Singh et al., 2010; Gurav et al., 2015). Subsequently it became apparent that the modulation of the mucosal immune system by gut microbes is not limited solely to facilitation of their co-existence with the host; it also has significant impact on colonic health protecting against inflammation and carcinogenesis. These observations led to a major paradigm shift in the field, making it obvious that the relationship between the host and the gut microbes is not “commensal” but actually “symbiotic.” Despite this inter-dependent mutually beneficial relationship, the gut microbes are still referred often times as “commensals,” thus inadvertently denying them the well-deserved recognition of their beneficial effects on the host. An important outcome of this paradigm shift is the appreciation of the importance of diet in this process because the constituents of dietary fiber serve as the raw material for bacterial fermentation to generate SCFAs as the effector molecules. Gut microbes consist of 800–1,000 species with different metabolic capabilities; they rely on different constituents of dietary fiber for fermentation and consequently their fermentation products vary depending on the microbial species. As a result, diet is an important determinant of the composition of microbial species in the gut, which differs from individual to individual and can be altered in a given individual. Dysbiosis (i.e., quantitative changes in relative proportion of different microbial species, thus altering their normal balance and their fermentation products) is often associated with various intestinal diseases such as inflammatory bowel disease.
It has become increasingly clear in recent years that the impact of gut microbes and their metabolites on the mucosal immune system has consequences well beyond the local environment and affects biological processes elsewhere in the body (Kuhn and Stappenbeck, 2013). This is neither surprising nor unexpected given the fact that the intestinal tract harbors almost two-thirds of the immune cells found in the body and that these immune cells do not stay stagnant in the gut but migrate to other parts of the body, including the brain. As the differentiation and function of immune cells are subject to modulation by gut microbes while residing in the gut, the subtype and cytokine profiles of the immune cells that emigrate from the gut depend on the microbial composition as well as diet. One can thus expect dietary constituents and gut microbes to impact on immune-related processes outside the gut. Haghikia and colleagues (2015) demonstrate this phenomenon in the context of autoimmunity in the central nervous system. It is already known that diet and gut microbes influence systemic autoimmune responses in diseases such as type 1 diabetes, rheumatoid arthritis, systemic lupus erythematosus, and multiple sclerosis (Longman and Littman, 2015; Vieira et al., 2014), but little is known on this phenomenon at molecular level. The study by Haghikia et al. (2015) delves into the molecular mechanisms by which gut microbes and their metabolites impact the progression of an experimentally induced T cell-mediated autoimmune disease in the central nervous system and also into the role of dietary fatty acids in the process. These studies consist of in vitro and in vivo experiments. There are three salient features of the in vitro experiments. First, medium-chain fatty acids (MCFAs; carbon chain length 6–12) and long-chain fatty acids (LCFAs; carbon chain length 14–18) promote the differentiation of murine and human CD4+ naive T cells into T helper 1 (Th1) and Th17 cells with corresponding production of the pro-inflammatory cytokines interferon-γ (IFN-γ) and interleukin-17A (IL-17A); MCFAs also suppress the conversion of CD4+ naive T cells into FOXP3+ regulatory (Treg) cells with a decrease in expression of the anti-inflammatory cytokine IL-10. Second, short-chain fatty acids (SCFAs; carbon chain length 3–4) suppress the production of Th17 cells and promote the production of Treg cells from CD4+ naive T cells. Third, MCFAs elicit their effects via activation of the p38 MAPK pathway, whereas SCFAs elicit their effects via activation of lipin2-JIP2 pathway. These data demonstrate that MCFAs and LCFAs promote inflammation whereas SCFA suppress inflammation via their differential effects on the differentiation of naive CD4+ T cells into Th1, Th17, and Treg cells. The investigators then used a murine central nervous system autoimmune disease model, experimental autoimmune encephalomyelitis, which is a surrogate for human multiple sclerosis, and examined the influence of dietary lauric acid (MCFAs), palmitic acid (LCFAs), or propionic acid (SCFAs) on the progression of the disease. The findings of these in vivo experiments include two main points. First, dietary MCFAs and LCFAs aggravate the disease progression due to an increase in Th17 cells in the brain and spleen, and second, SCFAs ameliorate the disease progression as a result of an increase in Treg cells and IL-10 production. The study then focused on the anatomic site in which dietary fatty acids elicit their effects in terms of T cell differentiation. These experiments show that dietary MCFAs increase the amount of MCFAs and LCFAs but decrease the amount of SCFAs in the feces along with an increase in Th1 and Th17 cells in the lamina propria of the small intestine, and that these effects are not seen in germfree mice. The conclusion of these studies is that dietary MCFAs and LCFAs modulate gut microbes and their metabolism such that naive CD4+ T cells in the lamina propria are exposed to increased concentrations of MCFAs and LCFAs and decreased concentrations of SCFAs, thus tilting the T cell repertoire more toward the pro-inflammatory type and less toward the immunosuppressive type; when these cells emigrate from the intestinal tract to the brain, they affect the progression of autoimmunity (Figure 1). Taken collectively, these findings demonstrate an interesting and important role for bacteria-derived SCFA in autoimmunity; these bacterial metabolites promote differentiation of the CD4+ naive T cells in the gut into immunosuppressive cell types and block differentiation into pro-inflammatory cell types to guard against autoimmunity outside the gut.
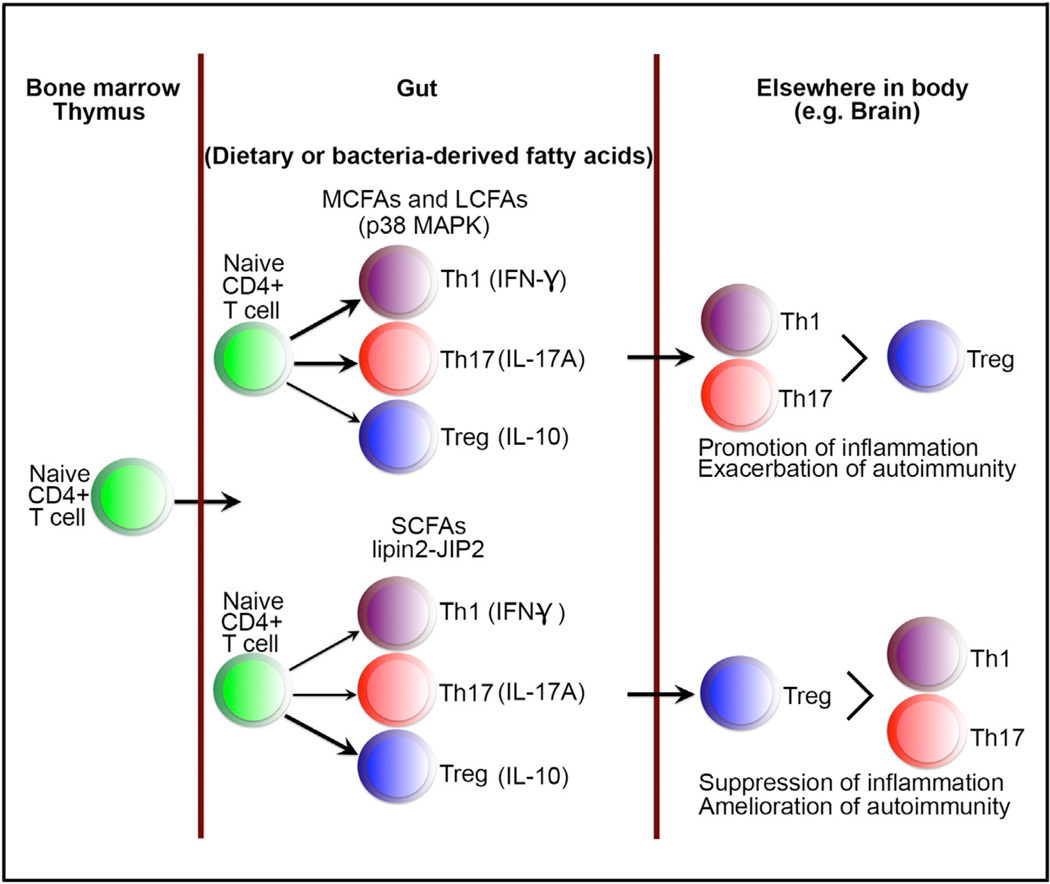
Figure 1. Gut Fatty Acids Drive Intestinal T Cell Differentiation that Influences Development of Autoimmunity in other Distal Tissues.
Naive CD4+ T cells that originate in the bone marrow and thymus migrate to the lamina propria of the gut, where they differentiate into various T helper subtypes under the influence of fatty acids derived from the diet or bacteria. In the presence of MCFAs or LCFAs, T cell differentiation is skewed toward inflammatory subtypes (Th1 and Th17), whereas in the presence of SCFAs, differentiation is skewed toward regulatory subtypes (Treg cells). These fatty-acid-influenced T cells drive pathogenic or protective responses in other tissues such as the central nervous system and modulate autoimmunity. Abbreviations are as follows: MCFAs, medium-chain fatty acids; LCFAs, long-chain fatty acids; SCFAs, short-chain fatty acids.
The exact molecular targets for MCFAs and LCFAs, and SCFAs to bring about their differential effects on the differentiation of naive CD4+ T cells remain unknown. Yes, the studies described by Haghikia et al. (2015) have shown that MCFAs and LCFAs act through activation of p38MAPK and that SCFAs act through lipin2-JIP2, but what triggers these signaling pathways upstream when the naive CD4+ T cells get exposed to the fatty acids has not been identified. The authors of the study seem to implicate cell-surface receptors as the most likely targets. This might well be true, but the possibility exists that these fatty acids might elicit their biological effects independent of cell-surface receptors. MCFAs and LCFAs might enter the cells and modulate signaling pathways via covalent modification of specific proteins (e.g., myristylation, palmitoylation, or stearoylation) (Magee and Seabra, 2005). This mechanism as an upstream element for activation of p38MAPK cannot be entirely ruled out because dietary lauric acid that aggravates autoimmunity in the brain does in fact increase the levels of myristic acid, palmitic acid, and stearic acid in the small intestinal mucosa. Similarly, SCFAs (propionic acid and butyric acid) function as inhibitors of histone deacetylases independent of their known cell-surface receptors; the involvement of this function upstream of lipin2-JIP2 pathway in the actions of SCFA cannot be ruled out either.
The clinical and therapeutic implications of this study are readily apparent. The findings indicate that modification of gut microbes and their metabolism are viable targets for treatment of not only multiple sclerosis but potentially also other autoimmune diseases. The goal of such a strategy is to promote generation of SCFAs in the gut so as to drive the differentiation of naive CD4+ T cells into Treg cells and not into Th1 and Th17 cells. This can be achieved with the use of appropriate types of dietary fiber that have the ability to support the growth and proliferation of SCFA-producing gut microbes. Prebiotics such as fructo-oligosaccharides are known to increase the levels of SCFAs in the gut. Probiotics containing selective species of bacteria known to produce SCFAs as the major fermentation products can also be used to achieve this goal. Oral delivery of SCFAs might be problematic because these fatty acids are metabolized extensively in the upper portions of the intestinal tract with only a minor fraction of the original oral dose entering the ileum and colon where majority of mucosal immune cells reside. But, SCFAs can be chemically modified (e.g., SCFAs esterified to starch) and then administered orally, an approach that is likely to result in delivery of appreciable amounts of SCFAs to the distal portions of the intestinal tract. The studies by Haghikia and colleagues (2015) provide convincing evidence in support of therapeutic potential of such approaches for prevention and/or treatment of autoimmune diseases.
Acknowledgments
This work was supported in part by the National Institutes of Health Grant CA190710 and by the Welch Endowed Chair in Biochemistry, Grant No. BI-0028, at Texas Tech University Health Sciences Center.
REFERENCES
- Ganapathy V, Thangaraju M, Prasad PD, Singh N. Curr. Opin. Pharmacol. 2013;13:869–874. doi: 10.1016/j.coph.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Gurav A, Sivaprakasam S, Bhutia YD, Boettger T, Singh N, Ganapathy V. Biochem. J. 2015;469:267–278. doi: 10.1042/BJ20150242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghikia A, Jorg S, Duscha A, Berg J, Manzel A, Waschbisch A, Hammer A, Lee D-H, May C, Wilck N, et al. Immunity. 2015;43:817–829. doi: 10.1016/j.immuni.2015.09.007. this issue. [DOI] [PubMed] [Google Scholar]
- Kim CH, Park J, Kim M. Immune Netw. 2014;14:277–288. doi: 10.4110/in.2014.14.6.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn KA, Stappenbeck TS. Semin. Immunol. 2013;25:364–369. doi: 10.1016/j.smim.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longman RS, Littman DR. Curr. Opin. Rheumatol. 2015;27:381–387. doi: 10.1097/BOR.0000000000000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee T, Seabra MC. Curr. Opin. Cell Biol. 2005;17:190–196. doi: 10.1016/j.ceb.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Singh N, Thangaraju M, Prasad PD, Martin PM, Lambert NA, Boettger T, Offermanns S, Ganapathy V. J. Biol. Chem. 2010;285:27601–27608. doi: 10.1074/jbc.M110.102947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira SM, Pagovich OE, Kriegel MA. Lupus. 2014;23:518–526. doi: 10.1177/0961203313501401. [DOI] [PMC free article] [PubMed] [Google Scholar]