Abstract
Background
Congenital cytomegalovirus (cCMV) infection contributes to considerable long-term sequelae in neonates and children all over the world. The association between viral genotypes and severity of clinical cytomegalovirus (CMV) infection is yet to be defined. The objective of this study was to find the impact of active CMV infection during pregnancy and the clinical significance of genotypes in neonates with congenital cytomegalovirus infections in Pakistan.
Methods
A total of 409 blood samples from pregnant women seeking health care services at the two antenatal hospitals of Islamabad during January to December 2012 were tested by ELISA and nested-PCR. Pregnant women with active infection (detected as IgM positive, PCR positive or positive on both assays) were followed until delivery, to detect the outcome of overt cCMV infection in neonates. Genetic characterization of CMV strains was performed by sequence analysis of envelope glycoproteins: gB, gN and gH to detect the contributing CMV genotypes.
Results
The seroprevalence of anti-CMV IgG and IgM was 97.5% (399 out of 409) and 12.7% (52 out of 409), respectively, while 20% (82/409) pregnant women were found positive for CMV DNA by PCR. Logistic regression analysis showed a significant association of active infection with parity [OR = 2.56, 95% CI = 1.82–2.62, p = 0.04], febrile illness [OR = 1.84, 95% CI = 1.76–3.65, p = 0.01] and jaundice [OR = 22.5, 95% CI = 4.53–85.02, p = 0.002]. We were able to isolate virus in 41 out of 70 neonates; 36.6% (15 out of 41) of them were symptomatic at birth while 63.4% (26 out of 41) were asymptomatic. The most prominent clinical feature observed in symptomatic neonates was hepatosplenomegaly (26.6%; 4 out of 15). All three genotypes gB, gN and gH were found with the highest frequency of gB1 genotype, found in 75% infants with hepatic damage. Phylogenetic analysis of Pakistani strains showed 96%-100% homology to their prototype strains.
Conclusions
Active CMV infection during pregnancy is a major cause of congenital CMV infection with comparable distribution of all three genotypes: gB, gN and gH in symptomatic and asymptomatic neonates. Our findings emphasize to conduct a comprehensive large scale survey and introduction of country wide routine screening at maternity clinics for early diagnosis of CMV to reduce its associated devastating outcomes.
Introduction
Human cytomegalovirus (HCMV) belongs to the beta herpesvirinae family, and humans are its only natural hosts. HCMV, also called human herpesvirus-5 (HHV-5), is one of the 8 human herpesviruses. HCMV is a common cause of congenital cytomegalovirus (cCMV) infections in developed as well as developing countries. Cytomegalovirus (CMV) infection may be acquired prenatally (congenital) through transplacental acquisition of either a primary or a recurrent maternal infection or during the perinatal/postnatal phase—at the time of birth or immediately after, due to exposure to infected cervical secretions, breast milk, or blood products [1]. The severity of congenital infection depends on maternal primary infection or re-activation, with primary infection more likely to result in severe sequelae. In developing countries, mostly congenitally infected neonates born to women with recurrent infections are clinically asymptomatic [2].
HCMV is a large, genetically diverse virus with over 200 open reading frames [3]. Genotyping of HCMV is principally based on variation in surface glycoprotein sequences, which show frequent genetic polymorphism. The HCMV genome encodes numerous glycoproteins; gB, gH and gN are the most abundant and have been studied extensively. Glycoprotein B (gpUL55) is a polymorphic glycoprotein and is a component of envelope complex gB-I with four genotypes (gB1-gB4) [4, 5]. The gH glycoprotein, an 86 kDa protein, is encoded by the UL75 gene and has two major variants—gH1 and gH2, based on the variability in the 37 amino acid N-terminal domain [6]. Another HCMV surface glycoprotein, gpUL73 (gN), encoded by UL73 has four genomic variants termed gN-1, gN-2, gN-3 and gN-4, with gN-3 sub-divided into gN-3a and gN-3b while the gN-4 genotype has three subgroups (gN-4a, gN-4b and gN-4c), respectively[7].
Several studies have been carried out to verify an association between genotype and disease manifestation, but heterogeneous findings from the various reports failed to define clear linkages [8, 9]. Approximately 10% of infants with cCMV exhibit clinical symptoms at birth, including intrauterine growth retardation (IUGR), jaundice, hepatosplenomegaly, retinitis, purpura, seizures and thrombocytopenia [10, 11]. Among the remaining 90% of infants that are asymptomatic at birth, 8% to 15% later develop complications, mainly neuro-developmental defects and deafness [12]. Thus, there is a need for early diagnosis, close monitoring, and timely therapeutic interventions to avoid the development of serious consequences in these asymptomatic children. Furthermore, HCMV infections can be a predisposing factor for bacterial and fungal infections due to reduced immune response and may have a role in graft rejection [13].
The prevalence of congenital CMV infection is usually higher in developing countries with higher maternal seroprevalence and varies within and between countries, with some reports as high as 6–14% [14]. There is currently no published data regarding cCMV infection in Pakistan. The aim of the present study was to identify cCMV infection, determine prevalent genotypes and assess possible relationships between genotypes and clinical features of the neonates born to actively infected mothers.
Materials and Methods
Study design
Pregnant women seeking health services at two major antenatal public sector hospitals of Islamabad, Federal Government Services Hospital and Pakistan Institute of Medical Sciences, during January to December 2012 were enrolled in this study. Furthermore, pregnant women with active infections were followed up to identify cCMV infection and genetic variability among circulating genotypes in neonates. Inclusion criteria of this study were: (1) Maternal active infection documented by either serum CMV-IgM antibody and/or PCR positive results; (2) Congenital CMV infection demonstrated by virus isolation within 3 weeks of age for neonates.
Demographic and clinical data were collected from the medical reports of newborns and from parents with their permission. The purpose of study was explained, and written consent was obtained from all participants. For minors and children, an informed written consent from the parents, caretakers, and/or guardians was obtained after explaining the study details and outcomes. The laboratory diagnostic and sequencing work was carried out at the Department of Virology, National Institute of Health (NIH), Islamabad. The study design and concept was approved by the ethical committee of respective Hospitals and of the Internal Review Board of the National Institute of Health (NIH), Pakistan.
Sample Collection and Processing
At the time of enrollment, blood samples were collected from all enrolled pregnant women (n = 409). During the follow-up visits, five cc of blood sample was collected from mothers. From neonates, two types of samples, urine and saliva, were collected within the fifteen days of their birth. Saliva was collected from all enrolled infants (n = 70) and urine was collected from 18 neonates within fifteen days of their birth.
Urine samples were collected in sterile urine bags attached to the perineum and transported on ice to the laboratory. Saliva samples were collected by swabbing the inside of neonates mouth using sterile polyester fiber tipped applicator and transported on ice to the laboratory. The saliva samples, when received, were incubated at 55°C for 1 hour with lysis buffer containing proteinase K, followed by denaturation of the enzyme at 95°C for 10 minutes.
Serology
To determine the immune status (IgG and IgM) of each participant and to calculate the anti-HCMV IgG avidity index, a commercial diagnostic ELISA kit (HUMAN Gesellschaft für Biochemica und Diagnostica mbH, Germany) was used. Blood samples were processed according to the manufacturer’s protocol, and results were interpreted accordingly.
Detection of HCMV by PCR
DNA was extracted from plasma, saliva and urine using a Nucleospin® Blood kit (MACHEREY-NAGEL GmbH & Co. KG, Germany) according to the manufacturer's protocol and stored at -20°C until further use. Nested PCR was carried out for screening in a 50 μl volume of 10X PCR buffer, 2.5 mM MgCl2, 200μM of each dNTPs, 5 units of Taq DNA polymerase and 10pmol of 2 primer sets specific for the 1st and 2nd round of amplification as described previously [15]. The cycling conditions for both rounds were as follows: 35 cycles at 94°C for 30 seconds, 58°C for 40 seconds and 72°C for 50 seconds and terminal extension at 72°C for 3 minutes. The amplified products were electrophoresed on a 2% agarose gel containing ethidium bromide and visualized under UV trans-illuminator.
Characterization of CMV gB, gN and gH genotypes
The samples positive for CMV were further subjected to nested PCR to amplify the UL55 (gB), UL73 (gN) and UL75 (gH) genes for genotyping [6, 16, 17]. All samples were tested for the detection of all three genotypes (gB, gH or gN). The primers and thermal conditions used for amplification are given in Table 1.
Table 1. Oligonucleotide primers and thermal conditions used for PCR and sequencing of cytomegalovirus.
| Target gene-Primer name | Sequence (5'-3') | Initial denaturation | Annealing temperature | Elongation time | Reference |
|---|---|---|---|---|---|
| UL55(gB)-Outer Forward | TCCGAAGCCGAAGACTCGTA | 94°C/5min | 58°C /1 min | 72°C/5 min | [16] |
| UL55(gB)-Outer Reverse | CATTCCTCAGTGCGGTGGTT | ||||
| UL55(gB)-inner Forward* | TTTGGAGAAAACGCCGAC | 94°C/2 min | 60°C /1 min | 72°C/5 min | |
| UL55(gB)-inner Reverse* | CGCGCGGCAATCGGTTTGTTGTA | ||||
| UL73(gN)-outer Forward | GACAGTACCAGTTGAGAGTCG | 96°C/5 min | 55°C /1min | 72°C/2 min | [17] |
| UL73 (gN)-outer Reverse | GGAYTATCTAGACTCGCTGC | ||||
| UL73 (gN)-inner forward* | TGGTGTGATGGAGTGGAAC | 94°C/2 min | 60°C/2min | 72°C/1 min | |
| UL73 (gN)-inner reverse* | TAGCCTTTGGTGGTGGTTGC | ||||
| UL75(gH)-outer Forward | CCTTCTCTCGGGTGTAAGC | 96°C/5 min | 55°C /1min | 72°C/2min | [6] |
| UL75(gH) outer Reverse | GTAGGTGTTAAGTCTCTG | ||||
| UL75(gH) inner Forward* | CCACCTGGATCACGCCGCTG | 94°C/2 min | 60°C/2min | 72°C/1 min | |
| UL75(gH) inner Reverse* | TGGTGTTTTCACGCAGGAA |
Y = C+T (Based on the CMV strain AD169 sequence)
* Primers used for sequencing
Phylogenetic analysis
The amplicons of UL55, UL73 and UL75 were purified using a QIAquick PCR Purification kit (Qiagen) and sequenced in both directions using a BigDye Terminator v3.1 Cycle Sequencing kit (Perkin Elmer-Applied Biosystems, Inc., Foster City, CA, USA). The sequences were analyzed on an ABI Prism Genetic Analyzer (3130xl, Applied Bio systems) and edited by Sequencher v.4.1 (Gene Codes Inc., Ann Arbor, MI, USA). Phylogenetic analysis of the UL55, UL73 and UL75 genes were performed using strains of different genotypes and of different geographical regions as retrieved from GenBank. Evolutionary tree and distances were generated by the neighbor-joining method using MEGA 5.0 [18].
Statistical analysis
The analysis was carried out using SPSS v.18.0. The mean (± Standard Deviation) values were calculated for age, and frequencies and percentages were calculated for HCMV positive and negative subjects. Various parameters were compared between HCMV positive and negative cases. Student’s t test was used for comparison of continuous variables, and the Pearson chi-square test was used for comparison of dichotomous variables. Logistic regression analysis along with calculation of odds ratio (OR) and 95% confidence intervals (CI) were performed to determine the association between the characteristics of subjects and HCMV seropositivity or active infection. Level of statistical significance was p < 0.05 (two-sided).
Results
A total of 409 pregnant women were enrolled in this study with over half (67.2%) of them residing in rural areas of Islamabad. The majority (96.82%) of pregnant women enrolled in this study were housewives while 3.18% were working women. The mean age (±SD) was 26.8 (±3.8) years with a range of 16–40 years.
Socio-demographic, obstetrical and clinical features of pregnant women
One hundred and eighteen (28.8%) women had a past abortion and thirty-four (8.31%) reported a history of jaundice. At the time of enrollment, 45 (11%) women were in the first trimester, 160 (39.1%) were in their second trimester, while 204 (49.87%) women were in their third trimester. One hundred and forty-eight (36%) of the women were pregnant for the first time (primigravida) while two hundred and sixty-one (64%) were multiparous. Logistic regression analysis of the various factors showed that high parity (>3 deliveries) [OR = 2.56, 95% CI = 1.82–2.62, p = 0.04] had a significant association with active CMV infection in these women. However, other parameters such as maternal age, area of residence, education level, trimester of pregnancy, and history of previous miscarriages were found without a significant effect (Table 2).
Table 2. Logistic regression analysis of demographic and clinical features of mothers infected with active CMV infection.
| Features | Odds ratioa | 95% confidence interval | P-value |
|---|---|---|---|
| Age (years) | |||
| 16–30 | 0.45 | 0.89–3.32 | 0.18 |
| 31–40 | 0.04 | 0.97–2.80 | 0.15 |
| More than 3 children | 2.56 | 1.82–2.62 | 0.04 |
| History of jaundice | 22.5 | 4.53–85.02 | 0.002 |
| Febrile illness | 1.84 | 1.76–3.65 | 0.01 |
| Gestational trimester | 1.08 | 0.64–1.25 | 0.1 |
| History of abortion | 0.78 | 0.45–1.08 | 0.2 |
| Education level | 0.26 | 0.87–1.58 | 0.3 |
| Residence | 0.87 | 0.36–1.67 | 0.5 |
| History of abortion | 0.63 | 0.58–1.42 | 0.09 |
| Hemoglobin | 0.49 | 0.96–1.36 | 0.4 |
a Adjusted by the features included in this table
OR = Odd Ratio
CI = Confidence Interval
Detection of IgM, IgG and HCMV DNA
Among the 409 pregnant women enrolled in the study, 399 (97.55%) were positive for IgG antibodies while IgM antibodies were detected in 52 (12.71%) women. Using molecular testing, HCMV DNA was detected in only 82/409 (20%) of the women. Eighty two (n = 82; 20) women were found with active infection (either positive on IgM ELISA and/or PCR) as given in Table 3.
Table 3. Results of IgM, IgG and PCR testing for diagnosis of CMV infection in pregnant women.
| Serology Results | |||||
|---|---|---|---|---|---|
| PCR results | IgM+ IgG+ n(%) | IgM+ IgG- n(%) | IgM- IgG+ n(%) | IgM- IgG- n(%) | Total n(%) |
| PCR +ve | 48 (58.5) | 2 (2.4%) | 32 (39%) | 0 | 82 (20%) |
| PCR -ve | 2 (0.6%) | 0 | 315 (96.3%) | 10 (3%) | 327 (80%) |
| Total | 50 | 2 | 347 | 10 | 409 |
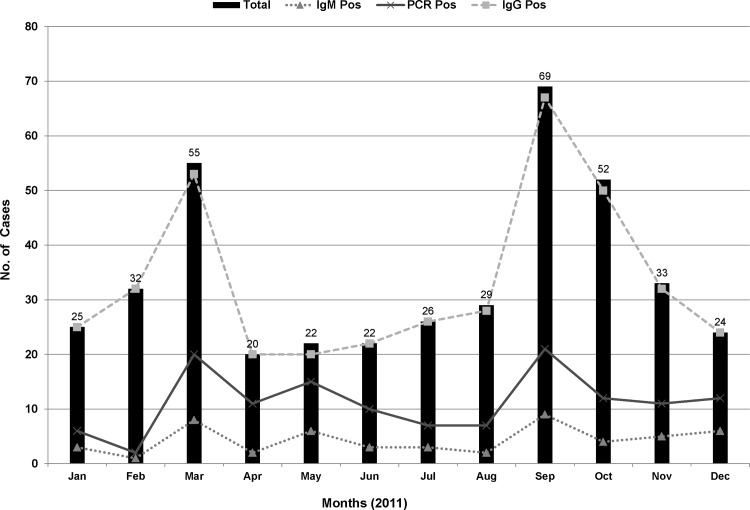
Patients with active infection were defined as “those with either IgM Positive only, or PCR positive only or both IgM and PCR positive” as demarcated by CDC, USA (http://www.cdc.gov/cmv/clinical/lab-tests.html). For the clinical characteristics, (fever) [OR = 1.84, 95% CI = 1.76–3.65, p = 0.01] and history of jaundice [OR = 22.5, 95% CI = 4.53–85.02, p = 0.002] had significant associations with active CMV infection in pregnant women. Other clinical features such as lymphadenopathy, sore throat and flu showed no significant association with active CMV infection. No seasonal trend of CMV infection was observed; however, it was more frequent in March and September of the study year (Fig 1). The pregnant women who were seronegative (2.4%) were unprotected and fall within the group in which infection is likely to occur.
Fig 1. The distribution of HCMV positive cases in study population during 2012.
The months are given on X-axis. The data labels on Y-axis indicate the number of total and positive cases across the year. The solid black line indicates total number of cases while the number of positive cases for each assay indicated with different bar-style.
IgG avidity assay was performed on 52 IgM positive samples and found that six samples showed low HCMV IgG avidity (<50%), two samples fell in grey zone (54% index) and 42 samples presented with high avidity (>60%). These findings confirmed that six women showed primo-infection and 44 (84.6%) had secondary infection.
Outcomes of congenital CMV infection
Eighty-two (n = 82) pregnant women showing active infections were followed up to study congenital infection in neonates. Out of these, five (n = 5) pregnancies were aborted or intrauterine fetal deaths occurred, while seven cases were missed as we were unable to follow up or refused to participate further. Seventy (n = 70) pregnancies were followed up successfully until deliveries for the congenital CMV infection study. Out of these cases, the virus was isolated from forty-one (n = 41) neonates within three weeks of age. Fifteen (n = 15) neonates were symptomatic while twenty-six (n = 26) were asymptomatic at birth. There were 6/10 (60%) symptomatic neonates born to mothers with first trimester infections compared with 9/60 (15%) born to mothers with infections in the second and third trimester, indicating significant association between the stage of mothers exposure and the clinical infections in newborns (Table 4). The proportion of clinically infected newborns whose mothers got infected during the first trimester was higher than those whose mothers were found CMV positive during second and third trimester thus indicating a strong association of the gestational age of mothers’s infection with the appearance of clinical disease in the newborns.
Table 4. Association of clinical infection in newborns compared to the mother’s gestational stage of CMV exposure.
| Maternal CMV exposure | Diseased | Not Diseased | Total | Odd ratio | 95% CI | P-value |
|---|---|---|---|---|---|---|
| First trimester | 6 | 4 | 10 | 8.5 | 1.993–36.25 | 0.0048 |
| Second +Third trimester | 9 | 51 | 60 | |||
| Total | 15 | 55 | 70 |
When clinical manifestations of symptomatic newborns were compiled, it was found that hepatosplenomegaly (4/15; 26.6%) was the most common feature followed by neonatal jaundice (3/15; 20%) and premature delivery (Table 5). No significant differences were found between asymptomatic and symptomatic infants with respect to maternal clinical and demographic parameters. Among the clinical samples collected and tested for CMV infection in neonates, high frequency of CMV infections were detected through saliva samples compared to urine. Out of 18 urine samples, 5 (27.7%) were positive while 100% of saliva samples were positive for CMV.
Table 5. Clinical Abnormalities found in Newborns with Symptomatic Congenital CMV Infection.
| Findings/Abnormality | Positive/Total Examined (%) |
|---|---|
| Bronchopneumonia | 1/15 (6.6) |
| Congenital Cataracts | 1/15 (6.6) |
| Developmental Delay | 1/15 (6.6) |
| Hepatosplenomegaly | 4/15 (26.6) |
| Hydrocephaly | 1/15 (6.6) |
| Thrombocytopenia (Platelet count <100000/mm3) | 2/15 (13.3) |
| Microcephaly | 1/15 (6.6) |
| Neonatal Jaundice | 2/15 (13.3) |
| Respiratory Distress | 1/15 (6.6) |
| Petechiae | 1/15 (6.6) |
| Petechiae with Jaundice | 1/15 (6.6) |
Distribution of HCMV genotypes and phylogenetic analysis
A total of 41 PCR positive newborns were further analyzed for genotype determination. The three major genotypes were detected in 36 cases while PCR analysis for HCMV genotype was negative in five cases. Sequence data on representative strains of the various genogroups were retrieved from GenBank and included in the sequence alignment and phylogenetic analysis. All four gB genotypes (1–4), six gN sub-genotypes (1, 3a, 3b, 4a, 4b, 4c) out of 7 and both gH genotypes (1 and 2) were detected in the study population, with variable proportions as described in Table 6. Majority of the isolates were identified as the gN (15; 41.6%) genotype while 11 were identified as gH (30.5%) and 10 as gB (27.7%).
Table 6. Genotype distribution of CMV in neonates born with congenital infection.
| Infants | gB1 | gB2 | gB3 | gB4 | gH1 | gH2 | gN1 | gN-3a | gN-3b | gN-4a | gN -4b | gN-4c | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bronchopneumonia | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Congenital Cataracts | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| Developmental Delay | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Hepatosplenomegaly | 3 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| Hydrocephaly | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Thrombocytopenia | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Microcephaly | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Neonatal Jaundice | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Respiratory Distress | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Petechiae | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 |
| Petechiae with Jaundice | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Symptomatic (Total) | 3 | 2 | 1 | 1 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 0 | 16 |
| Asymptomatic (Total) | 1 | 0 | 2 | 0 | 5 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 20 |
| Total (Symptomatic+Asymptomatic) | 4 | 2 | 3 | 1 | 7 | 4 | 3 | 3 | 3 | 3 | 2 | 1 | 36 |
The overall distribution of individual sub-genotypes in this study cohort was as follows: gH1 (7/11; 63.7%), gH2 (4/11; 36.3%), gB1 (4/10; 40%), gB2 (2/10; 20%), gB3 (3/10; 30%), gB4 (1/10; 10%); gN1, gN3-(a, b, c) and gN-4a (3/15; 20%), respectively; gN-4b (2/15; 13.3%) and gN-4c (1/15; 6.7%) as in Table 6.
The distribution of HCMV genotypes between symptomatic and asymptomatic children was analyzed to assess the possible association of genotypes with clinical signs and symptoms. Our findings revealed that compared to other, only gB1 genotype was significantly associated with hepatic damage when compared between symptomatic and asymptomatic neonates.
The phylogenetic analysis showed that gB genotypes have 98.5 to 100% similarity with reference strains, gN genotypes showed 94.2 to 99.4% similarity and gH 95.3 to 99.7%, as shown in Fig 2.
Fig 2. Phylogenetic analysis of gB, gN and gH genotypes of HCMV.
The reference strains and isolates detected through BLAST are given for genetic comparison. The phylogenetic tree with 1000 bootstrap replicates was reconstructed using neighbor joining method and Kimura two-parameter distances model incorporated in MEGA v5.0. The number at the nodes indicates bootstrap values shown above 50. Fig 2A, 2B and 2C represent genetic relationships of gB, gH and gN genotypes of CMV respectively. The strains detected in this study are represented by sample identification with codes mentioned as PAK: Pakistan, NIH: National Institute of Health Islamabad.
Discussion
HCMV is a frequent cause of congenital infection, the most common being sensorineural hearing loss, mental retardation, motor deficits, seizures, and chorioretinitis [19, 20]. The reason why only some children develop SNHL or other sequelae after congenital CMV infection is not well understood but could be related to both host and viral factors. At birth, it is essential to test for congenital CMV infection. Although congenital CMV infection occurs in 0.2 to 1% of live births worldwide, there has been no population-based HCMV clinical or epidemiological study in Pakistan. Majority of the Pakistani population lives below poverty level, and because health care facilities are not available, women are likely to be exposed to CMV infections. The main objectives of the present study were to investigate HCMV seroprevalence among expectant women, to explore the correlation of active infection to congenital infection in newborns and to determine the prevalent HCMV genotypes.
The seroprevalence of HCMV IgG in pregnant women in this study (97.55%) was comparable to a regional study carried out in the KPK province of Pakistan [21] as well as other countries such as Qatar [22], Egypt [23], Tunisia [24], Iraq [25], Iran [26], Brazil [27] and Turkey [28]. All these studies have reported higher seroprevalence rates than developed countries such as the United Kingdom [24] and Germany [29].
The testing of ELISA and PCR for CMV IgM antibodies and DNA revealed the positivity rates of 12.71% (n = 52/409) and 20% (n = 82/409) respectively. This may attribute to the selection of specific high risk group i.e. pregnant women. In addition, CMV positivity rate is inevitably high in the areas with high population density and low-resource settings like Pakistan [30]. It is likely that in developed countries, pregnant women are generally better informed of good hygienic practices such as hand washing and sanitation, thus accounting for a reduced risk of acquiring CMV infection. In the present study, only four (0.9%) of 409 women interviewed had heard about CMV infection previously. A lack of awareness about CMV infection among the public is a major barrier to controlling the disease. The high prevalence rate in our settings indicates the endemicity of infection and can be linked with socio-economic and climatic factors. In addition, the high frequency of women positive for HCMV DNA by PCR compared to ELISA reflects the higher sensitivity of nested PCR as reported previously [31].
In the current study, we observed that fever; jaundice and high parity were associated with active CMV infection. However, given the numerous causes of fever and jaundice, these represent non-specific indicators of maternal CMV infection. High parity increased the susceptibility to acquisition of maternal CMV infection, perhaps through direct contact with contagious secretions from their own children or poor hygiene practices [32, 33]. We did not demonstrate that active infection leads to congenital CMV infection, either symptomatic or asymptomatic, and did not clearly identify a relationship between the severity of disease and genotypes in neonates.
It is not entirely clear whether, over the course of the pregnancy, vertical transmission to the fetus after maternal viremia occurs either as a single event or through multiple transplacental transfer events. In this study, it was observed that CMV active infection during pregnancy led to intrauterine transmission and occurrence of congenital CMV infection as documented and reported by Boppana et al [34]. The majority of symptomatic cCMV infections were due to first trimester maternal infection, and the rate of sequelae in infected offspring is lower with maternal infection later in pregnancy. Daiminger et al [35] reported an association between earlier gestation maternal or fetal infection and poor outcomes for the fetus. Nevertheless, second and even third trimester maternal infections are also capable of causing hearing defects. However, the relationship between HCMV genotypes and the consequences of infection in terms of pathogenesis and long term outcomes in children with congenital HCMV infection are not yet clearly understood.
Classification of genotypes was performed by sequence comparison of glycoprotein gene from Pakistani strains using online BLAST (Basic Local Alignment Search Tool) analysis. Genotype gB1 was recently found to be associated with severe manifestations of CMV disease in infants with congenital CMV infection [36]. Other studies suggest that the gB, gN and gH genotypes of CMV may result in neurological dysfunction or hearing loss in congenitally infected infants [37, 38]. Our findings also support these studies and further demonstrate that gN and gH genotypes may be associated with congenital infection [39, 40]. Pignatelli et al [17] described the distribution of gN (UL73) variants gN-1, gN-3 and gN-4 and their subtypes in four geographical regions; Europe, China, Australia and North America with different frequencies of gN-2 (rarest genotype), which was found in North America and Europe but was not identified in Asian countries and Australia. Likewise, we did not find any gN-2 genotype in study confirming the previous findings from this region.
Urine and saliva specimens have been suggested for the detection of congenital HCMV infection as sample collection from neonates is simpler as compared to blood; higher volumes can be easily obtained and the procedure can be repeated easily if needed as parent compliance is higher. Out of the total urine samples collected from 18 neonates, 5 were negative although saliva from all of these neonates was positive. Thus our study confirms previous findings that testing saliva is more sensitive than urine for CMV testing [41].
The prevalence of different genotypes in symptomatic and asymptomatic infants was not significantly different, and none of the genotypes were associated with virulence. Although no significant genotype specific-association with specific clinical features could be established, all the infants with hepatosplenomegaly carried the gB1 genotype, which has been reported globally by other groups [36, 42]. However, our study contradicts other publications which reported that only gB genotypes were associated with virulence [36, 42, 43]. This may be due to differences in geographical location, population selection or other factors such as culture-propagated viral isolates which could select for a single virus strain and thus decrease the chances of identifying multiple viral strains or genotypes [17, 44]. Many other studies have also described HCMV infection with multiple viral strains in different populations, including children attending day care centers, HIV-infected individuals, allograft recipients, and infants with congenital HCMV infection [44–47]. Together, these findings suggest that infections with multiple CMV strains occur frequently.
Our study thus represents the first report of CMV infection and its circulating genotypes in Pakistani population focusing the primary risk group i.e. pregnant women. This may underestimate the true burden of HCMV in indigenous Pakistani population. Likewise, we were able to target only two major hospitals in Islamabad that may not represent the true disease burden and outcomes in terms of congenital infections for the entire Pakistani population. Due to limited number of samples within a given population, the comprehensive epidemiology of HCMV genotypes and their clinical impact cannot be truly established. Thus the study findings emphasize continuing large scale epidemiological surveys in the other areas of the country. Public awareness programs must been introduced and scaled-up targeting the population at risk and those in the regions with high population density and low hygiene and socio-economic status.
Conclusion
Active CMV infection during pregnancy was a major cause of cCMV infection, and distribution of gB, gN and gH genotypes were similar in symptomatic and asymptomatic neonates. Currently, no therapeutics or vaccines are available for CMV; therefore, minimizing the number of cases of CMV by informing women about their CMV serostatus before and during pregnancy and educating them about preventive hygienic measures to avoid exposure to CMV is crucial. It is difficult to estimate the true epidemiological profile of CMV infections and prevalent genotypes in our population due to absence of routine screening and surveillance studies. Indeed, there is a dire need for early diagnosis of CMV to reduce congenital CMV infections and to determine the true burden of the disease. The availability of current information on CMV prevalence and implementation of disease control measures will ultimately enable us limit exposure to the virus and reduce childhood mortality as envisaged for the Millennium Development Goals.
Acknowledgments
The study was supported by the indigenous scholarship award to Ghulam Mujtaba for his Ph.D research work.
Data Availability
All relevant data are within the paper.
Funding Statement
The authors have no support or funding to report.
References
- 1.Cannon MJ. Congenital cytomegalovirus (CMV) epidemiology and awareness. J Clin Virol. 2009;46 Suppl 4:S6–10. Epub 2009/10/06. 10.1016/j.jcv.2009.09.002 S1386-6532(09)00418-1 [pii]. . [DOI] [PubMed] [Google Scholar]
- 2.Ornoy A, Diav-Citrin O. Fetal effects of primary and secondary cytomegalovirus infection in pregnancy. Reprod Toxicol. 2006;21(4):399–409. Epub 2006/04/04. S0890-6238(05)00052-3 [pii] 10.1016/j.reprotox.2005.02.002 . [DOI] [PubMed] [Google Scholar]
- 3.Murphy E, Shenk T. Human cytomegalovirus genome. Curr Top Microbiol Immunol. 2008;325:1–19. Epub 2008/07/22. . [DOI] [PubMed] [Google Scholar]
- 4.Chou SW, Dennison KM. Analysis of interstrain variation in cytomegalovirus glycoprotein B sequences encoding neutralization-related epitopes. J Infect Dis. 1991;163(6):1229–34. Epub 1991/06/01. . [DOI] [PubMed] [Google Scholar]
- 5.Fries BC, Chou S, Boeckh M, Torok-Storb B. Frequency distribution of cytomegalovirus envelope glycoprotein genotypes in bone marrow transplant recipients. J Infect Dis. 1994;169(4):769–74. Epub 1994/04/01. . [DOI] [PubMed] [Google Scholar]
- 6.Chou S. Molecular epidemiology of envelope glycoprotein H of human cytomegalovirus. J Infect Dis. 1992;166(3):604–7. Epub 1992/09/01. . [DOI] [PubMed] [Google Scholar]
- 7.Pignatelli S, Dal Monte P, Landini MP. gpUL73 (gN) genomic variants of human cytomegalovirus isolates are clustered into four distinct genotypes. J Gen Virol. 2001;82(Pt 11):2777–84. Epub 2001/10/17. 10.1099/0022-1317-82-11-2777 . [DOI] [PubMed] [Google Scholar]
- 8.Pignatelli S, Lazzarotto T, Gatto MR, Dal Monte P, Landini MP, Faldella G, et al. Cytomegalovirus gN genotypes distribution among congenitally infected newborns and their relationship with symptoms at birth and sequelae. Clin Infect Dis. 2010;51(1):33–41. Epub 2010/05/28. 10.1086/653423 . [DOI] [PubMed] [Google Scholar]
- 9.Lisboa LF, Tong Y, Kumar D, Pang XL, Asberg A, Hartmann A, et al. Analysis and clinical correlation of genetic variation in cytomegalovirus. Transpl Infect Dis. 2012;14(2):132–40. Epub 2011/11/19. 10.1111/j.1399-3062.2011.00685.x . [DOI] [PubMed] [Google Scholar]
- 10.Ludwig A, Hengel H. Epidemiological impact and disease burden of congenital cytomegalovirus infection in Europe. Euro Surveill. 2009;14(9):26–32. Epub 2009/03/26. 19140 [pii]. . [PubMed] [Google Scholar]
- 11.Demmler GJ. Infectious Diseases Society of America and Centers for Disease Control. Summary of a workshop on surveillance for congenital cytomegalovirus disease. Rev Infect Dis. 1991;13(2):315–29. Epub 1991/03/01. . [DOI] [PubMed] [Google Scholar]
- 12.Fowler KB, McCollister FP, Dahle AJ, Boppana S, Britt WJ, Pass RF. Progressive and fluctuating sensorineural hearing loss in children with asymptomatic congenital cytomegalovirus infection. J Pediatr. 1997;130(4):624–30. Epub 1997/04/01. S0022-3476(97)70248-8 [pii]. . [DOI] [PubMed] [Google Scholar]
- 13.Kusne S, Shapiro R, Fung J. Prevention and treatment of cytomegalovirus infection in organ transplant recipients. Transpl Infect Dis. 1999;1(3):187–203. Epub 2001/06/29. tid010307 [pii]. . [DOI] [PubMed] [Google Scholar]
- 14.Zhang XW, Li F, Yu XW, Shi XW, Shi J, Zhang JP. Physical and intellectual development in children with asymptomatic congenital cytomegalovirus infection: a longitudinal cohort study in Qinba mountain area, China. J Clin Virol. 2007;40(3):180–5. Epub 2007/10/09. S1386-6532(07)00328-9 [pii] 10.1016/j.jcv.2007.08.018 . [DOI] [PubMed] [Google Scholar]
- 15.Halwachs-Baumann G, Wilders-Truschnig M, Enzinger G, Eibl M, Linkesch W, Dornbusch HJ, et al. Cytomegalovirus diagnosis in renal and bone marrow transplant recipients: the impact of molecular assays. J Clin Virol. 2001;20(1–2):49–57. Epub 2001/02/13. S1386-6532(00)00155-4 [pii]. . [DOI] [PubMed] [Google Scholar]
- 16.Tarrago D, Quereda C, Tenorio A. Different cytomegalovirus glycoprotein B genotype distribution in serum and cerebrospinal fluid specimens determined by a novel multiplex nested PCR. J Clin Microbiol. 2003;41(7):2872–7. Epub 2003/07/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pignatelli S, Dal Monte P, Rossini G, Chou S, Gojobori T, Hanada K, et al. Human cytomegalovirus glycoprotein N (gpUL73-gN) genomic variants: identification of a novel subgroup, geographical distribution and evidence of positive selective pressure. J Gen Virol. 2003;84(Pt 3):647–55. Epub 2003/02/27. 10.1099/vir.0.18704-0 . [DOI] [PubMed] [Google Scholar]
- 18.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular biology and evolution. 2011;28(10):2731–9. Epub 2011/05/07. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berenberg W, Nankervis G. Long-term follow-up of cytomegalic inclusion disease of infancy. Pediatrics. 1970;46(3):403–10. Epub 1970/09/01. . [PubMed] [Google Scholar]
- 20.Adler SP, Nigro G, Pereira L. Recent advances in the prevention and treatment of congenital cytomegalovirus infections. Semin Perinatol. 2007;31(1):10–8. Epub 2007/02/24. S0146-0005(07)00003-1 [pii] 10.1053/j.semperi.2007.01.002 . [DOI] [PubMed] [Google Scholar]
- 21.Ali S, Khan FA, Mian AA, Afzal MS. Seroprevalence of cytomegalovirus, herpes simplex virus and rubella virus among pregnant women in KPK province of Pakistan. Journal of infection in developing countries. 2014;8(3):389–90. 10.3855/jidc.3854 . [DOI] [PubMed] [Google Scholar]
- 22.Abu-Madi MA, Behnke JM, Dabritz HA. Toxoplasma gondii seropositivity and co-infection with TORCH pathogens in high-risk patients from Qatar. Am J Trop Med Hyg. 2010;82(4):626–33. Epub 2010/03/30. 10.4269/ajtmh.2010.09-0530 82/4/626 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.el-Nawawy A, Soliman AT, el Azzouni O, Amer el S, Karim MA, Demian S, et al. Maternal and neonatal prevalence of toxoplasma and cytomegalovirus (CMV) antibodies and hepatitis-B antigens in an Egyptian rural area. J Trop Pediatr. 1996;42(3):154–7. Epub 1996/06/01. . [DOI] [PubMed] [Google Scholar]
- 24.Hannachi N, Marzouk M, Harrabi I, Ferjani A, Ksouri Z, Ghannem H, et al. [Seroprevalence of rubella virus, varicella zoster virus, cytomegalovirus and parvovirus B19 among pregnant women in the Sousse region, Tunisia]. Bull Soc Pathol Exot. 2011;104(1):62–7. Epub 2011/01/19. 10.1007/s13149-010-0119-z . [DOI] [PubMed] [Google Scholar]
- 25.Omer AR, Salih JI, Al-Nakshabandi AA. Frequency of blood-borne viral infections among leukemic patients in central Iraq. Saudi Med J. 2011;32(1):55–61. Epub 2011/01/08. 0' [pii]. . [PubMed] [Google Scholar]
- 26.Tabatabaee M, Tayyebi D. Seroepidemiologic study of human cytomegalovirus in pregnant women in Valiasr Hospital of Kazeroon, Fars, Iran. J Matern Fetal Neonatal Med. 2009;22(6):517–21. Epub 2009/04/08. 10.1080/14767050902801678 910259521 [pii]. . [DOI] [PubMed] [Google Scholar]
- 27.Almeida LN, Azevedo RS, Amaku M, Massad E. Cytomegalovirus seroepidemiology in an urban community of Sao Paulo, Brazil. Rev Saude Publica. 2001;35(2):124–9. Epub 2001/05/19. S0034-89102001000200004 [pii]. . [DOI] [PubMed] [Google Scholar]
- 28.Uyar Y, Balci A, Akcali A, Cabar C. Prevalence of rubella and cytomegalovirus antibodies among pregnant women in northern Turkey. New Microbiol. 2008;31(4):451–5. Epub 2009/01/07. . [PubMed] [Google Scholar]
- 29.Ocak S, Zeteroglu S, Ozer C, Dolapcioglu K, Gungoren A. Seroprevalence of Toxoplasma gondii, rubella and cytomegalovirus among pregnant women in southern Turkey. Scand J Infect Dis. 2007;39(3):231–4. Epub 2007/03/17. 771165636 [pii] 10.1080/00365540600978880 . [DOI] [PubMed] [Google Scholar]
- 30.Manicklal S, Emery VC, Lazzarotto T, Boppana SB, Gupta RK. The "silent" global burden of congenital cytomegalovirus. Clinical microbiology reviews. 2013;26(1):86–102. 10.1128/CMR.00062-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Enan KA, Rennert H, El-Eragi AM, El Hussein AR, Elkhidir IM. Comparison of Real-time PCR to ELISA for the detection of human cytomegalovirus infection in renal transplant patients in the Sudan. Virology journal. 2011;8:222 10.1186/1743-422X-8-222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kramer A, Schwebke I, Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC infectious diseases. 2006;6:130 Epub 2006/08/18. 10.1186/1471-2334-6-130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dowd JB, Haan MN, Blythe L, Moore K, Aiello AE. Socioeconomic gradients in immune response to latent infection. Am J Epidemiol. 2008;167(1):112–20. Epub 2007/09/18. kwm247 [pii] 10.1093/aje/kwm247 . [DOI] [PubMed] [Google Scholar]
- 34.Boppana SB, Rivera LB, Fowler KB, Mach M, Britt WJ. Intrauterine transmission of cytomegalovirus to infants of women with preconceptional immunity. N Engl J Med. 2001;344(18):1366–71. Epub 2001/05/03. 10.1056/NEJM200105033441804 . [DOI] [PubMed] [Google Scholar]
- 35.Daiminger A, Bader U, Enders G. Pre- and periconceptional primary cytomegalovirus infection: risk of vertical transmission and congenital disease. BJOG. 2005;112(2):166–72. Epub 2005/01/25. BJO00328 [pii] 10.1111/j.1471-0528.2004.00328.x . [DOI] [PubMed] [Google Scholar]
- 36.Yu ZS, Zou CC, Zheng JY, Zhao ZY. Cytomegalovirus gB genotype and clinical features in Chinese infants with congenital infections. Intervirology. 2006;49(5):281–5. Epub 2006/05/23. 93458 [pii] 10.1159/000093458 . [DOI] [PubMed] [Google Scholar]
- 37.Paradowska E, Jablonska A, Studzinska M, Suski P, Kasztelewicz B, Zawilinska B, et al. Distribution of cytomegalovirus gN variants and associated clinical sequelae in infants. J Clin Virol. 2013;58(1):271–5. Epub 2013/06/29. 10.1016/j.jcv.2013.05.024 S1386-6532(13)00204-7 [pii]. . [DOI] [PubMed] [Google Scholar]
- 38.Paradowska E, Jablonska A, Studzinska M, Kasztelewicz B, Zawilinska B, Wisniewska-Ligier M, et al. Cytomegalovirus glycoprotein H genotype distribution and the relationship with hearing loss in children. J Med Virol. 2014;86(8):1421–7. Epub 2014/03/13. 10.1002/jmv.23906 . [DOI] [PubMed] [Google Scholar]
- 39.Ross SA, Novak Z, Pati S, Patro RK, Blumenthal J, Danthuluri VR, et al. Mixed infection and strain diversity in congenital cytomegalovirus infection. J Infect Dis. 2011;204(7):1003–7. Epub 2011/09/02. 10.1093/infdis/jir457 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pati SK, Pinninti S, Novak Z, Chowdhury N, Patro RK, Fowler K, et al. Genotypic diversity and mixed infection in newborn disease and hearing loss in congenital cytomegalovirus infection. Pediatr Infect Dis J. 2013;32(10):1050–4. Epub 2013/05/23. 10.1097/INF.0b013e31829bb0b9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ross SA, Ahmed A, Palmer AL, Michaels MG, Sanchez PJ, Bernstein DI, et al. Detection of congenital cytomegalovirus infection by real-time polymerase chain reaction analysis of saliva or urine specimens. The Journal of infectious diseases. 2014;210(9):1415–8. 10.1093/infdis/jiu263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lukacsi A, Tarodi B, Endreffy E, Babinszki A, Pal A, Pusztai R. Human cytomegalovirus gB genotype 1 is dominant in congenital infections in South Hungary. J Med Virol. 2001;65(3):537–42. Epub 2001/10/12. 10.1002/jmv.2070 [pii]. . [DOI] [PubMed] [Google Scholar]
- 43.Barbi M, Binda S, Caroppo S, Primache V, Dido P, Guidotti P, et al. CMV gB genotypes and outcome of vertical transmission: study on dried blood spots of congenitally infected babies. J Clin Virol. 2001;21(1):75–9. Epub 2001/03/20. S1386-6532(00)00188-8 [pii]. . [DOI] [PubMed] [Google Scholar]
- 44.Rasmussen L, Hong C, Zipeto D, Morris S, Sherman D, Chou S, et al. Cytomegalovirus gB genotype distribution differs in human immunodeficiency virus-infected patients and immunocompromised allograft recipients. J Infect Dis. 1997;175(1):179–84. Epub 1997/01/01. . [DOI] [PubMed] [Google Scholar]
- 45.Coaquette A, Bourgeois A, Dirand C, Varin A, Chen W, Herbein G. Mixed cytomegalovirus glycoprotein B genotypes in immunocompromised patients. Clin Infect Dis. 2004;39(2):155–61. Epub 2004/08/13. 10.1086/421496 CID32984 [pii]. . [DOI] [PubMed] [Google Scholar]
- 46.Arav-Boger R, Willoughby RE, Pass RF, Zong JC, Jang WJ, Alcendor D, et al. Polymorphisms of the cytomegalovirus (CMV)-encoded tumor necrosis factor-alpha and beta-chemokine receptors in congenital CMV disease. J Infect Dis. 2002;186(8):1057–64. Epub 2002/10/02. JID020054 [pii] 10.1086/344238 . [DOI] [PubMed] [Google Scholar]
- 47.Bale JF Jr, Petheram SJ, Souza IE, Murph JR. Cytomegalovirus reinfection in young children. J Pediatr. 1996;128(3):347–52. Epub 1996/03/01. S0022-3476(96)70279-2 [pii]. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.