Abstract
The sessile plants have evolved diverse intrinsic mechanisms to control their proper development under variable environments. In contrast to plastic vegetative development, reproductive traits like floral identity often show phenotypic robustness against environmental variations. However, it remains obscure about the molecular basis of this phenotypic robustness. In this study, we found that eg1 (extra glume1) mutants of rice (Oryza savita L.) showed floral phenotypic variations in different growth locations resulting in a breakdown of floral identity robustness. Physiological and biochemical analyses showed that EG1 encodes a predominantly mitochondria-localized functional lipase and functions in a high temperature-dependent manner. Furthermore, we found that numerous environmentally responsive genes including many floral identity genes are transcriptionally repressed in eg1 mutants and OsMADS1, OsMADS6 and OsG1 genetically act downstream of EG1 to maintain floral robustness. Collectively, our results demonstrate that EG1 promotes floral robustness against temperature fluctuation by safeguarding the expression of floral identify genes through a high temperature-dependent mitochondrial lipid pathway and uncovers a novel mechanistic insight into floral developmental control.
Author Summary
Various mechanisms have evolved to ensure proper organ formation under variable environments in order to complete one organism’s life cycle. In angiosperms, vegetative and reproductive organs show a differential plastic development between varied environments, with a low plasticity or high robustness for flower formation, but little is known about its intrinsic mechanism. Here we report that gene EG1 (EXTRA GLUME1) can enhance the floral robustness against temperature fluctuation in rice. EG1 encodes a predominantly mitochondria-localized functional lipase and its loss of function disrupts floral development in a high temperature-dependent manner. In consistent, both EG1 and its lipase activity are positively induced by high temperature. Transcriptomic and genetic analyses revealed that EG1 functions upstream of several floral identity genes, eg, OsMADS1, OsMADS6 and OsG1. Taken together, our results uncover a novel mitochondria-mediated lipid metabolic pathway to promote floral developmental robustness. Our findings may help to genetically improve floral traits of rice to maintain a stable yield when planted in different locations and/or under heat stress conditions.
Introduction
The sessile plants have evolved various exquisite adaptive strategies to cope with environmental changes [1,2]. Among them, phenotypic plasticity is the ability of a single genotype capable of producing different phenotypes in response to varying environments [3–6]. For an integral high fitness, morphologies of vegetative organs of a single plant, such as roots, leaves and stems, require a high phenotypic plasticity [7–10], whereas that of reproductive organs, such as flowers, fruits and seeds, are always associated with low plasticity also known as phenotypic robustness/stability [11–15]. Thus, plants must coordinate the developments of these organs.
Compared with progresses in understanding the molecular mechanisms of high phenotypic plasticity [10,16–19], very little is known about the molecular basis of phenotypic robustness [20,21]. Recent studies have shown that there are a group of specific genes regulating the degree of phenotypic plasticity and determining the reaction norm of a trait among various environments, which are termed plasticity genes [22–24]. However, most of the identified plasticity genes are high plasticity-associated [16,25–27], only few promote phenotypic robustness [28–30]. Members of HSP90 (HEAT SHOCK PROTEIN 90) family, as central hubs of numerous biological pathways, are required for maintenance of phenotypic robustness in both animals and plants [28,31–34]. MSH1 (MutS HOMOLOG1), a homolog of bacterial mismatch repair protein MutS, has been reported to repress the developmental plasticity of plant architecture, leaf morphology and flowering time in several dicot and monocot plants [29,35]. A nuclear protein RPL1 (RICE PLASTICITY1) in rice also appears to promote the relatively stable plant architecture and panicle structure between different environments [30]. Despite these discoveries, we still know very little about the molecular mechanisms of phenotypic robustness, especially that of plant reproductive traits. In addition to the known epigenetics-dependent transcriptional regulation and hormone signaling [20,29–31,35], lipid homeostasis is also known to influence phenotypic robustness [36,37]. Coordinated regulations of cellular lipid homeostasis are crucial to organisms’ adaptive robustness under severe temperatures [37–40]. Furthermore, lipid-related synthetases and lipases can also be regulated at transcriptional and posttranslational levels to influence lipid homeostasis [41,42]. Among them, mitochondria-associated lipid metabolism is key to the lipid homeostasis [43]. For instance, Arabidopsis seedlings with decreased cardiolipin in mitochondrial membrane are easier to turn yellow and necrotic under extended darkness or heat due to a failure of mitochondrial morphogenesis, showing a lowered stability [41,44,45]. However, it remains unclear whether mitochondria also mediate the phenotypic robustness in plant reproductive organs.
Flower morphology, as a gold standard in plant taxonomy, has the most remarkable robustness within and between individuals of the same population [11,46], making it an ideal trait for studies on the molecular basis of phenotypic robustness against environmental fluctuation. Nevertheless, so far no gene has been identified to regulate the phenotypic robustness of floral identity, although several environment-dependent floral mutants have been reported [47–52]. We previously found that a rice floral mutant eg1 (extra glume1) exhibited a floral variation in different growth conditions [53], implying that EG1 is likely involved in floral robustness. Recent studies have shown that EG1 encodes a putative lipase regulating rice floral identity and meristem determinacy [53]. It also functions in JA (jasmonic acid) biosynthesis to promote the expression of floral identity gene OsMADS1 through an EG2/OsJAZ1, OsCOIb and OsMYC2- mediated JA signaling pathway [54], similar to its homologous genes AtDAD1 (DEFECTIVE IN ANTHER DEHISCENCE1) and AtDGL (DONGLE) in Arabidopsis [55,56]. In this study, we find that EG1 is a predominantly mitochondria-localized functional lipase and promotes floral robustness against temperature fluctuation in a high temperature-dependent manner. Collectively, our results reveal a novel molecular mechanism underlying floral phenotypic robustness.
Results
eg1 shows high plasticity in floral identity through an interaction of genotype and environment
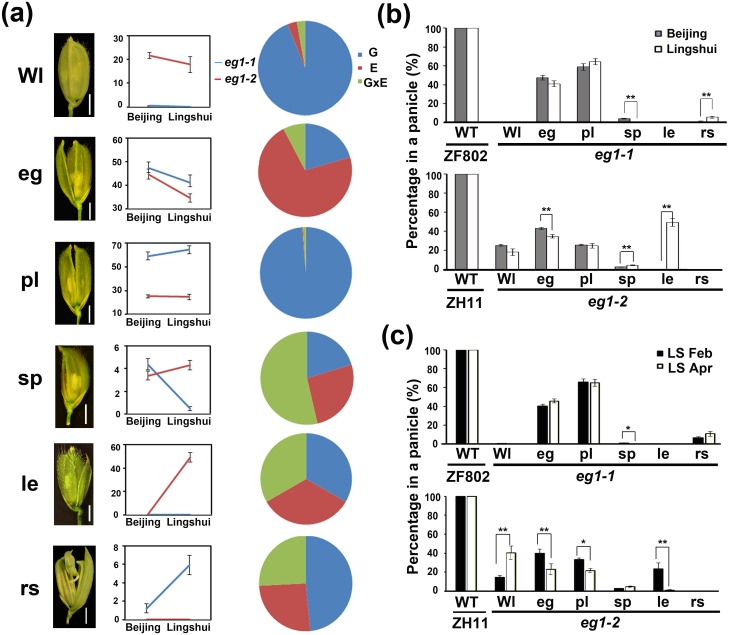
Previously, we found that eg1 displayed a floral identity variation possibly influenced by growth conditions [53]. To examine if this variability was mainly due to the environmental alterations, we analyzed the spikelet phenotypes of eg1-1 (in indica ZF802 background) and eg1-2 (in japonica ZH11 background) in two groups of separate environments (Fig 1) and found that the floral phenotypic variability of eg1 is likely caused by both genotype and environment. To define the phenotypic variability, we divided the spikelet phenotypes of eg1 into six groups, which were called variable phenotypes, including Wl (WT-like), eg (extra glume), pl (palea to lemma), sp (smaller pa), le (long empty glumes) and rs (reiterated spikelets) (Fig 1A, S1 Fig and S1 Table). The results showed that sp and rs of eg1-1 as well as Wl, le, eg and sp of eg1-2 exhibited significant plasticity between two environments, especially le of eg1-2, which displayed the highest plasticity (Fig 1B and 1C), suggesting that environment also contributes to the variations of floral phenotypes of eg1. To further examine the relationships of genotype (G), environment (E), genotype-environment interaction (GxE) and the variable phenotypes, we calculated their effects on phenotypes by two-factor ANOVA and found that Wl and pl were affected mainly by G, eg by both E and G but rarely by GxE, sp, le and rs by all three factors, and among them, le could serve as a marker for the phenotypic plasticity of eg1-2 due to its large proportion in a panicle and opposite phenotypes between two environments (Fig 1A). Taken together, these results showed that eg1 shows higher floral plasticity, suggesting that EG1 promotes the floral robustness in rice.
Fig 1. Phenotypic plasticity of floral identities of eg1.
(a) Morphology and plasticity analysis of six variable phenotypes of eg1 spikelets. Floral structures and two-factor plots with percentages per panicle in the y axis of all variable phenotypes are shown in the left and middle columns, respectively. Effects of G, E and GxE on each variable phenotype shown in the pie charts (right column) were analyzed by two-way ANOVA. Bar = 2 mm in floral structures. (b) Statistical analysis of the phenotypic plasticity of eg1 and wild-type spikelets in two different planting locations. Beijing, Beijing summer. Lingshui, Lingshui winter. (c) Statistical analysis of the phenotypic plasticity of eg1 and wild-type spikelet in two different planting seasons. LS Feb, Lingshui Feb. LS Apr, Lingshui Apr. Wl, WT-like; eg, extra glume; pl, palea to lemma; sp, smaller pa; le, long empty glumes; rs, reiterated spikelets. Values are means ± SE, number of analyzed panicles ≥ 20 in (b), ≥ 10 in (c). Significant difference was determined by ANOVA, *P < 0.05, **P < 0.01.
Allelic eg1 and their genetic backgrounds together regulate the eg1 floral plasticity
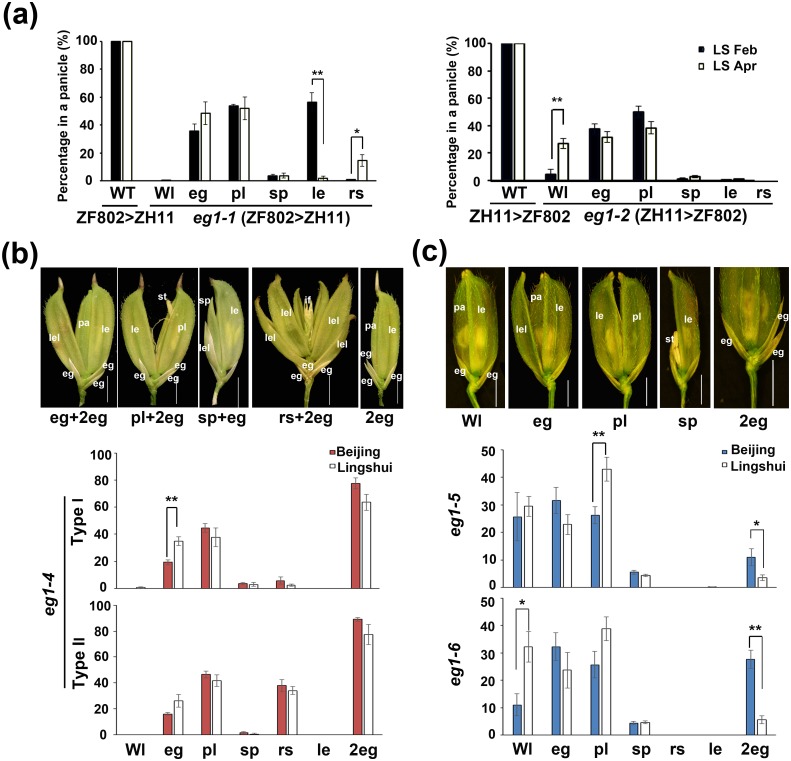
To further examine the influence of genotype on the floral plasticity of eg1, we swapped the genetic backgrounds of two eg1 alleles. eg1-1 in a largely japonica background showed high phenotypic plasticity especially for le and rs phenotypes, similar to eg1-2 (ZH11), whereas eg1-2 in an indica-dominant background showed low floral plasticity similar to eg1-1 (ZF802) (Fig 2A), indicating that genetic backgrounds also influence the phenotypic plasticity of eg1 spikelets.
Fig 2. Subspecific variations of eg1 floral plasticity.
(a) Floral plasticity of eg1 alleles in two exchanged backgrounds. eg1-1 (ZF802>ZH11) and eg1-2 (ZH11>ZF802) show eg1-1 and eg1-2 backcrossed into ZH11 or ZF802 backgrounds, respectively. (b) Floral plasticity of eg1-4 in indica Dular background. Statistical analysis of two types of panicles according to rs (Type I and Type II) are shown. (c) Floral plasticity of eg1-5 and -6 in japonica Nipponbare background. Statistical analysis of the two independent lines are shown. LS Feb, Lingshui Feb. LS Apr, Lingshui Apr. Beijing, Beijing summer. Lingshui, Lingshui winter. Variable phenotypes of spikelets are defined as in S1 Table, and percentages of them in a panicle are shown in the y axis. le, lemma; pa, palea; st, stamen; eg, empty glume; if, inflorescence primordia; sp, smaller pa; lel, lemma-like organ; pl, palea-lemma mosaic organ. Bars = 2 mm. Values are means ± SE, number of analyzed panicles ≥ 5, and significant difference was determined by ANOVA, *P < 0.05, **P < 0.01.
To verify this finding, we further used CRISPR/Cas9 technology to construct eg1 alleles in Nipponbare (japonica) and Dular (indica) backgrounds. Two types of spikelet phenotypes were found in eg1-4 allele with Dular background and both showed low plasticity (Fig 2B), while eg1-5 and -6 alleles in Nipponbare background showed relatively higher plasticity than alleles in two indica backgrounds ZF802 and Dular, similar to that in japonica ZH11 (Fig 2C and S2 Fig), suggesting that the floral plasticity of eg1 alleles in japonica backgrounds tend to be higher than that in indica backgrounds. In another aspect, eg1 alleles in indica backgrounds had severer floral disturbance than that in japonica concerning Wl and rs phenotypes (Fig 2 and S1 Fig), suggesting that EG1 has functions in both floral robustness and identity, which are differentiated in two subspecies. To explore the possible causes of these differentiation, we compared the cis-elements and expressional level of EG1 in several japonica and indica varieties and discovered the correlative differences in both cis-elements and expressional levels between japonica and indica (two types) varieties (S3 Fig and S2 Table), which implied that transcriptional differences may be a crucial cause of functional differentiation of EG1 in subspecies. All these results indicated that both eg1 allelic variations and their genetic backgrounds regulate the floral plasticity of eg1.
Temperature is a major environmental factor mediating the plastic development of eg1 spikelets
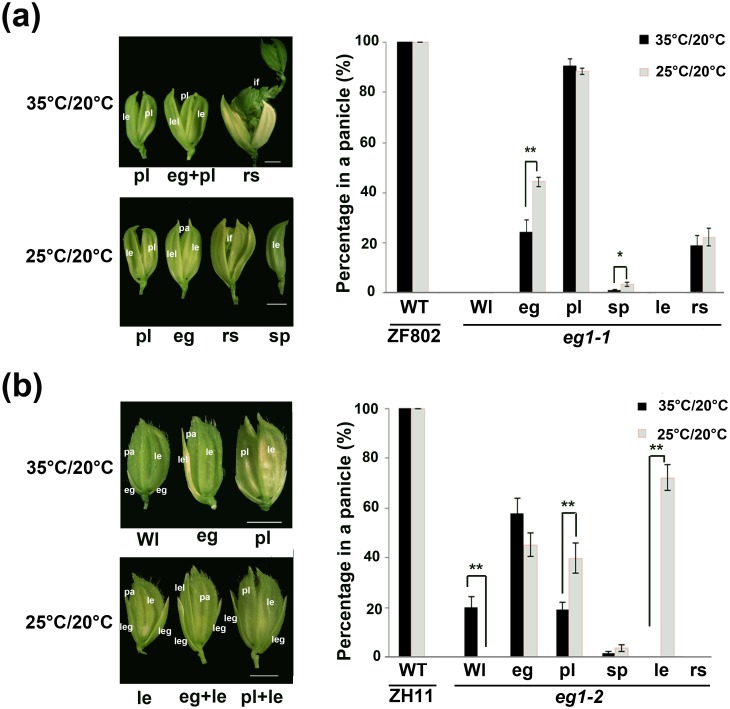
In order to find out the environmental factors mediating the plastic development of eg1 spikelets, we first compared the growing conditions for phenotypic analysis and found a marked difference in daily high temperatures of two environments (S4 Fig), suggesting that the temperature variation between two environments could be a major environmental factor influencing the eg1 plasticity. To verify this prediction, floral plasticity of wild-types and eg1 alleles were examined in two artificial growth chambers with 35°C light 12 hr / 20°C dark 12 hr and 25°C light 12 hr / 20°C dark 12 hr respectively, while other growth conditions were kept identical. The low plasticity of eg1-1 and nearly 70% le phenotypes of eg1-2 showed that the floral plasticity of eg1 in the chambers was similar to and even higher than that under natural growth conditions (Fig 3). These results showed that temperature is a major environmental factor mediating the floral plasticity of eg1.
Fig 3. Temperature-dependent floral plasticity of eg1.
(a) Floral plasticity of eg1-1 (ZF802) under two different temperature conditions. (b) Floral plasticity of eg1-2 (ZH11) under two different temperature conditions. Spikelet phenotypes and statistical analysis are shown in the left and right of (a) and (b). Variable phenotypes of spikelets are defined as in S1 Table. le, lemma; pa, palea; eg, empty glume; if, inflorescence primordia; lel, lemma-like organ; pl, palea-lemma mosaic organ; leg, long empty glume in spikelet structures. Bars = 2 mm. Values are means ± SE, number of analyzed panicles >10, and significant difference was determined by ANOVA, *P < 0.05, **P < 0.01.
EG1 encodes a predominately mitochondria-localized functional lipase
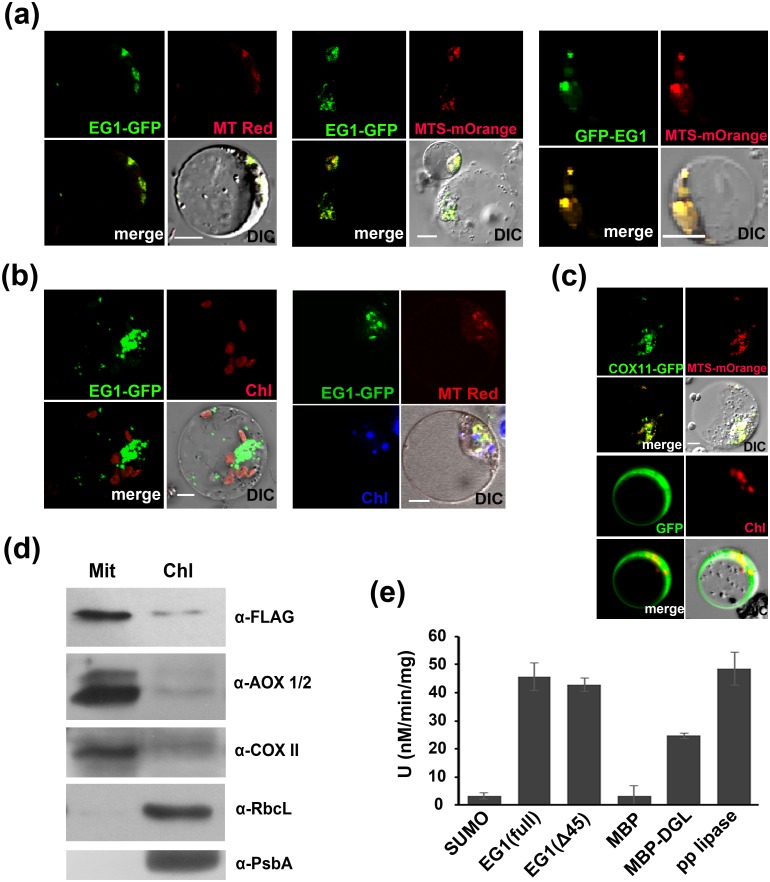
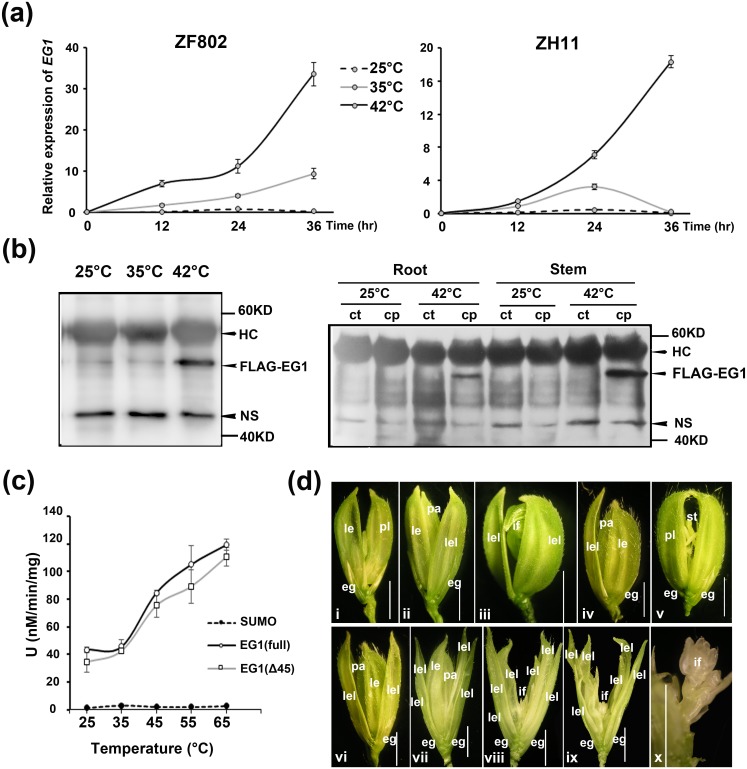
Previously, EG1 was shown to be localized in chloroplasts in transient expression assays [54]. However, EG1-like lipases appear to have variable subcellular locations [56–59]. To examine the subcellular localization of EG1 in vivo, two different EG1 and GFP fusion proteins driven by 35S promoter were first expressed in rice protoplasts and were found to be co-localized with both mitochondrial specific dye Mito Tracker Red and mitochondrial maker protein MTS-mOrange [60] but hardly with chloroplast auto-fluorescence, and an EG1-GFP fusion protein driven by native promoter was also detected in mitochondria (Fig 4A and 4B), suggesting that EG1 protein is mainly, if not all, localized in mitochondria. To compare this finding with the previous one, the reported vector pCAMBIA1301-Pro35S:EG1-GFP [54] was also examined in our transient system and a similar localization was detected (S5 Fig). To further verify the EG1 localization, subcellular fractionations of one-week seedlings of eg1-2 complementation lines with FLAG-EG1 (S6 Fig) were successively carried out and the EG1 fusion protein was predominately co-fractionated with mitochondria (Fig 4D), confirming the mitochondrial localization of EG1 in vivo. Taken together, these results showed that EG1 encodes a protein predominately localized in mitochondria. To explore the biochemical function of EG1, we tested its lipase activity [53] in vitro and found that both the full-length EG1 and truncated EG1 without predicted targeting peptides showed significant lipase activity (Fig 4E), indicating that EG1 encodes a functional lipase. Taken together, our results showed that EG1 functions as a predominately mitochondria-localized lipase.
Fig 4. EG1 encodes a functional lipase predominately localized in mitochondria.
(a) Co-localization of EG1-GFP or GFP-EG1 fusion protein with mitochondria in rice protoplasts. Mitochondria are marked by dye Mito Tracker Red (MT Red) or MTS-mOrange protein. (b) Localization analysis of EG1-GFP and chloroplasts in rice protoplasts. An EG1-GFP driven by 35S or native promoter is shown in the left and right, respectively. Chloroplasts are detected by its auto-fluorescence. Mitochondria are marked by Mito Tracker Red (MT Red). (c) Localization of mitochondrial (35SPro:COX11-GFP) [60] (up) and cellular (35SPro:GFP) (bottom) controls of rice protoplasts. DIC, pictures photographed by differential interference contrast microscope. Bar = 10 μm. (d) Subcellular fractionation assay. Mit, mitochondria fraction; Chl, chloroplasts fraction; α-FLAG, antibody of FLAG-EG1; α-AOX1/2 and α-COXII, specific antibodies of mitochondrial proteins AOX1/2 and COXII; α-RbcL and α-PsbA, specific antibodies of chloroplast proteins RbcL and PsbA. (e) Lipase activity of EG1 in vitro with P-nPB as a substrate at 30°C. EG1 (Full) and EG1 (Δ45) respectively refer to fusion proteins of full-length or no N-terminal (45 aa) protein of EG1 and SUMO peptide. DGL and pp lipase (porcine pancreatic lipase) were used as positive controls. Values are means ± SE for three independent experiments.
EG1 functions in a high temperature-dependent manner in the floral robustness regulation
The dependence of the eg1 floral plasticity on environmental temperature raised a possibility that either EG1 or its product or both are likewise regulated by temperature. To examine these possibilities, some heat/cold responsive cis-elements were discovered in the 2 kb genomic sequence upstream of the start codon of EG1 (S3 Table), implying that its expression could be induced by extreme temperatures. To examine this possibility, one-week wild-type seedlings were treated under different temperatures and the EG1 transcript was found to accumulate gradually, to an extremely high extent under heat shock (42°C) as well as usual high temperature 35°C for rice (Fig 5A), but to some extent suppressed under cold stress (4°C) (S7A Fig), indicating the high temperature-induced expression of EG1. A similar result was obtained by using young inflorescences in which EG1 has a high expression (S7B Fig). To examine whether EG1 protein was also influenced by high temperatures, accumulation of FLAG-EG1 fusion protein in eg1-2 complementation lines, with a temperature-insensitive promoter (S7C and S7E Fig), was detected under different temperatures and found it was significantly induced at extreme high temperature 42°C than 25°C and 35°C (Fig 5B and S7D Fig), indicating a stabilization of EG1 protein under heat stress. Furthermore, we detected that lipase activity of EG1 fusion proteins increase as temperature rising (Fig 5C), consistent with the assumption of EG1’s function required under high temperatures. Additionally, we also examined the effect of high temperatures on EG1 subcellular localization, and found no obvious translocation in protoplast system (S8A Fig), while failed to detect EG1 protein in the subcellular fractions of complementation lines except under heat stress for its minute amount (S8B Fig), suggesting that temperature does not significantly influence the subcellular localization of EG1. The increased the transcriptional level, protein stability and lipase activity of EG1 under high temperatures, implying its more significant role under high temperatures. To verify this hypothesis, we observed the floral phenotypes of eg1 mutants under extremely high temperatures and found much severer spikelets in eg1 mutants especially in eg1-2, with multilayer lemma-like organs and undetermined inflorescence meristem, which have never been found in other temperature conditions (Fig 5D), showing a more significant function of EG1 at higher temperatures in floral robustness. eg1 was also found to grow faster than wild-type during primary growing days [54], and we detected this phenotype was much severer under extremely high temperature than others compared with wild-type, which was consistent with the floral phenotype (S9 Fig). Therefore, we concluded that EG1 functions in a high temperature-dependent manner to regulate the floral robustness.
Fig 5. High temperature-dependent manner of EG1 in floral robustness regulation.
(a) RT-qPCR analysis of EG1 expression induced by high temperatures in two wild-types. Values are means ± SE (n = 3), and significant difference was determined by ANOVA, *P < 0.05, **P < 0.01, and rice α-TUBULIN as the reference. (b) Western blot analysis of FLAG-EG1 protein accumulation under different temperatures and different tissues in the EG1 complementation lines for 24 hr. Cp, Complementation lines; Ct, non-transgenic wild-type control. HC, Heavy chain of IgG; NS, Nonspecific band (as a loading control). (c) Temperature-dependent lipase activity of EG1. EG1 (Full) and EG1 (Δ45) respectively refer to full-length and no N-terminal (45 AA) protein of EG1 fused to SUMO peptide. Values are means ± SE for three independent experiments. (d) Floral phenotypes of eg1 mutants in a condition of 40°C light 12 hr / 30°C dark 12 hr. Spikelets of eg1-1 with pl, eg and rs phenotypes are shown on i, ii and iii, respectively. Spikelets of eg1-2 with eg and pl phenotypes are shown on iv and v, and with multilayer lemma-like glumes (lel) and/or undetermined inflorescences primordia are on vi to x. x is the inside of ix. le, lemma; pl, palea-lemma mosaic organ; eg, empty glume; lel, lemma-like organ; pa, palea; st, stamen; if, inflorescence primordia. Bars = 2 mm.
EG1 mediates the transcriptional responses of downstream genes and pathways to environmental fluctuation
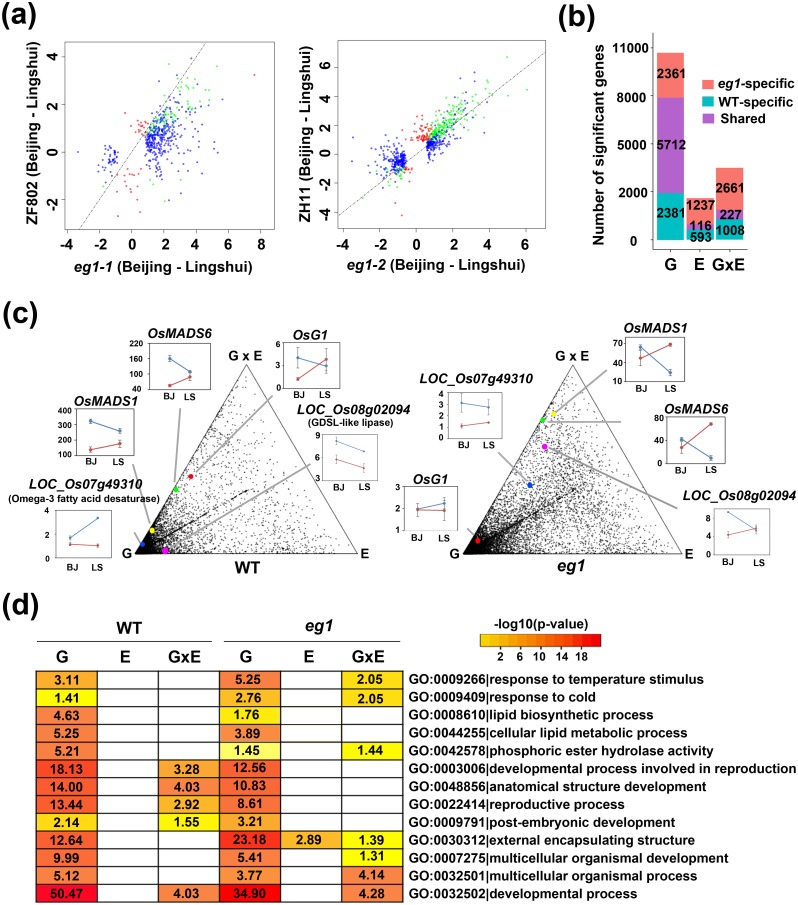
Since the floral plasticity of eg1 was influenced by both genotype and environment, to examine the molecular mechanism of genotype-environment interaction in floral plasticity, transcriptomes of inflorescences of two eg1 alleles (eg1-1 and eg1-2) and their wild-types (ZF802 and ZH11) in Beijing and Lingshui were analyzed. First, to evaluate the reliability of the transcriptomic data, we divided all transcripts into 33 modules by co-expression network analysis and analyzed their correlations with six variable phenotypes, and it turned out that the relationships among the variable phenotypes derived from these correlations were quite similar to their morphological correlations (S10 Fig), showing a good reliability of the transcriptomic data. Second, through overall comparisons of all transcriptomes, we discovered that the expression patterns of floral transcriptomes of eg1 alleles between two environments were significantly different from their wild-types (S11A Fig), indicating a role of EG1 in regulating expressions of environmentally responsive genes. The numbers of environmentally responsive genes in eg1 mutants were much larger than that of wild-types, in contrast to the similar numbers between two wild-types or two eg1 alleles (Fig 6A and S11B Fig), implying that EG1 negatively regulates the responses of its downstream genes to environment. To verify this finding, we analyzed the effects of G, E, and GxE on transcriptomes of eg1 and wild-type by two-way ANOVA and found that the number of genes significantly affected by E and GxE in eg1 were significantly larger than that in wild-types (Fig 6B, S11C Fig and S4 Table), displaying a switch of many genes from G-affected to E/GxE-affected ones (Fig 6C), indicating that EG1 represses its downstream genes not only to respond to, but also to interact with environment. Furthermore, the effects of the three factors on several important pathways varied significantly between eg1 and wild-type, including the pathways related to temperature response, lipid metabolism and floral development (Fig 6D and S5 Table), indicating that EG1 mediates a crosstalk of these pathways with environment. Since EG1 was reported to regulate JA biosynthesis [54], in order to analyze its effect on the floral robustness control, we examined the expressional patterns of JA biosynthesis and signaling associated genes in our transcriptome data, and found that the transcriptional responsive patterns to environment or transcriptional level of several JA signaling genes (JAZ7 and JAZ8) and JA biosynthesis genes (four methyltransferase genes) are varied in eg1 mutants (S12 Fig), implying a possible role of JA in the EG1-associated floral robustness regulation but different from previously reported [54]. Taken together, these results revealed that EG1 mediates the transcriptional responses of downstream genes and pathways to environmental fluctuation.
Fig 6. Genotype (G), environment (E) and genotype-environment interaction (GxE)-dependent gene expressional variations of eg1.
(a) Scatterplots of comparisons of environmentally responsive genes between eg1 alleles and wild-types. x and y axes are values of log2 [ratios of gene expression in Beijing to that in Lingshui] of two genotypes respectively. Points represent wild-type-specific (red), eg1-specific (blue) and shared (green) genes. Dotted lines indicate y = x lines. (b) Comparisons of genes significantly affected by G, E and GxE in wild-type and eg1. Effects were analyzed by two-way ANOVA. (c) Triangular scatterplots of distributions of genes significantly affected by G, E and GxE in wild-type and eg1. Each dot indicates a gene, and the three vertexes of triangle indicate three factors G, E, GxE respectively. The closer distance between a gene and a vertex means the stronger effect of the factor on the gene. Insets show expressions of some representative genes in ZF802/eg1-1 (blue lines) and ZH11/eg1-2 (red lines) of Beijing (BJ) and Lingshui (LS). Effects were analyzed by two-way ANOVA. (d) Comparisons of thirteen major pathways affected by G, E and GxE in wild-type and eg1. Numbers in boxes indicate -log10 (P-value) of the pathway enrichment tested by Fisher's exact test with Bonferroni correction and blank boxes no statistical significance.
OsMADS1, OsMADS6 and OsG1 act downstream of EG1 to mediate floral robustness regulation
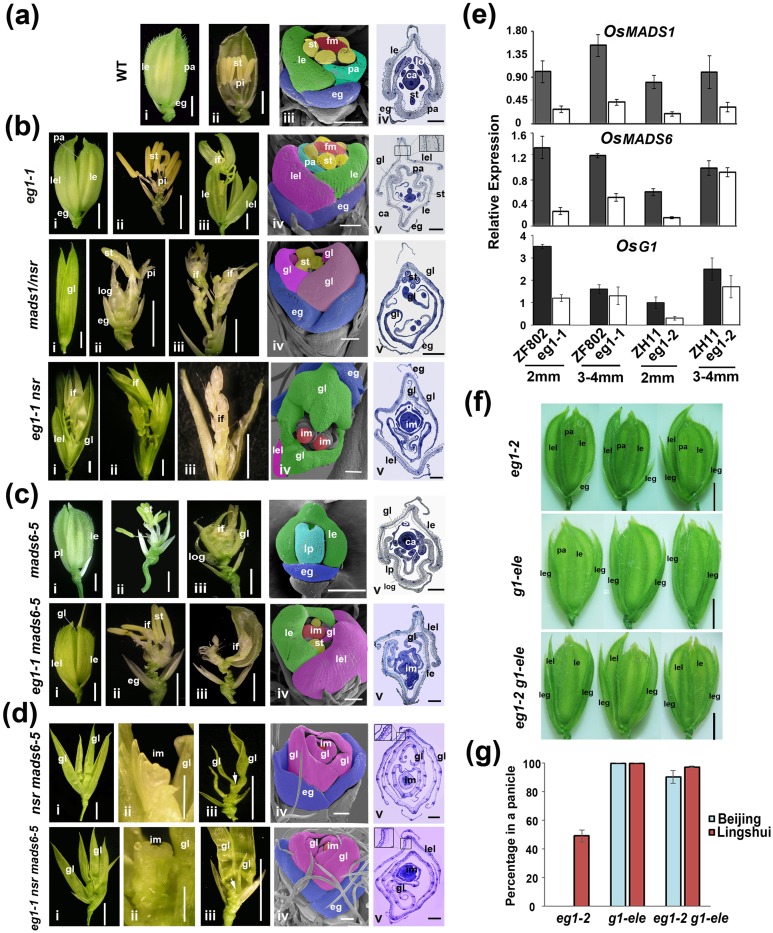
The significant transcriptional effects on the floral development pathways based on the G, E and GxE analysis in eg1 mutant suggested that floral identity genes are likely involved in EG1-dependent floral robustness regulation. To examine this, expressional variation of thirteen floral identity genes between two environments were further analyzed, and seven (OsMADS1, OsMADS6, OsG1, OsMADS4, OsMADS7, OsMADS8, OsMADS58) of them showed both varied environment-dependent expression patterns and repressed expression levels in eg1 (S13 Fig), indicating their positive regulatory roles in the floral robustness. To further examine this possibility, genetic analysis between EG1 and three of them (OsMADS1, OsMADS6 and OsG1) were performed. OsMADS1 and OsMADS6 are two major genes regulating glume identity and floral determinacy in rice [61–69], and their expressions were significantly varied in eg1 (Fig 7E and S13 Fig), indicating that EG1 is required for their expressions. To examine their genetic relationships with EG1, a double mutant of OsMADS1 mutant allele nsr [61] and eg1-1 was generated and it exhibited longer and leafy lemmas/paleas similar to nsr, with all inner three whorls replaced by half-developed inflorescences or inflorescence primordia, which is severer than both single mutants (Fig 7B), indicating that OsMADS1 functions downstream of EG1 in lemma/palea identity and they may together regulate the determinacy of inner three whorls. In addition, the double mutant of eg1-1 and OsMADS6 mutant allele osmads6-5 [66] showed abnormal paleas, with all transformed into one or two small lemma-like glumes and mostly with inflorescence primordia inside the spikelets (Fig 7C), indicating that OsMADS6 functions downstream of EG1 in specifying palea but may also regulate floral determinacy together with EG1. osmads6-5 exhibited weaker floral disturbance in the F2 population when crossed with eg1-1 (ZF802), with lemma-palea mosaic paleas and usually normal inner whorls (Fig 7C), showing its floral phenotype is also greatly influenced by genetic backgrounds. To further examine the relationships among EG1, OsMADS1 and OsMADS6 especially in floral determinacy, nsr osmads6-5 and eg1-1 nsr osmads6-5 were successively generated. The floral meristems of these two mutants similarly generated continuous glume primordia or became inactive before inner three whorls developed (Fig 7D), which were more dedifferentiated than the inflorescence primordia of eg1-1 nsr and eg1-1 osmads6-5, supporting the findings that both OsMADS1 and OsMADS6 act downstream of EG1 and they together control the floral differentiation of inner three whorls. Compared with eg1-1, the rs plasticity of eg1-1 nsr osmads6-5 totally disappeared when these two MADS genes were both deficient (Fig 7B and 7D), supporting the important roles of OsMADS1 and OsMADS6 in the rs plasticity regulation. Additionally, the spikelet phenotype of eg1-1 nsr [54] and nsr osmads6-5 [68,69] were consistent to the previously reported, and eg1 was linked with the lemma-like (lel) structure, and not affected by the deficiency of OsMADS1 and OsMADS6 (Fig 7b–7d), implying that it is probably a special organ different from lemma and palea. Taken all these results together, we concluded that MADS1 and MADS6 together function downstream of EG1 to control the determinacy of inner three whorls of rice floret as well as to mediate the rs plasticity of eg1-1.
Fig 7. OsMADS1, OsMADS6 and OsG1 mediate the floral robustness regulation of EG1.
(a) Spikelets of wild-type rice. i-iv show outside (i), inside (ii), SEM (iii) and paraffin transverse section (iv) of wild-type spikelets, respectively. (b) Genetic analysis of eg1-1 and mads1/nsr. Photos show the outside (i), inside (ii-iii), SEM (iv) and paraffin transverse section (v) of eg1-1, mads1/nsr and eg1-1 nsr mutant spikelets, respectively. (c) Genetic analysis of eg1-1 and mads6-5. Photos of mads6-5 and eg1-1 mads6-5 are shown as (b). (d) Genetic analysis of eg1-1 and nsr mads6-5. Photos of nsr mads6-5 and eg1-1 nsr mads6-5 are shown as (b). Arrows on iii of nsr mads6-5 and eg1-1 nsr mads6-5 indicate the inactive growing points. (e) Relative expression levels of OsMADS1, OsMADS6 and OsG1 in wild-type and eg1 inflorescences of different growth stages by RT-qPCR. Results are means ± SE. Rice α-TUBULIN was used as the reference. (f) Genetic analysis of eg1 and g1-ele. Empty glume phenotypes of eg1-2 in Lingshui, g1-ele and eg1-2 g1-ele in Beijing/Lingshui are shown respectively. (g) The phenotypic plasticity of le in eg1-2, g1-ele and eg1-2 g1-ele. Values are means ± SE, number of analyzed panicles ≥ 5. le, lemma; pa, palea; st, stamen; pi, pistil; eg, empty glume; ca, carpel; fm, floral meristem; im, inflorescence meristem; if, inflorescence primordia; lel, lemma-like organ; gl, glume-like organ; lp, lemma-palea mosaic organ; log, lodicule-glume mosaic organ. leg, long empty glume. Bars = 2 mm, 200 μm and 200 μm in spikelet structures, SEMs and paraffin transverse sections respectively, except for 0.5 mm in ii of (d).
Furthermore, the le phenotype (long empty glume) of eg1-2 has the highest plasticity among all variable phenotypes (see Figs 1–3), and OsG1 (Long Sterile Lemma) and OsMADS19/34 are two crucial genes suppressing the elongation of empty glumes in rice [70–72]. The expression levels and patterns of OsG1 but not OsMADS19/34 appeared to be aberrant in eg1-2 (Fig 7E and S13 Fig), implying that OsG1 may contribute to the le phenotype of eg1-2. To confirm this, g1-ele allele of OsG1 [72] was used to obtain a double mutant by crossing with eg1-2, and it turned out that almost all empty glumes of eg1-2 g1-ele were elongated to lemma-like organs similar to g1-ele, exhibiting much lower plasticity compared with eg1-2 (Fig 7F and 7G), indicating that OsG1 functions downstream of EG1 in regulating empty glume development and contributes to the plastic le phenotype of eg1-2. Taken together, these results show that OsMADS1, OsMADS6 and OsG1 all act downstream of EG1 to mediate the floral robustness regulation.
Discussion
EG1 is a plasticity gene regulating the floral robustness against environmental fluctuation in rice
To our knowledge, no plasticity genes have been confirmed to function in floral robustness in flowering plants [47–52]. Given the sessile nature of plant species, uncovering this class of genes and dissecting their molecular mechanisms are crucial for understanding the biology of flower development and evolution. In this study, we have shown that EG1 encodes a mitochondria-localized lipase functioning as a plasticity gene to regulate the rice floral robustness by a coordinated transcriptional regulation of temperature, lipid metabolism and flower development pathways. First, eg1 alleles showed floral variations under both natural and artificial conditions and five eg1 alleles produced increased floral plasticity. Second, RNA expression, protein stability and lipase activity of EG1 can respond to environment enhancing its function significantly in severe conditions such as heat stress. Third, EG1 appears to possess a “buffering” function of repressing environmental stimuli to interfere the target genes, and when environmental pressure becomes severer such as heat stress, the strengthened EG1 function induced by heat is enough to buffer the stronger and more deleterious effect of heat on the responsive transcriptional pathways. Last, EG1 influences the expression of numerous floral identity genes, which are the direct contributors of plastic development of eg1 spikelets. Thus, EG1 is the first plasticity gene regulating plant floral robustness against environmental fluctuation. Our finding indicates that flowers retain a system containing EG1 to sense and respond to environmental stimuli to maintain its stable development, and suggests a mechanism of transition from high plasticity to robustness in flower by recruiting plasticity-repressing genes, which ensures the coordinated development of organs with different plasticity in one organism.
Mitochondria could serve as a hub for the lipid metabolism mediated-floral developmental robustness
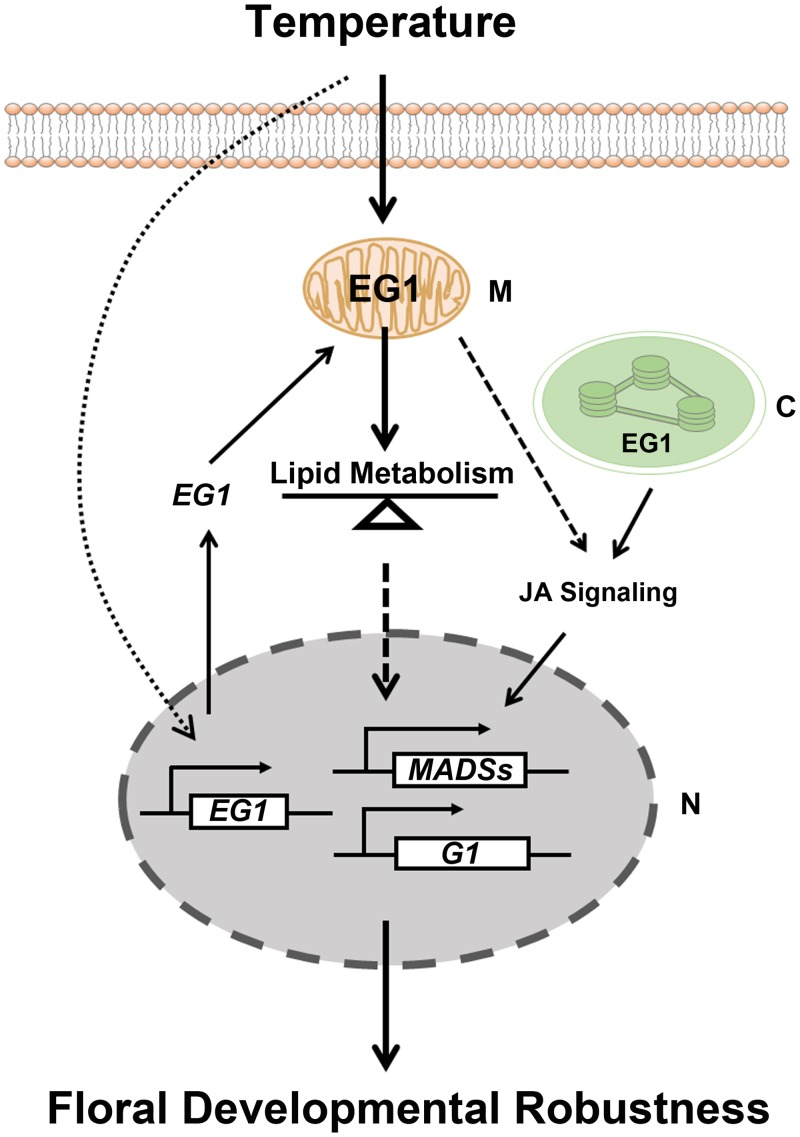
Lipid metabolisms are known to be involved in adaptive plasticity of organisms [36,37,41,42], and mitochondria, as the energy factory of eukaryotes and one of subcellular compartmentations of lipid metabolism, have been reported to be crucial to the adaptive stability of plant vegetative traits [41,43,44]. As EG1 is a predominately mitochondria-localized functional lipase, our results suggest that the mitochondria-mediated lipid metabolism plays an important role in the regulation of floral robustness against temperature fluctuation. However, it remains unclear how this could be carried out. It is likely that mitochondria-related lipid homeostasis could serve as a “buffer” to relieve the effect of environmental stimuli and mediate the temperature-dependent floral plasticity regulation (Fig 8). Recently, EG1 has been shown to influence JA synthesis, and JA signaling pathway regulates the transcription of floral identity gene OsMADS1 [54], showing that JA potentially mediates the crosstalk of EG1 and flower development related transcriptional factors. In fact, the most homologous gene of EG1 in Arabidopsis DGL has been verified to function in JA biosynthesis [55,56,73], though its chloroplast location was questioned [55], suggesting that a non-chloroplast localized lipase is likely to function in JA production (Fig 8). On the other side, we may fail to detect EG1 protein due to its minute amounts in chloroplasts. Furthermore, according to the much severer phenotype of eg1 and eg2-1D (a mutant allele of EG2/OsJAZ1) double mutant compared with two single [54], we noticed that JA signaling may be not the only pathway activated by EG1 to mediate the signal transduction from outside to inside of nucleus in the EG1-associated floral regulation, other lipid-related pathways and regulatory mechanisms are also possible (Fig 8).
Fig 8. A model for EG1 in regulating floral developmental robustness against temperature fluctuation in rice.
EG1 responses high temperature fluctuation at its transcriptional, posttranscriptional and lipase activity levels, and positively regulates the transcriptions of floral identity genes such as OsMADS1, OsMADS6 and OsG1, which are mediated by a mitochondria-associated lipid metabolism, resulting in floral developmental robustness against temperature fluctuation. Additionally, chloroplasts-localized EG1 has also been shown to regulate the transcription of floral identity gene OsMADS1 through JA signaling pathway [54].
Functional divergence of EG1 and its homologs in angiosperms
In our study, eg1 alleles showed a higher floral plasticity in japonica than that in indica varieties, while a severer floral disturbance in indica, revealing a functional divergence of EG1 in rice subspecies. No differences in coding sequences but cis-elements of EG1 in two subspecies were detected (S14 Fig), implying that this subspecific variation might be caused by an unknown differential responsive capability of promoters to environmental or endogenous stimuli. Besides, since genetic backgrounds are known to influence developmental outcomes via phenotypic modifiers [74,75], there also may be some indica-/japonica-specific modifiers to modulate EG1 function due to their different genetic backgrounds, which can be either epistatic genes or specific lipid substrates of EG1 required for floral developmental robustness. Genetic dissection of these subspecific modifiers of plasticity will provide further insights into the molecular mechanism of floral development. So far, all reported EG1 homologs in dicots have no apparent plasticity function in flowers [56–59], and EG1 homologs in monocots can be mainly divided into two clades, one similar to that in dicots and the other unique to monocot species based on the phylogenetic tree and predicted subcellular localizations (S13 Fig), we thus speculate that EG1 may have acquired a monocot-specific neofunctionalization in promoting floral robustness. Detailed genetic and biochemical analyses of these genes would provide additional clues to when and how the plasticity function and the subspecific divergence of EG1 arose in monocot species.
In conclusion, our results revealed a novel function of EG1 in floral developmental robustness against environmental fluctuation by mediating the mitochondrial lipid metabolism. Our finding also provide a genetic means to maintain the stable flower development under environmental stress ensuring grain yield stability in rice and potentially in other monocot species. Further studies should unlock the molecular crosstalk between mitochondria and nucleus in regulating floral developmental robustness.
Materials and Methods
Plant materials, growth conditions and spikelet phenotypic analysis
Five rice eg1 recessive alleles were used in this study. eg1-1 and eg1-2 were from our previous work [53], in backgrounds of O. sativa L. spp. indica Zhefu 802 (ZF802) and O. sativa L. spp. japonica Zhonghua 11 (ZH11) respectively. eg1-4 was generated from indica Dular, and eg1-5 and -6 from japonica Nipponbare by CRISPR/Cas9 technology [76,77]. Besides, ZF802>ZH11 and eg1-1 (ZF802>ZH11) were isolated from an F2 population of ZF802 wild-type or eg1-1 backcrossed with ZH11 three times, and ZH11>ZF802 and eg1-2 (ZH11>ZF802) were isolated from an F2 population of ZH11 wild-type or eg1-2 backcrossed with ZF802 once. Other rice mutants, nsr [61] and g1-ele [72] were kindly provided by Dr. Zhukuan Cheng, and osmads6-5 also from our previous work [66].
Plants were grown in the natural conditions of Beijing (China) from Mar. to Oct. and Lingshui (Hainan province, China) from Dec. to Apr. (year 2014~2015). Weather data of 2014.7.28~2014.8.17 of Beijing and 2014.3.15~2014.4.4 of Lingshui, and 2014.2.1~2014.2.20 and 2014.4.1~2014.4.20 of Lingshui were shown respectively, which were ranged from ten days before spikelet meristem formation (~2 mm) to ten days after that. Artificial conditions in chambers were 35°C light 12 hr / 20°C dark 12 hr, 25°C light 12 hr / 20°C dark 12 hr and 40°C light 12 hr / 30°C dark 12 hr, respectively, with identical light intensity 50 μmol m-2 s-1 and relative air humidity 60%.
Spikelets of eg1 were divided according to phenotypes of outer glumes (lemma, palea, empty glumes and extra glumes) following two rules: (1) the most significant mutant phenotype of eg1 is in the outer glumes; (2) outer glumes have linkages with inner organs: normal palea always linked with normal inner organs, lemma-like palea with increased stamens and pistils, smaller palea with decreased stamens, multilayer glumes linked with rs. Main panicles/inflorescences of plants at booting stage were used for phenotypic analysis. Percentages of all variable phenotypes in one panicle were calculated for comparisons, and in some cases more than one phenotype appeared in a single spikelet. Data were statistically analyzed by using one/two-way ANOVA (Excel). Effects of genotype, environment and genotype-environment interaction on all variable phenotypes were calculated using phenotypic statistic data of eg1 and wild-type grown in Beijing summer and Lingshui winter by two-way ANOVA. In CRISPR experiment, more than six independently homozygous lines were generated in each background, and their phenotypes were quite similar, thus only one/two lines were statistically analyzed and shown in our results.
Protein subcellular localization analyses
Full-length EG1 CDS was inserted into N- or C-terminal of GFP sequence of vector pBI221-35SPro:GFP, and full-length EG1 CDS with 1.5 kb native promoter into pCAMBIA1301-GFP. MTS-mOrange and Pro35S:COX11-GFP plasmids were kindly provided by Dr. Yaoguang Liu [60], and the reported vector pCAMBIA1301-35SPro:EG1-GFP was provided by Dr. Dabing Zhang [54]. All plasmids of high quality were prepared for protoplast transfection. Rice protoplast preparation from 2-week-seedlings grown in light and polyethylene glycol (PEG)-mediated transfections were performed as described by Bart et al. [78]. Images were captured by a confocal microscope (FluoView 1000, Olympus).
Floral disorder of eg1-2 was complemented by genetic transformation using vector pTCK303-ProUBIQUTIN:FLAG-EG1. One-week seedlings of EG1 complementation lines were used for subcellular fractionation. Fractionations of mitochondria and chloroplasts were performed as described by Rodiger et al. [79]. After precipitating the organelle fractions, western blots were performed with α-FLAG (Sigma), mitochondria specific antibodies α-AOX1/2 and α-COXII (Agrisera), and chloroplasts specific antibodies α-RbcL and α-PsbA (Agrisera). Fractionation assays were performed with two independent complementation lines.
Lipase activity analysis in vitro
Full-length EG1 CDS and a truncated EG1 CDS without predicted targeting sequence (135 bp) with engineered N-terminal SUMO tag were separately cloned into pET-30a (Novagen) to generate His6-SUMO-tagged fusion proteins. Mitochondrial targeting sequence was predicted with MitoProt II online [80]. DGL CDS without targeting sequence [81] was introduced into vector PMAL-C2X (NEB). All the fusion proteins were expressed in E. coli BL21 (DE3). The His6-SUMO-tagged fusion proteins were purified using Ni-NTA (Novagen) and eluted with buffer containing 25 mM Tris-HCl (pH 7.4), 150 mM NaCl and 250 mM imidazole. The MBP-tagged fusion proteins were purified using amylose resin (NEB) and eluted with buffer containing 50 mM Tris-HCl (pH 7.4) and 10 mM amylose. Imidazole in protein solutions was removed with desalting columns (Thermo Scientific) before lipase activity analysis.
p-nPB (p-nitrophenyl butyrate, Sigma) was used as the substrate of lipase analysis in vitro. Colorimetric assays for lipase activity of fusion proteins were performed as described by Seo et al. [82] with some modifications. A solution containing 1.11 mg/mL p-nPB (dissolved with isopropanol) and B solution containing 50 mM Tris-HCl (pH 7.4) and 0.1% Arabic gum were first prepared. Reactive solution was composed of 1 volume A solution and 9 volumes B solution with 2% Triton X-100. About 20 μl purified proteins (~5 μg) and 180 μl reactive solution were used for each reaction. After incubated under different temperatures for 30 min, p-nitrophenol formation from p-nitrophenyl butyrate was determined spectrophotometrically at 405 nm by an ELISA microplate reader. DGL and porcine pancreatic lipase (Sigma) were used for positive controls, and p-nitrophenol (Sigma) for the standard curve.
Physiological experiments
Cis-elements in the genomic sequence upstream of the start codon of EG1 was analyzed by PLACE online [83]. Wild-type ZF802, ZH11 and EG1 complementation lines were grown at 25°C for one week after germination, and wild-type ZH11 seedlings were grown in a consistent condition till 2 mm inflorescence meristem developed before being treated under different temperatures for different time. The transcripts of EG1 and FLAG-EG1 were analyzed by RT-QPCR or RT-PCR. The root and shoot phenotypes of seedlings were statistically analyzed after growing under consistent temperatures for six days after germination. Other condition parameters of physiological experiments were daylight 12 hr, light intensity 50 μmol m-2 s-1 and relative air humidity 60%. We use 42°C as an extremely high temperature for short treat but 40°C for long treat by considering the tolerance of plants.
FLAG-EG1 protein in EG1 complementation lines was enriched by immunoprecipitation for its small amount and detected by western blot with α-FLAG (Sigma).
Environmental transcriptome analysis
Two mm inflorescence meristems of eg1-1, eg1-2 and their wild-types ZF802 and ZH11 planted in Beijing summer and Lingshui winter were used for RNA sequencing, and two biological replicates were performed. Total RNAs were isolated with TRIzol kit (Invitrogen). Illumina sequencing libraries were prepared according to the manufacturer’s instructions (Illumina Part # 15026495Rev. D), and sequenced with Illumina system Hiseq2500. Analysis of RNA-seq data was conducted following the standard protocol as described by Trapnell et al. [84]. The raw reads of RNA-seq were mapped to MSU_IRGP_V7 (japonica) and Oryza_indicaASM465 v1.23 (indica) by Tophat [85]. Cuffdiff [85,86] was used to identify the differentially expressed genes between different genotypes or different environments. Co-expression network analysis was performed using R packages WGCNA. Enrichment pathways of genes significantly and specifically affected by G, E or GxE was analyzed and the -log10 (P-values) was tested by Fisher's exact test with Bonferroni correction as described by Lu et al. [87].
Evolutionary analysis
Amino acids sequences of EG1 homologs in different plants were aligned with CLUSTAL W and maximum likelihood tree was constructed with MEGA6.0. Subcellular localizations of proteins were predicted with five sorts of software online. Among them, TargetP [88] (http://www.cbs.dtu.dk/services/TargetP/) has the best consistency compared with MitoProt II-v1.101 [80] (https://ihg.gsf.de/ihg/mitoprot.html), iPSORT [89] (http://ipsort.hgc.jp/), ProtComp 9.0 (http://linux1.softberry.com/berry.phtmtopic=protcompan&group=help&subgroup=proloc) and WoLF PSORT (http://www.genscript.com/wolf-psort.html) online. Predicted localizations with TargetP were shown in our results, a/b in which means the protein is more likely in “a” location than in “b” although both are possible.
Supporting Information
Variable phenotypes of spikelets are defined as S1 Table. le, lemma; pa, palea; eg, empty glume; if, inflorescence primordia; sp, smaller pa; lel, lemma-like organ; pl, palea-lemma mosaic organ; leg, long empty glume in spikelet structures. Bars = 2 mm.
(TIF)
The predicted full-length CDS (1308 bp) of EG1 is shown. Horizontal red line indicates the sequence of lipase_3 domain, vertical black lines the mutation locations of alleles, and numbers in the bracket the numbers of bases from “A”.
(TIF)
Values are means ± SE.
(TIF)
High/low temperature (T) per day during rice booting stage. Weather data of summer of Beijing and winter of Lingshui (a), or February and April of Lingshui (b) are shown. Values are means ± SD.
(TIF)
Green and red colors indicate the fluorescence of EG1-GFP and MTS-mOrange, respectively. Blue color indicates auto-fluorescence emitted by chloroplasts. DIC, pictures photographed by differential interference contrast microscope. Bar = 10 μm.
(TIF)
Normal spikelet rate is the percentage of normal spikelets in a rice panicle, and seed set rate is the percentage of fully grown seeds in a panicle. Values are mean ± SE, number of analyzed panicles = 5.
(TIF)
(a) RT-qPCR analysis of EG1 expression in one-week seedlings of ZF802 and ZH11 wild-types treated under cold shock (4°C) for hours. Values are means ± SE (n = 3), and significant difference was determined by ANOVA, *P < 0.05, ** P < 0.01, and rice α-TUBULIN as the reference. (b) RT-qPCR analysis of EG1 expression in the young inflorescence of ZH11 wild-type treated under extremely high temperature (42°C) for hours. (c) RT-PCR analysis of FLAG-EG1 expression in one-week seedlings of EG1 complementation lines treated under different temperatures. Two pairs of FLAG-EG1 primers and three independent samples were used for analysis. Rice α-TUBULIN was for the reference. (d) Detection of FLAG-EG1 protein in EG1 complementation lines at 35°C for 0, 3, or 5 days. HC, Heavy chain of IgG; NS, Nonspecific band (as a loading control). (e) Detection of GFP protein under three temperatures (25°C, 35°Cand 42°C) in the ProUBIQUITIN-GFP transgenic plants by western blot. RbcL was used as a loading reference. (f) Detection of the peptides derived from FLAG-EG1 by mass spectrometry. FLAG-EG1 was purified from one-week seedlings of EG1 complementation lines and analyzed by MS. The peptides detected are shown in red letters.
(TIF)
(a) EG1-GFP localization under normal (28°C) or extremely high (40°C) temperatures in rice protoplasts. Mitochondria (Mit) and chloroplasts (Chl) are detected by Mito Tacker Red and auto-fluorescence. (b) Fractionation of mitochondria and chloroplasts in EG1 complementation line under different temperatures. Cp, Complementation lines; Ct, non-transgenic wild-type control. CB, Coomassie brilliant blue dyeing.
(TIF)
(a) Phenotypes of six-day-old ZF802 and eg1-1 seedlings. Bar = 1 cm. (b) Statistical analysis of root and shoot phenotypes of eg1-1 and ZF802 seedlings. Values are means ± SE (n >20). Labelled values are ratios of average value of root length or height of eg1-1 to that of ZF802 in the same condition. Significant difference was determined by ANOVA, *P < 0.05, ** P < 0.01.
(TIF)
(a) The graph of correlations between gene modules and variable phenotypes. Each color represents a gene module (y axis), and six variable phenotypes are traits (x axis). The deeper colors in the middle squares show stronger correlations between modules and traits with positive correlations in red color and negative in green. Numbers in the boxes are correlation factors and P-values (inside brackets). (b) The network for correlations of gene modules and variable phenotypes. (c) Morphological correlation analysis of six floral variable phenotypes of eg1 alleles.
(TIF)
(a) Comparisons of environmentally responsive transcriptomes between two different genotypes. Each point represents a transcript. x and y axes are values of log2 [ratios of gene expression in Beijing to that in Lingshui] of two genotypes respectively. Dotted lines indicate y = x lines and solid lines are the best fit lines by linear regression. (b) Comparisons of environmentally responsive genes between two eg1 alleles or two wild-types. Values of x and y axes are the same as (a). Points represent wild-type-specific (red), eg1-specific (blue) and shared (green) genes. Dotted lines indicate y = x lines. (c) Triangular scatterplot for distribution of total genes in wild-type and eg1 affected by G, E and GxE. Each dot indicates a gene, and the three vertexes of triangle indicate three factors G, E, GxE respectively. The closer distance between a gene and a vertex means the stronger effect of the factor on the gene.
(TIF)
Genes with boldface letters indicate genes with significant variations of expression pattern between eg1 and wild-type. Different colors indicate different samples of Beijing (BJ) and Lingshui (LS). Y axis indicates expression level of genes. Labelled values are log2 [ratios of gene mean expressions in BJ to that in LS] in the corresponding genotypes. “*” labels |log2|>1.
(TIF)
Column diagrams are described as in S12 Fig.
(TIF)
Proteins from dicots: Arabidopsis thaliana (AtDGL/At1g05800, At2g31690, AtDLAH/At1g30370, AtDSEL/At4g18550, AtDAD1/At2g44810), Glycine max (Glyma.08G362700, Glyma.18G299300), Ricinus communis (28424.t000001(Rc)) and Medicago truncatula (Medtr7g006400); from monocots: Oryza sativa (LOC_Os11g19340, LOC_Os11g19290, OsEG1, LOC_Os01g67450, LOC_Os02g42170), Brachypodium distachyon (Bradi4g20220, Bradi4g20230, Bradi2g57760), Zea mays (GRMZM2G117627_T0, GRMZM5G812425_T01, GRMZM2G406951_T01), Sorghum bicolor (Sobic.005G111400, Sobic.007G187000, Sobic.003G390900, Sobic.003G391000); and moss Physcomitrella patens (Phpat.022G001500). Gene IDs come from JGI website. chlo, chloroplast; mito, mitochondria; other, without a chlo/mito targeting peptide.
(TIF)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
We thank Dr. Zhukuan Cheng (Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, China) for providing seeds of g1-ele and nsr mutants, Dr. Yaoguang Liu (South China Agricultural University, China) for plasmids MTS-mOrange and 35SPro:COX11-GFP, and Dr. Dabing Zhang (Shanghai Jiao Tong University, China) for plasmid pCAMBIA1301-Pro35S:EG1-GFP.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by grants from Ministry of Science and Technology (2013CBA01402), Strategic Program of Chinese Academy of Sciences (XDA08010205) and National Natural Science Foundation of China (31221063). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Sultan SE. Plant developmental responses to the environment: eco-devo insights. Curr Opin Plant Biol. 2010; 13(1): 96–101. 10.1016/j.pbi.2009.09.021 [DOI] [PubMed] [Google Scholar]
- 2.Meinzer FC. Functional convergence in plant responses to the environment. Oecologia. 2003; 134(1): 1–11. [DOI] [PubMed] [Google Scholar]
- 3.Bradshaw AD. Unravelling phenotypic plasticity—why should we bother? New Phytol. 2006; 170(4): 644–648. [DOI] [PubMed] [Google Scholar]
- 4.Price TD, Qvarnstrom A, Irwin DE. The role of phenotypic plasticity in driving genetic evolution. Proc Biol Sci. 2003; 270(1523): 1433–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sultan SE. Evolutionary Implications of Phenotypic Plasticity in Plants. Evol Biol. 1987; 21: 127–178. [Google Scholar]
- 6.Sultan SE. Phenotypic plasticity and plant adaptation. Acta Bot Neerl. 1995; 44(4): 363–383. [Google Scholar]
- 7.Bell DL, Sultan SE. Dynamic phenotypic plasticity for root growth in Polygonum: A comparative study. Am J Bot. 1999; 86(6): 807–819. [PubMed] [Google Scholar]
- 8.Kembel SW, Cahill JF Jr. Plant phenotypic plasticity belowground: a phylogenetic perspective on root foraging trade-offs. Am Nat. 2005; 166(2): 216–230. [DOI] [PubMed] [Google Scholar]
- 9.Niinemets U, Valladares F, Ceulemans R. Leaf-level phenotypic variability and plasticity of invasive Rhododendron ponticum and non-invasive Ilex aquifolium co-occurring at two contrasting European sites. Plant Cell Environ. 2003; 26(6): 941–956. [DOI] [PubMed] [Google Scholar]
- 10.Yu P, White PJ, Hochholdinger F, Li C. Phenotypic plasticity of the maize root system in response to heterogeneous nitrogen availability. Planta. 2014; 240(4): 667–678. 10.1007/s00425-014-2150-y [DOI] [PubMed] [Google Scholar]
- 11.Armbruster WS, Di Stilio VS, Tuxill JD, Flores TC, Velasquez Runk JL. Covariance and decoupling of floral and vegetative traits in nine Neotropical plants: a re-evaluation of Berg's correlation-pleiades concept. Am J Bot. 1999; 86(1): 39–55. [PubMed] [Google Scholar]
- 12.Ghaderi A, Lower RL. Heterosis and Phenotypic Stability of F1 Hybrids in Cucumber under Controlled Environment. J Am Soc Hortic Sci. 1978; 103(2): 275–278. [Google Scholar]
- 13.Kaneko K. Relationship among phenotypic plasticity, phenotypic fluctuations, robustness, and evolvability; Waddington's legacy revisited under the spirit of Einstein. J Biosciences. 2009; 34(4): 529–542. [DOI] [PubMed] [Google Scholar]
- 14.Ibanez S, Dujardin G, Despres L. Stability of floral specialization in Trollius europaeus in contrasting ecological environments. J Evol Biol. 2009; 22(6): 1183–1192. 10.1111/j.1420-9101.2009.01731.x [DOI] [PubMed] [Google Scholar]
- 15.Pelabon C, Carlson ML, Hansen TF, Yoccoz NG, Armbruster WS. Consequences of inter-population crosses on developmental stability and canalization of floral traits in Dalechampia scandens (Euphorbiaceae). J Evol Biol. 2004; 17(1): 19–32. [DOI] [PubMed] [Google Scholar]
- 16.Nicotra AB, Atkin OK, Bonser SP, Davidson AM, Finnegan EJ, Mathesius U, et al. Plant phenotypic plasticity in a changing climate. Trends Plant Sci. 2010; 15(12): 684–692. 10.1016/j.tplants.2010.09.008 [DOI] [PubMed] [Google Scholar]
- 17.Smith H. Signal Perception, Differential Expression within Multigene Families and the Molecular-Basis of Phenotypic Plasticity—Introduction. Plant Cell Environ. 1990; 13(7): 585–594. [Google Scholar]
- 18.Schlichting CD, Wund MA. Phenotypic plasticity and epigenetic marking: an assessment of evidence for genetic accommodation. Evolution. 2014; 68(3): 656–672. 10.1111/evo.12348 [DOI] [PubMed] [Google Scholar]
- 19.Bourdeau PE, Butlin RK, Bronmark C, Edgell TC, Hoverman JT, Hollander J. What can aquatic gastropods tell us about phenotypic plasticity? A review and meta-analysis. Heredity. 2015; 115(4): 312–321. 10.1038/hdy.2015.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norouzitallab P, Baruah K, Vandegehuchte M, Van Stappen G, Catania F, Vanden Bussche J, et al. Environmental heat stress induces epigenetic transgenerational inheritance of robustness in parthenogenetic Artemia model. FASEB J. 2014; 28(8): 3552–3563. 10.1096/fj.14-252049 [DOI] [PubMed] [Google Scholar]
- 21.Oberti D, Biasini A, Kirschmann MA, Genoud C, Stunnenberg R, Shimada Y, et al. Dicer and HSP104 function in a negative feedback loop to confer robustness to environmental stress. Cell Rep. 2015; 10(1): 47–61. 10.1016/j.celrep.2014.12.006 [DOI] [PubMed] [Google Scholar]
- 22.Schlichting CD, Pigliucci M. Control of Phenotypic Plasticity Via Regulatory Genes. Am Nat. 1993; 142(2): 366–370. 10.1086/285543 [DOI] [PubMed] [Google Scholar]
- 23.Pigliucci M. The genetics of plasticity—Reply. Trends Ecol Evol. 1996; 11(9): 384–384. [DOI] [PubMed] [Google Scholar]
- 24.Pigliucci M. Modelling phenotypic plasticity.2. Do genetic correlations matter? Heredity. 1996; 77: 453–460. [DOI] [PubMed] [Google Scholar]
- 25.Soundararajan R, Paranjape AN, Barsan V, Chang JT, Mani SA. A novel embryonic plasticity gene signature that predicts metastatic competence and clinical outcome. Sci Rep-Uk. 2015; 5: 11766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin-Fairey CA, Nunez AA. Circadian modulation of memory and plasticity gene products in a diurnal species. Brain Res. 2014; 1581: 30–39. 10.1016/j.brainres.2014.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benito E, Urbanke H, Ramachandran B, Barth J, Halder R, Awasthi A, et al. HDAC inhibitor-dependent transcriptome and memory reinstatement in cognitive decline models. J Clin Invest. 2015; 125(9): 3572–3584. 10.1172/JCI79942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rutherford SL, Lindquist S. HSP90 as a capacitor for morphological evolution. Nature. 1998; 396(6709): 336–342. [DOI] [PubMed] [Google Scholar]
- 29.Xu YZ, Santamaria RD, Virdi KS, Arrieta-Montiel MP, Razvi F, Li SQ, et al. The Chloroplast Triggers Developmental Reprogramming When MUTS HOMOLOG1 Is Suppressed in Plants. Plant Physiol. 2012; 159(2): 710–720. 10.1104/pp.112.196055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang CC, Yuan WY, Zhang QF. RPL1, a Gene Involved in Epigenetic Processes Regulates Phenotypic Plasticity in Rice. Mol Plant. 2012; 5(2): 482–493. 10.1093/mp/ssr091 [DOI] [PubMed] [Google Scholar]
- 31.Salathia N, Queitsch C. Molecular mechanisms of canalization: HSP90 and beyond. J Biosciences. 2007; 32(3): 457–463. [DOI] [PubMed] [Google Scholar]
- 32.Krishna P. Plant HSP90 and its partner proteins. J Plant Biochem Biot. 2000; 9(2): 53–56. [Google Scholar]
- 33.Queitsch C, Sangster TA, Lindquist S. HSP90 as a capacitor of phenotypic variation. Nature. 2002; 417(6889): 618–624. [DOI] [PubMed] [Google Scholar]
- 34.Breiman A. Plant HSP90 and its Co-Chaperones. Curr Protein Pept Sc. 2014; 15(3): 232–244. [DOI] [PubMed] [Google Scholar]
- 35.Xu YZ, Arrieta-Montiel MP, Virdi KS, de Paula WBM, Widhalm JR, Basset GJ, et al. MutS HOMOLOG1 Is a Nucleoid Protein That Alters Mitochondrial and Plastid Properties and Plant Response to High Light. Plant Cell. 2011; 23(9): 3428–3441. 10.1105/tpc.111.089136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang X. A lipid pathway for heat adaptation. Sci China Life Sci. 2015; 58(7): 727–728. 10.1007/s11427-015-4880-x [DOI] [PubMed] [Google Scholar]
- 37.Chintalapati S, Kiran MD, Shivaji S. Role of membrane lipid fatty acids in cold adaptation. Cell Mol Biol (Noisy-le-grand). 2004; 50(5): 631–642. [PubMed] [Google Scholar]
- 38.Zhang YM, Rock CO. Membrane lipid homeostasis in bacteria. Nat Rev Microbiol. 2008; 6(3): 222–233. 10.1038/nrmicro1839 [DOI] [PubMed] [Google Scholar]
- 39.Strum JC, Ghosh S, Bell RM. Lipid second messengers—A role in cell growth regulation and cell cycle progression. Adv Exp Med Biol. 1997; 407: 421–431. [PubMed] [Google Scholar]
- 40.Falcone DL, Ogas JP, Somerville CR. Regulation of membrane fatty acid composition by temperature in mutants of Arabidopsis with alterations in membrane lipid composition. BMC Plant Biol. 2004; 4(1): 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pan RH, Jones AD, Hu JP. Cardiolipin-Mediated Mitochondrial Dynamics and Stress Response in Arabidopsis. Plant Cell. 2014; 26(1): 391–409. 10.1105/tpc.113.121095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen M, Thelen JJ. ACYL-LIPID DESATURASE2 is required for chilling and freezing tolerance in Arabidopsis. Plant Cell. 2013; 25(4): 1430–1444. 10.1105/tpc.113.111179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vamecq J, Dessein AF, Fontaine M, Briand G, Porchet N, Latruffe N, et al. Mitochondrial dysfunction and lipid homeostasis. Curr Drug Metab. 2012; 13(10): 1388–1400. [DOI] [PubMed] [Google Scholar]
- 44.Ostrander DB, Zhang M, Mileykovskaya E, Rho M, Dowhan W. Lack of mitochondrial anionic phospholipids causes an inhibition of translation of protein components of the electron transport chain—A yeast genetic model system for the study of anionic phospholipid function in mitochondria. J Biol Chem. 2001; 276(27): 25262–25272. [DOI] [PubMed] [Google Scholar]
- 45.Nowicki M, Muller F, Frentzen M. Cardiolipin synthase of Arabidopsis thaliana. Febs Lett. 2005; 579(10): 2161–2165. [DOI] [PubMed] [Google Scholar]
- 46.Brock MT, Weinig C. Plasticity and environment-specific covariances: an investigation of floral-vegetative and within flower correlations. Evolution. 2007; 61(12): 2913–2924. [DOI] [PubMed] [Google Scholar]
- 47.Maier AT, Stehling-Sun S, Wollmann H, Demar M, Hong RL, Haubeiss S, et al. Dual roles of the bZIP transcription factor PERIANTHIA in the control of floral architecture and homeotic gene expression. Development. 2009; 136(10): 1613–1620. 10.1242/dev.033647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sommer H, Beltran JP, Huijser P, Pape H, Lonnig WE, Saedler H, et al. Deficiens, a homeotic gene involved in the control of flower morphogenesis in Antirrhinum majus: the protein shows homology to transcription factors. EMBO J. 1990; 9(3): 605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zachgo S, Silva ED, Motte P, Trobner W, Saedler H, Schwarzsommer Z. Functional-Analysis of the Antirrhinum Floral Homeotic Deficiens Gene in vivo and in vitro by Using a Temperature-Sensitive Mutant. Development. 1995; 121(9): 2861–2875. [DOI] [PubMed] [Google Scholar]
- 50.Maier AT, Stehling-Sun S, Offenburger SL, Lohmann JU. The bZIP transcription factor PERIANTHIA: a multifunctional hub for meristem control. Front Plant Sci. 2011; 2: 79 10.3389/fpls.2011.00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sablowski RWM, Meyerowitz EM. Temperature-sensitive splicing in the floral homeotic mutant apetala3-1. Plant Cell. 1998; 10(9): 1453–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yi Y, Jack T. An intragenic suppressor of the Arabidopsis floral organ identity mutant apetala3-1 functions by suppressing defects in splicing. Plant Cell. 1998; 10(9): 1465–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li HG, Xue DW, Gao ZY, Yan MX, Xu WY, Xing Z, et al. A putative lipase gene EXTRA GLUME1 regulates both empty-glume fate and spikelet development in rice. Plant J. 2009; 57(4): 593–605. 10.1111/j.1365-313X.2008.03710.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cai Q, Yuan Z, Chen MJ, Yin CS, Luo ZJ, Zhao XX, et al. Jasmonic acid regulates spikelet development in rice. Nat Commun. 2014; 5: 3476 10.1038/ncomms4476 [DOI] [PubMed] [Google Scholar]
- 55.Ellinger D, Stingl N, Kubigsteltig II, Bals T, Juenger M, Pollmann S, et al. DONGLE and DEFECTIVE IN ANTHER DEHISCENCE1 Lipases Are Not Essential for Wound- and Pathogen-Induced Jasmonate Biosynthesis: Redundant Lipases Contribute to Jasmonate Formation. Plant Physiol. 2010; 153(1): 114–127. 10.1104/pp.110.155093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hyun Y, Choi S, Hwang HJ, Yu J, Nam SJ, Ko J, et al. Cooperation and functional diversification of two closely related galactolipase genes for jasmonate biosynthesis. Dev Cell. 2008; 14(2): 183–192. 10.1016/j.devcel.2007.11.010 [DOI] [PubMed] [Google Scholar]
- 57.Seo YS, Kim EY, Kim WT. The Arabidopsis sn-1-specific mitochondrial acylhydrolase AtDLAH is positively correlated with seed viability. J Exp Biol. 2011; 62(15): 5683–5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim EY, Seo YS, Kim WT. AtDSEL, an Arabidopsis cytosolic DAD1-like acylhydrolase, is involved in negative regulation of storage oil mobilization during seedling establishment. J Plant Physiol. 2011; 168(14): 1705–1709. 10.1016/j.jplph.2011.03.004 [DOI] [PubMed] [Google Scholar]
- 59.Padham AK, Hopkins MT, Wang TW, McNamara LM, Lo M, Richardson LGL, et al. Characterization of a plastid triacylglycerol lipase from Arabidopsis. Plant Physiol. 2007; 143(3): 1372–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luo DP, Xu H, Liu ZL, Guo JX, Li HY, Chen LT, et al. A detrimental mitochondrial-nuclear interaction causes cytoplasmic male sterility in rice. Nat Genet. 2013; 45(5): 573–577. 10.1038/ng.2570 [DOI] [PubMed] [Google Scholar]
- 61.Chen ZX, Wu JG, Ding WN, Chen HM, Wu P, Shi CH. Morphogenesis and molecular basis on naked seed rice, a novel homeotic mutation of OsMADS1 regulating transcript level of AP3 homologue in rice. Planta. 2006; 223(5): 882–890. [DOI] [PubMed] [Google Scholar]
- 62.Khanday I, Yadav SR, Vijayraghavan U. Rice LHS1/OsMADS1 controls floret meristem specification by coordinated regulation of transcription factors and hormone signaling pathways. Plant Physiol. 2013; 161(4): 1970–1983. 10.1104/pp.112.212423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Prasad K, Parameswaran S, Vijayraghavan U. OsMADS1, a rice MADS-box factor, controls differentiation of specific cell types in the lemma and palea and is an early-acting regulator of inner floral organs. Plant J. 2005; 43(6): 915–928. [DOI] [PubMed] [Google Scholar]
- 64.Jeon JS, Lee S, An G. Intragenic control of expression of a rice MADS box gene OsMADS1. Mol Cells. 2008; 26(5): 474–480. [PubMed] [Google Scholar]
- 65.Prasad K, Sriram P, Kumar CS, Kushalappa K, Vijayraghavan U. Ectopic expression of rice OsMADS1 reveals a role in specifying the lemma and palea, grass floral organs analogous to sepals. Dev Genes Evol. 2001; 211(6): 281–290. [DOI] [PubMed] [Google Scholar]
- 66.Duan Y, Xing Z, Diao Z, Xu W, Li S, Du X, et al. Characterization of Osmads6-5, a null allele, reveals that OsMADS6 is a critical regulator for early flower development in rice (Oryza sativa L.). Plant Mol Biol. 2012; 80(4–5): 429–442. 10.1007/s11103-012-9958-2 [DOI] [PubMed] [Google Scholar]
- 67.Zhang J, Nallamilli BR, Mujahid H, Peng Z. OsMADS6 plays an essential role in endosperm nutrient accumulation and is subject to epigenetic regulation in rice (Oryza sativa). Plant J. 2010; 64(4): 604–617. 10.1111/j.1365-313X.2010.04354.x [DOI] [PubMed] [Google Scholar]
- 68.Ohmori S, Kimizu M, Sugita M, Miyao A, Hirochika H, Uchida E, et al. MOSAIC FLORAL ORGANS1, an AGL6-like MADS box gene, regulates floral organ identity and meristem fate in rice. Plant Cell. 2009; 21(10): 3008–3025. 10.1105/tpc.109.068742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li H, Liang W, Jia R, Yin C, Zong J, Kong H, et al. The AGL6-like gene OsMADS6 regulates floral organ and meristem identities in rice. Cell Res. 2010; 20(3): 299–313. 10.1038/cr.2009.143 [DOI] [PubMed] [Google Scholar]
- 70.Gao X, Liang W, Yin C, Ji S, Wang H, Su X, et al. The SEPALLATA-like gene OsMADS34 is required for rice inflorescence and spikelet development. Plant Physiol. 2010; 153(2): 728–740. 10.1104/pp.110.156711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yoshida A, Suzaki T, Tanaka W, Hirano HY. The homeotic gene Long Sterile Lemma (G1) specifies sterile lemma identity in the rice spikelet. Proc Natl Acad Sci U S A. 2009; 106(47): 20103–20108. 10.1073/pnas.0907896106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hong LL, Qian Q, Zhu KM, Tang D, Huang ZJ, Gao L, et al. ELE restrains empty glumes from developing into lemmas. J Genet Genomics. 2010; 37(2): 101–115. 10.1016/S1673-8527(09)60029-1 [DOI] [PubMed] [Google Scholar]
- 73.Rudus I, Terai H, Shimizu T, Kojima H, Hattori K, Nishimori Y, et al. Wound-induced expression of DEFECTIVE IN ANTHER DEHISCENCE1 and DAD1-like lipase genes is mediated by both CORONATINE INSENSITIVE1-dependent and independent pathways in Arabidopsis thaliana. Plant Cell Rep. 2014; 33(6): 849–860. 10.1007/s00299-013-1561-8 [DOI] [PubMed] [Google Scholar]
- 74.Haluzik M, Colombo C, Gavrilova O, Chua S, Wolf N, Chen M, et al. Genetic background (C57BL/6J versus FVB/N) strongly influences the severity of diabetes and insulin resistance in ob/ob mice. Endocrinology. 2004; 145(7): 3258–3264. [DOI] [PubMed] [Google Scholar]
- 75.Threadgill DW, Dlugosz AA, Hansen LA, Tennenbaum T, Lichti U, Yee D, et al. Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science. 1995; 269(5221): 230–234. [DOI] [PubMed] [Google Scholar]
- 76.Miao J, Guo D, Zhang J, Huang Q, Qin G, Zhang X, et al. Targeted mutagenesis in rice using CRISPR-Cas system. Cell Res. 2013; 23(10): 1233–1236. 10.1038/cr.2013.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shan Q, Wang Y, Li J, Gao C. Genome editing in rice and wheat using the CRISPR/Cas system. Nat Protoc. 2014; 9(10): 2395–2410. 10.1038/nprot.2014.157 [DOI] [PubMed] [Google Scholar]
- 78.Bart R, Chern M, Park CJ, Bartley L, Ronald PC. A novel system for gene silencing using siRNAs in rice leaf and stem-derived protoplasts. Plant Methods. 2006; 2: 13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rodiger A, Baudisch B, Klosgen RB. Simultaneous isolation of intact mitochondria and chloroplasts from a single pulping of plant tissue. J Plant Physiol. 2010; 167(8): 620–624. 10.1016/j.jplph.2009.11.013 [DOI] [PubMed] [Google Scholar]
- 80.Claros MG, Vincens P. Computational method to predict mitochondrially imported proteins and their targeting sequences. Eur J Biochem. 1996; 241(3): 779–786. [DOI] [PubMed] [Google Scholar]
- 81.Seo YS, Kim EY, Kim JH, Kim WT. Enzymatic characterization of class I DAD1-like acylhydrolase members targeted to chloroplast in Arabidopsis. FEBS Lett. 2009; 583(13): 2301–2307. 10.1016/j.febslet.2009.06.021 [DOI] [PubMed] [Google Scholar]
- 82.Seo YS, Kim EY, Mang HG, Kim WT. Heterologous expression, and biochemical and cellular characterization of CaPLA1 encoding a hot pepper phospholipase A1 homolog. Plant J. 2008; 53(6): 895–908. [DOI] [PubMed] [Google Scholar]
- 83.Higo K, Ugawa Y, Iwamoto M, Higo H. PLACE: a database of plant cis-acting regulatory DNA elements. Nucleic Acids Res. 1998; 26(1): 358–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 2012; 7(3): 562–578. 10.1038/nprot.2012.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009; 25(9): 1105–1111. 10.1093/bioinformatics/btp120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010; 28(5): 511–515. 10.1038/nbt.1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lu ZF, Yu H, Xiong GS, Wang J, Jiao YQ, Liu GF, et al. Genome-Wide Binding Analysis of the Transcription Activator IDEAL PLANT ARCHITECTURE1 Reveals a Complex Network Regulating Rice Plant Architecture. Plant Cell. 2013; 25(10): 3743–3759. 10.1105/tpc.113.113639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Emanuelsson O, Nielsen H, Brunak S, von Heijne G. Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J Mol Biol. 2000; 300(4): 1005–1016. [DOI] [PubMed] [Google Scholar]
- 89.Bannai H, Tamada Y, Maruyama O, Nakai K, Miyano S. Extensive feature detection of N-terminal protein sorting signals. Bioinformatics. 2002; 18(2): 298–305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Variable phenotypes of spikelets are defined as S1 Table. le, lemma; pa, palea; eg, empty glume; if, inflorescence primordia; sp, smaller pa; lel, lemma-like organ; pl, palea-lemma mosaic organ; leg, long empty glume in spikelet structures. Bars = 2 mm.
(TIF)
The predicted full-length CDS (1308 bp) of EG1 is shown. Horizontal red line indicates the sequence of lipase_3 domain, vertical black lines the mutation locations of alleles, and numbers in the bracket the numbers of bases from “A”.
(TIF)
Values are means ± SE.
(TIF)
High/low temperature (T) per day during rice booting stage. Weather data of summer of Beijing and winter of Lingshui (a), or February and April of Lingshui (b) are shown. Values are means ± SD.
(TIF)
Green and red colors indicate the fluorescence of EG1-GFP and MTS-mOrange, respectively. Blue color indicates auto-fluorescence emitted by chloroplasts. DIC, pictures photographed by differential interference contrast microscope. Bar = 10 μm.
(TIF)
Normal spikelet rate is the percentage of normal spikelets in a rice panicle, and seed set rate is the percentage of fully grown seeds in a panicle. Values are mean ± SE, number of analyzed panicles = 5.
(TIF)
(a) RT-qPCR analysis of EG1 expression in one-week seedlings of ZF802 and ZH11 wild-types treated under cold shock (4°C) for hours. Values are means ± SE (n = 3), and significant difference was determined by ANOVA, *P < 0.05, ** P < 0.01, and rice α-TUBULIN as the reference. (b) RT-qPCR analysis of EG1 expression in the young inflorescence of ZH11 wild-type treated under extremely high temperature (42°C) for hours. (c) RT-PCR analysis of FLAG-EG1 expression in one-week seedlings of EG1 complementation lines treated under different temperatures. Two pairs of FLAG-EG1 primers and three independent samples were used for analysis. Rice α-TUBULIN was for the reference. (d) Detection of FLAG-EG1 protein in EG1 complementation lines at 35°C for 0, 3, or 5 days. HC, Heavy chain of IgG; NS, Nonspecific band (as a loading control). (e) Detection of GFP protein under three temperatures (25°C, 35°Cand 42°C) in the ProUBIQUITIN-GFP transgenic plants by western blot. RbcL was used as a loading reference. (f) Detection of the peptides derived from FLAG-EG1 by mass spectrometry. FLAG-EG1 was purified from one-week seedlings of EG1 complementation lines and analyzed by MS. The peptides detected are shown in red letters.
(TIF)
(a) EG1-GFP localization under normal (28°C) or extremely high (40°C) temperatures in rice protoplasts. Mitochondria (Mit) and chloroplasts (Chl) are detected by Mito Tacker Red and auto-fluorescence. (b) Fractionation of mitochondria and chloroplasts in EG1 complementation line under different temperatures. Cp, Complementation lines; Ct, non-transgenic wild-type control. CB, Coomassie brilliant blue dyeing.
(TIF)
(a) Phenotypes of six-day-old ZF802 and eg1-1 seedlings. Bar = 1 cm. (b) Statistical analysis of root and shoot phenotypes of eg1-1 and ZF802 seedlings. Values are means ± SE (n >20). Labelled values are ratios of average value of root length or height of eg1-1 to that of ZF802 in the same condition. Significant difference was determined by ANOVA, *P < 0.05, ** P < 0.01.
(TIF)
(a) The graph of correlations between gene modules and variable phenotypes. Each color represents a gene module (y axis), and six variable phenotypes are traits (x axis). The deeper colors in the middle squares show stronger correlations between modules and traits with positive correlations in red color and negative in green. Numbers in the boxes are correlation factors and P-values (inside brackets). (b) The network for correlations of gene modules and variable phenotypes. (c) Morphological correlation analysis of six floral variable phenotypes of eg1 alleles.
(TIF)
(a) Comparisons of environmentally responsive transcriptomes between two different genotypes. Each point represents a transcript. x and y axes are values of log2 [ratios of gene expression in Beijing to that in Lingshui] of two genotypes respectively. Dotted lines indicate y = x lines and solid lines are the best fit lines by linear regression. (b) Comparisons of environmentally responsive genes between two eg1 alleles or two wild-types. Values of x and y axes are the same as (a). Points represent wild-type-specific (red), eg1-specific (blue) and shared (green) genes. Dotted lines indicate y = x lines. (c) Triangular scatterplot for distribution of total genes in wild-type and eg1 affected by G, E and GxE. Each dot indicates a gene, and the three vertexes of triangle indicate three factors G, E, GxE respectively. The closer distance between a gene and a vertex means the stronger effect of the factor on the gene.
(TIF)
Genes with boldface letters indicate genes with significant variations of expression pattern between eg1 and wild-type. Different colors indicate different samples of Beijing (BJ) and Lingshui (LS). Y axis indicates expression level of genes. Labelled values are log2 [ratios of gene mean expressions in BJ to that in LS] in the corresponding genotypes. “*” labels |log2|>1.
(TIF)
Column diagrams are described as in S12 Fig.
(TIF)
Proteins from dicots: Arabidopsis thaliana (AtDGL/At1g05800, At2g31690, AtDLAH/At1g30370, AtDSEL/At4g18550, AtDAD1/At2g44810), Glycine max (Glyma.08G362700, Glyma.18G299300), Ricinus communis (28424.t000001(Rc)) and Medicago truncatula (Medtr7g006400); from monocots: Oryza sativa (LOC_Os11g19340, LOC_Os11g19290, OsEG1, LOC_Os01g67450, LOC_Os02g42170), Brachypodium distachyon (Bradi4g20220, Bradi4g20230, Bradi2g57760), Zea mays (GRMZM2G117627_T0, GRMZM5G812425_T01, GRMZM2G406951_T01), Sorghum bicolor (Sobic.005G111400, Sobic.007G187000, Sobic.003G390900, Sobic.003G391000); and moss Physcomitrella patens (Phpat.022G001500). Gene IDs come from JGI website. chlo, chloroplast; mito, mitochondria; other, without a chlo/mito targeting peptide.
(TIF)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.