Abstract
Background
Multiple sclerosis (MS) is the most common chronic autoimmune demyelinating and inflammatory disease of the central nervous system, afflicting both the body and mind. The risk of suffering from MS is 2.5–3.5 times greater in females than in males. While there is extant research on fatigue, depression, and cognitive impairment in patients with MS during its clinical course, there is a lack of research focusing on sleep, psychological functioning, and physical activity (PA) at the point of disease onset. The aims of the present study were therefore, to assess the markers of mental toughness (MT) as a dimension of psychological functioning, sleep disturbances (SD), and PA among patients at the moment of disease onset and to compare these with the corresponding values for healthy adolescents and young adults.
Methods
A total of 23 patients with MS at disease onset (mean age =32.31 years; 91% females), 23 healthy adolescents (mean age =17.43 years; 82% females), and 25 healthy young adults (mean age =20.72 years; 80% females) took part in the study. They completed questionnaires covering sociodemographic data, MT, SD, and PA.
Results
Patients with MS had similar scores for MT traits as those in healthy adolescents and healthy young adults, and equivalent levels of moderate-intensity PA and SD as young adults. MS patients reported lower levels of vigorous PA compared to both healthy adolescents and young adults.
Conclusion
The pattern of the results of the present study suggests that the onset of MS is not associated with poor MT, poor sleep, or reduced moderate-intensity PA. Lower levels of vigorous PA were observed in MS patients. Low levels of vigorous PA may lead to decreased cardiorespiratory fitness in patients with MS and, in the long run, to reduced cardiovascular health and degraded psychological functioning.
Keywords: multiple sclerosis, illness onset, mental toughness, sleep disturbances, physical activity, healthy controls
Introduction
Neurodegenerative conditions such as dementia, Parkinson’s disease, Huntington’s disease (HD), and multiple sclerosis (MS) are often characterized by significant psychological and physical deficits such as sleep disturbance (SD) and low physical activities that interfere significantly with patients’ abilities and their overall quality of life.1–7 In this context, MS is the most common chronic autoimmune demyelinating and inflammatory disease of the central nervous system. Demyelination in association with axonal damage results in slowing of nerve signals, leading to typical MS symptoms such as feeling tired (fatigue), pain, visual problems, paresthesia, and problems of movement and balance.8 MS is associated with several comorbidities including an increase in depressive symptoms, social withdrawal, reduced sexual drive, increased physical inactivity, and SD.9,10 Further, excessive fatigue severely reducing physical activity (PA) and exercising is experienced by at least two-thirds of MS patients.11
A broad range of medications is available to treat MS during the course of the disease.12–16 More specifically, the past two decades have witnessed remarkable advances in the treatment options for MS. New drugs have been developed on the basis of the knowledge of the pathobiology of MS,17 and as for now, medical treatment is the first-line treatment for MS.12,14–16 Accordingly, several disease-modifying drugs including interferon beta 1a and 1b, glatiramer acetate, natalizumab, mitoxantrone, and fingolimod are licensed worldwide to reduce the frequency of clinical attacks with the hope of slowing disability progression.17–19 Fingolimod is a once-daily oral medication approved for relapsing MS.17 Because interferon beta and glatiramer acetate have favorable long-term safety profiles and minimal monitoring requirements, they remain the common first-line choices despite competition from new oral therapies.17 Medication, though, was not the focus of concern in the present paper; instead, we examined psychological and sleep- and PA-related characteristics at illness onset.
Specifically, although research on the quality of life in MS patients has gained increasing interest, we decided to introduce a further psychological dimension, that is, we asked about patients’ mental toughness (MT). MT refers to an individual’s capacity to be consistently successful in coping with difficult life circumstances.20 Most studies in this field have focused on MT in elite athletes; these studies have shown that mentally tough athletes are able to cope with stress during a competition and to remain more focused and confident in the course of competition.20–23
Other studies have considered the association between MT and stress. For example, Gerber et al24,25 showed that adolescents with higher scores for MT were more resilient against stress. Accordingly, adolescents who were mentally tougher in a stressful situation were at a low risk of burnout compared to peers with lower scores for MT.26,27 The bot tom line appears to be that people with lower levels of MT experience greater stress when dealing with difficult and frustrating situations.
The concept of MT has been recognized recently for its psychological importance not just in coping with stress24 but also for its association with higher PA,28 and for its impact on both,29,30 and objective sleep quality.31 Consequently, there has been growing recognition of the applicability of MT to groups other than non-elite athletes, for example, healthy adolescents;29–31 healthy young adults;24 lower, middle, and senior managers; and clerical/administrative workers in early, middle, and late adulthood.32 However, to the best of our knowledge, MT traits have not so far been assessed in patients with MS, either at illness onset or during the course of the illness. Further aims of the present study were therefore to assess MT traits among patients with MS at disease onset and to compare the results from this group with those from healthy adolescents and young adults.
Overall, the concept of MT has been proven to be associated with a broad variety of cognitive, emotional, and behavioral dimensions. We chose to examine MT in patients with MS for the following reasons: 1) MT consists of four key factors: control (of own life and emotions), commitment, challenge, and confidence (in own abilities and in other people; Table 1), thus covering a range of cognitive–emotional processes closely involved in coping with stress, emotions, unexpected events, and social settings (confidence in other people); 2) MT is related to subjective and objective sleep; 3) MT is related to PA; 4) MT has not previously been assessed in patients with MS; 5) MT offers an excellent basis for both cross-sectional and longitudinal studies; 6) MT reflects a trait rather than a state marker; and 7) given that the Mental Toughness Questionnaire 48 (MTQ48)20 has been validated in different age and professional groups, data from previous studies (samples) are comparable with new samples.
Table 1.
Descriptive and inferential statistical overview of mental toughness traits, sleep disturbances, and physical activity of patients with diagnosed MS, healthy adolescents, and healthy young adults
| Demographics, mental toughness, sleep disturbaces, and physical activity | Groups
|
Statistics
|
|||
|---|---|---|---|---|---|
| MS | HA | HYAdu | Factor group | Post hoc tests | |
| N | 23 | 23 | 25 | ||
| Sex (female/male) | 21/2 | 19/4 | 20/5 | χ2(2) =1.39 | |
| Mean (SD) | Mean (SD) | Mean (SD) | F | ||
| Age (years) | 32.31 (7.04) | 17.43 (1.91) | 20.72 (2.53) | 72.15*** | MS > HYAdu > HA |
| Mental toughness | |||||
| Challenge | 26.47 (3.95) | 28.27 (2.93) | 31.60 (1.53) | 18.85*** | HYAdu > MS, HA |
| Commitment | 38.30 (5.78) | 38.11 (3.11) | 41.00 (2.75) | 3.81* | HYAdu > HA |
| Control emotion | 21.47 (4.04) | 20.52 (3.39) | 21.52 (1.41) | 0.77 | – |
| Control life | 23.35 (4.66) | 23.40 (1.80) | 25.56 (2.29) | 3.90* | HYAdu > HA |
| Control total score | 44.82 (7.28) | 43.93 (3.42) | 47.08 (2.45) | 2.78* | HYAdu > HA |
| Confidence abilities | 29.28 (5.33) | 30.52 (4.17) | 34.80 (2.36) | 10.50*** | HYAdu > MS, HA |
| Confidence interpersonal | 20.78 (3.26) | 21.66 (2.10) | 21.92 (1.75) | 1.41 | – |
| Confidence total score | 50.61 (5.93) | 52.18 (5.01) | 56.72 (1.81) | 11.78*** | HYAdu > MS, HA |
| Mental toughness total score | 160.21 (19.86) | 162.50 (5.94) | 176.40 (3.86) | 12.91*** | HYAdu > MS, HA |
| Sleep disturbances | 11.96 (4.99) | 6.57 (1.90) | 11.16 (1.95) | 18.70*** | HA < MS, HYAdu |
| Moderate physical activity (min/week) | 505.65 (145.74) | 251.30 (33.90) | 416.96 (137.37) | 3.71* | MS > HA HYAdu > HA |
| Vigorous physical activity (min/week) | 164.13 (91.48) | 409.48 (16.52) | 466.48 (97.54) | 19.64*** | HYAdu > HA > MS |
Notes: Degrees of freedom: always (2, 68);
P<0.05;
P<0.001. Post hoc tests after Bonferroni–Holm corrections for P-values: <, less than/lower than; >, more than/higher than.
Abbreviations: MS, multiple sclerosis; HA, healthy adolescents; HYAdu, healthy young adults; M, male; F, female.
We took all these considerations into account and asked about the MT profile of patients with MS at disease onset. Given that MT is considered a trait marker, we anticipated that scores would not differ from those of healthy controls immediately after a dramatic event such as the diagnosis of MS. Further, we applied MTQ48,20 thus allowing comparison with previous research.
A further core symptom of MS is fatigue, which means increased physical and psychological tiredness. Plausibly, fatigue might be associated with poor sleep though, surprisingly, the association between poor sleep and fatigue has been little studied so far. Only recently, Strober33 reviewed the state-of-the-art and concluded that the association between sleep and fatigue has been understudied and unrecognized, and that poor sleep is a significant contributor to fatigue. In the light of this observation, the first aim of the present study was to assess whether and to what extent SD might be precursors of MS at illness onset and to what extent any SD are comparable to those of healthy adolescents and young adults.
With regard to PA, there are three principal reasons why patients with MS may be less active than healthy people:34,35 fatigue, impairment, and lack of time. Whereas lack of time seems to be a common reason for people not to exercise, impairment and fatigue are potentially MS specific.34–36 With regard to impairment, there is some evidence of a negative feedback loop in that acute PA may lead to immediate impairment together with physical and mental discomfort. However, there is evidence that impairments and discomfort are due to an acute increase in body temperature and not to PA as such (Uhthoff’s phenomenon37). Further, the course of Huntington’s Disease, a neurodegenerative disorder, indicates that a less physically active lifestyle is a preclinical predictor of earlier disease onset.38 Thus, although it is well established that patients with MS report reduced PA during the course of the disease, and although in the case of another neurodegenerative disorder we have learnt that a less active lifestyle is associated with earlier illness onset, to the best of our knowledge, the level of PA at illness onset among patients with MS has yet to be studied. Accordingly, the second aim of the present study was to assess levels of moderate and vigorous PA of patients with MS at disease onset and to compare these levels with those of healthy adolescents and young adults.
Additionally, previous studies have shown that patients with MS reported increased symptoms of depression and anxiety during the course of the disease,5,39 while, more recently, the psychological dimension of quality of life has gained greater attention, thus shifting from a strictly psychiatric perspective to a more comprehensive psychological appraisal.40,41
To summarize, the aims of the present study were to explore to what extent psychological dimensions (here MT), sleep, and PA might be already affected at the onset of MS, thus providing indications of disease onset at psychological, sleep-related, and activity-related levels. To these ends, we assessed patients with MS at illness onset as well as healthy adolescents and healthy young adults. We believe that the pattern of results revealed in this study might be of practical importance, because until now, the only reliable state markers of the onset of MS were neurocognitive assessments to detect neurocognitive impairments42–45 and imaging techniques to assess cortical atrophy.46–50 These assessments, however, are time-consuming, expensive, require specialist expertise, and are not a part of annual routine medical checkups. By contrast, changes in sleep quality, PA, and cognitive, emotional, and behavioral frameworks are more easily detected both by individuals affected by MS and by their close family members and friends.
In the absence of existing evidence on patients with MS at illness onset, the following research questions were formulated in lieu of hypotheses: first, do the MT traits of patients with MS at illness onset differ significantly from those of healthy adolescents or healthy young adults? Second, are patients with MS at illness onset more likely to report SD than healthy adolescents and healthy young adults? Third, do patients with MS at illness onset report less moderate or less vigorous PA than healthy adolescents and healthy young adults?
Methods
Procedure
Three different populations were assessed and compared in the present study: patients with MS at disease onset, healthy adolescents, and healthy young adults. All the participants were informed about the voluntary character of participation and assured that all the data were gathered anonymously. Written informed consent was obtained from the participants or from their legal representatives in case they were minors (<18 years). All the participants completed a series of questionnaires covering sociodemographic data, MT, SD, and PA (see “Materials” section). Completion of the questionnaire took 30–40 minutes. The local ethics committee of the University of Basel (Basel, Switzerland) approved the study, which was performed in accordance with the principles laid down in the Declaration of Helsinki.
Samples
Patients with diagnosed MS
A total of 23 patients with diagnosed MS (mean age =32.31 years; standard deviation =7.04; 91.3% females) took part in the study. A trained neurologist not otherwise involved in the study diagnosed the patients as having MS 1–30 days before they were enrolled in the study. In other words, the diagnosis of MS was recent; these patients were new cases of MS. The patients completed the questionnaires individually during a routine checkup. A trained psychologist assisted the patients in case of questions.
Healthy adolescents
Data from 23 adolescents (mean age =17.43 years; standard deviation =1.91; 82.61% females) were derived from a larger sample, as already described by Brand et al.28 The participants were young adolescents with regular schedules such as attending school fulltime, individual sports activities and leisure time activities such as playing music, attending music events, and similar. Of the total sample of 1,475 adolescents, data of 23 participants were randomly selected to build a subgroup for the present study (data set) and to present a representative of the pattern of results of the total sample (for more details, refer the study by Brand et al). The participants filled in the questionnaires during a regular school lesson.
Healthy young adults
Data from 25 healthy young adults (mean age =20.72 years; standard deviation =2.53; 80% females) were derived from a larger sample, as already described in another study.24 The participants were young adult students from University of Basel (Switzerland), currently attending university classes fulltime. Of the total sample of 140 young adult students, the data of 25 participants were randomly selected to build a subgroup for the present study (data set) and to present a representative of the pattern of results for the sample as a whole. The participants completed the questionnaires during a regular lecture.
Materials
The participants completed a series of standardized questionnaires related to sociodemographic data, MT, SD, and PA.
Sociodemographic background
The participants reported their sex and age; additionally, patients with diagnosed MS reported time since onset of disease (in days).
Mental toughness
MT was assessed with MTQ48.20 It consists of 48 items, which are aggregated to the following dimensions: challenge (eg, “Challenges usually bring out the best in me”), commitment (eg, “I don’t usually give up under pressure”), emotional (eg, “Even when under considerable pressure I usually remain calm”) and life control (eg, “I generally feel in control”), interpersonal confidence (eg, “I usually take charge of a situation when I feel it is appropriate”), and confidence in ability (eg, “I am generally confident in my own abilities”). The factorial validity of MTQ48 has been proved in previous studies.24,32 Further, MTQ48 has a high test–retest reliability and a high internal consistency. Items are anchored on 5-point Likert scales from 1 (= strongly disagree) to 5 (= strongly agree), with higher scores reflecting greater MT. Additionally, responses across items were summed to obtain an overall MT index (Cronbach’s alpha =0.84–0.91).
Physical activity
PA was assessed with the short version of the International Physical Activity Questionnaire (IPAQ). IPAQ was developed by a working group initiated by the World Health Organization and the Centers for Disease Control and Prevention. Based on the results from 12 countries, reliability and validity of IPAQ were comparable to other self-reported measures of PA.51 The short form (self-administered, seven-item) of IPAQ was administered, asking about time spent in PA over the last 7 days. Minutes of sitting and walking as well as moderate-intensity (walking not included) and vigorous-intensity activities were calculated for the past week. The IPAQ questionnaires (short and long versions), including definitions of moderate and vigorous activity, are available at http://www.ipaq.ki.se.
Sleep disturbances
SD were assessed with the Insomnia Severity Index.52 This questionnaire is a seven-item screening measure for insomnia and an outcome measure for use in treatment research. The items, answered on 5-point rating scales ranging from 0 (= not at all) to 4 (= very much), refer in part to the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition)53 criteria for insomnia by assessing difficulty falling asleep, difficulty remaining asleep, early morning awakenings, impaired daytime performance, low satisfaction with sleep, and worry about sleep. The higher the overall score, the more the participant is assumed to suffer from insomnia (Cronbach’s alpha =0.87).
Statistical analysis
A chi-square test was performed to compare sex distributions between the three groups. Next, a series of univariate analysis of variance (ANOVA) was performed with group (MS patients, healthy adolescents, and healthy young adults) as an independent factor and age, MT, SD, and PA as dependent variables. The nominal level of significance was set at alpha P<0.05. All statistical computations were performed with SPSS® 22.0 (IBM Corporation, Armonk NY, USA) for Apple Mac®.
Results
Group differences in sociodemographic background
All descriptive and inferential statistical indices are reported in Table 1. Statistically significant group differences were found with regard to age, with MS patients having the highest age, followed by healthy young adults and healthy adolescents. Sex distribution did not differ between the three groups, P>0.05.
Group differences in MT and SD
With regard to MT, all descriptive and inferential statistics for patients with diagnosed MS, healthy adolescents, and healthy young adults are shown in Table 1. As can be seen from the ANOVAs, there were significant group effects for challenge, commitment, life control, confidence in own abilities, confi-dence total score, and MTQ48 total score. A nonsignificant trend (P<0.1) was observed for control total score. Post hoc analyses after Bonferroni–Holm corrections for P-values showed that healthy young adults had significantly higher scores for challenge, confidence in own abilities, confidence total score, and MTQ48 total score, compared to patients with MS and healthy adolescents (Figure 1). No significant group differences were observed for control of emotions or interpersonal confidence. Of note, no significant mean differences were observed between healthy adolescents and patients with MS.
Figure 1.
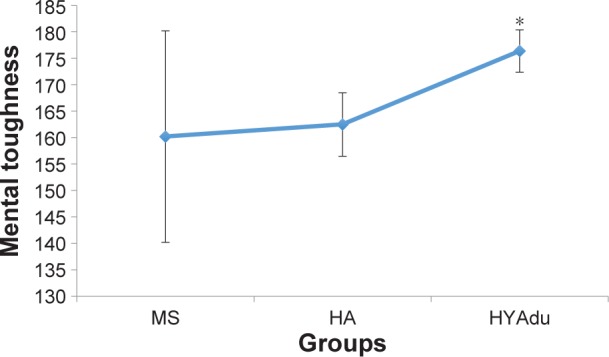
Mental toughness.
Notes: Mental toughness differed significantly between the groups, with significantly higher mental toughness among HYAdu, compared to patients with MS and HA. Points are mean values, and bars are standard deviation. *Significant mean difference compared to other mean values.
Abbreviations: MS, multiple sclerosis; HA, healthy adolescents; HYAdu, healthy young adults.
With regard to SD, there was a significant group effect (Table 1). Post hoc analyses after Bonferroni–Holm correction for P-values showed that healthy adolescents had the lowest level of SD, whereas SD did not differ between patients with MS and healthy young adults (Figure 2).
Figure 2.
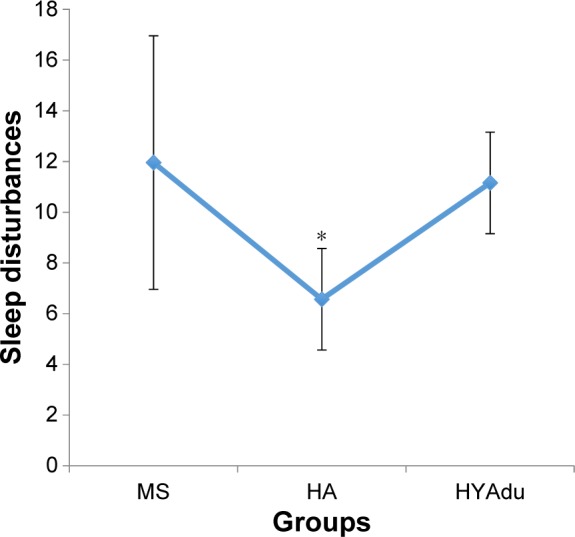
Sleep disturbances.
Notes: Sleep disturbances differed significantly between the groups, with significantly lower sleep disturbances among HA, compared to patients with MS and HYAdu. Points are mean values, and bars are standard deviation. *Significant mean difference compared to other mean values.
Abbreviations: MS, multiple sclerosis; HA, healthy adolescents; HYAdu, healthy young adults.
Group differences in PA
Table 1 also reports all descriptive and inferential statistical indices related to PA. ANOVAs showed significant effects of group for both moderate and vigorous PA. Post hoc analyses after Bonferroni–Holm corrections for P-values showed that healthy young adults and healthy adolescents reported significantly higher levels of vigorous PA than patients with MS. Furthermore, healthy adolescents also reported more vigorous PA than healthy young adults (Figure 3). Finally, patients with MS and young adults reported higher levels of moderate PA than did healthy adolescents, whereas no significant differences were found between patients with MS and health young adults (Figure 4).
Figure 3.
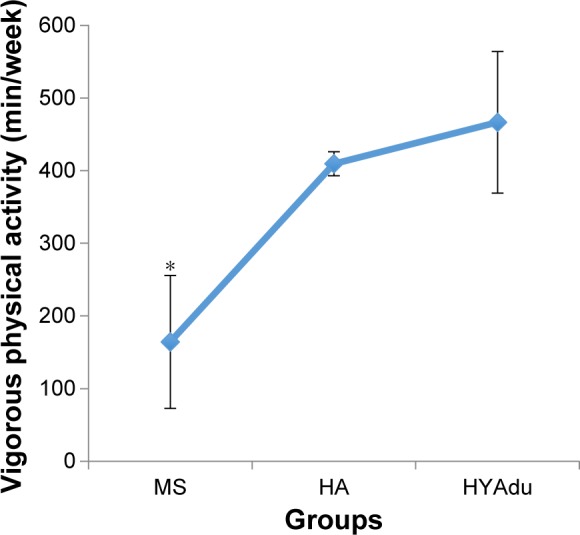
Vigorous physical activity.
Notes: Vigorous physical activity differed significantly between the groups, with significantly higher vigorous physical activity among HYAdu compared to HA and patients with MS. Further, HA reported significantly more vigorous PA compared to patients with MS. Points are mean values, and bars are standard deviation; *significant mean difference compared to other mean values.
Abbreviations: MS, multiple sclerosis; HA, healthy adolescents; HYAdu, healthy young adults.
Figure 4.
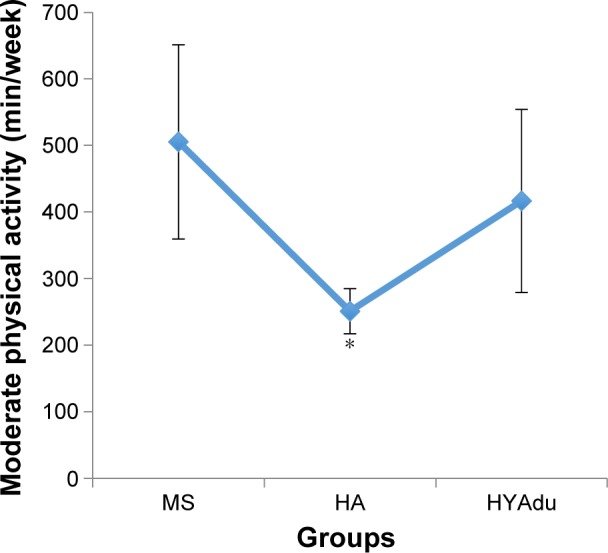
Moderate physical activity.
Notes: Moderate physical activity differed significantly between the groups, with significantly higher moderate physical activity among patients with MS compared to HA, and also higher moderate physical activity among HYAdu compared to HA, but there was no significant difference between patients with MS and HYAdu. Points are mean values, and bars are standard deviation; *significant mean difference compared to other mean values.
Abbreviations: MS, multiple sclerosis; HA, healthy adolescents; HYAdu, healthy young adults.
Discussion
The key findings of the present study were that MT levels on all dimensions of patients with MS at illness onset were similar to those of healthy adolescents and healthy young adults; patients with MS also had equal amounts of moderate PA and degrees of SD as young adults. However, patients reported lower levels of vigorous PA than either healthy adolescents or young adults. The present pattern of results adds to the current literature in showing that patients with MS at illness onset did not differ from healthy adolescents and healthy young adults in MT traits, SD, or moderate PA.
Three research questions were formulated and each of these is considered now in turn.
With the first research question, we focused on possible differences in MT traits between patients with MS at illness onset and healthy adolescents and young adults. The pattern of results in patients with MS showed a difference in overall MT score compared to healthy young adults, but not compared to healthy adolescents. Further inspection (Table 1) revealed that patients with MS at illness onset did not differ statistically from healthy young adults with regard to commitment, life control, emotion control, control overall score, or interpersonal confidence. Next, to further contextualize the present results, it should be noted that the sample of healthy young adults included sports students who, almost by definition, report higher levels of MT54 along with greater PA.
Overall, we believe that the present data add to the existing literature on MT in that this is the very first study on MT traits among patients with MS at illness onset. The study also adds to the current literature on MS in suggesting that at illness onset, MT levels of patients with MS are not different from those of healthy adolescents and young adults. Further and importantly, the present findings are at odds with those studies reporting increased symptoms of depression and anxiety5,39 and a lower quality of life.40,41
Our second research question concerned whether patients with MS at illness onset reported more SD than healthy adolescents or healthy young adults, and the answer was no. Therefore, again, we hold that the current pattern of results adds to the literature in showing that sleep at illness onset in patients with MS is not impaired compared to healthy young adults. Had this been the case, it then would have been possible that either alterations in the neuronal centers responsible for sleep-wake-generation and coordination55 or psychological issues (refer the study by Riemann et al56 for the hyperarousal model of SD) or both were responsible for poorer sleep. Further, the present results are at odds with those studies33,57,58 reporting impaired sleep patterns in patients with MS.
Third, we asked whether patients with MS at illness onset would report lower moderate, or vigorous PA, as compared to healthy adolescents and healthy young adults, and the answer was no. Specifically, they reported higher levels of moderate PA than healthy adolescents, and, descriptively, than healthy young adults, though these patients also reported lower levels of vigorous activity. Again, we hold that the present pattern of results adds to the current literature in showing that, at illness onset, PA does not appear to be impaired or reduced in patients with MS. Likewise, and in contrast to the findings from patients with Huntington’s Disease,38 PA patterns are not good predictors of preclinical symptoms. Further, the present pattern of results is at odds with those studies showing a decrease in PA among patients with MS.1,34,35
The novelty of the results should be balanced against the limitations of the study, which preclude overgeneralization of the findings. First, the sample sizes were small, though statistically significant mean differences were observed. Second, female participants predominated in the samples, though we note that females have a 2.5–3.5 times greater risk of suffering from MS than males. Third, no objective data were collected; objective assessments of PA and objective sleep measures, for example, would have provided a more complete picture and more reliable data on both PA and sleep. Fourth, it would have been interesting and enlightening to compare the present data with neurocognitive performance and imaging data in order to determine to what extent MT traits, SD, and PA are associated with neuronal changes and cognitive performance. Fifth, the present pattern of results might have emerged due to further latent but unassessed dimensions, which might have biased two or more dimensions in similar or opposite directions. Sixth, it would have been interesting to assess daytime sleepiness and fatigue, and to relate these to SD. This would have allowed a more thorough investigation of the association between poor sleep, tiredness, and fatigue, given that up to now there has been so little research on these associations. Seventh, it might be arguable to what extent it was useful to compare data of patients with MS at disease onset with data of healthy adolescents and healthy young adults (students in sport science). However, we followed Roberts et al,59 who showed a continuity of personality growth toward greater maturity from adolescence to young adulthood. Therefore, we wanted to explore to what extent continuity and change in personality would have been reflected in MT traits among these three different samples. In this view, the present data showed that such comparisons were reasonable, as patients with MS at disease onset reported very similar traits as did the healthy adolescents and healthy young adults. Eighth, the topic of MT gets increasingly assessed among nonelite athletes such as lower, middle, and senior managers, and clerical/administrative workers in early, middle, and later adulthood.32 Accordingly, we consider the present study as a contribution to the research on MT as a general set of personality traits covering dimensions such as stress management, self-esteem, confidence in social environment, and coping with changes. Lastly, the cross-sectional character of the study does not shed any light on the illness course; in this regard, it would be intriguing to know whether MT traits, SD, or amount of PA predict the future course of the illness along with quality of life.
Conclusion
The pattern of results suggests that patients with MS at illness onset show patterns of MT traits, SD, and PA comparable to those of healthy adolescents and young adults. We believe that the therapeutic challenge is to keep these features stable over the course of treatment and illness.
Acknowledgments
The authors thank Nick Emler (University of Surrey, Surrey, UK) for proofreading the manuscript. Further, we thank Ludwig Kappos (University of Basel, Basel, Switzerland) for helpful advice.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Razazian N, Yavari Z, Farnia V, et al. Exercising impacts on fatigue, depression, and paresthesia in female patients with MS. Med Sci Sports Exerc. 2016;48(5):796–803. doi: 10.1249/MSS.0000000000000834. [DOI] [PubMed] [Google Scholar]
- 2.Busse M, Quinn L, Dawes H, et al. Supporting physical activity engagement in people with Huntington’s disease (ENGAGE-HD): study protocol for a randomized controlled feasibility trial. Trials. 2014;15:487. doi: 10.1186/1745-6215-15-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iranzo A. Sleep in neurodegenerative diseases. Sleep Med Clin. 2016;11(1):1–18. doi: 10.1016/j.jsmc.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Garlovsky JK, Overton PG, Simpson J. Psychological predictors of anxiety and depression in Parkinson’s disease: a systematic review. J Clin Psychol. 2016 doi: 10.1002/jclp.22308. [DOI] [PubMed] [Google Scholar]
- 5.Fiest KM, Walker JR, Bernstein CN, et al. Systematic review and meta-analysis of interventions for depression and anxiety in persons with multiple sclerosis. Mult Scler Relat Disord. 2016;5(1):12–26. doi: 10.1016/j.msard.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Enrici I, Adenzato M, Ardito RB, et al. Emotion processing in Parkinson’s disease: a three-level study on recognition, representation, and regulation. PLoS One. 2015;10(6):e0131470. doi: 10.1371/journal.pone.0131470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavallo M, Cavanna AE, Harciarek M, Johnston H, Ostacoli L, Angilletta C. “Keep up the good work”: a case study of the effects of a specific cognitive training in Alzheimer’s disease. Neurocase. 2013;19(6):542–552. doi: 10.1080/13554794.2012.701643. [DOI] [PubMed] [Google Scholar]
- 8.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343(13):938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 9.Svendsen KB, Jensen TS, Hansen HJ, Bach FW. Sensory function and quality of life in patients with multiple sclerosis and pain. Pain. 2005;114(3):473–481. doi: 10.1016/j.pain.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 10.Veauthier C. Sleep disorders in multiple sclerosis. Review. Curr Neurol Neurosci Rep. 2015;15(5):21. doi: 10.1007/s11910-015-0546-0. [DOI] [PubMed] [Google Scholar]
- 11.Brañas P, Jordan R, Fry-Smith A, Burls A, Hyde C. Treatments for fatigue in multiple sclerosis: a rapid and systematic review. Health Technol Assess. 2000;4(27):1–61. [PubMed] [Google Scholar]
- 12.O’Connor P, Comi G, Freedman MS, et al. Long-term safety and efficacy of teriflunomide: nine-year follow-up of the randomized TEMSO study. Neurology. 2016;86(10):920–930. doi: 10.1212/WNL.0000000000002441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lublin F, Miller DH, Freedman MS, et al. Oral fingolimod in primary progressive multiple sclerosis (INFORMS): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2016;387(10023):1075–1084. doi: 10.1016/S0140-6736(15)01314-8. [DOI] [PubMed] [Google Scholar]
- 14.Kappos L, Wiendl H, Selmaj K, et al. Daclizumab HYP versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2015;373(15):1418–1428. doi: 10.1056/NEJMoa1501481. [DOI] [PubMed] [Google Scholar]
- 15.Kappos L, Radue EW, Chin P, Ritter S, Tomic D, Lublin F. Onset of clinical and MRI efficacy occurs early after fingolimod treatment initiation in relapsing multiple sclerosis. J Neurol. 2016;263(2):354–360. doi: 10.1007/s00415-015-7978-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kappos L, Kuhle J, Multanen J, et al. Factors influencing long-term outcomes in relapsing-remitting multiple sclerosis: PRISMS-15. J Neurol Neurosurg Psychiatry. 2015;86(11):1202–1207. doi: 10.1136/jnnp-2014-310024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wingerchuk DM, Carter JL. Multiple sclerosis: current and emerging disease-modifying therapies and treatment strategies. Mayo Clin Proc. 2014;89(2):225–240. doi: 10.1016/j.mayocp.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Lu E, Wang BW, Guimond C, Synnes A, Sadovnick D, Tremlett H. Disease-modifying drugs for multiple sclerosis in pregnancy: a systematic review. Neurology. 2012;79(11):1130–1135. doi: 10.1212/WNL.0b013e3182698c64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freedman MS, Hughes B, Mikol DD, et al. Efficacy of disease-modifying therapies in relapsing remitting multiple sclerosis: a systematic comparison. Eur Neurol. 2008;60(1):1–11. doi: 10.1159/000127972. [DOI] [PubMed] [Google Scholar]
- 20.Clough P, Earle K, Sewell D. Mental toughness: the concept and its measurement. Solutions Sport Psychol. 2002:32–43. [Google Scholar]
- 21.Mack MG, Ragan BG. Development of the mental, emotional, and bodily toughness inventory in collegiate athletes and nonathletes. J Athl Train. 2008;43(2):125–132. doi: 10.4085/1062-6050-43.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crust L, Clough PJ. Relationship between mental toughness and physical endurance. Percept Mot Skills. 2005;100(1):192–194. doi: 10.2466/pms.100.1.192-194. [DOI] [PubMed] [Google Scholar]
- 23.Antonini Philippe R, Sagar SS, Hauw D, Gerber M. Players perceptions of coaches’ contribution to their mental toughness. Int J Sports Sci Coaching. 2016;10(1):3–17. [Google Scholar]
- 24.Gerber M, Kalak N, Lemola S, et al. Are adolescents with high mental toughness levels more resilient against stress? Stress Health. 2013;29(2):164–171. doi: 10.1002/smi.2447. [DOI] [PubMed] [Google Scholar]
- 25.Gerber M, Brand S, Feldmeth AK, et al. Adolescents with high mental toughness adapt better to perceived stress: a longitudinal study with Swiss vocational students. Pers Individ Dif. 2013;54:808–814. [Google Scholar]
- 26.Gerber M, Feldmeth AK, Lang C, et al. The relationship between mental toughness, stress, and burnout among adolescents: a longitudinal study with Swiss vocational students. Psychol Rep. 2015;117(3):703–723. doi: 10.2466/14.02.PR0.117c29z6. [DOI] [PubMed] [Google Scholar]
- 27.Gerber M, Feldmeth AK, Elliot C, Brand S, Holsboer-Trachsler E, Pühse U. Mental health in Swiss vocational students: the moderating role of physical activity. J Res Adolesc. 2015;25:63–74. [Google Scholar]
- 28.Brand S, Kalak N, Gerber M, et al. During early to mid adolescence, moderate to vigorous physical activity is associated with restoring sleep, psychological functioning, mental toughness and male gender. J Sports Sci. 2016 Apr 1; doi: 10.1080/02640414.2016.1167936. Epub. [DOI] [PubMed] [Google Scholar]
- 29.Brand S, Kalak N, Gerber M, et al. During early and mid-adolescence, greater mental toughness is related to increased sleep quality and quality of life. J Health Psychol. 2016;21:905–915. doi: 10.1177/1359105314542816. [DOI] [PubMed] [Google Scholar]
- 30.Brand S, Gerber M, Kalak N, et al. “Sleep well, our tough heroes!” – in adolescence, greater mental toughness is related to better sleep schedules. Behav Sleep Med. 2014;12(6):444–454. doi: 10.1080/15402002.2013.825839. [DOI] [PubMed] [Google Scholar]
- 31.Brand S, Gerber M, Kalak N, et al. Adolescents with greater mental toughness show higher sleep efficiency, more deep sleep and fewer awakenings after sleep onset. J Adolesc Health. 2014;54(1):109–113. doi: 10.1016/j.jadohealth.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 32.Perry JL, Clough PJ, Crust L, Earle K, Nicholls AR. Factorial validity of the mental toughness questionnaire-48. Pers Individ Dif. 2013;54(5):587–592. [Google Scholar]
- 33.Strober LB. Fatigue in multiple sclerosis: a look at the role of poor sleep. Front Neurol. 2015;6:21. doi: 10.3389/fneur.2015.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Motl RW. Benefits, safety, and prescription of exercise in persons with multiple sclerosis. Expert Rev Neurother. 2014;14(12):1429–1436. doi: 10.1586/14737175.2014.983904. [DOI] [PubMed] [Google Scholar]
- 35.Asano M, Duquette P, Andersen R, Lapierre Y, Mayo NE. Exercise barriers and preferences among women and men with multiple sclerosis. Disabil Rehabil. 2013;35(5):353–361. doi: 10.3109/09638288.2012.742574. [DOI] [PubMed] [Google Scholar]
- 36.Lionetti E, Castellaneta S, Francavilla R, et al. Introduction of gluten, HLA status, and the risk of celiac disease in children. N Engl J Med. 2014;371(14):1295–1303. doi: 10.1056/NEJMoa1400697. [DOI] [PubMed] [Google Scholar]
- 37.Waschbisch A, Tallner A, Pfeifer K, Maurer M. Multiple sclerosis and exercise: effects of physical activity on the immune system. Nervenarzt. 2009;80(6):688–692. doi: 10.1007/s00115-008-2639-3. [DOI] [PubMed] [Google Scholar]
- 38.Trembath MK, Horton ZA, Tippett L, et al. A retrospective study of the impact of lifestyle on age at onset of Huntington disease. Mov Disord. 2010;25(10):1444–1450. doi: 10.1002/mds.23108. [DOI] [PubMed] [Google Scholar]
- 39.de Cerqueira AC, Semionato de Andrade P, Godoy Barreiros JM, Teixeira AL, Nardi AE. Psychiatric disorders in patients with multiple sclerosis. Compr Psychiatry. 2015;63:10–14. doi: 10.1016/j.comppsych.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 40.Baumstarck K, Boyer L, Boucekine M, Michel P, Pelletier J, Auquier P. Measuring the quality of life in patients with multiple sclerosis in clinical practice: a necessary challenge. Mult Scler Int. 2013;2013:524894. doi: 10.1155/2013/524894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baumstarck K, Boucekine M, Boyer L, et al. Quantification of relevance of quality of life assessment for patients with cognitive impairment: the suitability indices. BMC Neurol. 2014;14:78. doi: 10.1186/1471-2377-14-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tiemann L, Penner IK, Haupts M, Schlegel U, Calabrese P. Cognitive decline in multiple sclerosis: impact of topographic lesion distribution on differential cognitive deficit patterns. Mult Scler. 2009;15(10):1164–1174. doi: 10.1177/1352458509106853. [DOI] [PubMed] [Google Scholar]
- 43.Lensch E, Matzke M, Petereit HF, Scherer P, Schramm S, Calabrese P. Identification and management of cognitive disorders in multiple sclerosis – a consensus approach. J Neurol. 2006;253(Suppl 1):I29–I31. doi: 10.1007/s00415-006-1107-x. [DOI] [PubMed] [Google Scholar]
- 44.Calabrese P. Neuropsychology of multiple sclerosis – an overview. J Neurol. 2006;253(Suppl 1):I10–I15. doi: 10.1007/s00415-006-1103-1. [DOI] [PubMed] [Google Scholar]
- 45.Kalbe E, Calabrese P, Fengler S, Kessler J. DemTect, PANDA, EASY, and MUSIC: cognitive screening tools with age correction and weighting of subtests according to their sensitivity and specificity. J Alzheimers Dis. 2013;34(4):813–834. doi: 10.3233/JAD-122128. [DOI] [PubMed] [Google Scholar]
- 46.Calabrese M, Gajofatto A, Gobbin F, et al. Late-onset multiple sclerosis presenting with cognitive dysfunction and severe cortical/infratentorial atrophy. Mult Scler. 2015;21(5):580–589. doi: 10.1177/1352458514542363. [DOI] [PubMed] [Google Scholar]
- 47.Calabrese M, Favaretto A, Poretto V, et al. Low degree of cortical pathology is associated with benign course of multiple sclerosis. Mult Scler. 2013;19(7):904–911. doi: 10.1177/1352458512463767. [DOI] [PubMed] [Google Scholar]
- 48.Calabrese M, Favaretto A, Martini V, Gallo P. Grey matter lesions in MS: from histology to clinical implications. Prion. 2013;7(1):20–27. doi: 10.4161/pri.22580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Calabrese M, Atzori M, Bernardi V, et al. Cortical atrophy is relevant in multiple sclerosis at clinical onset. J Neurol. 2007;254(9):1212–1220. doi: 10.1007/s00415-006-0503-6. [DOI] [PubMed] [Google Scholar]
- 50.Calabrese M, Agosta F, Rinaldi F, et al. Cortical lesions and atrophy associated with cognitive impairment in relapsing-remitting multiple sclerosis. Arch Neurol. 2009;66(9):1144–1150. doi: 10.1001/archneurol.2009.174. [DOI] [PubMed] [Google Scholar]
- 51.Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 52.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 53.American Psychiatric Association . Diagnostic and Statistical manual of Mental Disorders 4th edition: DSM-IV-TR. Washington DC: American Psychiatric Association; 2000. [Google Scholar]
- 54.Gerber M, Kalak N, Lemola S, et al. Adolescents’ exercise and physical activity are associated with mental toughness. Ment Health Phys Act. 2012;5(1):35–42. [Google Scholar]
- 55.Cirelli C, Tononi G. Molecular neurobiology of sleep. Handb Clin Neurol. 2011;98:191–203. doi: 10.1016/B978-0-444-52006-7.00012-5. [DOI] [PubMed] [Google Scholar]
- 56.Riemann D, Spiegelhalder K, Feige B, et al. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev. 2010;14(1):19–31. doi: 10.1016/j.smrv.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 57.Strober LB, Arnett PA. An examination of four models predicting fatigue in multiple sclerosis. Arch Clin Neuropsychol. 2005;20(5):631–646. doi: 10.1016/j.acn.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 58.Arnett PA, Strober LB. Cognitive and neurobehavioral features in multiple sclerosis. Expert Rev Neurother. 2011;11(3):411–424. doi: 10.1586/ern.11.12. [DOI] [PubMed] [Google Scholar]
- 59.Roberts BW, Caspi A, Moffitt TE. The kids are alright: growth and stability in personality development from adolescence to adulthood. J Per Soc Psychol. 2001;81(4):670–683. [PubMed] [Google Scholar]


