Summary
The thiol-disulfide oxidoreductase CXXC catalytic domain of thioredoxin contributes to antioxidant defense in phylogenetically diverse organisms. We find that while the oxidoreductase activity of thioredoxin-1 protects Salmonella enterica serovar Typhimurium from hydrogen peroxide in vitro, it does not appear to contribute to Salmonella’s antioxidant defenses in vivo. Nonetheless, thioredoxin-1 defends Salmonella from oxidative stress resulting from NADPH phagocyte oxidase macrophage expression during the innate immune response in mice. Thioredoxin-1 binds to the flexible linker, which connects the receiver and effector domains of SsrB, thereby keeping this response regulator in the soluble fraction. Thioredoxin-1, independently of thiol-disulfide exchange, activates intracellular SPI2 gene transcription required for Salmonella resistance to both reactive species generated by NADPH phagocyte oxidase and oxygen-independent lysosomal host defenses. These findings suggest that the horizontally-acquired virulence determinant SsrB is regulated post-translationally by ancestrally-present thioredoxin.
Introduction
All aerobic, and many anaerobic, organisms experience oxidative stress at some point in their lifetime. Univalent or divalent reduction of molecular oxygen in the electron transport chain or in the flavin prosthetic groups of cytosolic enzymes are sources of endogenous oxidative stress (Boveris and Chance, 1973; Husain et al., 2008; Korshunov and Imlay, 2010). Steady-state oxidative stress resulting from these metabolic processes is, nonetheless, overshadowed by the high flux of reactive oxygen species (ROS) synthesized by the multisubunit NADPH phagocyte oxidase during the respiratory burst in macrophages and neutrophils (Babior, 1999). Salmonella enterica are able to survive activity of this flavohemoprotein in polymorphonuclear and mononuclear phagocytes (Burton et al., 2014; Vazquez-Torres et al., 2000a). The respiratory burst produced by the NADPH phagocyte oxidase is essential to the host defense against salmonellosis, as demonstrated by the prevalence of Salmonella infections in chronic granulomatous disease patients bearing autosomal or X-linked mutations in cytosolic and membrane-bound components of this enzymatic complex (Mouy et al., 1989). Mice deficient in the gp91phox or p47phox subunits of the NADPH phagocyte oxidase recapitulate the hypersusceptibility of patients with chronic granulomatous disease to Salmonella infection (Burton et al., 2014; Mastroeni et al., 2000; van Diepen et al., 2002).
Salmonella employ multiple strategies to combat oxidative stress resulting from NADPH phagocyte oxidase activity. Periplasmic Cu-Zn superoxide dismutase SodCI, glutathione and the ABC-type efflux pump MacAB defend this enteropathogen against cytotoxicity resulting from NADPH phagocyte oxidase (Bogomolnaya et al., 2013; De Groote et al., 1997; Song et al., 2013). In addition, the type III secretion system, encoded by the Salmonella pathogenicity island 2 (SPI2), reduces contact between Salmonella vacuoles and NADPH phagocyte oxidase-containing vesicles (Berger et al., 2010; Gallois et al., 2001; Vazquez-Torres et al., 2000b), thereby helping this bacterium maintain intracytoplasmic redox homeostasis in macrophages (van der Heijden et al., 2015). Despite the benefits associated with these antioxidant defenses, Salmonella suffer oxidative stress in phagocytic cells (Burton et al., 2014). Hydrogen peroxide (H2O2) is a critical effector of oxidative stress engendered in the respiratory burst of mononuclear phagocytes (Vazquez-Torres et al., 2000a). H2O2 leads to DNA double strand breaks in a ferrous iron-dependent manner. In addition to this mode I killing, H2O2 oxidizes both Feα of [4Fe-4S] prosthetic groups in dehydratases and thiol groups in cysteine residues of target proteins (Imlay, 2003). Disulfide bond formation between neighboring cysteine residues is a common H2O2-mediated modification. Thioredoxins and cognate thioredoxin reductases help maintain thiol-disulfide redox homeostasis (Holmgren, 1989). Thioredoxin-1 increases Salmonella fitness in a murine model of salmonellosis, but it does not seem to protect this enteropathogen from H2O2 killing (Bjur et al., 2006). It remains unknown if thioredoxin-1 is a component of Salmonella’s antioxidant toolbox.
Members of the highly conserved thioredoxin family contain 2 catalytic cysteine residues within the canonical CXXC sequence motif. During thiol-disulfide exchange, the catalytic cysteine residues in the CXXC sequence motif become oxidized. The resulting disulfide bond is reduced by thioredoxin reductase, a flavoprotein that is powered by electrons from NADPH (Arner and Holmgren, 2000). Thioredoxin-mediated thiol-disulfide exchange reactions control the activity of a range of biomolecules, including ribonucleotide reductase, phosphoadenosine-phosphosulfate reductase, methionine sulfoxide reductase, and arsenate reductase (Holmgren, 1989). Thioredoxin-1 has been shown to regulate Salmonella’s SPI2 type III secretion system (Negrea et al., 2009). How thioredoxin-1 controls SPI2 expression is currently unclear. Here we find that thioredoxin-1 promotes antioxidant defense of Salmonella against NADPH phagocyte oxidase-mediated oxidative stress in vivo independently of classical thiol-disulfide oxidoreductase. Rather, we find that thioredoxin-1, independent of its canonical thiol-disulfide oxidoreductase enzymatic activity, binds to and stabilizes the SPI2 master regulator SsrB, thereby helping Salmonella survive the antimicrobial activity of NADPH phagocyte oxidase activated during the innate immune response in primary macrophages and mice.
Results
The thioredoxin system protects Salmonella from the bacteriostatic activity of H2O2
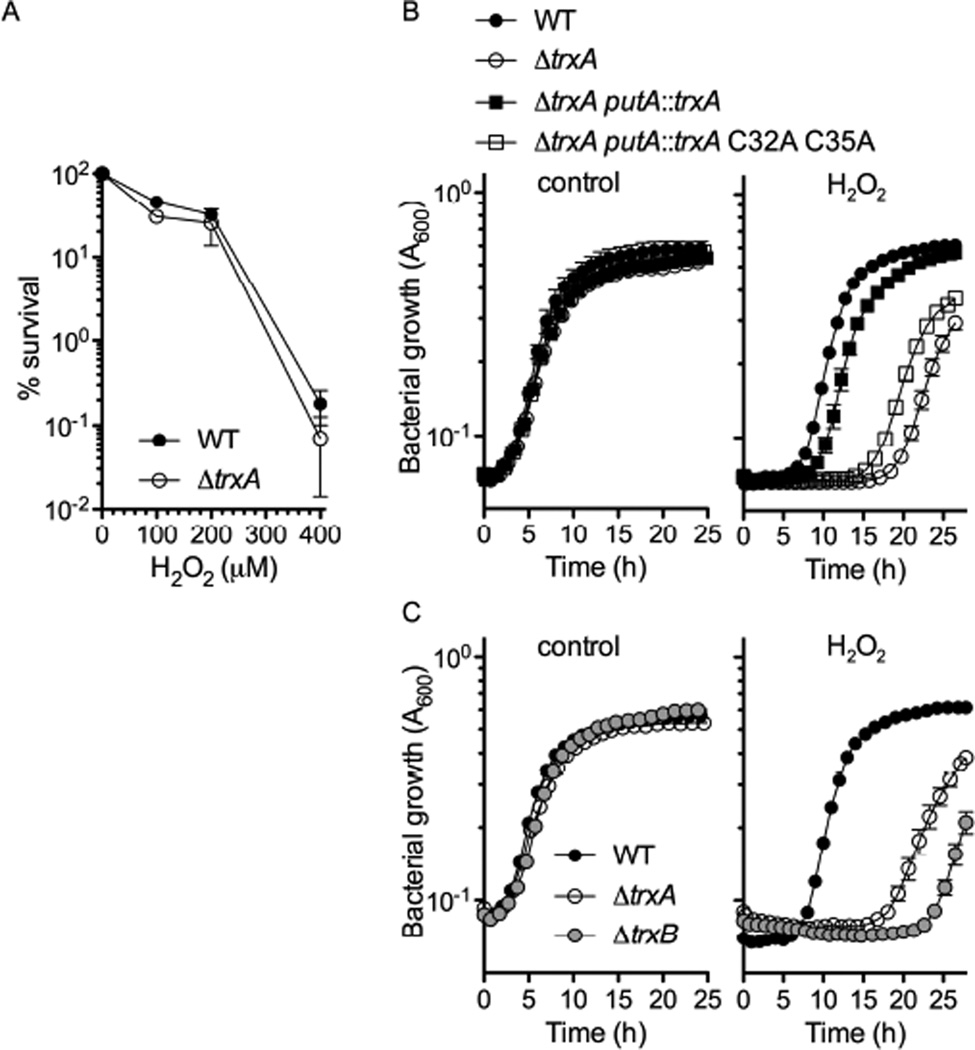
Despite its well-documented contributions to antioxidant defense (Carmel-Harel and Storz, 2000), thioredoxin-1 has yet to be identified as an important component of the antioxidant arsenal of Salmonella. In agreement with previous published data (Bjur et al., 2006), our investigations showed similar susceptibility of wild-type and trxA mutant Salmonella to H2O2 killing (Fig. 1A). Together, these investigations indicate that thioredoxin-1 does not protect Salmonella against the genotoxicity associated with mode I H2O2 killing (Imlay and Linn, 1986). Neither does thioredoxin-1 appear to defend Salmonella against the thiol-oxidizer diamide (Fig. S1A) or superoxide-mediated cytotoxicity of the redox cycling drug menadione (Fig. S1B). Although the NADPH phagocyte oxidase predominantly kills Salmonella during the initial phases of the infection, bacteriostasis appears to be the dominant antimicrobial activity associated with this flavohemoprotein as the infection proceeds (Grant et al., 2008). We therefore developed an in vitro system to test the effects of low concentrations of H2O2 on Salmonella growth. The addition of 100 µM H2O2 to exponentially growing Salmonella delayed bacterial replication to about 5 h (Fig. 1B, right panel). In contrast, 100 µM H2O2 extended the lag phase of ΔtrxA Salmonella by about 15 h. The profound H2O2-mediated cytotoxicity of thioredoxin-1-deficient Salmonella was reversed upon expression of trxA from the put intergenic region of the bacterial chromosome. The thiol-disulfide exchange reaction catalyzed by the thiolate of the attacking Cys32 and the thiol group of resolving Cys35 is responsible for thioredoxin-1 antioxidant defenses (Arner and Holmgren, 2000). To test whether thioredoxin-1 thiol-disulfide exchange antagonizes H2O2 bacteriostasis, ΔtrxA Salmonella were complemented with enzymatically inactive trxA C32A C35A. In contrast to the isogenic strain expressing the wild-type gene, Salmonella expressing the trxA C32A C35A allele were hypersusceptible to the bacteriostatic activity of 100 µM H2O2. These data indicate that the thioredoxin-1-mediated protection of Salmonella against the bacteriostatic activity of H2O2 relies on the thiol-disulfide exchange system. Further supporting this idea, a strain deficient in the trxB-encoded thioredoxin reductase, a flavoprotein that reduces disulfide-bonded thioredoxin-1, was also hypersusceptible to the bacteriostatic activity of 100 µM H2O2 (Fig. 1C).
Fig. 1. Thioredoxin-1-mediated resistance to H2O2.
The % survival of wild-type (WT) and ΔtrxA Salmonella was calculated 2 h after exposure to increasing concentrations of H2O2 (A). Growth (A600) of Salmonella in high-Mg2+ N salts medium in the presence of 100 µM H2O2 (B, C). PBS was used as control. The data are the mean ± SD of 5 independent experiments. See also Fig. S1.
Thioredoxin-1 protects Salmonella from the antimicrobial activity of the NADPH phagocyte oxidase
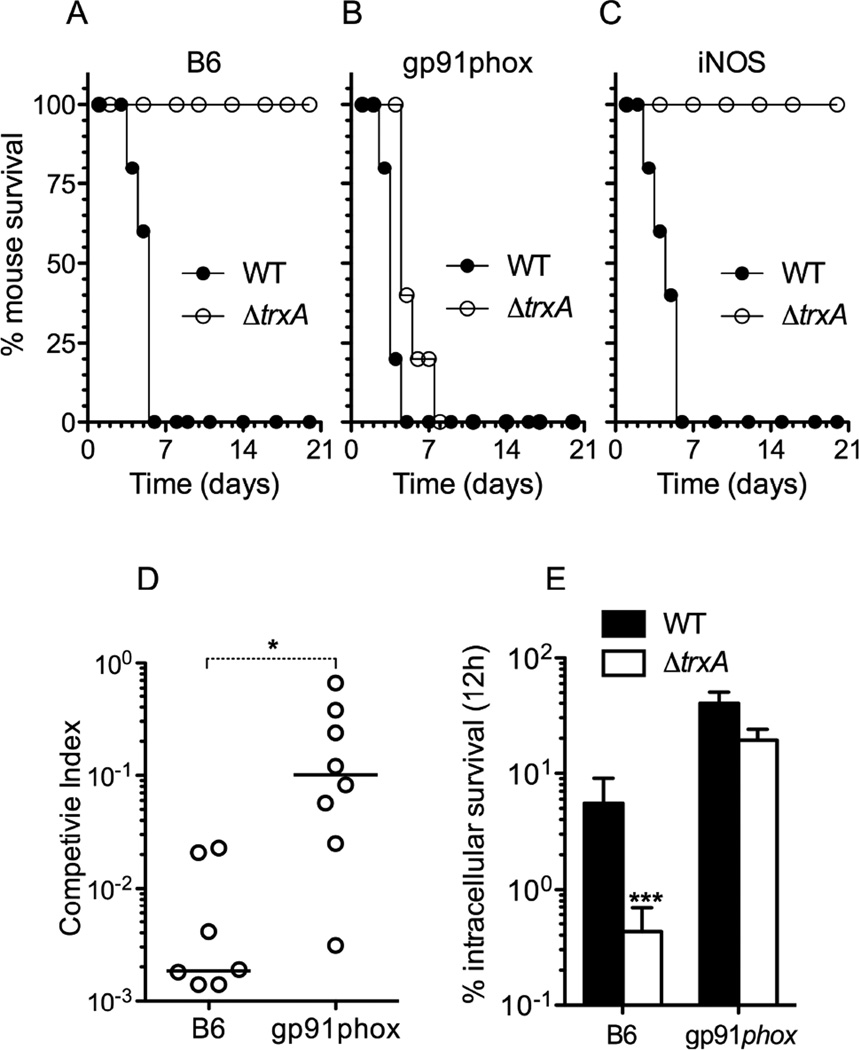
We used a C57BL/6 murine model of infection to begin testing the hypothesis that thioredoxin-1 protects Salmonella from ROS generated by the NADPH phagocyte oxidase (Vazquez-Torres et al., 2000a). C57BL/6 mice survived an oral challenge with ΔtrxA Salmonella but succumbed to infection with an isogenic wild-type control (Fig. 2A). The ΔtrxA mutant became as virulent as wild-type controls in immunodeficient mice lacking the gp91phox-encoded membrane-bound subunit of the NADPH phagocyte oxidase (Fig. 2B). The ΔtrxA mutant, however, remained attenuated in inducible nitric oxide (NO) synthase (iNOS)-deficient mice unable to synthesize NO in response to this intracellular pathogen (Fig. 2C). To analyze further the role played by thioredoxin-1 on antioxidant defense, the competitive index of wild-type and ΔtrxA∷::km Salmonella was defined in an intraperitoneal model of acute infection. The number of ΔtrxA::km Salmonella recovered from the livers of C57BL/6 mice 48 h after infection was 100 to 1000-fold lower when compared to wild-type controls. The competitive disadvantage of the ΔtrxA Salmonella was greatly diminished in gp91phox-deficient mice (Fig. 2D). These investigations indicate that thioredoxin-1 contributes to the antioxidant defenses that protect Salmonella against the enzymatic activity of the NADPH phagocyte oxidase during infection, but does not seem to be critical to the antinitrosative arsenal of this intracellular pathogen. In vivo, Salmonella are exposed to the antimicrobial activity of the NADPH phagocyte oxidase in macrophages (Burton et al., 2014). Thus, we examined the survival of ΔtrxA Salmonella in periodate-elicited peritoneal macrophages from C57BL/6 mice known to sustain a respiratory burst in response to Salmonella (Vazquez-Torres et al., 2000a). Salmonella lacking trxA survived 10-fold less than wild-type controls in macrophages from C57BL/6 mice (Fig. 2E). When compared to immunocompetent cells, a higher burden of wild-type Salmonella was recovered from gp91phox-deficient macrophages. Importantly, the ΔtrxA Salmonella survived as well as wild-type Salmonella in gp91phox-deficient macrophages. These results demonstrate that thioredoxin-1 protects Salmonella from the oxidative stress engendered within host cell macrophages upon the assembly of a functional NADPH phagocyte oxidase.
Fig. 2. Thioredoxin-1 protects Salmonella against the antimicrobial activity of the NADPH phagocyte oxidase.
C57BL/6 (B6) and congenic gp91phox- or iNOS-deficient mice were challenged orally with ~3 × 106 CFU/mouse of wild-type (WT) or ΔtrxA Salmonella (A–C). The survival of Salmonella-infected mice was scored over time. According to log-rank, Mantel-Cox survival test, ΔtrxA Salmonella are attenuated (p < 0.001) in B6 and iNOS-deficient mice. The competitive index was measured in the livers of C57BL/6 and gp91phox-deficient mice 48 h after i.p. inoculation with 500 CFU of a mixture containing equal numbers of ΔtrxA::km and WT Salmonella (D). *, p < 0.5. Survival of Salmonella in periodate-elicited peritoneal macrophages (E). ***, p < 0.001. The data are the mean ± SD of 9 biological repeats done on 3 independent days.
Salmonella virulence is co-dependent on thioredoxin-1 and the SPI2 type III secretion system
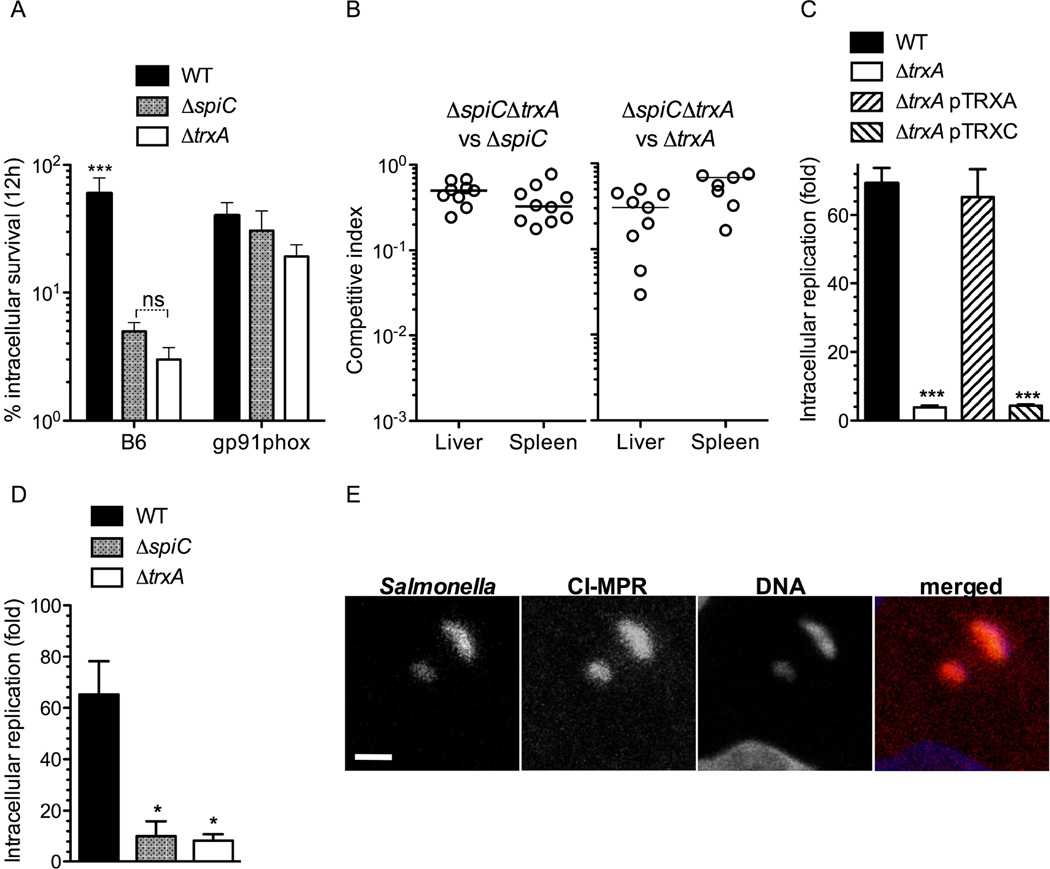
The SPI2 type III secretion system lessens exposure of Salmonella to the antimicrobial activity of the NADPH phagocyte oxidase (Berger et al., 2010; Gallois et al., 2001; van der Heijden et al., 2015; Vazquez-Torres et al., 2000b). To start investigating whether the contribution of thioredoxin-1 to Salmonella antioxidant defenses depends on SPI2, we compared the antimicrobial activity of primary macrophages against trxA-deficient Salmonella or a mutant lacking the spiC gene encoding a structural component and effector of the SPI2 type III secretion system (Uchiya et al., 1999; Yu et al., 2002). Both of these mutants showed similar hypersusceptibility to the bactericidal activity associated with the respiratory burst of primary macrophages (Fig. 3A). To quantify the interdependence of thioredoxin-1 and SPI2 in Salmonella pathogenesis, we performed competition assays between a ΔspiC ΔtrxA double mutant and ΔspiC or ΔtrxA single mutants in an i.p. model of acute Salmonella infection (Fig. 3B). These investigations showed that the ΔspiC ΔtrxA double mutant was recovered from C57BL/6 mice in similar numbers to ΔtrxA or ΔspiC single mutant Salmonella, suggesting that the contributions of thioredoxin-1 and SPI2 to Salmonella pathogenesis are codependent.
Fig. 3. Codependence of thioredoxin-1 and the SPI2 type III secretion system.
Survival of wild-type (WT) and mutant Salmonella in primary macrophages from C57BL/6 (B6) or gp91phox-deficient mice 12 h after challenge (A). p < 0.001 when compared to spiC or trxA mutant controls. Competitive index of ΔspiC ΔtrxA::km, ΔtrxA and ΔspiC Salmonella (B). The competitive index in livers and spleens was estimated 7 d after C57BL/6 mice (n= 7–10) were challenged i.p. with ~105 CFU of an equal mixture of the indicated strains. Intracellular growth of ΔtrxA (C) and ΔspiC (D) Salmonella in J774 cells. The effect of trxA- or trxC-expressing pTRXA or pTRXC plasmids on the intracellular growth of ΔtrxA Salmonella was also evaluated (C). The data are the mean ± SD of 3 independent experiments. *, p < 0.05. Confocal microscopy of GFP-expressing ΔtrxA Salmonella and CI-MPR+ lysosomes in HeLa cells (E). In the merged panel, Salmonella, lysosomes, and cell host nucleus are seen green, red, and blue, respectively. White scale bar = 1 µm. See also Fig. S2, and movies S1–S3.
In addition to antioxidant defense, the SPI2 type III secretion system contributes to the intracellular lifestyle of Salmonella by interfering with lysosomal trafficking and thus promoting intracellular replication (McGourty et al., 2012; Uchiya et al., 1999). To determine whether thioredoxin-1 adds to the defenses of Salmonella against ROS-independent host responses, the intracellular replication of ΔtrxA or ΔspiC Salmonella were measured in two macrophage-like murine cell lines. Whereas wild-type Salmonella replicated about 70–100-fold 18 h after infection, ΔtrxA and ΔspiC mutants replicated poorly in J774 (Fig. 3C & 3D) and RAW cells (Fig. S2A). The intracellular growth defect of ΔtrxA Salmonella could be complemented in trans with trxA but not the related trxC-encoded thioredoxin-2 (Fig. 3C). The bacteriostatic activity exerted by J774 and RAW cells against ΔtrxA Salmonella is unlikely to be mediated by the NADPH phagocyte oxidase, because the J744 and RAW cells used in the course of these investigations do not produce superoxide in response to Salmonella (Fig. S2B & C). As the SPI2 type III secretion system promotes intracellular replication by preventing fusion of Salmonella phagosomes with lysosomes (McGourty et al., 2012), we measured the interactions of vacuoles contain ΔtrxA Salmonella with mannose-6P receptor+ (MPR+) lysosomes. To facilitate visualization of vesicular trafficking, these investigations used HeLa cells (McGourty et al., 2012). Salmonella lacking trxA colocalized with MPR+ lysosomes (Fig. 3E & movie S1). A spiC deficient strain, but not the wild-type isogenic control, also colocalized with MPR+ lysosomes (movies S2 & S3). Collectively, these findings indicate that thioredoxin not only boosts Salmonella antioxidant defenses but also seems to help this intracellular pathogen avoid terminal stages of the degradative pathway. In addition, our investigations suggest that the thioredoxin-mediated resistance of Salmonella to oxygen-dependent and –independent innate responses relies on SPI2.
Thioredoxin optimizes intracellular SPI2 expression
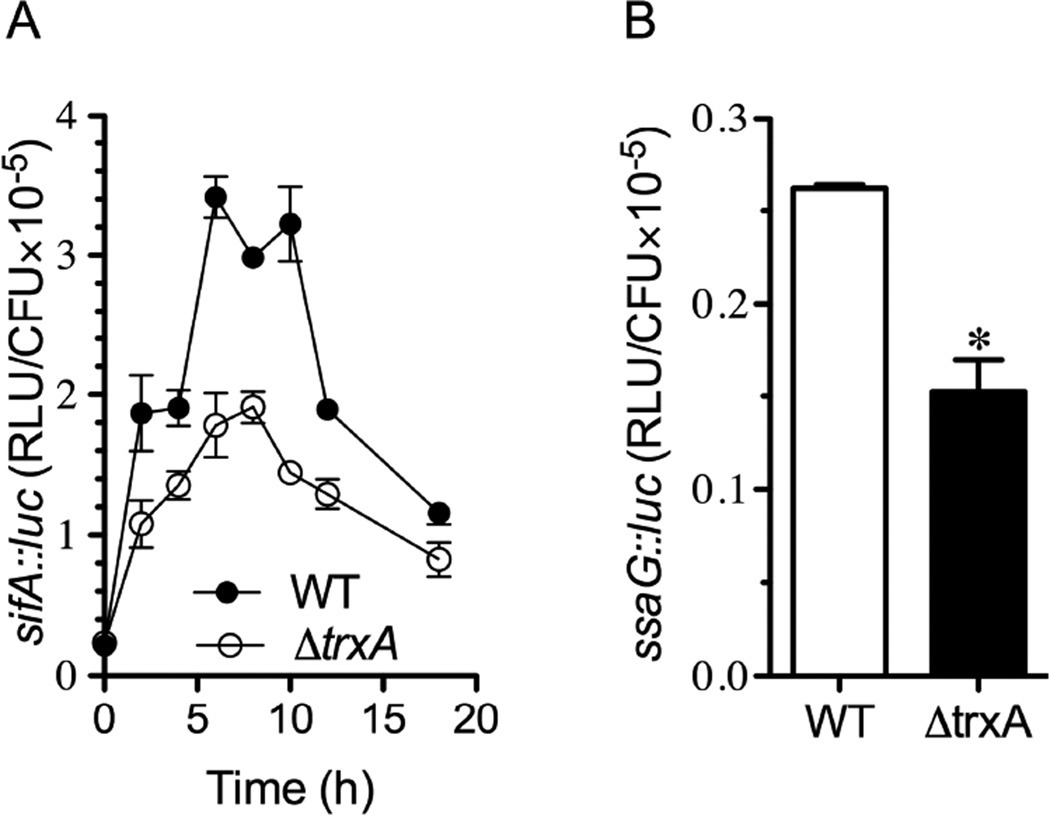
To get insights into a possible relationship between SPI2 and thioredoxin-1, we compared the intracellular SPI2 expression supported by wild-type and ΔtrxA mutant Salmonella. The absence of trxA resulted in decreased intracellular expression of both the SPI2 effector sifA (Fig. 4A) and the structural SPI2 gene ssaG (Fig. 4B). These findings suggest that thioredoxin-1 affects proper SPI2 function.
Fig. 4. Intracellular SPI2 gene expression in ΔtrxA Salmonella.
Intracellular transcription of sifA-luc (A) and ssaG-luc (B) genes as measured by luciferase activity in J774 cells infected with wild-type (WT) or ΔtrxA mutant Salmonella. The data are from 3 independent experiments.* p < 0.05.
SsrB is a substrate of both thioredoxin-1 thiol-disulfide oxidoreductase dependent and –independent activities
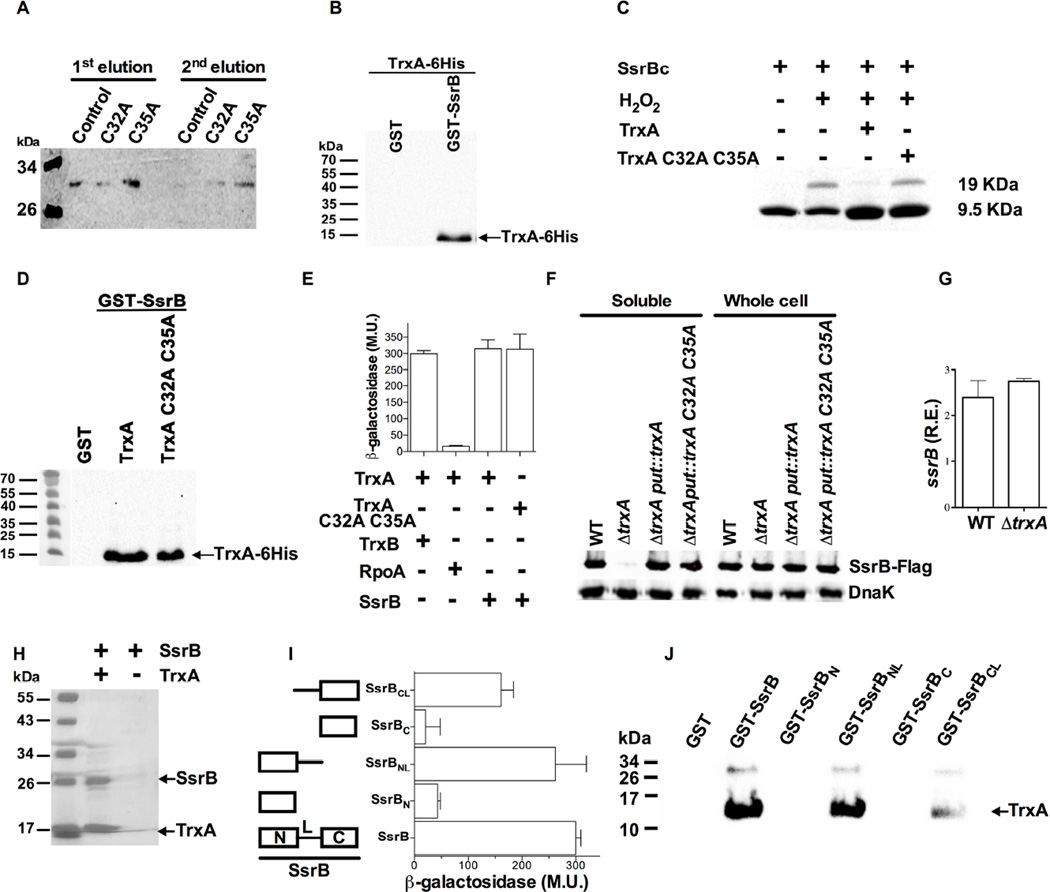
The SsrB response regulator, which controls overall SPI2 transcription, has a redox active cysteine at position 203 that is susceptible to oxidation (Husain et al., 2010). We therefore tested the possibility that SsrB Cys203 could be a substrate of thioredoxin-1. To identify possible interactions between TrxA and SsrB, we performed tandem-affinity purification using an C-terminal fusion of thioredoxin-1 with a calmodulin-binding peptide followed by tobacco etch virus protease cleavage site and Protein A. Tandem affinity purification showed that the TrxA C35A variant, which is unable to resolved mixed disulfides, and to a lesser extent TrxA C32A, interact with SsrB in stationary phase Salmonella (Fig. 5A). These investigations raise the interesting possibility that thioredoxin-1 may regulate SPI2 transcription through its interactions with the response regulator SsrB. The interaction of full-length SsrB and thioredoxin-1 was further examined in vitro with recombinant proteins (Fig. S3). Utilization of GST-SsrB as bate, but not GST, showed a direct interaction between SsrB and TrxA (Fig. 5B). Cys203 in the dimerization domain of SsrB undergoes S-nitrosation after exposure of Salmonella to reactive nitrogen species (Husain et al., 2010). Therefore, we investigated whether oxidized SsrB serves as substrate of thioredoxin-1 thiol-disulfide oxidoreductase activity. A fragment containing the C-terminus domain of SsrB dimerized upon exposure to 250 µM H2O2 (Fig. 5C). Addition of 25 µM TrxA, but not the TrxA C32A C35A variant, resolved the oxidized SsrB homodimer, demonstrating that the disulfide bond formed between SsrB Cys203 and SsrB Cys203’ is a substrate of thioredoxin-1 thiol-disulfide oxidoreductase activity. The biological relevance of the interaction of disulfide-bonded SsrB and thioredoxin-1 remains unknown. The thiol-disulfide oxidoreductase-dependent and –independent interactions of thioredoxin-1 with full-length SsrB were studied further with recombinant proteins in vitro (Fig. 5D). These studies showed interactions of SsrB with both TrxA and TrxA C32A C35A (Fig. 5D), suggesting that thioredoxin-1 can bind to SsrB independently of its CXXC catalytic motif. To gain more insights into the binding of TrxA and SsrB, we constructed a bacterial two-hybrid system that reconstitutes the enzymatic activity of adenylate cyclase through the interactions of T18-SsrB and T25-TrxA fusions. The bacterial two-hybrid system confirmed direct binding between SsrB and TrxA (Fig. 5E). As seen above with recombinant proteins, TrxA C32A C35A associated with SsrB as efficiently as wild-type TrxA, confirming that catalytic cysteine residues are dispensable for binding of thioredoxin-1 to SsrB.
Fig. 5. SsrB is a substrate of thioredoxin-1 thiol-disulfide oxidoreductase-dependent and –independent activities.
Western blotting of SsrB-3XFLAG after lysates of Salmonella containing pTRXATAP plasmids were purified sequentially with IgG (1st elution) and calmodulin (2nd elution) (A). Detection of the purified TrxA-6His proteins bound to the full-length of GST-SsrB by anti-6His immunoblot analysis after pull-down. The GST protein was used as control (B). Molecular markers are indicated. A recombinant C-terminal domain of SsrB separated by PAGE gel electrophoresis was visualized by Coomassie brilliant blue staining (C). Where indicated, 25 µM of the C-terminal domain of SsrB were oxidized with 250 µM H2O2. Some of the specimens exposed to H2O2 were treated with 25 µM of recombinant TrxA or TrxA C32A C35A. The sizes of SsrB monomers and dimers are indicated on the right. TrxA-6His and TrxA C32A C35A-6His proteins were visualized by Western blot using an anti-6His antibody after the pull-down with recombinant GST-SsrB (D). Interactions between wild-type or TrxA C32A C35A with full-length SsrB were studied in a bacterial two-hybrid system that reconstitutes the T18 and T25 domains of adenylate cyclase (E). Thioredoxin reductase (TrxB) and the RpoA α-subunit of the RNA polymerase were included as positive and negative controls, respectively. The activity of reconstituted adenylate cyclase is expressed in Miller units (M.U.). Abundance of TrxA in soluble and whole cell cytoplasmic extracts of stationary phase Salmonella was determined by Western blotting (F). The amount of ssrB mRNA was quantified in overnight cultures of WT and ΔtrxA Salmonella (G). The data are expressed relative to the rpoD house-keeping gene. Purified SsrB-6His was incubated with or without recombinant TrxA-6His at 24°C for 5 days (H). The proteins were resolved in SDS-PAGE and visualized by Coomassie Blue staining. Binding of SsrB fragments and TrxA was studied in a bacterial two-hybrid (I) and protein-protein reconstituted systems (J). TrxA proteins recovered in the pull-downs were visualized after Western-blotting of specimens separated by SDS-PAGE. The SsrB fragments containing N- and/or C-terminal domains +/− linker region (L) are shown on the left side of panel I. Data in A–D, F, H and J are representative of 2–4 blots run on independent days. The data in E, G and I are from 4–8 independent experiments collected in 2–3 days. See also Fig. S3.
We measured the abundance of SsrB protein in stationary phase wild-type or ΔtrxA Salmonella. In the absence of trxA, the intracellular concentration of SsrB protein in the cytoplasmic soluble fraction was dramatically diminished (Fig. 5F), even though the amount of ssrB mRNA was similar in wild-type and ΔtrxA Salmonella (Fig. 5G). Complementation of ΔtrxA Salmonella with either wild-type trxA or the trxA C32A C35A allele supported normal SsrB expression (Fig. 5F). When the amount of SsrB was measured in whole cell lysates, no differences were found between wild-type and ΔtrxA Salmonella, indicating that thioredoxin-1 helps maintain SsrB in the soluble fraction. To gain more insights into this possibility, recombinant full-length SsrB protein was incubated in vitro with or without equimolar amounts of thioredoxin-1. SsrB remained soluble for at least 5 days when incubated with thioredoxin-1 (Fig. 5H). In the absence of thioredoxin-1 full-length SsrB became insoluble.
To investigate in more detail the region of SsrB that interacts with thioredoxin-1, we use the bacterial two-hybrid described above and pull-downs of recombinant proteins. As shown in Fig. 5D and E, thioredoxin-1 bound to full-length SsrB (Fig. 5 I and J). Neither the receiver N-terminal domain of SsrB (i.e., SsrBN) nor the effector C-terminal domain (i.e., SsrBC) bound to thioredoxin-1. A fragment of SsrB encompassing the N-terminal receiver domain and the flexible linker (i.e., SsrBNL) appears to bind to thiredoxin-1 as effectively as full-length SsrB. A fragment of SsrB containing the linker and the C-terminal effector domain (SsrBCL) also bound to thioredoxin-1, although with seemingly less affinity than full-length SsrB or the SsrBNL fragment. These investigations indicate that thioredoxin-1 recognizes the flexible linker region of SsrB in the context of, in this order, receiver and effector domains.
Collectively, our investigations indicate that thioredoxin-1 can interact post-translationally with the SsrB response regulator through both thiol-disulfide oxidoreductase-dependent and –independent functions.
Thioredoxin-1, independently of thiol-disulfide oxidoreductase activity, promotes SPI2 expression, resistance to the NADPH phagocyte oxidase, and Salmonella virulence
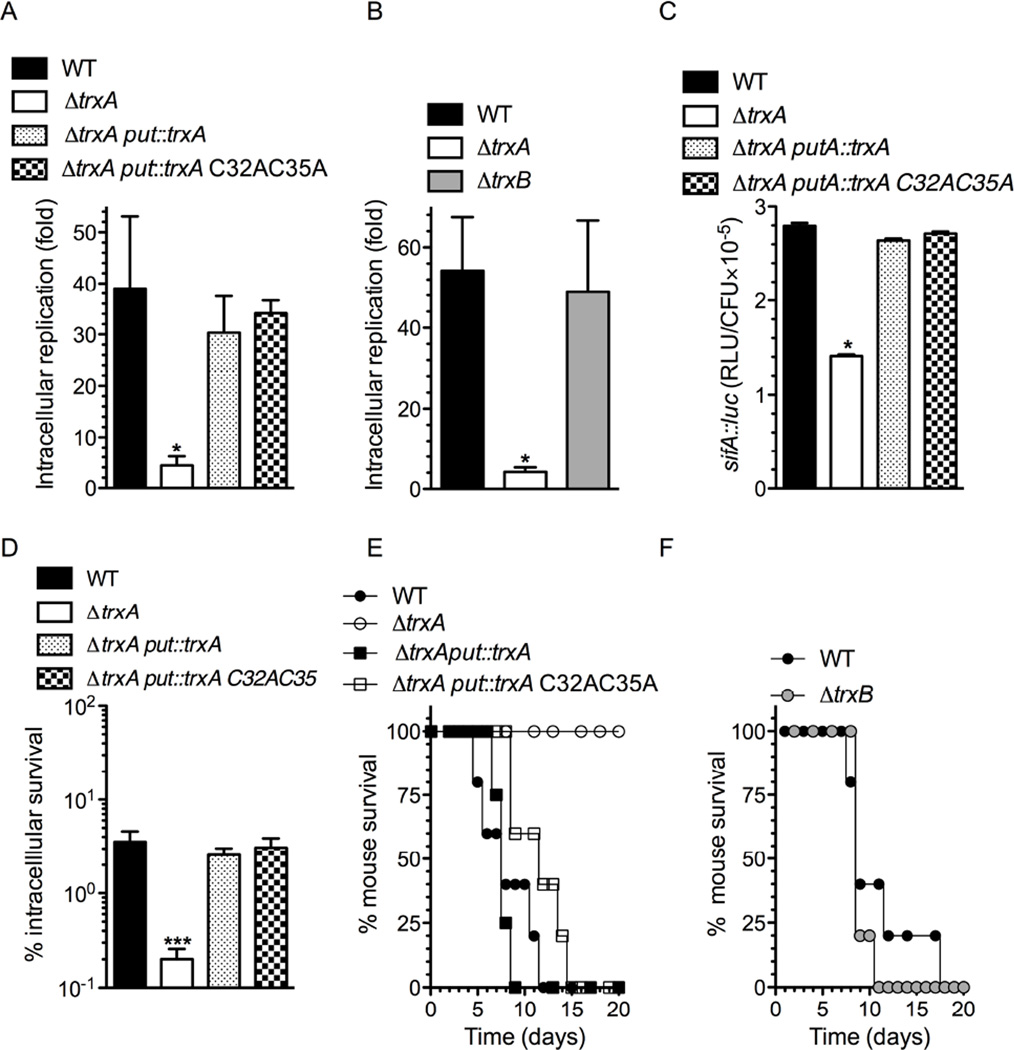
Our investigations have shown that i) most of the contribution of thioredoxin to Salmonella pathogenesis is co-dependent on SPI2 function, and ii) thioredoxin-1 binds to the SsrB response regulator in both thiol-disulfide oxidoreductase-dependent and –independent manners. The following experiments examined the relative contribution of thioredoxin-1 thiol-disulfide oxidoreductase-dependent and -independent activities to SPI2 function and Salmonella pathogenesis. Towards this end, ΔtrxA Salmonella was complemented with wild-type trxA or a trxA C32A C35A variant. Both wild-type or trxA C32A C35A supported growth of Salmonella in J774 cells (Fig. 6A), demonstrating that the thioredoxin-1 thiol-disulfide oxidoreductase activity is largely irrelevant for intracellular replication of Salmonella in these cells that do not sustain a respiratory burst. In support of this notion, ΔtrxB Salmonella lacking thioredoxin reductase replicated as efficiently as wild-type Salmonella in J774 cells (Fig. 6B). Moreover, wild-type Salmonella and controls expressing the trxA C32A C35A variant activated similar levels of sifA transcription in J774 macrophage-like cells (Fig. 6C). These investigations indicate that thioredoxin-1 does not depend on its well-characterized thiol-disulfide oxidoreductase to support SPI2 expression needed for the intracellular growth of Salmonella. These findings are consistent with previous published work that showed a role for TrxA in regulation of SPI2 function (Negrea et al., 2009). We also tested the survival of Salmonella expressing wild-type trxA or the trxA C32A C35A variant in primary macrophages producing large amounts of ROS through the enzymatic activity of the NADPH phagocyte oxidase (Fig. S2D). Expression of either wild-type trxA or the trxA C32A C35A variant restored the survival of ΔtrxA Salmonella in periodate-elicited macrophages from C57BL/6 mice (Fig. 6D). We find it remarkable that the canonical oxidoreductase activity of thioredoxin-1 seems to be dispensable against the cytotoxicity of ROS generated by the NADPH phagocyte oxidase in this population of primary macrophages. Lastly, we monitored the virulence of Salmonella expressing the trxA C32A C35A variant in a murine model of infection dominated by the innate response of the NADPH phagocyte oxidase. Salmonella expressing either of these two alleles killed C57BL/6 mice with similar kinetics to wild-type bacteria (p = 0.4). Together, these findings suggest that most contributions of thioredoxin-1 to Salmonella pathogenesis are independent of its thiol-disulfide oxidoreductase enzymatic activity. Accordingly, ΔtrxB Salmonella appear to be fully virulent in this model of experimental salmonellosis (Fig. 6F).
Fig. 6. Contribution of thioredoxin-1 thiol-disulfide oxidoreductase-dependent and –independent activities to SPI2 function and Salmonella pathogenesis.
Replication of trxA variants (A) and ΔtrxB (B) Salmonella in J774 cells. Wild-type (WT) Salmonella were used as controls. Activity of the sifA::luc chromosomal reporter in J774 cells as measured by luciferase luminescence in Salmonella expressing the indicated trxA variants (C). Survival of ΔtrxA Salmonella in periodate-elicited macrophages from C57BL/6 mice (D). ***, p < 0.001. Survival of C57BL/6 mice challenged orally with ~3 × 106 CFU/mouse of ΔtrxA (E) or ΔtrxB (F) Salmonella. Data are from 3–6 biological replicates.
Discussion
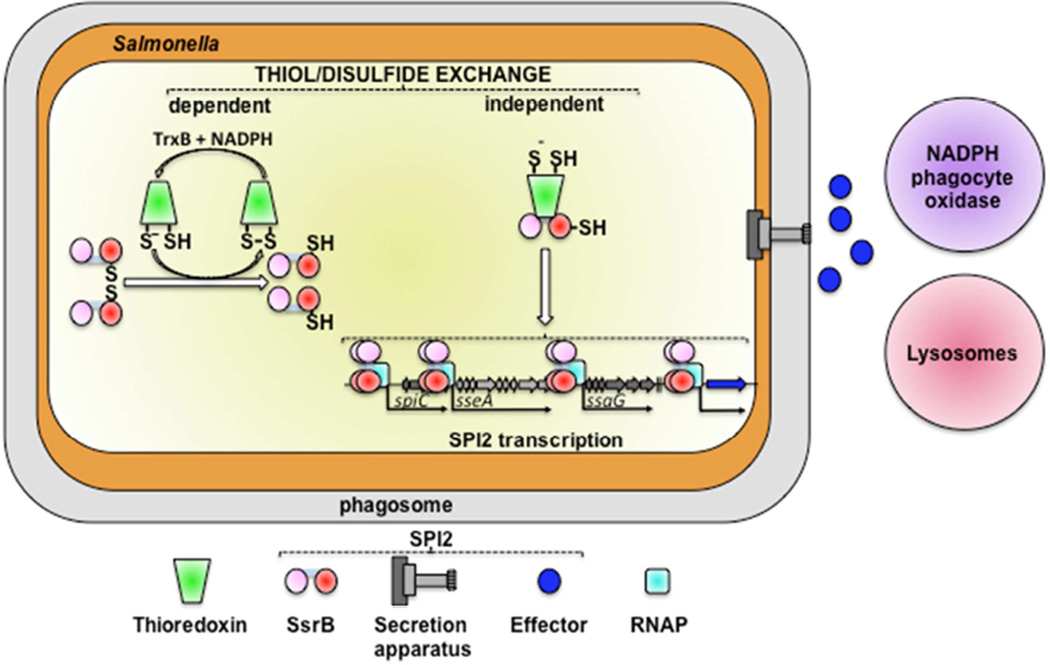
Members of the thioredoxin family contribute to the antioxidant defenses of phylogenetically diverse organisms including bacteria and humans (Carmel-Harel and Storz, 2000). By directing electrons from NADPH and thioredoxin reductase to disulfide bonds in target proteins, thioredoxins maintain thiol redox homeostasis. Regulation of SPI2 expression by thioredoxin-1 is critical to Salmonella pathogenesis (Bjur et al., 2006; Negrea et al., 2009), but the molecular mechanism by which thioredoxin-1 promotes SPI2 expression and Salmonella virulence is incompletely understood. The classical oxidoreductase activity of thioredoxin-1 promotes antioxidant defenses of Salmonella in vitro, but appears to be largely dispensable in vivo. Nonetheless, thioredoxin-1, independent of canonical thiol-disulfide oxidoreductase, protects Salmonella against the NADPH phagocyte oxidase. Thioredoxin-1 binds to SsrB, thereby stimulating intracellular SPI2 expression, facilitating growth in professional phagocytes, and ultimately protecting Salmonella against the oxidative stress emanating from the enzymatic activity of the NADPH phagocyte oxidase in primary macrophages and a murine model of acute systemic infection (Fig. 7).
Fig. 7. Model for post-translational regulation of SsrB by thioredoxin-1 thiol-disulfide oxidoreductase-dependent and -independent activities.
Oxidation of SsrB results in reversible disulfide bond formation between Cys203 and Cys203’ in the homodimer. The disulfide bond in the SsrB homodimer is attacked by the thiolate (-S−) of thioredoxin-1 Cys32; the resulting mixed disulfide is resolved by thioredoxin-1 Cys35 (-SH). Disulfide-bonded thioredoxin-1 is repaired by the enzymatic activity of thioredoxin reductase TrxB, using NADPH as reducing power. In addition to serving as a substrate of thiol-disulfide oxidoreductase activity, thioredoxin-1 binds to SsrB independently of its thiol-disulfide oxidoreductase activity, resulting in stabilization of SsrB. By doing so, thioredoxin-1 aids with the activation of the SPI2 type III secretion system, thus lessening the cytotoxicity of the NADPH phagocyte oxidase in the innate response of macrophages while minimizing interactions of Salmonella-containing vacuoles with lysosomes. Receiver (pink) and effector (red) domains of SsrB, thioredoxin-1 (green), SPI2 apparatus (grey), SPI2 effectors (blue), and RNA polymerase (RNAP, cyan).
Reactive oxygen and nitrogen species generated by the enzymatic activity of NADPH phagocyte oxidase and iNOS flavohemoproteins are critical components of the anti-Salmonella arsenal of human and murine macrophages (Stevanin et al., 2002; Vazquez-Torres et al., 2000a). We find that thioredoxin-1-deficient Salmonella become virulent in gp91phox-deficient macrophages and mice lacking the membrane-bound subunit of the NADPH phagocyte oxidase, but remain attenuated in iNOS-deficient mice. This indicates that thioredoxin-1 contributes to the antioxidant defenses that protect this intracellular pathogen against the respiratory burst of professional phagocytic cells, but appears to be dispensable for the antinitrosative defenses of Salmonella. It should be noted that the recovery of fitness of ΔtrxA Salmonella in gp91phox-deficient mice is substantial but not complete, suggesting that the thioredoxin-dependent regulation of SsrB protects Salmonella against oxygen-independent host defenses as well. According to this idea, ΔtrxA Salmonella fail to grow intracellularly in J774 cells unable to sustain a productive respiratory burst. Halting the final steps of the degradative pathway (Uchiya et al., 1999) and intersection of vesicles from the trans-Golgi network (Kuhle et al., 2006; Salcedo and Holden, 2003) are additional mechanisms by which the thioredoxin-1-dependent regulation of SsrB function may contribute to Salmonella virulence. In fact, the lack of fusion of Salmonella-containing vesicles with MPR+ lysosomes is dependent on thioredoxin-1.
The enzymatic activity of periplasmic Cu-Zn superoxide dismutase and the MacAB multidrug efflux pump protect Salmonella extracytoplasmic molecular targets from the oxidative stress of macrophages (Bogomolnaya et al., 2013; De Groote et al., 1997). In addition, the concerted action of glutathione, catalases, and hydroperoxidases boost the antioxidant defenses of intracellular Salmonella (Hebrard et al., 2009; Song et al., 2013). Our investigations have identified thioredoxin-1 as an additional component of the antioxidant toolbox of intracellular Salmonella in macrophages and mice. In contrast to its previously described roles in antioxidant defense of prokaryotic and eukaryotic organisms (Carmel-Harel and Storz, 2000), the protection afforded by thioredoxin-1 against the NADPH phagocyte oxidase occurs independently of its canonical thiol-disulfide oxidoreductase enzymatic activity. By controlling the expression of SPI2, a type III secretion system that reduces contact of phagosomes with incoming NADPH phagocyte oxidase-containing vesicles (Berger et al., 2010; Gallois et al., 2001; Suvarnapunya and Stein, 2005; van der Heijden et al., 2015; Vazquez-Torres et al., 2001; Vazquez-Torres et al., 2000b), thioredoxin protects Salmonella from the oxidative stress generated during the innate immune response in macrophages.
ROS generated at the early stages of the Salmonella infection are bactericidal (Grant et al., 2008). Our investigations, as well as previously published work (Bjur et al., 2006), indicate that thiol-disulfide exchange reactions of thioredoxin-1 do not protect Salmonella against the microbicidal activity of authentic H2O2. Failure of thioredoxin-1 to protect against the bactericidal activity of H2O2 can be explained if we consider that this ROS kills bacteria such as Salmonella by Fenton-mediated chemistry, in which ferrous iron reduces H2O2 to generate highly genotoxic hydroxyl radicals (Imlay and Linn, 1988). Nonetheless, the thiol-disulfide oxidoreductase activity of thioredoxin-1 ameliorates the bacteriostatic effects of H2O2 in vitro. The importance of classical thioredoxin-1-thioredoxin reductase in resistance of Salmonella to the antimicrobial activity of the NADPH phagocyte oxidase remains uncertain, as indicated by the fact that strains lacking thioredoxin reductase or expressing the TrxA C32A C35A variant survive normally the respiratory burst of primary macrophages. It seems unlikely that H2O2 produced in the respiratory burst of macrophages does not oxidize cysteine residues in Salmonella proteins. Glutathione, glutathione peroxidase, and glutaredoxins may maintain thiol homeostasis in the absence of the thioredoxin/thioredoxin reductase system.
Thioredoxin-1 participates in the post-translational regulation of SsrB. Biochemical and genetic lines of evidence have shown that thioredoxin-1 interacts with SsrB independently of its thiol-disulfide oxidoreductase. In analogy to the binding of E. coli’s thioredoxin to a flexible loop in the thumb of gene 5 protein DNA polymerase of bacteriophage T7 (Doublie et al., 1998), thioredoxin-1 seems to interact with the linker region joining receiver and effector domains of SsrB. Based on crystal structures of response regulators (Buckler et al., 2002; Menon and Wang, 2011), the linker region of SsrB is likely to be disorganized. The initial interaction of SsrB with the unorganized linker region seems to promote interactions with globular parts of the receiver and effector domains, keeping SsrB in the soluble fraction. The associations between thioredoxin-1 and SsrB might be generalizable to other response regulators, as suggested by the pull-down of RcsB and OmpR with thioredoxin-1 in a proteomic screen done in E. coli (Kumar et al., 2004). Future work will need to test if the associations discovered here between thioredoxin-1 and SsrB apply to other response regulators. Thioredoxin-1 can also bind to disulfide-bonded SsrB in vitro through its thiol-disulfide oxidoreductase catalytic domain. The biological relevance of this interaction, however, remains unknown.
In summary, by regulating SPI2 expression, thioredoxin-1 antagonizes a variety of oxygen-dependent and –independent host defenses. Our work indicates that the horizontally-acquired virulence determinant SsrB is post-translationally regulated by the ancestral protein thioredoxin-1. Because thioredoxins are ubiquitous in the bacterial kingdom, the interactions established between thioredoxin and SsrB in ancestral bacteria may have been conserved after lateral gene transfer of the SPI2 pathogenicity island into the Salmonella lineage.
Experimental Procedures
Bacterial strains
Supplementary Tables 1 and 2 list the Salmonella strains, as well as plasmids and primers used in this manuscript. Supplementary Experimental Procedures describe the construction of the strains of Salmonella enterica serovar Typhimurium used here.
Mouse virulence
Six- to 8-week old C57BL/6 and congenic gp91phox- (Pollock et al., 1995) or iNOS-deficient (MacMicking et al., 1995) mice bred in our animal facility according to Institutional Animal Care and Use Committee guidelines were used for live/dead and competition assays as described in Supplementary Information.
H2O2 killing
Salmonella grown for 20 h in LB broth at 37°C in a shaker inc ubator were diluted in PBS to a final concentration of 5 × 105 CFU/ml. The bacteria were challenged at 37°C with increasing concentrations o f H2O2, menadione, or diamide for 2 h. The cultures were then serially diluted in PBS and spotted on LB agar plates. The number of bacteria capable of forming a colony was quantified after overnight culture. The % of surviving bacteria was calculated according to the formula (CFU from treated sample/CFU from untreated sample) × 100.
Bacterial growth in response to H2O2
Salmonella grown in LB broth for 18 h were inoculated into high-Mg2+ N salts medium [5 mM KCl, 7.5 mM (NH4)SO4, 0.5 mM K2SO4, 1 mM KH2PO4, 38 mM glycerol, 0.1% casamino acids supplemented with 10 mM MgCl2 and 100 mM Tris-HCl, pH 7.6]. Salmonella were then grown for 4 h at 37°C in a shaking incubator until the cultures reached A600 of 0.5. Bacteria were adjusted to a final concentration of 5 × 106 CFU/ml and challenged with 100 µM H2O2. Bacterial growth was measured as A600 every 15 min for 25 h in a Bioscreen-C Growth analyzer (Oy Growth Curves AB Ltd, Helsinki, Finland).
Intracellular survival
J774 cells and primary macrophages were infected at MOI of 10 and 2, respectively, with Salmonella grown for 20 h in LB broth at 37°C in a shaker incubator. The intracellular number of bacteria was determined on LB agar plates after lysing host cells with 0.25% deoxycholate.
Immunofluorescence microscopy
HeLa cells were infected with late log phase Salmonella expressing the PrpsM-GFP construct. Specimens collected 8 h after infection were fixed with 3.7% paraformaldehyde and stained with anti-CI MPR antibody (Developmenal Studies Hybridoma Bank) followed an anti-mouse IgG antibody conjugated with Texas Red (Rockland). Co-localization of GFP-expressing Salmonella with MPR+ lysosomes was visualized on a Leica TCS SP8 Confocal Laser Scanning Microscope.
Transcriptional analysis
Intracellular SPI2 expression by sifA-luc- or expression of ssaG-luc-expressing Salmonella in J774 was determined by recording luminescence as previously described (Gerlach et al., 2007) and explained in detail in supplementary information.
Tandem-affinity purification
Protein partners of thioredoxin-1 were identified by tandem-affinity purification using a trxA construct in plasmid pFA6a-CTAP (Tasto et al., 2001) that allows for sequential purification of calmodulin-binding peptide and Protein A. This procedure is described in Supplementary Information.
Binding of thioredoxin-1 and SsrB
Recombinant TrxA-6His, TrxA C32A C35A-6His, and GST-SsrB proteins were purified as described in Supplementary Information. Binding of recombinant TrxA-6His or TrxA C32A C35A-6His to GST-SsrB was analyzed as described previously (Henard et al., 2014). Briefly, 1 nmol of GST-SsrB proteins were incubated with 200 µl of Glutathione-Sepharose 4B beads (BioWorld, Dubin, OH) at 4°C for 2 h, washed with 20 bed volume of 50 mM Tris-HCl, pH 7.5, and then incubated with 2 nmole of TrxA-6His or TrxA C32A C35A-6His proteins at 4°C for 2 h with agitation. After washing with 30 mM NaCl buffer, samples were eluted with 500 mM NaCl, precipitated with 10% TCA, and loaded into a 15% SDS-PAGE gels to detect the TrxA-6His or TrxA C32A C35A-6His proteins by immunoblot analysis. Electro-blotted proteins were treated with a 1/1,000 dilution of anti-6His antibody (Rockland, Limerick, PA), followed by a 1/10,000 dilution of goat anti-rabbit IgG (Pierce, Rockford, IL) conjugated with horseradish peroxidase. TrxA-6His-tagged proteins were visualized using a ECL prime Western blotting detection reagent by GE. Purified GST protein was used as a negative control.
Thioredoxin-1 thiol-disulfide oxidoreductase activity
Full-length TrxA and a C-terminal SsrB fragment encompassing residues 137–212 expressed as GST fusions from pGEX6P1 (GE Healthcare Biosciences, Fairfield, CT) were purified as described previously for DksA (Henard et al., 2014). Where indicated, 25 µM recombinant SsrB was treated with 250 µM H2O2 for 1 h at 37°C. Selected samples were treated wit h 25 µM TrxA for 30 min. The specimens were mixed with 3× Red loading buffer (New England Biolabs, Ipswich, MA) in the absence of reducing agents. The samples were loaded into 4–20% SDS-PAGE gels (Bio-Rad) and electrophoresed at 125V on ice. Proteins were visualized by Coomassie blue staining.
SsrB stability by TrxA
Recombinant TrxA-6His and SsrB-6His proteins were purified as described in Supplementary Information. To determine the SsrB stability with and without TrxA-6His protein, 100 pmol of the purified SsrB-6His proteins in 50 mM Tris-HCl (pH 7.5) were incubated at room temperature with and without 100 pmole of TrixA-6His proteins. After 5 days, samples were loaded onto a 12% SDS-PAGE gels to assess the TrxA-6His or SsrB-6His proteins by Coomassie brilliant blue staining.
Bacterial two-hybrid system
The trxA or trxA C32A C35A genes were cloned into the pUT18 vector of a bacterial two-hybrid system to produce fusions to the N-terminus of the T18 subunit of adenlyate cyclase (Euromedox, Souffelweyersheim Cedex, France). The resulting plasmids were electroplated into E. coli expressing pKNT25-ssrB encoding SsrB fused to the N-terminus of the T25 subunit of adenlyate cyclase. Binding of TrxA to SsrB was measured by following β-galactosidase in overnight cultures using o-nitrophenol-β-galactoside. The results are expressed in Miller units.
Statistical Analysis
One-way analysis of variance (ANOVA) followed by a Bonferroni post-test helped determine statistical significance between multiple comparisons. Data were considered statistically significant when p < 0.05.
Supplementary Material
Highlights.
Thioredoxin defends Salmonella against the NADPH phagocyte oxidase
Thioredoxin promotes antioxidant defense by facilitating SPI2 transcription
Thioredoxin binds to the SsrB linker, stabilizing this SPI2 response regulator
Thioredoxin regulates SsrB independently of its CXXC catalytic motif
Acknowledgments
Research supported by the NIH grant AI54959, the Veterans Administration grant BX002073, and the Burroughs Wellcome Fund. We thank members of the Vazquez-Torres’ lab for stimulating discussions, and Timothy Tapscott for providing the Slider Image. We thank Ronald Bouchard (Veterans Affairs Eastern Colorado Health Care System) for help with the confocal microscopy, and Dr. Michael Hensel (Mikrobiologisches Institut, Universitätsklinikum Erlangen, Germany) for his generous gift of the sifA-luc construct.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
Conceptualization and writing - original draft, M.S., J.S.K., and A.V.T.; Investigation, M.S., J.S.K., L.L., M.H., and A.V.T.; Funding Acquisition, Supervision, and Writing - Review & Editing, A.V.T.
References
- Arner ES, Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem. 2000;267:6102–6109. doi: 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- Babior BM. NADPH oxidase: an update. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- Berger SB, Romero X, Ma C, Wang G, Faubion WA, Liao G, Compeer E, Keszei M, Rameh L, Wang N, et al. SLAM is a microbial sensor that regulates bacterial phagosome functions in macrophages. Nat Immunol. 2010;11:920–927. doi: 10.1038/ni.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuzon CR, Meresse S, Unsworth KE, Ruiz-Albert J, Garvis S, Waterman SR, Ryder TA, Boucrot E, Holden DW. Salmonella maintains the integrity of its intracellular vacuole through the action of SifA. Embo J. 2000;19:3235–3249. doi: 10.1093/emboj/19.13.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjur E, Eriksson-Ygberg S, Aslund F, Rhen M. Thioredoxin 1 promotes intracellular replication and virulence of Salmonella enterica serovar Typhimurium. Infect Immun. 2006;74:5140–5151. doi: 10.1128/IAI.00449-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogomolnaya LM, Andrews KD, Talamantes M, Maple A, Ragoza Y, Vazquez-Torres A, Andrews-Polymenis H. The ABC-type efflux pump MacAB protects Salmonella enterica serovar typhimurium from oxidative stress. MBio. 2013;4:e00630–e00613. doi: 10.1128/mBio.00630-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler DR, Zhou Y, Stock AM. Evidence of intradomain and interdomain flexibility in an OmpR/PhoB homolog from Thermotoga maritima. Structure. 2002;10:153–164. doi: 10.1016/s0969-2126(01)00706-7. [DOI] [PubMed] [Google Scholar]
- Burton NA, Schurmann N, Casse O, Steeb AK, Claudi B, Zankl J, Schmidt A, Bumann D. Disparate impact of oxidative host defenses determines the fate of Salmonella during systemic infection in mice. Cell Host Microbe. 2014;15:72–83. doi: 10.1016/j.chom.2013.12.006. [DOI] [PubMed] [Google Scholar]
- Carmel-Harel O, Storz G. Roles of the glutathione- and thioredoxin-dependent reduction systems in the Escherichia coli and saccharomyces cerevisiae responses to oxidative stress. Annu Rev Microbiol. 2000;54:439–461. doi: 10.1146/annurev.micro.54.1.439. [DOI] [PubMed] [Google Scholar]
- De Groote MA, Ochsner UA, Shiloh MU, Nathan C, McCord JM, Dinauer MC, Libby SJ, Vazquez-Torres A, Xu Y, Fang FC. Periplasmic superoxide dismutase protects Salmonella from products of phagocyte NADPH-oxidase and nitric oxide synthase. Proc Natl Acad Sci U S A. 1997;94:13997–14001. doi: 10.1073/pnas.94.25.13997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doublie S, Tabor S, Long AM, Richardson CC, Ellenberger T. Crystal structure of a bacteriophage T7 DNA replication complex at 2.2 A resolution. Nature. 1998;391:251–258. doi: 10.1038/34593. [DOI] [PubMed] [Google Scholar]
- Gallois A, Klein JR, Allen LA, Jones BD, Nauseef WM. Salmonella pathogenicity island 2-encoded type III secretion system mediates exclusion of NADPH oxidase assembly from the phagosomal membrane. J Immunol. 2001;166:5741–5748. doi: 10.4049/jimmunol.166.9.5741. [DOI] [PubMed] [Google Scholar]
- Gerlach RG, Holzer SU, Jackel D, Hensel M. Rapid engineering of bacterial reporter gene fusions by using Red recombination. Appl Environ Microbiol. 2007;73:4234–4242. doi: 10.1128/AEM.00509-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant AJ, Restif O, McKinley TJ, Sheppard M, Maskell DJ, Mastroeni P. Modelling within-host spatiotemporal dynamics of invasive bacterial disease. PLoS biology. 2008;6:e74. doi: 10.1371/journal.pbio.0060074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebrard M, Viala JP, Meresse S, Barras F, Aussel L. Redundant hydrogen peroxide scavengers contribute to Salmonella virulence and oxidative stress resistance. J Bacteriol. 2009;191:4605–4614. doi: 10.1128/JB.00144-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henard CA, Tapscott T, Crawford MA, Husain M, Doulias PT, Porwollik S, Liu L, McClelland M, Ischiropoulos H, Vazquez-Torres A. The 4-cysteine zinc-finger motif of the RNA polymerase regulator DksA serves as a thiol switch for sensing oxidative and nitrosative stress. Mol Microbiol. 2014;91:790–804. doi: 10.1111/mmi.12498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin and glutaredoxin systems. J Biol Chem. 1989;264:13963–13966. [PubMed] [Google Scholar]
- Husain M, Bourret TJ, McCollister BD, Jones-Carson J, Laughlin J, Vazquez-Torres A. Nitric oxide evokes an adaptive response to oxidative stress by arresting respiration. J Biol Chem. 2008;283:7682–7689. doi: 10.1074/jbc.M708845200. [DOI] [PubMed] [Google Scholar]
- Husain M, Jones-Carson J, Song M, McCollister BD, Bourret TJ, Vazquez-Torres A. Redox sensor SsrB Cys203 enhances Salmonella fitness against nitric oxide generated in the host immune response to oral infection. Proc Natl Acad Sci U S A. 2010;107:14396–14401. doi: 10.1073/pnas.1005299107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imlay JA. Pathways of oxidative damage. Annu Rev Microbiol. 2003;57:395–418. doi: 10.1146/annurev.micro.57.030502.090938. [DOI] [PubMed] [Google Scholar]
- Imlay JA, Linn S. Bimodal pattern of killing of DNA-repair-defective or anoxically grown Escherichia coli by hydrogen peroxide. J Bacteriol. 1986;166:519–527. doi: 10.1128/jb.166.2.519-527.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imlay JA, Linn S. DNA damage and oxygen radical toxicity. Science. 1988;240:1302–1309. doi: 10.1126/science.3287616. [DOI] [PubMed] [Google Scholar]
- Korshunov S, Imlay JA. Two sources of endogenous hydrogen peroxide in Escherichia coli. Mol Microbiol. 2010;75:1389–1401. doi: 10.1111/j.1365-2958.2010.07059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhle V, Abrahams GL, Hensel M. Intracellular Salmonella enterica redirect exocytic transport processes in a Salmonella pathogenicity island 2-dependent manner. Traffic. 2006;7:716–730. doi: 10.1111/j.1600-0854.2006.00422.x. [DOI] [PubMed] [Google Scholar]
- Kumar JK, Tabor S, Richardson CC. Proteomic analysis of thioredoxin-targeted proteins in Escherichia coli. Proc Natl Acad Sci U S A. 2004;101:3759–3764. doi: 10.1073/pnas.0308701101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMicking JD, Nathan C, Hom G, Chartrain N, Fletcher DS, Trumbauer M, Stevens K, Xie QW, Sokol K, Hutchinson N, et al. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81:641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- Mastroeni P, Vazquez-Torres A, Fang FC, Xu Y, Khan S, Hormaeche CE, Dougan G. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. II. Effects on microbial proliferation and host survival in vivo. J Exp Med. 2000;192:237–248. doi: 10.1084/jem.192.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGourty K, Thurston TL, Matthews SA, Pinaud L, Mota LJ, Holden DW. Salmonella inhibits retrograde trafficking of mannose-6-phosphate receptors and lysosome function. Science. 2012;338:963–967. doi: 10.1126/science.1227037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon S, Wang S. Structure of the response regulator PhoP from Mycobacterium tuberculosis reveals a dimer through the receiver domain. Biochemistry. 2011;50:5948–5957. doi: 10.1021/bi2005575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouy R, Fischer A, Vilmer E, Seger R, Griscelli C. Incidence, severity, and prevention of infections in chronic granulomatous disease. J Pediatr. 1989;114:555–560. doi: 10.1016/s0022-3476(89)80693-6. [DOI] [PubMed] [Google Scholar]
- Negrea A, Bjur E, Puiac S, Ygberg SE, Aslund F, Rhen M. Thioredoxin 1 participates in the activity of the Salmonella enterica serovar Typhimurium pathogenicity island 2 type III secretion system. J Bacteriol. 2009;191:6918–6927. doi: 10.1128/JB.00532-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock JD, DA W, Gifford MA, Li LL, Du X, Fisherman J, Orkin SH, Doerschuk CM, Dinauer MC. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet. 1995;9:202–209. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- Salcedo SP, Holden DW. SseG, a virulence protein that targets Salmonella to the Golgi network. Embo J. 2003;22:5003–5014. doi: 10.1093/emboj/cdg517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M, Husain M, Jones-Carson J, Liu L, Henard CA, Vazquez-Torres A. Low-molecular-weight thiol-dependent antioxidant and antinitrosative defences in Salmonella pathogenesis. Mol Microbiol. 2013;87:609–622. doi: 10.1111/mmi.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevanin TM, Poole RK, Demoncheaux EA, Read RC. Flavohemoglobin Hmp protects Salmonella enterica serovar typhimurium from nitric oxide-related killing by human macrophages. Infect Immun. 2002;70:4399–4405. doi: 10.1128/IAI.70.8.4399-4405.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvarnapunya AE, Stein MA. DNA base excision repair potentiates the protective effect of Salmonella Pathogenicity Island 2 within macrophages. Microbiology. 2005;151:557–567. doi: 10.1099/mic.0.27555-0. [DOI] [PubMed] [Google Scholar]
- Tasto JJ, Carnahan RH, McDonald WH, Gould KL. Vectors and gene targeting modules for tandem affinity purification in Schizosaccharomyces pombe. Yeast. 2001;18:657–662. doi: 10.1002/yea.713. [DOI] [PubMed] [Google Scholar]
- Uchiya K, Barbieri MA, Funato K, Shah AH, Stahl PD, Groisman EA. A Salmonella virulence protein that inhibits cellular trafficking. Embo J. 1999;18:3924–3933. doi: 10.1093/emboj/18.14.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heijden J, Bosman ES, Reynolds LA, Finlay BB. Direct measurement of oxidative and nitrosative stress dynamics in Salmonella inside macrophages. Proc Natl Acad Sci U S A. 2015;112:560–565. doi: 10.1073/pnas.1414569112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Diepen A, van der Straaten T, Holland SM, Janssen R, van Dissel JT. A superoxide-hypersusceptible Salmonella enterica serovar Typhimurium mutant is attenuated but regains virulence in p47(phox−/−) mice. Infect Immun. 2002;70:2614–2621. doi: 10.1128/IAI.70.5.2614-2621.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Torres A, Fantuzzi G, Edwards CK, 3rd, Dinarello CA, Fang FC. Defective localization of the NADPH phagocyte oxidase to Salmonella-containing phagosomes in tumor necrosis factor p55 receptor-deficient macrophages. Proc Natl Acad Sci U S A. 2001;98:2561–2565. doi: 10.1073/pnas.041618998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Torres A, Jones-Carson J, Mastroeni P, Ischiropoulos H, Fang FC. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. I. Effects on microbial killing by activated peritoneal macrophages in vitro. J Exp Med. 2000a;192:227–236. doi: 10.1084/jem.192.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Torres A, Xu Y, Jones-Carson J, Holden DW, Lucia SM, Dinauer MC, Mastroeni P, Fang FC. Salmonella pathogenicity island 2-dependent evasion of the phagocyte NADPH oxidase. Science. 2000b;287:1655–1658. doi: 10.1126/science.287.5458.1655. [DOI] [PubMed] [Google Scholar]
- Yu XJ, Ruiz-Albert J, Unsworth KE, Garvis S, Liu M, Holden DW. SpiC is required for secretion of Salmonella Pathogenicity Island 2 type III secretion system proteins. Cell Microbiol. 2002;4:531–540. doi: 10.1046/j.1462-5822.2002.00211.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.