Abstract
Background
Gastric cancer is a malignant tumor with a high morbidity and mortality. MicroRNAs are important regulators of gene expression, influencing the progression of gastric cancer. This study aimed to reveal the role of microRNA-140 (miR-140) in gastric cancer cell proliferation and its potential mechanisms.
Material/Methods
Gastric cancer tissues and cell lines BGC-823, SGC-7901, and HGC-27 were used to analyze miR-140 levels compared to normal tissues and cell line GES-1. In HGC-27 cells transfected with miR-140 mimic, we performed MTT, colony formation assay, and cell cycle assay by flow cytometry. SOX4, a predicted target of miR-140, was mutated to verify its regulation by miR-140, and was overexpressed to analyze its function in cell proliferation. Doxorubicin treatment was performed to investigate the effect of miR-140 on drug resistance.
Results
miR-140 was down-regulated in gastric cancer tissues and cell lines, with the lowest expression level in HGC-27. miR-140 overexpression inhibited HGC-27 cell viability and colony formation and resulted in G0/G1 arrest. miR-140 suppressed SOX4 expression via binding to the 3′ untranslated region, while the mutant SOX4 could not be regulated. Overexpressing SOX4 led to promoted cell viability, colony formation, and cell cycle progress. miR-140 overexpression also improved the anti-viability effects of doxorubicin, suggesting its potential in reducing the drug resistance of gastric cells.
Conclusions
These findings suggest that miR-140 directly inhibits SOX4, which might be one of its mechanisms in suppressing gastric cancer cell proliferation. This study provides a promising therapeutic strategy for treating gastric cancer and facilitates microRNA research in various diseases.
MeSH Keywords: Cell Proliferation, Drug Resistance, MicroRNAs, SOXC Transcription Factors, Stomach Neoplasms
Background
Gastric cancer is a malignant tumor originating from the glandular epithelium of the gastric mucosa. It has high morbidity and mortality, especially in China, severely affecting the health of patients [1,2]. Risk factors include unhealthy eating habits, alcohol, smoking, bacteria, and viruses [3]. Gastric cancer cells are usually promoted in their proliferation and cell cycle progression, resulting in the spread and metastasis of gastric cancer. The accurate staging of tumor depth invasion (T), regional lymph mode invasion (N), and distant metastases (M) in gastric cancer is crucial for improving the outcome of treatment [4]. For gastric cancer patients, surgery remains the only effective therapy, with the assistance of chemotherapy and chemoradiation [5]. Gastric cancer treatment is still a thorny issue, with multidrug resistance being one of the major obstacles [6,7].
MicroRNAs are small non-coding RNA molecules that regulate gene expression through controlling mRNA stability or degradation, gene translation, and other processes. These biological features of microRNAs have provided possibilities for treating various diseases, including gastric cancer. A number of microRNAs are aberrantly expressed in gastric cancer tissues [8,9], and some of them are promising fingerprints for diagnosis [10], and others are potential therapeutic targets for treating gastric cancer [11]. For example, microRNA-7 (miR-7) is an inhibitor of gastric cancer metastasis due to its regulation of insulin-like growth factor-1 receptor [12], while miR-223 promotes gastric cancer metastasis by targeting tumor suppressor genes [13]. Thus, studies on microRNAs are of great significance not only for the improvement in gastric cancer treatment, but also for a more profound understanding of their regulatory mechanism in diseases.
Previous studies indicated that miR-140 suppresses cancer cell formation, invasion, and metastasis in breast cancer, non-small cell lung cancer, and other cancers [14,15]. However, its role in gastric cancer remains elusive. This study investigated the impact of miR-140 on gastric cancer cell proliferation and its potential mechanism. We analyzed the expression pattern of miR-140 in gastric cancer tissues and different gastric cancer cell lines. Cell viability, colony formation, and cell cycle assays were performed to reveal the effects of miR-140 mimic transfection on HGC-27 cell proliferation. Sex-determining region (SRY)-box 4 (SOX4) was predicted to be a target of miR-140, which was verified by mutation of the binding site in SOX4 3′ untranslated region (3′UTR) and luciferase reporter assay. Moreover, the influence of miR-140 on drug resistance was analyzed. Our study shows the anti-proliferative role of miR-140 in gastric cancer and provides possibilities for its use in treating gastric cancer.
Material and Methods
Human tissue samples and cell culture
Human gastric cancer tissue and the adjacent normal tissue samples were obtained during surgery from 20 gastric cancer patients (10 males and 10 females) aged from 42 to 81 years (60.3±9.8). These patients were admitted to the hospital from April 2014 to March 2015, and their gastric cancer degrees fell into IA (4 cases), IB (7 cases), IIA (3 cases), IIB (2 cases), and IIIA (4 cases) according to the 7th edition of the AJCC TNM staging system. No patients had received any adjuvant treatment before the surgery. The samples were frozen immediately and stored at −80°C for RNA extraction. The sampling process was agreed to by the patients and was performed according to the instructions of our institute under the supervision of the Ethics Committee.
Human gastric cancer cell lines HGC-27, BGC-823, and SGC-7901 and normal gastric mucosa epithelial cell line GES-1 (ATCC, Manassas, VA) were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium supplemented with 10% fetal bovine serum (Gibco, Carlsbad, CA). The cells were incubated in a humidified atmosphere with 5% CO2 at 37°C.
Cell transfection
HGC-27 cells were seeded in 24-well plates at a density of 30% and cultured for 24 h before the transfection. The cells were transfected with miR-140 mimic or mimic control (50 nM, Sangon Biotech, Shanghai, China) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. For SOX4 overexpression, the coding sequence of human SOX4 was cloned into pcDNA3.1 vector (Thermo Scientific, Carlsbad, CA) and the correct product was screened by PCR and sequencing. Then the vector was transfected into HGC-27 cells using Lipofectamine 2000, and cell samples were collected at 24, 48, and 72 h after transfection for further analysis.
Cell viability assay
HGC-27 cell viability was analyzed with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method using the Cell Proliferation Kit I (Roche, Basel, Switzerland) according to the manufacturer’s instructions. The cell samples collected in logarithmic phase were seeded in 96-well plates (5×103 cells per well). For doxorubicin treatment, doxorubicin (Sigma-Aldrich, Shanghai, China) of various concentrations (0, 0.05, 0.1, and 0.2 μg/mL) was added to the medium and cultured for 12 h. MTT assay was then performed and the optical density (OD) was measured at 570 nm. The OD of the samples was compared to the control group (untransfected cells).
Colony formation assay
HGC-27 cells in the logarithmic phase were digested into single-cell suspension and added to the culture dishes (6 cm in diameter), with 1×103 cells in each dish. The dishes were incubated at 37°C for 14 days. The supernatant was discarded. The colonies were washed twice with phosphate-buffered saline and fixed in methanol for 15 min. Then the methanol was discarded and the colonies were stained with Giemsa (Sigma-Aldrich) for 30 min. Colony numbers were counted under an optical microscope (Leica Microsystems, Wetzlar, Germany). The colony formation efficiency was calculated as (the colony formation number/the seeded cell number) × 100%.
Cell cycle analysis
Cell cycle distribution was analyzed using the Cell Cycle and Apoptosis Analysis Kit (Leagene, Beijing, China). The transfected HGC-27 cells in the logarithmic phase were collected and washed. Prodium iodide (PI) buffer prepared according to the instructions was added to the cells for incubation in the dark for 30 min at 37°C. Then the samples were analyzed immediately by cytometry (BD Biosciences, San Jose, CA).
Luciferase reporter assay
TargetScanHuman 7.0 (www.targetscan.org) predicted that SOX4 was a potential target of miR-140. The wild-type and mutant 3′UTR fragments of SOX4 synthesized using QuikChange Multi Site-Directed Mutagenesis Kit (Agilent Technologies, Santa Clara, CA) were separately cloned into the luciferase reporter vector using the Firefly and Renilla Luciferase Assay Kit (Biotium, Shanghai, China). These vectors were co-transfected with miR-140 mimic or mimic control using Lipofectamine 2000. Transfected cells were collected after 48 h to detect the fluorescence intensity using cytometry (BD Biosciences).
Real-time quantitative PCR (qPCR)
Tissue or cell samples were lysed in RNAiso for Small RNA (TaKaRa, Dalian, China) for miRNA extraction, and cell samples were lysed in TRIzol (Invitrogen) for total RNA extraction according to the manufacturer’s instructions. Total RNAs and miRNAs were reverse-transcribed into complementary DNAs (cDNAs) using PrimeScript Reverse Transcriptase (TaKaRa). qPCR was performed on a LightCycler 480 (Roche) with 20 ng cDNAs and the specific primers for miR-140 and the other tested genes (Table 1). U6 and GAPDH were used as the internal control. This experiment was repeated 3 times and data were analyzed with 2−ΔΔCt method.
Table 1.
Primers used in qPCR.
| Primer | Sequence (5′ to 3′) |
|---|---|
| miR-140-RT | CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCTACCATA |
| miR-140-Fw | ACACTCCAGCTGGGCAGTGGTTTTACCCTA |
| miR-140-Rv | TGGTGTCGTGGAGTCG |
| U6-Fw | CTCGCTTCGGCAGCACA |
| U6-Rv | AACGCTTCACGAATTTGCGT |
| GAPDH-Fw | GAAGGTGAAGGTCGGAGTC |
| GAPDH-Rv | GAAGATGGTGATGGGATTG |
| SOX4-Fw | GGCCTGTTTCGCTGTCGGGT |
| SOX4-Rv | GCCTGCATGCAACAGACTGGC |
| CCND1-Fw | CCTGTCCTACTACCGCCTCA |
| CCND1-Rv | TCCTCCTCTTCCTCCTCCTC |
| CDK2-Fw | CCTCCTGGGCTGCAAATA |
| CDK2-Rv | CAGAATCTCCAGGGAATAGGG |
Western blot
Cells were lysed in the lysis buffer for protein extraction (Beyotime, Shanghai, China). Protein samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane (Roche). After being blocked in 5% skim milk for 2 h at room temperature, the membrane was incubated in the specific primary antibodies (ABcam, Cambridge, UK) overnight at 4°C. The membrane was washed and then incubated in horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. Signals were developed using ECL Plus Western Blotting Substrate (Thermo Scientific) and analyzed by ImageJ 1.49 (National Institute of Health, Bethesda, MD). GAPDH was used as the internal control.
Statistical analysis
All the experiments were repeated 5 times unless otherwise specified. Results are indicated as the mean ± standard deviation. Data were analyzed by t test and one-way ANOVA using SPSS (IBM, New York, USA). Differences were considered statistically significant at P<0.05.
Results
miR-140 is down-regulated in gastric cancer and inhibits HGC-27 cell proliferation
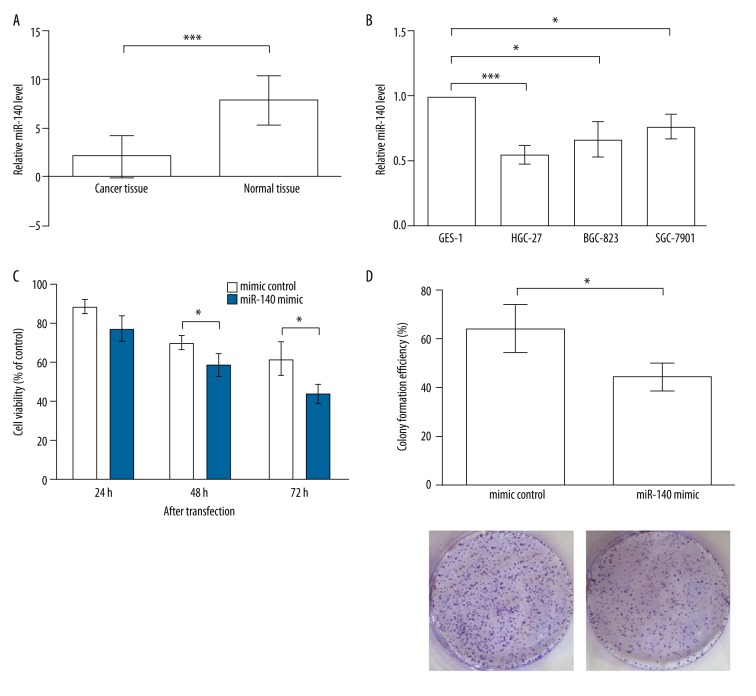
The miR-140 level in tissue samples was detected by qPCR, and results indicated a significant down-regulation of miR-140 in gastric cancer tissues compared to normal tissues (P<0.001, Figure 1A), which led us to suspect the role of miR-140 in modulating gastric cancer cells. The miR-140 level was also detected in the normal gastric cells GES-1, as well as in gastric cancer cells, including HGC-27 (undifferentiated), BGC-823 (lightly differentiated), and SGC-7901 (moderately differentiated), which differed in their degree of cellular differentiation (Figure 1B). We found that the miR-140 expression was down-regulated in all of the detected gastric cancer cells (P<0.05), and the HGC-27 cells possessed the lowest miR-140 expression (P<0.001), in line with its undifferentiated feature. It was likely that the gastric cells with a lower differentiation degree exhibited more significant differences from normal cells, which would be more suitable to use in studying miR-140 functions. Therefore, the following experiments were performed in the HGC-27 cells.
Figure 1.
miR-140 is down-regulated in gastric cancer and inhibits cell proliferation. (A) miR-140 is down-regulated in gastric cancer tissues compared to normal tissues. (B) miR-140 is down-regulated in gastric cancer cell lines, including HGC-27, BGC-823, and SGC-7901, compared to normal gastric cell line GES-1. (C) MTT results indicate that HGC-27 cells transfected with miR-140 mimic possess lower viability than mimic control. Significant differences are found at 48 and 72 h after transfection. (D) Colony formation experiment shows the HGC-27 cells transfected with miR-140 mimic possess lower colony formation efficiency after 2 weeks of incubation. * P<0.05; *** P<0.001.
The roles of miR-140 in gastric cancer cells were reflected by its influence on HGC-27 cell viability and proliferation, analyzed by MTT and colony formation, respectively. MTT results showed that the cells transfected with miR-140 mimic had significant lower viability than those transfected with mimic control at 48 and 72 h after transfection (P<0.05, Figure 1C). No significant difference was found at 24 h after transfection (P>0.05). These data indicate that overexpression of miR-140 in HGC-27 cells was able to inhibit cell viability; in accordance with this, the cells transfected with miR-140 formed fewer colonies after 2 weeks of incubation (P<0.05, Figure 1D), suggesting the miR-140 overexpression can also inhibit HGC-27 cell proliferation.
miR-140 leads to G0/G1 arrest in HGC-27
Since miR-140 was able to suppress cell proliferation, it was possible that its anti-proliferative role was related to the hindered cell cycle, which was to be investigated. Flow cytometry results showed that the percentage of HGC-27 cells in the G0/G1 phase was increased when miR-140 was overexpressed (Figure 2A, 2B), while cells in the S and G2/M phases were decreased, indicating that the HGC-27 cells overexpressing miR-140 were arrested in the G0/G1 phase compared to the mimic control.
Figure 2.
miR-140 leads to G0/G1 arrest of HGC-27 cells. (A) Flow cytometry results show the cell percent in the G0/G1 phase is increased, while that in S and G2/M phases is decreased by miR-140 overexpression. (B) Histogram reflects the cell cycle distribution in (A). (C) CCND1 and CDK2 mRNA levels are inhibited by miR-140 overexpression. GAPDH is used as the internal control. * P<0.05. (D) CCND1 and CDK2 protein levels are inhibited by miR-140 overexpression. GAPDH is used as the internal control. CCND1 – cyclin D1. CDK2 – cyclin-dependent kinase 2.
Both cyclin D1 (CCND1) and cyclin-dependent kinase 2 (CDK2) are vital controllers of the cell cycle. CDK2 can form complexes with cyclins and be activated in the late G1 phase, thus promoting G1/S transition [16]. CCND1 is a regulator of CDKs, and its knockdown results in G1 arrest [17]. Therefore, these 2 factors were used in this study to verify the G0/G1 cell arrest. In accordance with the flow cytometry results, CCND1 and CDK2 mRNAs were both inhibited in HGC-27 cells transfected with miR-140 mimic (P<0.05, Figure 2C), and levels of their proteins were also down-regulated (Figure 2D), suggesting these 2 cell cycle modulators were inhibited by miR-140. Together with the flow cytometry results, it could be speculated that miR-140 overexpression is capable of arresting HGC-27 cells in the G0/G1 phase.
miR-140 directly inhibits SOX4
The potential regulatory mechanism of miR-140 was analyzed by investigating its target mRNA, SOX4, which was predicted to be a target of miR-140 by the online database TargetScanHuman 7.0, with the sequence AACCACU in SOX4 3′UTR being the potential binding site (Figure 3A). To verify whether SOX4 mRNA was a direct target of miR-140, we mutated 3 nucleotides in the binding site, and the generated mutant type was not supposed to be targeted by miR-140. Indeed, luciferase activity assay showed that the luciferase activity of vector containing wild-type SOX4 3′UTR could be suppressed by miR-140 overexpression (P<0.05, Figure 3B), while miR-140 failed to inhibit mutant type of SOX4 3′UTR (P>0.05), suggesting that this binding site in SOX4 3′UTR was essential for the regulation by miR-140. Together with the prediction results that SOX4 was a potential target for miR-140, it could be deduced that miR-140 can directly regulate SOX4 mRNA.
Figure 3.
SOX4 is directly inhibited by miR-140. (A) SOX4 mRNA is predicted to be a target for miR-140, with the binding sequence AACCACU in the 3′UTR (position 2132 to 2138). Multi-site mutation is performed to mutate 3 nucleotides (underlined) in the binding site. Mut-SOX4 3′UTR, mutant type of SOX4 3′UTR. (B) Luciferase activity assay indicates that the binding sequence in SOX4 3′UTR is necessary for regulation by miR-140. HGC-27 cells overexpressing miR-140 are co-transfected with the reporter vectors of wild-type or mutant-type of SOX4 3′UTR (SOX4 3′UTR or mut-SOX4 3′UTR). The activity of mutant-type cannot be regulated by miR-140. (C) SOX4 mRNA level is down-regulated by miR-140 overexpression. (D) SOX4 protein level is suppressed by miR-140 overexpression. * P<0.05. SOX4 – sex-determining region Y (SRY)-box 4.
The inhibitory effect of miR-140 on SOX4 mRNA was analyzed at mRNA and protein levels. qPCR results showed that SOX4 mRNA was down-regulated by miR-140 overexpression (P<0.05, Figure 3C), possibly due to the effects of miR-140 on SOX4 mRNA stability. SOX4 protein level was also down-regulated (P<0.05, Figure 3D), which was caused by the down-regulated mRNA level or the inhibited translation process by miR-140. Taken together, these results indicate that miR-140 can directly inhibit SOX4 by targeting the binding sequence in the 3′UTR of SOX4 mRNA.
SOX4 promotes HGC-27 cell proliferation
Was the inhibition of SOX4 by miR-140 associated with HGC-27 cell proliferation? To answer this question, we performed SOX4 overexpression by transfecting its overexpression vector to investigate the changes in cell viability, proliferation, and cell cycle. Cell viability was analyzed by MTT method and results showed that SOX4 overexpression could significantly promote HGC-27 cell viability at 24, 48, and 72 h after transfection (P<0.05, Figure 4A). Similarly, overexpressing SOX4 also increased the number of colonies formed after 2 weeks of incubation (P<0.05, Figure 4B), indicating the promotion of cell proliferation. Further, the percentage of cells in the G0/G1 phase was decreased by SOX4 overexpression compared to the control group (Figure 4C), which was in accordance to the up-regulated CCND1 and CDK2 protein levels (Figure 4D), implying that SOX4 might help to promote the G1/S transition. Taken together, these data suggest the roles of SOX4 in promoting HGC-27 cell viability, proliferation, and cell cycle processes, which were consistent with the above results that the anti-proliferative miR-140 inhibited SOX4. Therefore, the inhibition of SOX4 might be a potential mechanism by which miR-140 suppresses cell proliferation.
Figure 4.
SOX4 promotes HGC-27 cell viability, proliferation, and cell cycle process. (A) MTT data show that cell viability is promoted by SOX4 overexpression when detected at 24, 48, and 72 h after transfection. (B) Colony formation assay shows the colony is increased by SOX4 overexpression after 2 weeks of incubation. (C) Histogram reflecting the flow cytometry result indicates that the cell percent in the G0/G1 phase is decreased by SOX4 overexpression. (D) Western blot shows CCND1 and CDK2 protein levels are up-regulated by SOX4 overexpression. GAPDH is used as the internal control. * P<0.05. SOX4 – sex-determining region Y (SRY)-box 4. CCND1 – cyclin D1. CDK2 – cyclin-dependent kinase 2.
miR-140 reduces the drug resistance of HGC-27 cells
The resistance of gastric cancer cells to drugs is a thorny problem in treating gastric cancer. Based on the above results, miR-140 was likely to be a protective factor against gastric cancer, reflected in its anti-proliferative roles in HGC-27 cells. Therefore, we analyzed its impact on the sensitivity of HGC-27 to doxorubicin, a widely used drug in treating gastric cancer. During the MTT experiment, various doses of doxorubicin were added to the medium for a 12-h treatment. Because we wanted the impact of miR-140 on cell viability to be minimal, the duration of treatment was shorter than 24 h, when no significant viability change could be detected based on the above results (Figure 1C).
Results showed that doxorubicin treatments at 0.05, 0.1, or 0.2 μg/mL all resulted in the inhibition of cell viability compared to cells without treatment (P<0.05, Figure 5A). No significant viability change was detected between cells overexpressing miR-140 and the mimic control when no doxorubicin was used. Importantly, in cells treated by the same dose of doxorubicin, cells overexpressing miR-140 had lower viability than the mimic control (P<0.05), suggesting that their sensitivity to doxorubicin was increased by miR-140 overexpression, which indicates a lower drug resistance. To verify this result, we further tested the expression changes in resistance-associated factors – ATP-binding cassette sub-family C member 1 (ABCC1, alias MRP1) and sub-family G member 2 (ABCG2) – both of which are multidrug resistance-associated proteins broadly applied in studies on drug resistance and playing vital roles in resistance to doxorubicin in cancers [18,19]. Western blot analysis showed that both ABCC1 and ABCG2 were inhibited by miR-140 overexpression (treated by 0.05 μg/mL doxorubicin, Figure 5B), suggesting that miR-140 can suppress the expression of resistance-associated factors, which indicated the inhibited drug resistance of the transfected cells.
Figure 5.
miR-140 reduces the drug resistance of HGC-27 cells. (A) MTT shows that the effects of doxorubicin (0.05, 0.1, or 0.2 μg/mL) are more significant in cells overexpressing miR-140 than in the mimic control. * P<0.05. (B) Western blot shows the drug resistance-associated factors ABCC1 and ABCG2 are inhibited by miR-140 overexpression when treated with 0.05 μg/mL doxorubicin. GAPDH is used as the internal control. DOX – doxorubicin. ABCC1, ATP-binding cassette sub-family C member 1. ABCG2 – ATP-binding cassette sub-family G member 2.
Discussion
MicroRNAs are small but powerful molecules regulating genes involved in developmental and pathological processes. In the present study, miR-140 was found to be down-regulated in gastric cancer tissues and cell lines BGC-823, SGC-7901, and HGC-27. Its overexpression can inhibit HGC-27 proliferation via targeting SOX4, an important transcription factor that promotes HGC-27 proliferation. miR-140 may also help to reduce the drug resistance of HGC-27 cells to doxorubicin.
The most significant finding of this study was the anti-proliferative role of miR-140 in gastric cancer cell line HGC-27. The inhibition of cell proliferation by miR-140 was shown by the suppressed cell viability, the decreased cell colony formation, and the hindered cell cycle by miR-140 mimic. miR-140 has been reported to regulate cell activities, such as in undifferentiated human mesenchymal stem cells, where it suppresses osteogenic differentiation [20], and in esophageal cancer, where it inhibits epithelial-mesenchymal transition (EMT) and cell invasion [21]. Some of these studies also discussed the cell cycle change leading to suppressed proliferation and indicating that miR-140 overexpression can result in cell cycle arrest in the G0/G1 phase, as was observed in HGC-27 cells of this study. Cell cycle mediators CCND1 and CDK2, 2 inducers of G1/S transition, were suppressed by miR-140, further verifying that miR-140 can lead to G0/G1 arrest and thus inhibit gastric cancer cell proliferation.
Next, we investigated the underlying mechanism by verifying the predicted target of miR-140. SOX4 is a member of the SRY-related HMG box (SOX) transcription factor family, with earlier studies focusing on its significance in lymphocytes [22]. It is up-regulated in various cancer cells [23], where it functions as a facilitator of EMT, tumorigenesis, and metastasis, thus being defined as an oncogenic target [24–26]. In HGC-27 cells, SOX4 was also shown to promote cell viability, colony formation, and cell cycle progression, which suggests its roles in promoting cell proliferation, in contrast with the function of miR-140 in HGC-27 cells. Whether miR-140 could directly modulate and suppress SOX4 was also analyzed by the mutation in the binding site and luciferase reporter assay. Taken together, it could be deduced that inhibition of SOX4 by miR-140 might be one of the regulatory mechanisms of miR-140 in suppressing gastric cancer cell proliferation.
miR-140 is capable of regulating many target mRNAs, some of which are validated by luciferase reporter assay and qPCR. Besides SOX4, histone deacetylase 4 (HDAC4) is one of the valid targets of miR-140 in osteosarcoma and colon cancer cell lines, whose protein expression was inhibited by miR-140, but the mRNA level remains almost unchanged [27], implying that miR-140 may affect HDAC4 expression through controlling the translation process. In the present study, SOX4 was inhibited by miR-140 at mRNA and protein levels, possibly due to the alteration of its mRNA stability. In addition, other mRNAs, including the SOX family members SOX2 and SOX9, which are involved in cancer cell survival and invasion, are also valid targets of miR-140 [21,28]. These findings show that the regulation of cancer cell activities by miR-140 is likely to involve multiple factors. A thorough understanding of this network is essential for future application of miR-140 in treating gastric cancer.
Knowledge of the histological type of gastric cancer is useful for estimating the disease progression and outcomes. Studies indicate that the 5-year survival rate for patients with well-differentiated gastric cancer is higher than that for patients with poorly differentiated gastric cancer [29]. Cancer stem cell markers like ABCB1, ABCG2, and CD133 are expressed at higher levels in more malignant gastric cancer cells [30]. Consistent with these findings, the miR-140 expression revealed in this study was decreased with the increase of gastric cancer cell malignancy, with the undifferentiated gastric cancer cell line HGC-27 possessing the lowest miR-140 level. It is necessary to perform these tests in other gastric cancer cells in future studies to determine whether miR-140 can indicate gastric cancer malignancy.
We also investigated the effect of miR-140 on drug resistance in gastric cancer cells. The resistance of cancer cells to drugs is a common problem, severely impacting the therapeutic outcomes [7]. In gastric cancer, microRNAs like miR-497 and miR-508-5p can modulate drug resistance via targeting related factors, including B-cell CLL/lymphoma 2, ATP-binding cassette sub-family B member 1, and zinc ribbon domain containing 1 [31,32]. In this study, results showed that with the overexpression of miR-140, the effects of doxorubicin on inhibiting HGC-27 cell viability were more evident, suggesting that miR-140 has promise in decreasing drug resistance of gastric cancer cells. We also found the down-regulation of related factors ABCC1 and ABCG2 by miR-140, but the regulatory relationship needs further investigation.
Conclusions
In summary, miR-140 is capable of suppressing gastric cancer cell proliferation via inhibiting its target, SOX4, and shows the potential for reducing drug resistance of gastric cancer cells, which is a promising therapeutic strategy for gastric cancer treatment. Further research is needed to provide a deeper understanding of the role of microRNAs in gastric cancer.
Footnotes
Conflicts of interest
There are no conflicts of interest
Source of support: Departmental sources
References
- 1.Ferro A, Peleteiro B, Malvezzi M, et al. Worldwide trends in gastric cancer mortality (1980–2011), with predictions to 2015, and incidence by subtype. Eur J Cancer. 2014;50:1330–44. doi: 10.1016/j.ejca.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Zhang S, et al. Annual report on status of cancer in China, 2010. Chin J Cancer Res. 2014;26:48–58. doi: 10.3978/j.issn.1000-9604.2014.01.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guggenheim DE, Shah MA. Gastric cancer epidemiology and risk factors. J Surg Oncol. 2013;107:230–36. doi: 10.1002/jso.23262. [DOI] [PubMed] [Google Scholar]
- 4.Seevaratnam R, Cardoso R, McGregor C, et al. How useful is preoperative imaging for tumor, node, metastasis (TNM) staging of gastric cancer? A meta-analysis. Gastric Cancer. 2012;15(Suppl 1):S3–18. doi: 10.1007/s10120-011-0069-6. [DOI] [PubMed] [Google Scholar]
- 5.Orditura M, Galizia G, Sforza V, et al. Treatment of gastric cancer. World J Gastroenterol. 2014;20:1635–49. doi: 10.3748/wjg.v20.i7.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang S, Chen M, Shen Y, et al. Inhibition of activated Stat3 reverses drug resistance to chemotherapeutic agents in gastric cancer cells. Cancer Lett. 2012;315:198–205. doi: 10.1016/j.canlet.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 7.Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: An evolving paradigm. Nat Rev Cancer. 2013;13:714–26. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 8.KaTada T, Ishiguro H, Kuwabara Y, et al. microRNA expression profile in undifferentiated gastric cancer. Int J Oncol. 2009;34:537–42. [PubMed] [Google Scholar]
- 9.Ueda T, Volinia S, Okumura H, et al. Relation between microRNA expression and progression and prognosis of gastric cancer: A microRNA expression analysis. Lancet Oncol. 2010;11:136–46. doi: 10.1016/S1470-2045(09)70343-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu R, Zhang C, Hu Z, et al. A five-microRNA signature identified from genome-wide serum microRNA expression profiling serves as a fingerprint for gastric cancer diagnosis. Eur J Cancer. 2011;47:784–91. doi: 10.1016/j.ejca.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 11.Li J, Guo Y, Liang X, et al. MicroRNA-223 functions as an oncogene in human gastric cancer by targeting FBXW7/hCdc4. J Cancer Res Clin Oncol. 2012;138:763–74. doi: 10.1007/s00432-012-1154-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao X, Dou W, He L, et al. MicroRNA-7 functions as an anti-metastatic microRNA in gastric cancer by targeting insulin-like growth factor-1 receptor. Oncogene. 2013;32:1363–72. doi: 10.1038/onc.2012.156. [DOI] [PubMed] [Google Scholar]
- 13.Li X, Zhang Y, Zhang H, et al. miRNA-223 promotes gastric cancer invasion and metastasis by targeting tumor suppressor EPB41L3. Mol Cancer Res. 2011;9:824–33. doi: 10.1158/1541-7786.MCR-10-0529. [DOI] [PubMed] [Google Scholar]
- 14.Li Q, Yao Y, Eades G, et al. Downregulation of miR-140 promotes cancer stem cell formation in basal-like early stage breast cancer. Oncogene. 2014;33:2589–600. doi: 10.1038/onc.2013.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuan Y, Shen Y, Xue L, Fan H. miR-140 suppresses tumor growth and metastasis of non-small cell lung cancer by targeting insulin-like growth factor 1 receptor. PLoS One. 2013;8:e73604. doi: 10.1371/journal.pone.0073604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neganova I, Zhang X, Atkinson S, Lako M. Expression and functional analysis of G1 to S regulatory components reveals an important role for CDK2 in cell cycle regulation in human embryonic stem cells. Oncogene. 2009;28:20–30. doi: 10.1038/onc.2008.358. [DOI] [PubMed] [Google Scholar]
- 17.Masamha CP, Benbrook DM. Cyclin D1 degradation is sufficient to induce G1 cell cycle arrest despite constitutive expression of cyclin E2 in ovarian cancer cells. Cancer Res. 2009;69:6565–72. doi: 10.1158/0008-5472.CAN-09-0913. [DOI] [PubMed] [Google Scholar]
- 18.Asano T. Indomethacin overcomes doxorubicin resistance by inhibiting Multi-drug resistance protein 1 (MRP1/ABCC1) promoter activity. Cancer Research. 2005;65:125. doi: 10.1007/s00280-005-0162-9. [DOI] [PubMed] [Google Scholar]
- 19.Lopez JP, Wang-Rodriguez J, Chang C, et al. Gefitinib inhibition of drug resistance to doxorubicin by inactivating ABCG2 in thyroid cancer cell lines. Arch Otolaryngol Head Neck Surg. 2007;133:1022–27. doi: 10.1001/archotol.133.10.1022. [DOI] [PubMed] [Google Scholar]
- 20.Hwang S, Park SK, Lee HY, et al. miR-140-5p suppresses BMP2-mediated osteogenesis in undifferentiated human mesenchymal stem cells. FEBS Lett. 2014;588:2957–63. doi: 10.1016/j.febslet.2014.05.048. [DOI] [PubMed] [Google Scholar]
- 21.Li W, Jiang G, Zhou J, et al. Down-regulation of miR-140 induces EMT and promotes invasion by targeting Slug in esophageal cancer. Cell Physiol Biochem. 2014;34:1466–76. doi: 10.1159/000366351. [DOI] [PubMed] [Google Scholar]
- 22.van de Wetering M, Oosterwegel M, van Norren K, Clevers H. Sox-4, an Sry-like HMG box protein, is a transcriptional activator in lymphocytes. EMBO J. 1993;12:3847–54. doi: 10.1002/j.1460-2075.1993.tb06063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhodes DR, Yu J, Shanker K, et al. Large-scale meta-analysis of cancer microarray data identifies common transcriptional profiles of neoplastic transformation and progression. Proc Natl Acad Sci USA. 2004;101:9309–14. doi: 10.1073/pnas.0401994101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J, Liang Q, Lei Y, et al. SOX4 induces epithelial-mesenchymal transition and contributes to breast cancer progression. Cancer Res. 2012;72:4597–608. doi: 10.1158/0008-5472.CAN-12-1045. [DOI] [PubMed] [Google Scholar]
- 25.Vervoort SJ, van Boxtel R, Coffer PJ. The role of SRY-related HMG box transcription factor 4 (SOX4) in tumorigenesis and metastasis: Friend or foe? Oncogene. 2012;32:3397–409. doi: 10.1038/onc.2012.506. [DOI] [PubMed] [Google Scholar]
- 26.Zhang H, Alberich-Jorda M, Amabile G, et al. Sox4 is a key oncogenic target in C/EBPalpha mutant acute myeloid leukemia. Cancer Cell. 2013;24:575–88. doi: 10.1016/j.ccr.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song B, Wang Y, Xi Y, et al. Mechanism of chemoresistance mediated by miR-140 in human osteosarcoma and colon cancer cells. Oncogene. 2009;28:4065–74. doi: 10.1038/onc.2009.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Eades G, Yao Y, et al. Estrogen receptor alpha signaling regulates breast tumor-initiating cells by down-regulating miR-140 which targets the transcription factor SOX2. J Biol Chem. 2012;287:41514–22. doi: 10.1074/jbc.M112.404871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adachi Y, Yasuda K, Inomata M, et al. Pathology and prognosis of gastric carcinoma: Well versus poorly differentiated type. Cancer. 2000;89:1418–24. [PubMed] [Google Scholar]
- 30.Jiang Y, He Y, Li H, et al. Expressions of putative cancer stem cell markers ABCB1, ABCG2, and CD133 are correlated with the degree of differentiation of gastric cancer. Gastric Cancer. 2012;15:440–50. doi: 10.1007/s10120-012-0140-y. [DOI] [PubMed] [Google Scholar]
- 31.Shang Y, Zhang Z, Liu Z, et al. miR-508-5p regulates multidrug resistance of gastric cancer by targeting ABCB1 and ZNRD1. Oncogene. 2014;33:3267–76. doi: 10.1038/onc.2013.297. [DOI] [PubMed] [Google Scholar]
- 32.Zhu W, Zhu D, Lu S, et al. miR-497 modulates multidrug resistance of human cancer cell lines by targeting BCL2. Med Oncol. 2012;29:384–91. doi: 10.1007/s12032-010-9797-4. [DOI] [PubMed] [Google Scholar]