Abstract
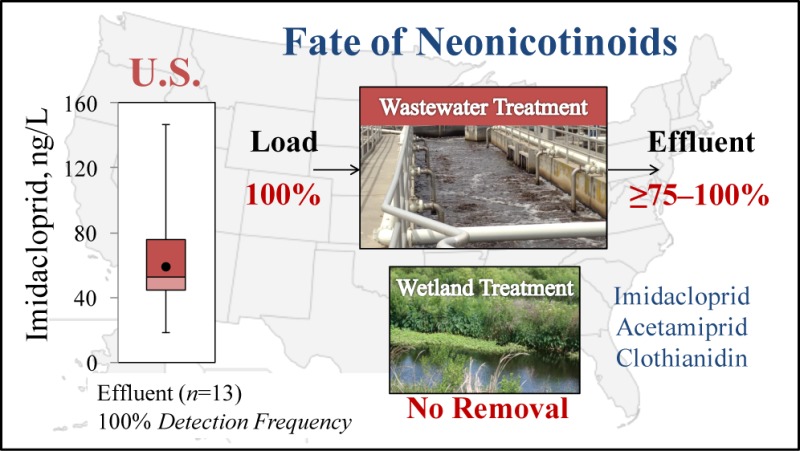
Occurrence and removal of six high-production high-volume neonicotinoids was investigated in 13 conventional wastewater treatment plants (WWTPs) and one engineered wetland. Flow-weighted daily composites were analyzed by isotope dilution liquid chromatography tandem mass spectrometry, revealing the occurrence of imidacloprid, acetamiprid, and clothianidin at ng/L concentrations in WWTP influent (60.5 ± 40.0; 2.9 ± 1.9; 149.7 ± 289.5, respectively) and effluent (58.5 ± 29.1; 2.3 ± 1.4; 70.2 ± 121.8, respectively). A mass balance showed insignificant removal of imidacloprid (p = 0.09, CI = 95%) and limited removal of the sum of acetamiprid and its degradate, acetamiprid-N-desmethyl (18 ± 4%, p = 0.01, CI = 95%). Clothianidin was found only intermittently, whereas thiamethoxam, thiacloprid, and dinotefuran were never detected. In the wetland, no removal of imidacloprid or acetamiprid was observed. Extrapolation of data from 13 WWTPs to the nation as a whole suggests annual discharges on the order of 1000–3400 kg/y of imidacloprid contained in treated effluent to surface waters nationwide. This first mass balance and first United States nationwide wastewater reconnaissance identified imidacloprid, acetamiprid, and clothianidin as recalcitrant sewage constituents that persist through wastewater treatment to enter water bodies at significant loadings, potentially harmful to sensitive aquatic invertebrates.
Introduction
Neonicotinoids are the world’s most widely used insecticides, with global production valued at US$2.5 billion and registrations in more than 120 countries for commercial use on more than 140 crops.1 These insecticides are used for control of aphids, whiteflies, planthoppers, lepidoptera, and some coleopteran and other pests, where they function as powerful neurotoxins.1−3
In December, 2013, the European Commission introduced a two year moratorium on clothianidin, imidacloprid, and thiamethoxam, following reports by the European Food Safety Authority (EFSA) of these substances posing an “acute risk” to honeybees essential to farming and natural ecosystem.4 Temporal and expired restrictions allow for potential current and future uses of neonicotinoids in these settings. Adverse effects from widespread use of neonicotinoids have been reported recently for many nontarget organisms like phloem-feeding insects,5 pollinators and bees,6 and aquatic invertebrates.7 Median lethal dose values (LD50) of neonicotinoids for bees vary from 5 to 70 ng/bee.8 Sublethal doses have been shown to cause ATP synthesis inhibition,9 resulting in weakening of foraging success, memory and learning, damage to the central nervous system,6 and increased susceptibility to diseases.10 A recent review based on 214 toxicity tests of 48 species suggested that average individual environmental concentrations of greater than 35 ng/L may severely affect sensitive aquatic invertebrates populations.7 Another study indicated that aquatic macrofauna populations dropped sharply at concentrations between 13 and 67 ng/L.11 Insectivorous birds are also susceptible to exposure through the food chain.12 A study in The Netherlands observed a decline in the insectivorous bird population after the introduction of imidacloprid, the highest production volume insecticide in the world; imidacloprid concentrations of greater than 20 ng/L correlated with 3.5% average annual declines in bird populations.13 Imidacloprid is moderately toxic to fish communities;14 oxidative stress and DNA damage have been reported in zebrafish.15 Furthermore, co-occurrence of multiple neonicotinoids is known to impart synergistic toxic effects.16
During the past decades global contamination with neonicotinoids has been observed in surface waters, many of which receiving treated effluent from wastewater treatment plants. In a nationwide assessment of United States streams, at least one neonicotinoid was detected in 53% of the samples analyzed (n = 38).17 In California, imidacloprid was detected in 89% of surface water samples collected from agricultural regions (n = 75) in which 19% of the samples exceeded concentrations of 1.05 μg/L, the chronic invertebrate Aquatic Life Benchmark value established by the United States Environmental Protection Agency (USEPA).18 In Canadian wetlands of the central-eastern region of Saskatchewan, neonicotinoids were detected frequently in 2012–2013 (48%; n = 440) at a total average concentration of 51.8 ng/L, with higher detection frequencies being observed in spring and higher mean concentrations in summer.19 In several rivers around Sydney, Australia, the average total neonicotinoid concentration was 118 ng/L; imidacloprid was the most common neonicotinoid, detected in 93% of samples (n = 15).20 Clothianidin was detected with a detection frequency of 46.6% in groundwater and surface water samples (n = 58) collected in Germany.21
Wastewater constitutes a potential source of neonicotinoids in the environment that has not received much attention yet. Neonicotinoids are widely used in nonindustrial agricultural applications such as pet flea treatment, horticulture, and household pest control products. Thus, these usages may contribute to neonicotinoid loadings detectable in sewage. A few studies have detected imidacloprid in wastewater, showing that treated effluent can inadvertently contribute to neonicotinoid discharge into receiving water bodies. Indeed, a nationwide assessment of United States streams showed a positive correlation between neonicotinoid occurrence and urban land usage but not with agricultural use.17 In Oregon, effluent samples from 52 wastewater treatment plants (WWTPs) analyzed for imidacloprid showed detections in 9.8% samples (n = 102), with an average concentration of 270 ng/L.22 In Spain, imidacloprid was detected in wastewater influent and effluent samples at concentrations ranging from 1.4–165.7 ng/L (59.4%; n = 32).23 In another related Spanish study, imidacloprid was detected in river water receiving WWTP effluent at a maximum concentration of 19.2 ng/L, identifying sewage treatment facilities as a source of neonicotinoids in the environment.24 In Beijing, China, imidacloprid was detected in WWTP influent and effluent at concentrations of 45–100 and 45–106 ng/L, respectively, with no further information being provided on the removal rate.25
With wastewater representing a likely source of neonicotinoids in the United States aquatic freshwater environment, the goal of the present study was to conduct a first mass balance assessing the fate of six neonicotinoids (listed in order of decreasing global annual turnover: imidacloprid, thiamethoxam, clothianidin, acetamiprid, thiacloprid, and dinotefuran1) during conventional wastewater treatment and wetland treatment and to obtain through a nationwide reconnaissance a first national emission estimate by monitoring additional treatment facilities from across the United States.
Materials and Methods
Chemicals and Reagents
Organic solvent of high performance liquid chromatography (HPLC) grade and formic acid of American Chemical Society (ACS) grade (98%) were purchased from Sigma-Aldrich Corp., St. Louis, MO, U.S.A. Ultrapure LC-MS grade water was purchased from Thermo Fisher Scientific, Waltham, MA, U.S.A. Analytical standards for six neonicotinoids, an acetamiprid degradate, and deuterated labeled standards for imidacloprid (imidacloprid-d4), acetamiprid (acetamiprid-d3), and clothianidin (clothianidin-d3) were obtained from Sigma-Aldrich Corp., St. Louis, MO, U.S.A. (CAS numbers provided in Table S1). Stock solutions of analytical standards (1 ppb to 10 ppm) and their mixtures were prepared in acetonitrile and stored at −20 °C.
Sample Collection
Sampling for this mass balance assessment was conducted at two levels. One plant and wetland were studied in great detail to obtain general information on the fate of neonicotinoids, and then additional plants were sampled to see whether the information obtained is more broadly applicable to treatment facilities in the United States. In early December 2014 for a period of five consecutive days (Thursday through Monday), a large activated sludge sewage treatment plant with an engineered wetland downstream was sampled extensively. The plant is located in the southwestern region of the United States and designed to serve a population of up to 2.5 million with design capacity of 870 million L/d (MLD), receiving sewage comprised of 94% domestic wastewater and 6% industrial wastewater. The treatment facility produces Class B+ reclaimed water discharged into a river and Class B sludge used for land application. The highest-flow treatment train was selected for detailed studies on plant performance. Unit processes performed at the WWTP include screening, grit removal, primary sedimentation, activated sludge biological treatment, secondary clarification, disinfection treatment by chlorination, thickening of primary sludge, waste-activated sludge by centrifugation, anaerobic sludge digestion, and dewatering of digested sludge by centrifugation. Primary sludge and waste activated sludge are digested at 35 °C, with an average solids retention time of 21 days. Effluents from a total of five parallel treatment trains are combined, and a portion of this total flow is directed into an engineered wetland located immediately downstream and featuring a hydraulic retention time (HRT) of about 4.7 days, an average water depth of about 1.5 m, total suspended solids (TSS) concentrations in wetland influent and effluent of 10–15 mg/L, and average wastewater flow received and discharged around 280 and 250 MLD, respectively. Average values of carbonaceous biochemical oxygen demand (cBOD) for plant influent and wetland effluent were 288 ± 23 and 7 ± 1 mg/L, respectively, demonstrating cBOD removal of approximately 98%. Average TSS values in plant influent and wetland effluent were 437 ± 160 and 14 ± 3 mg/L, respectively, achieving TSS removal of 96 ± 1%.
The treatment train selected for sampling received wastewater at a flow rate averaging 230 MLD. Seven portable automated samplers (6712 full-size portable sampler, Teledyne Isco, Lincoln, NE, U.S.A.) were programmed based on three-week average hourly daily flow rate data to collect 2.5 L of flow-weighted composite samples of primary influent, primary effluent, secondary effluent, waste activated sludge, disinfection basin effluent, wetland influent, and wetland effluent over a period of 24 h for 5 consecutive days. Detailed information on the sample programming and flow diagram of WWTP (Figure S1) is provided in the Supporting Information (SI). Samples were collected in precleaned (acetone washed and heated at 500 °C for 5 h) amber 2.5 L wide-mouth glass bottles. Grab samples of primary sludge and dewatered sludge were collected in precleaned amber 1 L glass bottles and amber 40 mL volatile organic analysis (VOA) glass vials, respectively.
After collection, samples were placed into coolers and shipped to the laboratory, where 600 mg/L of Kathon CG-ICP preservative and 80–100 mg/L of sodium thiosulfate were added to disinfect and dechlorinate the samples and to prevent biological and chemical degradation of analytes to take place during storage (see SI for additional information). Then, 500 mL of aliquots of water were fortified with 200 ng of the deuterated surrogate standards to account for losses during storage, extraction, and analysis. Solid samples were dried and fortified with labeled standards to a nominal concentration of 400 ng/g (dry weight solids). All samples were stored at 4 °C prior to processing.
For the expanded nationwide reconnaissance, 12 additional United States WWTPs voluntarily collected 24 h flow-adjusted samples that were provided to the study team in the year of 2015 as a composited sample. The WWTPs, who requested anonymity as a prerequisite of study participation, are located in different regions of the country as described in the Discussion section. Typically, only one composite each was provided of raw influent and treated effluent collected simultaneously on a random workday. Four facilities provided effluent only; three facilities performed tertiary treatment by filtration, three facilities performed UV disinfection instead of chlorination; all other facilities performed conventional treatment (secondary treatment followed by chlorine disinfection). Samples were stored at −20 °C prior to processing.
Sample Preparation and Analysis
Extraction of Water Samples
An automatic solid-phase extraction instrument (Dionex AutoTrace 280, Thermo Scientific, Waltham, MA, U.S.A.) was used to concentrate and elute analytes from water samples from the sorbent bed for analysis. Following screening of extraction efficiency of a combination of sorbents and sample volumes, reverse phase functionalized polymeric styrene divinylbenzene sorbent (Strata X and XL, 500 mg/3 mL, Phenomenex, Torrance, CA, U.S.A.) was selected and loaded with 500 mL of wastewater sample. Before loading, cartridges were conditioned with 3 mL of methanol, followed by 3 mL of water. Then, 500 mL of wastewater samples spiked with 200 ng of the deuterated surrogate standards were loaded onto the cartridges at a flow rate of 2 mL/min, washed with water, and dried with nitrogen gas for 5 min. Two consecutive elutions were performed, each with 4 mL of a mixture (95:5, v/v) of methanol and formic acid. Equal volumes of serial eluates were combined, evaporated, and reconstituted to half the volume of water and methanol solution (80:20, v/v) in 0.1% formic acid for LC-MS analysis. Waste activated sludge and primary sludge samples featuring a TSS content of approximately 2% and 6%, respectively were spun in a centrifuge at 7500g for 10 min. Resultant supernatants were extracted as described above for water samples, whereas the solids separated from the samples were extracted separately as described below.
Extraction of Solid Samples
Solid samples were dried under nitrogen using an evaporator (Reacti-Therm TS-18821, Thermo Scientific, Waltham, MA, U.S.A.). One gram aliquots of solids samples (dry weight) spiked with 400 ng of the deuterated surrogate standards were transferred into 40 mL VOA vials, extracted with 10 mL acetone, placed on a shaker for 24 h, and sonicated for 1 h. Extracts were spun in a centrifuge at 3000g for 5 min, and the supernatants were transferred into new vials. The solids were extracted a second time with acetone, vortexed for a minute, and centrifuged, and the supernatants were combined with the first extracts. After two extractions in sequence, the resultant acetone extracts were dried under a stream of nitrogen, and analytes were reconstituted in 6 mL of hexane, following which the resultant extract was cleaned up by solid phase extraction (similar to USEPA Method 3620C) with a sorbent bed featuring a blend of magnesium oxide and silica gel (Sep-Pak Vac Florisil Cartridge 6 cc containing 1 g of sorbent, Waters Corporation, Milford, MA, U.S.A.). Before loading, the sorbent was conditioned successively with 6 mL of dichloromethane (DCM), 6 mL of acetone, and 6 mL of hexane. Extracts in hexane were loaded onto the cartridges, the resin bed washed with 6 mL of hexane, and analytes eluted subsequently with 4 mL of DCM and 4 mL of acetone. Aliquots of 1 mL of each serial extract (acetone and DCM) were transferred and combined into 2 mL LC analysis vials, dried under a gentle stream of nitrogen, and reconstituted with 1 mL of a solution of water, methanol, and formic acid (80/20/0.1, v/v/v) for analysis.
Liquid Chromatography Separation and Tandem Mass Spectrometry Analysis
Separation was carried out using a Shimadzu Ultra Performance Liquid Chromatography (UPLC) system, equipped with the SIL-20AC autosampler and 20-AD solvent delivery system (Shimadzu Scientific Instruments, Inc., Columbia, MD, U.S.A.). Simultaneous chromatographic separation of the six neonicotinoids plus one degradate was performed by reverse phase liquid chromatography using a 4.6 mm × 150 mm C8 column (XBridge, Waters Corporation Milford, MA, U.S.A.) with 3.5 μm bridged ethylene hybrid (BEH) particles. A binary gradient with 0.1% formic acid in water and methanol at a total flow rate of 0.5 mL/min was applied. The injection volume was 100 μL, and the mobile phase consisted of 20% organic with an initial 1 min ramp of 10% solvent content increased per min, followed by a 6 min ramp of 10.8% per min to 95% organic, where it was held for 3.5 min, for a total run time of 14 min. Identification and quantitation were performed using an API 4000 tandem mass spectrometer (ABSciex, Framingham, MA, U.S.A.) in positive electrospray (ESI+) mode by monitoring the first and second most abundant ion transitions for quantification and confirmation, respectively. Mass spectrometry was performed at a source heating temperature of 700 °C, ion spray voltage of 4500 V, curtain gas (nitrogen) pressure of 50 psi, nebulizer gas pressure of 90 psi, heater gas pressure of 75 psi, and dwell time of 70 ms. Analyst software, version 1.5 (ABSciex, Framingham, MA, U.S.A.) was used for LC-MS/MS system control and data analysis. Information on calibration curves, method validation, quality assurance, and quality control can be found in the SI.
Mass Balance Calculations
An analyte mass balance was performed for the full-scale wastewater treatment train over a period of 5 consecutive days (to account for the hydraulic residence time), combining primary, activated sludge, and disinfection treatment, using the following equation:
| 1 |
where, ṁtransformed = mass input of neonicotinoids lost to transformation or unaccounted for (g/day), Qinf = flow rate of influent to primary clarifier (L/day), Cinf = concentration of neonicotinoids in influent entering primary clarifier (g/L), Qeff = flow rate of effluent after chlorine disinfection (L/day), Ceff = concentration of neonicotinoids in effluent leaving treatment plant (g/L), MDWS = mass of dewatered sludge produced (kg/day), and CDWS = concentration of neonicotinoids in dewatered sludge (g/kg).
Individual mass balance for primary treatment, activated sludge treatment, disinfection treatment, and constructed wetland were calculated similarly (see SI). A paired two tailed t-test was performed (α = 0.05) to compare mean daily masses between treatment streams. Differences were determined at the p < 0.05 significance level.
Determination of Sludge Water Partitioning Coefficient (Distribution Coefficient, KD)
To determine the sorption affinity of analytes onto sludge particulates, a partitioning study was conducted.26 Ten milliliters of aliquots of water having 1, 10, and 100 ppm of all six neonicotinoids was added to 1 g of dewatered sludge, and after 10 days of shaking in the dark at 22 °C, water and solids were analyzed to establish the partitioning behavior. Sludge was inactivated prior to shaking by addition of 600 mg Kathon CG/ICP and 300 mg of sodium azide to prevent any possible biotransformation. To determine KD values, the sorbed concentration was plotted against bulk concentration remaining after sorption, and eq 2 was used:
| 2 |
where KD = distribution coefficient, L/kg dry weight; CS = sorbed concentration on the solid particulates, mg/kg dry weight of dewatered solids; CD = bulk concentration remaining after sorption, mg/L.
Results and Discussion
Method Performance
The tandem mass spectrometry method developed for this study targeted six neonicotinoids and one degradate simultaneously at part-per-trillion levels by monitoring two ion transitions via multiple reaction monitoring (MRM). Mass spectrometry parameters optimized for detection are summarized in Table S2 of the SI.
Limits of detection of analytes in different matrices are shown in Table 1 (see SI for information on data analysis and reporting methods). To ensure the quality and validity of results, each analysis batch of environmental samples contained a field blank, method blank, and check samples. No false positive values suggesting postsample collection contamination were detected during the analysis of all samples. Values of relative percent deviation (RPD) were in an acceptable range for imidacloprid (25 ± 17%), acetamiprid (20 ± 17%), acetamiprid-N-desmethyl (28 ± 22%), and clothianidin (18 ± 22%), as summarized in Table S3.
Table 1. Partitioning Properties, Method Detection Limits, and Detected Concentrations (mean ± SD) of Neonicotinoids in Wastewater Treatment and Wetland Streams.
| method detection
limit (MDL) |
detected concentration,
ng/L |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| partitioning properties |
wastewater |
biosolids |
WWTPa process streams |
wetland |
||||||||||||
| compound | log KOW | log KD | MDL, ng/L | LOQ, ng/L | absolute recovery, % | relative recovery, % | MDL, ng/g dw | LOQ, ng/g dw | absolute recovery, % | relative recovery, % | influent | primary effluent | secondary effluent | disinfection effluent | influent | effluent |
| imidacloprid | 0.57 | 1.20 | 0.6 | 1.8 | 82 ± 20 | 116 ± 10 | 1.1 | 3.3 | 86 ± 12 | 111 ± 19 | 54.7 ± 9.3 | 58.4 ± 12.6b | 48.6 ± 7.8 | 48.6 ± 8.4 | 48.2 ± 4.8 | 41.5 ± 11.5 |
| clothianidin | 0.91 | 1.20 | 0.9 | 2.7 | 90 ± 16 | 105 ± 10 | 1.4 | 4.2 | 95 ± 15 | 110 ± 8 | 149.7 ± 273.1c | 163.8 ± 195.9c | 131.3 ± 170.8c | 116.7 ± 144.9c | 124.8 ± 121.8 | 69.3 ± 53.9 |
| Acetamiprid (A) | 0.80 | 1.32 | 0.1 | 0.3 | 82 ± 3 | 95 ± 5 | 0.7 | 2.1 | 60 ± 3 | 97 ± 11 | 3.7 ± 0.8 | 3.4 ± 0.6 | 1.8 ± 0.4 | 1.7 ± 0.5 | 2.1 ± 0.5 | 2.0 ± 0.2 |
| A.-N-desmethyl | 0.65 | – | 0.6 | 1.8 | 87 ± 9 | N/A | 1.9 | 5.7 | 88 ± 9 | N/A | BDL | BDL | 1.3 ± 0.3 | 1.3 ± 0.4 | 1.4 ± 0.3 | 1.6 ± 0.3 |
| thiamethoxam | –0.13 | 0.37 | 0.3 | 0.9 | 99 ± 5 | N/A | 4.4 | 13.2 | 87 ± 12 | N/A | BDL | BDL | BDL | BDL | BDL | BDL |
| thiacloprid | 1.26 | 1.45 | 0.1 | 0.3 | 65 ± 4 | N/A | 1.6 | 4.8 | 69 ± 9 | N/A | BDL | BDL | BDL | BDL | BDL | BDL |
| dinotefuran | –0.55 | 0.34 | 32.6 | 97.8 | 31 ± 3 | N/A | 86.5 | 259.5 | 34 ± 5 | N/A | BDL | BDL | BDL | BDL | BDL | BDL |
WWTP, wastewater treatment plant.
Analyzed in triplicate.
80% detection frequency; N/A, not applicable (as isotope-labeled surrogate standard was not available); BDL, below detection limit; dw, dry weight; KOW, n-octanol–water partition coefficient; KD, sludge–water partition coefficient; LOQ, limit of quantification.
Occurrence and Fate of Neonicotinoids in the Wastewater Treatment Process
Over the sampling period of 5 consecutive days (Thursday through Monday) with 3 workweek days and 2 weekend days, consistent loading with imidacloprid (45–55 ng/L; 100% DF) and acetamiprid (3–5 ng/L; 100% DF), and erratic loading of clothianidin (<1–666 ng/L; 80% DF) was observed (Table 1). Also detected was acetamiprid-N-desmethyl (1–2 ng/L; 100% DF), a degradate of acetamiprid formed here as a result of activated sludge treatment. Neonicotinoids not detected in process streams included thiacloprid, thiamethoxam, and dinotefuran, with their corresponding method detection limits summarized in Table 1.
Mass Balance of Neonicotinoids in Aqueous WWTP Process Flows
During the 5 day sampling period, the average concentrations (mean ± standard deviation) of imidacloprid, acetamiprid, and clothianidin detected in plant influent were 54.7 ± 9.3, 3.7 ± 0.8, and 149.7 ± 273.1 ng/L, respectively. These neonicotinoids entered the primary clarifier in which settling occurred, diverting 1% of the total volumetric flow away as sludge featuring a TSS content 17 times higher than that of the clarifier effluent. Resultant daily composite effluent samples of primary treatment contained similar levels to those found in raw sewage (influent) during the 5 day sampling period. Secondary treatment consisted of an activated sludge unit operation, a biological process aimed at breaking down organic compounds primarily by microbial degradation. Average concentrations of imidacloprid and clothianidin in secondary effluent were 48.6 ± 7.8 and 131.3 ± 170.8 ng/L, implying no discernible removal by processes including microbial degradation, hydrolysis, and oxidation in the aeration basin. Prior lab studies also had shown insignificant transformation of imidacloprid in both acidic and neutral water.27 However, acetamiprid undergoes relatively fast dissipation in a neutral environment having an aqueous dissipation half-life of 4.7 days,27 and corresponding results were observed during secondary treatment, with effluent concentration of acetamiprid (1.8 ± 0.4 ng/L) cut in half compared to influent, and the formation of acetamiprid-N-desmethyl being observed, thereby confirming transformation of acetamiprid in the aeration basin, presumably mediated in part by aerobic microorganisms. Secondary effluent showed average daily concentrations of acetamiprid-N-desmethyl of 1.3 ± 0.3 ng/L. Concentrations of acetamiprid-N-desmethyl in primary influent and primary effluent were below the detection limit (<0.5 ng/L). To meet microbial removal criteria, the wastewater facility examined herein uses chlorination at a chlorine dosage of 2.5 mg/L. Although chlorine has the potential to oxidize organic compounds, no change in concentrations of imidacloprid, acetamiprid, acetamiprid-N-desmethyl, and clothianidin were observed during this disinfection treatment process.
Concentration (Table 1) data on neonicotinoids in aqueous process streams were used in conjunction with corresponding flow rate (Table S4) information to compute pesticide mass flow through the facility. On the basis of the daily average flow received by the treatment train, the total mass of analytes passing through the facility during the monitoring period was determined (Figure 1). Error values on the total mass are derived from maximum and minimum values of detected concentrations from two experimental replicates.
Figure 1.
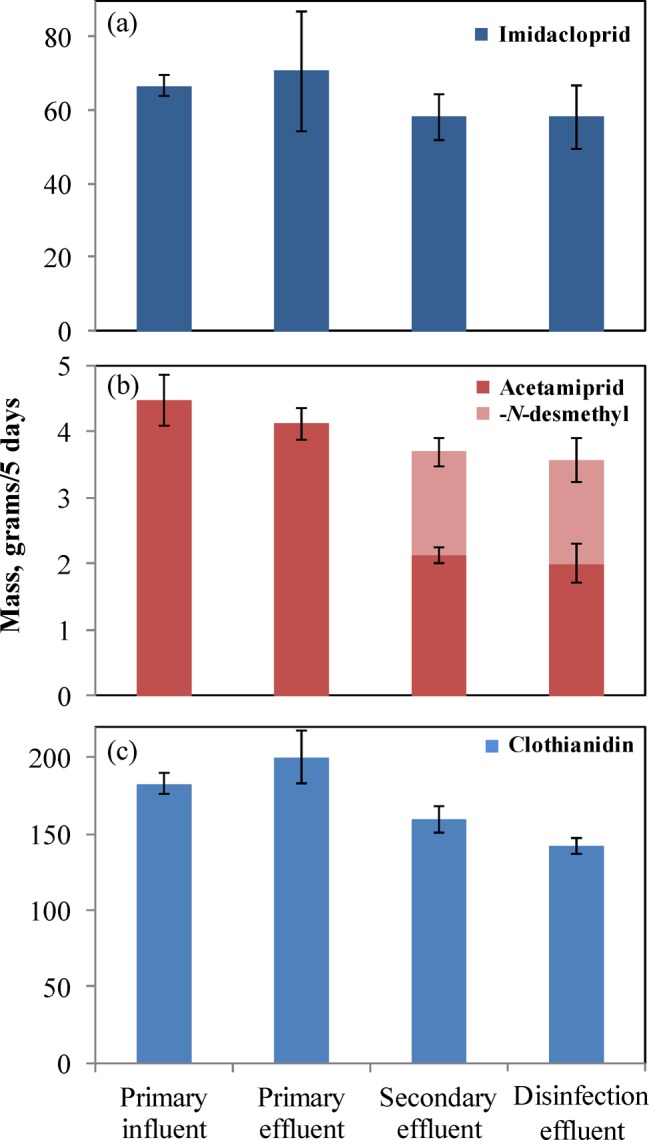
Total mass of imidacloprid (a), acetamiprid (b), and clothianidin (c) in wastewater unit operation flows over a 5 day period. Whiskers represent maximum and minimum values from two experimental replicates.
Mass in raw sewage of imidacloprid, acetamiprid, and clothianidin corresponded to 66.7 ± 3.0, 4.5 ± 0.4 g, and 183.0 ± 7.3 g/5 days, respectively. After primary treatment, the total mass leaving the primary clarifier in effluent was similar to those in influent (Figure 1), implying insignificant sorption of neonicotinoids onto sludge particulates, with the analytes persisting during primary treatment. The mass of imidacloprid and clothianidin leaving the secondary clarifier was 58.3 ± 6.3 and 159.6 ± 8.9 g/5 days, respectively. These data indicate persistence of both compounds during secondary treatment. The mass of acetamiprid leaving the secondary clarifier in the form of the parent compound was 2.1 ± 0.1 g/5 days, indicating a 53 ± 3% loss of acetamiprid in the aeration basin. The acetamiprid degradate, acetamiprid-N-desmethyl, accounted for 1.6 ± 0.2 g/5 days in effluent, which reduced the total mass removal estimate for acetamiprid and its major degradate to 18 ± 4%. Whereas relevant information on the toxicity of acetamiprid-N-desmethyl is unavailable, in theory this degradate could still impart toxicity to nontarget organisms via its cyano group, which is known to interact with the nAChR receptor of insects.28,29 Similarly, a mass balance on chlorination treatment of imidacloprid, acetamiprid, acetamiprid-N-desmethyl, and clothianidin showed resistance of each of these compounds to oxidation under real-world conditions.
Paired t-tests were performed to compare the influent and effluent concentrations of the three analytes. The mean and standard deviation for imidacloprid for influent and effluent were 13.3 ± 2.4 and 11.7 ± 2.1, respectively. The mean daily influent and effluent mass loadings of imidacloprid detected over the sampling period were statistically indistinguishable (t = 1.88, p = 0.09, CI = 95%). The mean and standard deviation for acetamiprid for influent and effluent were 0.90 ± 0.21 and 0.73 ± 0.09, respectively. A mass balance over the WWTP showed total acetamiprid removal of 18 ± 4% (t = 3.31, p = 0.01, CI = 95%), with 45 ± 4% of the initial mass being discharged as acetamiprid and 37 ± 4% as its degradate, acetamiprid-N-desmethyl. Strong variations in the loading of clothianidin during the sampling period stood in the way of conducting a firm mass balance; nevertheless, notable persistence (>70%) of the compound during treatment was firmly established.
Neonicotinoids in Sludges and Biosolids
As primary sludge and waste activated sludge represented 2% of the total facility flow, the mass of neonicotinoids accumulated in sludge was assessed as part of the mass balance analysis. Partitioning of neonicotinoids to wastewater solids was not a major factor for their fate during treatment, however. Levels of imidacloprid, acetamiprid, acetamiprid-N-desmethyl, and clothianidin were all below their respective MDLs of 1.1, 0.7, 1.9, and 1.4 μg/kg dry weight sludge. Despite these nondetect values, refined concentration estimates were obtained by analyzing the decanted liquid of sludges and using the established partition coefficients (Table 1) to calculate the approximate neonicotinoid concentrations on dry weight solids. As shown in Table S6, the resultant concentrations were low and inconsequential for the mass balance analysis (<1% of total mass).
Fate of Neonicotinoids in a Constructed Wetland
Availability of sunlight, an average water depth of only about 1.5 m, low TSS concentrations (10–15 mg/L), and a HRT of about 4.7 days made the constructed wetland a location of potential photolysis of neonicotinoids. Imidacloprid concentrations entering and leaving the engineered wetland after 5 days were 54.4 ± 3.4 and 49.9 ± 14.6 ng/L, respectively, with corresponding mass loading and discharge of 15.1 ± 0.9 and 11.4 ± 3.3 g/day. Though lab studies have shown that the photolysis half-life of imidacloprid in water is less than 1 day,30 no significant removal of imidacloprid was observed. During the sampling period (5 days), average concentrations of imidacloprid entering and leaving the engineered wetland were 48.2 ± 4.8 and 41.5 ± 11.5 ng/L, respectively; the corresponding average daily mass loading and output values were 13.6 ± 1.1 and 10.2 ± 2.7 g/day. Thus, no significant removal of imidacloprid was observed in the wetland regardless of whether average concentrations or daily concentrations off set by the HRT were compared. Similar results were found for acetamiprid and acetamiprid-N-desmethyl (Figure S3). Notable changes in loading of clothianidin made it impossible to draw any firm conclusions about potential losses in the wetland (Table S3).
Environmental Emissions and Potential Impacts of Discharged Neonicotinoids
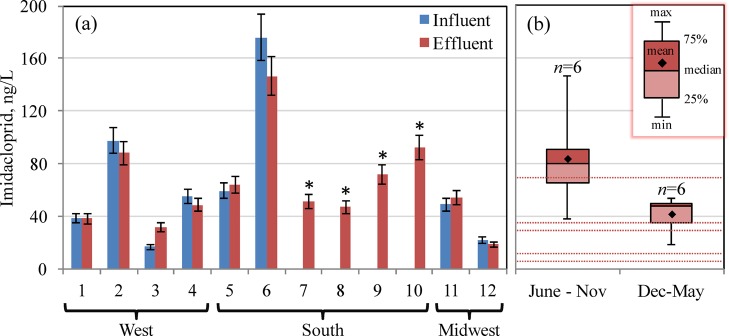
Considering the high toxicity of neonicotinoids to aquatic communities at low concentrations, it is necessary to consider WWTP effluent as a source of pesticides to the environment. Therefore, to better define the discharge of neonicotinoids into United States surface waters nationwide and to confirm that the observed behavior is not plant specific, composite wastewater samples were collected from 12 United States WWTPs between January and December 2015 and analyzed. The WWTPs analyzed were located in the western (n = 4), southern (n = 6), and midwestern (n = 2) regions of the United States, featuring diverse microbial communities, suspended solids, sludge age, and hydraulic retention time. Influent and effluent concentrations (Figure 2a) of neonicotinoids coincided with the conducted mass balance. Facilities 2, 5, and 12 performed tertiary treatment by filtration, and facilities 2, 6, and 12 performed UV disinfection instead of chlorination. All other facilities performed conventional treatment, i.e., secondary treatment followed by chlorine disinfection. Regardless of treatment strategy investigated, neonicotinoids persisted in each case without notable differences. The average concentrations discharged (including information on minimum, maximum, and median values in ng/L as well as detection frequency) were 62.6 ng/L (18.5, 146.4, 52.7, 100%) for imidacloprid, 1.9 ng/L (0.6, 5.7, 1.3, 67%) for acetamiprid, and 12.1 ng/L (9.9, 13.4, 12.5, 33%) for clothianidin. Thiamethoxam, thiacloprid, and dinotefuran were not detected in any of the samples examined, with MDLs of 0.3, 0.1, and 32.6 ng/L, respectively. On the basis of the detected concentration of neonicotinoids in influent and the population served by the studied treatment facilities, the total neonicotinoid annual loading in sewage is estimated to range from 3.1 to 10.7 mg/person/y, a value reflecting both known domestic and unknown agricultural insecticide uses in the respective sewersheds. Accordingly, the mass of neonicotinoids discharged into United States surface waters nationwide is estimated to be on the order of approximately 1.0–3.4 t of imidacloprid [United States population is considered 318.9 million (2014) (Source: United States Census Bureau)]. No estimates are provided for acetamiprid and clothianidin here because of low concentrations (<10 ng/L) and relatively low detection frequencies. The nationwide estimate provided here could be improved upon by future studies featuring a larger number of seasonal samples taken at a greater number of plants.
Figure 2.
Imidacloprid concentrations detected in 12 United States wastewater treatment plants (a); for WWTPs 7–10 (∗), only effluent was analyzed. Also shown is a comparison of published ecological toxicity benchmark values for chronic and acute exposure (red dotted lines) with discharged effluent concentration of imidacloprid at different times of year (b). Appropriate in-stream dilution factors for receiving surface water bodies need to be considered for risk assessment and may be as small as unity in effluent-dominated streams.
The international regulatory framework for neonicotinoids is still immature. In the United States, there currently are no binding regulations in place for neonicotinoid residues in treated wastewater. The Dutch government has established maximum permissible risk threshold levels for ecosystems ranging from 8 to 13 ng/L,7 and other published ecological reference values7,11,31−34 for aquatic invertebrates are about 30–40 ng/L. The imidacloprid concentrations in discharged treated wastewater established in this study (18.5–146.4 ng/L) exceed the above-mentioned thresholds (Figure 2b). Risk posed by wastewater-borne neonicotinoids will be most pervasive in situations where the discharge receiving stream is effluent-dominated, as is the case in the southern locations examined here. Fate of discharged neonicotinoids will be influenced by vegetation downstream, water depth, and pH, among other factors.35 In this study, the fate of the discharged neonicotinoids was traced with a comprehensive sampling campaign at one WWTP only, and significant persistence was observed. Whether WWTP effluent-borne neonicotinoids pose related threats to plants and wildlife in wetlands and aquatic ecosystems downstream of WWTP discharge locations is currently unknown and deserves further study. Aside from posing direct toxicity to aquatic species, these systemic pesticides also can be taken up by plants and circulated throughout the plant tissues;5 this represents a potential pathway for exposure of pollinator species and other susceptible, nontarget organisms upon accumulation of insecticide mass in pollen and nectar.36 During this one time sampling event at each facility, it was observed that relatively higher concentrations were discharged in the period of June to November when compared to the December to May time frame; however, regional time series analysis is required to confirm and elucidate this phenomenon.
Consistent loading of imidacloprid (influent concentration of 54.7 ± 9.3 ng/L) during sampling for 5 consecutive days (not coinciding with seasonal pesticide applications in the region) and 100% detection frequency at various locations throughout the year suggest that nonagricultural neonicotinoid uses also should be considered as contributors. Neonicotinoids have been detected in urine samples of Japanese adults and children without occupational spraying histories, suggesting exposure from daily lives and consumables.37−39 In recent years, nonagricultural applications of neonicotinoids have expanded. Some of the best selling canine and feline flea control products in United States contain around 10% imidacloprid as an active ingredient. Termicide products often contain up to 25% acetamiprid. Neonicotinoids also are being used in household sectors as fly bait, roach bait, and ant bait, and to eradicate bed bugs. These uses could potentially contribute to the loadings in sewage observed here. However, lack of inventory and application rate for such nonagricultural usage of these active ingredients is a major knowledge gap to study their contribution, transport, and impact on nontarget organisms.40
In summary, the present work adds much needed data to the occurrences and fates of neonicotinoid pesticides in the built water environment. Imidacloprid, thiamethoxam, clothianidin, and acetamiprid are frequently detected neonicotinoids in global surface waters.7,17−21,41−43 According to a recent study, 74% of global surface waters exhibited concentrations of individual neonicotinoids exceeding 35 ng/L (n = 17).7 Yet, the role of real-world, conventional pollution control infrastructure in attenuating sewage-borne neonicotinoids was until now ill defined.
This study adds to the present state of knowledge by furnishing the first mass balance for three neonicotinoids—namely, imidacloprid, acetamiprid and clothianidin—in a full-scale, conventional wastewater treatment plant and constructed wetland in the United States, using previously established methods to obtain reliable data.44,45 Adding to prior fate studies including a recent nationwide assessment of neonicotinoids in United States streams,46,17 we here provide the first nationwide reconnaissance on the occurrence and fate of neonicotinoid insecticides during wastewater treatment. Acetamiprid-N-desmethyl was identified as a major degradate formed during activated sludge treatment. The present work establishes the presence of neonicotinoids in urban sewersheds, demonstrates significant recalcitrance of these compounds during conventional and advanced wastewater treatment, and indicates risk to the effluent-dominated ecosystems. An order-of-magnitude estimate of the discharge load to surface waters in the United States indicates that successful management of risks posed by neonicotinoid compounds will have to take sewage sources into consideration, even for urban, nonagricultural geographical settings.
Acknowledgments
This project was supported in part by Award Number R01ES020889 from the National Institute of Environmental Health Sciences (NIEHS) and by Award Number LTR 05/01/12 of the Virginia G. Piper Charitable Trust. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIEHS or the National Institutes of Health (NIH). We thank Heather Finden, Larry Westerman, Ron Elkins, David Epperson, Tamara Saunders, Dr. Dan Childers, Dr. Arjun Venkatesan, and Edward Reyes for their help with the sampling campaign, and Jing Chen and Joshua Steele for their help with the data analysis.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.est.6b01032.
Information as mentioned in the text. (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Jeschke P.; Nauen R.; Schindler M.; Elbert A. Overview of the status and global strategy for neonicotinoids. J. Agric. Food Chem. 2011, 59 (7), 2897–2908. 10.1021/jf101303g. [DOI] [PubMed] [Google Scholar]
- Matsuda K.; Buckingham S. D.; Kleier D.; Rauh J. J.; Grauso M.; Sattelle D. B. Neonicotinoids: insecticides acting on insect nicotinic acetylcholine receptors. Trends Pharmacol. Sci. 2001, 22 (11), 573–580. 10.1016/S0165-6147(00)01820-4. [DOI] [PubMed] [Google Scholar]
- Tomizawa M.; Casida J. E. Neonicotinoid insecticide toxicology: mechanisms of selective action. Annu. Rev. Pharmacol. Toxicol. 2005, 45 (1), 247–268. 10.1146/annurev.pharmtox.45.120403.095930. [DOI] [PubMed] [Google Scholar]
- Commission implementing regulation (EU) No 485/2013 of 24 May 2013 amending implementing regulation (EU) No 540/2011, as regards the conditions of approval of the active substances clothianidin, thiamethoxam and imidacloprid, and prohibiting the use and sale of seeds treated with plant protection products containing those active substances. Official Journal of the European Union, Volume L 139, 2013; pp 12–26.
- Bonmatin J. M.; Giorio C.; Girolami V.; Goulson D.; Kreutzweiser D. P.; Krupke C.; Liess M.; Long E.; Marzaro M.; Mitchell E. A. D.; Noome D. A.; Simon-Delso N.; Tapparo A. Environmental fate and exposure: neonicotinoids and fipronil. Environ. Sci. Pollut. Res. 2015, 22 (1), 35–67. 10.1007/s11356-014-3332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Sluijs J. P.; Simon-Delso N.; Goulson D.; Maxim L.; Bonmatin J.-M.; Belzunces L. P. Neonicotinoids, bee disorders and the sustainability of pollinator services. Curr. Opin. Environ. Sustainability 2013, 5 (3–4), 293–305. 10.1016/j.cosust.2013.05.007. [DOI] [Google Scholar]
- Morrissey C. A.; Mineau P.; Devries J. H.; Sanchez-Bayo F.; Liess M.; Cavallaro M. C.; Liber K. Neonicotinoid contamination of global surface waters and associated risk to aquatic invertebrates: A review. Environ. Int. 2015, 74 (0), 291–303. 10.1016/j.envint.2014.10.024. [DOI] [PubMed] [Google Scholar]
- Suchail S.; Guez D.; Belzunces L. P. Discrepancy between acute and chronic toxicity induced by imidacloprid and its metabolites in Apis mellifera. Environ. Toxicol. Chem. 2001, 20 (11), 2482–2486. 10.1002/etc.5620201113. [DOI] [PubMed] [Google Scholar]
- Nicodemo D.; Maioli M. A.; Medeiros H. C. D.; Guelfi M.; Balieira K. V. B.; De Jong D.; Mingatto F. E. Fipronil and imidacloprid reduce honeybee mitochondrial activity. Environ. Toxicol. Chem. 2014, 33 (9), 2070–2075. 10.1002/etc.2655. [DOI] [PubMed] [Google Scholar]
- Di Prisco G.; Cavaliere V.; Annoscia D.; Varricchio P.; Caprio E.; Nazzi F.; Gargiulo G.; Pennacchio F. Neonicotinoid clothianidin adversely affects insect immunity and promotes replication of a viral pathogen in honey bees. Proc. Natl. Acad. Sci. U. S. A. 2013, 110 (46), 18466–18471. 10.1073/pnas.1314923110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk T. C.; Van Staalduinen M. A.; Van der Sluijs J. P. Macro-invertebrate decline in surface water polluted with imidacloprid. PLoS One 2013, 8 (5), e62374. 10.1371/journal.pone.0062374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulson D. Pesticides linked to bird declines. Nature 2014, 511 (7509), 295–296. 10.1038/nature13642. [DOI] [PubMed] [Google Scholar]
- Hallmann C. A.; Foppen R. P. B.; van Turnhout C. A. M.; de Kroon H.; Jongejans E. Declines in insectivorous birds are associated with high neonicotinoid concentrations. Nature 2014, 511 (7509), 341–343. 10.1038/nature13531. [DOI] [PubMed] [Google Scholar]
- Fishel F. M.Pesticide toxicity profile Neonictinoid pesticides IFAS Ext.; Publication PI-80; IFAS Extension, University of Florida, 2009.PI-80
- Ge W.; Yan S.; Wang J.; Zhu L.; Chen A.; Wang J. Oxidative stress and dna damage induced by imidacloprid in zebrafish (danio rerio). J. Agric. Food Chem. 2015, 63 (6), 1856–1862. 10.1021/jf504895h. [DOI] [PubMed] [Google Scholar]
- van der Sluijs J. P.; Amaral-Rogers V.; Belzunces L. P.; Bijleveld van Lexmond M. F. I. J.; Bonmatin J. M.; Chagnon M.; Downs C. A.; Furlan L.; Gibbons D. W.; Giorio C.; Girolami V.; Goulson D.; Kreutzweiser D. P.; Krupke C.; Liess M.; Long E.; McField M.; Mineau P.; Mitchell E. A. D.; Morrissey C. A.; Noome D. A.; Pisa L.; Settele J.; Simon-Delso N.; Stark J. D.; Tapparo A.; Van Dyck H.; van Praagh J.; Whitehorn P. R.; Wiemers M. Conclusions of the worldwide integrated assessment on the risks of neonicotinoids and fipronil to biodiversity and ecosystem functioning. Environ. Sci. Pollut. Res. 2015, 22 (1), 148–154. 10.1007/s11356-014-3229-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hladik M. L.; Kolpin D. W. First national-scale reconnaissance of neonicotinoid insecticides in streams across the USA. Environ. Chem. 2016, 13 (1), 12–20. 10.1071/EN15061. [DOI] [Google Scholar]
- Starner K.; Goh K. S. Detections of the neonicotinoid insecticide imidacloprid in surface waters of three agricultural regions of California, USA, 2010–2011. Bull. Environ. Contam. Toxicol. 2012, 88 (3), 316–21. 10.1007/s00128-011-0515-5. [DOI] [PubMed] [Google Scholar]
- Main A. R.; Headley J. V.; Peru K. M.; Michel N. L.; Cessna A. J.; Morrissey C. A. Widespread use and frequent detection of neonicotinoid insecticides in wetlands of Canada’s Prairie Pothole region. PLoS One 2014, 9 (3), e92821. 10.1371/journal.pone.0092821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Bayo F.; Hyne R. V. Detection and analysis of neonicotinoids in river waters – Development of a passive sampler for three commonly used insecticides. Chemosphere 2014, 99 (0), 143–151. 10.1016/j.chemosphere.2013.10.051. [DOI] [PubMed] [Google Scholar]
- Reemtsma T.; Alder L.; Banasiak U. Emerging pesticide metabolites in groundwater and surface water as determined by the application of a multimethod for 150 pesticide metabolites. Water Res. 2013, 47 (15), 5535–5545. 10.1016/j.watres.2013.06.031. [DOI] [PubMed] [Google Scholar]
- Hope B. K.; Pillsbury L.; Boling B. A state-wide survey in Oregon (USA) of trace metals and organic chemicals in municipal effluent. Sci. Total Environ. 2012, 417–418, 263–272. 10.1016/j.scitotenv.2011.12.028. [DOI] [PubMed] [Google Scholar]
- Campo J.; Masiá A.; Blasco C.; Picó Y. Occurrence and removal efficiency of pesticides in sewage treatment plants of four Mediterranean River Basins. J. Hazard. Mater. 2013, 263, 146–157. 10.1016/j.jhazmat.2013.09.061. [DOI] [PubMed] [Google Scholar]
- Masiá A.; Campo J.; Vázquez-Roig P.; Blasco C.; Picó Y. Screening of currently used pesticides in water, sediments and biota of the Guadalquivir River Basin (Spain). J. Hazard. Mater. 2013, 263, 95–104. 10.1016/j.jhazmat.2013.09.035. [DOI] [PubMed] [Google Scholar]
- Qi W.; Singer H.; Berg M.; Müller B.; Pernet-Coudrier B.; Liu H.; Qu J. Elimination of polar micropollutants and anthropogenic markers by wastewater treatment in Beijing, China. Chemosphere 2015, 119, 1054–1061. 10.1016/j.chemosphere.2014.09.027. [DOI] [PubMed] [Google Scholar]
- Site Characterization for Subsurface Remediation; EPA/625/4-91/026;Office of Research and Development, U.S. EPA, 1991.
- PPDB: Pesticide Properties Database. http://sitem.herts.ac.uk/aeru/ppdb/en/index.htm (accessed May 1, 2016).
- Tomizawa M.; Casida J. E. Selective toxicity of neonicotinoids attributable to specificity of insect and mammalian nicotinic receptors. Annu. Rev. Entomol. 2003, 48, 339–64. 10.1146/annurev.ento.48.091801.112731. [DOI] [PubMed] [Google Scholar]
- Simon-Delso N.; Amaral-Rogers V.; Belzunces L. P.; Bonmatin J. M.; Chagnon M.; Downs C.; Furlan L.; Gibbons D. W.; Giorio C.; Girolami V.; Goulson D.; Kreutzweiser D. P.; Krupke C. H.; Liess M.; Long E.; McField M.; Mineau P.; Mitchell E. A. D.; Morrissey C. A.; Noome D. A.; Pisa L.; Settele J.; Stark J. D.; Tapparo A.; Van Dyck H.; Van Praagh J.; Van der Sluijs J. P.; Whitehorn P. R.; Wiemers M. Systemic insecticides (neonicotinoids and fipronil): trends, uses, mode of action and metabolites. Environ. Sci. Pollut. Res. 2015, 22 (1), 5–34. 10.1007/s11356-014-3470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamhoff H.; Schneider V. Photodegradation of Imidacloprid. J. Agric. Food Chem. 1999, 47 (4), 1730–1734. 10.1021/jf980820j. [DOI] [PubMed] [Google Scholar]
- Roessink I.; Merga L. B.; Zweers H. J.; Van den Brink P. J. The neonicotinoid imidacloprid shows high chronic toxicity to mayfly nymphs. Environ. Toxicol. Chem. 2013, 32 (5), 1096–1100. 10.1002/etc.2201. [DOI] [PubMed] [Google Scholar]
- Mineau P.; Palmer C.. Neonicotinoid Insecticides and Birds: The Impact of the Nation’s Most Widely Used Insecticides on Birds; American Bird Conservancy, 2013. [Google Scholar]
- Environmental Risk Limits for Imidacloprid. Posthuma-Doodeman C. J. A. M., Ed.; National Institute for Public Health and the Environment: Bilthoven, The Netherlands, 2008. [Google Scholar]
- Water Quality Standards for Imidacloprid: Proposal for an Update According to the Water Framework Directive; National Institute for Public Health and the Environment: Bilthoven, The Netherlands, 2014. [Google Scholar]
- Main A.; Michel N.; Headley J.; Peru K.; Morrissey C. Ecological and landscape drivers of neonicotinoid insecticide detections and concentrations in canada’s prairie wetlands. Environ. Sci. Technol. 2015, 49 (14), 8367–8376. 10.1021/acs.est.5b01287. [DOI] [PubMed] [Google Scholar]
- Pisa L. W.; Amaral-Rogers V.; Belzunces L. P.; Bonmatin J. M.; Downs C. A.; Goulson D.; Kreutzweiser D. P.; Krupke C.; Liess M.; McField M.; Morrissey C. A.; Noome D. A.; Settele J.; Simon-Delso N.; Stark J. D.; Van der Sluijs J. P.; Van Dyck H.; Wiemers M. Effects of neonicotinoids and fipronil on non-target invertebrates. Environ. Sci. Pollut. Res. 2015, 22 (1), 68–102. 10.1007/s11356-014-3471-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueyama J.; Nomura H.; Kondo T.; Saito I.; Ito Y.; Osaka A.; Kamijima M. Biological monitoring method for urinary neonicotinoid insecticides using LC-MS/MS and its application to Japanese adults. J. Occup. Health 2014, 56 (6), 461–468. 10.1539/joh.14-0077-OA. [DOI] [PubMed] [Google Scholar]
- Osaka A.; Ueyama J.; Kondo T.; Nomura H.; Sugiura Y.; Saito I.; Nakane K.; Takaishi A.; Ogi H.; Wakusawa S.; Ito Y.; Kamijima M. Exposure characterization of three major insecticide lines in urine of young children in Japan—neonicotinoids, organophosphates, and pyrethroids. Environ. Res. 2016, 147, 89–96. 10.1016/j.envres.2016.01.028. [DOI] [PubMed] [Google Scholar]
- Ueyama J.; Harada K. H.; Koizumi A.; Sugiura Y.; Kondo T.; Saito I.; Kamijima M. Temporal Levels of Urinary Neonicotinoid and Dialkylphosphate Concentrations in Japanese Women Between 1994 and 2011. Environ. Sci. Technol. 2015, 49 (24), 14522–14528. 10.1021/acs.est.5b03062. [DOI] [PubMed] [Google Scholar]
- Goulson D.; Kleijn D. Review: An overview of the environmental risks posed by neonicotinoid insecticides. J. Appl. Ecol. 2013, 50 (4), 977–987. 10.1111/1365-2664.12111. [DOI] [Google Scholar]
- Schaafsma A.; Limay-Rios V.; Baute T.; Smith J.; Xue Y. Neonicotinoid insecticide residues in surface water and soil associated with commercial maize (corn) fields in Southwestern Ontario. PLoS One 2015, 10 (2), e0118139. 10.1371/journal.pone.0118139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hladik M. L.; Calhoun D. L.. Analysis of the Herbicide Diuron, Three Diuron Degradates, and Six Neonicotinoid Insecticides in Water—Method Details and Application to Two Georgia Streams; Scientific Investagation Report 2012-5206; U.S. Geological Survey, 2012.
- Hladik M. L.; Kolpin D. W.; Kuivila K. M. Widespread occurrence of neonicotinoid insecticides in streams in a high corn and soybean producing region, USA. Environ. Pollut. 2014, 193 (0), 189–196. 10.1016/j.envpol.2014.06.033. [DOI] [PubMed] [Google Scholar]
- Heidler J.; Halden R. U. Meta-analysis of mass balances for monitoring chemical fate during wastewater treatment. Environ. Sci. Technol. 2008, 42 (17), 6324–6332. 10.1021/es703008y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidler J.; Sapkota A.; Halden R. U. Partitioning, persistence, and accumulation in digested sludge of the topical antiseptic triclocarban during wastewater treatment. Environ. Sci. Technol. 2006, 40 (11), 3634–3639. 10.1021/es052245n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena A.; Rodriguez-Liebana J. A.; Mingorance M. D. Persistence of two neonicotinoid insecticides in wastewater, and in aqueous solutions of surfactants and dissolved organic matter. Chemosphere 2011, 84 (4), 464–470. 10.1016/j.chemosphere.2011.03.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.