Abstract
In humans, microbial cells (including bacteria, archaea, and fungi) greatly outnumber host cells. Candida albicans is the most prevalent fungal species of the human microbiota; this species asymptomatically colonizes many areas of the body, particularly the gastrointestinal and genitourinary tracts of healthy individuals. Alterations in host immunity, stress, resident microbiota, and other factors can lead to C. albicans overgrowth, causing a wide range of infections, from superficial mucosal to hematogenously disseminated candidiasis. To date, most studies of C. albicans have been carried out in suspension cultures; however, the medical impact of C. albicans (like that of many other microorganisms) depends on its ability to thrive as a biofilm, a closely packed community of cells. Biofilms are notorious for forming on implanted medical devices, including catheters, pacemakers, dentures, and prosthetic joints, which provide a surface and sanctuary for biofilm growth. C. albicans biofilms are intrinsically resistant to conventional antifungal therapeutics, the host immune system, and other environmental perturbations, making biofilm-based infections a significant clinical challenge. Here, we review our current knowledge of biofilms formed by C. albicans and closely related fungal species.
Keywords: fungi, pathogen, microbiota, microbial community, infection, transcriptional regulation
INTRODUCTION
Biofilms are the predominant growth state of many microorganisms. Over the last 20 years, the definition of biofilm has evolved from a stringent set of specific criteria to a broader description of microbial community structures observed in both natural and laboratory environments. In the most general sense, a biofilm is a community of adherent cells with properties that are distinct from those of free-floating (planktonic) cells (91, 92, 111). Although biofilms are often attached to solid surfaces, they can also form in other environments, for example, liquid-air interfaces. A near-universal characteristic of biofilms, compared with their free-floating counterparts, is the greater resistance of their cells to chemical and physical insults (31, 32).
It is now recognized that, for many microorganisms, the biofilm state is probably more relevant to their “natural” settings than to suspension cultures. To name just a few, these settings include aquatic environments (e.g., intertidal sediments that are home to cyanobacteria biofilms), artificial structures (e.g., pipelines where algal biofilms flourish), biomaterials (e.g., implanted heart valves where Staphylococcus epidermidis biofilms develop), plant tissues (e.g., root legumes colonized by rhizobia biofilms), and mammalian tissues (where Candida albicans biofilms are frequently found). Local effects of biofilms on their environments and/or hosts are complex; they may be favorable, harmful, or benign, and these effects can change over time. The National Institutes of Health estimate that biofilms are responsible, in one way or another, for over 80% of all microbial infections in the United States (61). In this review, we focus on C. albicans biofilms, which colonize implanted medical devices and mucosal surfaces, from which they can seed systemic infections in humans (46, 47, 64, 89, 97, 161, 172).
C. albicans is one of the very few fungal species causing disease in humans—millions of others do not. It is a member of the healthy microbiota, asymptomatically colonizing the gastrointestinal (GI) tract, reproductive tract, oral cavity, and skin of most humans (1, 64, 87, 97, 99). In individuals with healthy immune systems, C. albicans is often harmless, kept in balance with other members of the local microbiota. However, alterations in the host microbiota (e.g., due to antibiotics), changes in the host immune response (e.g., during stress, infection by another microbe, or immunosuppressant therapy), or variations in the local environment (e.g., shifts in pH or nutritional content) can enable C. albicans to overgrow and cause infection. These infections range from superficial mucosal and dermal infections, such as thrush, vaginal yeast infections, and diaper rash, to hematogenously disseminated infection with sizable mortality rates (approaching 40% in some cases) (21, 148, 202). Candida infections are especially serious in immunocompromised individuals (such as those with AIDS or those undergoing anticancer or immunosuppression therapies) and healthy people with implanted medical devices (96, 201).
C. albicans produces highly structured biofilms composed of multiple cell types (i.e., round, budding yeast-form cells; oval pseudohyphal cells; and elongated, cylindrical hyphal cells) encased in an extracellular matrix (23, 61, 158, 161). Accounting for 15% of all hospital-acquired sepsis cases, species within the CTG clade (predominantly C. albicans, but including several closely related species as well) are the fourth most frequent cause of bloodstream infections in clinical settings and are the predominant fungal species isolated from medical device infections (43, 152, 202, 204). Urinary and central venous catheters, pacemakers, mechanical heart valves, joint prostheses, contact lenses, and dentures are all susceptible to C. albicans biofilms (22, 45, 90, 175). Once it forms on an implanted medical device, a Candida biofilm has the potential to seed disseminated bloodstream infections and to lead to invasive systemic infections of tissues and organs. Over five million central venous catheters are placed each year in the United States (data obtained from 1992 to 2001) (90, 129). Currently—even with recently improved clinical approaches—biofilm infection occurs in over 50% of these catheters. Responsible for an estimated 100,000 deaths and $6.5 billion in excess expenditure annually in the United States alone, these infections have serious health and economic consequences. Because fungal biofilms are largely resistant to current antifungal drugs, high antifungal doses together with removal of the colonized medical device are generally required to treat infections (6, 29, 104, 112, 121). Removal of some devices (e.g., artificial heart valves and joints) is costly and, in some cases, dangerous, and administration of high doses of antifungal agents (typically given intravascularly) can cause complications, including kidney and liver damage. Oftentimes, these treatments are not possible, as many critically ill patients are unable to tolerate them.
IN VITRO AND IN VIVO DEVELOPMENT OF C. ALBICANS BIOFILMS
C. albicans biofilm formation in the laboratory can be readily observed on a solid surface, for example, on silicone, the common material used for intravascular catheters. Typically, a small silicone square is added to a culture of C. albicans (in any of a wide variety of growth media). An initial incubation is performed to allow the C. albicans cells to adhere to the silicone square; nonadherent cells are then washed away, and a biofilm is allowed to form. Throughout the process, the cells are constantly shaken to prevent settling; alternatively, a continuous flow of liquid across the biofilm can be imposed, mimicking exposure of an implanted catheter to blood flows. A mature biofilm typically forms within 24 hours and can be visualized by the unaided eye as a cloudy surface structure on top of the silicone square and with a microscope as an organized collection of different cell types. C. albicans biofilm formation comprises four temporal stages (10, 23, 47, 75, 142, 194) (Figure 1): (a) adherence to a surface (spherical yeast cells), (b) proliferation to form a basal layer of anchoring cells, (c) growth of pseudohyphae (ellipsoid cells joined end to end) and hyphae (chains of cylindrical cells) concomitant with production of extracellular matrix material, and (d) slow dispersal of yeast-form cells from the biofilm to seed new sites. In the laboratory, C. albicans biofilms can develop on several substrates and in many types of media, indicating an inherent robustness of biofilm development to changes in external conditions.
Figure 1.
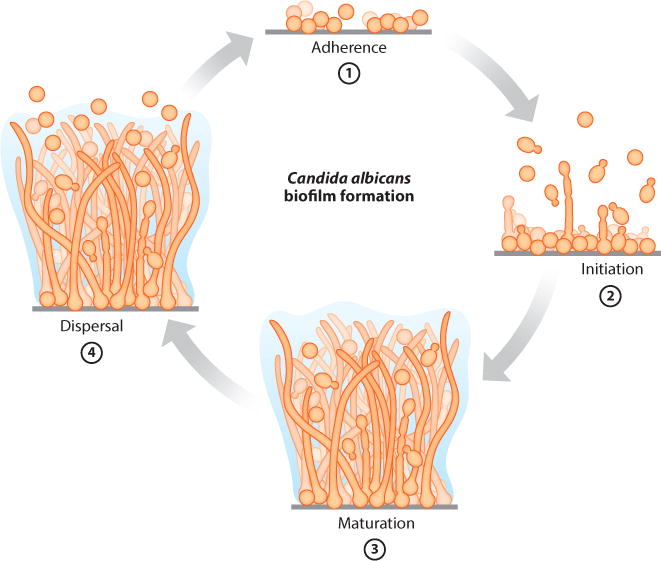
Stages of Candida albicans biofilm formation. ➀ Adherence of yeast-form cells to a surface. ➁ Initiation of cell proliferation, forming a basal layer of anchoring cells. ➂ Maturation, including growth of hyphae concomitant with the production of extracellular matrix material. ➃Dispersal of yeast-form cells from the biofilm to seed new sites.
In vitro biofilm formation has, for the most part, correlated well with in vivo and ex vivo biofilm models. For example, Candida biofilms obtained from patients with denture stomatitis and from patients with infected intravascular catheters confirm the presence of yeast, hyphae and extracellular matrix (114, 162). Biofilm architectures in rat and rabbit central venous and indwelling urinary catheter models and in rat denture stomatitis models also include numerous yeast cells in the basal region, and hyphae and extracellular matrix extending throughout the biofilm (5, 84, 131, 134, 166, 173, 199). Vaginal mucosal mouse models, both in vivo (live mice inoculated with C. albicans on the vaginal mucosa) and ex vivo (vaginas excised from euthanized mice and then inoculated with C. albicans in tissue culture plates), show similar biofilm architectures, with clear yeast, hyphae, and extracellular matrix evident throughout the biofilms formed on top of the mucosal layers (72). Other animal models for monitoring biofilm formation include rodent oral mucosal, oropharyngeal, subcutaneous, and burn wound models (28, 44, 53, 166). Development of newer systems is under way to visualize the temporal and spatial progression of biofilm infections in live animals using bioluminescence imaging. For example, a vulvovaginal candidiasis model using a codon-optimized C. albicans luciferase bioreporter was used to observe biofilm formation in real time in the vaginal lumen (48). Other C. albicans bioluminescent biofilm models include oropharyngeal, cutaneous, subcutaneous, and implanted catheter models (52, 124, 153, 197, 198).
GENETICS OF C. ALBICANS BIOFILM FORMATION
Although C. albicans is not genetically tractable in the conventional sense (its parasexual cycle is cumbersome to use in the lab), its genome is relatively simple to alter using recombinant DNA technologies. For example, nearly 1,000 gene knockout mutants of this diploid organism have been constructed (out of ~6,000 genes total), and many of these deletion mutants have been screened for biofilm formation. Other approaches include genome-wide transcriptional profiling and proteomics techniques to identify genes and proteins expressed specifically in biofilms (115, 191, 209). These studies have revealed that many hundreds of mRNAs and proteins are differentially expressed between biofilms and planktonic cells.
In this section we review these genetic studies with particular emphasis on the master regulators that orchestrate biofilm formation as well as some of the important nonregulatory genes that have been genetically validated to play important roles in biofilm formation. We exclude genes whose deletions cause broad phenotypes (such as slow growth), as their effects on biofilm formation are likely to be indirect. Because the available deletion libraries are enriched for knockouts of transcriptional regulators, we probably know more about the transcriptional regulation of biofilm production than we do about the process itself. Based on the current literature, we count 50 transcriptional regulators (Table 1) and 101 nonregulatory genes (Table 2) that have functionally validated roles in biofilm formation.
Table 1.
C. albicans transcriptional regulators with roles in biofilm formation
| ORF No. | Name | Biofilm phenotypea | Integrated in core network?b | Reference(s) | |
|---|---|---|---|---|---|
| In vitro | In vivo | ||||
| orf19.6124 | ACE2 | Defective | Defective | No | 57, 86 |
| orf19.2331 | ADA2 | Defective | NA | No | 57 |
| orf19.7381 | AHR1 | Defective | NA | Yes | 8 |
| orf19.4766 | ARG81 | Defective | NA | No | 57 |
| orf19.723 | BCR1 | Defective | Defective | Yes | 140, 141 |
| orf19.6874 | BPR1 | Defective | NA | Yes | 59 |
| orf19.4056 | BRG1 | Defective | Defective | Yes | 140 |
| orf19.4670 | CAS5 | Defective | NA | Yes | 57 |
| orf19.2356 | CRZ2 | No effect | Defective | Yes | 57 |
| orf19.3127 | CZF1 | Defective | NA | Yes | 57 |
| orf19.3252 | DAL81 | Defective | NA | No | 57 |
| orf19.610 | EFG1 | Defective | Defective | Yes | 140, 163 |
| orf19.3193 | FCR3 | Defective | NA | Yes | 57 |
| orf19.6680 | FGR27 | Defective | NA | No | 57 |
| orf19.1093 | FLO8 | Defective | Defective | Yes | 59 |
| orf19.5338 | GAL4 | Enhanced | Enhanced | Yes | 59 |
| orf19.1358 | GCN4 | Defective | NA | Yes | 66 |
| orf19.2842 | GZF3 | Defective | NA | Yes | 59 |
| orf19.4225 | LEU3 | Defective | NA | No | 57 |
| orf19.5312 | MET4 | Defective | NA | No | 57 |
| orf19.6309 | MSS11 | Defective | NA | Yes | 193 |
| orf19.2119 | NDT80 | Defective | Defective | Yes | 140, 178 |
| orf19.2012 | NOT3 | Defective | NA | No | 57 |
| orf19.7150 | NRG1 | Defective | NA | Yes | 195 |
| orf19.7247 | RIM101 | Defective | No effect | Yes | 59 |
| orf19.2823 | RFG1 | Defective | No effect | Yes | 59 |
| orf19.4590 | RFX2 | Enhanced | Enhanced | Yes | 59 |
| orf19.4662 | RLM1 | Defective | Defective | No | 136 |
| orf19.4998 | ROB1 | Defective | Defective | Yes | 140 |
| orf19.5871 | SNF5 | Defective | Defective | No | 57 |
| orf19.7319 | SUC1 | Defective | NA | No | 57 |
| orf19.798 | TAF14 | Defective | NA | No | 57 |
| orf19.5908 | TEC1 | Defective | Defective | Yes | 140, 141 |
| orf19.4062 | TRY2 | Defective | NA | No | 57 |
| orf19.1971 | TRY3 | Defective | NA | No | 57 |
| orf19.5975 | TRY4 | Defective | NA | Yes | 57 |
| orf19.3434 | TRY5 | Defective | NA | Yes | 57 |
| orf19.6824 | TRY6 | Defective | NA | Yes | 57 |
| orf19.4941 | TYE7 | Defective | NA | Yes | 17 |
| orf19.7317 | UGA33 | Defective | NA | No | 57 |
| orf19.1822 | UME6 | Defective | NA | Yes | 194, 195 |
| orf19.1035 | WAR1 | Defective | NA | No | 57 |
| orf19.3794 | ZAP1 | Enhanced | Enhanced | Yes | 57, 63, 144 |
| orf19.1718 | ZCF8 | Defective | NA | Yes | 57 |
| orf19.4767 | ZCF28 | No effect | Defective | No | 57 |
| orf19.5924 | ZCF31 | Defective | NA | Yes | 57 |
| orf19.6182 | ZCF34 | Defective | NA | No | 57 |
| orf19.7583 | ZCF39 | Defective | NA | No | 57 |
| orf19.6781 | ZFU2 | No effect | Defective | No | 57 |
| orf19.3187 | ZNC1 | Defective | NA | No | 57 |
Abbreviations: NA, not available; ORF, open reading frame.
Indicates biofilm-related phenotype of mutation, where mutation can cause a defect or an enhancement.
Indicates that the gene is bound by any of the six “master” biofilm regulators (140).
Table 2.
C. albicans nonregulatory genes with roles in biofilm formation
| Biofilm process | Genes | References |
|---|---|---|
| Adhesion | ALS1, ALS2, ALS3, ALS5, EAP1, ECM33, HWP1, MP65, MSB2, PBR1, PGA1, PGA7, PGA10, PGA13, PGA26, PSA2 | 65, 74, 102, 106, 138, 150, 155, 168, 170, 171, 177, 183, 213 |
| Filamentation/cell wall | AMS1, CAS4, CBK1, CHK1, CSA1, DSE1, GAL10, GAL102, GWT1, HGC1, HWP2, HYR1, KEM1, KIC1, MDS3, MED20, MFG1, MKC1, MOB2, NOT4, PMT1, PMT2, PMT4, PMT6, RBT1, RBT5, RHR2, SMI1, SPF1, SPT20, SUN41, SUR7, TPK1, TPK2, YAK1 | 15, 37, 41, 49, 51, 55, 67, 68, 71, 93, 94, 98, 107, 136, 146, 149, 150, 165, 169, 177, 179, 182, 190, 196, 200, 208 |
| Drug resistance | CDR1, CDR2, MDR1, QDR1, QDR2, QDR3 | 119, 126, 180 |
| Extracellular matrix | ADH1, ADH5, BGL2, CSH1, GCA1, GCA2, GSC1, IFD6, PHR1, XOG1 | 7, 127, 144, 189 |
| Dispersion | HOS2, HSP90, PES1, SET3, SIF2, SNT1, YWP1 | 70, 139, 167, 177, 194 |
| Unknown | AQY1, CAT2, GUP1, HSP21, HSP104, IPT1, NDH51, NUP85, OBPa, OBPalpha, ORF19.2175, ORF19.5412, PAP1, PAPalpha, PDX1, PIKa, PIKalpha, RIX7, SHA3, SOH1, SRB9, SSN3, SUV3, VAM3, VPS1, VPS4, YVC1 | 14, 56, 58, 83, 103, 108, 109, 120, 146, 147, 165, 177, 184, 186, 188, 192, 196, 207 |
Regulatory Control of C. albicans Biofilm Development
In 2012, a large transcriptional network that controls the development of C. albicans biofilms was described (140). This network consists of six master transcriptional regulators (Efg1, Tec1, Bcr1, Ndt80, Brg1, and Rob1), each of which is required for biofilm development in both in vitro and in vivo rat catheter and rat denture models (140). Each master regulator also controls the expression of the other master regulators, resulting in a complex, intertwined regulatory network. Together, these six master regulators directly bind to the promoters of and very likely regulate the expression of approximately 1,000 target genes, some of which are additional transcriptional regulators. Work carried out in both planktonic and biofilm conditions has indicated that various target genes play roles in hyphal formation, adhesion, drug resistance, and matrix production (see 61 for a summary), all of which are important characteristics of biofilms. However, the majority of newly identified target genes in the biofilm network have not yet been studied; many have no overt sequence similarity to any previously characterized genes from any organism. Orthology mapping indicates that the entire set of target genes is significantly enriched for “young” genes, suggesting that the ability of C. albicans to form biofilms evolved relatively recently with respect to evolutionary timescales. This inference provides an explanation as to why C. albicans and closely related species are only a few of the many fungal species able to form biofilms within a mammalian host. With the outline of the biofilm network (which is undoubtedly incomplete), it is now possible to systematically study the role of the nonregulatory target genes in biofilm development.
In addition to the 6 master transcriptional regulators discussed above, another 44 transcriptional regulators have been identified; if any of these is deleted, some aspect of C. albicans biofilm formation is affected under at least one condition (Table 1; revised from 61 and 18). The majority of these regulators are directly bound by at least one of the 6 master biofilm network regulators, indicating that they have direct regulatory connections to the core biofilm circuit (Table 1).
The transcriptional network controlling biofilm development in C. albicans may seem overly complex, but it is typical of many other transcriptional networks, particularly those in eukaryotic organisms. For example, mammalian stem cell maintenance, Drosophila eye development, and Arabidopsis circadian clock rhythms are all controlled by multiple transcriptional regulators that regulate each other and many additional target genes (3, 110, 203, 206). Complex transcriptional circuits also control pseudohyphal growth and the response to osmotic stress in baker’s yeast (19, 137) as well as the white-opaque cell-type switch in C. albicans (76). Although its significance in any transcriptional network is not fully understood, this high degree of complexity does seem to be a common feature of networks that coordinate morphological changes. In the following sections, we combine genetic results with genomic analyses and break down the biofilm circuit into smaller pieces, each of which contributes to biofilm formation and maintenance.
Adherence
The ability of cells in a biofilm to adhere to each other and to surfaces, which can be hard (a medical device) or soft (a mucosal layer), is important for all stages of C. albicans biofilm development. For example, the biofilm master regulator Bcr1 and some of its downstream targets, including the cell wall proteins Als1, Als3, and Hwp1, are all required for adherence during biofilm formation (23, 138, 141, 143, 145, 212). Many additional transcriptional regulators have been implicated in adherence (49, 57, 123, 143, 212; Table 1), the majority having been discovered in a study that screened a library of transcriptional regulator mutants using an in vitro flow cell assay (57). Here, 30 transcriptional regulators were identified as important for adherence to a silicone substrate under these conditions. Of these 30, 4 (Bcr1, Ace2, Snf5, and Arg81) were also required for biofilm formation under common conditions (shaking in microtiter plates) for in vitro biofilm formation. It is clear that biofilm formation by C. albicans can occur over a broad range of conditions and that the genetic requirements likely vary from one condition to the next.
Hyphae
C. albicans is distinguished from many other fungal species by its ability to form both yeast cells and hyphae under many different environmental conditions. (This is the basis of the early classification of C. albicans as dimorphic.) Although they readily form in planktonic cultures, hyphae are an important structural component of C. albicans biofilms; thus, it is not surprising that genes required for hyphal growth in suspension cultures are also necessary for proper biofilm formation. These include the transcriptional regulators Efg1, Tec1, Ndt80, and Rob1 (140, 163, 174). The hyphae in biofilms contribute to the overall architectural stability of the biofilm, acting as a support scaffold for yeast cells and other hyphae. Thus, the ability to form hyphae and the ability of these hyphae to adhere to one another and to yeast cells are critical for normal biofilm development and maintenance. Indeed, the master regulator Bcr1 is not needed for hyphal formation per se, but it is needed for the hyphae to adhere to one another in biofilms (141).
Extracellular Matrix
Mature C. albicans biofilms are encased in a complex material known as the extracellular matrix. Two known transcriptional regulators of biofilm matrix production in C. albicans are Rlm1 and Zap1. Deletion of RLM1 causes a reduction in matrix levels (136), whereas deletion of ZAP1 leads to an increase in the accumulation of extracellular matrix material, probably by upregulating the glucoamylases Gca1 and Gca2 (144).
Dispersal
Dispersal of cells within a C. albicans biofilm into the environment is another important stage in the biofilm life cycle. Cells are dispersed continuously throughout biofilm formation, and they are thought to be primarily (if not exclusively) in the round yeast form (194). Although dispersed cells morphologically resemble yeast cells in the planktonic mode of growth, they have distinct characteristics. These include (relative to planktonic cells) increased adherence properties, the ability to form biofilms more efficiently, and enhanced virulence in mouse models of infection (194). Two transcriptional regulators of C. albicans biofilm dispersal have been identified, Nrg1 and Ume6; transcriptional overexpression of either regulator increased the number of dispersed cells actively released from the biofilm (194, 195). Nrg1 probably acts through a Set3 chromatin-modifying complex (77, 139). Indeed, mutant strains of individual Set3 complex members formed extra strong biofilms that were incapable of normal biofilm dispersal and especially recalcitrant to mechanical perturbation (139). Nrg1 and Set3 complex mutants are hyperfilamentous, consistent with their inability to generate yeast-form cells (77, 195).
The molecular chaperone Hsp90 has also been implicated in C. albicans biofilm dispersal, as depletion of Hsp90 markedly reduces the number of dispersed cells from a biofilm (167). Depletion of Hsp90 also induces filamentation by relieving Hsp90-mediated repression of the cAMP-PKA signaling pathway (181). The cell wall protein Ywp1 is also important for biofilm dispersal, as deletion of YWP1 leads to decreased biofilm dispersal and increased biofilm adhesiveness (69, 70). It is possible that any mutation that favors filamentous cells over yeast-form cells reduces biofilm dispersal. Thus, both types of cells are required for fully functioning biofilms.
Drug Resistance and the Extracellular Matrix
The inherent resistance of C. albicans biofilms to antimicrobial agents is a key feature of the biofilm mode of growth. This resistance is due to the upregulation of efflux pumps, the presence of the extracellular matrix, and the presence of recalcitrant persister cells. In C. albicans, two major classes of efflux pumps modulate drug exportation: the ATP-binding cassette transporter superfamily (including CDR1 and CDR2) and the major facilitator class (including MDR1) (4, 33, 156). In planktonic cells, these efflux pumps are typically upregulated in response to antifungal drugs; in biofilms, however, they become upregulated within the first few hours of surface contact and remain upregulated throughout biofilm development, whether or not an antifungal drug is present (119, 126, 133, 140, 156, 205). This seemingly automatic upregulation of efflux pumps clearly contributes to the recalcitrance of biofilms to treatment with antifungal agents; it may be a manifestation of small-molecule warfare between C. albicans and other microbial species that occupy the same environmental niches.
Secreted extracellular matrix also contributes to biofilm drug resistance, both acting as a physical barrier to drug penetration and directly contributing to the overall structural integrity of the biofilm (2, 11, 130, 135). One known constituent of the biofilm matrix that contributes to its drug-resistance properties is the polysaccharide β-1,3-glucan (130). Thus, treatment of biofilms with β-1,3-glucanase increases the susceptibility of biofilms to fluconazole (130), and addition of exogenous β-1,3-glucans increases the tolerance of planktonic cells to fluconazole (122). Genes involved in production and delivery of β-1,3-glucans include the synthase-encoding gene FKS1 and the genes BGL2, XOG1, and PHR1, which encode proteins involved in the modification and transport of β-glucans (132, 189). The transcriptional regulator Rlm1 (discussed above) contributes to antifungal drug resistance by regulating the expression of FKS1 (136). Finally, extracellular DNA is also a component of the matrix in C. albicans and contributes, probably indirectly, to drug resistance (117). For example, one study reported that treatment of biofilms with DNAse enhanced the activity of caspofungin and amphotericin B in disrupting mature C. albicans biofilms (116).
Understanding how the matrix contributes to drug resistance in C. albicans has been challenging, in part because of difficulties in identifying the key matrix constituents. Significant progress has been made in comprehensive analysis of its macromolecular content (209). The C. albicans biofilm matrix is predominantly composed of proteins and glycoproteins (55%), carbohydrates (25%), lipids (15%), and nucleic acids (5%). Over 500 proteins were identified in the matrix. Most of these were predicted to be enzymes, including hydrolyzing enzymes, suggesting that the matrix may play an active role in breaking down biopolymers. It is intriguing to consider the biofilm matrix as an extracellular, enzymatically active element of a C. albicans biofilm—one that can break down molecules both as a protective response and to access a nutrient source. Polysaccharides make up the second major fraction of the matrix, consisting largely of mannan-glucan complexes made predominantly of α-1,6-linked mannan and α-1,2-linked side chains complexed to β-1,6-glucan (209). Thus, although β-1,3-glucans contribute to drug resistance and are the major cell wall polysaccharide (130), we now know that they constitute only a small fraction of the total polysaccharides present, implying that the matrix is not constructed simply of released cell wall constituents.
Persister Cells
Persister cells are another contributor to the drug-resistance properties of biofilms. A minor subset of metabolically dormant yeast cells that stochastically arise as phenotypical variants within biofilms, persister cells are extremely resistant to antifungal drugs (100). Although we know little about the formation and roles of persister cells in C. albicans biofilms, we do know that the drug resistance of persister cells is independent of cell membrane composition and efflux pump expression; rather, it is the result of the metabolically dormant state of the cells (88, 100). We know little about how persister cells are regulated and controlled, despite their importance to the drug resistance of C. albicans biofilms.
BIOFILM FORMATION BY OTHER ASCOMYCOTA SPECIES
As mentioned, C. albicans is not the only fungal species that can form biofilms in a mammalian host. The closely related species Candida dubliniensis, Candida parapsilosis, Candida tropicalis, and Candida krusei have all been implicated in biofilm-associated infections (73, 157, 187). Each of these species can form a biofilm in vitro, but the thickness, strength, and robustness in different environmental conditions decrease as species diverge phylogenetically away from C. albicans. Thus, among the CTG clade, C. albicans is probably the best biofilm former in the sense that it can form relatively thick biofilms under many different environmental conditions. It is also the most extensively studied.
Outside the CTG clade, numerous fungal species have been reported to form biofilms in vitro and in vivo. It should be emphasized that biofilm formation is often loosely defined; in some cases, simply adhering to plastic or glass is considered a sufficient criterion. The type of biofilm formed by C. albicans is probably unique to the CTG clade; given that the C. albicans biofilm circuit is a recent evolutionary innovation, it is likely that biofilms from more distantly related fungal species were also relatively recent innovations that independently evolved along those clades. Thus, it is unlikely that biofilms from fungal species hundreds of millions of years distant from C. albicans are similar in detail. Below, we briefly survey the literature on biofilm formation outside of the CTG clade.
C. albicans is a member of the phylum Ascomycota, which includes the genera Acremonium, Aspergillus, Blastomyces, Blastoschizomyces, Candida, Cladosporium, Coccidioides, Fusarium, Histoplasma, Paracoccidioides, Pneumocystis, Saccharomyces and Scedosporium. For some of these genera, biofilm formation has been implicated in pathogenesis in humans, but for others this connection is less clear. For example, a number of these biofilm formers (Table 3) have been tested for drug susceptibility in vitro, and cells in biofilms have been found to be more resistant to drugs compared with cells grown in suspension culture. These include (in addition to Candida) members from Acremonium, Aspergillus, Cladosporium, Fusarium, and Pneumocystis (9, 20, 35, 42, 79, 160, 176). Candida glabrata (which, despite the name, lies well outside the CTG clade phylogenetically) forms thin biofilms in vitro (without hyphal cells) on biotic and abiotic surfaces associated with the human host (157).
Table 3.
Non–C. albicans biofilm formers in the Ascomycota phylum
| Species | Reference(s) |
|---|---|
| Candida dubliniensis | 73, 157, 187 |
| Candida parapsilosis | 73, 157 |
| Candida tropicalis | 73, 157 |
| Candida krusei | 73, 157 |
| Candida glabrata | 95, 157 |
| Acremonium implicatum | 211 |
| Aspergillus fumigatus | 125, 158, 176 |
| Blastoschizomyces capitatus | 16, 38, 39 |
| Cladosporium sphaerospermum | 211 |
| Coccidioides immitis | 40 |
| Fusarium solani | 211 |
| Histoplasma capsulatum | 154 |
| Pneumocystis jirovecii | 35, 36 |
| Saccharomyces cerevisiae | 26, 50, 128, 164 |
| Scedosporium prolificans | 30 |
Saccharomyces cerevisiae, or baker’s yeast, rarely causes infections in humans and is generally not considered a pathogen. In a few case studies, however, S. cerevisiae has been implicated in catheter-associated infections with mixed-species biofilms in patients in intensive care units (ICUs), and it is able to form a thin biofilm consisting of round, budding yeast-form cells and pseudohyphal cells in vitro (26, 50, 128, 164). Blastoschizomyces capitatus is another rare cause of infection in humans, although more cases have been emerging in highly immunocompromised patients in ICUs. B. capitatus can cause catheter-associated bloodstream infections and has the capacity to form a biofilm both in vitro and in vivo (16, 38).
Aspergillus is a fungal pathogen of great medical importance that can form biofilms both in vitro and in vivo (125, 159). Aspergillus fumigatus is a common invasive colonizer of the respiratory tract in individuals with cystic fibrosis or other conditions that compromise the immune system (176). During infection, airborne spores enter the host via the airway and undergo a morphological switch to the filamentous growth form upon contact with host tissues. A. fumigatus then continues to proliferate along the lining of the respiratory tract, forming a dense network of hyphal cells. Aspergillomas, or dense hyphal balls, may also form as A. fumigatus continues to proliferate in the respiratory tract; these structures share many architectural characteristics with biofilms formed by other fungal species (159).
Other Ascomycota members also infect the respiratory tract of mammals. For example, Pneumocystis jirovecii causes frequently fatal pneumonia in mammals. Morphological examination of lung tissue infected by P. jirovecii reveals a biofilm-like growth structure consisting of round yeast-form clusters that can be recapitulated in vitro under certain laboratory conditions (35, 36). Scedosporium prolificans, a recently emerging human fungal pathogen of the respiratory system, may also form biofilms, given that fungus balls, resembling biofilm structures, have been observed in some clinical cases (30). Histoplasma capsulatum is another opportunistic pathogen that infects the respiratory system of mammals, causing histoplasmosis (85). H. capsulatum typically exists in a filamentous form in the environment outside of the host but is usually found as yeast-form cells within the host. There is at least one report of H. capsulatum forming biofilms consisting of dense clusters of yeast-form cells in vitro, suggesting that H. capsulatum may exist in biofilms in vivo (154). Along with H. capsulatum, Coccidioides immitis, Paracoccidioides brasiliensis, and Blastomyces dermatitidis are all sources of mycoses in immunocompromised individuals and have also caused endemic outbreaks in specific geographical regions (62, 113). All infect the respiratory system but are capable of causing invasive infections. Although rare, C. immitis has been reported to form biofilms on medical devices (40). Whether B. dermatitidis and P. brasiliensis form biofilms in vitro or in vivo is unknown.
Finally, the ascomycete species Fusarium solani, Acremonium implicatum, and Cladosporium sphaerospermum can cause keratitis in mammals, a serious infection of the cornea of the eye, as well as other less-common infections. Keratitis is generally thought of as a biofilm infection and has been associated with the use of contact lenses. In fact, a Fusarium keratitis outbreak occurred in the United States as a result of a contact lens multipurpose solution lacking sufficient antifungal efficacy against F. solani (24, 105). Recent work in vitro has shown that F. solani, A. implicatum, and C. sphaerospermum can all form biofilms under laboratory conditions (211). Though biofilm formation has been most extensively studied for C. albicans, it is clear—even from this brief discussion—that biofilm formation by an evolutionarily broad range of fungal species has enormous impact on human health.
MULTISPECIES BIOFILMS WITH C. ALBICANS
Microbial infections are often thought of, and treated, as if a single microbial species were acting alone. Yet they occur in the presence of the human microbiota—the collection of microbes that inhabit the body. This microbiota includes members from three major branches of life: bacteria, archaea, and fungi. Outnumbering human cells by at least a factor of ten, these microbes form a complex ecosystem, one whose balance can be affected by the host’s diet, genetic background, and alterations in immunity and by transient environmental perturbations, such as changes in pH, viscosity of mucosal layers, and the use of broad-spectrum antibiotics. Thus, expanding our understanding of biofilm formation in the context of other microbial members is an important goal. Although C. albicans is the fungal pathogen most frequently isolated from human infections, other Candida species have been found together with C. albicans in polymicrobial biofilms from patients (27). These species include C. dubliniensis, C. tropicalis, C. parapsilosis, C. guillermondii, C. krusei, and C. glabrata (80, 90, 118, 151). We know virtually nothing about the interactions among these Candida species in polymicrobial biofilms.
However, limited progress has been made in understanding the dual-species biofilms C. albicans forms with a few common bacterial species likely to interact with it in humans. Several studies have investigated such dual-species biofilms comprising C. albicans and a bacterium commonly isolated from denture stomatitis, periodontitis, or dental caries, such as Streptococcus mutans, Streptococcus gordonii, Actinomyces viscosus, or Fusobacterium species (12, 81, 82). C. albicans also interacts with several bacterial species found in the gut, such as Enterococcus and Escherichia species; those found in the vagina, such as Lactobacillus species; and those found in the lungs of patients with cystic fibrosis, such as Pseudomonas aeruginosa (13, 34, 54, 78). From these studies, we have learned that C. albicans and bacteria can interact with each other by secretion of signaling molecules that influence the behavior of one species toward the other, by direct cell-cell physical contact, and by alterations of the local environment that influence the other species (e.g., alterations in pH and oxygen concentrations). For example, P. aeruginosa secretes a 12-carbon acyl homoserine lactone that modulates hyphal growth by C. albicans (78). Another study examined dual-species biofilms C. albicans forms with one of five prevalent members of the human gut microbiota: Bacteroides fragilis, Clostridium perfringens, Escherichia coli, Klebsiella pneumoniae, or Enterococcus faecalis (60). In this study, biofilms formed by C. albicans provided a hypoxic microenvironment that could support the growth of the two strictly anaerobic bacteria, B. fragilis and C. perfringens—even though the biofilms were grown under oxygen-rich conditions. Thus, a C. albicans biofilm can act as a safe haven for anaerobic pathogens in oxygen-rich environments. Moreover, when cultured together with C. albicans in ambient oxygen, these anaerobes could induce the latter to form mini-biofilms, which, in turn, could protect the bacteria and allow them to proliferate under otherwise toxic conditions. Interactions occurring between different species in polymicrobial biofilms and their relevance to human health are clearly of great interest and importance.
PERSPECTIVES FOR DEVELOPING THERAPEUTICS FOR C. ALBICANS BIOFILM INFECTIONS
No biofilm-specific drugs exist today for C. albicans (or any microbe), making treatment of biofilm-based infections particularly problematic. However, a better understanding of the molecular mechanisms underlying biofilm formation and maintenance could lead to the development of new antifungals that specifically target the biofilm state. For example, identification of the molecular mechanisms behind biofilm dispersal could lead to a drug-based strategy that prevents it—or enhances it to the extent that a stable biofilm cannot form. A better understanding of adherence—both between C. albicans cells and a surface and between cells within the biofilm—could lead to strategies to prevent biofilm formation or to disrupt mature biofilms. Finally, a better understanding of the molecular basis of metabolic dormancy of subpopulations of cells, such as persister cells, might lead to strategies to reverse the physiology of these cells. These are only a few of the possible mechanism-based strategies that could be exploited to develop new biofilm-specific therapeutics.
Screens of chemical libraries have identified compounds that disrupt formation and/or maintenance of biofilms (25, 101, 185, 210). Although results from this approach are encouraging, it remains to be seen whether it will lead to useful therapeutics. Finally, any strategies that weaken C. albicans biofilm formation or maintenance could render biofilms susceptible to conventional antifungal drugs, making combination therapies effective, at least in principle. Current antifungal drugs have only two specific targets (ergosterol biosynthesis and 1,3-β-D-glucan synthesis), and it is widely believed that antifungal drugs with new mechanisms of action are needed (4, 18, 79). Our developing mechanistic knowledge of both the biofilm and the planktonic states (as well as the transition between the two) will hopefully be translated into new therapies to effectively clear biofilm-based infections and reduce relapse infection rates.
Acknowledgments
We thank Nairi Hartooni for drawing Figure 1. This study is supported by National Institutes of Health grants R00AI100896 and R01AI083311.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Clarissa J. Nobile, Email: cnobile@ucmerced.edu.
Alexander D. Johnson, Email: ajohnson@cgl.ucsf.edu.
LITERATURE CITED
- 1.Achkar JM, Fries BC. Candida infections of the genitourinary tract. Clin Microbiol Rev. 2010;23:253–73. doi: 10.1128/CMR.00076-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Fattani MA, Douglas LJ. Biofilm matrix of Candida albicans and Candida tropicalis: chemical composition and role in drug resistance. J Med Microbiol. 2006;55:999–1008. doi: 10.1099/jmm.0.46569-0. [DOI] [PubMed] [Google Scholar]
- 3.Alabadi D, Oyama T, Yanovsky MJ, Harmon FG, Mas P, Kay SA. Reciprocal regulation between TOC1 and LHY/CCA1 within the Arabidopsis circadian clock. Science. 2001;293:880–83. doi: 10.1126/science.1061320. [DOI] [PubMed] [Google Scholar]
- 4.Anderson JB. Evolution of antifungal-drug resistance: mechanisms and pathogen fitness. Nat Rev Microbiol. 2005;3:547–56. doi: 10.1038/nrmicro1179. [DOI] [PubMed] [Google Scholar]
- 5.Andes D, Nett J, Oschel P, Albrecht R, Marchillo K, Pitula A. Development and characterization of an in vivo central venous catheter Candida albicans biofilm model. Infect Immun. 2004;72:6023–31. doi: 10.1128/IAI.72.10.6023-6031.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andes DR, Safdar N, Baddley JW, Playford G, Reboli AC, et al. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: a patient-level quantitative review of randomized trials. Clin Infect Dis. 2012;54:1110–22. doi: 10.1093/cid/cis021. [DOI] [PubMed] [Google Scholar]
- 7.Angiolella L, Stringaro AR, De Bernardis F, Posteraro B, Bonito M, et al. Increase of virulence and its phenotypic traits in drug-resistant strains of Candida albicans. Antimicrob Agents Chemother. 2008;52:927–36. doi: 10.1128/AAC.01223-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Askew C, Sellam A, Epp E, Mallick J, Hogues H, et al. The zinc cluster transcription factor Ahr1p directs Mcm1p regulation of Candida albicans adhesion. Mol Microbiol. 2011;79:940–53. doi: 10.1111/j.1365-2958.2010.07504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bachmann SP, VandeWalle K, Ramage G, Patterson TF, Wickes BL, et al. In vitro activity of caspofungin against Candida albicans biofilms. Antimicrob Agents Chemother. 2002;46:3591–96. doi: 10.1128/AAC.46.11.3591-3596.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baillie GS, Douglas LJ. Role of dimorphism in the development of Candida albicans biofilms. J Med Microbiol. 1999;48:671–79. doi: 10.1099/00222615-48-7-671. [DOI] [PubMed] [Google Scholar]
- 11.Baillie GS, Douglas LJ. Matrix polymers of Candida biofilms and their possible role in biofilm resistance to antifungal agents. J Antimicrob Chemother. 2000;46:397–403. doi: 10.1093/jac/46.3.397. [DOI] [PubMed] [Google Scholar]
- 12.Bamford CV, d’Mello A, Nobbs AH, Dutton LC, Vickerman MM, Jenkinson HF. Streptococcus gordonii modulates Candida albicans biofilm formation through intergeneric communication. Infect Immun. 2009;77:3696–704. doi: 10.1128/IAI.00438-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bandara HM, Yau JY, Watt RM, Jin LJ, Samaranayake LP. Escherichia coli and its lipopolysaccharide modulate in vitro Candida biofilm formation. J Med Microbiol. 2009;58:1623–31. doi: 10.1099/jmm.0.012989-0. [DOI] [PubMed] [Google Scholar]
- 14.Bernardo SM, Khalique Z, Kot J, Jones JK, Lee SA. Candida albicans VPS1 contributes to protease secretion, filamentation, and biofilm formation. Fungal Genet Biol. 2008;45:861–77. doi: 10.1016/j.fgb.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernardo SM, Lee SA. Candida albicans SUR7 contributes to secretion, biofilm formation, and macrophage killing. BMC Microbiol. 2010;10:133. doi: 10.1186/1471-2180-10-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Birrenbach T, Bertschy S, Aebersold F, Mueller NJ, Achermann Y, et al. Emergence of Blastoschizomyces capitatus yeast infections, Central Europe. Emerg Infect Dis. 2012;18:98–101. doi: 10.3201/eid1801.111192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonhomme J, Chauvel M, Goyard S, Roux P, Rossignol T, d’Enfert C. Contribution of the glycolytic flux and hypoxia adaptation to efficient biofilm formation by Candida albicans. Mol Microbiol. 2011;80:995–1013. doi: 10.1111/j.1365-2958.2011.07626.x. [DOI] [PubMed] [Google Scholar]
- 18.Bonhomme J, d’Enfert C. Candida albicans biofilms: building a heterogeneous, drug-tolerant environment. Curr Opin Microbiol. 2013;16:398–403. doi: 10.1016/j.mib.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Borneman AR, Leigh-Bell JA, Yu H, Bertone P, Gerstein M, Snyder M. Target hub proteins serve as master regulators of development in yeast. Genes Dev. 2006;20:435–48. doi: 10.1101/gad.1389306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bowyer P, Moore CB, Rautemaa R, Denning DW, Richardson MD. Azole antifungal resistance today: focus on Aspergillus. Curr Infect Dis Rep. 2011;13:485–91. doi: 10.1007/s11908-011-0218-4. [DOI] [PubMed] [Google Scholar]
- 21.Calderone RA, Fonzi WA. Virulence factors of Candida albicans. Trends Microbiol. 2001;9:327–35. doi: 10.1016/s0966-842x(01)02094-7. [DOI] [PubMed] [Google Scholar]
- 22.Cauda R. Candidaemia in patients with an inserted medical device. Drugs. 2009;69(Suppl 1):33–38. doi: 10.2165/11315520-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 23.Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, Ghannoum MA. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J Bacteriol. 2001;183:5385–94. doi: 10.1128/JB.183.18.5385-5394.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang DC, Grant GB, O’Donnell K, Wannemuehler KA, Noble-Wang J, et al. Multistate outbreak of Fusarium keratitis associated with use of a contact lens solution. JAMA. 2006;296:953–63. doi: 10.1001/jama.296.8.953. [DOI] [PubMed] [Google Scholar]
- 25.Chavez-Dozal AA, Jahng M, Rane HS, Asare K, Kulkarny VV, et al. In vitro analysis of flufenamic acid activity against Candida albicans biofilms. Int J Antimicrob Agents. 2014;43:86–91. doi: 10.1016/j.ijantimicag.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chitasombat MN, Kofteridis DP, Jiang Y, Tarrand J, Lewis RE, Kontoyiannis DP. Rare opportunistic (non-Candida, non-Cryptococcus) yeast bloodstream infections in patients with cancer. J Infect. 2012;64:68–75. doi: 10.1016/j.jinf.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chitnis AS, Magill SS, Edwards JR, Chiller TM, Fridkin SK, Lessa FC. Trends in Candida central line-associated bloodstream infections among NICUs, 1999–2009. Pediatrics. 2012;130:e46–52. doi: 10.1542/peds.2011-3620. [DOI] [PubMed] [Google Scholar]
- 28.Cole MF, Bowen WH, Zhao XJ, Cihlar RL. Avirulence of Candida albicans auxotrophic mutants in a rat model of oropharyngeal candidiasis. FEMS Microbiol Lett. 1995;126:177–80. doi: 10.1111/j.1574-6968.1995.tb07413.x. [DOI] [PubMed] [Google Scholar]
- 29.Cornely OA, Bassetti M, Calandra T, Garbino J, Kullberg BJ, et al. ESCMID guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect. 2012;18(Suppl 7):19–37. doi: 10.1111/1469-0691.12039. [DOI] [PubMed] [Google Scholar]
- 30.Cortez KJ, Roilides E, Quiroz-Telles F, Meletiadis J, Antachopoulos C, et al. Infections caused by Scedosporium spp. Clin Microbiol Rev. 2008;21:157–97. doi: 10.1128/CMR.00039-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Costerton JW, Cheng KJ, Geesey GG, Ladd TI, Nickel JC, et al. Bacterial biofilms in nature and disease. Annu Rev Microbiol. 1987;41:435–64. doi: 10.1146/annurev.mi.41.100187.002251. [DOI] [PubMed] [Google Scholar]
- 32.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–22. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 33.Cowen LE. The evolution of fungal drug resistance: modulating the trajectory from genotype to phenotype. Nat Rev Microbiol. 2008;6:187–98. doi: 10.1038/nrmicro1835. [DOI] [PubMed] [Google Scholar]
- 34.Cruz MR, Graham CE, Gagliano BC, Lorenz MC, Garsin DA. Enterococcus faecalis inhibits hyphal morphogenesis and virulence of Candida albicans. Infect Immun. 2013;81:189–200. doi: 10.1128/IAI.00914-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cushion MT, Collins MS. Susceptibility of Pneumocystis to echinocandins in suspension and biofilm cultures. Antimicrob Agents Chemother. 2011;55:4513–18. doi: 10.1128/AAC.00017-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cushion MT, Collins MS, Linke MJ. Biofilm formation by Pneumocystis spp. Eukaryot Cell. 2009;8:197–206. doi: 10.1128/EC.00202-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daher JY, Koussa J, Younes S, Khalaf RA. The Candida albicans Dse1 protein is essential and plays a role in cell wall rigidity, biofilm formation, and virulence. Interdiscip Perspect Infect Dis. 2011;2011:504280. doi: 10.1155/2011/504280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.D’Antonio D, Mazzoni A, Iacone A, Violante B, Capuani MA, et al. Emergence of fluconazole-resistant strains of Blastoschizomyces capitatus causing nosocomial infections in cancer patients. J Clin Microbiol. 1996;34:753–55. doi: 10.1128/jcm.34.3.753-755.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.D’Antonio D, Parruti G, Pontieri E, Di Bonaventura G, Manzoli L, et al. Slime production by clinical isolates of Blastoschizomyces capitatus from patients with hematological malignancies and catheter-related fungemia. Eur J Clin Microbiol Infect. 2004;23:787–89. doi: 10.1007/s10096-004-1207-4. [DOI] [PubMed] [Google Scholar]
- 40.Davis LE, Cook G, Costerton JW. Biofilm on ventriculo-peritoneal shunt tubing as a cause of treatment failure in coccidioidal meningitis. Emerg Infect Dis. 2002;8:376–79. doi: 10.3201/eid0804.010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Desai JV, Bruno VM, Ganguly S, Stamper RJ, Mitchell KF, et al. Regulatory role of glycerol in Candida albicans biofilm formation. mBio. 2013;4:e00637–12. doi: 10.1128/mBio.00637-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Desai JV, Mitchell AP, Andes DR. Fungal biofilms, drug resistance, and recurrent infection. Cold Spring Harb Perspect Med. 2014;4:a019729. doi: 10.1101/cshperspect.a019729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dominic RM, Shenoy S, Baliga S. Candida biofilms in medical devices: evolving trends. Kathmandu Univ Med J. 2007;5:431–36. [PubMed] [Google Scholar]
- 44.Dongari-Bagtzoglou A, Kashleva H, Dwivedi P, Diaz P, Vasilakos J. Characterization of mucosal Candida albicans biofilms. PLOS ONE. 2009;4:e7967. doi: 10.1371/journal.pone.0007967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167–93. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Douglas LJ. Medical importance of biofilms in Candida infections. Rev Iberoamericana Micol. 2002;19:139–43. [PubMed] [Google Scholar]
- 47.Douglas LJ. Candida biofilms and their role in infection. Trends Microbiol. 2003;11:30–36. doi: 10.1016/s0966-842x(02)00002-1. [DOI] [PubMed] [Google Scholar]
- 48.Doyle TC, Nawotka KA, Kawahara CB, Francis KP, Contag PR. Visualizing fungal infections in living mice using bioluminescent pathogenic Candida albicans strains transformed with the firefly luciferase gene. Microb Pathog. 2006;40:82–90. doi: 10.1016/j.micpath.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 49.Dwivedi P, Thompson A, Xie Z, Kashleva H, Ganguly S, et al. Role of Bcr1-activated genes Hwp1 and Hyr1 in Candida albicans oral mucosal biofilms and neutrophil evasion. PLOS ONE. 2011;6:e16218. doi: 10.1371/journal.pone.0016218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Enache-Angoulvant A, Hennequin C. Invasive Saccharomyces infection: a comprehensive review. Clin Infect Dis. 2005;41:1559–68. doi: 10.1086/497832. [DOI] [PubMed] [Google Scholar]
- 51.Ene IV, Bennett RJ. Hwp1 and related adhesins contribute to both mating and biofilm formation in Candida albicans. Eukaryot Cell. 2009;8:1909–13. doi: 10.1128/EC.00245-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Enjalbert B, Rachini A, Vediyappan G, Pietrella D, Spaccapelo R, et al. A multifunctional, synthetic Gaussia princeps luciferase reporter for live imaging of Candida albicans infections. Infect Immun. 2009;77:4847–58. doi: 10.1128/IAI.00223-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fader RC, Nunez D, Unbehagen J, Linares HA. Experimental candidiasis after thermal injury. Infect Immun. 1985;49:780–84. doi: 10.1128/iai.49.3.780-784.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Falagas ME, Betsi GI, Athanasiou S. Probiotics for prevention of recurrent vulvovaginal candidiasis: a review. J Antimicrob Chemother. 2006;58:266–72. doi: 10.1093/jac/dkl246. [DOI] [PubMed] [Google Scholar]
- 55.Fanning S, Xu W, Beaurepaire C, Suhan JP, Nantel A, Mitchell AP. Functional control of the Candida albicans cell wall by catalytic protein kinase A subunit Tpk1. Mol Microbiol. 2012;86:284–302. doi: 10.1111/j.1365-2958.2012.08193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ferreira C, Silva S, Faria-Oliveira F, Pinho E, Henriques M, Lucas C. Candida albicans virulence and drug-resistance requires the O-acyltransferase Gup1p. BMC Microbiol. 2010;10:238. doi: 10.1186/1471-2180-10-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Finkel JS, Xu W, Huang D, Hill EM, Desai JV, et al. Portrait of Candida albicans adherence regulators. PLOS Pathog. 2012;8:e1002525. doi: 10.1371/journal.ppat.1002525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fiori A, Kucharikova S, Govaert G, Cammue BP, Thevissen K, Van Dijck P. The heat-induced molecular disaggregase Hsp104 of Candida albicans plays a role in biofilm formation and pathogenicity in a worm infection model. Eukaryot Cell. 2012;11:1012–20. doi: 10.1128/EC.00147-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fox EP, Bui CK, Nett JE, Hartooni N, Mui MM, et al. An expanded regulatory network temporally controls Candida albicans biofilm formation. Mol Microbiol. 2015;96:1226–39. doi: 10.1111/mmi.13002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fox EP, Cowley ES, Nobile CJ, Hartooni N, Newman DK, Johnson AD. Anaerobic bacteria grow within Candida albicans biofilms and induce biofilm formation in suspension cultures. Curr Biol. 2014;24:2411–16. doi: 10.1016/j.cub.2014.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fox EP, Nobile CJ. A sticky situation: untangling the transcriptional network controlling biofilm development in Candida albicans. Transcription. 2012;3:315–22. doi: 10.4161/trns.22281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fridkin SK, Jarvis WR. Epidemiology of nosocomial fungal infections. Clin Microbiol Rev. 1996;9:499–511. doi: 10.1128/cmr.9.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ganguly S, Bishop AC, Xu W, Ghosh S, Nickerson KW, et al. Zap1 control of cell-cell signaling in Candida albicans biofilms. Eukaryot Cell. 2011;10:1448–54. doi: 10.1128/EC.05196-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ganguly S, Mitchell AP. Mucosal biofilms of Candida albicans. Curr Opin Microbiol. 2011;14:380–85. doi: 10.1016/j.mib.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garcia MC, Lee JT, Ramsook CB, Alsteens D, Dufrene YF, Lipke PN. A role for amyloid in cell aggregation and biofilm formation. PLOS ONE. 2011;6:e17632. doi: 10.1371/journal.pone.0017632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garcia-Sanchez S, Aubert S, Iraqui I, Janbon G, Ghigo JM, d’Enfert C. Candida albicans biofilms: a developmental state associated with specific and stable gene expression patterns. Eukaryot Cell. 2004;3:536–45. doi: 10.1128/EC.3.2.536-545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Giacometti R, Kronberg F, Biondi RM, Passeron S. Candida albicans Tpk1p and Tpk2p isoforms differentially regulate pseudohyphal development, biofilm structure, cell aggregation and adhesins expression. Yeast. 2011;28:293–308. doi: 10.1002/yea.1839. [DOI] [PubMed] [Google Scholar]
- 68.Goyard S, Knechtle P, Chauvel M, Mallet A, Prevost MC, et al. The Yak1 kinase is involved in the initiation and maintenance of hyphal growth in Candida albicans. Mol Biol Cell. 2008;19:2251–66. doi: 10.1091/mbc.E07-09-0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Granger BL. Insight into the antiadhesive effect of yeast wall protein 1 of Candida albicans. Eukaryot Cell. 2012;11:795–805. doi: 10.1128/EC.00026-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Granger BL, Flenniken ML, Davis DA, Mitchell AP, Cutler JE. Yeast wall protein 1 of Candida albicans. Microbiology. 2005;151:1631–44. doi: 10.1099/mic.0.27663-0. [DOI] [PubMed] [Google Scholar]
- 71.Gutierrez-Escribano P, Zeidler U, Suarez MB, Bachellier-Bassi S, Clemente-Blanco A, et al. The NDR/LATS kinase Cbk1 controls the activity of the transcriptional regulator Bcr1 during biofilm formation in Candida albicans. PLOS Pathog. 2012;8:e1002683. doi: 10.1371/journal.ppat.1002683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Harriott MM, Lilly EA, Rodriguez TE, Fidel PL, Jr, Noverr MC. Candida albicans forms biofilms on the vaginal mucosa. Microbiology. 2010;156:3635–44. doi: 10.1099/mic.0.039354-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hasan F, Xess I, Wang X, Jain N, Fries BC. Biofilm formation in clinical Candida isolates and its association with virulence. Microbes Infect. 2009;11:753–61. doi: 10.1016/j.micinf.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hashash R, Younes S, Bahnan W, El Koussa J, Maalouf K, et al. Characterisation of Pga1, a putative Candida albicans cell wall protein necessary for proper adhesion and biofilm formation. Mycoses. 2011;54:491–500. doi: 10.1111/j.1439-0507.2010.01883.x. [DOI] [PubMed] [Google Scholar]
- 75.Hawser SP, Douglas LJ. Biofilm formation by Candida species on the surface of catheter materials in vitro. Infect Immun. 1994;62:915–21. doi: 10.1128/iai.62.3.915-921.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hernday AD, Lohse MB, Fordyce PM, Nobile CJ, Derisi JL, Johnson AD. Structure of the transcriptional network controlling white-opaque switching in Candida albicans. Mol Microbiol. 2013;90:22–35. doi: 10.1111/mmi.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hnisz D, Bardet AF, Nobile CJ, Petryshyn A, Glaser W, et al. A histone deacetylase adjusts transcription kinetics at coding sequences during Candida albicans morphogenesis. PLOS Genet. 2012;8:e1003118. doi: 10.1371/journal.pgen.1003118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hogan DA, Vik A, Kolter R. A Pseudomonas aeruginosa quorum-sensing molecule influences Candida albicans morphology. Mol Microbiol. 2004;54:1212–23. doi: 10.1111/j.1365-2958.2004.04349.x. [DOI] [PubMed] [Google Scholar]
- 79.Jabra-Rizk MA, Falkler WA, Meiller TF. Fungal biofilms and drug resistance. Emerg Infect Dis. 2004;10:14–19. doi: 10.3201/eid1001.030119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jabra-Rizk MA, Falkler WA, Jr, Merz WG, Baqui AA, Kelley JI, Meiller TF. Retrospective identification and characterization of Candida dubliniensis isolates among Candida albicans clinical laboratory isolates from human immunodeficiency virus (HIV)-infected and non-HIV-infected individuals. J Clin Microbiol. 2000;38:2423–26. doi: 10.1128/jcm.38.6.2423-2426.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jack AA, Daniels DE, Jepson MA, Vickerman MM, Lamont RJ, et al. The Streptococcus gordonii comCDE (competence) operon modulates biofilm formation with Candida albicans. Microbiology. 2014;161:411–21. doi: 10.1099/mic.0.000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jarosz LM, Deng DM, van der Mei HC, Crielaard W, Krom BP. Streptococcus mutans competence-stimulating peptide inhibits Candida albicans hypha formation. Eukaryot Cell. 2009;8:1658–64. doi: 10.1128/EC.00070-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Johnson C, Kweon HK, Sheidy D, Shively CA, Mellacheruvu D, et al. The yeast Sks1p kinase signaling network regulates pseudohyphal growth and glucose response. PLOS Genet. 2014;10:e1004183. doi: 10.1371/journal.pgen.1004183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Johnson CC, Yu A, Lee H, Fidel PL, Jr, Noverr MC. Development of a contemporary animal model of Candida albicans-associated denture stomatitis using a novel intraoral denture system. Infect Immun. 2012;80:1736–43. doi: 10.1128/IAI.00019-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev. 2007;20:115–32. doi: 10.1128/CMR.00027-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kelly MT, MacCallum DM, Clancy SD, Odds FC, Brown AJ, Butler G. The Candida albicans CaACE2 gene affects morphogenesis, adherence and virulence. Mol Microbiol. 2004;53:969–83. doi: 10.1111/j.1365-2958.2004.04185.x. [DOI] [PubMed] [Google Scholar]
- 87.Kennedy MJ, Volz PA. Ecology of Candida albicans gut colonization: inhibition of Candida adhesion, colonization, and dissemination from the gastrointestinal tract by bacterial antagonism. Infect Immun. 1985;49:654–63. doi: 10.1128/iai.49.3.654-663.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Khot PD, Suci PA, Miller RL, Nelson RD, Tyler BJ. A small subpopulation of blastospores in Candida albicans biofilms exhibit resistance to amphotericin B associated with differential regulation of ergosterol and beta-1,6-glucan pathway genes. Antimicrob Agents Chemother. 2006;50:3708–16. doi: 10.1128/AAC.00997-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim J, Sudbery P. Candida albicans, a major human fungal pathogen. J Microbiol. 2011;49:171–77. doi: 10.1007/s12275-011-1064-7. [DOI] [PubMed] [Google Scholar]
- 90.Kojic EM, Darouiche RO. Candida infections of medical devices. Clin Microbiol Rev. 2004;17:255–67. doi: 10.1128/CMR.17.2.255-267.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kolter R. Biofilms in lab and nature: a molecular geneticist’s voyage to microbial ecology. Int Microbiol. 2010;13:1–7. doi: 10.2436/20.1501.01.105. [DOI] [PubMed] [Google Scholar]
- 92.Kolter R, Greenberg EP. Microbial sciences: the superficial life of microbes. Nature. 2006;441:300–2. doi: 10.1038/441300a. [DOI] [PubMed] [Google Scholar]
- 93.Krueger KE, Ghosh AK, Krom BP, Cihlar RL. Deletion of the NOT4 gene impairs hyphal development and pathogenicity in Candida albicans. Microbiology. 2004;150:229–40. doi: 10.1099/mic.0.26792-0. [DOI] [PubMed] [Google Scholar]
- 94.Kruppa M, Krom BP, Chauhan N, Bambach AV, Cihlar RL, Calderone RA. The two-component signal transduction protein Chk1p regulates quorum sensing in Candida albicans. Eukaryot Cell. 2004;3:1062–65. doi: 10.1128/EC.3.4.1062-1065.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kucharikova S, Tournu H, Lagrou K, Van Dijck P, Bujdakova H. Detailed comparison of Candida albicans and Candida glabrata biofilms under different conditions and their susceptibility to caspofungin and anidulafungin. J Med Microbiol. 2011;60:1261–69. doi: 10.1099/jmm.0.032037-0. [DOI] [PubMed] [Google Scholar]
- 96.Kullberg BJ, Oude Lashof AM. Epidemiology of opportunistic invasive mycoses. Eur J Med Res. 2002;7:183–91. [PubMed] [Google Scholar]
- 97.Kumamoto CA. Candida biofilms. Curr Opin Microbiol. 2002;5:608–11. doi: 10.1016/s1369-5274(02)00371-5. [DOI] [PubMed] [Google Scholar]
- 98.Kumamoto CA. A contact-activated kinase signals Candida albicans invasive growth and biofilm development. PNAS. 2005;102:5576–81. doi: 10.1073/pnas.0407097102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kumamoto CA. Inflammation and gastrointestinal Candida colonization. Curr Opin Microbiol. 2011;14:386–91. doi: 10.1016/j.mib.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.LaFleur MD, Kumamoto CA, Lewis K. Candida albicans biofilms produce antifungal-tolerant persister cells. Antimicrob Agents Chemother. 2006;50:3839–46. doi: 10.1128/AAC.00684-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.LaFleur MD, Lucumi E, Napper AD, Diamond SL, Lewis K. Novel high-throughput screen against Candida albicans identifies antifungal potentiators and agents effective against biofilms. J Antimicrob Chemother. 2011;66:820–26. doi: 10.1093/jac/dkq530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Laforet L, Moreno I, Sanchez-Fresneda R, Martinez-Esparza M, Martinez JP, et al. Pga26 mediates filamentation and biofilm formation and is required for virulence in Candida albicans. FEMS Yeast Res. 2011;11:389–97. doi: 10.1111/j.1567-1364.2011.00727.x. [DOI] [PubMed] [Google Scholar]
- 103.Lattif AA, Mukherjee PK, Chandra J, Roth MR, Welti R, et al. Lipidomics of Candida albicans biofilms reveals phase-dependent production of phospholipid molecular classes and role for lipid rafts in biofilm formation. Microbiology. 2011;157:3232–42. doi: 10.1099/mic.0.051086-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lepak A, Andes D. Fungal sepsis: optimizing antifungal therapy in the critical care setting. Crit Care Clin. 2011;27:123–47. doi: 10.1016/j.ccc.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 105.Levy B, Heiler D, Norton S. Report on testing from an investigation of Fusarium keratitis in contact lens wearers. Eye Contact Lens. 2006;32:256–61. doi: 10.1097/01.icl.0000245556.46738.14. [DOI] [PubMed] [Google Scholar]
- 106.Li F, Svarovsky MJ, Karlsson AJ, Wagner JP, Marchillo K, et al. Eap1p, an adhesin that mediates Candida albicans biofilm formation in vitro and in vivo. Eukaryot Cell. 2007;6:931–39. doi: 10.1128/EC.00049-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lin CH, Kabrawala S, Fox EP, Nobile CJ, Johnson AD, Bennett RJ. Genetic control of conventional and pheromone-stimulated biofilm formation in Candida albicans. PLOS Pathog. 2013;9:e1003305. doi: 10.1371/journal.ppat.1003305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lindsay AK, Morales DK, Liu Z, Grahl N, Zhang A, et al. Analysis of Candida albicans mutants defective in the Cdk8 module of Mediator reveal links between metabolism and biofilm formation. PLOS Genet. 2014;10:e1004567. doi: 10.1371/journal.pgen.1004567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu G, Vellucci VF, Kyc S, Hostetter MK. Simvastatin inhibits Candida albicans biofilm in vitro. Pediatr Res. 2009;66:600–4. doi: 10.1203/PDR.0b013e3181bd5bf8. [DOI] [PubMed] [Google Scholar]
- 110.Locke JC, Southern MM, Kozma-Bognar L, Hibberd V, Brown PE, et al. Extension of a genetic network model by iterative experimentation and mathematical analysis. Mol Syst Biol. 2005;1:2005.0013. doi: 10.1038/msb4100018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lopez D, Vlamakis H, Kolter R. Biofilms. Cold Spring Harb Perspect Biol. 2010;2:a000398. doi: 10.1101/cshperspect.a000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lortholary O, Petrikkos G, Akova M, Arendrup MC, Arikan-Akdagli S, et al. ESCMID guideline for the diagnosis and management of Candida diseases 2012: patients with HIV infection or AIDS. Clin Microbiol Infect. 2012;18(Suppl 7):68–77. doi: 10.1111/1469-0691.12042. [DOI] [PubMed] [Google Scholar]
- 113.Marques SA. Paracoccidioidomycosis. Clin Dermatol. 2012;30:610–15. doi: 10.1016/j.clindermatol.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 114.Marrie TJ, Costerton JW. Scanning and transmission electron microscopy of in situ bacterial colonization of intravenous and intraarterial catheters. J Clin Microbiol. 1984;19:687–93. doi: 10.1128/jcm.19.5.687-693.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Martinez-Gomariz M, Perumal P, Mekala S, Nombela C, Chaffin WL, Gil C. Proteomic analysis of cytoplasmic and surface proteins from yeast cells, hyphae, and biofilms of Candida albicans. Proteomics. 2009;9:2230–52. doi: 10.1002/pmic.200700594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Martins M, Henriques M, Lopez-Ribot JL, Oliveira R. Addition of DNase improves the in vitro activity of antifungal drugs against Candida albicans biofilms. Mycoses. 2012;55:80–85. doi: 10.1111/j.1439-0507.2011.02047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Martins M, Uppuluri P, Thomas DP, Cleary IA, Henriques M, et al. Presence of extracellular DNA in the Candida albicans biofilm matrix and its contribution to biofilms. Mycopathologia. 2010;169:323–31. doi: 10.1007/s11046-009-9264-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Masala L, Luzzati R, Maccacaro L, Antozzi L, Concia E, Fontana R. Nosocomial cluster of Candida guillermondii fungemia in surgical patients. Eur J Clin Microbiol Infect Dis. 2003;22:686–88. doi: 10.1007/s10096-003-1013-4. [DOI] [PubMed] [Google Scholar]
- 119.Mateus C, Crow SA, Jr, Ahearn DG. Adherence of Candida albicans to silicone induces immediate enhanced tolerance to fluconazole. Antimicrob Agents Chemother. 2004;48:3358–66. doi: 10.1128/AAC.48.9.3358-3366.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Melo AS, Padovan AC, Serafim RC, Puzer L, Carmona AK, et al. The Candida albicans AAA ATPase homologue of Saccharomyces cerevisiae Rix7p (YLL034c) is essential for proper morphology, biofilm formation and activity of secreted aspartyl proteinases. Genet Mol Res. 2006;5:664–87. [PubMed] [Google Scholar]
- 121.Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mitchell KF, Taff HT, Cuevas MA, Reinicke EL, Sanchez H, Andes DR. Role of matrix β-1,3 glucan in antifungal resistance of non-albicans Candida biofilms. Antimicrob Agents Chemother. 2013;57:1918–20. doi: 10.1128/AAC.02378-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Monniot C, Boisrame A, Da Costa G, Chauvel M, Sautour M, et al. Rbt1 protein domains analysis in Candida albicans brings insights into hyphal surface modifications and Rbt1 potential role during adhesion and biofilm formation. PLOS ONE. 2013;8:e82395. doi: 10.1371/journal.pone.0082395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mosci P, Pericolini E, Gabrielli E, Kenno S, Perito S, et al. A novel bioluminescence mouse model for monitoring oropharyngeal candidiasis in mice. Virulence. 2013;4:250–54. doi: 10.4161/viru.23529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mowat E, Butcher J, Lang S, Williams C, Ramage G. Development of a simple model for studying the effects of antifungal agents on multicellular communities of Aspergillus fumigatus. J Med Microbiol. 2007;56:1205–12. doi: 10.1099/jmm.0.47247-0. [DOI] [PubMed] [Google Scholar]
- 126.Mukherjee PK, Chandra J, Kuhn DM, Ghannoum MA. Mechanism of fluconazole resistance in Candida albicans biofilms: phase-specific role of efflux pumps and membrane sterols. Infect Immun. 2003;71:4333–40. doi: 10.1128/IAI.71.8.4333-4340.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mukherjee PK, Mohamed S, Chandra J, Kuhn D, Liu S, et al. Alcohol dehydrogenase restricts the ability of the pathogen Candida albicans to form a biofilm on catheter surfaces through an ethanol-based mechanism. Infect Immun. 2006;74:3804–16. doi: 10.1128/IAI.00161-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Munoz P, Bouza E, Cuenca-Estrella M, Eiros JM, Perez MJ, et al. Saccharomyces cerevisiae fungemia: an emerging infectious disease. Clin Infect Dis. 2005;40:1625–34. doi: 10.1086/429916. [DOI] [PubMed] [Google Scholar]
- 129.Natl. Nosocom. Infect. Surveill. Sys. National Nosocomial Infections Surveillance (NNIS) System report, data summary from January 1992-June 2001, issued August 2001. Am J Infect Control. 2001;29:404–21. doi: 10.1067/mic.2001.119952. [DOI] [PubMed] [Google Scholar]
- 130.Nett J, Lincoln L, Marchillo K, Massey R, Holoyda K, et al. Putative role of beta-1,3 glucans in Candida albicans biofilm resistance. Antimicrob Agents Chemother. 2007;51:510–20. doi: 10.1128/AAC.01056-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nett JE, Brooks EG, Cabezas-Olcoz J, Sanchez H, Zarnowski R, et al. Rat indwelling urinary catheter model of Candida albicans biofilm infection. Infect Immun. 2014;82:4931–40. doi: 10.1128/IAI.02284-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nett JE, Crawford K, Marchillo K, Andes DR. Role of Fks1p and matrix glucan in Candida albicans biofilm resistance to an echinocandin, pyrimidine, and polyene. Antimicrob Agents Chemother. 2010;54:3505–8. doi: 10.1128/AAC.00227-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nett JE, Lepak AJ, Marchillo K, Andes DR. Time course global gene expression analysis of an in vivo Candida biofilm. J Infect Dis. 2009;200:307–13. doi: 10.1086/599838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nett JE, Marchillo K, Spiegel CA, Andes DR. Development and validation of an in vivo Candida albicans biofilm denture model. Infect Immun. 2010;78:3650–59. doi: 10.1128/IAI.00480-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nett JE, Sanchez H, Cain MT, Andes DR. Genetic basis of Candida biofilm resistance due to drug-sequestering matrix glucan. J Infect Dis. 2010;202:171–75. doi: 10.1086/651200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nett JE, Sanchez H, Cain MT, Ross KM, Andes DR. Interface of Candida albicans biofilm matrix-associated drug resistance and cell wall integrity regulation. Eukaryot Cell. 2011;10:1660–69. doi: 10.1128/EC.05126-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ni L, Bruce C, Hart C, Leigh-Bell J, Gelperin D, et al. Dynamic and complex transcription factor binding during an inducible response in yeast. Genes Dev. 2009;23:1351–63. doi: 10.1101/gad.1781909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nobile CJ, Andes DR, Nett JE, Smith FJ, Yue F, et al. Critical role of Bcr1-dependent adhesins in C. albicans biofilm formation in vitro and in vivo. PLOS Pathog. 2006;2:e63. doi: 10.1371/journal.ppat.0020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nobile CJ, Fox EP, Hartooni N, Mitchell KF, Hnisz D, et al. A histone deacetylase complex mediates biofilm dispersal and drug resistance in Candida albicans. mBio. 2014;5:e01201–14. doi: 10.1128/mBio.01201-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nobile CJ, Fox EP, Nett JE, Sorrells TR, Mitrovich QM, et al. A recently evolved transcriptional network controls biofilm development in Candida albicans. Cell. 2012;148:126–38. doi: 10.1016/j.cell.2011.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nobile CJ, Mitchell AP. Regulation of cell-surface genes and biofilm formation by the C. albicans transcription factor Bcr1p. Curr Biol. 2005;15:1150–55. doi: 10.1016/j.cub.2005.05.047. [DOI] [PubMed] [Google Scholar]
- 142.Nobile CJ, Mitchell AP. Genetics and genomics of Candida albicans biofilm formation. Cell Microbiol. 2006;8:1382–91. doi: 10.1111/j.1462-5822.2006.00761.x. [DOI] [PubMed] [Google Scholar]
- 143.Nobile CJ, Nett JE, Andes DR, Mitchell AP. Function of Candida albicans adhesin Hwp1 in biofilm formation. Eukaryot Cell. 2006;5:1604–10. doi: 10.1128/EC.00194-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nobile CJ, Nett JE, Hernday AD, Homann OR, Deneault JS, et al. Biofilm matrix regulation by Candida albicans Zap1. PLOS Biol. 2009;7:e1000133. doi: 10.1371/journal.pbio.1000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nobile CJ, Schneider HA, Nett JE, Sheppard DC, Filler SG, et al. Complementary adhesin function in C. albicans biofilm formation. Curr Biol. 2008;18:1017–24. doi: 10.1016/j.cub.2008.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Norice CT, Smith FJ, Jr, Solis N, Filler SG, Mitchell AP. Requirement for Candida albicans Sun41 in biofilm formation and virulence. Eukaryot Cell. 2007;6:2046–55. doi: 10.1128/EC.00314-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Palanisamy SK, Ramirez MA, Lorenz M, Lee SA. Candida albicans PEP12 is required for biofilm integrity and in vivo virulence. Eukaryot Cell. 2010;9:266–77. doi: 10.1128/EC.00295-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pappas PG, Rex JH, Sobel JD, Filler SG, Dismukes WE, et al. Guidelines for treatment of candidiasis. Clin Infect Dis. 2004;38:161–89. doi: 10.1086/380796. [DOI] [PubMed] [Google Scholar]
- 149.Peltroche-Llacsahuanga H, Goyard S, d’Enfert C, Prill SK, Ernst JF. Protein O-mannosyltransferase isoforms regulate biofilm formation in Candida albicans. Antimicrob Agents Chemother. 2006;50:3488–91. doi: 10.1128/AAC.00606-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Perez A, Pedros B, Murgui A, Casanova M, Lopez-Ribot JL, Martinez JP. Biofilm formation by Candida albicans mutants for genes coding fungal proteins exhibiting the eight-cysteine-containing CFEM domain. FEMS Yeast Res. 2006;6:1074–84. doi: 10.1111/j.1567-1364.2006.00131.x. [DOI] [PubMed] [Google Scholar]
- 151.Perlroth J, Choi B, Spellberg B. Nosocomial fungal infections: epidemiology, diagnosis, and treatment. Med Mycol. 2007;45:321–46. doi: 10.1080/13693780701218689. [DOI] [PubMed] [Google Scholar]
- 152.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20:133–63. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pietrella D, Enjalbert B, Zeidler U, Znaidi S, Rachini A, et al. A luciferase reporter for gene expression studies and dynamic imaging of superficial Candida albicans infections. Methods Mol Biol. 2012;845:537–46. doi: 10.1007/978-1-61779-539-8_39. [DOI] [PubMed] [Google Scholar]
- 154.Pitangui NS, Sardi JC, Silva JF, Benaducci T, Moraes da Silva RA, et al. Adhesion of Histoplasma capsulatum to pneumocytes and biofilm formation on an abiotic surface. Biofouling. 2012;28:711–18. doi: 10.1080/08927014.2012.703659. [DOI] [PubMed] [Google Scholar]
- 155.Puri S, Kumar R, Chadha S, Tati S, Conti HR, et al. Secreted aspartic protease cleavage of Candida albicans Msb2 activates Cek1 MAPK signaling affecting biofilm formation and oropharyngeal candidiasis. PLOS ONE. 2012;7:e46020. doi: 10.1371/journal.pone.0046020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ramage G, Bachmann S, Patterson TF, Wickes BL, Lopez-Ribot JL. Investigation of multidrug efflux pumps in relation to fluconazole resistance in Candida albicans biofilms. J Antimicrob Chemother. 2002;49:973–80. doi: 10.1093/jac/dkf049. [DOI] [PubMed] [Google Scholar]
- 157.Ramage G, Martinez JP, Lopez-Ribot JL. Candida biofilms on implanted biomaterials: a clinically significant problem. FEMS Yeast Res. 2006;6:979–86. doi: 10.1111/j.1567-1364.2006.00117.x. [DOI] [PubMed] [Google Scholar]
- 158.Ramage G, Mowat E, Jones B, Williams C, Lopez-Ribot J. Our current understanding of fungal biofilms. Crit Rev Microbiol. 2009;35:340–55. doi: 10.3109/10408410903241436. [DOI] [PubMed] [Google Scholar]
- 159.Ramage G, Rajendran R, Gutierrez-Correa M, Jones B, Williams C. Aspergillus biofilms: clinical and industrial significance. FEMS Microbiol Lett. 2011;324:89–97. doi: 10.1111/j.1574-6968.2011.02381.x. [DOI] [PubMed] [Google Scholar]
- 160.Ramage G, Rajendran R, Sherry L, Williams C. Fungal biofilm resistance. Int J Microbiol. 2012;2012:528521. doi: 10.1155/2012/528521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ramage G, Saville SP, Thomas DP, Lopez-Ribot JL. Candida biofilms: an update. Eukaryot Cell. 2005;4:633–38. doi: 10.1128/EC.4.4.633-638.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ramage G, Tomsett K, Wickes BL, Lopez-Ribot JL, Redding SW. Denture stomatitis: a role for Candida biofilms. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:53–59. doi: 10.1016/j.tripleo.2003.04.002. [DOI] [PubMed] [Google Scholar]
- 163.Ramage G, VandeWalle K, Lopez-Ribot JL, Wickes BL. The filamentation pathway controlled by the Efg1 regulator protein is required for normal biofilm formation and development in Candida albicans. FEMS Microbiol Lett. 2002;214:95–100. doi: 10.1111/j.1574-6968.2002.tb11330.x. [DOI] [PubMed] [Google Scholar]
- 164.Reynolds TB, Fink GR. Bakers’ yeast, a model for fungal biofilm formation. Science. 2001;291:878–81. doi: 10.1126/science.291.5505.878. [DOI] [PubMed] [Google Scholar]
- 165.Richard ML, Nobile CJ, Bruno VM, Mitchell AP. Candida albicans biofilm-defective mutants. Eukaryot Cell. 2005;4:1493–502. doi: 10.1128/EC.4.8.1493-1502.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ricicova M, Kucharikova S, Tournu H, Hendrix J, Bujdakova H, et al. Candida albicans biofilm formation in a new in vivo rat model. Microbiology. 2010;156:909–19. doi: 10.1099/mic.0.033530-0. [DOI] [PubMed] [Google Scholar]
- 167.Robbins N, Uppuluri P, Nett J, Rajendran R, Ramage G, et al. Hsp90 governs dispersion and drug resistance of fungal biofilms. PLOS Pathog. 2011;7:e1002257. doi: 10.1371/journal.ppat.1002257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rouabhia M, Semlali A, Chandra J, Mukherjee P, Chmielewski W, Ghannoum MA. Disruption of the ECM33 gene in Candida albicans prevents biofilm formation, engineered human oral mucosa tissue damage and gingival cell necrosis/apoptosis. Mediat Inflamm. 2012;2012:398207. doi: 10.1155/2012/398207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ryan O, Shapiro RS, Kurat CF, Mayhew D, Baryshnikova A, et al. Global gene deletion analysis exploring yeast filamentous growth. Science. 2012;337:1353–56. doi: 10.1126/science.1224339. [DOI] [PubMed] [Google Scholar]
- 170.Sahni N, Yi S, Daniels KJ, Srikantha T, Pujol C, Soll DR. Genes selectively up-regulated by pheromone in white cells are involved in biofilm formation in Candida albicans. PLOS Pathog. 2009;5:e1000601. doi: 10.1371/journal.ppat.1000601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sandini S, Stringaro A, Arancia S, Colone M, Mondello F, et al. The MP65 gene is required for cell wall integrity, adherence to epithelial cells and biofilm formation in Candida albicans. BMC Microbiol. 2011;11:106. doi: 10.1186/1471-2180-11-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sardi JC, Scorzoni L, Bernardi T, Fusco-Almeida AM, Mendes Giannini MJ. Candida species: current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J Med Microbiol. 2013;62:10–24. doi: 10.1099/jmm.0.045054-0. [DOI] [PubMed] [Google Scholar]
- 173.Schinabeck MK, Long LA, Hossain MA, Chandra J, Mukherjee PK, et al. Rabbit model of Candida albicans biofilm infection: liposomal amphotericin B antifungal lock therapy. Antimicrob Agents Chemother. 2004;48:1727–32. doi: 10.1128/AAC.48.5.1727-1732.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Schweizer A, Rupp S, Taylor BN, Rollinghoff M, Schroppel K. The TEA/ATTS transcription factor CaTec1p regulates hyphal development and virulence in Candida albicans. Mol Microbiol. 2000;38:435–45. doi: 10.1046/j.1365-2958.2000.02132.x. [DOI] [PubMed] [Google Scholar]
- 175.Seddiki SM, Boucherit-Otmani Z, Boucherit K, Badsi-Amir S, Taleb M, Kunkel D. Assessment of the types of catheter infectivity caused by Candida species and their biofilm formation: first study in an intensive care unit in Algeria. Int J Gen Med. 2013;6:1–7. doi: 10.2147/IJGM.S38065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Seidler MJ, Salvenmoser S, Muller FM. Aspergillus fumigatus forms biofilms with reduced anti-fungal drug susceptibility on bronchial epithelial cells. Antimicrob Agents Chemother. 2008;52:4130–36. doi: 10.1128/AAC.00234-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sellam A, Al-Niemi T, McInnerney K, Brumfield S, Nantel A, Suci PA. A Candida albicans early stage biofilm detachment event in rich medium. BMC Microbiol. 2009;9:25. doi: 10.1186/1471-2180-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sellam A, Askew C, Epp E, Tebbji F, Mullick A, et al. Role of transcription factor CaNdt80p in cell separation, hyphal growth, and virulence in Candida albicans. Eukaryot Cell. 2010;9:634–44. doi: 10.1128/EC.00325-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sen M, Shah B, Rakshit S, Singh V, Padmanabhan B, et al. UDP-glucose 4, 6-dehydratase activity plays an important role in maintaining cell wall integrity and virulence of Candida albicans. PLOS Pathog. 2011;7:e1002384. doi: 10.1371/journal.ppat.1002384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Shah AH, Singh A, Dhamgaye S, Chauhan N, Vandeputte P, et al. Novel role of a family of major facilitator transporters in biofilm development and virulence of Candida albicans. Biochem J. 2014;460:223–35. doi: 10.1042/BJ20140010. [DOI] [PubMed] [Google Scholar]
- 181.Shapiro RS, Uppuluri P, Zaas AK, Collins C, Senn H, et al. Hsp90 orchestrates temperature-dependent Candida albicans morphogenesis via Ras1-PKA signaling. Curr Biol. 2009;19:621–29. doi: 10.1016/j.cub.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Singh V, Satheesh SV, Raghavendra ML, Sadhale PP. The key enzyme in galactose metabolism, UDP-galactose-4-epimerase, affects cell-wall integrity and morphology in Candida albicans even in the absence of galactose. Fungal Genet Biol. 2007;44:563–74. doi: 10.1016/j.fgb.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 183.Srikantha T, Daniels KJ, Pujol C, Kim E, Soll DR. Identification of genes upregulated by the transcription factor Bcr1 that are involved in impermeability, impenetrability, and drug resistance of Candida albicans a/α biofilms. Eukaryot Cell. 2013;12:875–88. doi: 10.1128/EC.00071-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Srikantha T, Daniels KJ, Pujol C, Sahni N, Yi S, Soll DR. Nonsex genes in the mating type locus of Candida albicans play roles in a/αbiofilm formation, including impermeability and fluconazole resistance. PLOS Pathog. 2012;8:e1002476. doi: 10.1371/journal.ppat.1002476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Srinivasan A, Gupta CM, Agrawal CM, Leung KP, Lopez-Ribot JL, Ramasubramanian AK. Drug susceptibility of matrix-encapsulated Candida albicans nano-biofilms. Biotechnol Bioeng. 2014;111:418–24. doi: 10.1002/bit.25120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Strijbis K, van Roermund CW, Visser WF, Mol EC, van den Burg J, et al. Carnitine-dependent transport of acetyl coenzyme A in Candida albicans is essential for growth on nonfermentable carbon sources and contributes to biofilm formation. Eukaryot Cell. 2008;7:610–18. doi: 10.1128/EC.00017-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sullivan DJ, Westerneng TJ, Haynes KA, Bennett DE, Coleman DC. Candida dubliniensis sp nov.: phenotypic and molecular characterization of a novel species associated with oral candidosis in HIV-infected individuals. Microbiology. 1995;141(Part 7):1507–21. doi: 10.1099/13500872-141-7-1507. [DOI] [PubMed] [Google Scholar]
- 188.Sun X, Lu H, Jiang Y, Cao Y. CaIPF19998 reduces drug susceptibility by enhancing the ability of biofilm formation and regulating redox homeostasis in Candida albicans. Curr Microbiol. 2013;67:322–26. doi: 10.1007/s00284-013-0366-x. [DOI] [PubMed] [Google Scholar]
- 189.Taff HT, Nett JE, Zarnowski R, Ross KM, Sanchez H, et al. A Candida biofilm-induced pathway for matrix glucan delivery: implications for drug resistance. PLOS Pathog. 2012;8:e1002848. doi: 10.1371/journal.ppat.1002848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Tan X, Fuchs BB, Wang Y, Chen W, Yuen GJ, et al. The role of Candida albicans SPT20 in filamentation, biofilm formation and pathogenesis. PLOS ONE. 2014;9:e94468. doi: 10.1371/journal.pone.0094468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Thomas DP, Bachmann SP, Lopez-Ribot JL. Proteomics for the analysis of the Candida albicans biofilm lifestyle. Proteomics. 2006;6:5795–804. doi: 10.1002/pmic.200600332. [DOI] [PubMed] [Google Scholar]
- 192.Thomas DP, Lopez-Ribot JL, Lee SA. A proteomic analysis of secretory proteins of a pre-vacuolar mutant of Candida albicans. J Proteomics. 2009;73:342–51. doi: 10.1016/j.jprot.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Tsai PW, Chen YT, Yang CY, Chen HF, Tan TS, et al. The role of Mss11 in Candida albicans biofilm formation. Mol Genet Genomics. 2014;289:807–19. doi: 10.1007/s00438-014-0846-0. [DOI] [PubMed] [Google Scholar]
- 194.Uppuluri P, Chaturvedi AK, Srinivasan A, Banerjee M, Ramasubramaniam AK, et al. Dispersion as an important step in the Candida albicans biofilm developmental cycle. PLOS Pathog. 2010;6:e1000828. doi: 10.1371/journal.ppat.1000828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Uppuluri P, Pierce CG, Thomas DP, Bubeck SS, Saville SP, Lopez-Ribot JL. The transcriptional regulator Nrg1p controls Candida albicans biofilm formation and dispersion. Eukaryot Cell. 2010;9:1531–37. doi: 10.1128/EC.00111-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Uwamahoro N, Qu Y, Jelicic B, Lo TL, Beaurepaire C, et al. The functions of Mediator in Candida albicans support a role in shaping species-specific gene expression. PLOS Genet. 2012;8:e1002613. doi: 10.1371/journal.pgen.1002613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Vande Velde G, Kucharikova S, Schrevens S, Himmelreich U, Van Dijck P. Towards non-invasive monitoring of pathogen-host interactions during Candida albicans biofilm formation using in vivo bioluminescence. Cell Microbiol. 2014;16:115–30. doi: 10.1111/cmi.12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Vande Velde G, Kucharikova S, Van Dijck P, Himmelreich U. Bioluminescence imaging of fungal biofilm development in live animals. Methods Mol Biol. 2014;1098:153–67. doi: 10.1007/978-1-62703-718-1_13. [DOI] [PubMed] [Google Scholar]
- 199.Wang X, Fries BC. A murine model for catheter-associated candiduria. J Med Microbiol. 2011;60:1523–29. doi: 10.1099/jmm.0.026294-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Watanabe NA, Miyazaki M, Horii T, Sagane K, Tsukahara K, Hata K. E1210, a new broad-spectrum antifungal, suppresses Candida albicans hyphal growth through inhibition of glycosylphosphatidylinositol biosynthesis. Antimicrob Agents Chemother. 2012;56:960–71. doi: 10.1128/AAC.00731-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Weig M, Gross U, Muhlschlegel F. Clinical aspects and pathogenesis of Candida infection. Trends Microbiol. 1998;6:468–70. doi: 10.1016/s0966-842x(98)01407-3. [DOI] [PubMed] [Google Scholar]
- 202.Wenzel RP. Nosocomial candidemia: risk factors and attributable mortality. Clin Infect Dis. 1995;20:1531–34. doi: 10.1093/clinids/20.6.1531. [DOI] [PubMed] [Google Scholar]
- 203.Wilson NK, Foster SD, Wang X, Knezevic K, Schutte J, et al. Combinatorial transcriptional control in blood stem/progenitor cells: genome-wide analysis of ten major transcriptional regulators. Cell Stem Cell. 2010;7:532–44. doi: 10.1016/j.stem.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 204.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–17. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 205.Yeater KM, Chandra J, Cheng G, Mukherjee PK, Zhao X, et al. Temporal analysis of Candida albicans gene expression during biofilm development. Microbiology. 2007;153:2373–85. doi: 10.1099/mic.0.2007/006163-0. [DOI] [PubMed] [Google Scholar]
- 206.Young RA. Control of the embryonic stem cell state. Cell. 2011;144:940–54. doi: 10.1016/j.cell.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Yu Q, Wang F, Zhao Q, Chen J, Zhang B, et al. A novel role of the vacuolar calcium channel Yvc1 in stress response, morphogenesis and pathogenicity of Candida albicans. Int J Med Microbiol. 2014;304:339–50. doi: 10.1016/j.ijmm.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 208.Yu Q, Wang H, Xu N, Cheng X, Wang Y, et al. Spf1 strongly influences calcium homeostasis, hyphal development, biofilm formation and virulence in Candida albicans. Microbiology. 2012;158:2272–82. doi: 10.1099/mic.0.057232-0. [DOI] [PubMed] [Google Scholar]
- 209.Zarnowski R, Westler WM, Lacmbouh GA, Marita JM, Bothe JR, et al. Novel entries in a fungal biofilm matrix encyclopedia. mBio. 2014;5:e01333–14. doi: 10.1128/mBio.01333-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zeidler U, Bougnoux ME, Lupan A, Helynck O, Doyen A, et al. Synergy of the antibiotic colistin with echinocandin antifungals in Candida species. J Antimicrob Chemother. 2013;68:1285–96. doi: 10.1093/jac/dks538. [DOI] [PubMed] [Google Scholar]
- 211.Zhang X, Sun X, Wang Z, Zhang Y, Hou W. Keratitis-associated fungi form biofilms with reduced antifungal drug susceptibility. Invest Ophthalmol Visual Sci. 2012;53:7774–78. doi: 10.1167/iovs.12-10810. [DOI] [PubMed] [Google Scholar]
- 212.Zhao X, Daniels KJ, Oh SH, Green CB, Yeater KM, et al. Candida albicans Als3p is required for wild-type biofilm formation on silicone elastomer surfaces. Microbiology. 2006;152:2287–99. doi: 10.1099/mic.0.28959-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zhao X, Oh SH, Yeater KM, Hoyer LL. Analysis of the Candida albicans Als2p and Als4p adhesins suggests the potential for compensatory function within the Als family. Microbiology. 2005;151:1619–30. doi: 10.1099/mic.0.27763-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


