Abstract
Marine sponges have been considered as a drug treasure house with respect to great potential regarding their secondary metabolites. Most of the studies have been conducted on sponge’s derived compounds to examine its pharmacological properties. Such compounds proved to have antibacterial, antiviral, antifungal, antimalarial, antitumor, immunosuppressive, and cardiovascular activity. Although, the mode of action of many compounds by which they interfere with human pathogenesis have not been clear till now, in this review not only the capability of the medicinal substances have been examined in vitro and in vivo against serious pathogenic microbes but, the mode of actions of medicinal compounds were explained with diagrammatic illustrations. This knowledge is one of the basic components to be known especially for transforming medicinal molecules to medicines. Sponges produce a different kind of chemical substances with numerous carbon skeletons, which have been found to be the main component interfering with human pathogenesis at different sites. The fact that different diseases have the capability to fight at different sites inside the body can increase the chances to produce targeted medicines.
Keywords: Sponges, Pharmacokinetics, Antitumor, Antiviral, Pathogenesis, Microbes
INTRODUCTION
Sponges are spineless animals belong to phylum, “the pore bearers” (Porifera), serve as most primitive multicelled animals, existing for millions of year ago. Marine sponges are soft bodied, sessile and filter feeders assembling small particles of food from sea water rising through their bodies (Hadas et al., 2009; Ramel, 2010). All over the world, marine sponges are the member of benthic communities of a marine environment, including its biomass as well as its ability to promote pelagic and benthic processes (Maldonado et al., 2005), also provide habitat for other organisms (Hultgren and Duffy, 2010). Marine life is a massive source for the synthesis of novel molecules and it need to be studied. According to evolutionary history, marine microorganisms are more diversified than terrestrial microorganisms. Marine sponges frequently produce bioactive compounds as compared to other living microorganisms. Because sponges cannot move and lack physical defenses, they are highly susceptible to marine predators such as fish, turtles, and invertebrates. Thus, it is not surprising that sponges have developed a wide suite of defensive chemicals to deter predators (Thomas et al., 2010). They also use their defensive chemicals to keep the offspring of small plants and animals (fouling organisms) from settling onto their outer surfaces (Mol et al., 2009; Hertiani et al., 2010). These sessile animals are a prolific source of a huge diversity of secondary metabolites that has been discovered over the past 50 years (Faulkner, 2002; Blunt et al., 2005; Laport et al., 2009; Hertiani et al., 2010; Proksch et al., 2010). The bioactive compounds are very diverse in both structure and bioactivity. The known species of sponges are more than 8000 (Van soest et al., 2014) widely distributed in sea and freshwater environment (Hooper and van Soest, 2002).
In the early 1950s, pharmaceutical interest among sponges have been started and it has started by the investigation of the nucleosides spongouridine and spongothymidine in the marine sponge i.e. Cryptotethya crypta (Bergmann and Feeney, 1950; 1951). These nucleosides were the basic root for the synthesis of ara-A, an antiviral drug and ara-C, the first marine-derived anticancer agent (Proksch et al., 2002). Currently, ara-C used in the treatment of lymphoma and leukemia, a part of this one of its fluorinated derivative also permitted for the treatment of lung, pancreatic (Momparler, 2013), breast and bladder cancer (Schwartsmann, 2000). On the other hand, it also been revealed that lower invertebrates have more lipid components such as sterols, fatty acids and other unsaponifiable elements as compared to vertebrate animals (Bergmann and Swift, 1951; Piel, 2004). Up till now approximately 20,000 bioactive compounds have been found in marine organisms (Hu et al., 2011). However, most of these biologically active compounds, which are predominantly terpenoids and alkaloids, have been isolated from sponges (Leal et al., 2012). Regarding the diversity of marine compounds, sponges are the most important producer. Every year around 5300 different natural products and new compounds have been isolated from marine sponges (Faulkner, 2000; 2001; 2002). Sponges are most abundantly produce novel compounds, including more than 200 novel metabolites, every year (Blunt et al., 2006; Turk et al., 2013). About 300 novel compounds were reported in 2011 from the phylum Porifera (Blunt et al., 2013). Moreover, some of the sponge-derived substances are however in a process of a clinical and pre-clinical trial (e.g., as anti-inflammatory or anticancer agents) in comparison of those substances that derived from different marine phylum (Blunt et al., 2005: Martins et al., 2014).
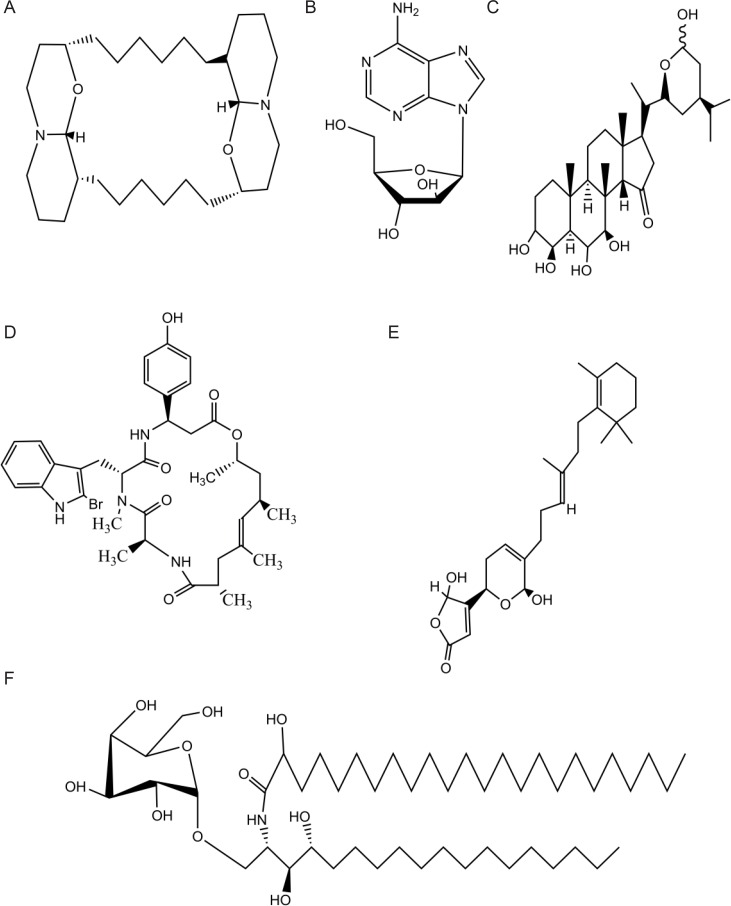
Sponge-derived or other marine microorganism’s associated bioactive substances have possessed antibacterial, antiviral, antifungal, antimalarial, anthelminthic, immunosuppressive, muscle relaxants and anti-inflammatory activities. Sponge substances have remarkable chemical diversity. A part of uncommon nucleosides, marine sponges also able to produce other classes of amino acid derivatives including cyclic peptides, alkaloids, sterols, terpenes, fatty acids, peroxides, etc. (Fig. 1) (Donia and Hamann, 2003; Blunt et al., 2005, 2006; Sipkema et al., 2005; Piel, 2006). Although few representatives from sponges are approved as drugs, hundreds of new compounds with interesting pharmacological activities are discovered from sponges every year. Several sponge-derived compounds are already in clinical trials as agents against cancer, microbial infections, inflammation and other diseases. However, in many cases drug development is severely hampered by the limited supply of the respective compounds, as they are often present only in minute amounts in the sponge tissue. These reasons have moved the pharmaceutical drug discovery programs away from natural products in favor of synthetic approaches. However, the abundance of synthetic compounds with similar chemical functional groups and, therefore, limited chemical diversity has renewed interest in nature as a good resource for finding new fascinating leads to be applied to design the next generation of drugs.
Fig. 1.
The chemical structure of sponge derived molecules. (A) Xestospongin C (Xestospongia sp./macrocyclic bis-oxaquinolizidine. (B) Ara-A (Cryptotethia crypta/unusal nucleoside). (C) Contignasterol (Petrosia contignata/oxygenated sterol). (D) Jaspamide (Hemiastrella minor/macrocyclic lactam/lactone). (E) Manolide (Sesterterpenoids/Luffariella variabilis sp). (F) Agelasphin (Agelas mauritianus/agalactosy-ceramide).
In most cases development and production of sponge-derived drugs is hindered by environmental concerns and technical problems associated with harvesting large amounts of sponges. The presence of possibly producing microbial symbionts is therefore especially intriguing, as a sustainable source of sponge-derived drug candidates could be generated by establishing a symbiont culture or by transferring its biosynthetic genes into culturable bacteria. For example, Manzamine alkaloids, the promising leads for extended preclinical assessment against malaria, tuberculosis and HIV, have been previously isolated from sponge Acanthostrongylophora sp. and have also been isolated from the associated microorganism Micromonospora sp. (Hill et al., 2005). A dinoflagellate Prorocentrum lima produces okadaic acid (Morton et al., 1998), first isolated from the host sponge Halichondria okadai (Kobayashi and Ishibashi, 1993). A Vibrio sp. produces peptide, andrimid and brominated biphenyl ethers (Maria et al., 2011) that was purified from the sponge Hyatella sp. extract (Oclarit et al., 1994) and sponge Dysidea sp. (Elyakov et al., 1991).Thus, the microbial association that occurs on or in sponges could be of great interest as a solution of the supply problem of most of pharmaceutical compounds produced by sponges.
Therefore, the main focus of this review is to highlight the survey of discoveries of products derived from marine sponges which displayed in vivo potency or effective in vitro activity against infectious and parasitic diseases, including protozoal, bacterial, fungal and viral infections and their mode of action by which they interpose with the pathogenesis of human diseases. The knowledge of mechanisms of actions is very necessary for the development of the drug from a bioactive compound. For example, many secondary metabolites inhibit the growth of cancer cell lines or show the highest degree of antibiosis activity, but they do not prove that they are fit as anti-cancer or anti-microbial agents because they may exhibit severe adverse effects. Our objective was to highlight the compounds by disease type, their mode of action and the greatest potential to drive towards clinically useful treatments.
ANTIBACTERIAL ACTIVITY
At the beginning of the twenty century, the first antibiotics detection left the scientific and social society untrained, when the antibiotic-resistant bacteria emerged. This antibiotic-resistance bacterium has multiplied very swiftly and creates a considerable problem while both Staphylococcus aureus and some pathogenic bacteria are involved in causing the infections. According to Davies and Davies (Rice, 2006), lately vancomycin became ineffective to cure the infections caused by methicillin-resistant S. aureus (MRSA). The importance of drug-resistant bacterial infection has produced an imperative requirement for the quick and sustained development of new antibiotics classes, which may keep pace with the varying face of bacterial antibiotic vulnerability. Therefore, the first precedence of a biochemical research community is the innovation and improvement of new antibiotics.
The marine sponges crude extracts exhibited a low level of anti-bacterial activity against marine bacteria while a high level of antibacterial activity was exhibited against terrestrial bacteria (Amade et al., 1982; 1987; McCaffrey and Endeau, 1985; Uriz et al., 1992; Xue et al., 2004). The antimicrobial screenings of crude extracts from 101 Arctic sponges against bacteria associated with opportunistic infections showed that about 10% of the sponges yielded significant antimicrobial activities, with IC50 values from 0.2 to 5 μg/mL (Turk et al., 2013). In every year, some new molecules containing antibiotic properties are introduced, but in marine sponges, their ubiquity is on the top.
After an early screening test by Burkholder and Xue (Burkholder and Ruetzler, 1969; Xue et al., 2004), it was noted that 18 sponges out of 31 exhibited antibacterial effect while some of them were very strong against Gram-positive and Gram-negative bacteria. It was observed that marine sponges screening test for an antibacterial activity directed to both the isolation and characterization of a wide range of active substances, containing some promising therapeutic (Mayer and Hamann, 2004; Moura et al., 2006; Mayer et al., 2011). Marine sponges produced up to 800 antibiotic substances (Torres et al., 2002), while some other antibacterial agents have also been identified from sponges by marine natural products community. However, no antibacterial product was reported yet in the discovered marine natural product but many of them are under investigation in current research. More or less isolated marine sponges substances with antibacterial activity are shown in (Table 1). Manoalide, one of the first sesterterpenoids to be isolated from a marine sponge Luffariella variabilis, was found to be an antibiotic (Fig. 1) (de Silva and Scheuer, 1980). This is the only example of antibiotic sesterterpenoid discovered so far.
Table 1.
Examples of antibacterial compounds
| Substance | Chemistry | Species | Activity Spectrum | MIC Value | References |
|---|---|---|---|---|---|
| Discodermins B, C and D | Cyclic peptide | Discodermia kiiensis/Lithistida | Antibacterial (B. subtilis) | 3 μg/ml | Matsunaga et al., 1985 |
| Arenosclerins A–C | Alkyl pepridine alkaloid | Arenosclera brasiliensis | S. aureus, P. aeruginosa, M. tuberculosis | 16 μg/ml*, 30 μg/ml** | Torres et al., 2002 |
| Haliclona cyclamine E | Alkylpiperidine alkaloids | Arenosclera brasiliensis | S. aureus, P. aeruginosa | 8 μg/ml* | Torres et al., 2002 |
| CvL | Lectine | Cliona varians | S. aureus, B. subtilis | 16 μg/ml* | Moura et al., 2006 |
| Axinellamines B–D | Imidazo-azolo-imidazole alkaloid | Axinella sp./Halichondrida | H. Pylori, M. Luteus | 16.7 μg/ml*** | Urban et al., 1999 |
| Caminosides A–D | Glycolipids | Caminus sphaeroconia | E. coli, S. aureus | 16 μg/ml* | Linington et al., 2006 |
| 6-hydroxymanzamine E | Alkaloid | Acanthostrongylophora sp. | M. tuberculosis | 0.9 μg/ml** | Rao et al., 2004 |
| Cribrostatin 3 | Alkaloid | Cribrochalina sp. | N.gonorrheae (antibiotic resistant strain) | - | Petit and Knight, 2002 |
| Cribrostatin 6 | Alkaloid | Cribrochalina sp. | S. pneumoniae (anitibiotic resistant strain) | ≤2 | Pettit et al., 2004 |
| Isojaspic acid, cacospongin D and jaspaquinol | Meroditerpenes | Cacospongia sp. | S. epidermidis | 20 μg/ml | Rubio et al., 2007 |
| Isoaaptamine | Alkaloid | Aaaptos aaptos | S. aureus | 3.7 μg/ml | Jang et al., 2007 |
| (−)-Microcionin-1 | Terpenoid | Fasciospongia sp | M. Luteus | 6 μg/ml | Gaspar et al., 2008 |
S. aureus
M. tuberculosis,
M. luetus
ANTIVIRAL ACTIVITY
The officially approved antiviral drug armamentarium for clinical use contains approximately 40 substances and most of them were discovered recently. It was reported that half of the recently discovered substances are used for the human immunodeficiency virus (HIV) infection treatment (De Clercq, 2004; Yasuhara-Bell and Lu, 2010). The significance of new antiviral agents development help to increase the number of available drugs becomes clear. It was observed that the adenovirus serotype 5 (AdV-5) is much constant in the environment for long time, and connected to respiratory infections with no special cure (Wiedbrauk and Johnston, 1992; Sipkema et al., 2005). There are some viruses such as rotaviruses, which are mainly responsible for sever gastroenteritis in human and animals. The treatment of diarrhea is only possible by symptomatic, which may cause the infection of children and immune compromised patients even it can lead to death (White and Fenner, 1986; Grimwood and Lambert, 2009).
Some new approaches being use to introduce new antiviral agents from marine sources and many promising therapeutic leads because sponges are one of the rich source of antiviral property compounds (Table 2). Maximum quantities of HIV-inhibiting compounds were introduced, while they do not reflect greater potential of sponges to fight against AIDS compared with other viral diseases. Researchers use screening techniques for anti-HIV activity has led to introducing of different compounds, although the system of inhibition is still not clear. It has been reported recently by many researchers that HIV-inhibiting compounds were produced by different sponges (Ford et al., 1999; Qureshi and Faulkner, 1999; Yasuhara-Bell and Lu, 2010; Sagar et al., 2010). For instance, avarol is a compound which inhibits the progression of HIV infection up to some extent. The data form in vitro experiment and animal show that avarol combines have very useful properties and increase humoral immune response (Muller et al., 1987; Amigó et al., 2007). HIV inhibits completely by avarol and blocking the production of natural UAG suppressor glutamine transfer tRNA. After viral infection, the production of tRNA is up-regulated, which is necessary for the viral protease and viral proliferation synthesis. The low Concentration of avarol 0.3 and 0.9 μM resulted in 50 and 80% of inhibition of virus released from infected cells (Muller et al., 1987). Moreover, the derivatives of avarol such as 6′-hydroxy avarol and 3′-hydroxy avarone were noted as very strong inhibitors of HIV reverse transcriptase (Fig. 2). Avarol play very important role during the early stages of HIV infection and it also has a specific target for antiviral drugs, while it convert the viral genomic RNA into pro-viral double-stranded DNA, and later on it integrated into the host chromosomal DNA (Loya and Hizi, 1990).
Table 2.
Examples of antiviral compounds
| Substances | Chemistry | Species | Action spectrum | References |
|---|---|---|---|---|
| 4-Methylaaptamine | Alkaloid | Aaptos aaptos | Anti-viral (HSV-1) | Souza et al., 2007 |
| Papuamides A–D | Cyclic depsipeptides | Theonella sp. | Anti-viral (HIV-1) | Ford et al., 1999 |
| Ara-A | Nucleoside | Cryptotethya crypta | HSV-1, HSV-2, VZV | Faulkner, 2002 |
| Avarol | Sesquiterpene hydroquinone | Dysidea avara | HIV-1,UAG suppressor Glutamine tRNA inhibitor | Muller et al., 1987 |
| Haplosamates A and B | Sulfamated steroid | Xestospongia sp./Haplosclerida | Anti-viral (HIV-1Integrase inhibitor) | Qureshi and Faulkner, 1999 |
| Dragmacidin F | Alkaloid | Halicortex sp. | HIV-1 | Cutignano et al., 2000 |
| Hamigeran B | Phenolic Macrolide | Hamigera tarangaensis | Anti-viral (herpes and polio virus) | Wellington et al., 2000 |
| Mycalamide A–B | Nucleosides | Mycale sp. | A59 coronavirus, (HSV-1) | Perry et al., 1990 |
| Mirabamides A, C and D | Peptide | Siliquariaspongia mirabilis | Antiviral (HIV-1) | Plaza et al., 2007 |
| Oroidin | Alkaloid | Stylissa carteri | Antiviral (HIV-1) | O’Rourke et al., 2016 |
Fig. 2.
Molecular structures of avarol (a: R1 = H) and 6¢- hydroxy avarol (A: R1 = OH) and avarone (B: R1 = H) and 3¢- hydroxy avarone (B: R1 = OH).
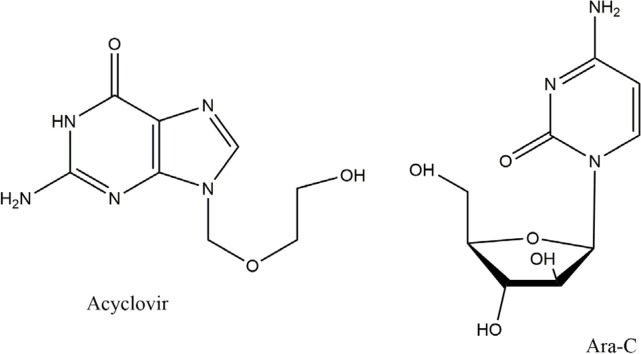
Another important antiviral discovery from marine source reported is the nucleoside ara A (vidarabine) which was isolated from Cryptotethya crypta sponge and was first synthesized in 1960 (Walter, 2005). Ara-A is an arabinosyl nucleosides which inhibits viral DNA synthesis (Bergmann and Swift, 1951; Blunt et al., 2006; Sagar et al., 2010). Research proved that our biological systems can recognize nucleoside base just after sugar moiety modifications, then chemists started to replace the pentoses by acyclic entities or with sugar molecules, it lead to the development of azidothymidine (zidovudine) drug. An examples of semisynthetic arabinosyl nucleosides modification are Ara-A, acyclovir, ara-C (Fig. 1, 3) and azidothymidine are in clinical use (De Clercq et al., 2002; Sagar et al., 2010).
Fig. 3.
Molecular structure of Acyclovir and Ara-c (Acyclovir is a drug of choice for Herpes virus).
ANTIFUNGAL ACTIVITY
In the last decades, the fungal infection (especially invasive mycoses) dramatically increased in those individuals suffering from AIDS, immune depressants, hematological malignancies, and transplant recipients, increased the need of new antifungals (García-Ruiz et al., 2004; Pontón et al., 2000). Fungal infection remains a major direct cause of death for those patients who are treated for malignant disease (Sandven, 2000; Ellis et al., 2000). Fungal causing malignant diseases are a major cause of life threatening diseases as well as resistance to them is a major problem (García-Ruiz et al., 2004; Giusiano et al., 2004; Walsh et al., 2004; Giusiano et al., 2005). Immunocompromised patients are mainly infected by Candida, Aspergillus, Cryptococcus and other opportunistic fungi. Currently using fungicides are less diversified than antimicrobial substances and their use is restricted because of biological system toxicity (Rahden-Staron, 2002).
Jaspamide is the first example of cyclodepsipeptide 19-membered macrocyclic depsipeptide (Fig. 1) isolated from the sponges Jaspis sp has a selective in vitro antifungal activity with MIC of 25 μg/ml against C. albicans while in vivo topical activity of a 2% solution against Candida vaginal infection in mice (Zabriskie et al., 1986; Ebada et al., 2009). The other examples of important antifungals examined in vitro with MIC values have been listed (Table 3).
Table 3.
Examples of antiviral compounds
| Substances | Chemistry | Species | Action spectrum | MIC value | References |
|---|---|---|---|---|---|
| Jaspamide | Macrocyclic depsipeptide | Jaspis sp | C.albicans | 25 μg/ml* | Zabriskie et al., 1986 |
| Eurysterols A–B | Sterols | Euryspongia sp | C.albicans, Amphoterician B-resistant | 62.5 μg/ml*, 15.6 μg/ml | Boonlarppradab and Faulkner, 2007 |
| Naamine D | Imidazole alkaloid | Leucetta cf. chagosensis | C.neoformans | 6.25 μg/ml** | Dunbar et al., 2000 |
| Mirabilin B | Tricyclic guanidine alkaloid | Monanchora unguifera | C.neoformans | 7.0 μg/ml** | Hua et al., 2004 |
| Hamacanthin A | Indole alkaloid | Spongosorities sp. | C.albicans | 6.25 μg/ml* | Oh et al., 2006 |
| Macanthins A–B | Indole alkaloid | Spongosorities sp. | C.albicans, C.neoformans | 1.6 μg/ml*, 6.2 μg/ml** | Oh et al., 2006 |
| Agelasines and agelasimines | Purine derivative | Agelas sp. | C.krusei | 15.6 μg/ml | Vik et al., 2007 |
MIC: Minimum Inhibitory Concentration,
C. albicans,
C. neoformans.
ANTIMALARIAL PROPERTIRES
In sub- Saharan Africa, malaria is a predominant disease including that it is also serious public health problem in some areas of South America and Southeast Asia. Most of the malaria related deaths are caused by Plasmodium falciparum parasite (Mishra et al., 1999; Caraballo and King, 2014; WHO, 2015). Recently, most widely disseminated malarial species all over the world is Plasmodium vivax. P. vivax is the predominant specie in the Asia and America, while in Brazil this species represents around 80% of clinical issue annually (Brazilian Health Ministry, 2002). Sub-Saharan Africa carries a disproportionately high share of the global malaria burden. In 2015, the region was home to 88% of malaria cases and 90% of malaria deaths (Baird, 2013; WHO, 2015). During last decades, some of the antimicrobial compounds have been derived from sponges (Table 4, Fig. 4). Increasing resistance among Plasmodium strains created a need to discover new antimalarial compounds. Plasmodium falciparum has become resistant toward chloroquinone, pyrimethamine, and sulfadoxine (Bwijo et al., 2003). In vitro, selective antimalarial activity against Plasmodium falciparum has been recorded by different isonitrilese, isothiocyanates and terpenoid isocyanates from Cymbastela hooperi (Konig et al., 1996). Including that a number of free carboxylic acids (Diacarnus levii) after esterification were used as precursors to synthesize some new cyclic norditerpene peroxides. These epidioxy-substituted norsesterterpenes and norditerpenes endoperoxides from marine sponge Diacarnus megaspinorhabdosa showed antimalarial activity against both chloroquine-resistant P. falciparum and chloroquine-sensitive (D Ambrosio et al., 1998; Yang et al., 2014).
Table 4.
Examples of anti-malarial compounds
| Substances | Chemistry | Species | Action spectrum | IC50 value | References |
|---|---|---|---|---|---|
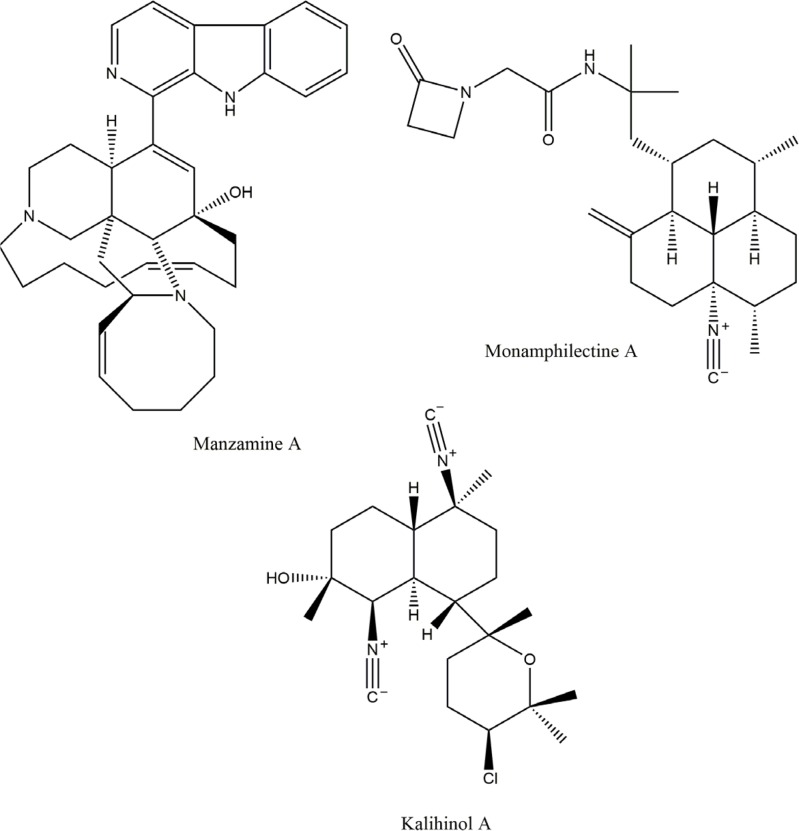
| Monamphilectine A | Antimalarial β-lactam | Hymeniacidon sp | P. falciparum | 0.6 μM*** | Avilés and Rodriguez, 2010 |
| Manzamine A | Alkaloids |
e.g., Haliclona sp./ Haplosclerida
Cymbastela hooperi/Halichondrida Diacarnus levii/Poecilosclerida |
T. gondii, P. berghei, P. falciparum | 4.5 ng/ml*** | D Ambrosio et al., 1998 |
| Kalihinol A | Isonitril-containing kalihinane diterpenoid | Acanthella sp./Halichondrida | P. falciparum | 0.0005 μg/ml** | Ang et al., 2001 |
| Diisocyanoadociane | Tetracyclic diterpene | Cymbastela hooperi | P. falciparum | 0.005 μg/ml** | Miyaoka et al., 1998 |
| Halichondramide | Macrolides | P. falciparum | 0.002 μg/ml** | Konig et al., 1996 | |
| Sigmosceptrellin-B | Norsesterterpene acid | Diacarnus erythraeanus | T. gondii, P. falciparum | 1200 ng/ml* | Konig et al., 1996 |
| (E)-Oroidin | Alkaloids | Agelas oroides | P. falciparum | 0.30 μg/ml** | Yousaf et al., 2002 |
| Plakortin and dihydroplakortin | Cycloperoxidase | Plakortis simplex | P. falciparum | 1263-1117 nM* | Tasdemir et al., 2007 |
IC50: Inhibitory Concentration,
P. falciparum (D10),
P. falciparum (D6 clone),
Chloroquine-resistant P. falciparum (W2). [h] Fattorusso et al., 2002.
Fig. 4.
Structure of Antimalarial compounds; Manzamine A; Monamphilectine A; Kalihinol A.
The most capable and promising antimalarial compound, manzamines have been isolated from a number of sponges (Sakai et al., 1986; Yousaf et al., 2002; Fattorusso and Taglialatela, 2009). Manzamine A displayed a potent in vitro activity against P. falciparum (D6clone), with MIC of 0.0045 μg/ml (Sakai et al., 1986; Ashok et al., 2014). According to research antimalarial activity of manzamine A is due to enhancing immune response (Ang et al., 2001).
ANTI-INFLAMMATORY ACTIVITY
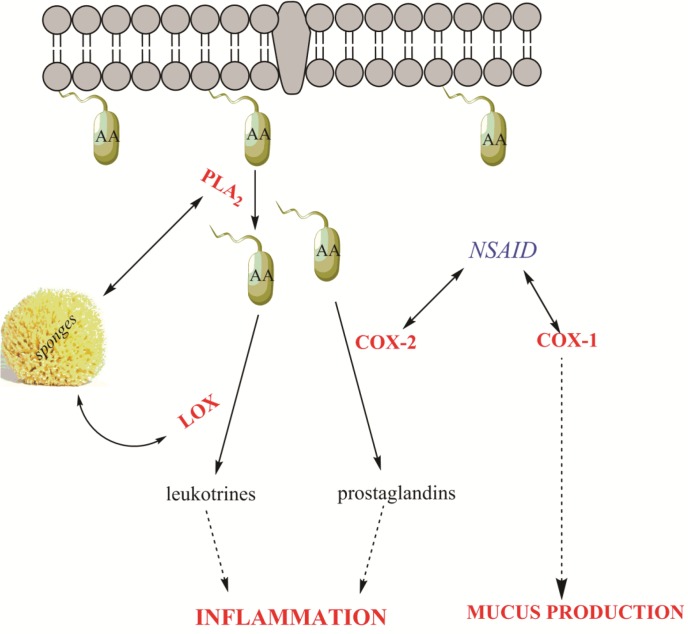
Body inflammation is caused by physical or chemical damage or due to infection. In this case, blood is oozing out from blood vessels into tissues (Tan et al., 1997; Franceschi and Campisi, 2014). Manolide is the first sesterterpenoids anti-inflammatory drug derived from marine sponges with several other pharmaceutical properties (Mayer and Jacobs, 1998). Its Anti-inflammatory action is basically an irreversible inhibition of the release of arachidonic acid from phospholipid membrane by the mechanism of preventing the phospholipase A2 enzyme from the binding to the membrane of phospholipid, the reason is that it increases the concentration of intracellular arachidonic acid that results in the upregulation of the inflammation mediators synthesis as a leukotrienes and prostaglandins. The Mode of action of sponge-derived anti-inflammatory substances has different from other non-steroidal anti-inflammatory drugs (NSAIDS). Only a few of sponge-derived substances have the capability to inhibit lipoxygenase, another enzyme which is involved in the inflammatory response (Carroll et al., 2001) (Fig. 5).
Fig. 5.
Diagrammatic process of Inflammatory cascade inside the cell. Phospholipase A2 (PLA2) catalyzes the release of membrane-bound arachidonic acid (AA) to free arachidonic acid. Arachidonic acid is then converted to leukotrienes and prostaglandins by lipoxygenase (LOX) and cyclooxygenase-2 (COX-2), respectively. Sponge derived anti-inflammatory substances are mainly inhibitors of PLA2 or LOX, while nonsteroidal anti-inflammatory drugs (NSAID) inhibit COX-2, but also the constitutive COX-1.
ANTITUMOR ACTIVITY
In the tumor development protein kinase C (PKC) is an essential factor (Bradshaw et al., 1993; Kang, 2014). Many of the sponge-derived substances are PKC inhibitors. Worldwide, PKC inhibitors have attracted interest because of providing evidence, that extreme levels of PKC enzymes are involved in the pathogenesis of psoriasis, arthritis and especially in the development of tumor (Bradshaw et al., 1993; Kang, 2014). PKC serve as a receptor for tumor-promoting phorbol esters by the binding prevention of carcinosarcoma cells with Endothelium (Liu et al., 1991; Kang, 2014).
Fucosyltransferase inhibitors, like octa and nonaprenylhydroquinone sulfates, which were derived from Sarcotragus sp. (Wakimoto et al., 1999), may be capable candidates for regulating inflammatory processes like arthritis or for opposing tumor growth.
There are many other sponge derived compounds having anti-tumor potency with different kind of mechanisms of actions (Table 5). These are divided into three types.
Table 5.
Examples of anti-tumor compounds
| Compound | Chemistry | Species/order | Mode of action | References |
|---|---|---|---|---|
| Isoaaptamine | Benzonaphthyridine alkaloid | Aaptos aaptos/Hadromerida | Protein kinase C inhibitor | Fedoreev et al., 1988 |
| Debromohymenialdisine | Pyrrole-guanidine alkaloid, prenylhydroquinone derivative | Hymeniacidonaldis/Halichondrida | Protein kinase C inhibitor | Kitagawa et al., 1983 |
| Adociasulfates | Triterpenoid hydroquinones | Sarcotragus sp./ Dictyoceratida Haliclona (aka Adocia) sp./Haplosclerida | A1, 3-fucosyltransferase inhibitor Kinesin motor protein inhibitors | Zapolska-Downar et al., 2001 |
| Discodermolide | Linear tetraene lactone | Discodermia dissolute/ Lithistida | Stabilization of microtubules | Ter Haar et al., 1996 |
| Peloruside A | Macrocyclic lactone | Mycdle hentschett/ Poecilosclerida | Stabilization of microtubules | Hood et al., 2002 |
| Elenic acid | Alkylphenol | Plakinastrella sp./ Homosclerophorida | Topoisomerase II inhibitor | Hood et al., 2002 |
| Naamine D | Imidazole alkaloid | Leucetta cf. chagosensis | Nitric oxide synthetase inhibitor | Juagdan et al., 1995 |
| Agelasphin (KRN7000) | a-Galactosylceramide | Agelas mauritianus / Agelasida | NKT cell activator | Shimosaka, 2002 |
| Crambescidins 1–4 | Pentacyclic guanidine derivative | Crambe crambe/Poecilosclerida | Ca2+/channel blocker | Jares-Erijman et al., 1991 |
| Discorhabdin D | Fused pyrrolophenanthroline alkaloid | Latrunculia brevis/Poecilosclerida; Prianos sp./Haplosclerida | Unknown | Perry et al., 1990 |
| Glaciasterols A and B | 9, 11-Secosterol | Aplysilla glacialis/Dendroceratida | Unknown | Pika et al., 1992 |
| Durumolides A–C | Terpenoid | Lobophytum duru | Inducible NOS and COX-2 inhibition | Cheng et al., 2008 |
| Plakortide P | Polyketide | Plakortis angulospiculatus | TXB2 inhibition | Kossuga et al., 2008 |
| 24-methoxypetrosaspongia C | Sesterterpenes | Hyrtios erectus | Unknown | Elhady et al., 2016 |
Non-specific inhibitors
Nonspecific anti-tumor inhibitors are important compounds to treat cancer but in unusual conditions because these compounds also have toxic effects on healthy cells of a body. Example is adociasulfates (titerpenoid hydroquinones), isolated from Haticlona sp. Etc (Blackburn et al., 1999; Zapolska-Downar et al., 2001) and they are protein inhibitors by binding to microtubule binding site “locking up” protein motor function and there by blocking cell division.
Specific inhibitors
Specific inhibitors are specifically active against the tumor. For example, agosterol A derived from marine spongia can reverse the over appearance of membrane glycoproteins. These proteins are responsible for multidrug resistance in human carcinoma cells. Another example belongs to these inhibitors group is salicylihamide A. The first natural isolated from cinachyrella spp. is 6- hydroximino-4-en-3 (Griffith and Gross, 1996).
Inhibitors of a cancer cell of certain types
Growth inhibitory active compounds against tumor cell line have been isolated but its mechanism of action is still unknown. For example Discorhabdin D (Perry et al., 1990) etc.
IMMUNE SUPPRESSIVE ACTIVITY
Nitric oxide synthetase inhibitors, as anti-cancer agents are also responsible for the immune system suppression by downregulating the T-cells (Griffith and Gross, 1996). The ratio of Immune system suppression is very highly desired in case of hypersensitivity to antigens (e.g. allergies) medicines or organ transplantations. The cases in which patients receive any donor organ have to persist on life-long medication to prevent rejection by the body immune system as a foreign agent, and for that reasons, it is very important that these medicines should be specific suppressors. To prevent this autoimmune body defensive response and rejection of the donor organ, therefore, now it is a very crucial need for new specific immunosuppressors. A number of new biomolecules with strong immunosuppressive activities, which interfere at different sites of the immune response system have been discovered in marine sponges.
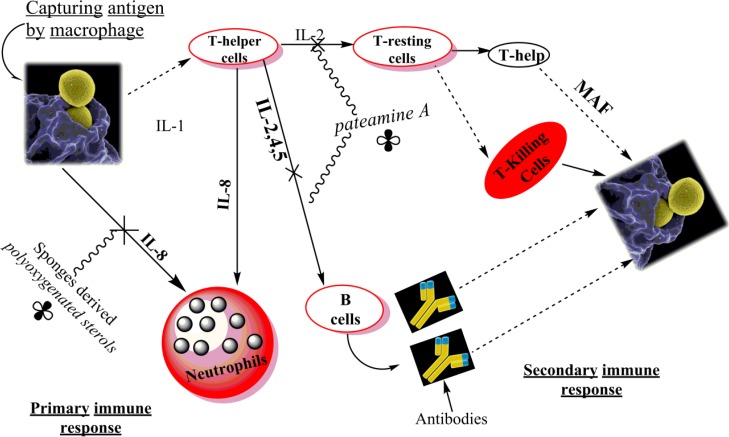
Dysidea sp have a large contribution in the portion of biomolecules (Mayer et al., 2000; 2004; 2011). 3 polyoxygenated sterols derived from Dysidea sp. in North Australia having a strong selective immunosuppressive capability of blocking the binding of interleukin 8 (IL-8), a cytokine that attracts neutrophil into tissue injury site, to the IL-8 receptor (de Almeida Leone et al., 2000). Thus, these polyoxygenated sterols have a specific selective inhibition on primary immune response (Fig. 6). Correspondingly, Pateamine A derived from Mycale sp., are the selective inhibitors of the production of interleukin 2 (IL-2), IL-2 helps in activation of B cells and T resting cells leading to cause antigen-antibody reaction and produce Secondary immune response.(Romo et al., 1998; Pattenden et al., 2004). Some examples for these suppressants are mentioned in Table 6, Fig. 6.
Fig. 6.
Diagrammatic representation of body immune response towards antigen capturing by Macrophages. The macrophages and T-helper cells secrete many interleukins (IL-x) or macrophage activation factor (MAF), to trigger primary immune response with the help of neutrophils, or the secondary immune response by activating the B and resting T-cells. The activated B cells secrete antibodies which bind to macrophages that already have phagocytized an antigen, and then killed by T-killer cells. The
 sign shows the sponge derived substances.
sign shows the sponge derived substances.
Table 6.
Examples of immunosuppressive compounds
| Compounds | Chemistry | Species/ order | Mode of action | References |
|---|---|---|---|---|
| Simplexides | Glycolopids | Plakortis simplex/Homosclerophorida | Inhibitors of T cell proliferation | Costantino et al., 1999 |
| Polyoxygenated sterols | Sterol | Dysidea sp./Dendroceratida | IL 8 inhibitor | de Almeida Leone et al., 2000 |
| Contignasterol | Oxygenated sterol | Petrosia contignata/Haplosclerida | Histamine release inhibitor | Takei et al., 1994 |
| Pateamine A | Thiazole macrolide | Mycale sp./Poecilosclerida | IL-2 inhibitor | Northcote et al., 1991 |
| Iso-iantheran A | Polyketide | Ianthella quadrangulata | Ionotropic P2Y11 receptor activation | Greve et al., 2007 |
CARDIOVASCULAR AGENTS
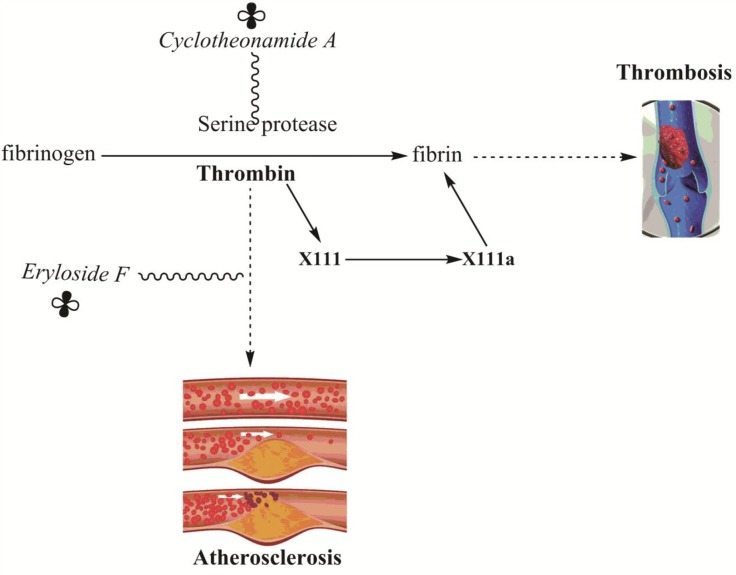
Some of the very common blood-related diseases like diabetes, thrombosis, atherosclerosis etc. have been treated by some marine sponge’s derived substances (Table 7, Fig. 7). The mechanism of blood coagulation is managed by a complex photolytic cascade that leads to the production of fibrin. Fibrin, a major component responsible for blood coagulation has been generated by the peptide cleaving of fibrinogen by thrombin (Kołodziejczyk and Ponczek, 2013). Cyclotheonamide A, isolated from marine sponges Theonella sp (Maryanoff et al., 1993) is an unusual class of Serine protease (an enzyme responsible for the conversion of fibrinogen into fibrin) inhibitor and is a drug of choice for thrombosis (Maryanoff et al., 1993; Schaschke and Sommerhoff, 2010). Eryloside F derived from Eryltus formosus sp. was found to be a potent Thrombin-receptor antagonist (Shuman et al., 1993; Stead et al., 2000; Kalinin et al., 2012). Thrombin receptor plays a central role not only in thrombosis but also the main agent to cause atherosclerosis (Fig. 7) (Chackalamannil, 2001; Ikenaga et al., 2016). Atherosclerosis is a disease in which plaque (fats, cholesterol, and calcium etc.) builds up layer by layer inside the arteries and resulting by narrowing of the arteries, causing a barrier to blood circulation leading to serious problems including heart attack, stroke or maybe death (Zapolska-Downar et al., 2001; Ikenaga et al., 2016).
Table 7.
Cardiovascular compound examples
| Compounds | Chemistry | Species/ order | Mode of action | References |
|---|---|---|---|---|
| Cyclotheonamide A | Cyclic pentapeptide | Theonella sp./Lithistida | Serine protease inhibitor | Maryanoff et al., 1993 |
| Eryloside F | Penasterol disaccharide | Eryltus formosus/Astrophorida | Thrombin receptor antagonist | Stead et al., 2000 |
| Halichlorine | Cyclic aza Polyketide | Halichondria okadai/Halichondria | VCAM 1* inhibitor | Arimoto et al., 1998 |
VCAM: vascular cell adhesion molecule.
Fig. 7.
Blood coagulation (Thrombosis) and atherosclerosis (arterial disease characterized by the deposition of plaques of fatty material on their inner walls) pathway in vivo showing central role played by Thrombin. X111 represent fibrin stabilizing factor (enzyme responsible for blood coagulation). The
 Sign shows the sponge derived compounds.
Sign shows the sponge derived compounds.
ANTIHELMINTHIC ACTIVITY
A new macrocyclic polyketide lactam tetramic acid, geodin A Magnesium salt, isolated from the marine sponge Geodia sp. exhibited a remarkable nematocidal activity with (LD99=14 μg/ml) against Haemonchus contortus (Capon et al., 1999). The mode of action of the pure Geodin A is not explored yet. Two more studies contributed to the search of novel anthelmintic marine sponge derived products during 2005–6. Two novel alkaloidal betaines (−)-echinobetaine A (1) and (+)-echinobetaine B (2), isolated from marine sponge Echinodictyum sp proved to be a nematocidal with (LD99=83 and 8.3 μg/ mL, respectively) against commercial livestock parasite Haemonchus contortus (Capon et al., 2005). Unfortunately, the mode of action of these compounds was also undetermined. (+)-echinobetaine B’s nematocidal potency was comparable to that of “two commercially available synthetic anthelmintic, closantel and levamisole” (Capon et al., 2005).
MUSCLE RELAXANT
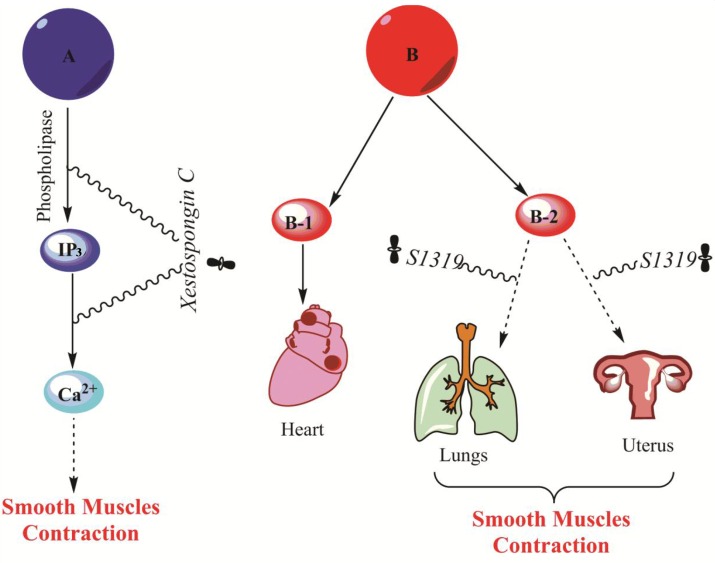
Continuous muscles activation caused by disturbances in the neuromuscular communication that result in muscular stress (Lundberg et al., 1995; Edgar et al., 2002; Hibbs and Zambon, 2011). Muscle relaxants are divided into two parts; centrally and peripherally active. Centrally active can mediate neuromuscular communication while peripherally relaxants are used for local muscle relaxation like stroke or during surgery (Frakes, 2001; Hibbs and Zambon, 2011) Xestospongin C (Fig. 1) isolated from marine sponge Xestospongia sp is a potent α-receptor’s IP3 (Inositol triphosphate) inhibitor and Ca2+ (calcium channel) blocker (Quinn et al., 1980; Gafni et al., 1997; Miyamoto et al., 2000). IP3 is a secondary messenger molecule used in signal transduction and it diffuses throughout the cell and increases the Ca2+ level and resulting cause’s smooth muscles contraction (Fig. 8) (Quinn et al., 1980; Nausch et al., 2010). S1319 isolated from a Dysidea sp. (Suzuki et al., 1999) is another substance with a remarkable muscle relaxing capability. Its mechanism of action is to agonist the β-Adrenoreceptor. β-Adrenoreceptors are of two types β-1 and β-2. β-1 receptors are available in heart increases heart rate, myocardial contractility and increases conduction velocity while β-2 receptors are available in lungs and uterus responsible for dilation of bronchial smooth muscles, dilation of blood vessels in skeletal smooth muscles and relaxation of uterus muscles (Dennedy et al., 2002; Barrese and Taglialatela, 2013). S1319 have the uterus relaxing capability which can be therapeutically used at infant’s delivery time (Dennedy et al., 2002) and bronchodilation property which can be used as antiasthmatic (Suzuki et al., 1999). However, because of their low selectivity, they have some side effects like activation of β-1 receptors resulting arterial hypertension, tachycardia and coronary heart disease (Borchard, 1998). Therefore, there is a desired continued research in interest to find selective β-agonists.
Fig. 8.
The mechanism of adrenergic receptors. A represent α-receptors and trigger the IP3 (Inositol triphosphate) which then increase the Ca2+ level in cytoplasm and causing muscles contraction. B represents β-adrenoreceptors. The
 represents Marine compounds. Xestospongin C inhibit the phospholipase enzyme which play a key role in activation of IP3 (Inositol triphosphate) and block Ca2+ channels. S1319 B-2 receptor agonist resulting Bronchodilation and uterus relaxation.
represents Marine compounds. Xestospongin C inhibit the phospholipase enzyme which play a key role in activation of IP3 (Inositol triphosphate) and block Ca2+ channels. S1319 B-2 receptor agonist resulting Bronchodilation and uterus relaxation.
CONCLUSION
Sponge-derived substances span a wide range of chemistry (e.g., alkaloid, peptide, terpenoid and polyketides) with an equally variety of biotechnological properties (e.g., Antibacterial, antifungal, antiviral, immunosuppressive, cardiovascular and anti-parasitic) (Ang et al., 2001; Torres et al., 2002). The relationship between the chemistry of the secondary metabolites originated from marine sponges and their mode of action on disease in vivo is mostly not obvious (interaction with DNA to combat tumors, or inhibition of α/β receptors to provide muscle relaxation). Moreover, in drug discovery, it is frequently observed that a certain series of compounds that exhibited the most potent inhibitors in vitro turned out not to be the drug of choice in vivo. It is likely that for every compound prior to coming out to the market, its profile should be with a distinct chemistry, improved bioavailability with lesser side effects.
Now, there are some significant reports of activities from a particular class of metabolites, the manzamines from marine sponges as potential drugs that might be effective against HIV (Muller et al., 1987), malaria (Konig et al., 1996), tuberculosis (Schwartsmann, 2000) and some other diseases. Other substances with best anti-pathogenic profiles like ara-A, ara-C, acyclovir are in clinical use and are all examples of products originated from marine sponges (Muller et al., 1987).
The potency of sponge-derived medicines lies in the fact that each of these thousands of metabolites and their derivatives has its own specific dose-related efficacy, inhibitory effect, and potential side effects that determine its suitability for medicinal use. Unfortunately, these secondary metabolites are usually present in very trace amounts, and natural stocks are too small which is one of the major obstacles in sustaining the development of widely available medicines. An example is avarol (D.avara sponge), a potent anti-HIV drug (Muller et al., 1987), that was in preclinical assessment. However, further studies on this natural product stopped due to an insufficient amount of sponge for its isolation (Müller et al., 2004). In addition, the active core or skeleton of these compounds may be used as a vehicle to generate derivatives with their own distinct efficacy and side effects. Therefore, the most significant challenge in the transformation of bioactive molecules into medicines is now to screen the drug treasure house of sponges and elect those that illustrate a precise mode of action with the desired characteristics towards a disease. A major question for the future still persists, how to actually prepare the potential novel drugs in a bulk quantity.
REFERENCES
- Amade P, Charroin G, Baby C, Vacelet J. Antimicrobial activity of marine sponges of Mediterranean. Sea Mar Biol. 1987;94:271–275. doi: 10.1007/BF00392940. [DOI] [Google Scholar]
- Amade PH, Pesando D, Chevolot L. Antimicrobial activities of marine from French Polynesia and Brittany. Mar Biol. 1982;70:223–228. doi: 10.1007/BF00396840. [DOI] [Google Scholar]
- Amigó M, Terencio MC, Payá M, Iodice C, De Rosa S. Synthesis and evaluation of diverse thio avarol derivatives as potential UVB photoprotective candidates. Bioorg Med Chem Lett. 2007;17:2561–2565. doi: 10.1016/j.bmcl.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Ang KK, Holmes MJ, Kara UA. Immunemediated parasite clearance in mice infected with Plasmodium berghei following treatment with manzamine A. Parasitol Res. 2001;87:715–721. doi: 10.1007/s004360000366. [DOI] [PubMed] [Google Scholar]
- Arimoto H, Hayakawa I, Kuramoto M, Uemura D. Absolute stereochemistry of halichlorine; a potent inhibitor of VCAM-1 induction. Tetrahedron Lett. 1998;39:861–862. doi: 10.1016/S0040-4039(97)10714-6. [DOI] [Google Scholar]
- Ashok P, Ganguly S, Murugesan S. Manzamine alkaloids: isolation, cytotoxicity, antimalarial activity and SAR studies. Drug Discovr. Today. 2014;19:1781–1791. doi: 10.1016/j.drudis.2014.06.010. [DOI] [PubMed] [Google Scholar]
- Avilés E, Rodríguez AD. Monamphilectine A, a Potent Antimalarial β-Lactam from Marine Sponge Hymeniacidon sp: Isolation, Structure, Semisynthesis, and Bioactivity. Org Lett. 2010;12:5290–5293. doi: 10.1021/ol102351z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird JK. Evidence and implications of mortality associated with acute Plasmodium vivax malaria. Clin Microbiol Rev. 2013;26:36–57. doi: 10.1128/CMR.00074-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrese V, Taglialatela M. New advances in beta-blocker therapy in heart failure. Front Physiol. 2013;4:323. doi: 10.3389/fphys.2013.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann W, Feeney RJ. The isolation of a new thymine pentoside from sponges. J Am Chem Soc. 1950;72:2809–2810. doi: 10.1021/ja01162a543. [DOI] [Google Scholar]
- Bergmann W, Feeney RJ. Contribution to the study of marine products. J Org Chem. 1951;16:981–987. doi: 10.1021/jo01146a023. [DOI] [Google Scholar]
- Bergmann W, Swift AN. Contributions to the study of marine products. XXX. Component acids of lipids sponges. I. J Org Chem. 1951;16:1206–1221. doi: 10.1021/jo50002a005. [DOI] [Google Scholar]
- Blackburn CL, Hopmann C, Sakowicz R, Berdelis MS, Goldstein LSB, Faulkner DJ. Adociasulfates 1–6 inhibitors of kinesin motor proteins from the sponge Haliclona (aka Adocia) sp. J Org Chem. 1999;64:5565–5570. doi: 10.1021/jo9824448. [DOI] [PubMed] [Google Scholar]
- Blunt JW, Copp BR, Keyzers RA, Munroa MH, Prinsep MR. Marine natural products. Nat Prod Rep. 2013;30:237–323. doi: 10.1039/C2NP20112G. [DOI] [PubMed] [Google Scholar]
- Blunt JW, Copp BR, Munro MH, Northcote PT, Prinsep MR. Marine natural products. Nat Prod Rep. 2005;22:15–61. doi: 10.1039/b415080p. [DOI] [PubMed] [Google Scholar]
- Blunt JW, Copp BR, Munro MH, Northcote PT, Prinsep MR. Marine natural products. Nat Prod Rep. 2006;23:26–78. doi: 10.1039/b502792f. [DOI] [PubMed] [Google Scholar]
- Boonlarppradab C, Faulkner DJ. Eurysterols A and B, cytotoxic and antifungal steroidal sulfates from a marine sponge of the genus Euryspongia. J Nat Prod. 2007;70:846–848. doi: 10.1021/np060472c. [DOI] [PubMed] [Google Scholar]
- Borchard U. Pharmacological properties of b-adrenoreceptor blocking drugs. J Clin Basic Cardiol. 1998;1:5–9. [Google Scholar]
- Bradshaw D, Hill CH, Nixon JS, Wilkinson SE. Therapeutic potential of protein kinase C inhibitors. Agents Actions. 1993;38:135–147. doi: 10.1007/BF02027225. [DOI] [PubMed] [Google Scholar]
- Brazilian Health Ministry Epidemiological survey of malaria in Brazil, Funasa, Brasília. 2002 Available from: http://www.funasa.gov.br/.
- Burkholder PR, Ruetzler K. Antimicrobial activity of some marine sponges. Nature. 1969;222:983–984. doi: 10.1038/222983a0. [DOI] [PubMed] [Google Scholar]
- Capon RJ, Skene C, Lacey E, Gill JH, Wadsworth D, Friedel T. Geodin A magnesium salt: a novel nematocide from a southern Australian marine sponge, Geodia. J Nat Prod. 1999;62:1256–1259. doi: 10.1021/np990144v. [DOI] [PubMed] [Google Scholar]
- Capon RJ, Vuong D, McNally M, Peterle T, Trotter N, Lacey E, Gill JH. (+)-Echinobetaine B: isolation, structure elucidation, synthesis and preliminary SAR studies on a new nematocidal betaine from a southern Australian marine sponge, Echinodictyum sp. Org Biomol Chem. 2005;3:118–122. doi: 10.1039/b414839h. [DOI] [PubMed] [Google Scholar]
- Caraballo H, King K. Emergency department management of mosquito-borne illness: malaria, dengue, and west nile virus. Emerg Med Pract. 2014;16:1–23. [PubMed] [Google Scholar]
- Carroll J, Johnsson EN, Ebel R, Hartman MS, Holman TR, Crews P. Probing sponge-derived terpenoids for human 15-L-lipoxygenase inhibitors. J Org Chem. 2001;66:6847–6851. doi: 10.1021/jo015784t. [DOI] [PubMed] [Google Scholar]
- Chackalamannil S, Xia Y. Thrombin receptor (PAR-1) antagonists as novel antithrombotic agents. Expert Opin Ther Pat. 2006;16:493–505. doi: 10.1517/13543776.16.4.493. [DOI] [PubMed] [Google Scholar]
- Cheng S, Wen Z, Chiou S, Hsu C, Wang S, Dai C, Chiang MY, Duh C. Durumolides A–E, anti-inflammatory and antibacterial cembranolides from the soft coral Lobophytum durum. Tetrahedron. 2008;64:9698–9704. doi: 10.1016/j.tet.2008.07.104. [DOI] [Google Scholar]
- Costantino V, Fattorusso E, Mangoni A, Di Rosa M, Ianaro A. Glycolipids from sponges, VII: simplexides, novel immuno-suppressive glycolipids from the Caribbean sponge Plakortis simplex. Bioorg Med Chem Lett. 1999;9:271–276. doi: 10.1016/S0960-894X(98)00719-7. [DOI] [PubMed] [Google Scholar]
- Cutignano A, Bifulco G, Bruno I, Casapullo A, Gomez-Paloma L, Riccio R. Dragmacidin F: A New Antiviral Bromoindole Alkaloid from the Mediterranean Sponge Halicortex sp. Tetrahedron. 2000;56:3743–3748. doi: 10.1016/S0040-4020(00)00281-7. [DOI] [Google Scholar]
- D Ambrosio M, Guerriero A, Deharo E, Debitus C, Munoz V, Pietra F. New types of potentially antimalarial agents: epidioxy-substituted norditerpene and norsesterpenes from the marine sponge Diacarnuslevii. Helv. Chim. Acta. 1998;81:1285–1292. doi: 10.1002/hlca.19980810539. [DOI] [Google Scholar]
- de Almeida Leone P, Redburn J, Hooper JN, Quinn RJ. Polyoxygenated dysidea sterols that inhibit the binding of [I125] IL-8 to the human recombinant IL-8 receptor type A. J Nat Prod. 2000;63:694–697. doi: 10.1021/np9904657. [DOI] [PubMed] [Google Scholar]
- De Clercq E. New anti-HIV agents and targets. Med Res Rev. 2002;22:531–565. doi: 10.1002/med.10021. [DOI] [PubMed] [Google Scholar]
- De Clercq E. Antiviral drugs in current clinical use. J Clin Virol. 2004;30:115–133. doi: 10.1016/j.jcv.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Dennedy MC, Houlihan DD, McMillan H, Morrison JJ. b2- and b3-Adrenoreceptor agonists: human myometrial selectivity and effects on umbilical artery tone. Am J Obstet Gynecol. 2002;187:641–647. doi: 10.1067/mob.2002.125277. [DOI] [PubMed] [Google Scholar]
- de Silva ED, Scheuer PJ. Manoalide, an antibiotic sesterterpenoid from the marine sponge luffariella variabilis (polejaeff) Tetrahedron. Lett. 1980;21:1611–1614. doi: 10.1016/S0040-4039(00)77766-5. [DOI] [Google Scholar]
- Donia M, Hamann MT. Marine natural products and their potential applications as anti-infective agents. Lancet Infect Dis. 2003;3:338–348. doi: 10.1016/S1473-3099(03)00655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar DC, Rimoldi JM, Clark AM, Kelly M, Hamann MT. Anti-cryptococcal and nitric oxide synthase inhibitory imidazole alkaloids from the calcareous sponge Leucetta cf chagosensis. Tetrahedron. 2000;56:8795–8798. doi: 10.1016/S0040-4020(00)00821-8. [DOI] [Google Scholar]
- Ebada SS, Wray V, de Voogd NJ, Deng Z, Lin W, Proksch P. Two new jaspamide derivatives from the marine sponge Jaspis splendens. Mar. Drugs. 2009;7:435–444. doi: 10.3390/md7030435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar VA, Cremaschi GA, Sterin-Borda L, Genaro AM. Altered expression of autonomic neurotransmitter receptors and proliferative responses in lymphocytes from a chronic mild stress model of depression: effects of fluoxetine. Brain Behav Immun. 2002;16:333–350. doi: 10.1006/brbi.2001.0632. [DOI] [PubMed] [Google Scholar]
- Elhady SS, El-Halawany AM, Alahdal AM, Hassanean HA, Ahmed SA. A new bioactive metabolite isolated from the red sea marine sponge Hyrtios erectus. Molecules. 2016;21:82. doi: 10.3390/molecules21010082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elyakov GB, Kuznetsova T, Mikhailov VV, Maltsev II, Voinov VG, Fedoreyev SA. Brominated diphenyl ethers from a marine bacterium associated with the sponge Dysidea sp. Experientia. 1991;47:632–633. doi: 10.1007/BF01949894. [DOI] [Google Scholar]
- Faulkner DJ. Marine natural products. Nat Prod Rep. 2000;17:7–55. doi: 10.1039/a809395d. [DOI] [PubMed] [Google Scholar]
- Faulkner DJ. Marine natural products. Nat Prod Rep. 2001;18:1–49. doi: 10.1039/b006897g. [DOI] [PubMed] [Google Scholar]
- Faulkner DJ. Marine natural products. Nat Prod Rep. 2002;19:1–48. doi: 10.1039/b009029h. [DOI] [PubMed] [Google Scholar]
- Fedoreev SA, Prokof’eva NG, Denisenko VA, Rebachuk NM. Cytotoxic activity of aaptamines from suberitid marine sponges. Pharm Chem J. 1988;22:615–618. doi: 10.1007/BF00763625. [DOI] [Google Scholar]
- Ford PW, Gustafson KR, McKee TC, Shigematsu N, Maurizi LK, Pannell LK, Williams DE, De Silva ED, Lassota P, Alien TM, Van Soest R, Andersen RJ, Boyd MR. Papuamides A–D, HIV-inhibitory and cytotoxic depsipeptides from the sponges Theonella mirabilis and Theonella swinhoei collected in Papua New Guinea. J Am Chem Soc. 1999;121:5899–5909. doi: 10.1021/ja990582o. [DOI] [Google Scholar]
- Frakes MA. Muscle relaxant choices for rapid sequence induction. Air Med J. 2001;20:20–21. doi: 10.1016/S1067-991X(01)70074-0. [DOI] [PubMed] [Google Scholar]
- Gafni J, Munsch JA, Lam TH, Catlin MC, Costa LG, Molinski TF, Pessah IN. Xestospongins: potent membrane permeable blockers of the inositol 1,4,5-triphosphate receptor. Neuron. 1997;19:723–733. doi: 10.1016/S0896-6273(00)80384-0. [DOI] [PubMed] [Google Scholar]
- García-Ruiz JC, Amutio E, Pontón J. Invasive fungal infection in immunocompromised patients. Rev Iberoam Micol. 2004;21:55–62. [PubMed] [Google Scholar]
- Gaspar H, Santos S, Carbone M, Rodrigues AS, Rodrigues AI, Uriz MJ, Savluchinske Feio SM, Melck D, Humanes M, Gavagnin M. Isomeric furanosesquiterpenes from the Portuguese marine sponge Fasciospongia sp. J Nat Prod. 2008;71:2049–2052. doi: 10.1021/np800346c. [DOI] [PubMed] [Google Scholar]
- Giusiano G, Mangiaterra M, Rojas F, Gámez V. Yeasts species distribution in Neonatal Intensive Care Units in northeast Argentina. Mycoses. 2004;47:300–303. doi: 10.1111/j.1439-0507.2004.00993.x. [DOI] [PubMed] [Google Scholar]
- Giusiano G, Mangiaterra M, Rojas F, Gámez V. Azole Resistance in Neonatal Intensive Care Units in Argentina. J Chemother. 2005;17:347–350. doi: 10.1179/joc.2005.17.3.347. [DOI] [PubMed] [Google Scholar]
- Greve H, Meis S, Kassack MU, Kehraus S, Krick A, Wright AD, Konig GM. New iantherans from the marine sponge Ianthella quadrangulata: novel agonists of the P2Y(11) receptor. J Med Chem. 2007;50:5600–5607. doi: 10.1021/jm070043r. [DOI] [PubMed] [Google Scholar]
- Griffith OW, Gross SS. Inhibitors of nitric oxide synthases. In: Stamler J, Feelish M, editors. Methods in nitric oxide research. Wiley & Sons; New York: 1996. pp. 187–208. [Google Scholar]
- Grimwood K, Lambert SB. Rotavirus vaccines: opportunities and challenges. Hum Vaccin. 2009;5:57–69. doi: 10.4161/hv.5.2.6924. [DOI] [PubMed] [Google Scholar]
- Hadas E, Shpigel M, Ilan M. Particulate organic matter as a food source for a coral reef sponge. J Exp Biol. 2009;212:3643–3650. doi: 10.1242/jeb.027953. [DOI] [PubMed] [Google Scholar]
- Hertiani T, Edrada-Ebel R, Ortlepp S, van Soest RW, de Voogd NJ, Wray V, Hentschel U, Kozytska S, Muller WE, Proksch P. From anti-fouling to biofilm inhibition: New cytotoxic secondary metabolites from two Indonesian Agelas sponges. Bioorg Med Chem. 2010;18:1297–1311. doi: 10.1016/j.bmc.2009.12.028. [DOI] [PubMed] [Google Scholar]
- Hibbs RE, Zambon AC. Control of muscle spasms and rigidity. Agents acting at the neuromuscular junction and autonomic ganglia. In: Brunton LL, Chabner BA, Knollman BC, editors. Goodman & Gilman’s the pharmacological basis of therapeutics. McGraw-Hill; New York: 2011. pp. 266–276. [Google Scholar]
- Hill RT, Hamann M, Peraud O, Kasanah N. University of Maryland Biotechnology Institute, assignee. Manzamine-producing actinomycetes. 2005 Nov 3; inventors. United States patent US 20050244938 A1. [Google Scholar]
- Hood KA, West LM, Rouwe B, Northocote PT, Berridge MV, Wakefield SJ, Miller JH. Peloruside A, a novel antimitotic agent with paclitaxel-like microtubule-stabilizing activity. Cancer Res. 2002;62:3356–3360. [PubMed] [Google Scholar]
- Hooper JNA, van Soest RWM. Systema porifera: a guide to the classification of sponges. Kluwer Academic/Plenum Publishers; New York: 2002. [DOI] [Google Scholar]
- Hu GP, Yuan J, Sun L, She ZG, Wu JH, Lan XJ, Zhu X, Lin YC, Chen SP. Statistical research on marine natural products based on data obtained between 1985 and 2008. Mar Drugs. 2011;9:514–525. doi: 10.3390/md9040514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua HM, Peng J, Fronczek FR, Kelly M, Hamann MT. Crystallographic and NMR studies of antiinfective tricyclic guanidine alkaloids from the sponge Monanchora unguifera. Bioorg Med Chem. 2004;12:6461–6464. doi: 10.1016/j.bmc.2004.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultgren KM, Duffy JE. Sponge host characteristics shape the community structure of their shrimp associates. Mar Ecol Prog Ser. 2010;407:1–12. doi: 10.3354/meps08609. [DOI] [Google Scholar]
- Ikenaga M, Higaki Y, Saku K, Uehara Y. High-Density Lipoprotein Mimetics: a Therapeutic Tool for Atherosclerotic Diseases. J Atheroscler Thromb. 2016;23:385–394. doi: 10.5551/jat.33720. [DOI] [PubMed] [Google Scholar]
- Jang KH, Chung SC, Shin J, Lee SH, Kim TI, Lee HS, Oh KB. Aaptamines as sortase A inhibitors from the tropical sponge Aaptos aaptos. Bioorg Med Chem Lett. 2007;17:5366–5369. doi: 10.1016/j.bmcl.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Jares-Erijman EA, Sakai R, Rinehart KL. Crambescidins: new antiviral and cytotoxic compounds from the sponge Crambe crambe. J Org Chem. 1991;56:5712–5715. doi: 10.1021/jo00019a049. [DOI] [Google Scholar]
- Juagdan EG, Kalindindi RS, Scheuer PJ, Kelly-Borges M. Elenic acid, an inhibitor of topoisomerase II, from a sponge, Plakinastrella sp. Tetrahedron Lett. 1995;36:2905–2908. doi: 10.1016/0040-4039(95)00432-C. [DOI] [Google Scholar]
- Kalinin VI, Ivanchina NV, Krasokhin VB, Makarieva TN, Stonik VA. Glycosides from marine sponges (Porifera, Demospongiae): structures, taxonomical distribution, biological activities and biological roles. Mar. Drugs. 2012;10:1671–1710. doi: 10.3390/md10081671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J.-H. Protein kinase C (PKC) isozymes and cancer. New J Sci. 2014;2014:231418. doi: 10.1155/2014/231418. [DOI] [Google Scholar]
- Kitagawa I, Kobayashi M, Kitanaka K, Kido M, Kyogoku Y. Marine natural products, XII: on the chemical constituents of the Okinawan marine sponge Hymeniacidon aldis. Chem Pharm Bull. 1983;31:2321–2328. doi: 10.1248/cpb.31.2321. [DOI] [Google Scholar]
- Kobayashi J, Ishibashi M. Bioactive metabolites of symbiotic marine microorganisms. Chem Rev. 1993;93:1753–1769. doi: 10.1021/cr00021a005. [DOI] [Google Scholar]
- Konig GM, Wright AD, Angerhofer CK. Novel potent antimalarial diterpene isocyanates, isothiocyanates, and isonitriles from the tropical marine sponge Cymbastela hooperi. J Org Chem. 1996;61:3259–3267. doi: 10.1021/jo952015z. [DOI] [Google Scholar]
- Kossuga MH, Nascimento AM, Reimao JQ, Tempone AG, Taniwaki NN, Veloso K, Ferreira AG, Cavalcanti BC, Pessoa C, Moraes MO, Mayer AM, Hajdu E, Berlinck RG. Antiparasitic, antineuroinflammatory, and cytotoxic polyketides from the marine sponge Plakortis angulospiculatus collected in Brazil. J NatProd. 2008;71:334–339. doi: 10.1021/np0705256. [DOI] [PubMed] [Google Scholar]
- Kołodziejczyk J, Ponczek MB. The role of fibrinogen, fibrin and fibrin (ogen) degradation products (FDPs) in tumor progression. Contemp. Oncol. (Pozn.) 2013;17:113–119. doi: 10.5114/wo.2013.34611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laport MS, Santos OC, Muricy G. Marine sponges: potential sources of new antimicrobial drugs. Curr Pharm Biotechnol. 2009;10:86–105. doi: 10.2174/138920109787048625. [DOI] [PubMed] [Google Scholar]
- Leal MC, Puga J, Serodio J, Gomes NCM, Calado R. Trends in the discovery of new marine natural products from invertebrates over the last two decades - where and what are we bioprospecting? PLoS ONE. 2012;7:e30580. doi: 10.1371/journal.pone.0030580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linington RG, Robertson M, Gauthier A, Finlay BB, MacMillan JB, Molinski TF, van Soest R, Andersen RJ. Caminosides BD, Antimicrobial Glycolipids Isolated from the Marine Sponge Caminus s phaeroconia. J Nat Prod. 2006;69:173–177. doi: 10.1021/np050192h. [DOI] [PubMed] [Google Scholar]
- Liu B, Timar J, Howlett J, Diglio CA, Honn KV. Lipoxygenase metabolites of arachidonic and linoleic acids modulate the adhesion of tumor cells to endothelium via regulation of protein kinase C. Cell Regul. 1991;2:1045–1055. doi: 10.1091/mbc.2.12.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loya S, Hizi A. The inhibition of human immunodeficiency virus type 1 reverse transcriptase by avarol and avarone derivatives. FEBS Lett. 1990;269:131–134. doi: 10.1016/0014-5793(90)81137-D. [DOI] [PubMed] [Google Scholar]
- Lundberg U. Methods and applications of stress research. Technol. Health Care. 1995;3:3–9. [PubMed] [Google Scholar]
- Maldonado M, Carmona C, Velasquez Z, Puig A, Cruzado A, Lopez A, Young CM. Siliceous sponges as a silicon sink: An overlooked aspect of the benthopelagic coupling in the marine silicon cycle. Limnol Oceanogr. 2005;50:799–809. doi: 10.4319/lo.2005.50.3.0799. [DOI] [Google Scholar]
- Maria M, Lone G, Thomas OL. Production of bioactive secondary metabolites by marine vibrionaceae. Mar. Drugs. 2011;9:1440–1468. doi: 10.3390/md9091440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins A, Vieira H, Gaspar H, Santos S. Marketed marine natural products in the pharmaceutical and cosmoceutical industries: tips for success. Mar. Drugs. 2014;12:1066–1101. doi: 10.3390/md12021066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maryanoff BE, Qiu X, Padmanabhan KP, Tulinsky A, Almond HR, Andrade-Gordon P, Greco MN, Kauffman JA, Nicolaou KC, Liu A, Brungs PH, Fusetani N. Molecular basis for the inhibition of human alpha-thrombin by the macrocyclic peptide cyclotheonamide A. Proc Natl Acad Sci USA. 1993;90:8048–8052. doi: 10.1073/pnas.90.17.8048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga S, Fusetani N, Konosu S. Bioactive marine metabolites, VII: structures of discodermins B, C, and D, antimicrobial peptides from the marine sponge Discodermia kiiensis. Tetrahedron Lett. 1985;26:855–856. doi: 10.1016/S0040-4039(00)61947-0. [DOI] [PubMed] [Google Scholar]
- Mayer AM, Rodriguez AD, Berlinck RG, Fusetani N. Marine pharmacology in 2007-8: Marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous system, and other miscellaneous mechanisms of action. Comp Biochem Physiol C Toxicol Pharmacol. 2011;153:191–222. doi: 10.1016/j.cbpc.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AM, Hamann MT. Marine pharmacology in 2000: marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiplatelet, antituberculosis, and antiviral activities; affecting the cardiovascular, immune, and nervous systems and other miscellaneous mechanisms of action. Mar Biotechnol. 2004;6:37–52. doi: 10.1007/s10126-003-0007-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AMS, Jacobs RS. Manoalide: an anti-inflammatory and analgesic marine natural product. Mem Calif Acad Sci. 1988;13:133. [Google Scholar]
- Mayer AMS, Lehmann VKB. Marine pharmacology in 1998: marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, anthelmintic, antiplatelet, antiprotozoal, and antiviral activities; with actions on the cardiovascular, endocrine, immune, and nervous systems; and other miscellaneous mechanisms of action. Pharmacologist. 2000;42:62–69. [Google Scholar]
- McCaffrey EJ, Endeau R. Antimicrobial activity of tropical and subtropical sponges. Mar Biol. 1985;89:1–8. doi: 10.1007/BF00392871. [DOI] [Google Scholar]
- Mishra SK, Satpathy SK, Mohanty S. Survey of malaria treatment and deaths. Bull World Health Organ. 1999;77:1020. [PMC free article] [PubMed] [Google Scholar]
- Miyamoto S, Izumi M, Hori M, Kobayashi M, Ozaki H, Karaki H. Xestospongin C, a selective and membrane-permeable inhibitor of IP3 receptor, attenuates the positive inotropic effect of α-adrenergic stimulation in guinea-pig papillary muscle. Br J Pharmacol. 2000;130:650–654. doi: 10.1038/sj.bjp.0703358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaoka H, Shimomura M, Kimura H, Yamada Y, Kim HS, Wataya Y. Antimalarial activity of kalahinol A and new relative diterpenoids from the Okinawan sponge, Acanthella sp. Tetrahedron. 1998;54:13467–13474. doi: 10.1016/S0040-4020(98)00818-7. [DOI] [Google Scholar]
- Mol VPL, Raveendran TV, Parameswaran PS. Antifouling activity exhibited by secondary metabolites of the marine sponge, Haliclona exigua (Kirkpatrick) Int Biodeterior Biodegrad. 2009;63:67–72. doi: 10.1016/j.ibiod.2008.07.001. [DOI] [Google Scholar]
- Momparler RL. Optimization of cytarabine (ARA-C) therapy for acute myeloid leukemia. Exp Hematol Oncol. 2013;2:20. doi: 10.1186/2162-3619-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton SL, Moeller PD, Young KA, Lanoue B. Okadaic acid production from the marine dinoflagellate Prorocentrum belizeanum Faust isolated from the Belizean coral reef ecosystem. Toxicon. 1998;36:201–206. doi: 10.1016/S0041-0101(97)00054-8. [DOI] [PubMed] [Google Scholar]
- Moura RM, Queiroz AF, Fook JM, Dias AS, Monteiro NK, Ribeiro JK, Moura GE, Macedo LL, Santos EA, Sales MP. CvL, a lectin from the marine sponge Cliona varians: Isolation, characterization and its effects on pathogenic bacteria and Leishmania promastigotes. Comp Biochem Physiol, Part A Mol Integr Physiol. 2006;145:517–523. doi: 10.1016/j.cbpa.2006.08.028. [DOI] [PubMed] [Google Scholar]
- Muller WG, Sobel C, Diehl-Seifert B, Maidhof A, Schroder HC. Influence of the antileukemic and anti-human immunodeficiency virus agent avarol on selected immune responses in vitro and in vivo. Biochem Pharmacol. 1987;36:1489–1494. doi: 10.1016/0006-2952(87)90115-8. [DOI] [PubMed] [Google Scholar]
- Müller WE, Schröder HC, Wiens M, Perovic-Ottstadt S, Batel R, Müller IM. Traditional and modern biomedical prospecting: Part II-the benefits. Evid Based Complement Alternat Med. 2004;1:133–144. doi: 10.1093/ecam/neh030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nausch B, Heppner TJ, Nelson MT. Nerve-released acetylcholine contracts urinary bladder smooth muscle by inducing action potentials independently of IP3-mediated calcium release. Am J Physiol Regul Integr Comp Physiol. 2010;299:R878–R888. doi: 10.1152/ajpregu.00180.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northcote PT, Blunt JW, Munro MHG. Pateamine: a potent cytotoxin from the New Zealand marine sponge, mycale sp. Tetrahedron Lett. 1991;32:6411–6414. doi: 10.1016/0040-4039(91)80182-6. [DOI] [Google Scholar]
- Oclarit JM, Okada H, Ohta S, Kaminura K, Yamaoka Y, Iizuka T, Miyashiro S, Ikegami S. Anti-bacillus substance in the marine sponge, Hyatella species, produced by an associated Vibrio species bacterium. Microbios. 1994;78:7–16. [PubMed] [Google Scholar]
- Oh KB, Mar W, Kim S, Kim JY, Lee TH, Kim JG, Shin D, Sim CJ, Shin J. Antimicrobial activity and cytotoxicity of bis (indole) alkaloids from the sponge Spongosorites sp. Biol Pharm Bull. 2006;29:570–573. doi: 10.1248/bpb.29.570. [DOI] [PubMed] [Google Scholar]
- O’Rourke A, Kremb S, Bader TM, Helfer M, Schmitt-Kopplin P, Gerwick WH, Brack-Werner R, Voolstra CR. Alkaloids from the sponge Stylissa carteri present prospective scaffolds for the inhibition of human immunodeficiency Virus 1 (HIV-1) Mar. Drugs. 2016;14:28. doi: 10.3390/md14020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattenden G, Critcher DJ, Remuiñán M. Total synthesis of ()-pateamine A, a novel immunosuppressive agent from Mycale sp. Can J Chem. 2004;82:353–365. doi: 10.1139/v03-199. [DOI] [Google Scholar]
- Perry NB, Blunt JW, Munro MHG, Thompson AM. Antiviral and antitumor agents from a New Zealand sponge, Mycale sp. 2. Structures and solution conformations of mycalamides A and B. J Org Chem. 1990;55:223–227. doi: 10.1021/jo00288a037. [DOI] [Google Scholar]
- Petit GR, Knight JC. Arizona Board of Regents, assignee. Cribrostatins 3–5. 2002 Aug 20; inventors. United States patent US 6437128 B1. [Google Scholar]
- Pettit RK, Fakoury BR, Knight JC, Weber CA, Pettit GR, Cage GD, Pon S. Antibacterial activity of the marine sponge constituent cribrostatin 6. J Med Microbiol. 2004;53:61–65. doi: 10.1099/jmm.0.05250-0. [DOI] [PubMed] [Google Scholar]
- Piel J. Metabolites from symbiotic bacteria. Nat Prod Rep. 2004;21:519–538. doi: 10.1039/b310175b. [DOI] [PubMed] [Google Scholar]
- Piel J. Bacterial symbionts: prospects for the sustainable production of invertebrate-derived pharmaceuticals. Curr Med Chem. 2006;13:39–50. doi: 10.2174/092986706775197944. [DOI] [PubMed] [Google Scholar]
- Pika J, Tischler M, Andersen RJ. Glaciasterols A and B, 9,11-secosteroids from the marine sponge Aplysilla glacialis. Can J Chem. 1992;70:1506–1510. doi: 10.1139/v92-186. [DOI] [Google Scholar]
- Plaza A, Gustchina E, Baker HL, Kelly M, Bewley CA. Mirabamides A–D, depsipeptides from the sponge Siliquari-aspongia mirabilis that inhibit HIV-1 fusion. J Nat Prod. 2007;70:1753–1760. doi: 10.1021/np070306k. [DOI] [PubMed] [Google Scholar]
- Pontón J, Rüchel R, Clemonds KV, Coleman DC, Grillot R, Guarro J, Aldebert D, Ambroise-Thomas P, Cano J, Carrillo-Muñoz AJ, Gené J, Pinel C, Stevens DA, Sullivan D. Emerging pathogens. Med Mycol. 2000;38:225–236. doi: 10.1080/mmy.38.s1.225.236. [DOI] [PubMed] [Google Scholar]
- Proksch P, Edrada RA, Ebel R. Drugs from the seas-current status and microbiological implications. Appl Microbiol Biotechnol. 2002;59:125–134. doi: 10.1007/s00253-002-1006-8. [DOI] [PubMed] [Google Scholar]
- Proksch P, Putz A, Ortlepp S, Kjer J, Bayer M. Bioactive natural products from marine sponges and fungal endophytes. Phytochem Rev. 2010;9:475–489. doi: 10.1007/s11101-010-9178-9. [DOI] [Google Scholar]
- Quinn RJ, Gregson RP, Cook AF, Bartlett AF. Isolation and synthesis of 1-methylisoguanisine, a potent pharmacologically active constituent from the marine sponge Tedania digitata. Tetrahedron Lett. 1980;21:567–568. doi: 10.1016/S0040-4039(01)85558-1. [DOI] [Google Scholar]
- Qureshi A, Faulkner DJ. Haplosamates A and B: new steroidal sulfamate esters from two haplosclerid sponges. Tetrahedron. 1999;55:8323–8330. doi: 10.1016/S0040-4020(99)00465-2. [DOI] [Google Scholar]
- Rahden-Staron I. The inhibitory effect of the fungicides captan and captafol on eukaryotic topoisomerases in vitro and lack of recombinagenic activity in the wing spot test of Drosophila melanogaster. Mutat Res. 2002;518:205–213. doi: 10.1016/S1383-5718(02)00107-9. [DOI] [PubMed] [Google Scholar]
- Ramel G. Phylum Porifera [cited 2013 Jan] 2010 Available from: http://www.earthlife.net/inverts/porifera.html/.
- Rao VK, Kasanah N, Wahyuono S, Tekwani BL, Schinazi RF, Hamann MT. Three new manzamine alkaloids from a common indonesian sponge and their activity against infectious and tropical parasitic diseases. J Nat Prod. 2004;67:1314–1318. doi: 10.1021/np0400095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice LB. Antimicrobial resistance in gram-positive bacteria. Am. J. Infect. Control. 2006;34:S11–S19. doi: 10.1016/j.ajic.2006.05.220. [DOI] [PubMed] [Google Scholar]
- Romo D, Rzasa RM, Shea HA, Park K, Langenhan JM, Sun L, Akhiezer A, Liu JO. Total synthesis and immuno-suppressive activity of (−)-pateamine A and related compounds: implementation of a b-lactambased macrocyclization. J Am Chem Soc. 1998;120:12237–12254. doi: 10.1021/ja981846u. [DOI] [Google Scholar]
- Rubio BK, van Soest RW, Crews P. Extending the record of meroditerpenes from Cacospongia marine sponges. J Nat Prod. 2007;70:628–631. doi: 10.1021/np060633c. [DOI] [PubMed] [Google Scholar]
- Sagar S, Kaur M, Minneman KP. Antiviral lead compounds from marine sponges. Mar. Drugs. 2010;8:2619–2638. doi: 10.3390/md8102619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai R, Higa T, Jefford CW, Bernardinelli G. Manzamin, A., a novel antitumor alkaloid from a sponge. J Am Chem Soc. 1986;108:6404–6405. doi: 10.1021/ja00280a055. [DOI] [Google Scholar]
- Sandven P. Epidemiology of candidemia. Rev Iberoam Micol. 2000;17:73–81. [PubMed] [Google Scholar]
- Schaschke N, Sommerhoff PC. Upgrading a natural product: inhibition of human β-tryptase by cyclotheonamide analogues. Chem Med Chem. 2010;5:367–370. doi: 10.1002/cmdc.200900484. [DOI] [PubMed] [Google Scholar]
- Schwartsmann G. Marine organisms and other novel natural sources of new cancer drugs. Ann Oncol. 2000;11:235–243. doi: 10.1093/annonc/11.suppl_3.235. [DOI] [PubMed] [Google Scholar]
- Shimosaka A. Role of NKT cells and a-galactosyl ceramide. Int J Hematol. 2002;76:277–279. doi: 10.1007/BF03165262. [DOI] [PubMed] [Google Scholar]
- Shuman RT, Rothenberger RB, Campell CS, Smith GF, Gifford-Moore DS, Gesellchen PD. Highly selective tripeptide thrombm inhibitors. J Med Chem. 1993;36:314–319. doi: 10.1021/jm00055a002. [DOI] [PubMed] [Google Scholar]
- Sipkema D, Osinga R, Schatton W, Mendola D, Tramper J, Wijffels RH. Large scale production of pharmaceuticals by marine sponges: Sea, cell, or biosynthesis. Biotechnol Bioeng. 2005;90:201–222. doi: 10.1002/bit.20404. [DOI] [PubMed] [Google Scholar]
- Souza TM, Abrantes JL, de A Epifanio R, Leite Fontes CF, Frugulhetti IC. The alkaloid 4-methylaaptamine isolated from the sponge Aaptos aaptos impairs Herpes simplex virus Type 1 penetration and immediate early protein synthesis. Planta Med. 2007;73:200–205. doi: 10.1055/s-2007-967109. [DOI] [PubMed] [Google Scholar]
- Stead P, Hiscox S, Robinson PS, Pike NB, Sidebottom PJ, Roberts AD, Taylor NL, Wright AE, Pomponi SA, Langley D. Eryloside F, a novel penasterol disaccharide possessing potent thrombin receptor antagonist activity. Bioorg Med Chem Lett. 2000;10:661–664. doi: 10.1016/S0960-894X(00)00063-9. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Shindo K, Ueno A, Miura T, Takei M, Sakakibara M, Fukamachi H, Tanaka J, Higa T. S1319: A novel β2-adrenoceptor agonist from a marine sponge Dysidea sp. Bioorg Med Chem Lett. 1999;9:1361–1364. doi: 10.1016/S0960-894X(99)00205-X. [DOI] [PubMed] [Google Scholar]
- Takei M, Burgoyne DL, Andersen RJ. Effect of contignasterol on histamine release induced by anti-immunoglobulin E from rat peritoneal mast cells. J Pharm Sci. 1994;83:1234–1235. doi: 10.1002/jps.2600830909. [DOI] [PubMed] [Google Scholar]
- Tan P, Luscinskas FW, Homer-Vanniasinkam S. Cellular and molecular mechanisms of inflammation and thrombosis. Eur J Vasc Endovasc Surg. 1997;17:373–389. doi: 10.1053/ejvs.1998.0759. [DOI] [PubMed] [Google Scholar]
- Tasdemir D, Topaloglu B, Perozzo R, Brun R, O’Neill R, Carballeira NM, Zhang X, Tonge PJ, Linden A, Rüedi P. Marine natural products from the Turkish sponge Agelas oroides that inhibit the enoyl reductases from Plasmodium falciparum, Mycobacterium tuberculosis and Escherichia coli. Bioorg Med Chem. 2007;15:6834–6845. doi: 10.1016/j.bmc.2007.07.032. [DOI] [PubMed] [Google Scholar]
- Ter Haar E, Kowalski RJ, Hamel E, Lin CM, Longley RE, Gunasekera SP, Rosenkranz HS, Day BW. Discodermolide, a cytotoxic marine agent that stabilizes microtubules more potently than taxol. Biochemistry. 1996;35:243–250. doi: 10.1021/bi9515127. [DOI] [PubMed] [Google Scholar]
- Thomas TR, Kavlekar DP, LokaBharathi PA. Marine drugs from sponge-microbe association-a review. Mar. Drugs. 2010;8:1417–1468. doi: 10.3390/md8041417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres YR, Berlink RG, Nascimento GG, Fortier SC, Pessoa C, de Moraes MO. Antibacterial activity against resistant bacteria and cytotoxicity of four alkaloid toxins isolated from the marine sponge Arenosclera brasiliensis. Toxicon. 2002;40:885–891. doi: 10.1016/S0041-0101(01)00286-0. [DOI] [PubMed] [Google Scholar]
- Turk T, Ambrožič Avguštin J, Batista U, Strugar G, Kosmina R, Čivovič S, Janussen D, Kauferstein S, Mebs D, Sepčič K. Biological activities of ethanolic extracts from deep-sea antarctic marine sponges. Mar. Drugs. 2013;11:1126–1139. doi: 10.3390/md11041126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban S, De Almeida Leone P, Carroll AR, Fechner GA, Smith J, Hooper JN, Quinn RJ. Axinellamines A–D, novel imidazo-azolo-imidazole alkaloids from the australian marine sponge Axinella sp. J Org Chem. 1999;64:731–735. doi: 10.1021/jo981034g. [DOI] [PubMed] [Google Scholar]
- Uriz MJ, Martin D, Rosell D. Relationships of biological and taxonomic characteristics to chemically mediated bioactivity in Mediterranean littoral sponges. Mar Biol. 1992;113:287–297. [Google Scholar]
- Vik A, Hedner E, Charnock C, Tangen LW, Samuelsen O, Larsson R, Bohlin L, Gundersen LL. Antimicrobial and cytotoxic activity of agelasine and agelasimine analogs. Bioorg Med Chem. 2007;15:4016–4037. doi: 10.1016/j.bmc.2007.03.086. [DOI] [PubMed] [Google Scholar]
- Wakimoto T, Maruyama A, Matsunaga S, Fusetani N, Shinoda K, Murphy PT. Octa- and nonaprenylhydroquinone sulfates, inhibitors of a1,3-fucosyltransferase VII, from an Australian marine sponge Sarcotragus sp. Bioorg Med Chem Lett. 1999;9:727–730. doi: 10.1016/S0960-894X(99)00059-1. [DOI] [PubMed] [Google Scholar]
- Walsh TJ, Groll A, Hiemenz J, Fleming R, Roilides E, Anaissie E. Infections due to emerging and uncommon medically important fungal pathogens. Clin Microbiol Infect. 2004;10:48–66. doi: 10.1111/j.1470-9465.2004.00839.x. [DOI] [PubMed] [Google Scholar]
- Walter S. Drug discovery: a history. Wiley; New York: 2005. p. 258. [Google Scholar]
- Wellington KD, Cambie RC, Rutledge PS, Bergquist PR. Chemistry of Sponges. 19. Novel Bioactive Metabolites from Hamigeratarangaensis. J Nat Prod. 2000;63:79–85. doi: 10.1021/np9903494. [DOI] [PubMed] [Google Scholar]
- White DE, Fenner FJ. Medical Virology. Academic Press; San Diego: 1986. [Google Scholar]
- WHO . World Malaria report. World Health Organization; Geneva: 2015. [Google Scholar]
- Wiedbrauk DL, Johnston SLG. Manual of Clinical Virology. Raven Press; New York: 1992. [Google Scholar]
- Xue S, Zhanga HT, Wua PC, Zhanga W, Yuana Q. Study on bioactivity of extracts from marine sponges in Chinese Sea. J Exp Mar Biol Ecol. 2004;298:71–78. doi: 10.1016/j.jembe.2003.08.004. [DOI] [Google Scholar]
- Yasuhara-Bell J, Lu Y. Marine compounds and their antiviral activities. Antiviral Res. 2010;86:231–240. doi: 10.1016/j.antiviral.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousaf M, El Sayed KA, Rao KV, Lim CW, Hu JF, Kelly M, Franzblau SG, Zhang F, Peraud O, Hill RT, Hamann MT. 12,34-Oxamanzamines, novel biocatalytic and natural products from rnanzamine producing Indo-Pacific sponges. Tetrahedron. 2002;58:7397–7402. doi: 10.1016/S0040-4020(02)00825-6. [DOI] [Google Scholar]
- Zabriskie TM, Klocke JA, Ireland CM, Marcus AH, Molinski TF, Faulkner DJ, Xu C, Clardy JC. Jaspamide, a modified peptide from a Jaspis sponge, with insecticidal and antifungal activity. J Am Chem Soc. 1986;108:3123–3124. doi: 10.1021/ja00271a062. [DOI] [Google Scholar]
- Zapolska-Downar D, Zapolska-Downar A, Markiewski M, Ciechanowicz M, Kaczmarczyk M, Naruszewicz M. Selective inhibition by procubol of vascular cell adhesion molecule 1 (VCAM-1) expression in human vascular endothelial cells. Atherosclerosis. 2001;155:123–130. doi: 10.1016/S0021-9150(00)00553-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.