Abstract
Store-operated calcium entry (SOCE), a major mode of extracellular calcium entry, plays roles in a variety of cell activities. Accumulating evidence indicates that the intracellular calcium ion concentration and calcium signaling are critical for the responses induced by oxidative stress. The present study was designed to investigate the potential effect of SOCE inhibition on H2O2-induced apoptosis in endothelial progenitor cells (EPCs), which are the predominant cells involved in endothelial repair. The results showed that H2O2-induced EPC apoptosis was reversed by SOCE inhibition induced either using the SOCE antagonist ML-9 or via silencing of stromal interaction molecule 1 (STIM1), a component of SOCE. Furthermore, SOCE inhibition repressed the increases in intracellular reactive oxygen species (ROS) levels and endoplasmic reticulum (ER) stress and ameliorated the mitochondrial dysfunction caused by H2O2. Our findings provide evidence that SOCE inhibition exerts a protective effect on EPCs in response to oxidative stress induced by H2O2 and may serve as a potential therapeutic strategy against vascular endothelial injury.
Keywords: Endothelial progenitor cells, Oxidative stress, SOCE, STIM 1, ML-9
INTRODUCTION
Endothelial dysfunction plays a central role in the development and progression of vascular diseases. Endothelial progenitor cells (EPCs), which are mobilized from bone marrow to the vascular endothelium, are vital for endothelial repair, functioning directly through differentiation and indirectly through paracrine effects. Oxidative stress caused by free radicals, oxygen anions and peroxides is closely associated with the initiation, development and progression of vascular diseases (Heitzer et al., 2001; Yoshii et al., 2006; Schleicher and Friess, 2007; Cachofeiro et al., 2008; Battelli et al., 2014) and has direct cytotoxic effects on the endothelium (Griendling and FitzGerald, 2003; Harrison et al., 2003) and EPCs (Hung et al., 2003; Urbich et al., 2005; Thum et al., 2006; Thum et al., 2007; Watson et al., 2008). However, the specific mechanism of these effects is not clear, and the roles of calcium and calcium signaling in this mechanism are undefined.
Calcium is an important ion that regulates a variety of activities, including muscle contraction, skeleton formation, and cell proliferation, migration and apoptosis. Store-operated calcium channels (SOCs; also known as SOCCs) mediate store-operated calcium entry (SOCE) and are expressed ubiquitously in non-excitable cells, such as EPCs (Lewis, 2007). Previous studies have demonstrated that SOCE in embryonic fibroblasts is critical for the oxidative stress response (Henke et al., 2012). Numerous studies have suggested that oxidative stress enhances the inward flow of calcium, leading to intracellular calcium overload (Caron et al., 2007) and ultimately, cell apoptosis (Henke et al., 2013). Therefore, we hypothesized that SOCE may play a significant role in oxidative stress-induced apoptosis in EPCs. Inhibition of SOCE may protect EPCs from oxidative stress-induced apoptosis, thus making SOCE a potential target for the treatment of various vascular diseases.
Stromal interaction molecule (STIM) proteins are localized to the endoplasmic reticulum (ER) and act as sensors of ER calcium. These proteins play a direct role in the ER-plasma membrane (PM) junction (Rebecchi and Pentyala, 2000; Liou et al., 2005). They regulate extracellular calcium via SOCE or capacitative calcium entry (Roos et al., 2005). Studies by our group have indicated that both shRNA (shSTIM1) and ML-9, an SOCE antagonist, protect EPCs from injuries induced by oxidative stress. Further work has suggested that this protection may involve interference with multiple factors associated with intracellular reactive oxygen species (ROS), ER stress and mitochondrial dysfunction.
MATERIALS AND METHODS
Cell culture and characterization of EPCs
Male Sprague-Dawley rats were sacrificed by injection of an overdose of sodium pentobarbital (100 mg/kg) into the peritoneal cavity (Zhao et al., 2008), according to the standards of the American Veterinary Medical Association Panel on Euthanasia. Bone marrow-derived EPCs (BM-EPCs) were isolated from the femurs and tibias of rats through density-gradient centrifugation (Sigma-aldrich, St. Louis, USA) according to established methods. Isolated EPCs were cultured in Dulbecco’s modified Eagle’s medium (Gibco, Grand Island, USA) at 37°C under 5% CO2 and 100% humidity. EPCs were cultured for 7 days before being utilized in experiments. To confirm the identities of EPCs, cells were incubated with acLDL-DiI (10 mg/ml) for 6 h and then fixed with 4% paraformaldehyde for 15 min, incubated with fluorescein isothiocyanate-labeled lectin (UEA-1; 10 mg/ml) for 1 h and examined under a laser confocal scanning microscope (Leica, Wetzlar, Germany) (Kuang et al., 2012). Cells that were double positive for acLDL-DiI and UEA-1 were identified as EPCs. Approximately 85% of cells were positive for both markers.
ShRNA transfection
STIM1 expression was silenced using a lentiviral vector carrying stromal interaction molecule 1 (STIM1) shRNA constructed by GenePharma (Shanghai, China). EPCs were cultured in 6-well plates 2 days before transfection. Cells were infected with the lentiviral vector at a multiplicity of infection of 100 for 48 h. The STIM1 shRNA sequence was GCGACTTCTGAAGAGTCTACC. The negative control shRNA sequence was TTCTCCGAACGTGTCACGT. The efficiency of shRNA transfection was confirmed by Western blot analysis.
Cell viability assay
A cell viability assay was performed using a Cell Counting Kit-8 (CCK-8) (Beyotime, Jiangsu, China) in accordance with the manufacturer’s protocol. After 7 days of culturing, EPCs were trypsinized (HyClone, Utah, USA) and seeded into 96-well plates (approximately 5,000 cells/well). Cell viability was quantified by measuring absorbance at 450 nm with a micro-plate reader (Molecular Devices, Sunnyvale, USA) after cells were treated with processing factors and incubated with CCK-8. The results were expressed as a percentage of the control group (Hou et al., 2007; Zhang and Sun, 2010).
Apoptosis assay
EPC apoptosis was evaluated with a FITC-Annexin V Apoptosis Detection Kit (Neo Bioscience, Beijing, China). After 7 days of culturing, EPCs were treated with processing factors and then trypsinized and treated with Annexin V-FITC and propidium iodide (PI). The treated cells were finally counted via flow cytometry (Beckman coulter, Indianapolis, USA) following the manufacturer’s protocol. The results were expressed as the percentages of cells in the second quadrant (early apoptosis) and third quadrant (late apoptosis).
Calcium imaging
Intracellular calcium concentration was measured with the Fluo-3AM calcium indicator. EPCs were pretreated with 50 μM ML-9 or shSTIM1, incubated with Fluo-3AM for 30 min in the dark, washed 3 times with phosphate-buffered saline (PBS), incubated in medium for 30 min, washed with PBS three times and placed in a calcium-containing or calcium-free solution. The cells were then observed under a laser scanning confocal microscope at an excitation wavelength of 488 nm and emission wavelengths alternating between 525 nm and 530 nm. The results were analyzed with specific software (Li et al., 2009).
Detection of ROS in EPCs
To detect intracellular ROS in EPCs, we used a Reactive Oxygen Species Assay Kit (Beyotime), which detects changes in intracellular ROS using the DCFH-DA fluorescent probe. EPCs were treated with processing factors and then incubated with DCFH-DA and observed under a laser scanning confocal microscope at a 488 nm excitation wavelength and a 525 nm emission wavelength. The results were evaluated based on the intensity of the intracellular fluorescence (Jia et al., 2006).
Measurement of the mitochondrial membrane potential (MMP)
The MMP was measured using the fluorescent dye rhodamine123 (Rh123) (Beyotime). Rh123 rapidly passes through the PM and is sequestered by active mitochondria within a few minutes. In our experiments, EPCs were cultured with 2 μM Rh123 for 30 min at 37°C in the dark and then washed with PBS. The results were estimated by determining the gray values of the cells.
Western blot analysis
After the various treatments were performed, proteins were extracted from EPCs using a lysis buffer (RIPA) containing phenylmethanesulfonyl fluoride (PMSF). Protein concentrations were determined with a BCA protein assay kit. Equal amounts of protein (30 μg per lane) were separated via sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were blocked with 5% non-fat milk in Tris-buffered saline containing 0.5% Tween 20 and then incubated with specific primary antibodies. The membranes were subsequently incubated with appropriate secondary antibodies and then visualized with a luminescent image analyzer (GE, Connecticut, USA).
Statistical analysis
Statistical analyses were performed, and the data were expressed as the mean ± standard deviation (SD) of five experiments. SPSS 16.0 was utilized for data analysis. The t-test was employed to compare two groups, and one-way analysis of variance (ANOVA) was used to compare multiple groups. A p-value<0.05 was regarded as statistically significant.
RESULTS
H2O2-induced cytotoxicity and apoptotic cell death involve disruption of intracellular calcium homeostasis
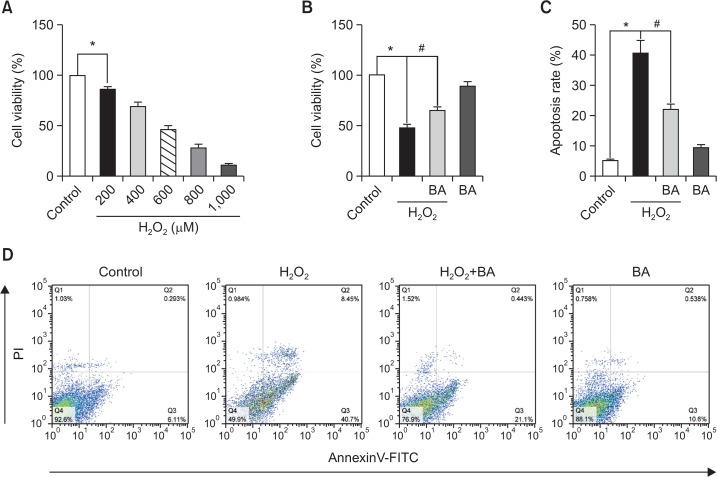
EPCs (cultured for 7 days) were exposed to various concentrations (200, 400, 600, 800 and 1000 μM) of H2O2 for 6 h, and cytotoxicity was determined with a CCK-8 kit. As shown in Fig. 1A, H2O2 induced cytotoxicity in a dose-dependent manner. When the H2O2 concentration was increased to 600 μM, cell viability was approximately 50%. This concentration was used in the following experiments. H2O2-induced apoptosis was detected via flow cytometry. To assess whether intracellular calcium was involved in the H2O2-induced cytotoxicity and apoptosis of EPCs, we pretreated EPCs with BAPTA-AM (BA), an intracellular free calcium chelator (Fig. 1B–1D). The addition of BA (10 μM) attenuated the H2O2-induced injury of EPCs, suggesting that H2O2-induced injury may be related to disruption of intracellular calcium homeostasis.
Fig. 1.
H2O2-induced cytotoxicity and apoptotic cell death involve disruption of intracellular calcium homeostasis. After treatment with different concentrations of H2O2 for 6 h, cell viability was measured using a CCK-8 kit (A). After pretreatment with BAPTA-AM (BA), cells were treated with H2O2 for 6 h, and cell viability was measured (B). EPCs were pretreated with BAPTA-AM (BA) before exposure to H2O2 for 6 h. The cells were then stained with Annexin V-FITC and propidium iodide (PI) and analyzed via flow cytometry (D). Quantitative data for Annexin V-FITC/PI staining is presented (C). *p<0.05; #p<0.05.
Inhibition of SOCE attenuates the detrimental effects of H2O2 on EPCs
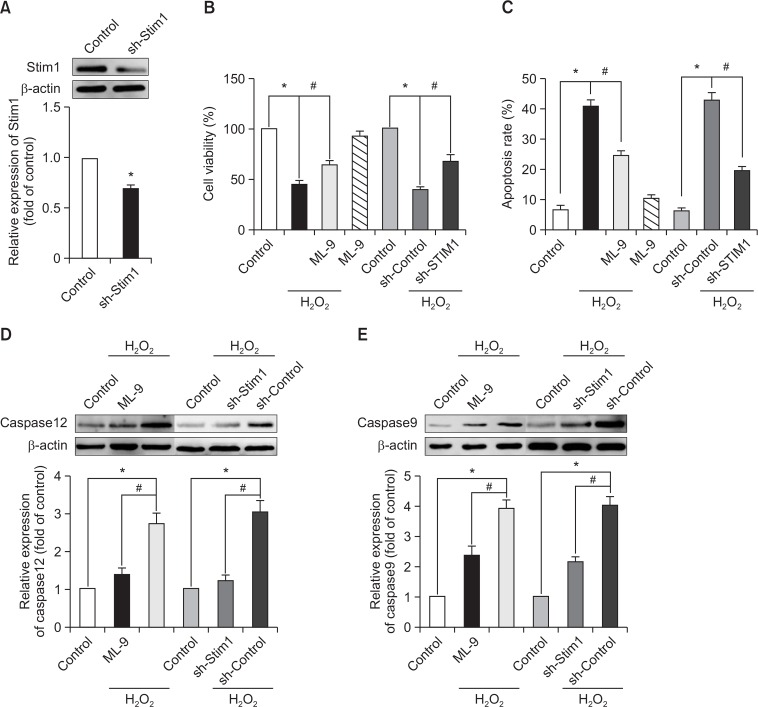
To investigate the effects of SOCE inhibition, we utilized ML-9 (50 μM), an antagonist of SOCE, and silenced the expression of STIM1 by shRNA (Fig. 2A). This method was used for SOCE in the following experiments. As hypothesized, the H2O2-induced decrease in EPC viability and increase in EPC apoptosis were reversed by the inhibition of SOCE (Fig. 2B, 2C). In addition, the H2O2-induced up-regulation of caspase 12, which is responsible for ER stress-induced apoptosis (Rasheva and Domingos, 2009), and caspase 9, which is closely related to the mitochondrial apoptotic pathway, was significantly attenuated by SOCE inhibition (Fig. 2D, 2E).
Fig. 2.
Inhibition of SOCE attenuates the detrimental effects of H2O2 on EPCs. Representative immunoblots (top) and quantification (bottom) of STIM1 expression 48 h after transfection (A). EPCs that had been cultured for 7 days were pretreated with ML-9 for 20 min or with STIM1 shRNA before the addition of H2O2 for 6 h. Cell viability (B) and apoptosis (C) were then measured. The relative expression levels of caspase 12 (D) and caspase 9 (E) were measured by Western blot analysis. *p<0.05; #p<0.05.
Inhibition of SOCE restores intracellular calcium homeostasis
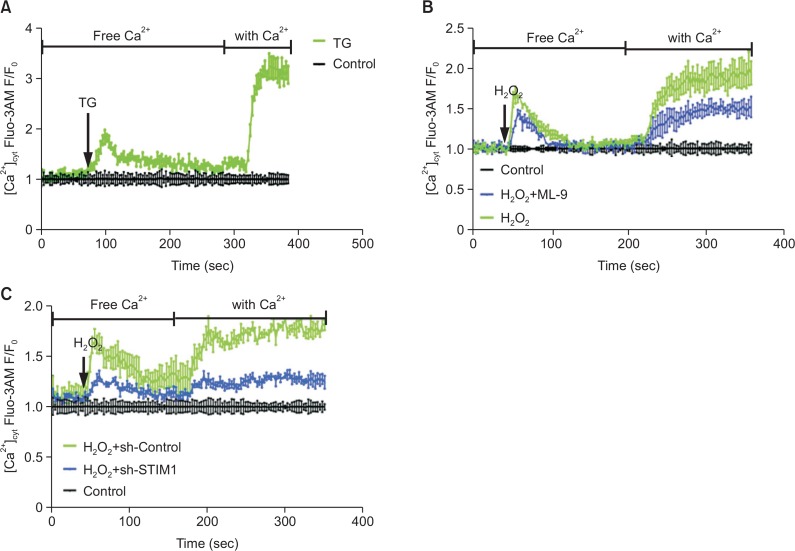
Numerous data have demonstrated that calcium entry through SOCE channels is a common and ubiquitous mechanism of regulating calcium entry and that SOCE is the main mode of calcium entry into EPCs. Thus, we performed calcium imaging to determine whether H2O2 affects calcium signaling in EPCs through SOCE. H2O2 stimulation of EPCs in a calcium-free buffer led to an increase in cytosolic calcium due to calcium release from the ER (left peaks in Fig. 3B, 3C). The addition of calcium after cytosolic calcium level slowed down resulted in calcium influx through the PM (right peaks in Fig. 3B, 3C) due to the depletion of ER stores. These results were considered typical calcium imaging results for SOCE and were similar to thapsigargin (TG; 2 μM)-induced calcium fluxes (Fig. 3A). Therefore, H2O2-induced calcium changes potentially occur mainly through SOCE. Furthermore, the inhibition of SOCE resulted in a significant decrease in cytosolic calcium in response to H2O2 insult (Fig. 3B, 3C).
Fig. 3.
Inhibition of SOCE restores intracellular calcium homeostasis. EPCs that had been cultured for 7 days were treated with thapsigargin (TG) (A) or pretreated with ML-9 for 20 min (B) or with STIM1 shRNA (C) before exposure to H2O2 in a calcium-free solution, followed by treatment with 2 mM CaCl2. The intracellular calcium concentration was then measured using Fluo-3AM, a calcium indicator.
Inhibition of SOCE prevents intracellular ROS accumulation
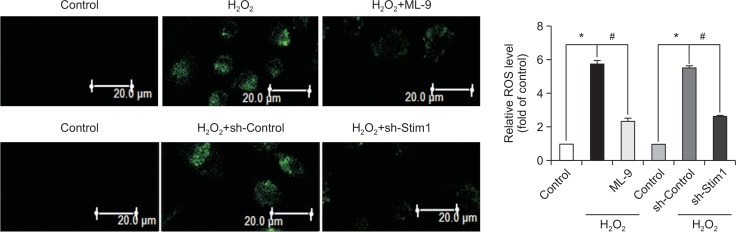
To investigate the role of ROS in the protective effects of SOCE inhibition, we used the fluorescent probe DCFH-DA to detect intracellular ROS production. Compared with the control group, the H2O2 treatment group exhibited significantly increased ROS accumulation, and the inhibition of SOCE markedly blunted this effect (Fig. 4).
Fig. 4.
Inhibition of SOCE prevents intracellular ROS accumulation. EPCs that had been cultured for 7 days were pretreated with ML-9 for 20 min or with STIM1 shRNA before exposure to H2O2 for 6 h, and intracellular ROS levels were evaluated using a fluorescence probe (DCFHDA). *p<0.05; #p<0.05.
Inhibition of SOCE protects EPCs from ER stress
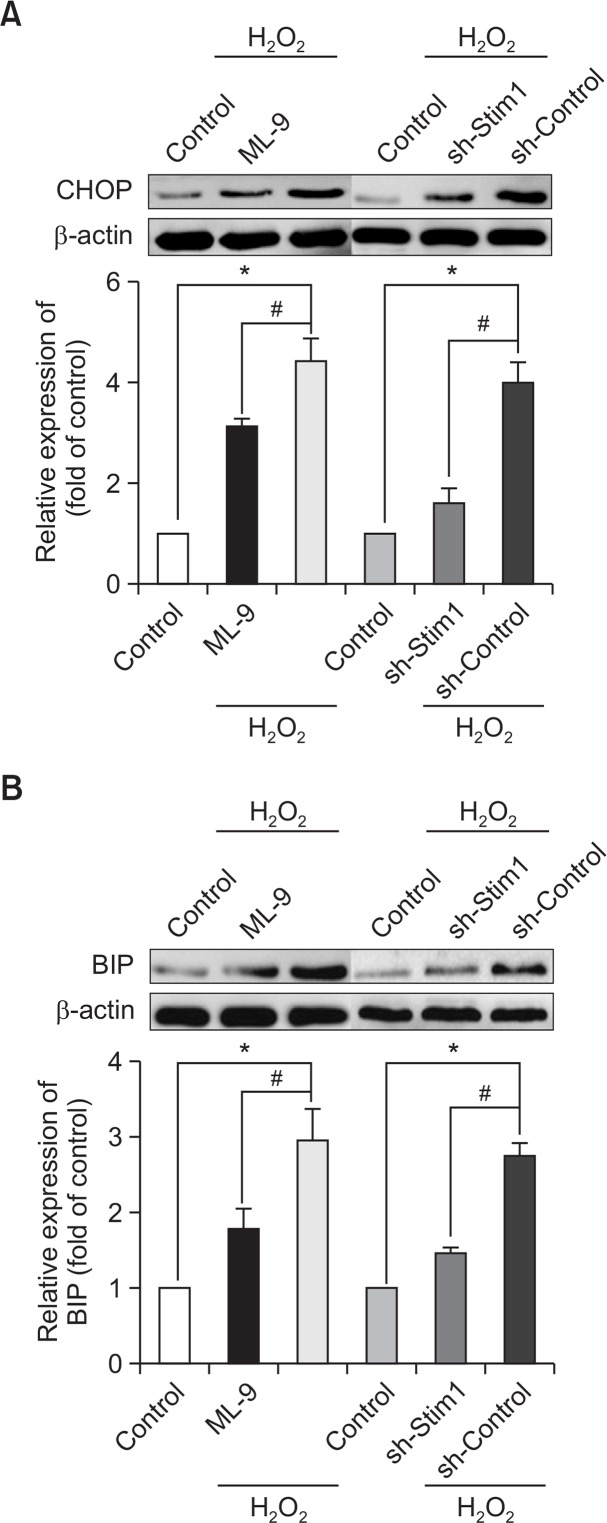
Many studies have demonstrated that ER stress is often accompanied by increased ROS production. To assess the protective effect of SOCE inhibition, we measured the expression of ER stress-related proteins. As shown in Fig. 5A, 5B, the expression levels of C/EBP homologous protein (CHOP) and CRP78/BIP were up-regulated by H2O2 stimulation compared with that of the control group. Moreover, the inhibition of SOCE suppressed the expression of ER stress-related proteins.
Fig. 5.
Inhibition of SOCE protects EPCs from ER stress. EPCs that had been cultured for 7 days were pretreated with ML-9 for 20 min or with STIM1 shRNA before treatment with H2O2 for 6 h. The expression of the ER stress-related proteins CHOP (A) and BIP (B) was evaluated by Western blot analysis. *p<0.05; #p<0.05.
Inhibition of SOCE ameliorates H2O2-induced mitochondrial dysfunction
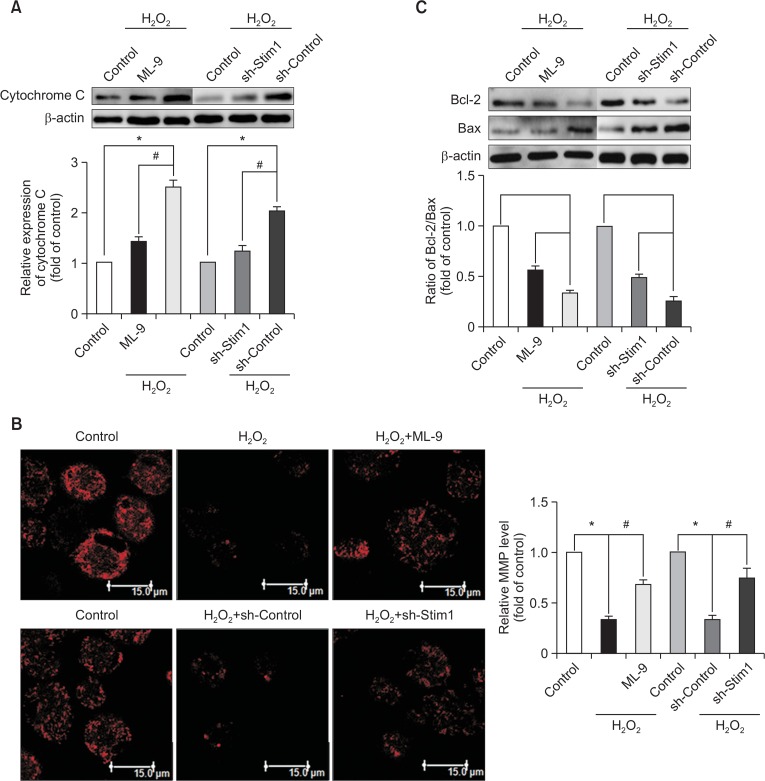
To demonstrate the effects of SOCE inhibition on the repair of mitochondrial dysfunction, we measured the MMP by Rh123 staining. After incubation with H2O2, the MMP was reduced compared with that of the control group, and this effect was ameliorated by SOCE inhibition (Fig. 6B). Moreover, the results of Western blot analysis suggested that H2O2 treatment up-regulated the cytosolic cytochrome C level compared with that of the control group (Fig. 6A). As predicted, SOCE inhibition significantly reduced the detrimental effects induced by H2O2. Furthermore, we observed the effects of SOCE inhibition on Bcl-2 family members, which play a vital role in the mitochondrial pro-apoptotic signaling pathway. Exposure to H2O2 led to a decrease in the Bcl-2/Bax ratio compared with that of the control group (Fig. 6C), and this decrease was ameliorated by SOCE inhibition.
Fig. 6.
Inhibition of SOCE ameliorates H2O2-induced mitochondrial dysfunction. EPCs that had been cultured for 7 days were pretreated with ML-9 for 20 min or with STIM1 shRNA before treatment with H2O2 for 6 h. The release of cytochrome C into the cytoplasm (A) and the Bcl-2/Bax ratio were measured by Western blot analysis (C). The MMP was measured via rhodamine 123 staining (B). *p<0.05; #p<0.05.
DISCUSSION
This study has shown for the first time that SOCE plays key roles in the H2O2-induced decrease in EPC cell viability and increase in EPC apoptosis. We have also demonstrated that SOCE inhibition attenuates H2O2-induced EPC cytotoxicity and apoptosis. First, H2O2 insult activated the process known as calcium release-activated calcium influx. Second, SOCE inhibition suppressed H2O2-induced calcium influx. Third, SOCE inhibition attenuated H2O2-induced EPC cytotoxicity and apoptosis. Finally, SOCE inhibition significantly reversed the H2O2-mediated induction of several key pro-apoptotic signaling pathways mediated by ER stress and mitochondrial dysfunction. Taken together, our results demonstrate that SOCE inhibition protects EPCs from H2O2-induced apoptosis.
It is generally recognized that H2O2 insult leads to calcium influx and disruption of intracellular calcium homeostasis, followed by an increase in ROS production (Ohyama et al., 2012). These changes further activate downstream apoptotic signaling pathways and ultimately result in cell death. Accumulating evidence suggests that oxidative stress-induced injuries might be attenuated by stabilizing intracellular calcium homeostasis (Godfraind, 2005; Ohyama et al., 2012). Moreover, some studies have indicated that antioxidants diminish increases in intracellular calcium levels (Pieper and Dondlinger, 1998; Su et al., 1999). Although the specific mechanism of the reduction of intracellular calcium following antioxidant application is still not clear, we hypothesized that the decrease in intracellular ROS production in response to antioxidant insult might lead to a change in the intracellular calcium level. In the present study, we demonstrated that H2O2 insult resulted in calcium influx and increase in intracellular ROS levels. In addition, we found that SOCE inhibition prevented intracellular ROS accumulation. To date, there have been no reports of the effect of antioxidants on calcium regulation in EPCs. Thus we will pay more attention to this aspect in our future study.
SOCE, which occurs ubiquitously in non-excitable cells, plays an essential role in the regulation of intracellular calcium homeostasis. Accumulating evidence has demonstrated that SOCE is involved in oxidative stress (Törnquist et al., 2000; Martín-Romero et al., 2008; Henke et al., 2013; Plomaritas et al., 2014). However, the effect of SOCE on oxidative stress remains to be elucidated. Several studies have demonstrated that H2O2 insult weakens SOCE (Törnquist et al., 2000; Plomaritas et al., 2014). Other studies have shown that H2O2 insult augments SOCE and the influx of calcium ions (Rao et al., 2013). These contradictory results may be explained by the activation of different signaling pathways in response to oxidative stress in different cell types. For example, the oxidative stress-induced changes in calcium influx and SOCE are mediated by the activation of protein kinase C (PKC) in thyroid FRTL-5 cells (Törnquist et al., 2000) and by regulation of STIM1 expression in the HT22 cells (Rao et al., 2013). In the present study, the H2O2 insult-induced disruption of intracellular calcium homeostasis was attenuated by inhibiting SOCE using ML-9 or via STIM1 silencing. These findings suggest that SOCE plays a critical role in oxidative stress.
Accumulating evidence supports the notion that calcium and calcium signaling are involved in ER and mitochondrial activities (Arnaudeau et al., 2001; Pizzo and Pozzan, 2007). The ER, a site of major calcium stores, plays a critical role in a variety of cellular activities, such as protein synthesis, post-translational modifications, protein folding and apoptosis. A prolonged decrease in the ER calcium level (Hooper et al., 2013) or accumulation of unfolded or misfolded proteins within the ER (Hammadi et al., 2013) leads to ER stress. Prolonged ER stress further promotes the expression of CHOP, a CEBP family transcription factor, and caspase 12, thereby activating downstream apoptotic pathways. Moreover, ER stress can result in sustained mitochondrial calcium accumulation (Deniaud et al., 2008). The mitochondria, which are also important calcium-buffering organelles, are physically and functionally interconnected with the ER. Mitochondria are often located near calcium release sites in the ER or at calcium influx channels at the PM, where they buffer intracellular calcium changes by modulating the rate of capacitative calcium entry through calcium release-activated channels (CRACs) or the rate of calcium release by IP3 receptors (Arnaudeau et al., 2001). Disruption of mitochondrial calcium homeostasis may result in dysfunction of the mitochondria, resulting in up-regulation of caspase 9 expression and enhanced release of cytochrome c from the mitochondria into the cytoplasm. Cytochrome c and caspase 9 form apoptotic bodies through the multiple polymerization of apoptotic protease activating factor 1 (Apaf-1), thereby activating downstream apoptotic pathways (Hikita et al., 2015). Several published reports have suggested that various apoptotic pathways are involved in the cross-talk between the ER and mitochondria (Wang and El-Deiry, 2004; Pizzo and Pozzan, 2007). Cytochrome c, which plays a vital role in this cross-talk, translocates to the ER to bind inositol 1,4,5-triphosphate receptors, leading to sustained calcium release. This calcium release in turn triggers the release of cytochrome c. Importantly, cytochrome c is involved in these apoptotic pathways. The present study demonstrated that SOCE inhibition restored intracellular calcium homeostasis and suppressed the pro-apoptotic effect by reducing ER stress and preserving mitochondrial function. Taken together, these data show that communication between the ER and mitochondria is involved in the protective effect of SOCE inhibition against H2O2 insult.
In conclusion, these findings have confirmed the role of SOCE in oxidative stress. H2O2-induced EPC apoptosis was shown to be alleviated by SOCE inhibition. The probable underlying mechanisms may be related to intracellular ROS levels, ER stress and mitochondrial dysfunction. Therefore, SOCE inhibition may serve as a potential therapeutic strategy for EPC injury in oxidative stress.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant 81370211). We would like to thank Prof. J.H.Z. and Prof. Y.Y. for the critical comments on this manuscript.
Footnotes
CONFLICTS OF INTEREST
All authors declare that there are no conflicts of interest.
REFERENCES
- Arnaudeau S, Kelley WL, Walsh JV, Jr, Demaurex N. Mitochondria recycle Ca2+ to the endoplasmic reticulum and prevent the depletion of neighboring endoplasmic reticulum regions. J Biol Chem. 2001;276:29430–29439. doi: 10.1074/jbc.M103274200. [DOI] [PubMed] [Google Scholar]
- Battelli MG, Polito L, Bolognesi A. Xanthine oxidore-ductase in atherosclerosis pathogenesis: not only oxidative stress. Atherosclerosis. 2014;237:562–567. doi: 10.1016/j.atherosclerosis.2014.10.006. [DOI] [PubMed] [Google Scholar]
- Cachofeiro V, Goicochea M, de Vinuesa SG, Oubiña P, Lahera V, Luño J. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int Suppl. 2008:S4–S9. doi: 10.1038/ki.2008.516. [DOI] [PubMed] [Google Scholar]
- Caron AZ, Chaloux B, Arguin G, Guillemette G. Protein kinase C decreases the apparent affinity of the inositol 1,4,5-trisphosphate receptor type 3 in RINm5F cells. Cell calcium. 2007;42:323–331. doi: 10.1016/j.ceca.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Deniaud A, Sharaf el dein O, Maillier E, Poncet D, Kroemer G, Lemaire C, Brenner C. Endoplasmic reticulum stress induces calcium-dependent permeability transition, mitochondrial outer membrane permeabilization and apoptosis. Oncogene. 2008;27:285–299. doi: 10.1038/sj.onc.1210638. [DOI] [PubMed] [Google Scholar]
- Godfraind T. Antioxidant effects and the therapeutic mode of action of calcium channel blockers in hypertension and atherosclerosis. Philos Trans R Soc Lond B, Biol Sci. 2005;360:2259–2272. doi: 10.1098/rstb.2005.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury: Part II: animal and human studies. Circulation. 2003;108:2034–2040. doi: 10.1161/01.CIR.0000093661.90582.c4. [DOI] [PubMed] [Google Scholar]
- Hammadi M, Oulidi A, Gackière F, Katsogiannou M, Slomianny C, Roudbaraki M, Dewailly E, Delcourt P, Lepage G, Lotteau S, Ducreux S, Prevarskaya N, Van Coppenolle F. Modulation of ER stress and apoptosis by endoplasmic reticulum calcium leak via translocon during unfolded protein response: involvement of GRP78. FASEB J. 2013;27:1600–1609. doi: 10.1096/fj.12-218875. [DOI] [PubMed] [Google Scholar]
- Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H. Role of oxidative stress in atherosclerosis. Am J Cardiol. 2003;91:7A–11A. doi: 10.1016/S0002-9149(02)03144-2. [DOI] [PubMed] [Google Scholar]
- Heitzer T, Schlinzig T, Krohn K, Meinertz T, Münzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–2678. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- Henke N, Albrecht P, Bouchachia I, Ryazantseva M, Knoll K, Lewerenz J, Kaznacheyeva E, Maher P, Methner A. The plasma membrane channel ORAI1 mediates detrimental calcium influx caused by endogenous oxidative stress. Cell Death Dis. 2013;4:e470. doi: 10.1038/cddis.2012.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke N, Albrecht P, Pfeiffer A, Toutzaris D, Zanger K, Methner A. Stromal interaction molecule 1 (STIM1) is involved in the regulation of mitochondrial shape and bioenergetics and plays a role in oxidative stress. J Biol Chem. 2012;287:42042–42052. doi: 10.1074/jbc.M112.417212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikita H, Kodama T, Tanaka S, Saito Y, Nozaki Y, Nakabori T, Shimizu S, Hayashi Y, Li W, Shigekawa M, Sakamori R, Miyagi T, Hiramatsu N, Tatsumi T, Takehara T. Activation of the mitochondrial apoptotic pathway produces reactive oxygen species and oxidative damage in hepatocytes that contribute to liver tumorigenesis. Cancer Prev. Res. (Phila.) 2015;8:693–701. doi: 10.1158/1940-6207.CAPR-15-0022-T. [DOI] [PubMed] [Google Scholar]
- Hooper R, Samakai E, Kedra J, Soboloff J. Multifaceted roles of STIM proteins. Pflugers Arch. 2013;465:1383–1396. doi: 10.1007/s00424-013-1270-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou G, Xue L, Lu Z, Fan T, Tian F, Xue Y. An activated mTOR/p70S6K signaling pathway in esophageal squamous cell carcinoma cell lines and inhibition of the pathway by rapamycin and siRNA against mTOR. Cancer Lett. 2007;253:236–248. doi: 10.1016/j.canlet.2007.01.026. [DOI] [PubMed] [Google Scholar]
- Hung YC, Sava VM, Blagodarsky VA, Hong MY, Huang GS. Protection of tea melanin on hydrazine-induced liver injury. Life Sci. 2003;72:1061–1071. doi: 10.1016/S0024-3205(02)02348-2. [DOI] [PubMed] [Google Scholar]
- Jia SJ, Jiang DJ, Hu CP, Zhang XH, Deng HW, Li YJ. Lysophosphatidylcholine-induced elevation of asymmetric dimethylarginine level by the NADPH oxidase pathway in endothelial cells. Vascul Pharmacol. 2006;44:143–148. doi: 10.1016/j.vph.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Kuang CY, Yu Y, Wang K, Qian DH, Den MY, Huang L. Knockdown of transient receptor potential canonical-1 reduces the proliferation and migration of endothelial progenitor cells. Stem Cells Dev. 2012;21:487–496. doi: 10.1089/scd.2011.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RS. The molecular choreography of a store-operated calcium channel. Nature. 2007;446:284–287. doi: 10.1038/nature05637. [DOI] [PubMed] [Google Scholar]
- Li B, Xiao L, Wang ZY, Zheng PS. Knockdown of STIM1 inhibits 6-hydroxydopamine-induced oxidative stress through attenuating calcium-dependent ER stress and mitochondrial dysfunction in undifferentiated PC12 cells. Free Radic Res. 2014;48:758–768. doi: 10.3109/10715762.2014.905687. [DOI] [PubMed] [Google Scholar]
- Li H, Wang L, Ye L, Mao Y, Xie X, Xia C, Chen J, Lu Z, Song J. Influence of Pseudomonas aeruginosa quorum sensing signal molecule N-(3-oxododecanoyl) homoserine lactone on mast cells. Med Microbiol Immunol. 2009;198:113–121. doi: 10.1007/s00430-009-0111-z. [DOI] [PubMed] [Google Scholar]
- Li X, Chen W, Zhang L, Liu WB, Fei Z. Inhibition of store-operated calcium entry attenuates MPP(+)-induced oxidative stress via preservation of mitochondrial function in PC12 cells: involvement of Homer1a. PLoS ONE. 2013;8:e83638. doi: 10.1371/journal.pone.0083638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Romero FJ, Ortíz-de-Galisteo JR, Lara-Laranjeira J, Domínguez-Arroyo JA, González-Carrera E, Alvarez IS. Store-operated calcium entry in human oocytes and sensitivity to oxidative stress. Biol Reprod. 2008;78:307–315. doi: 10.1095/biolreprod.107.064527. [DOI] [PubMed] [Google Scholar]
- Ohyama T, Sato K, Kishimoto K, Yamazaki Y, Horiguchi N, Ichikawa T, Kakizaki S, Takagi H, Izumi T, Mori M. Azelnidipine is a calcium blocker that attenuates liver fibrosis and may increase antioxidant defence. Br J Pharmacol. 2012;165:1173–1187. doi: 10.1111/j.1476-5381.2011.01599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieper GM, Dondlinger LA. Antioxidant pyrrolidine dithiocarbamate prevents defective bradykinin-stimulated calcium accumulation and nitric oxide activity following exposure of endothelial cells to elevated glucose concentration. Diabetologia. 1998;41:806–812. doi: 10.1007/s001250050991. [DOI] [PubMed] [Google Scholar]
- Pizzo P, Pozzan T. Mitochondria-endoplasmic reticulum choreography: structure and signaling dynamics. Trends Cell Biol. 2007;17:511–517. doi: 10.1016/j.tcb.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Plomaritas DR, Herbert LM, Yellowhair TR, Resta TC, Gonzalez Bosc LV, Walker BR, Jernigan NL. Chronic hypoxia limits H2O2-induced inhibition of ASIC1-dependent store-operated calcium entry in pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2014;307:L419–L430. doi: 10.1152/ajplung.00095.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao W, Zhang L, Su N, Wang K, Hui H, Wang L, Chen T, Luo P, Yang YF, Liu ZB, Fei Z. Blockade of SOCE protects HT22 cells from hydrogen peroxide-induced apoptosis. Biochem Biophys Res Commun. 2013;441:351–356. doi: 10.1016/j.bbrc.2013.10.054. [DOI] [PubMed] [Google Scholar]
- Rasheva VI, Domingos PM. Cellular responses to endoplasmic reticulum stress and apoptosis. Apoptosis. 2009;14:996–1007. doi: 10.1007/s10495-009-0341-y. [DOI] [PubMed] [Google Scholar]
- Rebecchi MJ, Pentyala SN. Structure, function, and control of phosphoinositide-specific phospholipase C. Physiol Rev. 2000;80:1291–1335. doi: 10.1152/physrev.2000.80.4.1291. [DOI] [PubMed] [Google Scholar]
- Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Veliçelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleicher E, Friess U. Oxidative stress, AGE, and atherosclerosis. Kidney Int Suppl. 2007;72:S17–S26. doi: 10.1038/sj.ki.5002382. [DOI] [PubMed] [Google Scholar]
- Su JY, Duffy S, Murphy TH. Reduction of H2O2-evoked, intracellular calcium increases in the rat N18-RE-105 neuronal cell line by pretreatment with an electrophilic antioxidant inducer. Neurosci Lett. 1999;273:109–112. doi: 10.1016/S0304-3940(99)00634-5. [DOI] [PubMed] [Google Scholar]
- Thum T, Fraccarollo D, Galuppo P, Tsikas D, Frantz S, Ertl G, Bauersachs J. Bone marrow molecular alterations after myocardial infarction: Impact on endothelial progenitor cells. Cardiovasc Res. 2006;70:50–60. doi: 10.1016/j.cardiores.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Thum T, Fraccarollo D, Schultheiss M, Froese S, Galuppo P, Widder JD, Tsikas D, Ertl G, Bauersachs J. Endothelial nitric oxide synthase uncoupling impairs endothelial progenitor cell mobilization and function in diabetes. Diabetes. 2007;56:666–674. doi: 10.2337/db06-0699. [DOI] [PubMed] [Google Scholar]
- Törnquist K, Vainio PJ, Björklund S, Titievsky A, Dugué B, Tuominen RK. Hydrogen peroxide attenuates store-operated calcium entry and enhances calcium extrusion in thyroid FRTL-5 cells. Biochem J. 2000;351:47–56. doi: 10.1042/bj3510047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbich C, Knau A, Fichtlscherer S, Walter DH, Brühl T, Potente M, Hofmann WK, de Vos S, Zeiher AM, Dimmeler S. FOXO-dependent expression of the proapoptotic protein Bim: pivotal role for apoptosis signaling in endothelial progenitor cells. FASEB J. 2005;19:974–976. doi: 10.1096/fj.04-2727fje. [DOI] [PubMed] [Google Scholar]
- Wang S, El-Deiry WS. Cytochrome c: a crosslink between the mitochondria and the endoplasmic reticulum in calcium-dependent apoptosis. Cancer Biol Ther. 2004;3:44–46. doi: 10.4161/cbt.3.1.740. [DOI] [PubMed] [Google Scholar]
- Watson T, Goon PK, Lip GY. Endothelial progenitor cells, endothelial dysfunction, inflammation, and oxidative stress in hypertension. Antioxid Redox Signal. 2008;10:1079–1088. doi: 10.1089/ars.2007.1998. [DOI] [PubMed] [Google Scholar]
- Yoshii T, Iwai M, Li Z, Chen R, Ide A, Fukunaga S, Oshita A, Mogi M, Higaki J, Horiuchi M. Regression of atherosclerosis by amlodipine via anti-inflammatory and anti-oxidative stress actions. Hypertens Res. 2006;29:457–466. doi: 10.1291/hypres.29.457. [DOI] [PubMed] [Google Scholar]
- Zhang HY, Sun H. Up-regulation of Foxp3 inhibits cell proliferation, migration and invasion in epithelial ovarian cancer. Cancer Lett. 2010;287:91–97. doi: 10.1016/j.canlet.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Zhao X, Huang L, Yin Y, Fang Y, Zhao J, Chen J. Estrogen induces endothelial progenitor cells proliferation and migration by estrogen receptors and PI3K-dependent pathways. Microvasc Res. 2008;75:45–52. doi: 10.1016/j.mvr.2007.02.009. [DOI] [PubMed] [Google Scholar]