Abstract
Silymarin from milk thistle (Silybum marianum) has been reported to show an anti-cancer activity. In previous study, we reported that silymarin induces cyclin D1 proteasomal degradation through NF-κB-mediated threonine-286 phosphorylation. However, mechanism for the inhibition of Wnt signaling by silymarin still remains unanswered. Thus, we investigated whether silymarin affects Wnt signaling in human colorectal cancer cells to elucidate the additional anti-cancer mechanism of silymarin. Transient transfection with a TOP and FOP FLASH luciferase construct indicated that silymarin suppressed the transcriptional activity of β-catenin/TCF. Silymarin treatment resulted in a decrease of intracellular β-catenin protein but not mRNA. The inhibition of proteasome by MG132 and GSK3β inhibition by SB216763 blocked silymarin-mediated downregulation of β-catenin. In addition, silymarin increased phosphorylation of β-catenin and a point mutation of S33Y attenuated silymarin-mediated β-catenin downregulation. In addition, silymarin decreased TCF4 and increased Axin expression in both protein and mRNA level. From these results, we suggest that silymarin-mediated downregulation of β-catenin and TCF4 may result in the inhibition of Wnt signaling in human colorectal cancer cells.
Keywords: Silymarin, Wnt signaling, Cancer chemoprevention, Human colorectal cancer
INTRODUCTION
Colorectal cancer is the second most leading cause of cancer related deaths worldwide (Siegel et al., 2014). The development of colorectal cancer has been regarded as a multi-step process accompanied by adenomatous polyps, acquiring a series of somatic mutation, and aberrant gene expression (Bos et al., 1987; Fearon et al., 1987).
Because the surgery and adjuvant therapy as currently available therapy against colorectal cancer have severe limitations (Chastek et al., 2013; Ahnen et al., 2014), the complementary and alternative medicines are gaining importance as chemopreventive and chemotherapeutic agents (Cassileth and Deng, 2004; Neergheen et al., 2010).
The abnormal regulation of Wnt signaling pathway is the most frequent cause in development of early stage of colorectal cancer (Fodde et al., 2001). In the absence of activated Wnt signaling, β-catenin locates at cytoplasm by forming the complex with Axin (axis inhibition protein), APC (adenomatous polysis coil) and GSK3β (glycogen synthase kinase 3β), which results in phosphorylation and subsequent proteasomal degradation (Reya and Clevers, 2005; Espada et al., 2009; Anderson et al., 2011). However, under activated Wnt signaling, β-catenin disaggregates from the complex, and subsequently translocates into the nucleus. Nuclear β-catenin forms a ternary complex with transcription factors TCF/Lef (T-cell factor, lymphoid enhancer factor) to activate genes involved in the colorectal tumorigenesis (Behrens et al., 1996; Tetsu and McCormick, 1999). Nuclear accumulation of β-catenin is observed in 80% of colorectal cancer (Sparks et al., 1998), which indicates that β-catenin is important for tumor initiation and later promotion to the colorectal carcinogenesis, and a key target for the preventive and therapeutic applications (Luu et al., 2004; Herbst and Kolligs, 2007). Therefore, some dietary anti-cancer compounds such as curcumin, epigallocatechin-3-gallate, quercetin, flavonoids and vitamin D are known to inhibit Wnt signaling (Narayan, 2004; Amado et al., 2011; Wang et al., 2012).
Silymarin found in the seeds of the milk thistle (Silybum marianum) is a complex of three flavonolignans (silybin, silydianin and silychristin) and two flavonoids (tamoxifen and quercetin) (Abenavoli et al., 2010). Silymarin has been used for treating liver diseases (Mereish et al., 1991) and reported to exert anticarcinogenic properties (Zi et al., 1998; Ramasamy and Agarwal, 2008; Cufí et al., 2013; Eo et al., 2015). Silybin and quercetin among the complex components of silymarin has been reported to inhibit Wnt signaling in human cancer cells (Lu et al., 2012; Novo et al., 2015), which indicates that silymarin may inhibit Wnt signaling. However, more detailed mechanism for silymarin-mediated inhibition of Wnt signaling still remains unanswered.
Here, we propose that silymarin may inhibit Wnt signaling through β-catenin proteasomal degradation and TCF4 transcriptional inhibition in human colorectal cancer cells.
MATERIALS AND METHODS
Reagents
Cell culture media, Dulbecco’s Modified Eagle medium (DMEM)/F-12 1:1 Modified medium (DMEM/F-12) was purchased from Lonza (Walkersville, MD, USA). SB216763, MG132 and silymarin were purchased from Sigma Aldrich (St. Louis, MO, USA). TOP/FOP FLASH luciferase constructs were purchased from Addgene (Cambridge, MA, USA). Antibodies against c-myc, β-catenin, p-β-catenin, TCF4, Axin, p-GSK3β, total-GSK3β, TBP and β-actin were purchased from Cell Signaling (Bervely, MA, USA). Cont- and Axin-siRNA were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). All chemicals were purchased from Fisher Scientific, unless otherwise specified.
Cell culture and treatment
Human colorectal cancer cell lines, HCT116 and SW480 were purchased from Korean Cell Line Bank (Seoul, Korea) and grown in DMEM/F-12 supplemented with 10% fatal bovine serum (FBS), 100 U/ml penicillin and 100 μg/ml streptomycin. The cells were maintained at 37°C under a humidified atmosphere of 5% CO2. Silymarin was dissolved in dimethyl sulfoxide (DMSO) and treated to cells. DMSO was used as a vehicle and the final DMSO concentration did not exceed 0.1% (v/v).
Reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNA was prepared using a RNeasy Mini Kit (Qiagen, Valencia, CA, USA) and total RNA (1 μg) was reverse-transcribed using a Verso cDNA Kit (Thermo Scientific, Pittsburgh, PA, USA) according to the manufacturer’s instruction for cDNA synthesis. PCR was performed using PCR Master Mix Kit (Promega, Madison, WI, USA) with human primers for β-catenin, TCF4, Axin and GAPDH as followed ; β-catenin: forward 5′-cccactaatgtccagcgttt-3′ and reverse 5′-aatccactggtgaaccaagc-3′, TCF4: forward 5′-ttcaaagacgacggcgaacag-3′ and reverse 5′-ttgctgtacgtgataagaggcg-3′, Axin: forward 5′-ac-cgaaagtacattcttgataac-3′ and reverse 5′-tccatacctgaactctct-gc-3′, GAPDH: forward 5′-acccagaagactgtggatgg-3′ and reverse 5′-ttctagacggcaggtcaggt-3′.
Isolation of cytosol and nucleus fraction
Cytosol and nuclear fractions were prepared according to the manufacturer’s instruction of a nuclear extract kit (Active Motif, Carlsbad, CA, USA). Briefly, after washing with ice-cold phosphate-buffered saline (PBS) containing phosphatase inhibitors, cells were harvested with 1× hypotonic buffer containing detergent and then incubated at 4°C for 15 min. The supernatants (cytoplasmic fraction) were collected after centrifugation at 14,000 g for 1 min at 4°C and stored at −80°C. Nuclear fractions were collected by suspending nuclear pellet with lysis buffer at 4°C for 30 min and centrifugation at 14,000 g for 10 min at 4°C.
SDS-PAGE and western blot
After silymarin treatment, cells were washed with 1×phosphate-buffered saline (PBS), and lysed in radioimmunoprecipitation assay (RIPA) buffer (Boston Bio Products, Ashland, MA, USA) supplemented with protease inhibitor cocktail (Sigma Aldrich) and phosphatase inhibitor cocktail (Sigma Aldrich), and centrifuged at 15,000×g for 10 min at 4°C. Protein concentration was determined by the bicinchoninic acid (BCA) protein assay (Pierce, Rockford, IL, USA). The proteins were separated on SDS-PAGE and transferred to PVDF membrane (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The membranes were blocked for non-specific binding with 5% non-fat dry milk in tris-buffered saline containing 0.05% Tween 20 (TBS-T) for 1h at room temperature and then incubated with specific primary antibodies in 5% non-fat dry milk at 4°C overnight. After three washes with TBS-T, the blots were incubated with horse radish peroxidase (HRP)-conjugated immunoglobulin G (IgG) for 1 h at room temperature and chemiluminescence was detected with ECL Western blotting substrate (Amersham Biosciences, Piscataway, NJ, USA) and visualized in Polaroid film.
Transient transfection and luciferase activity
Transient transfection was performed using the PolyJet DNA transfection reagent (SignaGen Laboratories, Ijamsville, MD, USA) according to the manufacturers’ instruction. Cells were plated in 12-well plates at a concentration of 2×105 cells/well. After growth overnight, plasmid mixtures containing 1 μg of TOP FLASH or FOP FLASH luciferase constructs and 0.1 μg of pRL-null vector were transfected for 24 h. The transfected cells were treated with silymarin for 24 h. The cells were then harvested in 1 × luciferase lysis buffer, and luciferase activity was normalized to the pRL-null luciferase activity using a dual-luciferase assay kit (Promega).
Transfection of small interference RNA (siRNA)
HCT116 cells were plated in 6-well plates and incubated overnight. HCT116 cells were transfected with control siRNA or Axin siRNA for 48 h at a concentration of 100 nM using TransIT-TKO transfection reagent (Mirus, Madison, WI, USA) according to the manufacturer’s instruction. Then the cells were treated with 100 μM of silymarin for 24 h.
Expression vectors
Wild type β-catenin and point mutation of S33Y β-catenin were provided from Addgene (Cambridge, MA, USA). Transient transfection of the vectors was performed using the PolyJet DNA transfection reagent (SignaGen Laboratories) according to the manufacturers’ instruction.
Statistical analysis
All the data are shown as mean ± SEM (standard error of mean). Statistical analysis was performed with one-way ANO-VA followed by Dunnett’s test. Differences with *p<0.05 were considered statistically significant.
RESULTS
Downregulation of β-catenin by silymarin treatment
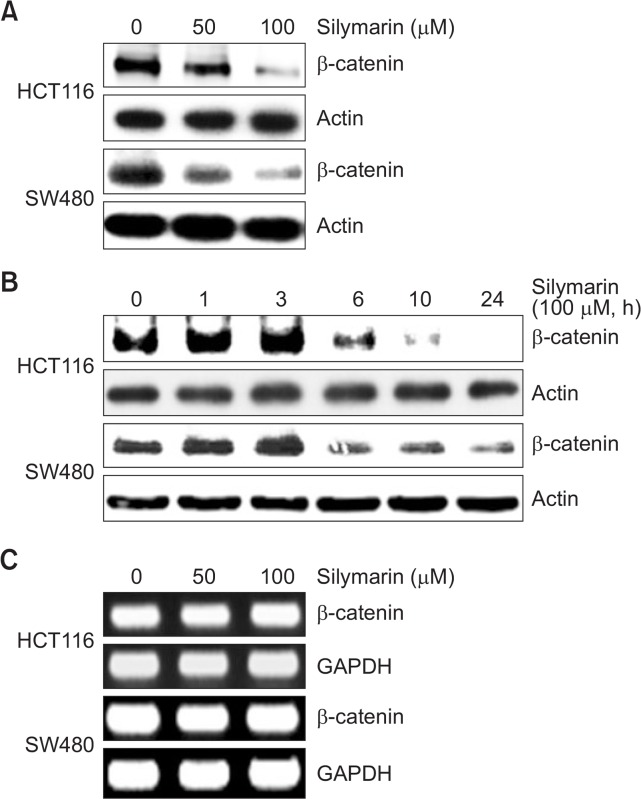
To test whether silymarin affects the protein levels of β-catenin, we performed Western blot in HCT116 and SW480 cells treated with 0, 50 and 100 μM of silymarin. As shown in Fig. 1A, silymarin treatment dose-dependently downregulated the protein levels of β-catenin compared with HCT116 and SW480 cells without silymarin treatment. In time-course experiment, β-catenin began to be decreased at 6 h after silymarin treatment in HCT116 and SW480 cells (Fig. 1B). To determine whether downregulation of β-catenin is responsible for transcriptional inhibition, we tested mRNA level in HCT116 and SW480 cells. As shown in Fig. 1C, mRNA level of β-catenin was not affected by silymarin treatment in HCT116 and SW480 cells. These results indicate that silymarin may downregulate the level of β-catenin through decreasing protein stability of β-catenin.
Fig. 1.
Decreased β-catenin level by silymarin treatment. (A) HCT116 and SW480 cells were treated with 0, 50 and 100 μM of silymarin for 24 h. Cell lysates were subjected to SDS-PAGE and then the Western blot was performed for β-catenin and actin. (B) HCT116 and SW480 cells were treated with 100 μM of silymarin for the indicated times. Cell lysates were subjected to SDS-PAGE and then the Western blot was performed for β-catenin and actin. (C) HCT116 and SW480 cells were treated with 0, 50 and 100 μM of silymarin for 24 h. total RNA was prepared after silymarin treatment for 24 h.
Silymarin-mediated proteasomal degradation of β-catenin
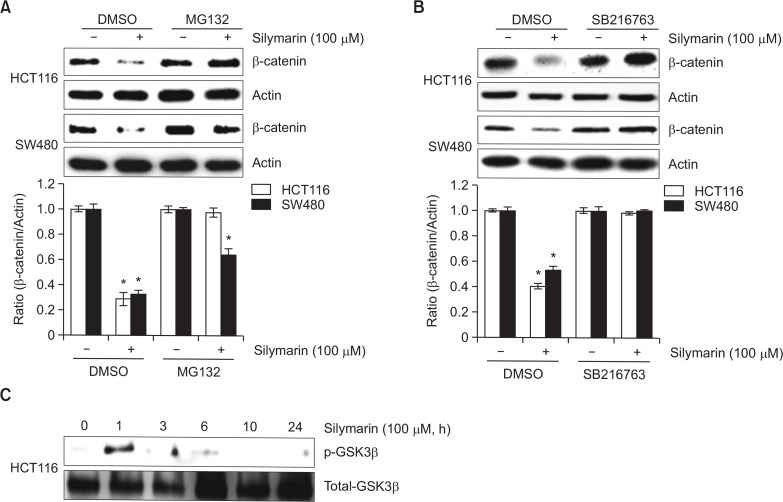
To confirm that silymarin affects the proteasomal degradation of β-catenin, HCT116 and SW480 cells were pretreated with MG132 as a proteasome inhibitor and then co-treated with silymarin. As a result, pretreatment of MG132 blocked silymarin-mediated downregulation of β-catenin in HCT116 and SW480 cells (Fig. 2A). To determine GSK3β is associated with silymarin-mediated proteasomal degradation of β-catenin, HCT116 and SW480 cells were pretreated with SB216763 (GSK3β inhibitor) and then co-treated with silymarin. As shown in Fig. 2B, silymarin-mediated proteasomal degradation of β-catenin was observed in DMSO-pretreated cells, while inhibition of GSK3β attenuated silymarin-induced downregulation of β-catenin in both HCT116 and SW480 cells. Because β-catenin downregulation by silymarin was dependent on GSK3β, we tested whether silymarin activates GSK3β. As shown in Fig. 2C, silymarin induced GSK3β phosphorylation as the active form of GSK3β at 1 h after silymarin treatment. These findings indicate that the GSK3β at least in part contributes to silymarin-mediated β-catenin proteasomal degradation.
Fig. 2.
GSK3β-mediated proteasomal degradation of β-catenin by silymarin treatment. (A, B) HCT116 and SW480 cells were pretreated with 10 μM of MG132 or 20 μM of SB216763 for 2 h and then co-treated with 100 μM of silymarin for 6 h. Cell lysates were subjected to SDS-PAGE and then the Western blot was performed for β-catenin and actin. (C) HCT116 cells were treated with 100 μM of silymarin for the indicated times. Cell lysates were subjected to SDS-PAGE and then the Western blot was performed for phospho-GSK3β and total-GSK3β. *p<0.05 compared to cells without silymarin.
Contribution of β-catenin phosphorylation by silymarin to proteasomal degradation of β-catenin
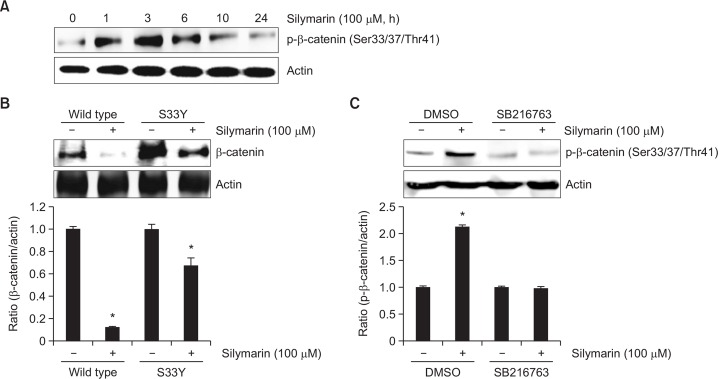
To test the effect of silymarin on the phosphorylation of β-catenin. As shown in Fig. 3A, β-catenin phosphorylation began to increase at 1 h in silymarin-treated HCT116 cell. To verify that β-catenin phosphorylation by silymarin results in β-catenin proteasomal degradation, HCT116 cells were transfected with HA-wild type β-catenin or S33Y β-catenin. As shown in Fig. 3B, silymarin induced β-catenin degradation in wild type β-catenin-transfected cells. However, it was partially attenuated in S33Y β-catenin-transfected cells. In addition, GSK3β inhibition by SB216763 reduced silymarin-mediated β-catenin phosphorylation in HCT116 cells (Fig. 3C). Overall, these data proposed that downregulation of β-catenin by silymarin depends on proteolytic proteasomal degradation via β-catenin phosphorylation.
Fig. 3.
Contribution of β-catenin phosphorylation by silymarin to proteasomal degradation of β-catenin. (A) HCT116 cells were plated overnight and then treated with 100 μM of silymarin for the indicated times. (B) HCT116 cells were transfected with wild type β-catenin or S33Y β-catenin, and then treated with 100 μM of silymarin. (C) HCT116 cells were pretreated with a selective inhibitor of GSK3β, SB216763 for 2 h and then co-treated with 100 μM of silymarin for 1 h. Cell lysates were subjected to SDS-PAGE and the Western blot was performed using antibodies against p-β-catenin or β-catenin. Actin was used as internal control. *p<0.05 compared to cells without silymarin.
Downregulation of TCF4 by silymarin treatment
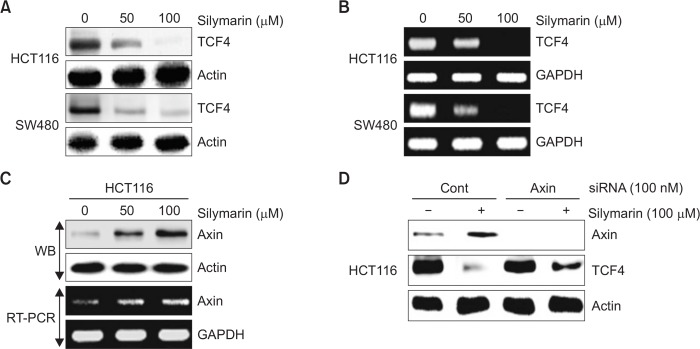
Because TCF4 has been regarded as one of the important regulators in Wnt signaling, we also evaluated whether silymarin affects TCF4 levels in HCT116 and SW480 cells. The cells were treated with 0, 50 and 100 μM of silymarin and we performed Western blot analysis. As shown in Fig. 4A, silymarin treatment dose-dependently downregulated the protein levels of TCF4. To determine whether downregulation of TCF4 results from the transcriptional inhibition, we tested mRNA level in HCT116 and SW480 cells. As shown in Fig. 4B, mRNA level of TCF4 was also attenuated by treatment of silymarin in HCT116 and SW480 cells. There is growing evidence that Axin downregulates TCF4 transcription (Yang et al., 2010). Thus, we tested the effect of silymarin on Axin expression in HCT116 cells. As a result (Fig. 4C), silymarin increased Axin expression in both protein and mRNA level. Next, to test whether silymarin-mediated Axin expression affects TCF4 downregulation, HCT116 cells were transfected with control or Axin siRNA for 48 h and then 100 μM of silymarin was treated for the additional 24 h. As shown in Fig. 4D, Axin knockdown by Axin siRNA attenuated silymarin-mediated TCF4 downregulation. These results indicate that silymarin may downregulates TCF4 expression through Axin-associated transcriptional inhibition of TCF4.
Fig. 4.
Decreased TCF4 level and increased Axin level by silymarin treatment. (A, B) HCT116 and SW480 cells were treated with 0, 50 and 100 μM of silymarin for 24 h. Cell lysates were subjected to SDS-PAGE and then the Western blot was performed for TCF4 and actin. For RT-PCR, total RNA was prepared after silymarin treatment for 24 h. (C) HCT116 cells were treated with 0, 50 and 100 μM of silymarin for 24 h. Cell lysates were subjected to SDS-PAGE and then the Western blot was performed for Axin and actin. For RT-PCR, total RNA was prepared after silymarin treatment for 24 h. (D) HCT116 cells were transfected with control- or Axin siRNA for 48 h and then co-treated with100 μM of silymarin for the further 24 h. Cell lysates were subjected to SDS-PAGE and then the Western blot was performed for Axin, TCF4 and actin.
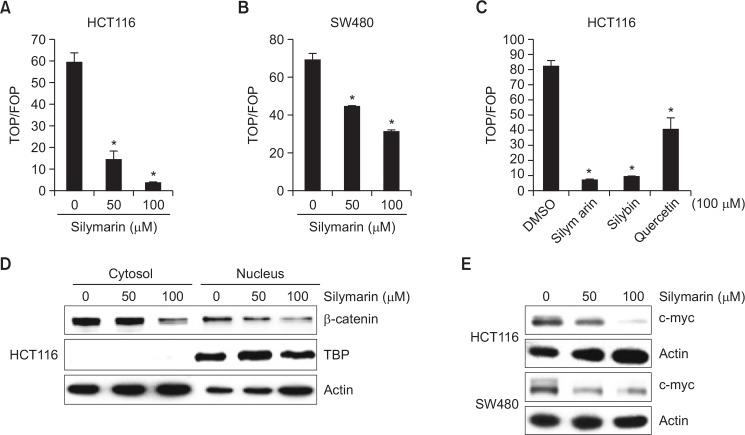
Regulation of silymarin on β-catenin-dependent transcriptional activity
To determine whether silymarin modulates β-catenin/TCF4-dependent activity, we performed a luciferase reporter assay using TOP-FLASH (wild-type TCF binding site) or FOP-FLASH (Mutated TCF binding site) in HCT116 and SW480 cells, respectively. Silymarin treatment significantly suppressed the TOP/FOP ratio in a dose-dependent manner in both HCT116 and SW480 cells (Fig. 5A, 5B). Next, we compared the inhibitory effect of silymarin and two compounds such as silybin and quercetin on β-catenin/TCF4-dependent activity. As shown in Fig. 5C, silymarin, silybin and quercetin suppressed β-catenin/TCF4-dependent activity by 91%, 89% and 51%, respectively. Nuclear accumulation of β-catenin allows interaction with TCF/LEF transcription factors and subsequently activates the transcription of the target genes such as cyclin D1 and c-myc. Thus, we evaluated whether inhibition of β-catenin-dependent transcriptional activity by silymarin results from the regulation of nuclear β-catenin accumulation and affects the expression of c-myc as a downstream β-catenin target genes. Silymarin treatment attenuated the level of nuclear β-catenin in HCT116 cells (Fig. 5D). In addition, the expression of c-myc was suppressed in silymarin-treated HCT116 and SW480 cells (Fig. 5E). These data indicates that the silymarin inhibits β-catenin/TCF-dependent signaling in human colorectal cancer cells.
Fig. 5.
Decreased transcriptional activity of β-catenin, nuclear accumulation and c-myc expression by silymarin treatment. (A, B, C) HCT116 or SW480 cells were co-transfected with TOP-FLASH or FOP-FLASH constructs containing wild-type or mutated TCF binding sites and pRL-null. The cells were treated with silymarin, silybin or quercetin at the indicated concentrations for 24 h. Luciferase activity for TOP-FLASH and FOP-FLASH was measured as a ratio of firefly luciferase signal/renilla luciferase signal using a dual luciferase assay kit. (D) HCT116 cells were treated with 0, 50 and 100 μM of silymarin for 24 h. Cytosol and nucleus extracts were prepared and subsequently western blot analysis was performed for β-catenin, TBP and actin. (E) HCT116 and SW480 cells were treated with 0, 50 and 100 μM of silymarin for 24 h. Cell lysates were harvested and subjected to SDS-PAGE. Western blot was performed for c-myc and actin. *p<0.05 compared to cells without silymarin.
DISCUSSION
Aberrant regulation of Wnt signaling associated with β-catenin and TCF4 is an important event in human colorectal cancer. Because of the mutation of APC or β-catenin genes, Wnt signaling is constitutively activated in most colorectal cancer cells, which suggests that Wnt signaling is one of the important molecular targets in the prevention of colorectal cancer.
In this study, we observed that silymarin significantly inhibits β-catenin/TCF4-dependent activity in HCT116 and SW480 cells. The level of β-catenin protein can be regulated by multiple mechanisms such as transcriptional regulation and the activation of proteasomal degradation. Indomethacin or activation of cGMP-dependent protein kinase downregulated β-catenin expression via transcriptional inhibition (Hawcroft et al., 2002; Kwon et al., 2010). Our data showed that silymarin did not affect β-catenin mRNA expression, which reveals that silymarin-mediated downregulation of β-catenin protein level may be independent on transcription. In presence of MG132 as a proteasome inhibitor, downregulation of β-catenin protein by silymarin treatment was blocked compared to silymarin-treated cells without MG132, which suggests that silymarin downregulates β-catenin through activating proteasomal degradation. Silybin as one of the major substances of silymarin mixture has been reported to decrease β-catenin protein level in SW480 cells, but no in HCT116 cells (Kaur et al., 2010). However, silymarin reduced β-catenin protein level in both HCT116 and SW480 cells, which indicates that another substance such as quercetin in silymarin mixture may affect β-catenin protein level in HCT116 cells.
The protein level of β-catenin has been known to be regulated by phosphorylation-dependent and ubiquitin-mediated degradation (Polakis, 2012). β-catenin is phosphorylated by GSK3β at Thr41, Ser37 (Liu et al., 2002) and Ser33, and subsequently is degraded via the proteasome pathway (Aberle et al., 1997). We found that silymarin induces β-catenin phosphorylation and GSK3β inhibition by SB216763 blocks the phosphorylation and proteasomal degradation of β-catenin by silymarin. In addition, the point mutation of S33Y attenuated silymarin-mediated β-catenin degradation. These results suggest that GSK3β may be an important upstream kinase for silymarin-mediated regulation of β-catenin.
Nuclear β-catenin forms a ternary complex with transcription factors, TCF4 for β-catenin/TCF4-dependent activation, which indicates that TCF4 is a central component of the Wnt signaling pathway. Also, there is growing evidence that capsaicin suppresses β-catenin/TCF4-dependent activity through downregulating TCF4 level but not β-catenin, which demonstrates the importance of TCF4 for β-catenin/TCF4-dependent activation. Thus, we evaluated whether silymarin affects TCF4 levels in HCT116 and SW480 cells. Silymarin treatment decreased TCF4 at both protein and mRNA level in HCT116 and SW480 cells, indicating that TCF4 may be an important molecular target for silymarin-mediated inhibition of Wnt signaling. Indeed, TCF4 alone may play a more significant role in tumorigenesis and TCF4 knockdown show better efficacy to induce growth arrest and apoptosis compared to β-catenin knockdown in human colorectal cancer cells (Xie et al., 2012).
Interestingly, we observed that silymarin activates Axin in protein and mRNA level in HCT116 cells. In addition, Axin knockdown attenuated TCF4 downregulation by silymarin. Axin has been reported to exert the downregulation of TCF4 transcription (Yang et al., 2010). These results indicate that Axin may be one of the positive effectors for silymarin-mediated inhibition of TCF4 transcription.
Indeed, there are some previous reports that a main component of silymarin such as silybin (silibinin) and quercetin can suppress β-catenin/TCF4-dependent activity (Lu et al., 2012; Novo et al., 2015). In addition, Vaid et al. has reported that silymarin blocks migration and invasion of human melanoma cells through suppressing β-catenin signaling (Vaid et al., 2011). Although we observed that silybin, quercetin and silymarin inhibits β-catenin/TCF4-dependent activity, there are some differences compared to previous reports. First, the molecular target of silybin for inhibition of Wnt/β-catenin signaling was Wnt co-receptor LRP6 (Lu et al., 2012), while we showed that silymarin targets β-catenin and TCF4 for inhibition of Wnt signaling. Second, silymarin has been reported to block β-catenin activation through decreasing the nuclear accumulation via β-catenin proteasomal degradation in melanoma cells (Vaid et al., 2011). However, in this study, we indicate that additional molecular target of silymarin for inhibition of Wnt signaling may be Axin-mediated inhibition of TCF4 transcription although the induction of β-catenin proteasomal degradation by silymarin is similar to a previous report (Vaid et al., 2011).
In conclusion, the current study provides information on molecular events for anti-cancer activity of silymarin. Silymarin induces GSK3β-dependent proteasomal degradation of β-catenin and Axin-dependent downregulation of TCF4. Decreased β-catenin and TCF4 by silymarin result in the inhibition of Wnt signaling in human colorectal cancer cells.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2014R1A1A2053448).
REFERENCES
- Abenavoli L, Capasso R, Milic N, Capasso F. Milk thistle in liver diseases: past, present, future. Phytother Res. 2010;24:1423–1432. doi: 10.1002/ptr.3207. [DOI] [PubMed] [Google Scholar]
- Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. β-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahnen DJ, Wade SW, Jones WF, Sifri R, Mendoza Silveiras J, Greenamyer J, Guiffre S, Axilbund J, Spiegel A, You YN. The increasing incidence of young-onset colorectal cancer: a call to action. Mayo Clin Proc. 2014;89:216–224. doi: 10.1016/j.mayocp.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Amado NG, Fonseca BF, Cerqueira DM, Neto VM, Abreu JG. Flavonoids: potential Wnt/beta-catenin signaling modulators in cancer. Life Sci. 2011;89:545–554. doi: 10.1016/j.lfs.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Anderson EC, Hessman C, Levin TG, Monroe MM, Wong MH. The role of colorectal cancer stem cells in metastatic disease and therapeutic response. Cancers (Basel) 2011;3:319–339. doi: 10.3390/cancers3010319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens J, von Kries JP, Kühl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of β-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- Bos JL, Fearon ER, Hamilton SR, Verlaan-de Vries M, van Boom JH, van der Eb AJ, Vogelstein B. Prevalence of ras gene mutations in human colorectal cancers. Nature. 1987;327:293–297. doi: 10.1038/327293a0. [DOI] [PubMed] [Google Scholar]
- Cassileth BR, Deng G. Complementary and alternative therapies for cancer. Oncologist. 2004;9:80–89. doi: 10.1634/theoncologist.9-1-80. [DOI] [PubMed] [Google Scholar]
- Chastek B, Kulakodlu M, Valluri S, Seal B. Impact of metastatic colorectal cancer stage and number of treatment courses on patient health care costs and utilization. Postgrad Med. 2013;125:73–82. doi: 10.3810/pgm.2013.03.2642. [DOI] [PubMed] [Google Scholar]
- Cufí S, Bonavia R, Vazquez-Martin A, Corominas-Faja B, Oliveras-Ferraros C, Cuyàs E, Martin-Castillo B, Barrajón-Catalán E, Visa J, Segura-Carretero A, Bosch-Barrera J, Joven J, Micol V, Menendez JA. Silibinin meglumine, a water-soluble form of milk thistle silymarin, is an orally active anti-cancer agent that impedes the epithelial-to-mesenchymal transition (EMT) in EGFR-mutant non-small-cell lung carcinoma cells. Food Chem Toxicol. 2013;60:360–368. doi: 10.1016/j.fct.2013.07.063. [DOI] [PubMed] [Google Scholar]
- Eo HJ, Park GH, Song HM, Lee JW, Kim MK, Lee MH, Lee JR, Koo JS, Jeong JB. Silymarin induces cyclin D1 proteasomal degradation via its phosphorylation of threonine-286 in human colorectal cancer cells. Int Immunopharmacol. 2015;24:1–6. doi: 10.1016/j.intimp.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Espada J, Calvo MB, Díaz-Prado S, Medina V. Wnt signalling and cancer stem cells. Clin Transl Oncol. 2009;11:411–427. doi: 10.1007/s12094-009-0380-4. [DOI] [PubMed] [Google Scholar]
- Fearon ER, Hamilton SR, Vogelstein B. Clonal analysis of human colorectal tumors. Science. 1987;238:193–197. doi: 10.1126/science.2889267. [DOI] [PubMed] [Google Scholar]
- Fodde R, Smits R, Clevers H. APC, signal transduction and genetic instability in colorectal cancer. Nat Rev Cancer. 2001;1:55–67. doi: 10.1038/35094067. [DOI] [PubMed] [Google Scholar]
- Hawcroft G, D’Amico M, Albanese C, Markham AF, Pestell RG, Hull MA. Indomethacin induces differential expression of β-catenin, γ-catenin and T-cell factor target genes in human colorectal cancer cells. Carcinogenesis. 2002;23:107–114. doi: 10.1093/carcin/23.1.107. [DOI] [PubMed] [Google Scholar]
- Herbst A, Kolligs FT. Wnt signaling as a therapeutic target for cancer. Methods Mol Biol. 2007;361:63–91. doi: 10.1385/1-59745-208-4:63. [DOI] [PubMed] [Google Scholar]
- Kaur M, Velmurugan B, Tyagi A, Agarwal C, Singh RP, Agarwal R. Silibinin suppresses growth of human colorectal carcinoma SW480 cells in culture and xenograft through down-regulation of β-catenin-dependent signaling. Neoplasia. 2010;12:415–424. doi: 10.1593/neo.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon IK, Wang R, Thangaraju M, Shuang H, Liu K, Dashwood R, Dulin N, Ganapathy V, Browning DD. PKG inhibits TCF signaling in colon cancer cells by blocking β-catenin ex pression and activating FOXO4. Oncogene. 2010;29:3423–3434. doi: 10.1038/onc.2010.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X. Control of β-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/S0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- Lu W, Lin C, King TD, Chen H, Reynolds RC, Li Y. Silibinin inhibits Wnt/β-catenin signaling by suppressing Wnt co-receptor LRP6 expression in human prostate and breast cancer cells. Cell Signal. 2012;24:2291–2296. doi: 10.1016/j.cellsig.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu HH, Zhang R, Haydon RC, Rayburn E, Kang Q, Si W, Park JK, Wang H, Peng Y, Jiang W, He TC. Wnt/β-catenin signaling pathway as a novel cancer drug target. Curr Cancer Drug Targets. 2004;4:653–671. doi: 10.2174/1568009043332709. [DOI] [PubMed] [Google Scholar]
- Mereish KA, Bunner DL, Ragland DR, Creasia DA. Protection against microcystin-LR-induced hepatotoxicity by Silymarin: biochemistry, histopathology, and lethality. Pharm Res. 1991;8:273–277. doi: 10.1023/A:1015868809990. [DOI] [PubMed] [Google Scholar]
- Narayan S. Curcumin, a multi-functional chemopreventive agent, blocks growth of colon cancer cells by targeting β-catenin-mediated transactivation and cell-cell adhesion pathways. J Mol Histol. 2004;35:301–307. doi: 10.1023/B:HIJO.0000032361.98815.bb. [DOI] [PubMed] [Google Scholar]
- Neergheen VS, Bahorun T, Taylor EW, Jen LS, Aruoma OI. Targeting specific cell signaling transduction pathways by dietary and medicinal phytochemicals in cancer chemoprevention. Toxicology. 2010;278:229–241. doi: 10.1016/j.tox.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Novo MC, Osugui L, dos Reis VO, Longo-Maugéri IM, Mariano M, Popi AF. Blockage of Wnt/β-catenin signaling by quercetin reduces survival and proliferation of B-1 cells in vitro. Immunobiology. 2015;220:60–67. doi: 10.1016/j.imbio.2014.09.001. [DOI] [PubMed] [Google Scholar]
- Polakis P. Wnt signaling in cancer. Cold Spring Harb Perspect Biol. 2012;4:a008052. doi: 10.1101/cshperspect.a008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy K, Agarwal R. Multitargeted therapy of cancer by silymarin. Cancer Lett. 2008;269:352–362. doi: 10.1016/j.canlet.2008.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- Sparks AB, Morin PJ, Vogelstein B, Kinzler KW. Mutational analysis of the APC/β-catenin/Tcf pathway in colorectal cancer. Cancer Res. 1998;58:1130–1134. [PubMed] [Google Scholar]
- Tetsu O, McCormick F. β-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- Vaid M, Prasad R, Sun Q, Katiyar SK. Silymarin targets β-catenin signaling in blocking migration/invasion of human melanoma cells. PLoS One. 2011;6:e23000. doi: 10.1371/journal.pone.0023000. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wang D, Wise ML, Li F, Dey M. Phytochemicals attenuating aberrant activation of β-catenin in cancer cells. PLoS One. 2012;7:e50508. doi: 10.1371/journal.pone.0050508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Xiang DB, Wang H, Zhao C, Chen J, Xiong F, Li TY, Wang XL. Inhibition of Tcf-4 induces apoptosis and enhances chemosensitivity of colon cancer cells. PLoS One. 2012;7:e45617. doi: 10.1371/journal.pone.0045617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang LH, Xu HT, Han Y, Li QC, Liu Y, Zhao Y, Yang ZQ, Dong QZ, Miao Y, Dai SD, Wang EH. Axin downregulates TCF-4 transcription via β-catenin, but not p53, and inhibits the proliferation and invasion of lung cancer cells. Mol Cancer. 2010;9:25. doi: 10.1186/1476-4598-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zi X, Feyes DK, Agarwa R. Anticarcinogenic effect of a flavonoid antioxidant, silymarin, in human breast cancer cells MDA-MB 468: induction of G1 arrest through an increase in Cip1/p21 concomitant with a decrease in kinase activity of cyclin-dependent kinases and associated cyclins. Clin Cancer Res. 1998;4:1055–1064. [PubMed] [Google Scholar]