Abstract
Quercetin is a flavonoid usually found in fruits and vegetables. Aside from its antioxidative effects, quercetin, like other flavonoids, has a various neuropharmacological actions. Quercetin-3-O-rhamnoside (Rham1), quercetin-3-O-rutinoside (Rutin), and quercetin-3-(2(G)-rhamnosylrutinoside (Rham2) are mono-, di-, and tri-glycosylated forms of quercetin, respectively. In a previous study, we showed that quercetin can enhance α7 nicotinic acetylcholine receptor (α7 nAChR)-mediated ion currents. However, the role of the carbohydrates attached to quercetin in the regulation of α7 nAChR channel activity has not been determined. In the present study, we investigated the effects of quercetin glycosides on the acetylcholine induced peak inward current (IACh) in Xenopus oocytes expressing the α7 nAChR. IACh was measured with a two-electrode voltage clamp technique. In oocytes injected with α7 nAChR copy RNA, quercetin enhanced IACh, whereas quercetin glycosides inhibited IACh. Quercetin glycosides mediated an inhibition of IACh, which increased when they were pre-applied and the inhibitory effects were concentration dependent. The order of IACh inhibition by quercetin glycosides was Rutin≥Rham1>Rham2. Quercetin glycosides-mediated IACh enhancement was not affected by ACh concentration and appeared voltage-independent. Furthermore, quercetin-mediated IACh inhibition can be attenuated when quercetin is co-applied with Rham1 and Rutin, indicating that quercetin glycosides could interfere with quercetin-mediated α7 nAChR regulation and that the number of carbohydrates in the quercetin glycoside plays a key role in the interruption of quercetin action. These results show that quercetin and quercetin glycosides regulate the α7 nAChR in a differential manner.
Keywords: Flavonoids, Quercetin, Quercetin glycosides, α7 nAChR
INTRODUCTION
Nicotinic acetylcholine receptors (nAChRs) are members of the Cys-loop family of ligand-gated ion channels. The Cys-loop family also includes serotonin (5-HT3), gamma-amino-butyric acid (GABAA), and glycine receptors (Jensen et al., 2005). nAChRs have been divided into two types: a muscle type and a neuronal type (Dani and Bertrand, 2007). Neuronal nAChRs are widely expressed in the human central and peripheral nervous systems. Eleven different nAChR subunits are currently known, and subunits of nAChR α (α2–10) and β (β2–4) have been identified (Nashmi and Lester, 2006). Neuronal nAChRs containing α2–6 subunits are usually expressed as heteromers in combination with β2–4 subunits (Boulter et al., 1987; Karlin, 2002) and are found throughout the whole nervous system (Gotti and Clementi, 2004). In contrast, the α7 and α9 subunits can form homomeric receptors (Couturier et al., 1990; Elgoyhen et al., 1994; Karlin, 2002). Homomeric α7 nAChRs are the major binding site for α-bungarotoxin in the central nervous system of mammals and are predominantly expressed in the cortical and the limbic areas including the hippocampus. Homomeric α7 nAChRs are known to play an important role in normal brain function and development (Gotti et al., 2000).
In previous reports, we have shown that the application of the flavonoid quercetin inhibits 5-HT- and glycine-induced peak inward currents (I5-HT and IGly) of mouse 5-HT3A and human glycine α receptor channels expressed in Xenopus laevis oocytes, respectively. The observed inhibition of I5-HT by quercetin was competitive and voltage-independent, whereas inhibition of IGly by quercetin appeared non-competitive and voltage-dependent (Lee et al., 2005; Lee et al., 2007). In addition, we have found that co- or pre-application of quercetin with acetylcholine (ACh) enhanced IACh in oocytes expressing human α7 nAChRs. This enhancement appeared independent of ACh concentration and voltage. Furthermore, quercetin enhanced Ca2+-mediated potentiation of IACh, which was observed to be dependent on extracellular Ca2+ concentration (Lee et al., 2010).
On the other hand, in addition to quercetin, quercetin glycosides are also compounds of low molecular weight and are mainly found in apples, tomatoes, gingko, other red fruits, and vegetables (Havsteen, 2002). In fruits and vegetables, quercetin naturally exists in glycosylated forms such as Rham 1, Rutin, Rham2, or other glycosidic forms (Azevedo et al., 2013). In previous studies using glycine and 5HT3 receptors, we have shown that quercetin glycosides can regulate ligand-gated ion channel activity in a differential manner with respect to quercetin. The inhibition of the glycine receptor channel activity by quercetin glycosides was noncompetitive and voltage-sensitive, whereas the inhibition of 5-HT3 receptor channel activity by quercetin glycosides was competitive and voltage-insensitive. Recently, we have also shown that quercetin glycosides inhibit GABAC receptor channel activity in a non-competitive and membrane voltage-insensitive manner. However, relatively little is known about the effects of quercetin glycosides on α7 nAChR channel activity.
In this study, we investigated the regulation of α7 nAChR channel activity expressed in Xenopus oocytes by quercetin glycosides. We first expressed neuronal human α7 nAChR copy RNAs (cRNAs) in Xenopus oocytes and examined the effect of quercetin and quercetin glycosides on IACh. This system was employed because (1) Xenopus laevis oocytes have been used widely as a tool to express the membrane proteins encoded by exogenously administered cDNAs or cRNAs including receptors, ion channels, and transporters (Dascal, 1987); and (2) nAChR channels expressed in Xenopus oocytes by the injection of nAChR subunit cRNAs have been well studied and characterized (Chavez-Noriega et al., 1997).
We found that co- or pre-application of quercetin with ACh enhanced IACh, whereas co- or pre-application of quercetin glycosides inhibited IACh. The observed inhibition of IACh by quercetin glycosides was ACh concentration- and voltage-independent. Interestingly, quercetin-induced enhancement of IACh was attenuated by co-treatment of quercetin glycosides. Here, we demonstrate that the quercetin glycosides-induced regulation of α7 nAChR channel activity is different from that of quercetin. We further discuss the role of the carbohydrate portion of quercetin glycosides in the differential regulation of α7 nAChR channel activity.
MATERIALS AND METHODS
Materials
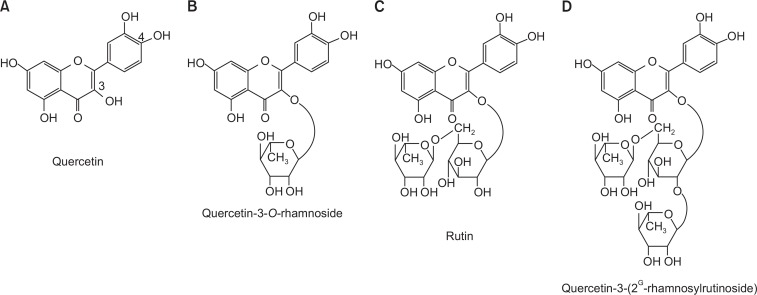
Human wild-type α7 nAChR cDNA was kindly provided by Dr. S. Heinemann (Salk Institute, California, USA). Quercetin (Fig. 1) and all other reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Fig. 1.
Chemical structures of quercetin and its glycosides. (A) Quercetin, (B) quercetin-3-O-rhamnoside (Rham1), (C) quercetin-3-O-rutinoside (Rutin), and (D) quercetin-3-(2G-rhamnosylrutinoside) (Rham2).
Preparation of Xenopus laevis oocytes and microinjection
X. laevis frogs were purchased from Xenopus I (Ann Arbor, MI, USA). Animal care and handling were in accordance with the highest standards of institutional guidelines. To isolate oocytes, frogs were anesthetized with an aerated solution of 3-amino benzoic acid ethyl ester, and the ovarian follicles were removed. The oocytes were separated with collagenase followed by agitation for 2 h in a Ca2+-free medium containing 82.5 mM NaCl, 2 mM KCl, 1 mM MgCl2, 5 mM HEPES, 2.5 mM sodium pyruvate, 100 units/ml penicillin, and 100 μg/ml streptomycin. Stage V–VI oocytes were collected and stored in a ND96 medium (96 mM NaCl, 2 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, and 5 mM HEPES, pH 7.5) supplemented with 50 μg/ml gentamicin. The solution containing the oocytes was maintained at 18°C with continuous gentle shaking and media was replaced daily. Electrophysiological experiments were performed five to six days after oocyte isolation, during which time the relevant chemicals were added to the media. α7 nAChR-encoding cRNAs (40 nL) were injected into the animal or vegetal pole of appropriate oocytes 1 day after isolation using a 10-μl microdispenser (VWR Scientific, West Chester, PA, USA) fitted with a tapered glass pipette tip (15–20 μm diameter) (Lee et al., 2005).
Data recording
A custom-made Plexiglas net chamber was used for two-electrode voltage-clamp recordings, as previously reported (Lee et al., 2005). A single oocyte was constantly superfused with ND96 media in the absence or presence of acetylcholine or quercetin during recording. The microelectrodes filled with 3 M KCl giving a resistance of 0.2–0.7 MΩ. Two-electrode voltage-clamp recordings were obtained at room temperature using an Oocyte Clamp (OC-725C, Warner Instrument) and digitized using Digidata 1200A (Molecular Devices, Sunnyvale, CA, USA). Both stimulation and data acquisition were controlled using pClamp 8 software (Molecular Devices). For most electrophysiological experiments, the oocytes were clamped at a holding potential of −80 mV, and 300 ms voltage steps were applied from −100 to +50 mV to assess the relationship between current and voltage. Linear leak and capacitance currents were corrected by means of the leak subtraction procedure. Because α7 nAChRs have a high relative permeability to Ca2+ (Séguéla et al., 1993; Castro and Albuquerque, 1995), oocytes were incubated in 100 μM 1,2-Bis(2-aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid tetrakis (acetoxymethyl ester) (BAPTA-AM) for 4 h before recording to avoid α7 nAChR-mediated endogenous Ca2+-activated Cl− currents.
Data analysis
To obtain the concentration-response curves for quercetin and quercetin glycosides on the inward peak IACh mediated by α7 AChR, the IACh peak was plotted at different concentrations of quercetin and its glycosides. Origin software (OriginLab Corp., Northampton, MA, USA) was used to fit the plot to the Hill equation: I/Imax=1/[1+(ED50/[A])nH], where Imax is maximal current obtained from each ED50 value of acetylcholine in wild-type receptors, ED50 is the concentration of quercetin or quercetin glycoside required to increase/decrease the response by 50%, [A] is the concentration of quercetin or quercetin glycoside, and nH is the Hill coefficient. All values are presented as means ± S.E.M. The differences between the means of control and treatment data were determined using the paired t-test or a one-way ANOVA followed by Tukey test. A value of p<0.05 was considered to be statistically significant.
RESULTS
Effects of quercetin or quercetin glycosides on IACh in oocytes expressing α7 nAChRs
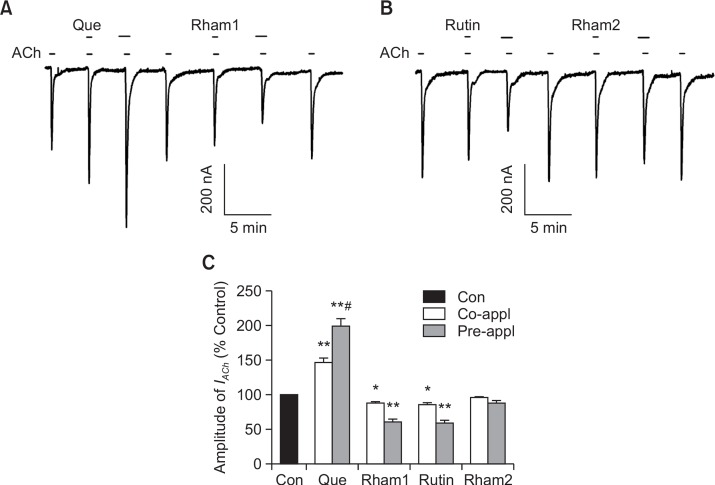
Treatment of ACh (200 μM) to oocytes injected with human α7 nAChR cRNA induced a large inward current (IACh) (Fig. 2A) but the application of ACh did not induce any inward current in H2O-injected control oocytes (data not shown) (Lee et al., 2010). Although quercetin (100 μM) itself had no effect on oocytes expressing α7 nAChRs at a holding potential of −80 mV (data not shown), the co-application of quercetin with ACh enhanced IACh in oocytes expressing α7 AChR (Fig. 2A, n=9 from three different frogs). The co-application of quercetin with ACh induced an enhancement of IACh by 46.5 ± 6.5% (Fig. 2B, **p<0.005 versus unexposed controls). In addition, pre-application of quercetin (100 μM) alone for 30 s before co-application with ACh (200 μM) induced a much larger enhancement of IACh in oocytes expressing α7 nAChRs than the enhancement observed after co-application as we previously demonstrated (Fig. 2A, #p<0.005, compared to co-treatment) (Lee et al., 2010). Next, we examined the effects of quercetin glycosides on IACh. Quercetin glycosides (100 μM each) themselves showed no effect on oocytes expressing the α7 nAChRs at a holding potential of −80 mV. Co-application of quercetin glycosides with ACh decreased the amplitude of IACh reversibly (13.2 ± 2.7%, 15.0 ± 2.9%, and 4.7 ± 1.2% inhibition by Rham1, Rutin, and Rham2, respectively) (Fig. 2C). Pre-application of quercetin glycosides alone for 30 s before co-application with ACh induced a much larger inhibitory effect on IACh (39.4 ± 3.5%, 42.1 ± 4.7%, and 13.1 ± 4.5% inhibition by Rham1, Rutin, and Rham2, respectively) (Fig. 2B, 2C, n=8–11 from three different frogs). Thus, the IACh inhibitory potency order appeared where Rutin≈Rham1>Rham2, also indicating that the regulatory pattern of quercetin glycosides on α7 nAChR channel activity is different from that of quercetin (Fig. 2).
Fig. 2.
Effects of quercetin and its glycosides on IACh in oocytes expressing human α7 nAChRs. (A–B) Acetylcholine (ACh; 200 μM) was applied first, followed by co- or pre-application of quercetin (Que) or quercetin glycosides (Rham1, Rutin, Rham2) and ACh. Co-application of 100 μM quercetin with ACh enhanced IACh and pre-application of 100 μM quercetin with ACh further enhanced IACh. Whereas, co-application of 100 μM of quercetin glycosides with ACh inhibited IACh and pre-application of 100 μM quercetin glycosides with ACh further inhibited IACh. Traces represent six separate oocytes from three different batches of frogs. (C) Summary of IACh enhancement by co- or pre-application of quercetin (*p<0.05, **p<0.005 compared to the control; #p<0.005, compared to the co-application of quercetin). Each point represents the means ± S.E.M. (n=9–12/group).
Quercetin enhances IACh, while quercetin glycosides inhibit IACh in a concentration-dependent manner
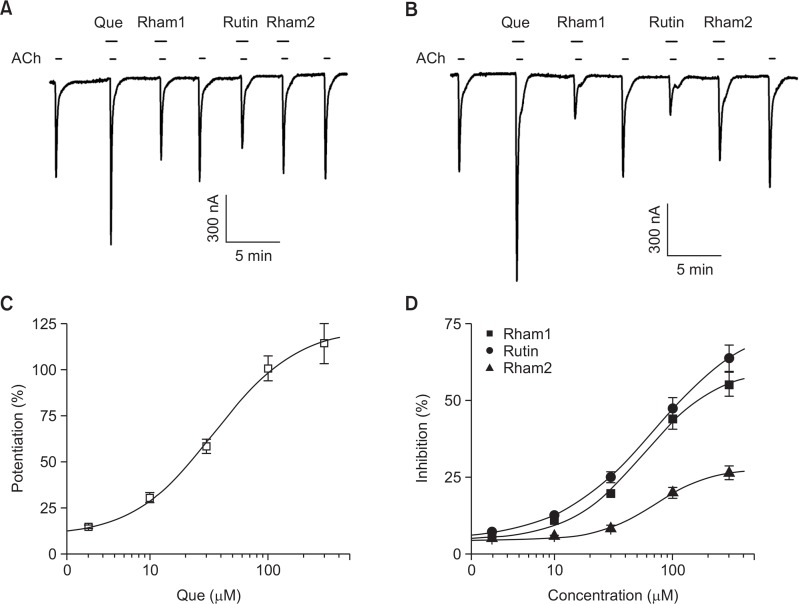
In concentration-dependent experiments with quercetin, pre-application with quercetin for 30 s enhanced IACh in a con centration-dependent manner in oocytes expressing α7 nA ChRs (Fig. 3C). Pre-application of quercetin at 3, 10, 30, 100, and 300 μM increased IACh by 5.9 ± 0.9, 23.1 ± 3.0, 52.9 ± 4.2, 98.7 ± 7.3, and 113.4 ± 11.9% in oocytes expressing α7 AChRs, respectively. Thus, the apparent EC50 of IACh for quercetin pre-application was 35.1 ± 3.8 μM (n=10–11, with samples taken from three different frogs for each point; Fig. 3C). In concentration-dependent experiments with quercetin glycosides, pre-application with quercetin glycosides for 30 s inhibited IACh in a concentration-dependent manner in oocytes expressing α7 nAChRs (Fig. 3D). For instance, pre-application of Rham1 inhibited IACh by 1.2 ± 0.3, 6.8 ± 0.8, 16.2 ± 1.1, 41.9 ± 3.5, and 53.9 ± 4.1% at 3, 10, 30, 100, and 300 μM in oocytes expressing α7 AChRs, respectively. Pre-application of Rutin inhibited IACh by 2.9 ± 0.9, 8.9 ± 0.8, 21.8 ± 1.8, 45.7 ± 3.7 and 63.1 ± 4.6% at 3, 10, 30, 100, and 300 μM in oocytes expressing α7 AChRs, respectively. Pre-application of Rham2 inhibited IACh by 0.7 ± 0.3, 1.3 ± 0.4, 4.1 ± 1.1, 16.3 ± 1.9 and 23.2 ± 2.6% at 3, 10, 30, 100, and 300 μM in oocytes expressing α7 AChRs, respectively. The apparent IC50s of IACh were 56.4 ± 8.4, 70.4 ± 3.6, 71.3 ± 6.7 μM for Rham1, Rutin, and Rham2 pre-application in oocytes expressing the α7 AChR receptor, respectively (n=10–11, with samples taken from three different frogs for each point; Fig. 3D). These results indicate that quercetin and quercetin glycosides regulate α7 nAChR channel activity in a differential manner.
Fig. 3.
Concentration-dependent effects of quercetin and its glycosides on IACh. (A) The representative trace of quercetin- or quercetin gly-coside- (30 μM each) mediated effects on IACh. IACh in oocytes expressing the α7 nAChR was elicited at a holding potential of −80 mV for 30 s in the presence of 200 μM ACh. Quercetin and its glycosides were pre-applied 30 s before ACh application. (B) The representative trace of quercetin- and quercetin glycoside- (300 μM each) mediated effects on IACh. IACh in oocytes expressing the α7 nAChRs was elicited at a holding potential of −80 mV for 30 s in the presence of 200 μM ACh. Quercetin and its glycosides were pre-applied 30 s before ACh application. Traces represent six separate oocytes from three different batches of frogs. (C–D) Concentration-dependent effects of quercetin and quercetin glycosides on IACh. The solid lines were fit using the Hill equation. Each point represents the mean ± S.E.M. (n=9–12/group).
Concentration-dependent effect of ACh and the relationship between current and voltage in the quercetin- or quercetin glycoside-mediated effects on IACh
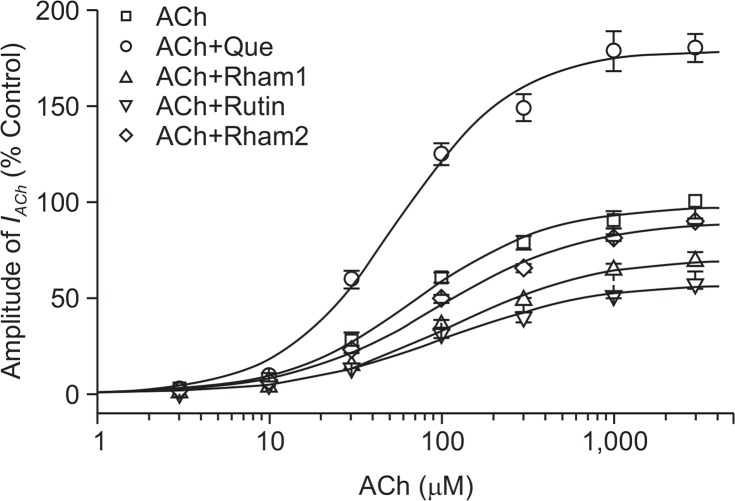
Similar to our previous report (Lee et al., 2010), when we analyzed the effect of quercetin on IACh evoked by different ACh concentrations, we found that pre-application of quercetin with different concentrations of ACh did not significantly shift the concentration-response curve of ACh to the left (EC50 values were changed from 70.8 ± 9.6 to 63.5 ± 7.5 μM, *p<0.08, while the Hill coefficient changed from 1.1 to 1.2) in oocytes expressing α7 nAChRs (Fig. 4), suggesting that quercetin did not affect the ACh binding.
Fig. 4.
Concentration-dependent effects of ACh on quercetin- and quercetin glycoside-mediated regulation of IACh. (A) Concentration-response relationships for oocytes expressing the α7 nAChRs treated with ACh (3–3000 μM) alone or with ACh plus 100 μM quercetin and 100 μM quercetin glycoside. The IACh of oocytes expressing α7 nAChRs was measured using the indicated concentration of ACh in the absence (□) or presence of quercetin (Que), Rham1, Rutin and Rham2. Oocytes were exposed to ACh alone or to ACh with quercetin and quercetin glycosides for 30 s before application. Oocytes were voltage-clamped at a holding potential of −80 mV.
Next, to further study the mechanism by which quercetin glycosides inhibit IACh, we first analyzed the effects of quercetin glycosides on IACh evoked by different ACh concentrations in oocytes expressing the α7 nAChR (Fig. 4). Pre-application of quercetin glycosides for 30 s with different concentrations of ACh did not significantly shift the dose-response curve of ACh. The apparent EC50 values were 70.8 ± 9.6, 78.6 ± 7.1, 76.1 ± 6.5, and 80.6 ± 6.5 μM for ACh alone, ACh + Rham1, ACh + Rutin, and ACh + Rham2, respectively, and the Hill coefficients were 1.1 ± 0.1, 1.0 ± 0.2, 1.0 ± 0.1, and 1.1 ± 0.1, respectively. Thus, Rham1, Rutin, and Rham2 significantly inhibited the IACh elicited, independent of ACh concentration (n=9–12 from three different frogs) (Fig. 4). These results show that quercetin increases IACh, whereas quercetin glycosides inhibit IACh and that these effects probably occur in a non-competitive manner.
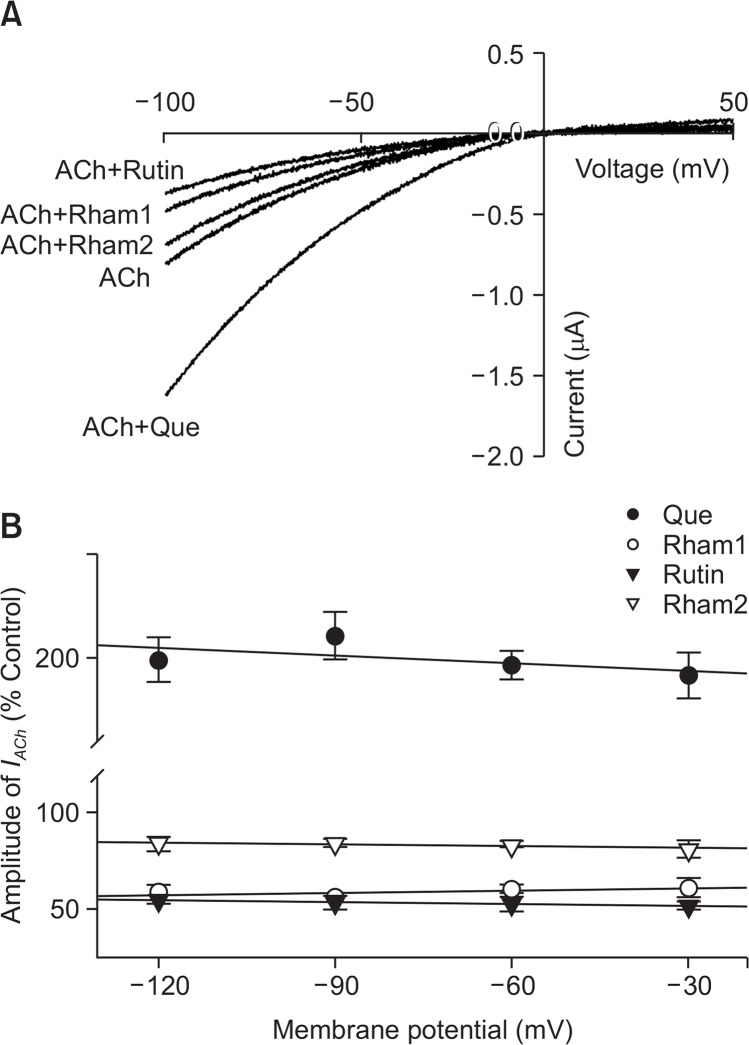
In the current-voltage relationship, the membrane potential, which was held at −80 mV, and a voltage ramp was applied from −100 to +50 mV for 300 ms. In the absence of ACh, the inward current (at −100 mV) and the outward current (at +50 mV) were negligible (data not shown). Treatment of ACh to in oocytes expressing the α7 nAChR induced a mainly inward current and outward current at negative- and positive voltages, respectively. Pre-application of quercetin with ACh enhanced both inward and outward currents. The reversal potential was near 0 mV for both ACh alone and for ACh with quercetin. This indicates that Na+ and Ca2+ are main charge carriers (Revah et al., 1991; Galzi et al., 1992). In addition, the pre-application of quercetin with ACh further increased currents but did not appear to affect α7 nAChR channel properties as quercetin addition did not change the reversal potential of the α7 nAChR (Fig. 5A). Pre-application of quercetin glycosides combined with ACh treatment gave greater inhibition of both inward and outward currents than those achieved when ACh and quercetin glycosides were applied together. The reversal potential was also near −0 mV when ACh was used alone, as well as in ACh+quercetin glycoside treatments (Fig. 5A). In addition, the enhancement of quercetin or the inhibitory effects of quercetin glycosides on IACh in oocytes expressing α7 nAChRs did not appear to be membrane voltage-sensitive. Quercetin increased IACh by 98.5 ± 5.4%, and 95.6 ± 6.3%, at −120 and −30 mV, respectively. Rham1, Rutin and Rham2 inhibited IACh by 41.2 ± 3.8%, 44.9 ± 2.2%, and 16.7 ± 3.7%, respectively, at −120 mV, and by 38.9 ± 4.9%, 48.1 ± 2.1%, and 18.7 ± 4.6%, respectively, at −30 mV (n=10–12, from three different frogs). These results indicate that quercetin enhances IACh, while quercetin glycosides inhibit IACh in a voltage-insensitive manner (n=10–12, from three different frogs; Fig. 5B).
Fig. 5.
Current-voltage relationships and voltage-independent inhibition by quercetin and its glycosides. (A) Current-voltage relationships of IACh regulation by quercetin in the oocytes expressing α7 nAChRs. Representative current-voltage relationships were obtained using voltage ramps of −100 to +50 mV for 300 ms at a holding potential of 80 mV. Voltage steps were applied before and after application of 200 μM ACh in the absence or presence of 100 μM quercetin, Rham1, Rutin, and Rham2. Each point represents the mean ± S.E.M. (n=7–9/group). (B) Voltage-independent regulation of IACh in oocytes expressing α7 nAChRs by quercetin and quercetin glycosides (100 μM each). The values were obtained in the presence of 200 μM ACh at the indicated membrane holding potentials. Each point represents the mean ± SEM (n=8–12/group).
Effects of quercetin glycosides on quercetin-induced IACh enhancement
The above results indicate that quercetin glycosides may be novel regulators of α7 nAChR channel activity and that their actions could be different from those of quercetin. Therefore, we investigated the effect of quercetin on IACh after co-treatment of quercetin glycosides. As shown in Fig. 6, the enhancing effect of quercetin on IACh was significantly attenuated in the presence of Rutin, Rham1 and Rham2. The order of potency for the quercetin attenuating effects was Rutin> Rham1>Rham2. The above results show that quercetin-mediated enhancement of IACh could be affected by the presence of quercetin glycosides.
Fig. 6.
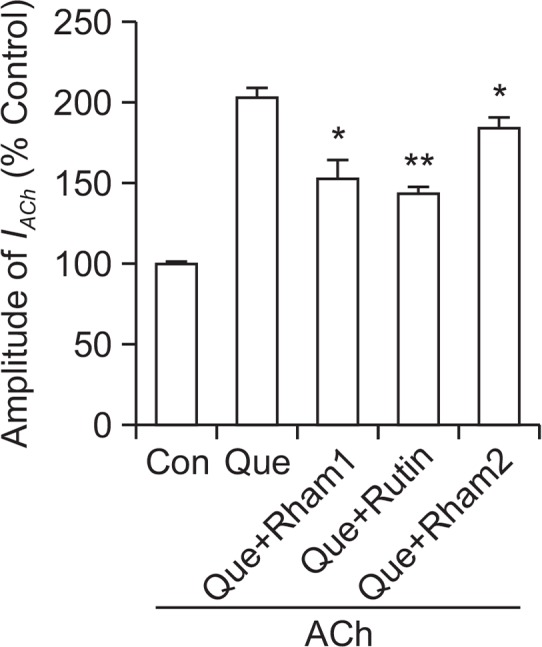
Effects of quercetin glycosides on quercetin-induced IACh enhancement. IACh in oocytes expressing α7 nAChRs was elicited at a holding potential of −80 mV for 30 s in the presence of 200 μM ACh. 100 μM quercetin (Que) alone or with 100 μM quercetin glycoside (Rham1, Rutin, Rham2) were applied for 30 s before ACh addition. Summary histograms are from three different frogs (n=7–12/group). Each point represents the mean ± S.E.M (*p<0.05, **p<0.005 compared to quercetin).
DISCUSSION
Quercetin is a flavonoid that shows diverse effects in nervous and non-nervous systems (Kandaswami and Middleton, 1994; Harborne and Williams, 2000). For example, quercetin protects the central nervous system against oxidative effects and exerts effects on analgesia, locomotor activity and sleep (Speroni and Minghetti, 1988; Picq et al., 1991; Oyama et al., 1994), as well as having anticonvulsant, sedative, and anxiolytic effects (Marder et al., 1996; Medina et al., 1997; Griebel et al., 1999; Yao et al., 2010). Quercetin glycosides are natural forms of quercetin that are found in colored fruit and vegetables (Fig. 1) (Murota and Terao, 2003; Nemeth and Piskula, 2007). In previous reports, we have shown that quercetin and quercetin glycosides can regulate the activity of several types of ligand-gated ion channels, but the relationship between quercetin and quercetin glycosides and α7 nAChR regulation has not been well characterized.
In the present study, we have investigated the effects of quercetin glycosides on human α7 nAChRs heterologously expressed in Xenopus oocytes. We found that: (1) pre-application of quercetin with ACh induced a large enhancement of IACh in a reversible and concentration-dependent manner; (2) quercetin glycoside pre-application with ACh inhibited IACh; (3) quercetin-mediated enhancement of IACh was non-competitive and membrane potential independent, while quercetin glycosides inhibit IACh in a non-competitive manner and are also membrane potential independent; and (4) quercetin-induced enhancement of IACh was attenuated in the presence of quercetin glycosides. These results indicate that quercetin and quercetin glycosides show opposite effects on α7 nAChR channel activity and quercetin glycosides are different from quercetin in their regulation of α7 nAChRs. Structural differences between quercetin and quercetin glycosides suggest that the differential regulation of α7 nAChR channel activity might be due to the carbohydrate components.
It will be questioned how structural differences between quercetin and quercetin glycosides induce differential regulations of α7 nAChR channel activity. One possibility is that the carbohydrates attached to quercetin might cause a different behavior in the regulations of α7 nAChRs. For example, quercetin enhances IACh, whereas quercetin glycosides inhibit IACh of α7 nAChR (Fig. 3). The other is that the number or different size of carbohydrate attached to quercetin might also induce differential effects on IACh of α7 nAChRs. Rham1 or Rutin with one or two carbohydrates more potently inhibited IACh than Rham2, which is tri-glycosylated forms of quercetin (Fig. 3). In addition, quercetin glycosides attenuated the quercetin-induced enhancement of IACh. Thus, although we found in the present study that carbohydrate component of quercetin could play important roles in the regulations of ligand-gated ion channel such as α7 nAChR, we do not know exactly how carbohydrate(s) attached to quercetin cause an opposite on IACh of α7 nAChRs or differential effects on IACh of α7 nAChRs and how quercetin glycosides decreases the quercetin-induced enhancement of IACh. Further study will be required to elucidate molecular mechanisms how carbohydrate(s) attached to quercetin contribute to quercetin-induced regulations of α7 nAChR.
The activation of the α7 nAChR is known to be linked to many physiological conditions (Khiroug et al., 2003; Gotti and Clementi, 2004; Gilbert et al., 2009). The α7 nAChR is widely expressed throughout the central nervous systems, including the cortical and limbic areas of the brain. Clearly, the α7 nAChR plays an important role in normal brain function because α7 nAChR dysfunction is associated with neurological disorders such as learning and memory loss, Alzheimer’s disease, schizophrenia, and epilepsy (Chini et al., 1994; Léna and Changeux, 1997; Weiland et al., 2000; Changeux and Edelstein, 2001). However, relatively little is known about the effects of quercetin glycosides on α7 nAChR function. In the present study, we found that quercetin glycosides inhibited IACh and furthermore, Rutin and Rham1 could attenuate the quercetin-induced enhancement of IACh. Interestingly, the inhibitory effects of quercetin glycosides on IACh as well as the attenuation of quercetin-induced enhancement of IACh, was observed to be more potent with pre-treatment before ACh addition. Currently we cannot explain the exact physiological or pharmacological role of quercetin glycosides or the reason for their behavior being different from that of quercetin in α7 nAChR regulation. It is known that dietary quercetin glycosides are usually metabolized in two ways. First, they are deglycosylated to the aglycone quercetin; and second, they remain as quercetin glycosides without further metabolism (Havsteen, 2002; Lee et al., 2010). Further studies will be required to elucidate the role of quercetin glycosides in the in vivo regulation of the α7 nAChR.
In previous studies, we have shown that quercetin inhibits 5-HT3 receptor-gated ion currents through interactions within the pre-transmembrane domain I. Furthermore, quercetin can inhibit or potentiate glycine receptor-gated ion currents through interactions with the amino acid Ser256 residue in transmembrane domain II (Lee et al., 2005; Lee et al., 2007). Examination of the effects of quercetin glycosides shows an inhibition of GABAC receptor channel activity in the order of Rutin>Rham1>Rham2 (Kim et al., 2015). In the present study, we found that quercetin glycosides also inhibited α7 nAChR-gated ion currents with the same order of efficacy. Thus, although the Cys-loop family of ligand-gated ion channels such as glycine, GABAC, 5-HT3, and α7 nAChR all form homomeric receptors, quercetin and quercetin glycosides regulate these homomeric receptors in a different manner.
In conclusion, we found that quercetin increased IACh, while quercetin glycosides inhibited IACh in Xenopus oocytes expressing α7 nAChRs. The present results indicate that quercetin and quercetin glycosides act differently in the regulation of α7 nAChRs. Finally, these results also suggest that quercetin and quercetin glycosides exhibit their differential regulation of α7 nAChR channel activity through their structural differences.
Acknowledgments
This research was supported by the Bio & Medical Technology Development Program of the NRF funded by the Korean government, MSIP(2015M3A9E3052336).
This paper was also written as part of Konkuk University’s research support program for its faculty on sabbatical leave in 2015 to S.M. Lee.
REFERENCES
- Azevedo MI, Pereira AF, Nogueira RB, Rolim FE, Brito GA, Wong DV, Lima-Júnior RC, de Albuquerque Ribeiro R, Vale ML. The antioxidant effects of the flavonoids rutin and quercetin inhibit oxaliplatin-induced chronic painful peripheral neuropathy. Mol Pain. 2013;9:53. doi: 10.1186/1744-8069-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter J, Connolly J, Deneris E, Goldman D, Heinemann S, Patrick J. Functional expression of two neuronal nicotinic acetylcholine receptors from cDNA clones identifies a gene family. Proc Natl Acad Sci USA. 1987;84:7763–7767. doi: 10.1073/pnas.84.21.7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro NG, Albuquerque EX. Alpha-bungarotoxin-sensitive hippocampal nicotinic receptor channel has a high calcium permeability. Biophys J. 1995;68:516–524. doi: 10.1016/S0006-3495(95)80213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J, Edelstein SJ. Allosteric mechanisms in normal and pathological nicotinic acetylcholine receptors. Curr Opin Neurobiol. 2001;11:369–377. doi: 10.1016/S0959-4388(00)00221-X. [DOI] [PubMed] [Google Scholar]
- Chavez-Noriega LE, Crona JH, Washburn MS, Urrutia A, Elliott KJ, Johnson EC. Pharmacological characterization of recombinant human neuronal nicotinic acetylcholine receptors hα2β2, hα2β4, hα3β2, hα3β4, hα4β2, hα4β4 and hα7 expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1997;280:346–356. [PubMed] [Google Scholar]
- Chini B, Raimond E, Elgoyhen AB, Moralli D, Balzaretti M, Heinemann S. Molecular cloning and chromosomal localization of the human alpha 7-nicotinic receptor subunit gene (CHR-NA7) Genomics. 1994;19:379–381. doi: 10.1006/geno.1994.1075. [DOI] [PubMed] [Google Scholar]
- Couturier S, Bertrand D, Matter JM, Hernandez MC, Bertrand S, Millar N, Valera S, Barkas T, Ballivet M. A neuronal nicotinic acetylcholine receptor subunit (alpha 7) is developmentally regulated and forms a homo-oligomeric channel blocked by alpha-BTX. Neuron. 1990;5:847–856. doi: 10.1016/0896-6273(90)90344-F. [DOI] [PubMed] [Google Scholar]
- Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- Dascal N. The use of Xenopus oocytes for the study of ion channels. CRC Crit Rev Biochem. 1987;22:317–387. doi: 10.3109/10409238709086960. [DOI] [PubMed] [Google Scholar]
- Elgoyhen AB, Johnson DS, Boulter J, Vetter DE, Heinemann S. α9: an acetylcholine receptor with novel pharmacological properties expressed in rat cochlear hair cells. Cell. 1994;79:705–715. doi: 10.1016/0092-8674(94)90555-X. [DOI] [PubMed] [Google Scholar]
- Galzi JL, Devillers-Thiéry A, Hussy N, Bertrand S, Changeux JP, Bertrand D. Mutations in the channel domain of a neuronal nicotinic receptor convert ion selectivity from cationic to anionic. Nature. 1992;359:500–505. doi: 10.1038/359500a0. [DOI] [PubMed] [Google Scholar]
- Gilbert D, Lecchi M, Arnaudeau S, Bertrand D, Demaurex N. Local and global calcium signals associated with the opening of neuronal alpha7 nicotinic acetylcholine receptors. Cell Calcium. 2009;45:198–207. doi: 10.1016/j.ceca.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Gotti C, Carbonnelle E, Moretti M, Zwart R, Clementi F. Drugs selective for nicotinic receptor subtypes: a real possibility or a dream? Behav Brain Res. 2000;113:183–192. doi: 10.1016/S0166-4328(00)00212-6. [DOI] [PubMed] [Google Scholar]
- Gotti C, Clementi F. Neuronal nicotinic receptors: from structure to pathology. Prog Neurobiol. 2004;74:363–396. doi: 10.1016/j.pneurobio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Griebel G, Perrault G, Tan S, Schoemaker H, Sanger DJ. Pharmacological studies on synthetic flavonoids: comparison with diazepam. Neuropharmacology. 1999;38:965–977. doi: 10.1016/S0028-3908(99)00026-X. [DOI] [PubMed] [Google Scholar]
- Harborne JB, Williams CA. Advances in flavonoid research since 1992. Phytochemistry. 2000;55:481–504. doi: 10.1016/S0031-9422(00)00235-1. [DOI] [PubMed] [Google Scholar]
- Havsteen BH. The biochemistry and medical significance of the flavonoids. Pharmacol Ther. 2002;96:67–202. doi: 10.1016/S0163-7258(02)00298-X. [DOI] [PubMed] [Google Scholar]
- Jensen ML, Schousboe A, Ahring PK. Charge selectivity of the Cys-loop family of ligand-gated ion channels. J Neurochem. 2005;92:217–225. doi: 10.1111/j.1471-4159.2004.02883.x. [DOI] [PubMed] [Google Scholar]
- Kandaswami C, Middleton E., Jr Free radical scavenging and antioxidant activity of plant flavonoids. Adv Exp Med Biol. 1994;366:351–376. doi: 10.1007/978-1-4615-1833-4_25. [DOI] [PubMed] [Google Scholar]
- Karlin A. Emerging structure of the nicotinic acetylcholine receptors. Nat Rev Neurosci. 2002;3:102–114. doi: 10.1038/nrn731. [DOI] [PubMed] [Google Scholar]
- Khiroug L, Giniatullin R, Klein RC, Fayuk D, Yakel JL. Functional mapping and Ca2+ regulation of nicotinic acetylcholine receptor channels in rat hippocampal CA1 neurons. J Neurosci. 2003;23:9024–9031. doi: 10.1523/JNEUROSCI.23-27-09024.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Lee BH, Choi SH, Jung SW, Kim HS, Lee JH, Hwang SH, Pyo MK, Kim HC, Nah SY. Differential effects of quercetin glycosides on GABAC receptor channel activity. Arch Pharm Res. 2015;38:108–114. doi: 10.1007/s12272-014-0409-2. [DOI] [PubMed] [Google Scholar]
- Lee BH, Jeong SM, Lee JH, Kim JH, Yoon IS, Lee JH, Choi SH, Lee SM, Chang CG, Kim HC, Han Y, Paik HD, Kim Y, Nah SY. Quercetin inhibits the 5-hydroxy-tryptamine type 3 receptor-mediated ion current by interacting with pre-transmembrane domain I. Mol Cells. 2005;20:69–73. [PubMed] [Google Scholar]
- Lee BH, Lee JH, Yoon IS, Lee JH, Choi SH, Pyo MK, Jeong SM, Choi WS, Shin TJ, Lee SM, Rhim H, Park YS, Han YS, Paik HD, Cho SG, Kim CH, Lim YH, Nah SY. Human glycine alpha1 receptor inhibition by quercetin is abolished or inversed by alpha267 mutations in trans-membrane domain 2. Brain Res. 2007;1161:1–10. doi: 10.1016/j.brainres.2007.05.057. [DOI] [PubMed] [Google Scholar]
- Lee BH, Choi SH, Shin TJ, Pyo MK, Hwang SH, Kim BR, Lee SM, Lee JH, Kim HC, Park HY, Rhim H, Nah SY. Quercetin enhances human α7 nicotinic acetylcholine receptor-mediated ion current through interactions with Ca2+ binding sites. Mol Cells. 2010;30:245–253. doi: 10.1007/s10059-010-0117-9. [DOI] [PubMed] [Google Scholar]
- Léna C, Changeux JP. Pathological mutations of nicotinic receptors and nicotine-based therapies for brain disorders. Curr Opin Neurobiol. 1997;7:674–682. doi: 10.1016/S0959-4388(97)80088-8. [DOI] [PubMed] [Google Scholar]
- Marder M, Viola H, Wasowski C, Wolfman C, Waterman PG, Cassels BK, Medina JG, Paladini AC. 6-Bromo-flavone, a high affinity ligand for the central benzodiazepine receptors is a member of a family of active flavonoids. Biochem Biophys Res Commun. 1996;223:384–389. doi: 10.1006/bbrc.1996.0903. [DOI] [PubMed] [Google Scholar]
- Medina JH, Viola H, Wolfman C, Marder M, Wasowski C, Calvo D, Paladini AC. Overview--flavonoids: a new family of benzodiazepine receptor ligands. Neurochem Res. 1997;22:419–425. doi: 10.1023/A:1027303609517. [DOI] [PubMed] [Google Scholar]
- Murota K, Terao J. Antioxidative flavonoid quercetin: implication of its intestinal absorption and metabolism. Arch Biochem Biophys. 2003;417:12–17. doi: 10.1016/S0003-9861(03)00284-4. [DOI] [PubMed] [Google Scholar]
- Nashmi R, Lester HA. CNS localization of neuronal nicotinic receptors. J Mol Neurosci. 2006;30:181–184. doi: 10.1385/JMN:30:1:181. [DOI] [PubMed] [Google Scholar]
- Nemeth K, Piskula MK. Food content, processing, absorption and metabolism of onion flavonoids. Crit Rev Food Sci Nutr. 2007;47:397–409. doi: 10.1080/10408390600846291. [DOI] [PubMed] [Google Scholar]
- Oyama Y, Fuchs PA, Katayama N, Noda K. Myricetin and quercetin, the flavonoid constituents of Ginkgo biloba extract, greatly reduce oxidative metabolism in both resting and Ca2+-loaded brain neurons. Brain Res. 1994;635:125–129. doi: 10.1016/0006-8993(94)91431-1. [DOI] [PubMed] [Google Scholar]
- Picq M, Cheav SL, Prigent AF. Effect of two flavonoid compounds on central nervous system. Analgesic activity. Life Sci. 1991;49:1979–1988. doi: 10.1016/0024-3205(91)90640-W. [DOI] [PubMed] [Google Scholar]
- Revah F, Bertrand D, Galzi JL, Devillers-Thiéry A, Mulle C, Hussy N, Bertrand S, Ballivet M, Changeux JP. Mutations in the channel domain alter desensitization of a neuronal nicotinic receptor. Nature. 1991;353:846–849. doi: 10.1038/353846a0. [DOI] [PubMed] [Google Scholar]
- Séguéla P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J Neurosci. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speroni E, Minghetti A. Neuropharmacological activity of extracts from Passiflora incarnata. Planta Med. 1988;54:488–491. doi: 10.1055/s-2006-962525. [DOI] [PubMed] [Google Scholar]
- Weiland S, Bertrand D, Leonard S. Neuronal nicotinic acetylcholine receptors: from the gene to the disease. Behav Brain Res. 2000;113:43–56. doi: 10.1016/S0166-4328(00)00199-6. [DOI] [PubMed] [Google Scholar]
- Yao Y, Han DD, Zhang T, Yang Z. Quercetin improves cognitive deficits in rats with chronic cerebral ischemia and inhibits voltage-dependent sodium channels in hippocampal CA1 pyramidal neurons. Phytother Res. 2010;24:136–140. doi: 10.1002/ptr.2902. [DOI] [PubMed] [Google Scholar]