Abstract
Age-related rotator cuff tendon degeneration is related to tenofibroblast apoptosis. Anthocyanins reduce oxidative stress-induced apoptotic cell death in tenofibroblasts. The current study investigated the presence of cell protective effects in cyanidin and delphinidin, the most common aglycon forms of anthocyanins. We determined whether these anthocyanidins have antiapoptotic and antinecrotic effects in tenofibroblasts exposed to H2O2, and evaluated their biomolecular mechanisms. Both cyanidin and delphinidin inhibited H2O2-induced apoptosis in a dose-dependent manner. However, at concentrations of 100 μg/ml or greater, delphinidin showed cytotoxicity against tenofibroblasts and a decreased antinecrotic effect. Cyanidin and delphinidin both showed inhibitory effects on the H2O2-induced increase in intracellular ROS formation and the activation of ERK1/2 and JNK. In conclusion, both cyanidin and delphinidin have cytoprotective effects on cultured tenofibroblasts exposed to H2O2. These results suggest that cyanidin and delphinidin are both beneficial for the treatment of oxidative stress-mediated tenofibroblast cell death, but their working concentrations are different.
Keywords: Cyanidin, Delphinidin, Apoptosis, Rotator cuff, Tenofibroblast
INTRODUCTION
Degenerative change in the rotator cuff tendon appears to be an inevitable pathophysiological concomitant of aging. This degenerative change leads to rotator cuff tear and, eventually, to degenerative arthritis (Neer et al., 1983). The incidence of partial or full thickness rotator cuff tear, which increases with age, reaching 80% among those 80 years and older, demonstrates this disease’s high morbidity and suggests its medical cost burdens (Milgrom et al., 1995; Tempelhof et al., 1999; Yamamoto et al., 2010). Exogenous and endogenous theories of the causes of this disease have been proposed; apoptosis-induced degenerative changes are currently receiving the most attention (Ozaki et al., 1988; Soslowsky et al., 2002; Nho et al., 2008). The increased incidence of apoptotic cell death in degenerative tendon tissue could affect the rates of collagen synthesis and repair, possibly weakening tendon tissue and increasing the risk of tendon rupture (Yuan et al., 2003b). The biomolecular mechanisms of the degenerative change leading to apoptotic cell death in tenofibroblasts have been identified as oxidative-stress-related cascade mechanisms (Yuan et al., 2002; Tuoheti et al., 2005). This finding indicates the necessity of developing strategies to intervene at one or more points in that oxidative-stress-related cascade.
Natural antioxidants have been reported to play a major role in blocking the oxidative stress induced by free radicals. The phytochemicals responsible for this antioxidant capacity are thought to be the phenolics, such as anthocyanins and other flavonoid compounds (Cao et al., 1997). Anthocyanins, which are found in a variety of highly pigmented fruits, potentially play a role in preventing human diseases related to oxidative stress (Tsuda, 2012). Hydrolyzed anthocyanins yield sugars and anthocyanidins, which are their common aglycon forms (Tsuda, 2012). Among the anthocyanidins are cyanidin and delphinidin, the natural anthocyanidins most commonly extracted from the edible parts of plants (Tsuda, 2012). A previous study demonstrated that anthocyanins from the black soybean seed coat (containing these anthocyanidins: cyanidin, delphinidin, and petunidin) block H2O2-induced apoptosis by inhibiting both the intracellular ROS production and the activation of ERK1/2 and JNK in tenofibroblasts (Park et al., 2010). That previous study used mixtures of anthocyanins, rather than discrete anthocyanidins.
Research on discrete, pure anthocyanidins is a necessary step toward their potential eventual use as health-enhancing compounds. Accordingly, the current study, which is a search for a single anthocyanidin involved in protection against oxidative stress, evaluated two common anthocyanidins: cyanidin and delphinidin. This study confirmed their cytoprotective effects against oxidative stress, examined the underlying mechanisms of their cytoprotective effects, and evaluated the possibility that they act synergistically.
MATERIALS AND METHODS
Materials and primary cell culture
Cyanidin and delphinidin were obtained from Enzo Life Sciences (Enzo life sciences Inc., Farmingdale, USA). Tenofibroblasts derived from the supraspinatus tendons of adult male Sprague-Dawley rats were prepared, as described previously (Park et al., 2010). Briefly, tissues were washed twice with PBS and then minced with a sterile scissors. A small pieces of tissue were placed in a 6-well tissue culture plate (Corning, NY, USA) with DMEM (Invitrogen, Carlsbad, CA, USA) supplemented with 30% FBS, 100 IU/mL penicillin, and 100 μg/mL streptomycin and grown at 37°C in a humidified atmosphere of 5% CO2 and 95% air. After reaching confluence, the cells were detached from the culture dishes with trypsin-EDTA (Invitrogen, Carlsbad, CA, USA) and expanded in a second passage. Cells from passages 3 to 6, inclusively, were used for the current study.
Measurement of apoptosis and necrosis by FACS analyses
To determine the extent of apoptosis and necrosis, cell death was analyzed by staining the cells with Annexin V-FITC and PI using Annexin-V-FLUOS Staining Kit (Roche diagnostics, Mannheim, Germany). For staining, cells (1×106 tenofibroblast cells/100 mm culture dish) were treated with H2O2, cyanidin or delphinidin. Pretreatment with cyanidin and delphinidin was performed 1 hr before H2O2 exposure. Cells were washed with cold PBS, centrifuged, and resuspended in a final volume of 100 μl Annexin V-FLUOS labeling solution (10 mM HEPES, pH 7.4, 140 mM NaCl, 2.5 mM CaCl2) containing 20 μl of Annexin V-FITC and PI (final concentration 1 μg/ml), as provided by the manufacturer. The cells were incubated at room temperature for 15 min and then 400 μl of PBS was added and the cells were analyzed using a FACSCalibur flow cytometer (BD Biosciences, San Diego, USA). For fluorescein detection, 488 nm excitation by an argon laser and 525-nm bandpass filter was used, and 560-nm bandpass filter was used for PI detection. A total of at least 10,000 cells were analyzed per sample. Fluorescence intensity was measured on a logarithmic scale. The amounts of apoptosis and necrosis were determined as percentages of Annexin V+/PI− and Annexin V+/PI+ cells, respectively. All experiments were independently conducted at least three times.
ROS measurement
Intracellular generation of ROS was measured using DCF-DA (Molecular Probes, Eugene, USA). The dye that integrated into the cells was deacetylated by intracellular esterases. Upon oxidation, DCF-DA is converted to highly fluorescent DCF. For the assay, tenofibroblast cells were cultured overnight in 6-well plates and then treated with H2O2 the presence or absence of cyanidin or delphinidin for 24 h. The ROS measurements were performed 15 min after the H2O2 treatment; that interval was based on the results of previous research on ROS production, which indicated that its peak is reached at 15 min after H2O2 exposure. The cells were incubated in the dark with 5 μM DCF-DA in serum-free medium for 10 min. After incubation, the dye-integrated cells were washed with serum-free DMEM. The DCF-induced fluorescence was detected using a laser-scanning confocal imaging system (Olympus IX70; Olympus, Tokyo, Japan) with excitation and emission settings at 488 nm and 530 nm, respectively. To quantify the production rates of ROS, the cells were stained with DCF-DA for 15 min, removed from the plate with trypsin-EDTA, and collected on a FACSCalibur flow cytometer (BD Biosciences, San Jose, USA). Data were analyzed using Cell Quest Pro software (BD Biosciences, San Jose, USA).
Analysis for intracellular MAPKs activation
Tenofibroblasts (1×106 cells/60 mm culture dish) were treated with the indicated concentrations of cyanidin or delphinidin for 1 h, and then exposed to 0.5 mM H2O2 for 1 h to target ERK1/2, JNK and p38. Following treatment, cells were washed with cold PBS, and total cell lysates were prepared by scrapping. In order to extract all the protein, the cells were placed in a lysis buffer solution (RIPA buffer 1 mL, protease inhibitor 10 μL, phosphatase inhibitor 10 μL, Thermo scientific, Waltham, MA, USA) for 30 min. The digested cells were then sonicated and centrifuged at 12,000×g for 10 min at 4°C to remove insoluble debris. The samples were resolved on 10% SDS-polyacrylamide gel, and then electrophoretically transferred onto a nitrocellulose membrane using the semidry technique as described previously (Park et al., 2010). After blocking for 1 h with 5% skimmed milk in a TBS-T buffer solution (10 mM Tris, 150 mM NaCl, and 0.1% Tween-20), the membrane was incubated with primary antibodies against ERK1/2, phospho-ERK1/2, JNK, phospho-JNK, p38, phospho-p38, (Cell Signaling Technology, Beverly, USA) in a TBS-T buffer containing 5% bovine serum albumin (BSA). Specific antibody binding was detected by horseradish peroxidase-conjugated secondary antibodies, and then visualized using an enhanced chemiluminescence detection reagent (Pierce, Rockford, USA).
Statistical analysis
All experiments were performed using triplicate cultures, with the results expressed in each case as the mean of the triplicate cultures. Each experiment was also performed at least three times, and representative data were reported. All statistical analyses were performed via one-way ANOVA, followed by Tukey’s multiple-comparison tests. Repeated-measures ANOVA was performed, first to determine the dose-dependent effects of cyanidin and delphinidin individually, and then to compare the dose-dependent effects of these anthocyanidins. Differences with a probability (p) of less than 0.05 were considered statistically significant. All statistical analysis was done by SPSS 17.0 for Windows (SPSS, Chicago, Illinois, USA).
RESULTS
Effects of cyanidin and delphinidin on cytotoxicity
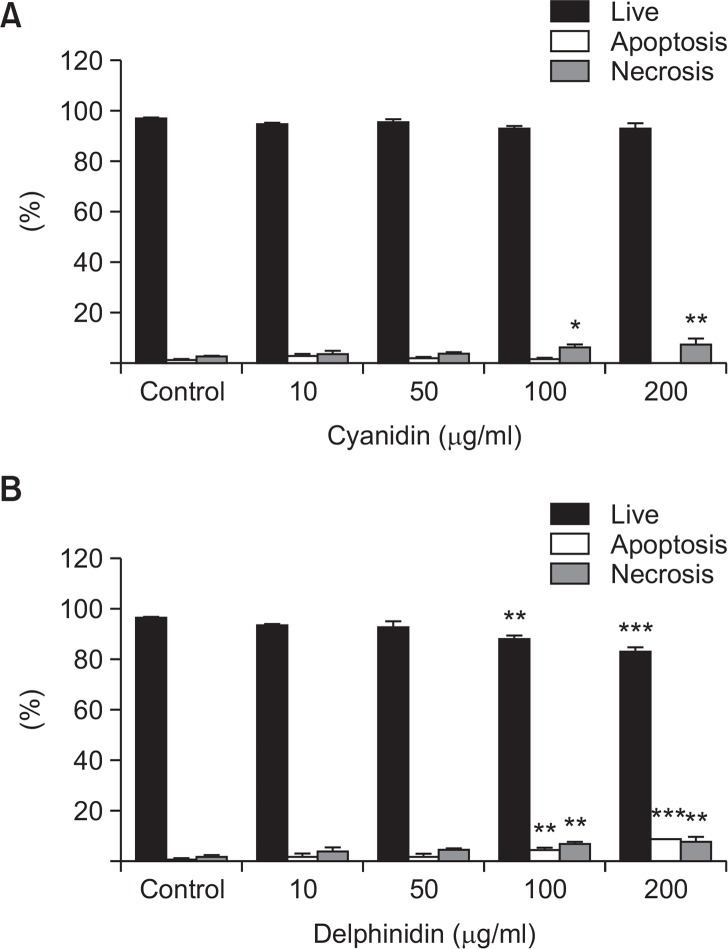
To determine the cytotoxicity of cyanidin and delphinidin, study subgroups of tenofibroblasts were treated with various concentrations (10, 50, 100, and 200 μg/ml) of cyanidin or delphinidin for 24 h. The viability of the cells was determined by the Annexin V and PI double staining method. As shown in Fig. 1A, there was no statistically significant difference in cell viability between the control group and the cyanidin subgroups at any of the studied concentrations. As shown in Fig. 1B, there was no statistically significant difference in cell viability between the control group and the delphinidin subgroups at concentrations of 10 and 50 μg/ml. However, significant reductions in cell viability were noted in the delphinidin concentrations of 100 μg/ml and 200 μg/ml.
Fig. 1.
Cell viability was assessed using Annexin V and PI double staining method. (A) There was no statistically significant difference in cell viability between the control group and the cyanidin subgroups at any of the studied concentrations (*p<0.05, as compared to the control). (B) There was no statistically significant difference in cell viability between the control group and the delphinidin subgroups at concentrations of 10 and 50 μg/ml. However, significant reductions in cell viability were noted in the delphinidin concentrations of 100 μg/ml and 200 μg/ml (**p<0.01 and ***p<0.001, as compared to the control).
Effects of cyanidin and delphinidin on cytotoxicity and H2O2-induced apoptosis
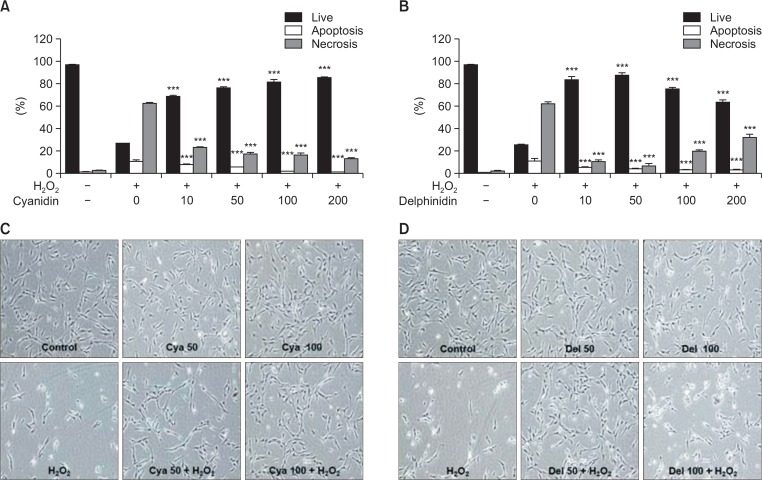
In a dose-dependent manner, pretreatment with cyanidin protected tenofibroblast cells exposed to oxidative stress. Exposure to 0.5 mM H2O2 decreased the viability to 26.79% (p<0.001) that of the untreated control. Pretreatment of the exposed cells with 10 μg/ml cyanidin yielded 69% viability; with 50 μg/ml, 76.45%; with 100 μg/ml, 81.64%; and with 200 μg/ml, 85.30% (Fig. 2A). Delphinidin showed its greatest protective effect at a concentration of 50 μg/ml, with a decreasing protective effect at concentrations of 100 μg/ml or greater. Pretreatment of the exposed cells with 10 μg/ml delphinidin yielded 83.74% viability; with 50 μg/ml, 88.25%; with 100 μg/ml, 75.94%; and with 200 μg/ml, 67.29% (Fig. 2B). As shown in Fig. 2C, 2D, the phase-contrast microscope findings indicated that cyanidin had a cytoprotective effect on H2O2-mediated cell death in a dose-dependent manner. Delphinidin also had a cytoprotective effect on H2O2-mediated cell death, although not in a dose-dependent manner in the same concentration range as cyanidin. Additionally, analysis of the apoptotic-cell rates indicated that both cyanidin and delphinidin exerted dose-dependent antiapoptotic effects (p<0.001), without significant differences (Fig. 2A, 2B). Analysis of the necrotic-cell rates indicated that cyanidin had a dose-dependent antinecrotic effect (p<0.001). In contrast, delphinidin showed its highest antinecrotic effect at a concentration of 50 μg/ml, with a decreasing antinecrotic effect at concentrations of 100 μg/ml and greater (Fig. 2A, 2B). Cyanidin and delphinidin were both shown to inhibit the apoptosis and necrosis of H2O2-exposed tenofibroblasts simultaneously. Delphinidin showed a significantly higher cytoprotective effect than cyanidin against H2O2 at concentrations of 10 μg/ml (p<0.001) and 50 μg/ml (p<0.001). However, delphinidin showed cytotoxicity at concentrations of 100 μg/ml and greater; that cytotoxicity was probably due to increased necrosis rather than increased apoptosis.
Fig. 2.
(A, B) The analyses of the apoptotic-cell rates indicate that both cyanidin and delphinidin exerted dose-dependent antiapoptotic effects (p<0.001). The analyses of necrotic-cell rates indicate that cyanidin showed dose-dependent antinecrotic effects (p<0.001). In contrast, delphinidin showed its highest antinecrotic effect at a concentration of 50 μg/ml, with decreasing antinecrotic effects at concentrations of 100 μg/ml and greater, as compared to the H2O2 group (***p<0.001). (C, D) According to the phase-contrast microscope analyses (×10 objective), cyanidin had protective effects on H2O2-mediated cytotoxicity in a dose-dependent manner. Delphinidin had cytoprotective effects on H2O2-mediated cell death, but not in a dose-dependent manner. Cya: Cyanidin, Del: Delphinidin.
Effects of cyanidin and delphinidin on intracellular ROS production
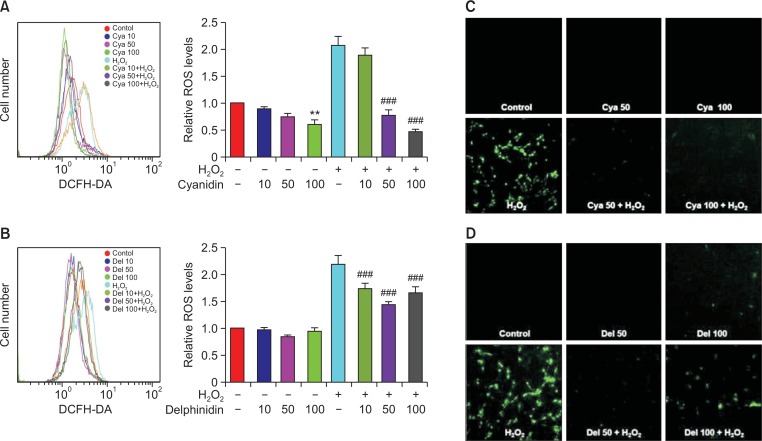
Flow cytometry analyses indicated that the levels of intracellular ROS production in the 50 and 100 ug/ml cyanidin-H2O2 (Fig. 3A) and in the 10, 50, and 100 μg/ml delphinidin-H2O2 subgroups (Fig. 3B) were all significantly lower than the level in the H2O2 group. These results demonstrated that both cyanidin and delphinidin have the ability to reduce H2O2-mediated intracellular ROS production. The amounts of intracellular ROS were shown to be lower, in a dose-dependent manner, in the groups pretreated with cyanidin than in the H2O2 group (Fig. 3A). The amounts of intracellular ROS were shown to be significantly lower in the groups pretreated with delphinidin than in the H2O2 group, but no dose-dependent significance was found (Fig. 3B). Specifically, the levels of intracellular ROS were shown to decrease until the dose of delphinidin reached 50 mg/ml. However, the decrease in those ROS levels was reversing once the dose of delphinidin reached 100 mg/ml. That corresponded to the cytotoxicity of delphinidin, as shown in Fig. 1B. Confocal microscope analyses showed that H2O2-induced intracellular ROS production was reduced by pretreatment with 50 and with 100 μg/ml doses of cyanidin and delphinidin. While intracellular ROS levels in all the subgroups treated with delphinidin were significantly lower than in the H2O2 group, the 100 μg/ml delphinidin subgroup showed an increase in ROS production over that of the 50 μg/ml subgroup (Fig. 3C, 3D).
Fig. 3.
(A, B) Intracellular ROS levels were significantly higher in the H2O2 group, as compared with the control. Intracellular ROS levels in the cyanidin-H2O2 and the delphinidin-H2O2 subgroups were lower than in the H2O2 group. The graph represents the mean ± SD of 3 independent experiments (**p<0.01 compared to control group; ###p<0.001 compared to H2O2 group). (C, D) According to the morphological analyses using a confocal microscope, intracellular ROS levels were higher in the H2O2 group than in the control; however, ROS levels were markedly lower in the cyanidin-H2O2 and the delphinidin-H2O2 subgroups than in the H2O2 group. Cya: Cyanidin, Del: Delphinidin.
Effects of cyanidin and delphinidin on MAPKs activation
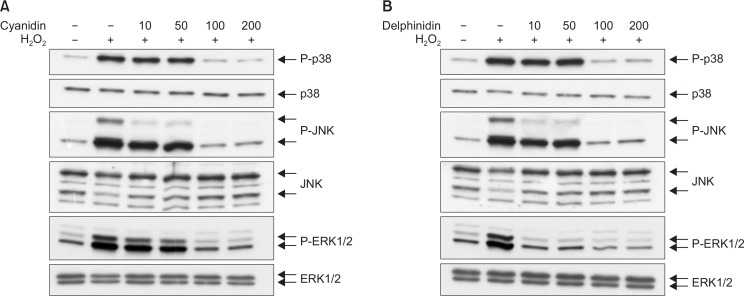
The western blot analyses indicated that 1 h of exposure to H2O2 induced phosphorylation of ERK1/2, JNK, and p38. Treatment with either cyanidin or delphinidin did not induce phosphorylation of ERK1/2, JNK, or p38. Pretreatment with cyanidin or delphinidin reduced the H2O2-induced phosphorylation of ERK1/2, JNK, and p38 (Fig. 4). Cyanidin inhibited the phosphorylation of ERK1/2 and JNK similarly (Fig. 4A). Delphinidin inhibited the phosphorylation of ERK1/2 more markedly than that of JNK (Fig. 4B).
Fig. 4.
MAPKs activation was assessed using western blot analyses. Western blot analyses demonstrated that expressions of phosphorylations of p38, JNK, and ERK were higher in the H2O2 group than in the control. Those expressions were lower in the cyanidin-H2O2 and delphinidin-H2O2 subgroups than in the H2O2 group.
DISCUSSION
The current study demonstrated that cyanidin and delphinidin had cytoprotective effects against oxidative-stress-induced cytotoxicity to rotator cuff tenofibroblasts; these effects were achieved by reducing intracellular ROS production and by inhibiting phosphorylation of ERK, JNK, and p38.
According to the cytotoxicity analyses, both cyanidin and delphinidin showed cytoprotective effects, which originated from their antiapoptotic and antinecrotic actions. At lower concentrations of up to 50 μg/ml, delphinidin showed greater cytoprotective effects on H2O2-induced tenofibroblast death than did cyanidin at the same concentration (p=0.000) (Fig. 2A). These effects may have resulted from the difference in antioxidant activity of the two anthocyanidins. Determined by oxidation potentials, the order of anthocyanidins by antioxidant activity is delphinidin > cyanidin > pelargonidin (Aaby et al., 2007). However, delphinidin showed increased cytotoxicity and a decreased cytoprotective effect on H2O2-exposed tenofibroblasts at concentrations of 100 μg/ml or greater (Fig. 2B). These effects probably arose because necrosis, rather than apoptosis, resulted from the high concentration of delphinidin (Fig. 2B).
The cause of rotator cuff tear is disputed. Although both exogenous and endogenous theories have been developed, none explains completely the etiology of rotator cuff tear. Recent molecular biological studies have focused on the role of apoptosis in rotator cuff tendinopathy, analyzing its key mediators and associated cellular changes (Yuan et al., 2002; Yuan et al., 2003b; Tuoheti et al., 2005). Although it is not known whether cellular necrosis is also involved in rotator cuff tendon degeneration, one of the common histological features of rotator cuff tendon degeneration is tissue necrosis (Fukuda et al., 1990; Tillander et al., 2002; Chillemi et al., 2011). This suggests that cellular necrosis also might be involved in rotator cuff tendon degeneration. Apoptosis and necrosis are both known to be mediated by oxidative stress, a condition in which ROS are overproduced (Gotoh et al., 1997; Fu et al., 2002; Arany et al., 2004). Lower levels of oxidative stress trigger apoptosis; higher levels mediate necrosis (Gotoh et al., 1997; Arany et al., 2004). The current study demonstrated that H2O2 stimulated intracellular ROS production; both cyanidin and delphinidin inhibited that intracellular ROS production.
This study demonstrated that H2O2 activated ERK, JNK, and p38; cyanidin and delphinidin inhibited their activation (Fig. 4A, 4B). Increased intracellular ROS production is also known to activate those MAPKs (Son et al., 2011; Siebel et al., 2013). Generally, ERK cascades sustain cell viability; JNK and p38 cascades promote apoptosis (Xia et al., 1995). The activation of these MAPKs’ signaling pathways depends on their cell types and the specific stimuli (Kong et al., 2000; Schroeter et al., 2002). Although ROS induce ERK activation in a variety of cell lines, the role of ERK in H2O2-induced cell death remains controversial. In studies of H2O2-exposed cells, some have shown ERK activation enhancing survival; others have shown it contributing to apoptosis (Wang et al., 1998; Bhat and Zhang, 1999; Tournier et al., 2000; Brand et al., 2001; Arany et al., 2004; Dong et al., 2004). JNK, whose cascade’s activation is considered an important intermediate trigger of apoptosis, has been recently implicated in mitochondrial death (Tournier et al., 2000; Petrosillo et al., 2003). Activation of p38 has been observed in cells undergoing apoptosis induced by diverse agents, including chemotherapeutics (Olson and Hallahan, 2004; Bradham and McClay, 2006).
This study examined apoptosis and necrosis induced by exposure to H2O2, although the involvement of H2O2 in the development of rotator cuff apoptosis has not yet been confirmed. However, H2O2 is known to be a major component of ROS in cells activated by various external stimuli that trigger internal substances (Ohba et al., 1994; Yim et al., 1994; Sundaresan et al., 1995; Bae et al., 1997). The super oxide anion (O2−), an ROS, is constantly being produced by metabolic reactions in all aerobic organisms; it is then spontaneously or enzymatically dismutated to H2O2 (Stadtman and Berlett, 1998). The hydroxyl radical (OH•), a well-known ROS, is intracellularly generated from H2O2, via the Fenton reaction(Stadtman and Berlett, 1998). Therefore, we postulated that H2O2 has a high probability of being involved in the apoptosis and necrosis processes of rotator cuff tenofibroblasts (Yuan et al., 2003a; Yuan et al., 2003b). Because this experiment was limited to demonstrating the effectiveness of cyanidin and delphinidin in reducing the apoptosis and necrosis induced only by H2O2, we also elaborated on the need for further research to determine the effectiveness of these two anthocyanins in suppressing the catabolic effects of other oxidants. Finally, because this is an in vitro study using concentrations of cyanidin and delphinidin which are within the range used in previous in vitro experiments (Oak et al., 2006; Chen et al., 2011; Guo et al., 2012; Seo et al., 2013), we suggest further study, using the animal overuse model, which is currently accepted for investigations of rotator cuff degeneration.
In conclusion, both cyanidin and delphinidin show cytoprotective effects on rotator cuff tenofibroblasts exposed to H2O2, through their antiapoptotic and antinecrotic properties. Both these anthocyanidins suppress intracellular ROS formation and the activation of ERK1/2 and JNK. Cyanidin and delphinidin are both beneficial for the treatment of oxidative stress-mediated tenofibroblast cell death, although at different ranges of concentrations.
Acknowledgments
This study was supported by a grant (313-2008-2-E00347) from the National Research Foundation of Korea. The authors have no potential conflicts to disclose.
REFERENCES
- Aaby K, Ekeberg D, Skrede G. Characterization of phenolic compounds in strawberry (Fragaria x ananassa) fruits by different HPLC detectors and contribution of individual compounds to total antioxidant capacity. J Agric Food Chem. 2007;55:4395–4406. doi: 10.1021/jf0702592. [DOI] [PubMed] [Google Scholar]
- Arany I, Megyesi JK, Kaneto H, Tanaka S, Safirstein RL. Activation of ERK or inhibition of JNK ameliorates H2O2 cytotoxicity in mouse renal proximal tubule cells. Kidney Int. 2004;65:1231–1239. doi: 10.1111/j.1523-1755.2004.00500.x. [DOI] [PubMed] [Google Scholar]
- Bae YS, Kang SW, Seo MS, Baines IC, Tekle E, Chock PB, Rhee SG. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J Biol Chem. 1997;272:217–221. doi: 10.1074/jbc.272.1.217. [DOI] [PubMed] [Google Scholar]
- Bhat NR, Zhang P. Hydrogen peroxide activation of multiple mitogen-activated protein kinases in an oligodendrocyte cell line: role of extracellular signal-regulated kinase in hydrogen peroxide-induced cell death. J Neurochem. 1999;72:112–119. doi: 10.1046/j.1471-4159.1999.0720112.x. [DOI] [PubMed] [Google Scholar]
- Bradham C, McClay DR. p38 MAPK in development and cancer. Cell Cycle. 2006;5:824–828. doi: 10.4161/cc.5.8.2685. [DOI] [PubMed] [Google Scholar]
- Brand A, Gil S, Seger R, Yavin E. Lipid constituents in oligodendroglial cells alter susceptibility to H2O2-induced apoptotic cell death via ERK activation. J Neurochem. 2001;76:910–918. doi: 10.1046/j.1471-4159.2001.00085.x. [DOI] [PubMed] [Google Scholar]
- Cao G, Sofic E, Prior RL. Antioxidant and prooxidant behavior of flavonoids: structure-activity relationships. Free Radic Biol Med. 1997;22:749–760. doi: 10.1016/S0891-5849(96)00351-6. [DOI] [PubMed] [Google Scholar]
- Chen CY, Yi L, Jin X, Zhang T, Fu YJ, Zhu JD, Mi MT, Zhang QY, Ling WH, Yu B. Inhibitory effect of delphinidin on monocyte-endothelial cell adhesion induced by oxidized low-density lipoprotein via ROS/p38MAPK/NF-κB pathway. Cell Biochem Biophys. 2011;61:337–348. doi: 10.1007/s12013-011-9216-2. [DOI] [PubMed] [Google Scholar]
- Chillemi C, Petrozza V, Garro L, Sardella B, Diotallevi R, Ferrara A, Gigante A, Di Cristofano C, Castagna A, Della Rocca C. Rotator cuff re-tear or non-healing: histopathological aspects and predictive factors. Knee Surg Sports Traumatol Arthrosc. 2011;19:1588–1596. doi: 10.1007/s00167-011-1521-1. [DOI] [PubMed] [Google Scholar]
- Dong J, Ramachandiran S, Tikoo K, Jia Z, Lau SS, Monks TJ. EGFR-independent activation of p38 MAPK and EG-FR-dependent activation of ERK1/2 are required for ROS-induced renal cell death. Am J Physiol Renal Physiol. 2004;287:F1049–F1058. doi: 10.1152/ajprenal.00132.2004. [DOI] [PubMed] [Google Scholar]
- Fu SC, Chan BP, Wang W, Pau HM, Chan KM, Rolf CG. Increased expression of matrix metalloproteinase 1 (MMP1) in 11 patients with patellar tendinosis. Acta Orthop Scand. 2002;73:658–662. doi: 10.3109/17453670209178031. [DOI] [PubMed] [Google Scholar]
- Fukuda H, Hamada K, Yamanaka K. Pathology and pathogenesis of bursal-side rotator cuff tears viewed from en bloc histologic sections. Clin Orthop Relat Res. 1990. pp. 75–80. [PubMed]
- Gotoh M, Hamada K, Yamakawa H, Tomonaga A, Inoue A, Fukuda H. Significance of granulation tissue in torn supraspinatus insertions: an immunohistochemical study with antibodies against interleukin-1 beta, cathepsin D, and matrix metalloprotease-1. J Orthop Res. 1997;15:33–39. doi: 10.1002/jor.1100150106. [DOI] [PubMed] [Google Scholar]
- Guo H, Liu G, Zhong R, Wang Y, Wang D, Xia M. Cyanidin-3-O-β-glucoside regulates fatty acid metabolism via an AMP-activated protein kinase-dependent signaling pathway in human HepG2 cells. Lipids Health Dis. 2012;11:10. doi: 10.1186/1476-511X-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong AN, Yu R, Chen C, Mandlekar S, Primiano T. Signal transduction events elicited by natural products: role of MAPK and caspase pathways in homeostatic response and induction of apoptosis. Arch Pharm Res. 2000;23:1–16. doi: 10.1007/BF02976458. [DOI] [PubMed] [Google Scholar]
- Milgrom C, Schaffler M, Gilbert S, van Holsbeeck M. Rotator-cuff changes in asymptomatic adults. The effect of age, hand dominance and gender. J Bone Joint Surg Br. 1995;77:296–298. [PubMed] [Google Scholar]
- Neer CS, 2nd, Craig EV, Fukuda H. Cuff-tear arthropathy. J Bone Joint Surg Am. 1983;65:1232–1244. [PubMed] [Google Scholar]
- Nho SJ, Yadav H, Shindle MK, Macgillivray JD. Rotator cuff degeneration: etiology and pathogenesis. Am J Sports Med. 2008;36:987–993. doi: 10.1177/0363546508317344. [DOI] [PubMed] [Google Scholar]
- Oak MH, Bedoui JE, Madeira SV, Chalupsky K, Schini-Kerth VB. Delphinidin and cyanidin inhibit PDGF(AB)-induced VEGF release in vascular smooth muscle cells by preventing activation of p38 MAPK and JNK. Br J Pharmacol. 2006;149:283–290. doi: 10.1038/sj.bjp.0706843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohba M, Shibanuma M, Kuroki T, Nose K. Production of hydrogen peroxide by transforming growth factor-beta 1 and its involvement in induction of egr-1 in mouse osteoblastic cells. J Cell Biol. 1994;126:1079–1088. doi: 10.1083/jcb.126.4.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson JM, Hallahan AR. p38 MAP kinase: a convergence point in cancer therapy. Trends Mol Med. 2004;10:125–129. doi: 10.1016/j.molmed.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Ozaki J, Fujimoto S, Nakagawa Y, Masuhara K, Tamai S. Tears of the rotator cuff of the shoulder associated with pathological changes in the acromion. A study in cadavera. J Bone Joint Surg Am. 1988;70:1224–1230. [PubMed] [Google Scholar]
- Park HB, Hah YS, Yang JW, Nam JB, Cho SH, Jeong ST. Antiapoptotic effects of anthocyanins on rotator cuff tenofibroblasts. J Orthop Res. 2010;28:1162–1169. doi: 10.1002/jor.21097. [DOI] [PubMed] [Google Scholar]
- Petrosillo G, Ruggiero FM, Paradies G. Role of reactive oxygen species and cardiolipin in the release of cytochrome c from mitochondria. FASEB J. 2003;17:2202–2208. doi: 10.1096/fj.03-0012com. [DOI] [PubMed] [Google Scholar]
- Schroeter H, Boyd C, Spencer JP, Williams RJ, Cadenas E, Rice-Evans C. MAPK signaling in neurodegeneration: influences of flavonoids and of nitric oxide. Neurobiol Aging. 2002;23:861–880. doi: 10.1016/S0197-4580(02)00075-1. [DOI] [PubMed] [Google Scholar]
- Seo BN, Ryu JM, Yun SP, Jeon JH, Park SS, Oh KB, Park JK, Han HJ. Delphinidin prevents hypoxia-induced mouse embryonic stem cell apoptosis through reduction of intracellular reactive oxygen species-mediated activation of JNK and NF-κB, and Akt inhibition. Apoptosis. 2013;18:811–824. doi: 10.1007/s10495-013-0838-2. [DOI] [PubMed] [Google Scholar]
- Siebel A, Cubillos-Rojas M, Santos RC, Schneider T, Bonan CD, Bartrons R, Ventura F, Rodrigues de Oliveira J, Rosa JL. Contribution of S6K1/MAPK signaling pathways in the response to oxidative stress: activation of RSK and MSK by hydrogen peroxide. PLoS One. 2013;8:e75523. doi: 10.1371/journal.pone.0075523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son Y, Cheong YK, Kim NH, Chung HT, Kang DG, Pae HO. Mitogen-activated protein kinases and reactive oxygen species: how can ROS activate MAPK pathways? J Signal Transduct. 2011;2011:792639. doi: 10.1155/2011/792639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soslowsky LJ, Thomopoulos S, Esmail A, Flanagan CL, Iannotti JP, Williamson JD, 3rd, Carpenter JE. Rotator cuff tendinosis in an animal model: role of extrinsic and overuse factors. Ann Biomed Eng. 2002;30:1057–1063. doi: 10.1114/1.1509765. [DOI] [PubMed] [Google Scholar]
- Stadtman ER, Berlett BS. Reactive oxygen-mediated protein oxidation in aging and disease. Drug Metab Rev. 1998;30:225–243. doi: 10.3109/03602539808996310. [DOI] [PubMed] [Google Scholar]
- Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- Tempelhof S, Rupp S, Seil R. Age-related prevalence of rotator cuff tears in asymptomatic shoulders. J Shoulder Elbow Surg. 1999;8:296–299. doi: 10.1016/S1058-2746(99)90148-9. [DOI] [PubMed] [Google Scholar]
- Tillander B, Franzén L, Norlin R. Fibronectin, MMP-1 and histologic changes in rotator cuff disease. J Orthop Res. 2002;20:1358–1364. doi: 10.1016/S0736-0266(02)00057-8. [DOI] [PubMed] [Google Scholar]
- Tournier C, Hess P, Yang DD, Xu J, Turner TK, Nimnual A, Bar-Sagi D, Jones SN, Flavell RA, Davis RJ. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science. 2000;288:870–874. doi: 10.1126/science.288.5467.870. [DOI] [PubMed] [Google Scholar]
- Tsuda T. Dietary anthocyanin-rich plants: biochemical basis and recent progress in health benefits studies. Mol Nutr Food Res. 2012;56:159–170. doi: 10.1002/mnfr.201100526. [DOI] [PubMed] [Google Scholar]
- Tuoheti Y, Itoi E, Pradhan RL, Wakabayashi I, Takahashi S, Minagawa H, Kobayashi M, Okada K, Shimada Y. Apoptosis in the supraspinatus tendon with stage II subacromial impingement. J Shoulder Elbow Surg. 2005;14:535–541. doi: 10.1016/j.jse.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Wang X, Martindale JL, Liu Y, Holbrook NJ. The cellular response to oxidative stress: influences of mitogen-activated protein kinase signalling pathways on cell survival. Biochem J. 1998;333:291–300. doi: 10.1042/bj3330291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Takagishi K, Osawa T, Yanagawa T, Nakajima D, Shitara H, Kobayashi T. Prevalence and risk factors of a rotator cuff tear in the general population. J Shoulder Elbow Surg. 2010;19:116–120. doi: 10.1016/j.jse.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Yim MB, Chae HZ, Rhee SG, Chock PB, Stadtman ER. On the protective mechanism of the thiol-specific antioxidant enzyme against the oxidative damage of biomacromolecules. J Biol Chem. 1994;269:1621–1626. [PubMed] [Google Scholar]
- Yuan J, Murrell GA, Wei AQ, Wang MX. Apoptosis in rotator cuff tendonopathy. J Orthop Res. 2002;20:1372–1379. doi: 10.1016/S0736-0266(02)00075-X. [DOI] [PubMed] [Google Scholar]
- Yuan J, Murrell GA, Trickett A, Wang MX. Involvement of cytochrome c release and caspase-3 activation in the oxidative stress-induced apoptosis in human tendon fibroblasts. Biochim Biophys Acta. 2003a;1641:35–41. doi: 10.1016/S0167-4889(03)00047-8. [DOI] [PubMed] [Google Scholar]
- Yuan J, Wang MX, Murrell GA. Cell death and tendinopathy. Clin Sports Med. 2003b;22:693–701. doi: 10.1016/S0278-5919(03)00049-8. [DOI] [PubMed] [Google Scholar]