Abstract
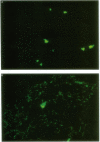
Although recombinant outer surface protein A (OspA) of Borrelia burgdorferi protects mice against injected Lyme disease spirochetes, the mode of protection has not yet been explored. Indeed, the efficacy of vaccine-induced immunity against a realistic vector-mediated challenge remains unexplored. Accordingly, we determined whether this immunogen protects mice against spirochetes delivered by nymphal Ixodes dammini ticks. Following challenge by tick bite, no spirochetes could be cultured from immunized mice, and no characteristic histopathology was found. The spirochete was not detected in ticks that fed on immunized animals and was present in virtually all ticks that fed on nonimmunized mice. We conclude that OspA-immunized mice are protected from spirochetal infection, at least in part, because the spirochete is destroyed in the infecting tick.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman S., Clare F. B., McGill T. W., Sonenshine D. E. Passage of host serum components, including antibody, across the digestive tract of Dermacentor variabilis (Say). J Parasitol. 1981 Oct;67(5):737–740. [PubMed] [Google Scholar]
- Azad A. F., Emala M. A. Suppression of Rickettsia typhi transmission in fleas maintained on murine typhus-immune rats. Am J Trop Med Hyg. 1987 Nov;37(3):629–635. doi: 10.4269/ajtmh.1987.37.629. [DOI] [PubMed] [Google Scholar]
- Barthold S. W., Beck D. S., Hansen G. M., Terwilliger G. A., Moody K. D. Lyme borreliosis in selected strains and ages of laboratory mice. J Infect Dis. 1990 Jul;162(1):133–138. doi: 10.1093/infdis/162.1.133. [DOI] [PubMed] [Google Scholar]
- Barthold S. W., Moody K. D., Terwilliger G. A., Duray P. H., Jacoby R. O., Steere A. C. Experimental Lyme arthritis in rats infected with Borrelia burgdorferi. J Infect Dis. 1988 Apr;157(4):842–846. doi: 10.1093/infdis/157.4.842. [DOI] [PubMed] [Google Scholar]
- Ben-Yakir D., Fox C. J., Homer J. T., Barker R. W. Quantification of host immunoglobulin in the hemolymph of ticks. J Parasitol. 1987 Jun;73(3):669–671. [PubMed] [Google Scholar]
- Brossard M., Rais O. Passage of hemolysins through the midgut epithelium of female Ixodes ricinus L. fed on rabbits infested or reinfested with ticks. Experientia. 1984 Jun 15;40(6):561–563. doi: 10.1007/BF01982330. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W., Hayes S. F., Benach J. L. Development of Borrelia burgdorferi in ixodid tick vectors. Ann N Y Acad Sci. 1988;539:172–179. doi: 10.1111/j.1749-6632.1988.tb31851.x. [DOI] [PubMed] [Google Scholar]
- Donahue J. G., Piesman J., Spielman A. Reservoir competence of white-footed mice for Lyme disease spirochetes. Am J Trop Med Hyg. 1987 Jan;36(1):92–96. doi: 10.4269/ajtmh.1987.36.92. [DOI] [PubMed] [Google Scholar]
- Fikrig E., Barthold S. W., Kantor F. S., Flavell R. A. Protection of mice against the Lyme disease agent by immunizing with recombinant OspA. Science. 1990 Oct 26;250(4980):553–556. doi: 10.1126/science.2237407. [DOI] [PubMed] [Google Scholar]
- Johnson R. C., Kodner C., Russell M. Active immunization of hamsters against experimental infection with Borrelia burgdorferi. Infect Immun. 1986 Dec;54(3):897–898. doi: 10.1128/iai.54.3.897-898.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Kodner C., Russell M. Passive immunization of hamsters against experimental infection with the Lyme disease spirochete. Infect Immun. 1986 Sep;53(3):713–714. doi: 10.1128/iai.53.3.713-714.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendis K. N., Munesinghe Y. D., de Silva Y. N., Keragalla I., Carter R. Malaria transmission-blocking immunity induced by natural infections of Plasmodium vivax in humans. Infect Immun. 1987 Feb;55(2):369–372. doi: 10.1128/iai.55.2.369-372.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M., Hirumi H., Moloo S. K. Suppression of Trypanosoma congolense, T. vivax and T. brucei infection rates in tsetse flies maintained on goats immunized with uncoated forms of trypanosomes grown in vitro. Parasitology. 1985 Aug;91(Pt 1):53–66. doi: 10.1017/s0031182000056511. [DOI] [PubMed] [Google Scholar]
- Piesman J., Mather T. N., Sinsky R. J., Spielman A. Duration of tick attachment and Borrelia burgdorferi transmission. J Clin Microbiol. 1987 Mar;25(3):557–558. doi: 10.1128/jcm.25.3.557-558.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro J. M., Makoul G. T., Levine J., Robinson D. R., Spielman A. Antihemostatic, antiinflammatory, and immunosuppressive properties of the saliva of a tick, Ixodes dammini. J Exp Med. 1985 Feb 1;161(2):332–344. doi: 10.1084/jem.161.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro J. M., Mather T. N., Piesman J., Spielman A. Dissemination and salivary delivery of Lyme disease spirochetes in vector ticks (Acari: Ixodidae). J Med Entomol. 1987 Mar;24(2):201–205. doi: 10.1093/jmedent/24.2.201. [DOI] [PubMed] [Google Scholar]
- Ribeiro J. M., Weis J. J., Telford S. R., 3rd Saliva of the tick Ixodes dammini inhibits neutrophil function. Exp Parasitol. 1990 May;70(4):382–388. doi: 10.1016/0014-4894(90)90121-r. [DOI] [PubMed] [Google Scholar]
- Schaible U. E., Kramer M. D., Eichmann K., Modolell M., Museteanu C., Simon M. M. Monoclonal antibodies specific for the outer surface protein A (OspA) of Borrelia burgdorferi prevent Lyme borreliosis in severe combined immunodeficiency (scid) mice. Proc Natl Acad Sci U S A. 1990 May;87(10):3768–3772. doi: 10.1073/pnas.87.10.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaible U. E., Kramer M. D., Museteanu C., Zimmer G., Mossmann H., Simon M. M. The severe combined immunodeficiency (scid) mouse. A laboratory model for the analysis of Lyme arthritis and carditis. J Exp Med. 1989 Oct 1;170(4):1427–1432. doi: 10.1084/jem.170.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. M., Schaible U. E., Kramer M. D., Eckerskorn C., Museteanu C., Müller-Hermelink H. K., Wallich R. Recombinant outer surface protein a from Borrelia burgdorferi induces antibodies protective against spirochetal infection in mice. J Infect Dis. 1991 Jul;164(1):123–132. doi: 10.1093/infdis/164.1.123. [DOI] [PubMed] [Google Scholar]
- Titus R. G., Ribeiro J. M. Salivary gland lysates from the sand fly Lutzomyia longipalpis enhance Leishmania infectivity. Science. 1988 Mar 11;239(4845):1306–1308. doi: 10.1126/science.3344436. [DOI] [PubMed] [Google Scholar]