Abstract
Background/Aims
Neurotensin is a gut-brain peptide with both inhibitory and excitatory actions on the colonic musculature; our objective was to understand the implications of this for motor patterns occurring in the intact colon of the rat.
Methods
The effects of neurotensin with concentrations ranging from 0.1–100 nM were studied in the intact rat colon in vitro, by investigating spatio-temporal maps created from video recordings of colonic motility before and after neurotensin.
Results
Low concentration of neurotensin (0.1–1 nM) inhibited propagating long distance contractions and rhythmic propagating motor complexes; in its place a slow propagating rhythmic segmental motor pattern developed. The neurotensin receptor 1 antagonist SR-48692 prevented the development of the segmental motor pattern. Higher concentrations of neurotensin (10 nM and 100 nM) were capable of restoring long distance contraction activity and inhibiting the segmental activity. The slow propagating segmental contraction showed a rhythmic contraction—relaxation cycle at the slow wave frequency originating from the interstitial cells of Cajal associated with the myenteric plexus pacemaker. High concentrations given without prior additions of low concentrations did not evoke the segmental motor pattern. These actions occurred when neurotensin was given in the bath solution or intraluminally. The segmental motor pattern evoked by neurotensin was inhibited by the neural conduction blocker lidocaine.
Conclusions
Neurotensin (0.1–1 nM) inhibits the dominant propulsive motor patterns of the colon and a distinct motor pattern of rhythmic slow propagating segmental contractions develops. This motor pattern has the hallmarks of haustral boundary contractions.
Keywords: Absorption, Colonic motility, Neurotensin, Peristalsis, Propulsion
Introduction
Neurotensin is a peptide present in the brain and gut with excitatory as well as inhibitory actions, it is found in synaptic vesicles of the central nervous system, in enteroendocrine cells and in the bloodstream.1,2
Neurotensin 1 receptors (NTR1) are abundantly present in the myenteric plexus, the submucosal plexus as well as within the musculature of the human colon, they co-localize with myenteric neurons3 but not interstitial cells of Cajal (ICC) in mice and humans.4 NTR1 were found on cultured ICC from mice and neurotensin modulated pacemaker currents via the activation of non-selective cation channels by intracellular Ca2+-release through NTR1.5
The laboratory of Rosa Serio studied the action of neurotensin on a 2 cm isolated segment of the rat colon using intraluminal pressure measurements. Neurotensin (100 nM) produced an inhibitory effect mediated by nitric oxide and an excitatory effect that was inhibited by atropine.6,7 Inhibitory and excitatory effects were also found in the human colon.8 Neurotensin might play a role in colon motor dysfunction in ulcerative colitis, where the colon produces an excessive amount of neurotensin.9 In mice, inhibitory and excitatory actions of neurotensin were shown to be mediated by the NTR1 receptor.10 Intravenous administration of neurotensin causes increased colonic motility in rats11 and humans.12
The present study deals with propulsive motor patterns in the rat colon. Colonic propulsive contractions are often referred to as “colonic migrating motor complexes (CMMCs)” described as such in the rat13,14 and mouse15–17 colon. We and others recently reported that the CMMC contains 2 distinct propulsive motor patterns, a pancolonic long distance contraction (LDC) and a rhythmic propagating motor complex (RPMC) that is primarily observed in the mid and distal colon.18–22
The objective of the present study was to understand how the inhibitory and excitatory actions of neurotensin translate into motor patterns of the whole colon in the rat. Interestingly, neurotensin consistently evoked a segmental motor pattern, slow propagating rhythmic haustral boundary contractions, that were rarely observed spontaneously. A haustrum is defined here as a pocket-like transient structure created by circumferential muscle contractions. They are prominent in the human23 and rabbit 3-taeniated colon24 and we show here that they appear in the rat colon in the presence of neurotensin. We applied neurotensin in the organ bath or intraluminally. Given in the bath solution, neurotensin will reach the NTR1 receptors in the myenteric plexus, similar to tetrodotoxin (TTX)22 or 5-hydroxytryptamine,19 which show immediate action upon bath application; neurotensin was applied in concentrations that mimicked plasma concentrations.25,26 Neurotensin given in the lumen may mimic the actions of the neuropeptide as secreted by enteroendocrine cells. The concentrations used in the present study are within the physiological range.25–27
Materials and Methods
Animals
We used 57 adult male Sprague-Dawley rats weighing 200–300 g. The study protocol was approved by the Ethic Committee of Renmin Hospital of Wuhan University School of Medicine (NSFC-81170249).
Preparation
After rats were killed by cervical dislocation, the entire colon was removed and placed in gassed (5% CO2 and 95% O2) Krebs at 37°C (pH 7.3–7.4). Krebs solution consisted of (mM) NaCl 118.1, KCl 4.8, NaHCO3 25, NaH2PO4 1.3, MgCl2·6H2O 1.2, Glucose 12.2, and CaCl2 2.5. All procedures, organ baths, and rat species were identical to those published previously.19,22 In short, the contents of the colon were gently washed out using warmed Krebs solution, and external connective tissue was removed because that interferes with spatio-temporal mapping. The proximal and distal ends were cannulated and fixed to the bottom of the organ bath. The proximal inflow tube (inner diameter 3 mm and outer diameter 4 mm) was connected to a 50 mL syringe and placed 15 cm above the level of the colon with phosphate buffered solution (PBS) (with 10 μM indomethacin, without glucose).22 Indomethacin was used in all solutions to avoid responses of prostaglandins released by the mucosa which may lead to changes in enteric nervous system (ENS) function.28 PBS consisted of NaCl 137.1 mM, KCl 2.7 mM, Na2HPO4 10 mM, and KH2PO4 2 mM. The distal outflow tube (inner diameter 3 mm, outer diameter 4 mm) was positioned in a narrow upright graduated cylinder filled with PBS. The fluid level in the cylinder determined the intraluminal pressure and the standard intraluminal pressure at the beginning was 5 cm H2O. After an LDC was completed, fluid flowed back into the colon. The colon was left to equilibrate for 20–30 minutes before the experiment started. Neurotensin was applied in the bath or via a small catheter into the lumen of the proximal colon, very slowly, taking care not to distend the colon. A video camera was mounted above the preparation and each experiment was recorded in its entirety. Data acquisition occurred through a Microsoft camera using Microsoft Life-Cam software (Microsoft Canada Co, Mississauga, ON, Canada). Drugs were given via the bath solution and intraluminally with an interval of 30 minutes using 5 concentrations.
Materials
The following drugs were used: neurotensin (Aladdin Industrial Co. Ltd, Shanghai, China), NTR1 antagonist SR-48692 (Sigma-Aldrich Co. Ltd, Zwijndrecht, Netherlands), lidocaine (Hualu Pharmaceutical Co. Ltd, Shandong, China), TTX (Baoman Biochemistry Co. Ltd, Shanghai, China). Krebs and PBS reagents were purchased from Sinopharm Chemical Reagent Co. Ltd, Shanghai, China.
Spatio-temporal Mapping
Data acquisition occurred through a Microsoft camera using Microsoft Lifecam software. Video recordings were analyzed by Image J aided by plugins written by Dr. Sean Parsons. A “spatio-temporal map” is an image which represents the motor activity based on the change in colon diameter. Diagonal streaks of black represent propagating contractions. Colon width (coded as image intensity, black to white) is calculated at each pixel along the colon’s length (image y-axis), for each video frame (image x-axis). Changes in diameter were quantified after calibrating distance, using dots at the bottom of the organ bath, which were separated by exactly 1 cm. The maps were made of the whole colon except for both ends, which were used to fix the colon onto the in and outflow tubes and the length was between 13 cm and 19 cm.
Statistical Methods
Frequencies were calculated over a 30-minute period. The maximum frequency was calculated from sections that showed the highest frequency in a regular rhythmic manner. Contraction duration (y-axis), length (x-axis), and velocity were calculated using Image J software (“analyze”).
One-way analysis of variance (ANOVA) was used to do statistical analysis among groups and least-significant difference was used to figure out difference between any 2 groups. Data were expressed as mean ± SD.
Results
Propagating Long Distance Contractions and Rhythmic Propagating Motor Complexes Are Replaced by Slow Propagating Segmental Contractions
Neurotensin was administered into the bath solution in a cumulative manner up to 0.1, 0.5, and 1.0 nM (n = 21). LDCs, always starting in the proximal colon, were inhibited in a dose dependent manner, assessed by the number of colons that lost all LDC activity (from 86% to 38%) and a significant reduction in frequency in the remainder (Fig. 1–3 and Table 1). Neurotensin also inhibited RPMC activity, a motor activity occurring in the mid-distal colon (Table 2). Replacing these 2 propulsive motor patterns22 emerged a motor pattern that originated in the proximal colon and propagated slowly as a rhythmically contracting circular muscle ring contraction (Fig. 1–3 and Table 3). The slow propagating contractions divided the colon into segments or pockets, and hence were called slow propagating segmental contractions (Fig. 2 and Supplementary Video 1). The slow propagating segmental contractions did not run from proximal to distal with a relatively constant force: rhythmic increases and decreases in amplitude occurred at a frequency of 1.6 ± 0.3 cpm. In addition, ripple activity often occurred within the pockets, often propagating retrogradely (Supplementary Video 1). Ripple activity is a low amplitude rhythmic motor pattern of the small intestine at 10–14 cpm.22
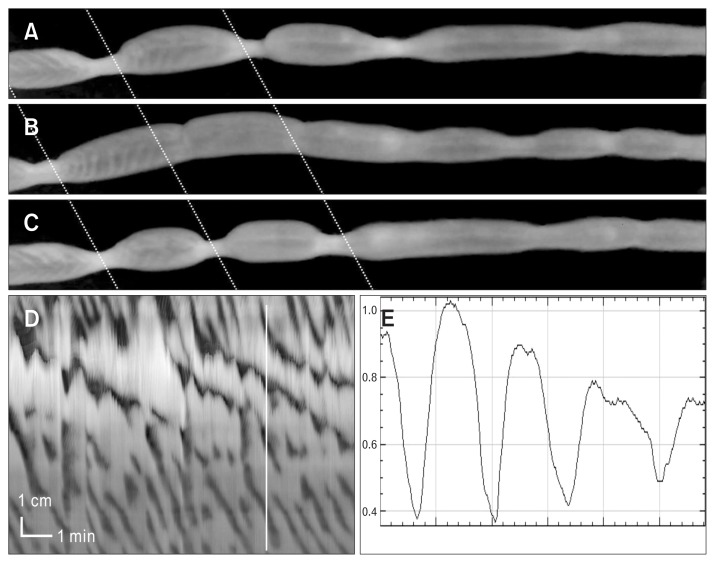
Figure 1.
The development of slow propagating segmental contractions in low concentrations of neurotensin.
Experiment 1: (A) The rat colon displayed strong long distance contraction (LDC) activity under control conditions. In all figures, the top shows the most proximal activity. (B) Neurotensin (0.1 nM) added to the bath solution, induces slow propagating segmental contractions (neurotensin is given 5 minutes before start of tracing). (C) Increasing the neurotensin concentration to 0.5 nM, the slow propagating segmental contractions are observed just distal to rhythmic proximal contractions.
Experiment 2: (D) The rat colon shows typical LDC and rhythmic propagating motor complex activity before addition of neurotensin at arrow. Neurotensin (0.1 nM), added to the bath, inhibits LDC activity and slow propagating segmental contractions start to develop. In the presence of neurotensin 0.5 nM (E) and 1 nM (F). The activity in 1 nM shows 4 slow propagating segmental contractions starting from the most proximal part of the colon.
Figure 2.
The segmental nature of the neurotensin induced contractions. In sequential video images (time between [A] and [B] is 88 seconds and time between [B] and [C] is 14 seconds) the segmental nature of the contractions is seen. The colon is divided into segments. The contractions are also seen to propagate and are seen to periodically relax, given the contractions an on/off/ on/off appearance. See Supplementary Video 1. The activity occurred in the presence of neurotensin (0.5 nM). (D) The spatio-temporal map (same as Fig. 1C) relates to the video images. The video images are moments in time, taken from Supplementary Video 1 whose spatiotemporal map is figure (D). The segmental nature can also be shown in an amplitude profile (E) made at the vertical line.
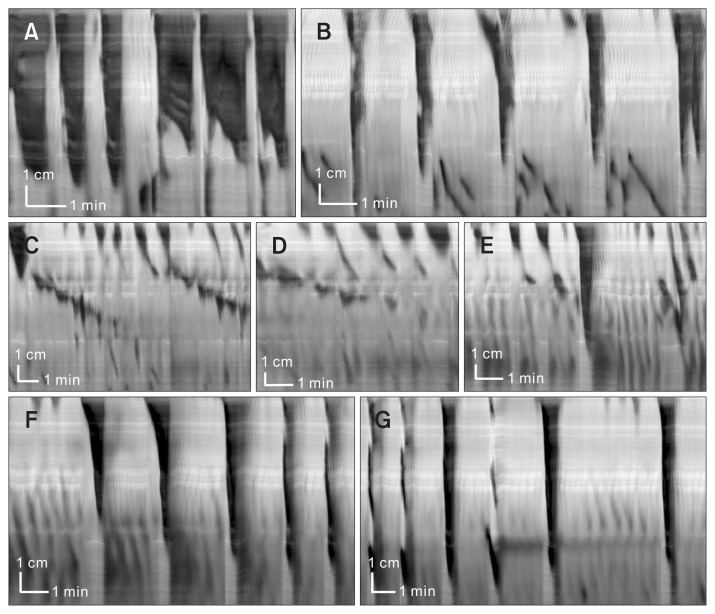
Figure 3.
Bath addition of neurotensin (0.1–1 nM) abolishes long distance contractions (LDCs) and induces slow propagating segmental contractions; neurotensin (10–100 nM) restores LDC activity. Here we show in one experiment the actions of low and high concentrations of neurotensin. (A) Control activity shows prominent rhythmic LDC activity. (B) Neurotensin (0.1nM) was added 10 minutes before recording, the LDC frequency and width decreased, some distal rhythmic propagating motor complexes are present. (C) Neurotensin (0.5 nM) was added, abolishing LDC activity and evoking slow propagating segmental rhythmic waves of contraction. Note the rhythmic changes in amplitude (the on/off pattern of the slow propagating segmental contractions) and the presence of regular proximal contractions. (D) Neurotensin (1 nM) was added, continuing the patterns observed in (C). (E) Neurotensin (10 nM) was added, a transition is seen from the segmental pattern in (C) to the LDC pattern in (F). (F, G) Neurotensin (100 nM) completely restores LDC activity. (F) and (G) are sequential in time.
Table 1.
Effects of Neurotensin, Added to the Bath Solution, on Long Distance Contractions
| Max Frequency (cpm) | Duration (sec) | Length (cm) | Velocity (cm/sec) | % colons with motor pattern present | ||
|---|---|---|---|---|---|---|
| 1 | Baseline | 1.19 ± 0.41a | 44.8 ± 13.2 | 9.2 ± 1.9 | 0.23 ± 0.11 | 86%, n = 18/21 |
| 2 | 0.1 nM | 1.10 ± 0.77a | 47.3 ± 20.1 | 10.2 ± 2.6a | 0.25 ± 0.11 | 71%, n = 15/21 |
| 3 | 0.5 nM | 0.59 ± 0.32a | 43.1 ± 19.4 | 8.7 ± 2.3 | 0.23 ± 0.10 | 38%, n = 8/21 |
| 4 | 1 nM | 0.63 ± 0.36a | 68.5 ± 42.1a | 7.8 ± 1.8a | 0.16 ± 0.10a | 38%, n = 8/21 |
| 5 | 10 nM | 0.91 ± 0.54 | 38.3 ± 17.6 | 8.8 ± 0.7 | 0.29 ± 0.20a | 41%, n = 5/12 |
| 6 | 100 nM | 1.36 ± 0.56a | 35.1 ± 6.2 | 8.2 ± 2.0 | 0.24 ± 0.05 | 25%, n = 3/12 |
| P-value | 0.038 | 0.106 | 0.177 | 0.45 | ||
| Baseline | 0.96 ± 0.21 | 49.3 ± 8.4 | 8.8 ± 3.6 | 0.19 ± 0.11 | 100%, n = 5/5 | |
| 7 | 10 nM | 1.34 ± 0.53 | 49.7 ± 23.2 | 10.3 ± 3.0 | 0.29 ± 0.10 | 100%, n = 5/5 |
| 8 | 100 nM | 1.26 ± 0.84 | 48.5 ± 7.8 | 9.9 ± 3.9 | 0.33 ± 0.27 | 80%, n = 4/5 |
| P-value | 0.543 | 0.993 | 0.791 | 0.444 |
Max Frequency: 1 vs 3, P = 0.011; 1 vs 4, P = 0.016; 2 vs 3, P = 0.034; 2 vs 4, P = 0.048; 3 vs 6, P = 0.04; 4 vs 6, P = 0.049; Duration: 1 vs 4, P = 0.014; 2 vs 4, P = 0.033; 3 vs 4, P = 0.026; 5 vs 4, P = 0.02; 6 vs 4, P = 0.03; Length: 2 vs 4, P = 0.012; Velocity: 4 vs 5, P = 0.049.
2–6 are given cumulatively. 7 and 8 are given in a separate series of experiments, hence without prior low concentrations.
Table 2.
Effects of Neurotensin, Added to the Bath Solution, on Rhythmic Propagating Motor Complexes
| Frequency (/min) Average |
Duration (sec) | Length (cm) | Velocity (cm/sec) | % colons with motor pattern present | ||
|---|---|---|---|---|---|---|
| 1 | baseline | 0.60 ± 0.19 | 16.6 ± 7.2a | 3.6 ± 1.0a | 0.25 ± 0.11a | 52%, n = 11/21 |
| 2 | 0.1 nM | 0.56 ± 0.27 | 20.6 ± 11.4a | 3.0 ± 0.7a | 0.19 ± 0.12 | 62%, n = 13/21 |
| 3 | 0.5 nM | 0.57 ± 0.24 | 34.7 ± 17.4a | 3.4 ± 0.9a | 0.13 ± 0.09a | 29%, n = 6/21 |
| 4 | 1 nM | 0.49 ± 0.18 | 36.7 ± 18.1a | 3.0 ± 0.3a | 0.10 ± 0.04a | 24%, n = 5/21 |
| 5 | 10 nM | 0.62 ± 0.26 | 15.4 ± 0.1a | 5.5 ± 0.9a | 0.35 ± 0.05a | 16%, n = 2/12 |
| 6 | 100 nM | -- | -- | -- | -- | n = 0/12 |
| P | 0.934 | 0.011 | 0.006 | 0.017 |
Duration: 1 vs 3, P = 0.007; 1 vs 4, P = 0.005; 2 vs 3, P = 0.027; 2 vs 4, P = 0.019; 4 vs 5, P = 0.048; Length: 1 vs 2, P = 0.029; 1 vs 5, P = 0.007; 2 vs 5, P < 0.001; 3 vs 5, P = 0.004; 4 vs 5, P = 0.001; Velocity: 1 vs 3, P = 0.032; 1 vs 4, P = 0.011; 3 vs 5, P = 0.014; 4 vs 5, P = 0.006.
Table 3.
Effects of Neurotensin, Added to the Bath Solution, on Slow Propagating Segmental Contractions
| Frequency (cpm) Average/Max |
Duration (sec) | Length (cm) | Velocity (cm/sec) | % colons with motor pattern present | ||
|---|---|---|---|---|---|---|
| 1 | Baseline | 0.03 0.03 |
159.1 | 6.6 | 0.04 | 5%, n = 1/21 |
| 2 | 0.1 nM | 0.24 ± 0.11 0.81 ± 0.47 |
179.4 ± 113.0 | 6.9 ± 2.2 | 0.05 ± 0.02 | 76%, n = 16/21 |
| 3 | 0.5 nM | 0.22 ± 0.10 0.63 ± 0.26 |
200.6 ± 74.7 | 7.4 ± 3.2 | 0.04 ± 0.01 | 57%, n = 12/21 |
| 4 | 1 nM | 0.25 ± 0.18 0.78 ± 0.25 |
174.3 ± 74.4 | 7.9 ± 2.4 | 0.05 ± 0.02 | 62%, n = 13/21 |
| 5 | 10 nM | 0.07 0.08 |
234.8 | 6.0 | 0.03 | 8%, n = 1/12 |
| 6 | 100 nM | 0.1 0.52 |
382.1 | 8.0 | 0.02 | 8%, n = 1/12 |
| P-value | 0.808 0.401 |
0.750 | 0.594 | 0.340 |
The maximal frequency of the slow propagating segmental contractions was similar to the LDC frequency at 0.5–1.5 cpm. If the frequency was low, the slow propagating segmental contractions could be interspersed with short proximal rhythmic contractions, the frequency of the proximal and segmental contractions combined was again in the range of 1 cpm.
In 12 out of 21 experiments, 10 nM and 100 nM concentrations of neurotensin were added to the lower concentrations, this caused a re-emergence of LDC activity in some of the colons and an inhibition of the slow propagating segmental contractions (Fig. 3 and Table 1). When 10 and 100 nM neurotensin were given cumulatively without prior addition of lower concentrations (n = 5), the LDC activity was not inhibited and the slow propagating segmental contractions did not emerge, except for one experiment where a few slow propagating segmental contractions intermingled with LDC activity (Table 1).
When neurotensin was given in the same concentration range as described above into the lumen of the colon, the results related to LDCs and slow propagating segmental contractions were similar (Fig. 4; Tables 4 and 5). RPMC activity was inhibited at the intraluminal neurotensin concentration of 0.1 nM; no RPMC activity was observed in the presence of 0.5 nM luminal neurotensin or higher.
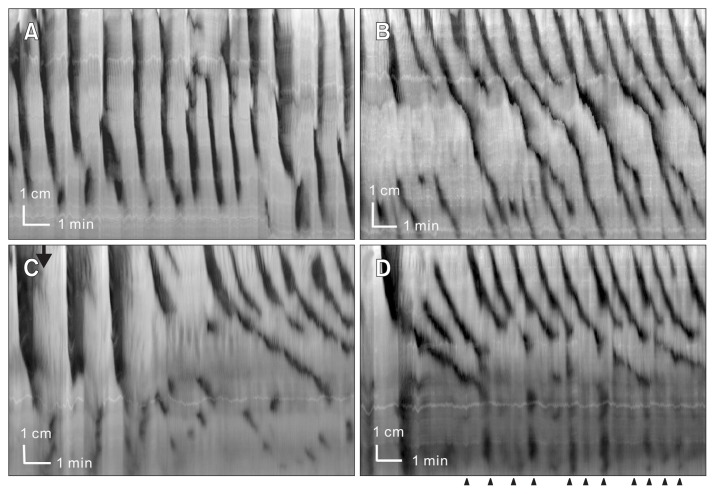
Figure 4.
Intraluminal neurotensin changes long distance contraction (LDC) activity into slow propagating segmental contractions.
Experiment 1: (A) Baseline activity shows regular rhythmic LDC activity at 0.6 cpm. (B) Neurotensin (0.1 nM) was added into the lumen 10 minutes before recording. LDCs changed into slow propagating segmental contractions. Strong proximal contractions remained at 0.6 cpm.
Experiment 2: (C) Neurotensin (0.1 nM) was added at arrow. LDCs change into slow propagating segmental contractions. Thereafter the concentration was increased to 0.5 nM neurotensin (D).
Table 4.
Effects of Neurotensin, Added Intraluminally, on Long Distance Contractions
| Frequency (/min) Average/Max |
Duration (sec) | Length (cm) | Velocity (cm/sec) | % colons with motor pattern present | ||
|---|---|---|---|---|---|---|
| 1 | Baseline | 0.36 ± 0.08a 1.06 ± 0.37a |
44.2 ± 14.4a | 9.4 ± 2.7 | 0.21 ± 0.11 | 87%, n = 13/15 |
| 2 | 0.1 nM | 0.17 ± 0.07a 0.76 ± 0.39 |
40.7 ± 7.5a | 9.0 ± 1.6 | 0.23 ± 0.08 | 67%, n = 10/15 |
| 3 | 0.5 nM | 0.18 ± 0.03a 0.66 ± 0.29 |
30.0 ± 10.3a | 8.5 ± 1.5 | 0.30 ± 0.09 | 27%, n = 4/15 |
| 4 | 1 nM | 0.09 ± 0.05a 0.47 ± 0.43a |
26.5 ± 1.3a | 8.4 ± 0.7 | 0.27 ± 0.16 | 20%, n = 3/15 |
| 5 | 10 nM | 0.26 ± 0.08a 1.03 ± 0.14 |
24.8 ± 8.3a | 6.8 ± 0.1 | 0.29 ± 0.09 | 20%, n = 3/15 |
| 6 | 100 nM | 0.23 ± 0.09a 0.8 ± 0.41 |
25.2 ± 1.0a | 7.4 ± 0.7 | 0.32 ± 0.01 | 40%, n = 6/15 |
| P-value | < 0.001 0.930 |
0.022 | 0.390 | 0.400 |
Average Frequency: 1 vs 2, P < 0.001; 1 vs 3, P < 0.001; 1 vs 4, P < 0.001; 1 vs 6, P = 0.035; 4 vs 5, P = 0.007; 4 vs 6, P = 0.034; Max frequency: 1 vs 4, P = 0.01; Duration: 1 vs 3, P = 0.036; 1 vs 4, P = 0.046; 1 vs 5, P = 0.012; 1 vs 6, P = 0.013; 2 vs 5, P = 0.045.
Table 5.
Effects of Neurotensin, Added Intraluminally, on Slow Propagating Segmental Contractions
| Frequency (cpm) Average/Max |
Duration (sec) | Length (cm) | Velocity (cm/sec) | % colons with motor pattern present | ||
|---|---|---|---|---|---|---|
| 1 | Baseline | 0.13 1.47 |
119.8 | 11.9 | 0.10 | 5%, n = 1/15 |
| 2 | 0.1 nM | 0.23 ± 0.12 1.04 ± 0.48a |
179.5 ± 104.6 | 8.9 ± 2.2 | 0.06 ± 0.03a | 60%, n = 9/15 |
| 3 | 0.5 nM | 0.20 ± 0.14 0.65 ± 0.17 |
237.5 ± 100.3 | 7.2 ± 2.7 | 0.03 ± 0.02a | 47%, n = 7/15 |
| 4 | 1 nM | 0.14 ± 0.03 0.42 ± 0.27a |
206.8 ± 101.7 | 6.5 ± 2.6 | 0.04 ± 0.03 | 27%, n = 4/15 |
| 5 | 10 nM | 0.31 0.38 |
130.5 | 9.0 | 0.07 | 5%, n = 1/15 |
| 6 | 100 nM | -- | -- | -- | -- | n = 0/15 |
| P-value | 0.491 0.026 |
0.548 | 0.211 | 0.088 |
Max frequency: 2 vs 4, P = 0.012; Velocity: 2 vs 3, P = 0.037.
The NTR1 antagonist SR-48692 did not affect baseline activity significantly but in 12 separate experiments, it prevented neurotensin (1 nM) from inhibiting LDC activity and the slow propagating segmental contractions did not develop (Fig. 5).
Figure 5.
The neurotensin receptor 1 (NTR1) antagonist SR-48692 inhibits the effect of neurotensin (1 nM). (A) Control activity shows rhythmic long distance contraction (LDC) activity. (B) SR-48692 (0.3 μM) was added 10 minutes before recording, LDC activity is maintained, the rhythmic propagating motor complex activity has become more prominent. (C) Neurotensin (1 nM) did not inhibit LDCs in the presence of SR-48692 and no slow propagating segmental contractions developed.
The slow propagating segmental contractions were rarely seen spontaneously, but Figure 6 shows an example where the motor pattern occurred without a deliberate stimulus, seen interspersed between LDCs. This experiment also shows that longitudinal muscle activity, which was not prominent in almost all experiments, can give the slow propagating segmental contractions a zigzag like appearance. The accompanying video (Supplementary Video 2) shows that this motor pattern divides the colon into segments. Again, ripple activity can be seen to propagate retrogradely in the segments.
Figure 6.
Spontaneous slow propagating segmental contractions. (A) and (B) show two time periods of a preparation that showed the rare occurrence of spontaneous rhythmic segmental contractions. They occurred in between LDC activity. (C) Video frames showing the segmented colon, as it changes over time; the contractions that create the segments are slowly propagating in anal direction. Time between top and middle image is 24 seconds, between middle and bottom image is 36 seconds. The length of the colon depicted is 14 cm. (D) Amplitude profile at one time point across the colon, at vertical line in (B), showing the segmental nature of the contraction pattern.
Propulsion
Propulsion was measured as outflow into the distal connecting tube which flowed into an upright graduated cylinder with a diameter of 2 cm. LDCs and RPMCs always resulted in an outflow of 1–3 mL, which in our experiments flowed back into the colon. The haustral boundary contractions induced by neurotensin did not cause a visible rise in the fluid level of the graduated cylinder.
Proximal Contractions
LDCs and the slow propagating segmental contractions were not seen in the presence of TTX (0.2 μM) or lidocaine (5.5 μM; n = 10; Fig. 7). Both motor patterns occurred within the frequency range of 0.3–1.6 cpm. In almost all experiments, rhythmic proximal activity remained in the presence of TTX or lidocaine within this frequency range but only propagating for 1–3 cm. To test the effect of neurotensin on this proximal activity, 4 colons were subjected to 5.5 μM lidocaine, this resulted in an average proximal contraction frequency of 0.93 ± 0.38 cpm with a maximal frequency in these preparations of 1.58 ± 0.70 cpm. Neurotensin (100 nM) significantly increased the average and maximal frequency to 1.69 ± 1.10 cpm and 2.25 ± 0.82 cpm respectively (P < 0.05).
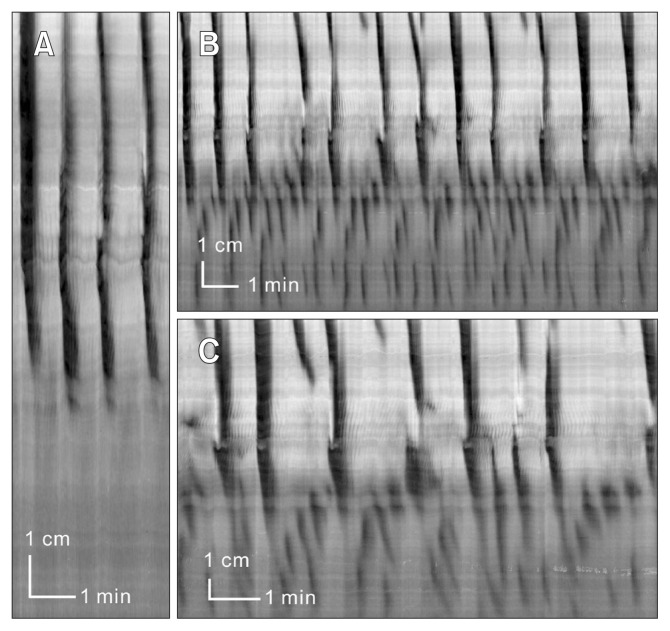
Figure 7.
Effect of neurotensin in the presence of nerve conduction blockade.
Experiment 1: (A) Typical activity in the presence of lidocaine (5.5 μM). Proximal rhythmic contractions are always prominent. (B) After addition of neurotensin (100 nM) the proximal rhythmic contractions increased in frequency and the contractions propagated across the colon.
Experiment 2: (C) Activity in the presence of lidocaine (5.5 μM). (D) In the presence of 100 nM neurotensin, strong rhythmic contractile activity occurs in both proximal and distal colon. In the distal colon it is predominantly retrograde.
Discussion
The present study shows that neurotensin causes a switch in neural programs in the rat colon. At concentrations between 0.1 and 1 nM, it inhibits pan-colonic propagating LDCs, as well as the RPMCs that mainly operate in the distal colon. At the same time, neurotensin at these concentrations evokes a unique motor pattern: rhythmic slow propagating segmental contractions of the circular muscle, inhibited by nerve conduction blockers. Supplementary Videos 1 and 2, and Figures 2 and 6 show that there are several contractions traversing the colon at the same time, dividing the colon into segments or pockets; their boundaries created by the circumferential circular muscle contractions. In the human23 and rabbit24 colon, such segments or pockets are called haustra; the neurotensin induced motor pattern can thus be referred to as haustral boundary contractions. When the concentration of neurotensin reaches 10 nM or 100 nM, LDC activity is promoted and no induction of the slow propagating segmental contractions occurs.
The slow propagating segmental contraction that is evoked by neurotensin is a ring contraction of the circular muscle. It occurs rhythmically at a frequency of ~0.2 cpm and a propagation velocity of 0.05 cm/sec. Consistent with the haustral boundary contractions in the rabbit,24 the ring contraction shows an on/off/on/off pattern at ~1.5 cpm. The on/off pattern is visualized in Figures 1–4 and 6 and Supplementary Videos 1 and 2. Hence, the boundaries of the segments are not a continuously moving sustained contraction and the propulsive nature of the contraction is therefore less effective because of its rhythmic contraction-relaxation cycle. This allows fluid to flow back when the contraction relaxes. The mixing within the segments is facilitated by the ripple activity, which is often seen to traverse the segment as high frequency retrograde propagating contractions (Supplementary Video 2).
The slow propagating segmental contractions have 2 intrinsic frequencies, the frequency of occurrence (~0.2 cpm) and the on/off frequency (~1.5 cpm). What is the origin of these rhythmicities? Smooth muscle cells contract when their membrane potential passes the threshold for activation of calcium channels. The membrane potential oscillates in much of the gut musculature because of pacemaker activity (electrical slow waves) generated by ICC that propagates into the musculature. Unlike the heart, gut pacemaker activity does not inevitably cause contractions. The most depolarized phase of the oscillations may not yet pass the threshold; this may need additional stimuli to further depolarize the musculature.29 Distinct ICC networks have omnipresent myogenic pacemaking activity such as the ICC-MP of the stomach and small intestine. The dog colon is also unambiguous; its omnipresent myogenic activity is in the ICC associated with the submuscular plexus (ICC-SMP).30 Also in the rat colon, the strongest myogenic pacemaker originates in the ICC-SMP, which is governing the ripples at 10–14 cpm, which can be seen to be part of all the motor patterns.19,21,22,31–33 They are clearly seen in the most proximal colon in between the LDCs in Figure 3B. They do not appear to be associated with the slow propagating segmental contractions. In the intestine and colon, a second network of ICC does not generate spontaneous rhythmicity; it is stimulus-dependent and hence functions “on demand.” In the small intestine, the classical segmentation motor activity is generated when the ICC associated with the deep muscular plexus (ICC-DMP) produce a stimulus-dependent rhythmic electrical activity.34,35 In the rat colon, it is the ICC-MP that generates a stimulus-dependent low frequency activity; the laboratories of Jimenez in the rat31 and Takaki in the mouse36 showed that ICC-MP are associated with a low frequency pacemaker that can drive contractions at frequencies from 0.3 to 2 cpm,19,21,22,31–33 hence the on/off pattern observed in the present study is likely to be associated with pacemaking from the ICC-MP. It is not known where the ultra-slow pacemaker that drives the slow propagating segmental contractions is located; it may be within the ENS or in the intramuscular ICC (ICC-IM). In the stomach, the ICC-IM harbor stimulus-dependent pacemaking activity,37 in that case induced by the vagus. If the ICC-IM are pacemaker cells in the rat colon, it may well be the ENS that supplies the stimulus to the ICC-IM consistent with the motor pattern being inhibited by nerve conduction blockers. The ICC-IM are heavily innervated by enteric nerves.38–40
Siegle and Ehrlein41 showed in the canine ileum, in vivo, that neurotensin changed a propulsive motor pattern into segmentation, a non-propagating motor activity. Strain gauges showed propulsive contractions that changed into stationary contractions after intravenous injection of neurotensin. Using spatio-temporal mapping, we showed that the segmentation motor pattern in the mouse intestine has a checkered appearance,35 identical to that described by Cannon.42 Segmentation in the small intestine is caused by interactions of 2 pacemaker activities.35 One is the myogenic pacemaker of the ICC-MP, the other a stimulus-dependent pacemaker of the ICC-DMP. We have shown that substance P can evoke the ICC-DMP pacemaker.43 Potential stimuli are not limited to neurotransmitters; fatty acids such as butyrate and decanoic acid can also evoke the low frequency pacemaker activity,34 and so can distention.44 Siegle and Ehrlein’s observation that neurotensin can evoke segmentation can be explained by the induction of ICC-DMP pacemaker activity.41 The segmentation motor pattern in the mouse intestine can be inhibited by TTX or it can be prominently present after blocking neural activity. It depends on the nature (neuronal or non-neuronal) of the stimulus that induces the pacemaking activity in concert with stimulation of the musculature. Our understanding of the stimulus-induced pacemaker is still limited and we do not know all the neuronal and non-neuronal physiological stimuli that can evoke it.45
Segmentation, as a motor pattern, is defined in the rat colon similar to its definition in the small intestine: short-lasting contractions, either stationary or propagating over very short distances.19,22 They can occur in an apparent random fashion or they can appear highly organized in a checkered pattern that is most prominently observed in the small intestine.35,46 The motor pattern described here as slow propagating segmental contraction is different in that it shows very clear rhythmicity and it shows propagation characteristics. We have also seen this pattern in response to granisetron, we described this as sequential segmental contractions19; from now on we will describe this motor pattern as slow propagating segmental contractions or haustral boundary contractions. Future research into the pacemakers and neural activities involved in these motor patterns will elucidate commonalities and mechanistic differences between these motor patterns.
The interaction between ICC and the ENS can take many forms. A well known example is the migrating motor complex in the small intestine which is a rhythmic motor pattern that is orchestrated by the ENS with input from the vagus; but the motor activity during phase III is driven by the slow wave activity, giving the individual contractions their frequency and propagation characteristics.47 In the mouse colon, excitatory nerves act on ICC-MP to evoke rhythmic depolarizations that underlie the propagating motor complexes.16,48 With respect to the slow propagating segmental contractions in the rat colon, further research has to reveal whether it is similar to the migrating motor complexes or the segmentation motor pattern in the intestine.
Neurotensin at 100 nM increased the frequency of the proximal low frequency rhythmic contractions. This may occur via direct action on ICC-MP. Neurotensin receptors are abundant in the neural plexuses and in the musculature, but no evidence was found of the receptor on ICC in the mouse and human.4 However, since the NTR1 is present on cultured ICC from mice where it modulates pacemaker currents,5 there is a possibility that neurotensin can influence or modulate pacemaker currents in ICC-MP in the rat colon.
In summary, neurotensin at 0.1–1 nM, switches programs that govern the motor patterns in the rat colon so that a rhythmic propulsive motor pattern changes into a rhythmic slow propagating segmental motor pattern that carries the hallmarks of haustral boundary contractions.
Supplementary Material
Footnotes
Supplementary Materials
Note: To access the supplementary videos mentioned in this article, visit the online version of Journal of Neurogastroenterology and Motility at http://www.jnmjournal.org/ and at http://dx.doi.org/10.5056/jnm15181.
Financial support: The study was financially supported by a grant from the National Natural Science Foundation of China (NSFC; Grant No. 81170249) to J.H.C. and from the Canadian Institutes of Health Research (CIHR; Grant No. MOP12874) to J.D.H.
Conflicts of interest: None.
Author contributions: Ji-Hong Chen and Jan D Huizinga supervised students, designed experimental work, supervised data interpretation and manuscript writing; Hongfei Li conducted and supervised the experiments, did most of the analysis, significantly contributed to data interpretation and manuscript writing; and Zixian Yang, Min Huang, Yuanjie Yu, Shiyun Tan, and Hesheng Luo contributed to experimental work, animal care, and data analysis.
References
- 1.Carraway R, Leeman SE. The isolation of a new hypotensive peptide, neurotensin, from bovine hypothalami. J Biol Chem. 1973;248:6854–6861. [PubMed] [Google Scholar]
- 2.O’Hara JR, Skinn AC, MacNaughton WK, Sherman PM, Sharkey KA. Consequences of Citrobacter rodentium infection on enteroendocrine cells and the enteric nervous system in the mouse colon. Cell Microbiol. 2006;8:646–660. doi: 10.1111/j.1462-5822.2005.00657.x. [DOI] [PubMed] [Google Scholar]
- 3.Rettenbacher M, Reubi JC. Localization and characterization of neuropeptide receptors in human colon. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:291–304. doi: 10.1007/s002100100454. [DOI] [PubMed] [Google Scholar]
- 4.Gromova P, Rubin BP, Thys A, Erneux C, Vanderwinden JM. Neurotensin receptor 1 is expressed in gastrointestinal stromal tumors but not in interstitial cells of Cajal. PLoS One. 2011;6:e14710. doi: 10.1371/journal.pone.0014710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee J, Kim YD, Park CG, et al. Neurotensin modulates pacemaker activity in interstitial cells of Cajal from the mouse small intestine. Mol Cells. 2012;33:509–516. doi: 10.1007/s10059-012-2290-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mulè F, Serio R. Inhibition of mechanical activity by neurotensin in rat proximal colon: involvement of nitric oxide. Am J Physiol. 1997;273(2 Pt 1):G491–G497. doi: 10.1152/ajpgi.1997.273.2.G491. [DOI] [PubMed] [Google Scholar]
- 7.Mulè F, Serio R. Mode and mechanism of neurotensin action in rat proximal colon. Eur J Pharmacol. 1997;319:269–272. doi: 10.1016/S0014-2999(96)00943-0. [DOI] [PubMed] [Google Scholar]
- 8.van der Veek PP, Schots ED, Masclee AA. Effect of neurotensin on colorectal motor and sensory function in humans. Dis Colon Rectum. 2004;47:210–218. doi: 10.1007/s10350-003-0029-2. [DOI] [PubMed] [Google Scholar]
- 9.Bassotti G, Antonelli E, Villanacci V, Baldoni M, Dore MP. Colonic motility in ulcerative colitis. United European Gastroenterol J. 2014;2:457–462. doi: 10.1177/2050640614548096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pettibone DJ, Hess JF, Hey PJ, et al. The effects of deleting the mouse neurotensin receptor NTR1 on central and peripheral responses to neurotensin. J Pharmacol Exp Ther. 2002;300:305–313. doi: 10.1124/jpet.300.1.305. [DOI] [PubMed] [Google Scholar]
- 11.Pellissier S, Eribon O, Chabert J, Gully D, Roche M. Peripheral neurotensin participates in the modulation of pre- and postprandial intestinal motility in rats. Neuropeptides. 1996;30:412–419. doi: 10.1016/S0143-4179(96)90002-5. [DOI] [PubMed] [Google Scholar]
- 12.Thor K, Rosell S. Neurotensin increases colonic motility. Gastroenterology. 1986;90:27–31. doi: 10.1016/0016-5085(86)90070-3. [DOI] [PubMed] [Google Scholar]
- 13.Spencer NJ, Bayguinov P, Hennig GW, et al. Activation of neural circuitry and Ca2+ waves in longitudinal and circular muscle during CMMCs and the consequences of rectal aganglionosis in mice. Am J Physiol Gastrointest Liver Physiol. 2007;292:G546–G555. doi: 10.1152/ajpgi.00352.2006. [DOI] [PubMed] [Google Scholar]
- 14.Heredia DJ, Gershon MD, Koh SD, Corrigan RD, Okamoto T, Smith TK. Important role of mucosal serotonin in colonic propulsion and peristaltic reflexes: in vitro analyses in mice lacking tryptophan hydroxylase 1. J Physiol. 2013;591(Pt 23):5939–5957. doi: 10.1113/jphysiol.2013.256230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fida R, Lyster DJ, Bywater RA, Taylor GS. Colonic migrating motor complexes (CMMCs) in the isolated mouse colon. Neurogastroenterol Motil. 1997;9:99–107. doi: 10.1046/j.1365-2982.1997.d01-25.x. [DOI] [PubMed] [Google Scholar]
- 16.Bayguinov PO, Hennig GW, Smith TK. Ca2+ imaging of activity in ICC-MY during local mucosal reflexes and the colonic migrating motor complex in the murine large intestine. J Physiol. 2010;588(Pt 22):4453–4474. doi: 10.1113/jphysiol.2010.196824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keating DJ, Spencer NJ. Release of 5-hydroxytryptamine from the mucosa is not required for the generation or propagation of colonic migrating motor complexes. Gastroenterology. 2010;138:659–670. e1–e2. doi: 10.1053/j.gastro.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 18.Kubota K, Ohtake N, Ohbuchi K, et al. Hydroxy-α sanshool induces colonic motor activity in rat proximal colon: a possible involvement of KCNK9. Am J Physiol Gastrointest Liver Physiol. 2015;308:G579–G590. doi: 10.1152/ajpgi.00114.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu Y, Chen JH, Li H, et al. Involvement of 5-HT3 and 5-HT4 receptors in colonic motor patterns in rats. Neurogastroenterol Motil. 2015;27:914–928. doi: 10.1111/nmo.12550. [DOI] [PubMed] [Google Scholar]
- 20.Kendig DM, Grider JR. Serotonin and colonic motility. Neurogastroenterol Motil. 2015;27:899–905. doi: 10.1111/nmo.12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huizinga JD, Martz S, Gill V, Wang XY, Jimenez M, Parsons S. Two independent networks of interstitial cells of Cajal work cooperatively with the enteric nervous system to create colonic motor patterns. Front Neurosci. 2011;5:93. doi: 10.3389/fnins.2011.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen JH, Zhang Q, Yu Y, et al. Neurogenic and myogenic properties of pan-colonic motor patterns and their spatiotemporal organization in rats. PLoS ONE. 2013;8:e60474. doi: 10.1371/journal.pone.0060474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bharucha AE, Brookes JH. Neurophysiologic mechanisms of human large intestine motility. In: Johnson LR, editor. Physiology of the gastrointestinal tract. Amsterdam: Elsevier Academic Press; 2012. pp. 977–1022. [DOI] [Google Scholar]
- 24.Chen JH, Yang Z, Yu Y, Huizinga JD. Haustral boundary contractions in the proximal 3-taeniated colon of the rabbit colon. Am J Physiol Gastrointest Liver Physiol. 2016;310:G181–G192. doi: 10.1152/ajpgi.00171.2015. [DOI] [PubMed] [Google Scholar]
- 25.Butler MG, Nelson TA, Driscoll DJ, Manzardo AM. High plasma neurotensin levels in children with Prader-Willi syndrome. Am J Med Genet A. 2015;167A:1773–1778. doi: 10.1002/ajmg.a.37103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sgourakis G, Papapanagiotou A, Kontovounisios C, et al. The value of plasma neurotensin and cytokine measurement for the detection of bowel ischaemia in clinically doubtful cases: a prospective study. Exp Biol Med. 2013;238:874–880. doi: 10.1177/1535370213494663. [DOI] [PubMed] [Google Scholar]
- 27.Dublineau I, Dudoignon N, Monti P, et al. Screening of a large panel of gastrointestinal peptide plasma levels is not adapted for the evaluation of digestive damage following irradiation. Can J Physiol Pharmacol. 2004;82:103–113. doi: 10.1139/y03-130. [DOI] [PubMed] [Google Scholar]
- 28.O’Hara JR, Lomax AE, Mawe GM, Sharkey KA. Ileitis alters neuronal and enteroendocrine signalling in guinea pig distal colon. Gut. 2007;56:186–194. doi: 10.1136/gut.2006.102780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huizinga JD, Thuneberg L, Vanderwinden JM, Rumessen JJ. Interstitial cells of Cajal as targets for pharmacological intervention in gastrointestinal motor disorders. Trends Pharmacol Sci. 1997;18:393–403. doi: 10.1016/S0165-6147(97)90668-4. [DOI] [PubMed] [Google Scholar]
- 30.Liu LW, Huizinga JD. Electrical coupling of circular muscle to longitudinal muscle and interstitial cells of Cajal in canine colon. J Physiol. 1993;470:445–461. doi: 10.1113/jphysiol.1993.sp019868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pluja L, Alberti E, Fernández E, Mikkelsen HB, Thuneberg L, Jimenez M. Evidence supporting presence of two pacemakers in rat colon. Am J Physiol Gastrointest Liver Physiol. 2001;281:G255–G266. doi: 10.1152/ajpgi.2001.281.1.G255. [DOI] [PubMed] [Google Scholar]
- 32.Gil V, Parsons SP, Gallego D, Huizinga J, Jimenez M. Effects of hydrogen sulphide on motility patterns in the rat colon. Br J Pharmacol. 2013;169:34–50. doi: 10.1111/bph.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mañé N, Gil V, Martínez-Cutillas M, Martín MT, Gallego D, Jiménez M. Dynamics of inhibitory co-transmission, membrane potential and pacemaker activity determine neuromyogenic function in the rat colon. Pflugers Arch. 2014;446:2305–2321. doi: 10.1007/s00424-014-1500-8. [DOI] [PubMed] [Google Scholar]
- 34.Pawelka AJ, Huizinga JD. Induction of rhythmic transient depolarizations associated with waxing and waning of slow wave activity in intestinal smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2015;308:G427–G433. doi: 10.1152/ajpgi.00409.2014. [DOI] [PubMed] [Google Scholar]
- 35.Huizinga JD, Chen JH, Zhu YF, et al. The origin of segmentation motor activity in the intestine. Nat Commun. 2014;5:3326. doi: 10.1038/ncomms4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoneda S, Takano H, Takaki M, Suzuki H. Properties of spontaneously active cells distributed in the submucosal layer of mouse proximal colon. J Physiol. 2002;542(Pt 3):887–897. doi: 10.1113/jphysiol.2002.018705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirst GD, Dickens EJ, Edwards FR. Pacemaker shift in the gastric antrum of guinea-pigs produced by excitatory vagal stimulation involves intramuscular interstitial cells. J Physiol. 2002;541(Pt 3):917–928. doi: 10.1113/jphysiol.2002.018614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wright GW, Parsons SP, Loera-Valencia R, Wang XY, Barajas-López C, Huizinga JD. Cholinergic signalling-regulated K7.5 currents are expressed in colonic ICC-IM but not ICC-MP. Pflugers Arch. 2014;466:1805–1818. doi: 10.1007/s00424-013-1425-7. [DOI] [PubMed] [Google Scholar]
- 39.Wang XY, Paterson C, Huizinga JD. Cholinergic and nitrergic innervation of ICC-DMP and ICC-IM in the human small intestine. Neurogastroenterol Motil. 2003;15:531–543. doi: 10.1046/j.1365-2982.2003.00429.x. [DOI] [PubMed] [Google Scholar]
- 40.Ward SM, Beckett EA, Wang X, Baker F, Khoyi M, Sanders KM. Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. J Neurosci. 2000;20:1393–1403. doi: 10.1523/JNEUROSCI.20-04-01393.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siegle ML, Ehrlein HJ. Neurotensin changes the motor pattern in canine ileum from propulsive to segmenting. Dig Dis Sci. 1989;34:1521–1527. doi: 10.1007/BF01537104. [DOI] [PubMed] [Google Scholar]
- 42.Cannon WB. The Movements of the Intestines studied by Means of the Rontgen Rays. J Med Res. 1902;7:72–75. [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu YF, Wang XY, Parsons SP, Huizinga JD. Stimulus-induced pacemaker activity in interstitial cells of Cajal associated with the deep muscular plexus of the small intestine (ICC-DMP) Neurogastroenterol Motil. doi: 10.1111/nmo.12808. Published Online First: 10 Mar 2016. [DOI] [PubMed] [Google Scholar]
- 44.Wang XY, Vannucchi MG, Nieuwmeyer F, Ye J, Faussone-Pellegrini MS, Huizinga JD. Changes in interstitial cells of Cajal at the deep muscular plexus are associated with loss of distention-induced burst-type muscle activity in mice infected by Trichinella spiralis. Am J Pathol. 2005;167:437–453. doi: 10.1016/S0002-9440(10)62988-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huizinga JD, Parsons SP, Chen JH, et al. Motor patterns of the small intestine explained by phase-amplitude coupling of two pacemaker activities; the critical importance of propagation velocity. Am J Physiol Cell Physiol. 2015;309:C403–C414. doi: 10.1152/ajpcell.00414.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith K. Neurogastroenterology. ICC act as pacemakers to control segmentation motor activity in the gut. Nat Rev Gastroenterol Hepatol. 2014;11:203. doi: 10.1038/nrgastro.2014.33. [DOI] [PubMed] [Google Scholar]
- 47.Hall KE, El-Sharkawy TY, Diamant NE. Vagal control of migrating motor complex in the dog. Am J Physiol. 1982;243:G276–G284. doi: 10.1152/ajpgi.1982.243.4.G276. [DOI] [PubMed] [Google Scholar]
- 48.Smith TK, Park KJ, Hennig GW. Colonic migrating motor complexes, high amplitude propagating contractions, neural reflexes and the importance of neuronal and mucosal serotonin. J Neurogastroenterol Motil. 2014;20:423–446. doi: 10.5056/jnm14092. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.