Abstract
Summer-active (continental) and summer-dormant (Mediterranean) tall fescue morphotypes are each adapted to different environmental conditions. Endophyte presence provides plant parasitic nematode resistance, but not with all endophyte strains and cultivar combinations. This study sought to compare effects of four nematode genera on continental and Mediterranean cultivars infected with common toxic or novel endophyte strains. A 6-mon greenhouse study was conducted with continental cultivars, Kentucky 31 (common toxic) and Texoma MaxQ II (novel endophyte) and the Mediterranean cultivar Flecha MaxQ (novel endophyte). Endophyte-free plants of each cultivar were controls. Each cultivar × endophyte combination was randomly assigned to a control, low or high inoculation rate of a mixed nematode culture containing stunt nematodes (Tylenchorhynchus spp.), ring nematodes (Criconemella spp.), spiral nematodes (Helicotylenchus spp.), and lesion nematodes (Pratylenchus spp.). Endophyte infection had no effect on nematode population densities. The cultivar × endophyte interaction was significant. Population densities of stunt nematode, spiral nematode, and ring nematodes were higher for Flecha MaxQ than other cultivar × endophyte combinations. Novel endophyte infection enhances suitability of Flecha MaxQ as a nematode host.
Keywords: endophyte, host-parasite relationship, lesion nematode (Pratylenchus spp.), ring nematode (Criconemella spp.), spiral nematode (Helicotylenchus spp.), stunt nematode (Tylenchorhynchus spp.), summer active, summer dormant, tall fescue
Tall fescue [Schedonorus arundinaceus (Schreb.) Dumont. = Lolium arundinaceum (Schreb.) Darbysh., formerly Festuca arundinacea Schreb.] is the dominant cool-season grass in the United States covering over 13 million ha from the Mississippi River to the Atlantic coast and from the Canadian border to South Georgia (Sleper and Buckner, 1995). Areas within this region generally receive annual rainfall of 450 mm or more (Burns and Chamblee, 1979). The annual rainfall requirement of tall fescue limits its adaptation and use west of the Mississippi River. Tall fescue digestibility (Bughrara et al., 1991), crude protein, and yield (Matches, 1979) are high. Yet despite high forage quality, weight gain of steers grazing tall fescue has been reported as low (Hoveland et al., 1983; Read and Camp, 1986; Crawford et al., 1989) as are cow (Schmidt et al., 1983; Danielson et al., 1986; Tucker et al., 1989) and equine performance (Smith et al., 2009). The contradictory aspects of tall fescue, adaptability, good nutritive value, and poor animal performance are solely due to the infection of tall fescue by an endophytic fungus, Epichloë coenophiala (Leuchtmann et al., 2014). Epichloë species form symbiotic relationships with many cool-season grasses such as tall fescue, and are capable of producing some or all of the bioactive alkaloids, peramine, lolines, ergot alkaloids, and indole-diterpenes that provide the host plant with insect and grazing animal resistance (Clay and Schardl, 2002). Peramine and lolines provide the plant with protection against insect predation and the ergot alkaloids and the indole-diterpene lolitrem B are best known for causing livestock toxicity, fescue toxicosis, and ryegrass staggers, respectively (Clay and Schardl, 2002).
Consumption of tall fescue containing ergot alkaloids by grazing animals (cattle, sheep, and horses) can create a host of animal disorders collectively referred to as “fescue toxicosis.” Fescue toxicosis causes reduction in serum prolactin levels, reduced animal weight gain and reduction in reproductive efficiency, rough hair coats, and general poor animal performance (Strickland et al., 2012). Early efforts to reduce the detrimental impacts of fescue toxicosis in grazing animals centered on the removal of the endophyte from tall fescue creating endophyte-free varieties, but endophyte-free tall fescue was not as persistent as endophyte-infected tall fescue (Bouton et al., 1993). A later, more successful approach has been the identification of endophytic fungi that do not produce the alkaloids causing livestock toxicity but continue to produce alkaloids for insect predation and improved persistence, which are termed “novel or selected” endophytes (Young et al., 2013; Johnson et al., 2013). These endophytes when placed into tall fescue cultivars result in plants infected with a novel endophyte that is persistent and does not cause tall fescue toxicosis (Bouton et al., 2002; Hopkins et al., 2010). The bulk of these novel endophyte-tall fescue associations are in cultivars that are summer active, originating from temperate climates, and are well adapted to regions in the eastern half of the United States. These temperate varieties are not well adapted to the Southern Great Plains region that is generally hotter and drier, resulting in high tiller mortality (Malinowski and Belesky, 2000). An alternative is tall fescue cultivars selected from Mediterranean climates that possess a summer dormancy trait that allows them to tolerate the adverse Southern Plains summer climate (Malinowski and Belsky, 2000; Malinowski and Pinchak, 2015).
It has been established that mutualistic endophyte infection improves persistence of summer-active, temperate tall fescue cultivars, but the benefits of endophyte infection of summer-dormant tall fescue is less understood but could impact tolerance to biotic stresses (Malinowski et al., 2012). In a tall fescue stockpile study that compared production and nutritive value of summer-dormant and summer-active cultivars, the cultivar Flecha MaxQ, a summer-dormant morphotype with a novel endophyte, was the only surviving entry after three harvest years. However, it had a reduction in stand count from 99.9% at the beginning of the study to 66% by the study’s conclusion (Rogers et al., 2014). In this stockpile study, stand reduction of all cultivars began in the first year. Plant-parasitic nematodes were suspected of having some impact on stand loss and the field was found to have stunt nematodes (Tylenchorhynchus spp.) present, though the population was low and unlikely to have been solely responsible for the stand losses. Several types of nematodes are known to affect tall fescue production, with root pruning, reduced forage yield, and plant losses reported (Timper, 2009). Ergot alkaloid–producing endophytes have been shown to reduce population densities of lesion nematode (Pratylenchus scribneri) (Panaccione et al., 2006; Bacetty et al., 2009), whereas novel endophyte resistance to lesion nematodes in summer-active cultivars was dependent on the endophyte strain and the tall fescue cultivar (Timper et al., 2005). Comparison of a transgenic nonergot alkaloid-producing strain (deletion of the dmaW gene encoding the first step of ergot alkaloid production) to the wild type strain showed endophyte-associated suppression of P. scribneri in perennial ryegrass was still evident suggesting other mechanisms might be responsible for the suppression (Panaccione et al., 2006). Reduction in root-knot nematode (Meloidogyne marylandi) population densities by ergot alkaloid-producing endophyte infected tall fescue plants has also been reported (Elmi et al., 1999). Each of these studies was conducted with summer-active tall fescue cultivars (Timper et al., 2005; Bacetty et al., 2009) or perennial ryegrass (Panaccione et al., 2006) and may not translate to effects seen with summer-dormant varieties. Therefore, our objective was to evaluate nematode population densities in novel endophyte-infected and endophyte-free summer-dormant and summer-active tall fescue compared to the common toxic endophyte-infected (Kentucky 31+) and endophyte-free summer-active tall fescue.
Materials and Methods
Three cultivars of tall fescue were tested in two sequential greenhouse trials in 2011 at the Samuel Roberts Noble Foundation in Ardmore, OK. Cultivars tested were Kentucky 31+ (Ky31+), a summer-active continental type, infected with a ergot alkaloid–producing common toxic endophyte; Texoma MaxQ II (TMQ+), a summer-active continental type infected with the novel endophyte strain AR584 (Hopkins et al., 2011); and Grasslands Flecha MaxQ (FL+) [Gentos SA (Buenos Aires, Argentina) and AgResearch Grasslands (Palmerston North, New Zealand)] summer-dormant, Mediterranean. Endophyte-free plants of each variety (Ky31−, TMQ−, and FL−) served as controls.
Subsequent to these trials, the FL+ seed stock that was used for this study and a number of field trials was shown to contain a mixture of two different endophytes, the expected AR542 strain and at lower levels AR502 (Young, unpubl. data). When this contamination issue was discovered in the field studies, the present greenhouse study had been terminated. Confirmation of a novel endophyte, lacking the dmaW gene that encodes the first step in ergot alkaloid production had been confirmed in FL+ prior to the start of the study and before assigning the plants to a specific replication. With the availability of new markers that can distinguish AR542 and AR502, we were able to go back to the DNA stocks from the original seedling samples and determined that AR502 represented 17% of the endophyte-infected FL+ plants. Unfortunately, no samples were available to test the plants at the end of the trials. AR542 and AR502 represent different endophyte species, E. coenophiala and Epichloë sp. FaTG-3, respectively, and have slightly different alkaloid profiles. Both endophytes are able to produce peramine and lolines and unable to produce ergot alkaloids, but only AR542 can produce indole-diterpenes such as terpendole C (Takach and Young, 2014). For the purpose of this study, FL+ will be considered endophyte-infected. Whether these two endophytes have different responses to nematodes will need to be further tested.
In February 2011, tall fescue plants were established from seed by placing one seed per cell in a 36-cell tray containing Metro-mix 830 potting soil (Sun Gro Horticulture, Vancouver, British Columbia, Canada). Seventy-two cells, each were established for Ky31+, Ky31−, TMQ+, and TMQ−. One hundred and eight cells, each were established for FL+ and FL− due to lower seed germination. Seeds were germinated in a starter room in the greenhouse facility at 23°C constant temperature and 60% relative humidity. Trays received moisture through a timed misting apparatus that provided uniform water distribution across the trays.
At 7 wk after emergence, a tiller was collected from each plant and tested for endophyte infection using PCR with primers designed to the endophyte genes tefA (primers tef1-exon1d-1 and tef1-exon5-1; Moon et al., 2002) and dmaW (primers dmaW-F4 and dmaW-6R; Takach et al., 2012). The PCR-based screen verified the presence or absence of the endophyte (tefA gene marker) and distinguished the novel endophyte from the common toxic endophyte based on the presence or absence of the dmaW gene marker (dmaW encodes the first step in ergot alkaloid biosynthesis) and thereby verified the endophyte–plant associations (Takach and Young, 2014; Young et al., 2014).
Following endophyte verification in late April, plants were transferred to 19 × 18 cm, 3.7-liter pots containing a 50:50 by volume mixture of a Renfrow silt loam (Fine, mixed, thermic Udertic Paleustolls) field soil and a Illite calcined clay (Profile Products, Buffalo Creek, IL). Prior to mixing with the clay, the field soil was steam autoclaved at 15 psi and 121°C for 40 min. A liner was placed in the bottom of each pot to prevent the sifting of soil from the pot, the pots were filled with the soil and clay mixture to within 2.5 cm of the top of each pot and then one plant representing one cultivar × endophyte combination was established per pot.
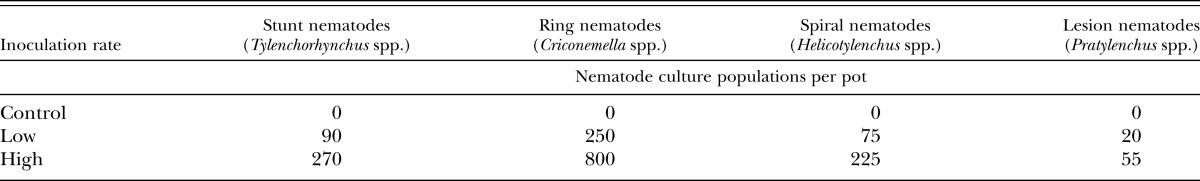
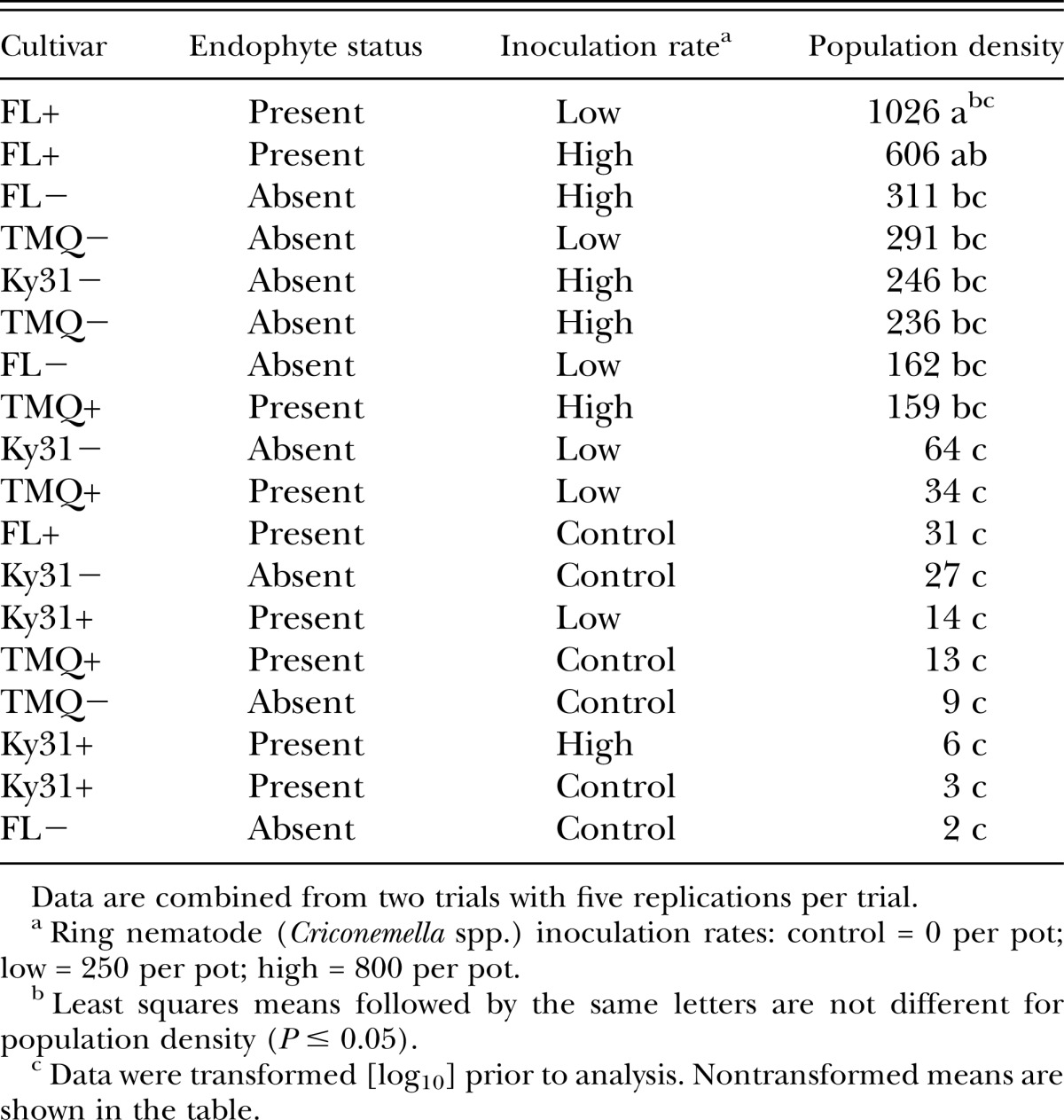
Each trial was a randomized complete block arranged in a factorial design with five replications and a total of 90 individual plants. Within a trial, a pot would contain one plant from one of six tall fescue cultivar × endophyte combinations (Ky31+, Ky31−, FL+, FL−, TMQ+, and TMQ−). On June 3, the cultivar × endophyte combinations were randomly assigned to one of three inoculation rates (control, low, or high) per pot of a mixed nematode culture to determine nematode population effect on carrying capacity of tall fescue cultivar. Nematode inoculum was obtained by collecting soil cores from an established creeping bentgrass (Agrostis stolonifera) putting green in Stillwater, OK, known to have high populations of several plant-parasitic nematode species. The soil cores were pooled and the nematodes were extracted using sieving and centrifugal floatation (Ayoub, 1980). Plant-parasitic nematodes were identified to genus and total populations were determined using a compound light microscope. The nematode inoculum contained the migratory ectoparasitic nematodes: stunt (Tylenchorhynchus spp.), ring (Criconemella spp.), spiral (Helicotylenchus spp.), and the migratory endoparasitic lesion nematode (Pratylenchus spp.). Nematode inoculation rates are listed in Table 1.
Table 1.
Nematode culture inoculation rates per pot that were applied to soil containing summer-dormant or summer-active tall fescue cultivars either infected or noninfected with an endophytic fungus.
Nematodes were inoculated by pipetting the treatment rate into three holes placed into the soil in the top of each pot and then covering the holes with soil following inoculation. The trials ran for a 6-mon period. Individual pots were watered twice per week and fertilized once per week with 20-10-20 at 100 ppm available N fertilizer solution (Jacks Pro Peat Lite; JR peters, Inc., Allentown, PA). Plants were routinely clipped to maintain a 10 cm height to prevent seed formation.
At the termination of the trials, plants were removed from the pots, soil shaken from the roots and bagged, then sent for nematode analysis. Plant roots and shoots were separated and placed into a freezer for future analysis. Roots and shoots were later dried and dry weights recorded. For nematode assays, a 100 cm3 soil subsample was taken from each pot and nematodes were recovered by wet sieving/sugar flotation and identified to genus using a compound microscope.
Nematode population data were analyzed for normality using the PROC UNIVARIATE procedure of SAS, Version 9.3 (SAS Institute, Cary, NC) and the Shapiro–Wilk test for normality. Nematode population data were found to not be from a normally distributed population (P < 0.01). Nematode population data were then transformed to log10 values and analyzed using the PROC analysis of variance procedure to test the interaction effects of trial × cultivar, trial × inoculation rate, and trial × endophyte. All interactions were nonsignificant (P > 0.05) and data from both trials were combined for further analysis.
Combined, transformed nematode data were then analyzed using the GLIMMIX general linear model procedure of SAS (SAS Institute). Trial and replication were considered to be random effects and cultivar, endophyte, inoculation rate, and their interactions fixed effects. The LSMEANS procedure was used with means adjusted by the Tukey–Kramer method to detect differences in fixed effects and interactions. Differences of LSMEANS were declared significant at P ≤ 0.05. Nontransformed means are reported in tables and discussion. Data for plant root and shoot mass were analyzed using the same procedures as was used for the nematode data.
Results
Lesion nematode population density:
Lesion nematode population numbers were not affected (P > 0.05) by cultivar, nematode treatment level, endophyte infection, or their interactions (Table 2). Lesion nematode results are difficult to interpret as they are an endoparasitic nematode and move from the soil into the plant roots where they cause root lesions, root stunting, and dieback, which results in a reduction in plant growth. Some visible root lesions on plant roots were observed but no extraction of lesion nematodes from the roots was done. A repeat of this study should include root extraction for lesion nematodes.
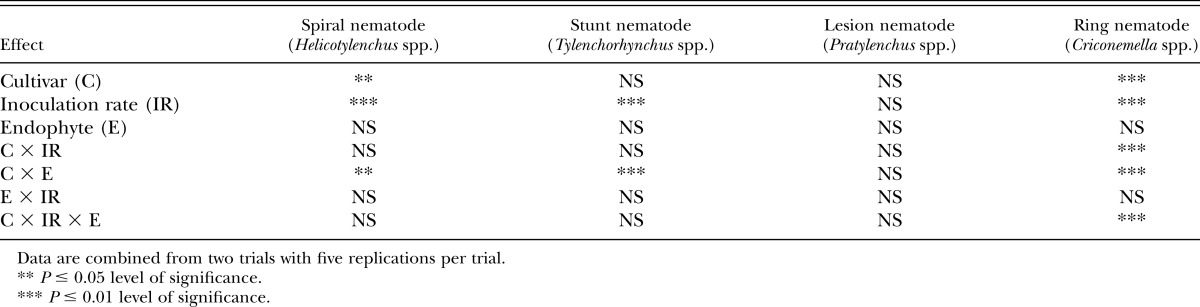
Table 2.
Analysis of variance (Type III test for fixed effects) for log10 transformed nematode population density counts from soil of pots containing tall fescue cultivars either infected or noninfected with an endophytic fungus and one of three nematode inoculation rates.
Spiral and stunt nematode population densities:
Nematode inoculation rate was significant (P < 0.05) for spiral nematode and stunt nematode (Table 2). The spiral nematode differences between the high inoculation rate (243/100 cm3 soil) and low inoculation rate (160/100 cm3 soil) were not significant (P > 0.05) but both inoculation rates were greater (P < 0.05) than the control (34/100 cm3 soil). The response for stunt nematodes was similar, the high inoculation rate (68/100 cm3 soil) and low inoculation rate (54/100 cm3 soil) were not significantly different (P > 0.05) but both were greater (P < 0.05) than the control (7/100 cm3 soil). The cultivar × inoculation rate interaction was significant (P < 0.05) only for ring nematode (Table 2).
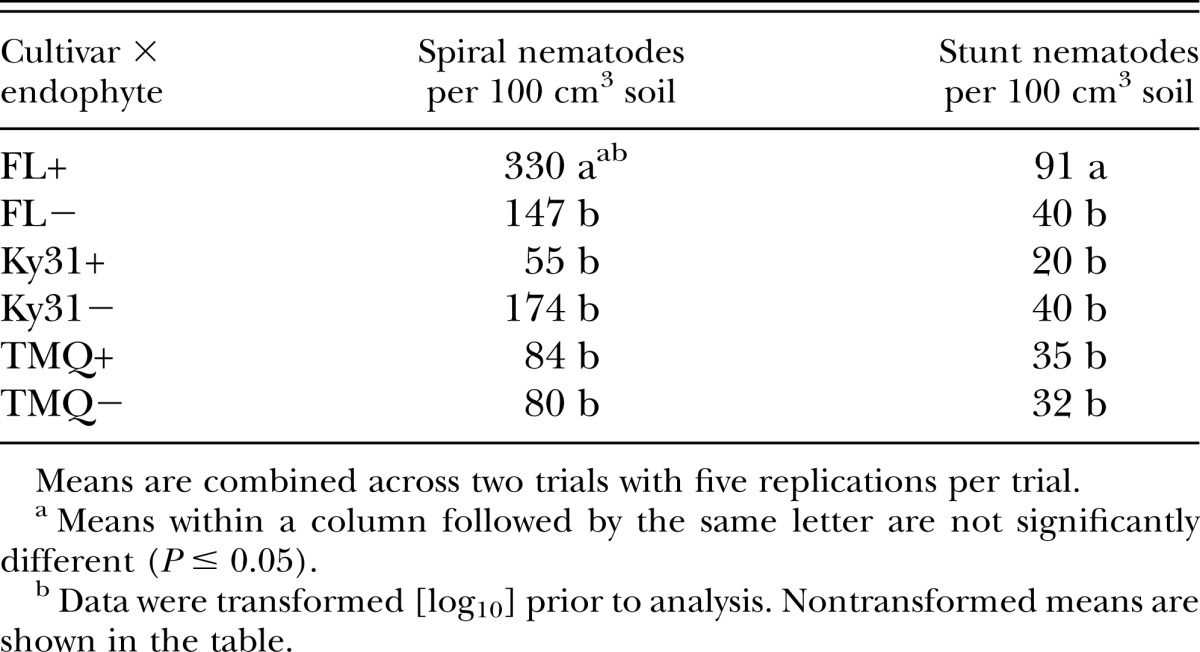
As a main effect, endophyte presence or absence had no effect on spiral and stunt nematode population densities. However, the cultivar × endophyte interaction was significant (P < 0.05) for both spiral and stunt nematodes (Table 2). FL+ had higher spiral and stunt nematode population densities than the other cultivar × endophyte combinations (Table 3). The remaining cultivar × endophyte combinations (FL−, Ky31+, Ky31−, TMQ+, TMQ−) were similar in populations densities of spiral and stunt nematodes.
Table 3.
Effect of tall fescue cultivar and endophyte infection status on mean spiral nematode (Helicotylenchus spp.) and stunt nematode (Tylenchorhynchus spp.) population counts per 100 cm3 soil volume.
Ring nematode population density:
Endophyte presence or absence had no effect (P < 0.05) on population density response of ring nematode as was also seen for spiral, stunt, and lesion nematodes (Table 2). Ring nematode population density was significantly affected (P < 0.05) by the three-way interaction of cultivar × inoculation rate × endophyte infection (Table 2). FL+ at low (250/100 cm3 soil) and high (800/100 cm3 soil) nematode inoculation rates were similar (P > 0.05) in ring nematode population density (Table 4). Ring nematode population density for FL+ at the low (250/100 cm3 soil) inoculation rate was higher (P < 0.05) than TMQ−, TMQ+, Ky31−, Ky31+, regardless of their inoculation rate (Table 4). No differences are noted in ring nematode population density numbers between TMQ+ and TMQ− implying that the novel endophyte strain AR584 had no effect on ring nematode population density of TMQ regardless of inoculation rate. No differences (P > 0.05) were noted across control inoculation rates (Table 4).
Table 4.
The effect of tall fescue cultivar, endophytic infection, and ring nematode inoculation rate on ring nematode (Criconemella spp.) population density means per 100 cm3 soil.
Reproductive factor:
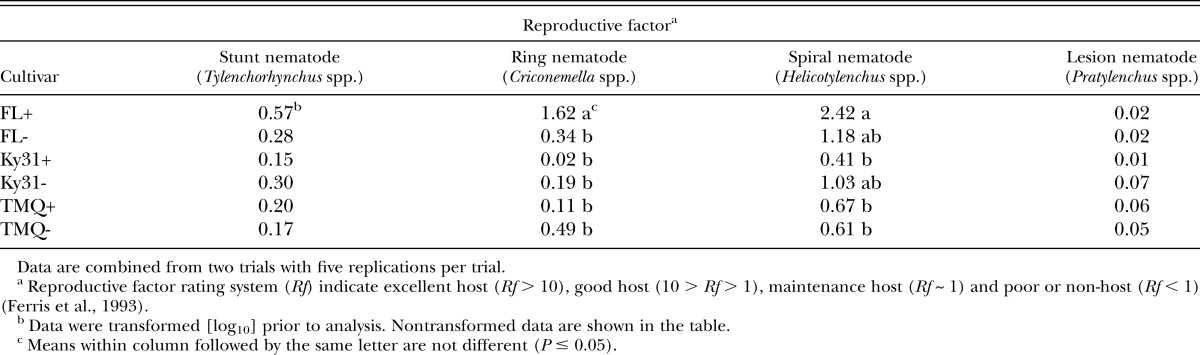
A reproductive factor (Rf) for each nematode species within each cultivar × endophyte combination in the study was calculated by taking the final population of each species and dividing by the initial nematode species population. Rf was then used to categorize the suitability of the tall fescue cultivars as a nematode host by a rating system (Ferris et al., 1993) of excellent host (Rf > 10), good host (10 > Rf > 1), maintenance host (Rf ∼ 1) and poor or nonhost (Rf < 1).
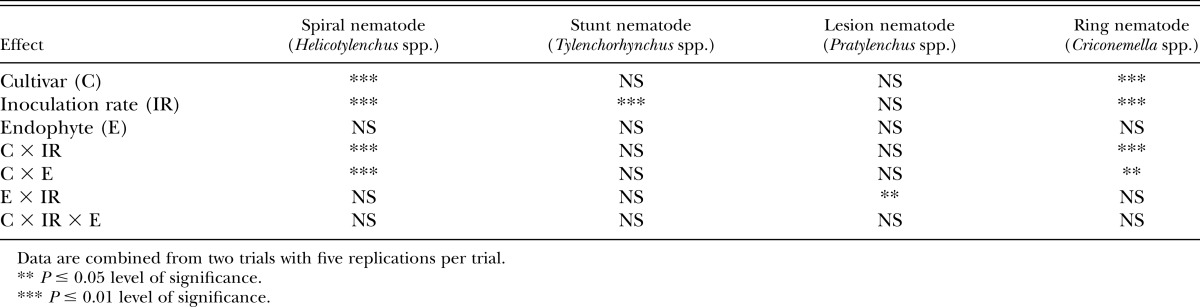
Nematode inoculation rate was significant (P < 0.05) for spiral nematode, stunt nematode, and ring nematode (Table 5). Rf factor was less than 1 for the stunt control, low, and high inoculation rates. A cultivar × inoculation rate was significant (P < 0.05) for spiral nematode and ring nematode (Table 5). FL low inoculation rate had an Rf of 3.8, significantly greater (P < 0.05) than other cultivar × inoculation rate combinations. This same effect was true for ring nematode. FL at low inoculation rate had an Rf of 2.38 for ring nematode compared to an Rf < 1 for all other cultivar × inoculation rate combinations.
Table 5.
Analysis of variance (Type III test for fixed effects) for log10 transformed nematode reproductive factor (final population density/initial population density) from tall fescue cultivars either infected or noninfected with an endophytic fungi and one of three nematode inoculation rates.
A cultivar × endophyte interaction had a significant (P < 0.05) effect on Rf for spiral nematode and ring nematode. The cultivar FL+ had a 1.62 Rf rating and would be characterized as a good host for ring nematodes compared to other cultivar × endophyte combinations (Table 6). FL+ (Rf 2.42) is a good spiral nematode host (P < 0.05) compared to Ky31+, TMQ+, and TMQ− all of which are rated as poor hosts for spiral nematodes (Table 6). FL+, FL−, and Ky31− have a similar (P > 0.05) Rf and would be characterized a poor host for spiral nematode.
Table 6.
Tall fescue endophyte by cultivar effect on the reproductive factor (population final/population initial) means of four nematode species per 100 cm3 soil.
Root and vegetative mass:
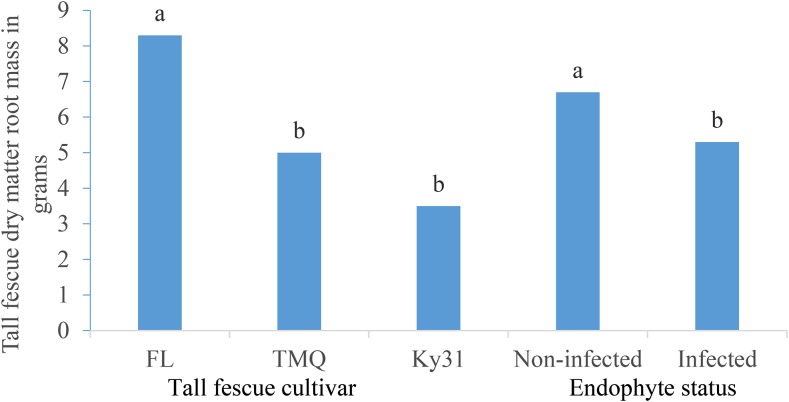
Cultivar and endophyte presence or absence had a significant effect (P < 0.05) on tall fescue root mass (Table 7). Other main effects and their interactions were not significant. The cultivar FL had greater mean root mass than TMQ or Ky31 (Fig. 1). Endophyte absence significantly increased (P < 0.05) root mass (Fig. 1).
Table 7.
Analysis of variance (Type II test for fixed effects) for vegetative and root mass from tall fescue cultivars either infected or noninfected with an endophytic fungi and three nematode inoculation rates.
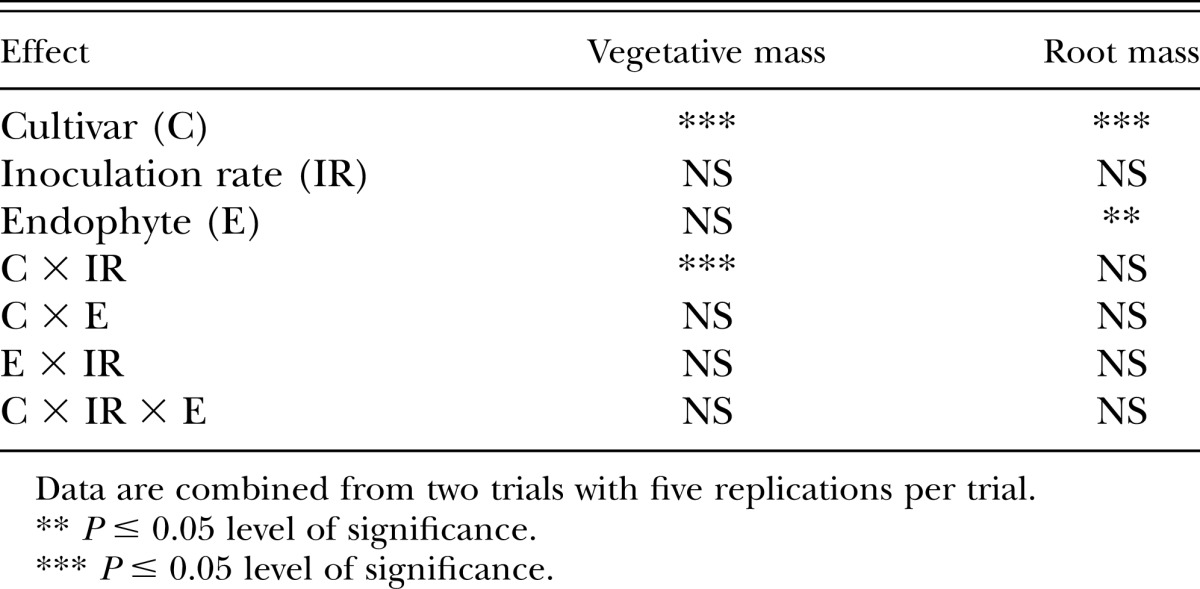
Fig. 1.
The main effects of tall fescue cultivar and endophyte (noninfected or infected) on tall fescue root mass (grams) means across two trials with five replications per trial. Means within tall fescue cultivar or endophyte status with the same letter are not significantly different (P ≤ 0.05).
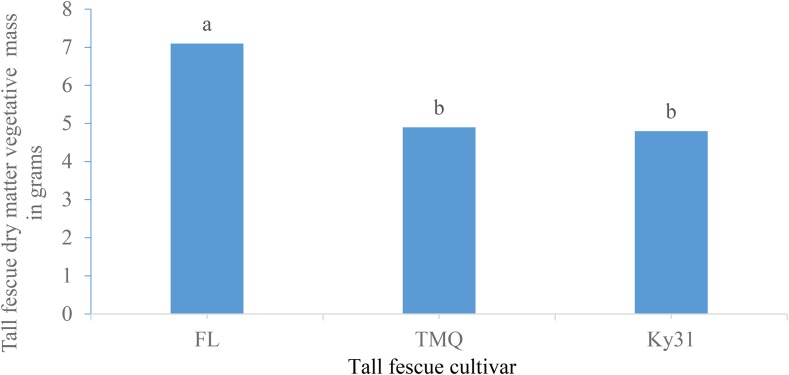
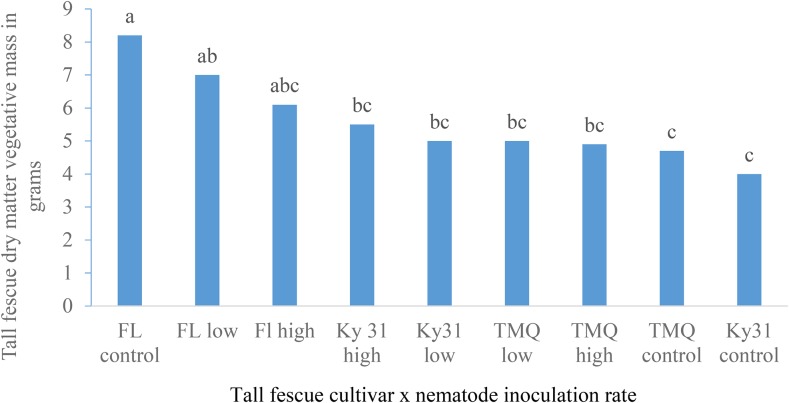
Results of vegetative mass production were similar to root mass as FL produced more vegetative mass than TMQ or Ky31 (Fig. 2). In a field study, FL produced similar forage yields as TMQ and Ky31 (Rogers et al., 2014), which supported these greenhouse vegetative mass results. A tall fescue cultivar × nematode inoculation rate interaction was significant for vegetative mass (Table 7). Within cultivars, vegetative mass was not effected by nematode inoculation rate (Fig. 3) though difference between cultivars and inoculation rates are noted.
Fig. 2.
The main effect of tall fescue cultivar on tall fescue vegetative mass means (grams) across two trials with five replications per trial. Tall fescue cultivar column means with the same letter are not significantly different (P ≤ 0.05).
Fig. 3.
The tall fescue cultivar × nematode inoculation rate on tall fescue vegetative mass means (grams) across two trials with five replications per trial. Column means with the same letter are not significantly different (P ≤ 0.05).
Discussion
The presence or absence of the tall fescue endophytic fungi E. coenophiala had no effect on nematode population densities in this greenhouse study. In a tall fescue study that included the MaxQ (AR524) novel endophyte, no endophyte status effect was shown for ring nematode reproduction when pots were inoculated with 1,000 adults and juvenile ring nematodes (Nyczepir, 2011). In addition, there was no tall fescue cultivar effect on ring nematode reproduction (Nyczepir, 2011). Our results found a cultivar by endophyte interaction influenced both spiral nematode and stunt nematode population densities. The novel endophyte-infected cultivar FL+ had significantly higher spiral nematode and stunt nematode population densities than FL−, Ky31+, Ky31−, TMQ+, and TMQ−, which contained similar nematode population densities. Cultivar × endophyte × nematode inoculation rate significantly affected ring nematode population densities. At the low inoculation rate, FL+ ring nematode was similar to FL+ at the high inoculation rate. However, FL+ ring nematode was greater than the remaining cultivar × endophyte × inoculation rates. FL+ had a higher Rf rating for ring nematode than other cultivar by endophyte combinations. FL+, FL−, and Ky31− had a similar Rf rating for spiral nematode, but FL+ Rf was higher than Ky31+, TMQ+, and TMQ−. From these data, we would then conclude that FL+ novel endophyte-infected cultivar is a more suitable host for ring nematode, spiral nematode, and stunt nematode. In earlier work with lesion nematode, the novel endophyte strain AR542 infecting the tall fescue cultivar Jesup did not confer lesion nematode resistance (Timper et al., 2005).
Within the continental tall fescue types (Ky31+, Ky31−, TMQ+, TMQ−), there were no differences in lesion, stunt, or ring nematode population densities regardless of endophyte infection. Rf data indicate Ky31+, TMQ+, and TMQ− are a poor host for stunt, ring, and spiral nematodes. Ky31− is a poor host for stunt and ring nematodes, but a good host for spiral nematode. In previous work, the wild type endophyte has been shown to suppress lesion nematode populations, (Timper et al., 2005; Panaccione et al., 2006; Bacetty et al., 2009; Timper and Bouton 2012), whereas Kimmons et al. (1990) found no difference in spiral nematode numbers in pots containing either endophyte-free or endophyte-infected (toxic) tall fescue. Results from this study support this previous study, but we also show no difference between TMQ+ (AR584 novel endophyte) and TMQ−.
An explanation to differences in root weights could be attributed to the origins of the cultivars. FL originated for the Mediterranean basin region and is summer dormant while the other cultivars are of European continental origin and are summer active or summer semidormant. The summer dormancy trait may impart emphasis on root growth and development as part of its survival mechanism to avoid and persist through periods of high temperature and drought.
Based on these data, the novel endophyte AR584 is conveying to the tall fescue cultivar TMQ similar nematode suppression of spiral, stunt, and ring nematodes as Ky31+ infected with a common toxic endophyte. In addition, the nematode host suitability appears to be enhanced for endophyte-infected FL+. This effect may be due to the plant genotype effect of suppressing endophyte growth or the endophyte may suppress the plant’s natural ability to suppress nematode populations. Meyer et al. (2013) reported that endophyte status of Jesup MaxQ did not appear to be the primary factor in root-knot nematode (Meloidogyne incognita) suppression, but rather root and shoot compounds produced by the cultivar are nemotostatic or nematotoxic to root-knot second stage juveniles. This could further help explain the cultivar by endophyte status effect seen for FL. Spiral, stunt, and ring nematode population densities were lower for FL− compared to FL+. FL− could be producing similar root and shoot exudates that are causing nematode suppression compared to FL+, which will need further investigation.
The role of E. coenophiala in its association with continental type tall fescue is understood and well documented whereas the role of endophyte associations in Mediterranean tall fescue is not (Malinowski et al., 2012). The main advantage of endophyte infection conferred to continental tall fescue is improved plant persistence during hot, dry summers. Since summer-dormant tall fescue types simply avoid these periods by going dormant, endophyte infection may not provide any additional drought tolerance and could possibly interfere with the plants ability to produce nematode suppressive exudates. Results of this study would indicate that interactions of tall fescue plant genotype × novel endophyte effects need additional study as to the suppression affect they may confer to plant parasitic nematodes and the impact on plant persistence and biomass production.
Literature Cited
- Ayoub SM. 1980. Plant nematology: An agricultural training aid. Sacramento, CA: NemaAid Publications. [Google Scholar]
- Bacetty AA, Snoopk ME, Glenn AE, Noe JP, Hill N, Culbreath A, Timper P, Nagabhyru P, Bacon CW. Toxicity of endophyte-infected tall fescue alkaloids and grass metabolites on Pratylenchus scribneri. Phytopathology. 2009;99:1336–1345. doi: 10.1094/PHYTO-99-12-1336. [DOI] [PubMed] [Google Scholar]
- Bouton JH, Gates RN, Belesky DP, Owsley M. Yield and persistence of tall fescue in the Southeastern Coastal Plain after removal of its endophyte. Agronomy Journal. 1993;85:52–55. [Google Scholar]
- Bouton JH, Latch GCM, Hill NS, Hoveland CS, McCann MA, Watson RH, Parish JA, Hawkins LL, Thompson FN. Reinfection of tall fescue cultivars with non-ergot alkaloid producing endophytes. Agronomy Journal. 2002;94:567–574. [Google Scholar]
- Bughrara SS, Sleper DA, Krause GF. Genetic variation in tall fescue digestibility estimated using a prepared cellulose solution. Crop Science. 1991;31:883–889. [Google Scholar]
- Burns JC, Chamblee DS. 1979. Adaptation. Pp. 9–30 in R. C. Buckner and L. P. Bush, eds. Tall fescue: American Society of Agronomy Monograph 20. Madison, WI: American Society of Agronomy, Crop Science Society of America, Soil Science Society of America.
- Clay K, Schardl C. Evolutionary origins and ecological consequences of endophyte symbiosis with grasses. The American Naturalist. 2002;160:S99–S127. doi: 10.1086/342161. [DOI] [PubMed] [Google Scholar]
- Crawford RJ, Forwood JR, Belyea RL, Garner GB. Relationship between level of endophyte infection and cattle gains on tall fescue. Journal of Production Agriculture. 1989;2:147–151. [Google Scholar]
- Danielson DA, Schmidt SP, King CC, Smith LA, Webster WB. Fescue toxicity and reproduction in beef heifers. Journal of Animal Science. 1986;63:296. (Abstr.). [Google Scholar]
- Elmi AA, West CP, Robbins RT, Kirkpatrick TL. Endophyte effects on reproduction of a root-knot nematode (Meloidogyne marylandi) and osmotic adjustment in tall fescue. Grass and Forage Science. 1999;55:166–172. [Google Scholar]
- Ferris H, Carlson HL, Viglierchio DR, Westerdahl BB, Wu FW, Anderson CE, Juurma A, Kirby DW. Host status of selected crops to Meloidogyne chitwoodi. Supplement to Journal of Nematology. 1993;25:849–857. [PMC free article] [PubMed] [Google Scholar]
- Hopkins AA, Young CA, Butler TJ, Bouton JH. Registration of ‘Texoma’ MaxQ II Tall Fescue. Journal of Plant Registrations. 2011;5:14–18. [Google Scholar]
- Hopkins AA, Young CA, Simpson WR, Panaccione DG, Mittal S, Bouton JH. Agronomic performance and lamb safety of tall fescue novel endophyte combinations in the south central USA. Crop Science. 2010;50:1552–1561. [Google Scholar]
- Hoveland CS, Schmidt SP, King CC, Odem JW, Clark EM, McGuire JA, Smith LA, Grimes HW, Hollman JL. Steer performance and association of Acremonium coenophialum fungal endophyte on tall fescue pasture. Agronomy Journal. 1983;75:821. [Google Scholar]
- Johnson LJ, de Bonth AC, Briggs LR, Caradus JR, Finch SC, Fleetwood DJ, Fletcher LR, Hume DE, Johnson RD, Popay AJ, Tapper BA, Simpson WR, Voisey CR, Card SD. The exploitation of Epichloeae endophytes for agricultural benefit. Fungal Diversity. 2013;60(1):171–188. [Google Scholar]
- Kimmons KA, Gwinn KD, Bernard EC. Nematode reproduction on endophyte-infected and endophyte-free tall fescue. Plant Disease. 1990;74:757–761. [Google Scholar]
- Leuchtmann A, Bacon CW, Schardl CL, White JF, Tadych M. Nomenclatural realignment of Neotyphodium species with genus Epichloë. Mycologia. 2014;106:202–215. doi: 10.3852/13-251. [DOI] [PubMed] [Google Scholar]
- Malinowski DP, Belesky DP. Adaptations of endophyte infected cool-season grasses to environmental stresses: Mechanisms of drought and mineral stress tolerance. Crop Science. 2000;40:923–940. [Google Scholar]
- Malinowski DP, Pinchak WE. Summer dormancy trait as a strategy to provide perennial cool-season grass forage alternatives in southern latitude environments affected by climate change. Agronomy Journal. 2015;107:1227–1234. [Google Scholar]
- Malinowski DP, West CP, Belesky DP. 2012. The role of endophytes in summer-dormant tall fescue. Pp. 104–106 in C. A. Young, G. E. Aiken, R. L. McCulley, J. R. Strickland, and C. L. Schardl, eds. Epichloae, endophytes of cool season grasses: Implications, utilization and biology. 1st ed. Ardmore, OK: The Samuel Roberts Noble Foundation.
- Matches AG. 1979. Management. Pp 171–99 in R. C. Buckner and L. P. Bush, eds. Tall fescue: American Society of Agronomy Monograph 20. Madison, WI: American Society of Agronomy, Crop Science Society of America, Soil Science Society of America.
- Meyer SLF, Nyczepir AP, Rupprecht SM, Mitchell AD, Martin PAW, Bush CW, Chitwood DJ, Vinyard BT. Tall fescue ‘Jesup (Max-Q)’: Meloidogyne incognita development in roots and nematotoxicity. Agonomy Journal. 2013;105:755–763. [Google Scholar]
- Moon CD, Miles CO, Jarlfors U, Schardl CL. The evolutionary origins of three new Neotyphodium endophyte species from grasses indigenous to the southern hemisphere. Mycologia. 2002;94:694–711. doi: 10.1080/15572536.2003.11833197. [DOI] [PubMed] [Google Scholar]
- Nyczepir AP. Host suitability of an endophyte-friendly tall fescue grass to Mesocriconema xenoplax and Pratylenchus vulnus. Nematropica. 2011;41:45–51. [Google Scholar]
- Panaccione DG, Kotcon JB, Schardl CL, Johnson RD, Morton JB. Ergot alkaloids are not essential for endophytic fungus-associated population suppression of the lesion nematode, Pratylenchus scribneri, on perennial ryegrass. Nematology. 2006;8:583–590. [Google Scholar]
- Read JC, Camp BJ. The effects of the fungal endophyte Acremonium coenophialum in tall fescue and animal performance, toxicity and stand maintenance. Agronomy Journal. 1986;78:848. [Google Scholar]
- Rogers JK, Young CA, Mosali J, Norton SL, Hopkins AA. 2014. Stockpiled forage yield and nutritive value of summer-dormant and summer-active tall fescue in a marginal environment. Forage and Grazinglands doi:10.2134/FG-2014-0065-RS.
- Schmidt SP, King CC, Jr, Hoveland CS, Clark EM, Smith LA, Grimes HW, Holliman JL. Cow-calf performance as affected by fungus infestation of Kentucky-31 tall fescue pastures. Journal of Animal Science. 1983;57(Suppl 1):295. (Abstr.). [Google Scholar]
- Sleper DA, Buckner RC. 1995. The fescues. Pp. 345–356. in R. F. Barnes, D. A. Miller, and C. J. Nelson, eds. Forages, 5th ed., Vol. I. Ames, IA: Iowa State University Press.
- Smith SR, Schwer L, Keene TC. 2009. Tall fescue toxicity for horses: Literature review and Kentucky’s successful pasture evaluation program. Forage and Grazinglands doi:10.1094/FG-2009-1102-02-RW.
- Strickland JR, Brown KR, Aiken GE, Klotz JL, Flythe MD. 2012. Ergot alkaloids: Toxicokinetics and vascular effects. Pp. 14–19 in C. A. Young, G. E. Aiken, R. L. McCulley, J. R. Strickland, and C. L. Schardl, eds. Epichloae, endophytes of cool season grasses: Implications, utilization and biology. 1st ed. Ardmore, OK: The Samuel Roberts Noble Foundation.
- Takach JE, Mittal S, Swoboda GA, Bright SK, Trammell MA, Hopkins AA, Young CA. Genotypic and chemotypic diversity of Neotyphodium endophytes in tall fescue from Greece. Applied and Environmental Microbiology. 2012;78:5501–5510. doi: 10.1128/AEM.01084-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takach JE, Young CA. Alkaloid genotype diversity of tall fescue endophytes. Crop Science. 2014;54:667–678. [Google Scholar]
- Timper P. 2009. Nematodes. Pp. 151–156 in H. A. Fribourg, D. B. Hannaway, and C. P. West, eds. Tall fescue for the twenty-first century. American Society of Agronomy Monograph 53. Madison, WI: American Society of Agronomy Publisher.
- Timper P, Bouton J. 2012. Variable response of non-ergot-producing strains of Neotyphodium coenophialum in tall fescue lesion nematodes. Pp. 40–43 in C. A. Young, G. E. Aiken, R. L. McCulley, J. R. Strickland, and C. L. Schardl, eds. Epichloae, endophytes of cool season grasses: Implications, utilization and biology. 1st ed. Ardmore, OK: The Samuel Roberts Noble Foundation.
- Timper P, Gates RN, Bouton JH. Response of Pratylenchus spp. in tall fescue infected with different strains of the fungal endophyte Neotyphodium coenophialum. Nematology. 2005;7:105–110. [Google Scholar]
- Tucker CA, Morrow RE, Gerrish JR, Nelson CJ, Garner GB, Jacobs VE, Hires WG, Shinkel JJ, Forwood JR. Forage systems for beef cattle: Effects of winter supplementation and forage system on reproductive performance of cows. Journal of Production Agriculture. 1989;2:217. [Google Scholar]
- Young CA, Charlton ND, Takach JE, Swoboda GA, Trammell MA, Huhman DV, Hopkins AA. Characterization of Epichloë coenophiala within the U.S.: Are all tall fescue endophytes created equal? Frontiers in Chemistry. Chemical Biology. 2014 doi: 10.3389/fchem.2014.00095. doi: 10.3389/fchem.2014.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young CA, Hume DE, McCulley R. Fungal endophytes of tall fescue and perennial ryegrass: Pasture friend or foe? Journal of Animal Science. 2013;91:2379–2394. doi: 10.2527/jas.2012-5951. [DOI] [PubMed] [Google Scholar]