Abstract
The main aims of this manuscript are to: i) determine the effect of commonly used antibiotics to treat osteoarticular infections on osteoblast viability, ii) study the dual release of the growth factor (BMP-7) and antibiotics (vancomycin and cefazolin) from chitosan microparticles iii) demonstrate the bioactivity of the antibiotics released in vitro on Staphylococcus epidermidis. The novelty of this work is dual delivery of growth factor and antibiotic from the chitosan microparticles in a controlled manner without affecting their bioactivity. Cefazolin and vancomycin have different therapeutic concentrations for their action in vivo and therefore, two different concentrations of the drugs were used. Osteoblast cytotoxicity test concluded that cefazolin concentrations of 50 and 100 μg/ml were found to have positive influence on osteoblast proliferation. A significant increase in osteoblast proliferation was observed in the presence of cefazolin and BMP-7 in comparison with BMP-7 alone group; indicating cefazolin might play a role in osteoblast proliferation. On the other hand, vancomycin concentration of 1000 μg/ml was found to significantly reduce (p < 0.01) osteoblast proliferation in comparison with controls. The microbial study indicated that cefazolin at a minimum concentration of 21.5 μg/ml could inhibit ~85% growth of S. epidermidis, whereas vancomycin at a concentration of 30 μg/ml was found to inhibit ~80% bacterial growth.
Keywords: Cefazolin, Vancomycin, Staphylococcus epidermidis, BMP-7, Chitosan microparticles, Osteoblast
1. Introduction
Gram-positive organisms are considered to be a major cause of bone infections, especially Staphylococci [1]. This genus of bacteria is a principal causative agent for mainly two types of bone infections namely, septic arthritis and osteomyelitis. These infections involve the inflammatory destruction of joint and bone [2–4]. Surveillance data from the Health Protection Agency on surgical site infection between 1997 and 2005 found Staphylococcus aureus to be the causative organism in 41.4% of hip prosthesis, 33.5% of knee prosthesis, 53% of open bone reduction of bone fracture and 59.1% of hip hemiarthroplasty infection. Staphylococcus epidermidis is the most common coagulase-negative Staphylococcus species in many types of infection, including osteomyelitis and infection of prosthetic joints [5].
In order to eradicate infection in bone and joints, it is essential to maintain antibiotics at the therapeutic concentration at the implantation site for an extended period of time. Parenteral administration of antibiotics is unsuccessful in the treatment of bone infections because of the insufficient local penetration of systemic administration. Traditionally, osteomyelitis has been treated with parenteral antibiotics for a period of 4–6 weeks after surgery. The high doses of systemic antibiotics above the minimum inhibitory concentration required at fracture site cause systemic toxicity [6,7]. Studies have shown that >80% of vancomycin is excreted unchanged in urine within 24 h after administration and cefazolin’s half-life is found to be approximately 4 h after IV injection [8]. Even after an intra-articular (IA) injection, half-life of the delivered vancomycin was just over 3 h, and the therapeutic level was maintained for 24 h in the joint serum [9]. Therefore, release of local antibiotic administration in a controlled fashion for extended period from a biodegradable scaffold will avoid risk of systemic toxicity and act as a prophylaxis measure against bone infections during the surgery [10].
The activity of bone healing also occurs at the same time and is accompanied by many growth factors, molecular signaling and various cellular activities [11,12]. These processes suggest that it would be beneficial to develop a system which could simultaneously and timely deliver both the growth factor and the drug in a sustained manner to help the above mentioned processes [13,14]. BMP-7 has been shown to possess the ability to differentiate mesenchymal stem cells and pre-osteoblasts into osteoblasts [15]. For drug delivery systems, there is a particular interest in developing microparticle system in which the growth factors and drugs are encapsulated in the microparticles for efficient release over a long period of time [16]. The core-shell structure of the microparticles can overcome the problem of burst release and at the same time protect the growth factor from harsh environmental conditions. Two main challenges in developing this system are to control the release behavior of the drug and growth factor simultaneously and to optimize the dosage of the drug and growth factor in order to observe efficient bone regeneration. Other parameters that need to be considered include the maintenance of effective concentration, prolong their bioactivity and to reduce the effect of high burst doses [17–20].
Chitosan has been demonstrated to possess antibacterial activity against many bacteria, filamentous fungi and yeast [21]. Research suggests that the antibacterial activities of chitosan rely on numerous intrinsic and extrinsic factors like pH, microorganism species, presence and absence of metal cations, pKa, molecular weight and degree of deacetylation [22–25]. Chitosan has a wide spectrum of activity against gram-positive and gram-negative bacteria, but lower toxicity against mammalian cells [26]. All these properties of chitosan such as biocompatibility, biodegradability, wound healing capabilities and antibacterial properties make it is an ideal scaffold materials to be used in bone tissue engineering. In order to enhance effectiveness of antibacterial properties in chitosan microparticles, antibiotics can be incorporated into them for controlled release over time.
Cephalosporins are the commonly used antibiotics to treat osteoarticular infections, given their broad range of activity spectrum [27]. Cefazolin is a semisynthetic cephalosporin with good in vitro activity against resistant strains of staphylococci (MIC90 1 mg/l), and having excellent tolerance and bone diffusion [28,29]. Several authors recommend the use of cefazolin for treating bone and joint infections, particularly those due to S. aureus [30,31]. Serious infections caused by resistant strains are treated with vancomycin which is a glycopeptide antibiotic with high activity against gram-positive bacteria [32].
In this present study, we intend to design dual drug and growth factor loaded scaffold system for bone tissue healing. Cefazolin or vancomycin was chosen to be incorporated into microparticles separately. BMP-7 was also separately encapsulated into different chitosan microparticles. The main objective of this study is to optimize the concentration of antibiotic to be incorporated based on the osteoblast viability and then to incorporate the respective amounts of vancomycin and cefazolin into chitosan-TPP microparticles. We studied controlled release kinetics of vancomycin and cefazolin incorporated microparticles individually and with BMP-7 encapsulated microparticles. We also intend to understand the interaction between the two compounds, antibiotic and growth factor, to ensure the antibacterial activity.
2. Materials and methods
2.1. Materials
Chitosan (85% deacetylated), sodium tripolyphosphate (TPP), acetic acid, tryptic soy agar and phosphate buffered saline (PBS) were purchased from Sigma Chemicals, USA. Bone morphogenetic protein 7 (BMP-7) was purchased from Peprotech and BMP-7 ELISA kit (DY354) was supplied by R & D systems, USA. Live/dead cell cytotoxicity/viability assay and α-MEM medium were purchased from Invitrogen, USA. Vancomycin hydrochloride and cefazolin sodium salt were purchased from MP Biomedicals, USA.
2.2. Effect of the drugs on osteoblast viability
Above certain concentrations, the antibiotics are harmful for the cells in the body. Therefore, in order to determine the optimum concentration of antibiotics for the growth and proliferation of osteoblasts, a live-dead cell assay was performed. For this assay, two different concentrations of each drug were used: 50 and 100 μg/ml of cefazolin; and 500 and 1000 μg/ml of vancomycin. The two drugs require different therapeutic levels for action in vivo and therefore accordingly two different concentrations have been chosen. A combination of growth factor BMP-7 and each drug concentration was also used to determine their effect on osteoblasts. Murine osteoblast cells (OB-6) were seeded at a density of 100,000 cells/ml in 24 well-plates. Cells were incubated at 37 °C with 5% CO2 atmosphere. Osteoblasts cultured in regular α-MEM media were used as a control. A live dead assay was performed on day 1 and 3 by removing all the media in the wells, washing it with PBS solution thoroughly and then adding 300 μl of D-PBS and 300 μl of live-dead cell assay solution containing calcein which stains the live healthy cells green and ethydium homodimer which stains the dead cells red. After 45 min, the plate was observed under fluorescence microscopy. For statistical analysis, each group had triplicates, and for live cell counting, 30 images were taken for each group and analyzed using ImageJ software.
2.3. Fabrication of chitosan microparticles loaded with antibiotics and a growth factor
2.3.1. Fabrication of blank microparticles
Microparticles were prepared by coacervation method using 2% w/v chitosan and 50% v/v tri-polyphosphate (TPP) [33,34]. Chitosan solution was prepared by dissolving chitosan in 2% acetic acid. The chitosan solution was then added drop-wise to the TPP solution which is continuously stirred at 300 rpm and was cross-linked for 5 h before it was air-dried overnight at room temperature.
2.3.2. Fabrication of antibiotic loaded microparticles
Two different amount of each antibiotic were incorporated into the microparticles. Vancomycin, 3 mg (represented by van 8e, 8c) and 5 mg (represented by van 16e, 16c), and cefazolin, 1.5 mg (represented by cef 4e, 4c) and 2.5 mg (represented by cef 8e, 8c) were used to prepare antibiotic encapsulated (represented by e) and coated (by surface adsorption) microparticles (represented by c). These concentrations were determined based on the minimum inhibitory concentration (MIC) of each for Staphylococcus strain [28,32]. To prepare antibiotic encapsulated microparticles, antibiotic was added to the chitosan solution before cross-linking, whereas for antibiotic coated microparticles, the antibiotic drug was added after drying the cross-linked microparticles. The microparticles were otherwise prepared in the same manner as mentioned above. In order to ensure that the same amount of drug was incorporated into the microparticles by the two techniques, we based our calculations of drug added per mg microparticles. Typically, 3 ml of chitosan solution yields 20 mg microparticles. In order to prepare a batch of microparticles using encapsulation technique, we added 3.05 mg of vancomycin (260 μl from 8 mg/ml stock solution) into 3 ml of chitosan solution. Finally from this solution, 20 mg microparticles containing antibiotic were obtained. So theoretically, we have 1.52 mg antibiotics/10 mg microparticles. So, if we are preparing 10 mg antibiotic coated microparticles, we soaked 10 mg microparticles in 130 μl of 8 mg/ml antibiotic solution in a closed vial. The microparticles were soaked till all the solution was adsorbed and dried. Therefore we assume that 100% of the antibiotic was incorporated into the microparticles.
2.3.3. Fabrication of growth factor loaded microparticles
BMP-7 encapsulated microparticles were prepared by dissolving 150 ng of BMP-7 into 2% chitosan solution and then adding it drop-wise into the 50% TPP solution. The particles were allowed to cross-link for 5 h before air drying. Previous study done in our lab indicated that BMP-7 being released from these microparticles is biologically active [33].
2.4. FTIR analysis
Antibiotic encapsulated microparticles were analyzed using Fourier transform infrared (FTIR) spectroscopy to determine the presence of drugs: vancomycin and cefazolin. Infrared chemical imaging was carried out using FTS 4000 FTIR spectrometer, coupled with a UMA 600 infrared microscope. The microparticles were analyzed via transmission or a germanium hemispherical attenuated total reflectance (micro-ATR) crystal. The germanium micro-ATR crystal tip was carefully placed in contact with the microparticles on the slide and a contact alert system (SpectraTech) was used to monitor the pressure applied. For ATR analysis, 256 co-added scans were collected at 8 cm−1 resolution. Background images were obtained from vacant areas on the slide. The spectral range collected was 800–4000 cm−1.
2.5. Release study of antibiotics from the microparticles
Either vancomycin or cefazolin was incorporated into the microparticles by encapsulation or coating method. These microparticles (20 mg) were suspended in 2 ml of PBS. The glass vials were incubated at 37 °C with continuous rotation at 25–30 rpm for 2 weeks. PBS in which the microparticles were suspended was collected at pre-determined time points and replaced with fresh 2 ml for every sample collection.
To quantify the amount of drug released, ultraviolet (UV) spectroscopy was used. A standard graph was plotted between known concentrations of the drug and absorbance at 280 nm for both vancomycin and cefazolin. Absorbance of the release samples collected at certain time points was determined and extrapolated on the standard graph to determine the concentration of the drug. Cumulative release graph was plotted to determine the total amount of drug released at the end of 2 weeks period. Encapsulation efficiency and loading efficiency of the each sample was also determined.
2.6. Dual release study of growth factor and the antibiotics from microparticles
BMP-7 encapsulated microparticles (20 mg containing 150 ng BMP-7) were added to 20 mg of cefazolin and vancomycin encapsulated microparticles, respectively. These microparticles were then suspended in 2 ml of PBS buffer solution in a glass vial incubated at 37 °C at constant rotation of 25–30 rpm. Sample collection process was similar to that mentioned above. Release of both the drug and the growth factor was determined from the collected sample.
To quantify the drugs, UV spectroscopy was used and to determine BMP-7, enzyme-linked immunosorbent assay (ELISA) was used. The protocol mentioned in the BMP-7 ELISA kit (DY354) was followed to quantify the BMP-7 amounts in the release samples. A standard graph was obtained by determining absorbance at 450 nm with wavelength correction at 540 nm. Unknown concentration in the samples was determined by using the standard graph.
2.7. Bioactivity of the released antibiotics
The drug released from the microparticles (dual release study experiment) were tested for ability to inhibit the growth and kill S. epidermidis (strain was kindly given to us by Dr. Robert Blumenthal, Department of Microbiology and Immunology, University of Toledo Health Science Campus). The ability of the released drug to inhibit bacterial growth was determined by turbidity test. To grow S. epidermidis, bacterial stock previously stored and frozen at −70 °C was resuscitated on tryptic soy solid medium. Two colonies were selected and cultured overnight in 10 ml tryptic soy liquid media in two separate conical flasks in shaking water bath at 37 °C. After overnight incubation, a second transfer was made to fresh tryptic soy media (1:10 dilution) and was grown to attain an OD600 nm = 0.05 for S. epidermidis (SPECTROstar Omega, USA). In the meantime, 100 μl of drug solution obtained for the release study were added to a 96-well plate (n = 3). As soon as an OD of 0.05 was obtained, 100 μl of the bacterial suspension was added to the wells and OD readings were taken at pre-determined time intervals for a period of 24 h. The growth curves plotted were compared against the growth of the control, which had only the bacterial culture in PBS.
| (1) |
where Ic and Is are the absorbance’s of control solution containing tryptic soy broth, and bacterial culture solution with and without drug release sample, respectively at 600 nm after 24 h [35].
2.8. Statistical analyses
Triplicates of each sample were used in all the studies. Two-way Analysis of Variance (ANOVA) was performed using IBM SPSS statistical software (version 19, IBM Company, Armonk, NY) to determine statistical difference. Post hoc Tukey’s test was performed to determine statistical difference between groups. A probability value of p < 0.05 was used to determine significance, unless otherwise mentioned.
3. Results
3.1. In vitro biocompatibility tests
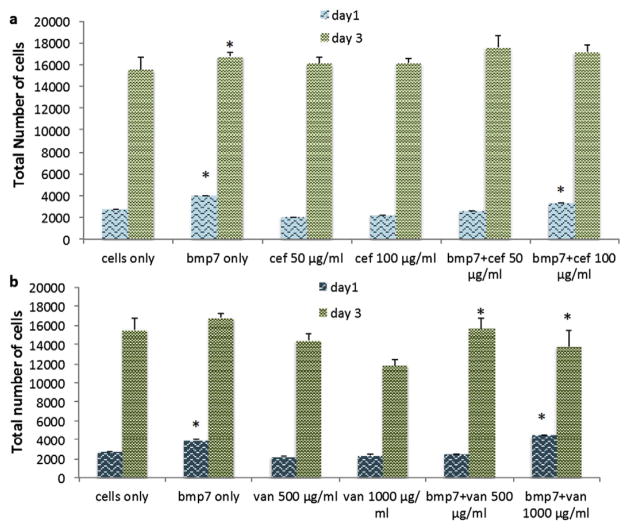
Pre-osteoblast viability and proliferation in the presence of cell culture medium alone (control), culture medium containing antibiotics or growth factor is shown in Fig. 1. On day 1, a post hoc Tukey’s HSD analysis indicated a significant difference (p < 0.001) in the proliferation of pre-osteoblasts (OB-6) grown in control and that with growth factor BMP-7, indicating that on day 1 itself, BMP-7 has an influence on the cell growth and proliferation (Fig. 1). In the antibiotics containing samples, a significant difference (p < 0.001) was observed between controls and vancomycin (1000 μg/ml), indicating that this concentration of vancomycin is inhibiting pre-osteoblast proliferation. In comparison with BMP-7 containing media, there was a significant difference (p < 0.001) observed between all the other samples except media containing BMP-7 + cefazolin (100 μg/ml) (Fig. 1a) and BMP-7 + vancomycin (1000 μg/ml) (Fig. 1b). This shows that higher concentrations of drugs are tolerable by the pre-osteoblasts in the early time period (day 1). In the medium containing cefazolin (50 μg/ml) only, a significant decrease (p < 0.05) is observed in comparison with medium containing BMP-7 and cefazolin (100 μg/ml). In the medium containing BMP-7 and cefazolin (100 μg/ml), a significant increase (p < 0.05) in proliferation was observed in comparison with the medium containing BMP-7 and vancomycin (500 μg/ml).
Fig. 1.
Effect of a) vancomycin b) cefazolin and growth factor on viability and proliferation of pre-osteoblasts OB-6 (n = 10). OB-6 cells were exposed to 100 μl of the respective drug concentrations for a period of 24 and 72 h.
On day 3 however, medium containing vancomycin (1000 μg/ml) showed significant decrease in the cell proliferation in comparison with all the samples except medium containing 500 μg/ml of vancomycin and a combination of vancomycin (1000 μg/ml) and BMP-7, indicating that both 500 μg/ml and 1000 μg/ml concentration of vancomycin inhibit pre-osteoblast proliferation. Medium containing 500 μg/ml vancomycin showed significant decrease (p < 0.05) in cell proliferation in comparison with medium containing BMP-7 and cefazolin (100 μg/ml and 50 μg/ml), indicating that cefazolin is more compatible for pre-osteoblast proliferation. A significant increase (p < 0.05) was also observed in proliferation in media containing BMP-7 and cefazolin (100 μg/ml or 50 μg/ml) over medium containing BMP-7 and vancomycin (1000 μg/ml). These results show that 50 μg/ml and 100 μg/ml cefazolin concentrations in the presence and absence of BMP-7 increases cell proliferation.
3.2. FTIR analysis
3.2.1. FTIR analysis of vancomycin encapsulated microparticles
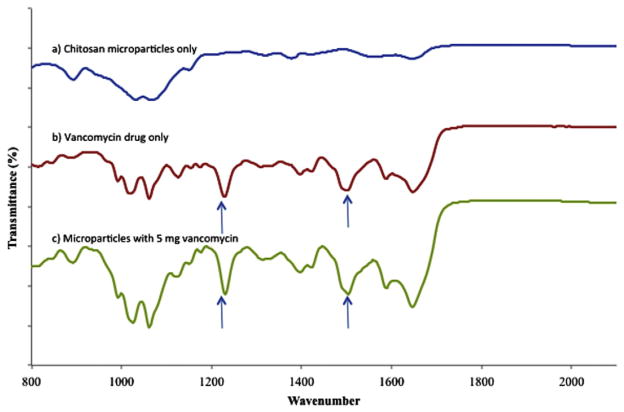
The presence of a distinct peaks at 1230 cm−1 (corresponding to the presence of aromatic ester) and 1550 cm−1 (skeletal vibration of C—O, C—N, C—C bonds in vancomycin) in vancomycin only and microparticles containing vancomycin clearly demonstrates the presence of vancomycin in the microparticles (compare Fig. 2a,b,c) [36,37].
Fig. 2.
FTIR spectra of (a) chitosan-TPP microparticles only (b) vancomycin drug only (c) chitosan-TPP microparticles with 5 mg of vancomycin.
3.2.2. FTIR analysis of cefazolin encapsulated microparticles
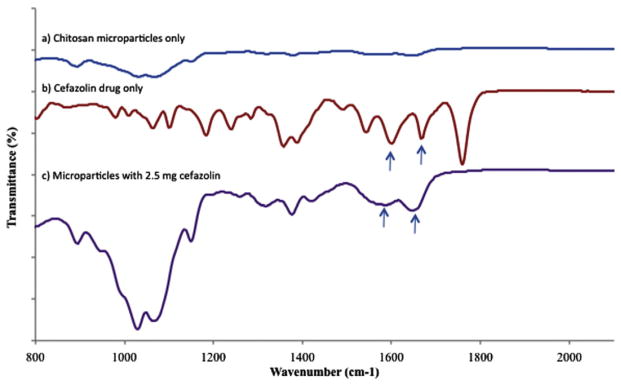
The P—O stretching in the FTIR spectra is distinctly seen at around at 1184 cm−1 for microparticles with and without the drug, which indicates no interaction of drug with TPP [38,39] (Fig. 3a). An FTIR spectrum of the cefazolin shows a sharp intense peak at 1759 cm−1 (which clearly shows presence of carbonyl group), which confirms the carboxylic group in cefazolin (Fig. 3b). Some of the other notable peaks seen in cefazolin containing samples include that at 1647 cm−1 and 1593 cm−1 indicating C=N stretching and the presence of amide. The proof of interaction between COOH group in cefazolin and NH2 group in chitosan is also indicated by the fact that the strong peak at 1759 cm−1 in the drug disappears in the drug with microparticle samples (Fig. 3c). The amide and C=N functional groups appear to have a shift in their wave number to 1666 cm−1 and 1585 cm−1 respectively indicating interaction between cefazolin and chitosan microparticles [39].
Fig. 3.
FTIR spectra of (a) chitosan-TPP microparticles only (b) cefazolin drug only (c) chitosan microparticles with 2.5 mg cefazolin.
3.3. Release kinetics
3.3.1. Cumulative release of vancomycin and cefazolin
Vancomycin and cefazolin encapsulated microparticles exhibited high encapsulation efficiencies (EE), indicating that most of the drug added to the microparticles was incorporated (Table 1). When antibiotics coated into microparticles, first we prepared the microparticles and then antibiotic solution coated on the microparticles allowing them to achieve complete incorporation, hence, coating efficiency of 100% was considered for calculations.
Table 1.
Encapsulation efficiency of various drug samples used in the experiments.
| Group | Amount of antibiotic added to 20 mg microparticles (mg) | Encapsulation efficiency (%) |
|---|---|---|
| Vancomycin concentration 8 mg/ml | 3.05 | 98.4 |
| Vancomycin concentration 16 mg/ml | 5.11 | 97.8 |
| Cefazolin concentration 4 mg/ml | 1.53 | 98.2 |
| Cefazolin concentration 8 mg/ml | 2.53 | 98.6 |
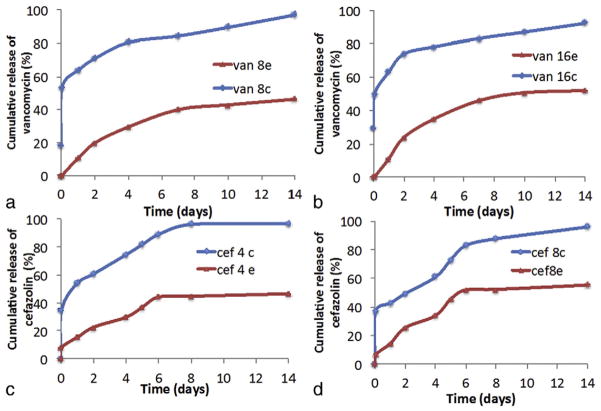
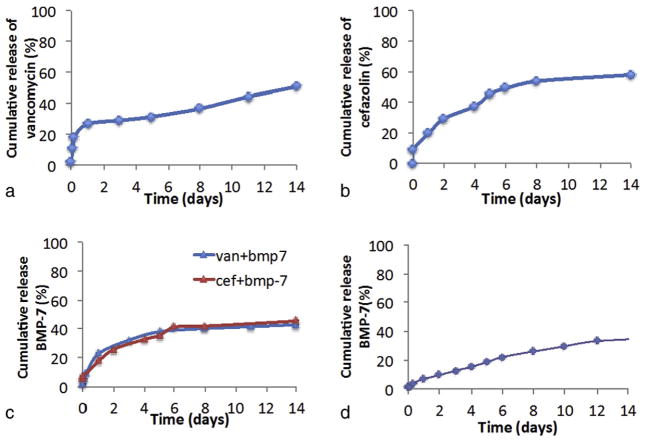
The release results of vancomycin and cefazolin are represented in Fig. 4. From all the release profiles, we can observe that when the drug is encapsulated, the release is much controlled and nearly just 50% of the drug encapsulated is released in 2 weeks time period, whereas in microparticles with drug coated onto it, approximately 95% release was observed in the same time period. In microparticles coated with either vancomycin or cefazolin, nearly >50% was released by day 1, which indicates burst release of the adsorbed drug. In contrast, microparticles with drug encapsulated into it showed minimal burst release, with only 10% cumulative release by day 1. Two different concentrations used for the two drugs (Fig. 4a,b and Fig. 4c,d), showed similar release profile, indicating that an increase in the amount of drug incorporated into the microparticles, increases the release amount proportionally. Statistical analysis indicated p < 0.01 for cumulative release amounts at different time intervals, which means that vancomycin and cefazolin release decreases significantly in drug encapsulated microparticles in comparison with drug coated microparticles till day 14.
Fig. 4.
Cumulative release profile of the drugs from microparticles containing (a) 3 mg vancomycin (b) 5 mg vancomycin (c) 1.5 mg cefazolin (d) 2.5 mg cefazolin, encapsulated (represented by e) and coated (represented by c) onto it. These microparticle were immersed in PBS buffer of pH 7.4 at 37 °C and samples were collected at pre-determined time intervals. Each data point is mean ± standard deviation (n = 3).
After studying the release profiles (Fig 4a,b,c,d), microparticles with 5 mg vancomycin and 2.5 mg cefazolin encapsulated were considered for the BMP-7 dual release study and in vitro antibacterial test. This was done so because we wanted vancomycin release above 8 μg/ml at each time point over the two-weeks. Similarly for cefazolin dual release study, microparticles with 2.5 mg of cefazolin was considered, since we wanted a release of above 4 μg/ml at each time point. These groups also show least burst release and longest release period.
3.3.2. Dual release of BMP-7 and the antibiotics
The release profile of vancomycin in the presence of BMP-7 was similar to the individual drug release profile. In the presence and absence of BMP-7, the cumulative release amount for the 5 mg vancomycin release study was 50.79% and 51.89% respectively, which indicates that the presence of BMP-7 does not affect the stability or release amounts of vancomycin (Fig. 5a). A similar observation was made for cefazolin release as well, with a cumulative release percent of 57.87% and 55.25% in the presence and absence of BMP-7 respectively (Fig. 5b). Statistically, there was no significant difference (p > 0.01) observed between the cumulative release amounts at the various time points, when a comparison is made between the drug release in the presence and absence of BMP-7.
Fig. 5.
Cumulative release profiles of (a) 5 mg vancomycin (b) 2.5 mg cefazolin (c) 5 mg vancomycin/BMP-7 and 2.5 mg cefazolin/BMP-7 encapsulated microparticles (d) BMP-7 release profile in the absence of drugs. Each data point is mean ± standard deviation (n = 3).
BMP-7 cumulative release was determined from the above release samples and the release profile was similar in the presence of vancomycin and cefazolin. Also when these profiles are compared with BMP-7 release alone, a similar result was observed, with the cumulative release percentage being 45.49%, 42.99%, and 36.45% in the presence of cefazolin, vancomycin (Fig. 5c) and BMP-7 alone (Fig. 5d) respectively, which indicates that the presence of drugs have no effect on the stability of BMP-7.
3.4. In vitro bacterial activity
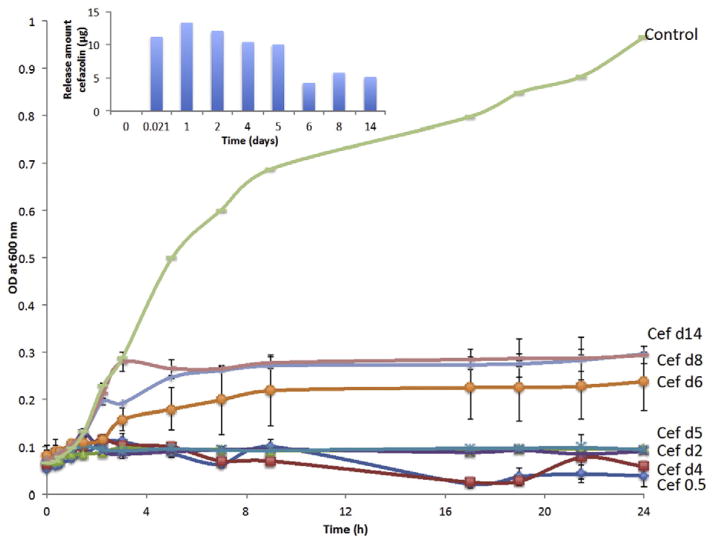
The amount of cefazolin and vancomycin released at the time points mentioned in Fig. 7 is the difference in the drug released between the two time points. This is because the method of sample collection for drug release was to collect the entire PBS in the vial and replace it with fresh amount of PBS. This approach for bacterial study is comparable with what happens in vivo as the drug released on day 1, would have dispersed from the defect site in some period of time.
Fig. 7.
Growth curve of Staphylococcus epidermidis plotted over 24 h in the presence of cefazolin release samples collected at various time points (n = 3).
3.4.1. Effect of vancomycin on S. epidermidis
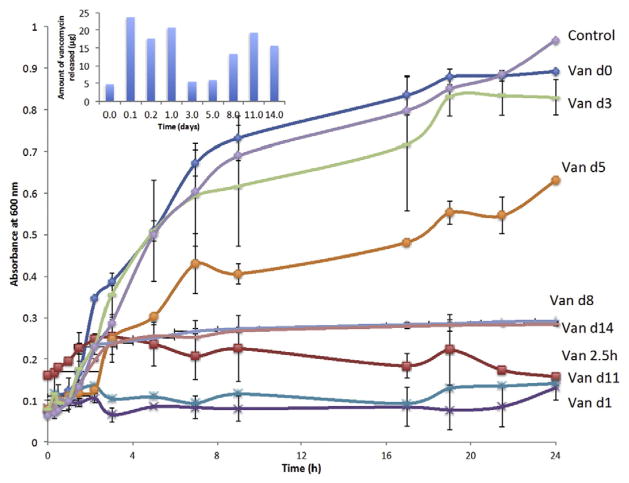
The data from the liquid bacterial culture (as shown in Fig. 6), demonstrated that the antibacterial function of vancomycin lasted for almost the complete 2 weeks, except at day 3 and 5, when partial inhibition of bacterial growth was observed (14.37% and 34.89% inhibition respectively). Average inhibition of 85% was observed for the release samples at other time points, suggesting vancomycin has a good antibacterial activity on S. epidermidis. There was a significant difference (p < 0.05) in the OD600 at 24 h between the prior mentioned release samples and the control, which consists of medium with 100 μl of PBS. It can be understood that the released concentrations between than 16–30 μg/ml is not adequate to completely inhibit their growth. These results also indicated that encapsulation of vancomycin in chitosan-TPP microparticles not only controlled the release rate and prolonged its release duration, but also retains its antibacterial activity.
Fig. 6.
Growth curve of Staphylococcus epidermidis plotted over 24 h in the presence of vancomycin release samples collected at various time points (n = 3).
3.4.2. Effect of cefazolin on S. epidermidis
Antibacterial study demonstrated that the cefazolin released from the microparticles can inhibit the growth of S. epidermidis completely during the study period. An average inhibition rate of 94% was observed for the release samples till day 5 and later an average inhibition rate of 72% was observed. The later inhibition rate corresponds to a cefazolin concentration of around 20 μg/ml.
The release profile of vancomycin from coated microparticles is similar to that of cefazolin (Fig. 7), but the amount of vancomycin encapsulated is higher than cefazolin. This decision was based on the MIC of cefazolin (1 μg/ml) and vancomycin (8–16 μg/ml).
4. Discussion
The main justification of the antibiotics we chose was to address the most commonly used antibiotics in clinical practice today, delivered either systemically or locally. Our lab has been involved in developing novel chitosan based scaffolds capable of delivering growth factors for bone regeneration. Since chitosan-TPP microparticles were prepared using coacervation procedure, it was easy to entrap hydrophilic small molecules of vancomycin and cefazolin into the microparticles in both the processes (encapsulation and coating procedure). High encapsulation efficiencies (Table 1) were obtained in this study in comparison with other studies where water-in-oil-in water (w/o/w) double emulsion procedures were used, wherein it is difficult to achieve high encapsulation efficiency due to high solubility of drug in the external phase, as a result majority of the drug diffuses to the external phase during emulsion and solvent evaporation process [40]. The drugs were incorporated in two ways - encapsulation and coating (adsorption) to determine their influence on the release profiles of both the drugs. We observed a similar trend in all the release studies-cefazolin, vancomycin and BMP-7; when these molecules were encapsulated, an average release of about 50% was observed by the end of 2 weeks and minimum burst initial burst release (15–20%) was observed in the release kinetics. In contrast, when these molecules are coated onto the microparticles (by the process of adsorption), we observed a burst initial release of about 50% by day 1 and by the end of 2 weeks period nearly 95% of the adsorbed protein was released. The huge difference in the release patterns between the two techniques can be attributed to the larger travel distance for the encapsulated drug to diffuse out of the microparticle, resulting in lower burst release during the early stages of release study. Also, since the viscosity of the chitosan solution is high, it reduced the diffusion of drug to the outer surface as a result least amount of drug was found on or near the surface of the microparticle, in contrast to the drug coated microparticles, where most of the drug was incorporated near to the outer surface, resulting in the burst release [41].
The high concentration of drugs inhibits the bone healing activity [42]. Our day 3 experiment of osteoblasts proliferation in presence of antibiotics and BMP-7 suggests that cefazolin concentration of 50 and 100 μg/ml did not significantly change the cell number in comparison with controls and also the presence of BMP-7 improved cell proliferation. This result indicates that cefazolin concentrations used are not toxic to osteoblasts and they also do not inhibit the activity of BMP-7. Our results are in agreement with other studies, which showed that at a concentration of 200 μg/ml cefazolin, they start to become toxic to osteoblasts, decreasing the cell number by >75% [43,44]. Fig. 5 also indicates an increase in the cell proliferation in cefazolin concentration of 50 and 100 μg/ml and BMP-7 in comparison with BMP-7 containing wells. This indicates that cefazolin itself improves osteoblast viability and proliferation. This observation is also in support with another study, which observed an increase in the alkaline phosphate activity in the presence of cephalosporins [45,46]. On the other hand, vancomycin concentration of 1000 μg/ml was found to be toxic to OB-6 cell line, even in presence of BMP-7, which indicates its toxicity [45]. Although others reported positive effects of vancomycin on osteoblasts until 2000 μg/ml [44], our observation clearly indicates a significant decrease (p < 0.001) in cell number in comparison with controls. Vancomycin belongs to glycopeptide family of drugs and cefazolin belongs to cephalosporin family and have indicated that there is a difference in with regards to their effect on cell viability and osteogenic potential.
Antibiotic loaded microparticles, antibiotics coated spacers and antibiotic coated implants may reduce infection, but they do little to improve bone regeneration, therefore, we studied the dual release of the drugs and growth factor to simultaneously promote bone growth and prevent infection. Even though individual effect of BMP-7 and drugs (vancomycin and cefazolin) has been studied, their combined effect on pre-osteoblasts (OB-6) and S. epidermidis, to our knowledge, never been established before [47–49]. BMP-7 released from microparticles and its activity in presence of pre-osteoblasts has been previously studied by our lab and therefore we were bound to the specific concentration of BMP-7 [33,34]. The drug release profiles in the presence of BMP-7 were similar to drug release alone, suggesting that no interaction occurred between drugs and growth factor. Since the growth factor and drugs were encapsulated into the microparticles, controlled release was observed, with about 50% cumulative release over the period of 2 weeks. Almost steady amount of drug is released over this period of time, minimizing peak/trough fluctuations, while maximizing the amount of time the drug concentrations remain at therapeutic levels. The justification for our choice of drug concentration to be incorporated were influenced from the osteoblast viability experiment, where we concluded that cefazolin concentration <100 μg/ml supported osteoblast proliferation and vancomycin concentration <500 μg/ml would be beneficial for their growth. The concentration of drugs incorporated into the microparticles were chosen such that the release concentration at any time point did not exceed those limits and in our release study we found that for drug encapsulated microparticles, the maximum release was 70 μg/ml and 120 μg/ml of cefazolin and vancomycin, respectively.
The antimicrobial experiment demonstrated that the biological function of vancomycin and cefazolin released from the chitosan-TPP microparticles for over a period of 2 weeks. In both the experiments, only about 50% of the total encapsulated drug is released and in that period, it was observed that cefazolin could inhibit bacterial growth to a greater extent (~85%) at lower concentrations in comparison with vancomycin (~80%). Minimum inhibitory concentration for vancomycin was found to be around 24 μg/ml, which is in agreement with other studies [50]. While the chitosan-TPP microparticles along with BMP-7 promote bone tissue regeneration, the vancomycin and cefazolin encapsulated in the scaffold will protect the tissue from microbial invasion.
This work has shown the capability of the chitosan-TPP microparticles for dual delivery of bioactive and stable drugs and growth factor in a controlled fashion over a prolonged period of time. The study also demonstrated that there is no interaction between the compounds during the dual release. The amount of drugs being incorporated into the microparticles should be optimized in order to inhibit bacterial growth and at the same time not negatively influence osteoblast proliferation and activity.
5. Conclusions
With this work, we begin to attempt to bridge the two main avenues in bone tissue engineering; improve bone healing process and avoid/ treat bone infection. While both areas are being individually targeted in clinical care, there still remains a need for refining the dual delivery techniques. This is the first of many studies which aims to streamline the dual-delivery system by considering optimum antibiotic concentration for infection treatment and bone repair, as it was noted from our study that increasing antibiotic concentration may help in faster infection treatment but may have detrimental effect on cell growth, thereby affecting bone repair. Our findings indicate that, individually, the growth factor BMP-7 and antibiotics, vancomycin and cefazolin, maintain their functionality respectively when delivered together. We have also established that the bioactivity of the antibiotics released is being maintained. As a whole, these results pave the way for future study in dual delivery of growth factor and antibiotics, where we plan to conduct more in vitro experiments designed to ensure that the quality of the cells grown in the presence of antibiotics is being maintained.
Acknowledgments
We would like to thank National Institute of Health (NIH) grant numbers R03DE019508 and R01DE023356 for providing financial support to accomplish this work.
References
- 1.Berbari EF, Steckelberg JM, Osmon DR. Osteomyelitis. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and Practice of Infectious Diseases. 6. Churchill Livingstone; New York: 2005. pp. 1323–1332. [Google Scholar]
- 2.Lazzarini L, Mader JT, Calhoun JH. Osteomyelitis in long bones. J Bone Joint Surg. 2004;86:2305–2318. doi: 10.2106/00004623-200410000-00028. [DOI] [PubMed] [Google Scholar]
- 3.Lew DP, Waldvogel FA. Osteomyelitis. Lancet. 2004;364:369–379. doi: 10.1016/S0140-6736(04)16727-5. [DOI] [PubMed] [Google Scholar]
- 4.Mantripragada VP, Lecka-Czernik B, Ebraheim NA, Jayasuriya AC. An overview of recent advances in designing orthopedic and craniofacial implants. J Biomed Mater Res A. 2013;101:3349–3364. doi: 10.1002/jbm.a.34605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.U.K. Health Protection Site Agency. Surgical Site Infection–National Aggregated Data on Surgical Infections for Hospitals That Have Participated in Surgical Site Infection Surveillance Scheme (SSISS) Between October 1997 and December 2005. 2008. [Google Scholar]
- 6.Gitelis S, Brebach GT. The treatment of chronic osteomyelitis with a biodegradable antibiotic impregnated implant. J Orthop Surg. 2002;10:53–60. doi: 10.1177/230949900201000110. [DOI] [PubMed] [Google Scholar]
- 7.Costerton JW, Lewandowski Z, Caldwell DE. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 8.Matzke GR, Zhanel GG, Guay DR. Clinical pharmacokinetics of vancomycin. Clin Pharmacokinet. 1986;11:257–282. doi: 10.2165/00003088-198611040-00001. [DOI] [PubMed] [Google Scholar]
- 9.Roy ME, Peppers MP, Whiteside LA, Lazear RM. Vancomycin concentration in synovial fluid: direct injection into the knee vs. intravenous infusion. J Arthroplast. 2014;29:564–568. doi: 10.1016/j.arth.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 10.Engelsman AF, Krom BP, Busscher HJ, van Dam GM, Ploeg RJ, van der Mei HC. Antimicrobial effects of an NO-realising poly(ethylene vinylacetate) coating on soft-tissue implants in vitro in a murine model. Acta Biomater. 2009;5:1905–1910. doi: 10.1016/j.actbio.2009.01.041. [DOI] [PubMed] [Google Scholar]
- 11.Marzona L, Pavolini B. Play and players in bone fracture healing match. Clin Cases Miner Bone Metab. 2009;6:159–162. [PMC free article] [PubMed] [Google Scholar]
- 12.Lienau J, Schmidt-Bleek K, Peters A, Weber H, Bail HJ, Duda G, Perka C, Schell H. Insight into the molecular pathophysiology of delayed bone healing in a sheep model. Tissue Eng A. 2010;16:191–199. doi: 10.1089/ten.TEA.2009.0187. [DOI] [PubMed] [Google Scholar]
- 13.Qiu LY, Bae YH. Self-assembled polyethylenimine–graft-poly(ε-caprolactone) micelles as potential dual carriers of genes and anticancer drugs. Biomaterials. 2007;28:4132–4142. doi: 10.1016/j.biomaterials.2007.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JS, Bae JW, Joung YK, Lee SJ, Han DK, Park KD. Controlled dual release of basic fibroblast growth factor and indomethacin from heparin-conjugated polymeric micelle. Int J Pharm. 2008;346:57–63. doi: 10.1016/j.ijpharm.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 15.Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233–241. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 16.Huang ZM, Yang AH. Encapsulation of pure drugs into the central part of polycaprolactone ultrafine fibers. Acta Polym Sin. 2006;1:48–52. [Google Scholar]
- 17.Martins A, Duarte ARC, Faria S, Marques AP, Reis RL, Neves NM. Osteogenic induction of hBMSCs by electrospun scaffolds with dexamethasone release functionality. Biomaterials. 2010;31:5875–5885. doi: 10.1016/j.biomaterials.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 18.Duarte AR, Mano JF, Reis RL. Dexamethasone-loaded scaffolds prepared by supercritical-assisted phase inversion. Acta Biomater. 2009;5:2054–2062. doi: 10.1016/j.actbio.2009.01.047. [DOI] [PubMed] [Google Scholar]
- 19.Schofer MD, Fuchs-Winkelmann S, Gräbedünkel C, Wack C, Dersch R, Rudisile M. Influence of poly(L-lactic acid) nanofibers and BMP-2-containing poly(L-lactic acid) nanofibers on growth and osteogenic differentiation of human mesenchymal stem cells. Sci World J. 2008;8:1269–1279. doi: 10.1100/tsw.2008.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao Y, Chen B, Lin H, Sun W, Zhao W, Zhang J, Dai J. The bone-derived collagen containing mineralized matrix for the loading of collagen-binding bone morphogenetic protein-2. J Biomed Mat Res A. 2009;88:725–734. doi: 10.1002/jbm.a.31928. [DOI] [PubMed] [Google Scholar]
- 21.Kong M, Chen XG, Xing K, Park HJ. Antimicrobial properties of chitosan and mode of action: a state of art review. Int J Food Microbiol. 2010;144:51–63. doi: 10.1016/j.ijfoodmicro.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 22.Jiang B, Li B. Polypeptide nanocoatings for preventing dental and orthopaedic device-associated infection: pH-induced antibiotic capture, release, and antibiotic efficacy. J Biomed Mat Res Part B. 2007;88B:332–338. doi: 10.1002/jbm.b.31021. [DOI] [PubMed] [Google Scholar]
- 23.Zivanovic S, Basurto CC, Chi S, Davidson PM, Weiss J. Molecular weight of chitosan influences antimicrobial activity in oil-in-water emulsions. J Food Prot. 2004;67:952–959. doi: 10.4315/0362-028x-67.5.952. [DOI] [PubMed] [Google Scholar]
- 24.Zivanovic S, Chi S, Draughon AF. Antimicrobial activity of essential oils incorporated in chitosan films. J Food Sci. 2005;70:45–51. [Google Scholar]
- 25.Zivanovic S, Li JJ, Davidson PM, Kit K. Physical, mechanical and antimicrobial properties of chitosan/PEO blend films. Biomacromolecules. 2007;8:1505–1510. doi: 10.1021/bm061140p. [DOI] [PubMed] [Google Scholar]
- 26.Takemono K, Sunamoto J, Akasi M. Polymer and Medical Care. Chapter IV Mita, Tokyo: 1989. [Google Scholar]
- 27.Ryu SJ, Jung H, Oh JM, Lee JK, Choy JH. Layered double hydroxide as novel antibacterial drug delivery system. J Phys Chem Solids. 2010;71:685–688. [Google Scholar]
- 28.Cunha BA, Gossling HR, Pasternak HS, Nightingale CH, Quintiliani R. The penetration characteristics of cefazolin, cephalothin, and cephradine into bone in patients undergoing total hip replacement. J Bone Joint Surg Am. 1977;59:856–859. [PubMed] [Google Scholar]
- 29.Cunha BA, Gossling HR, Pasternak HS, Nightingale CH, Quintiliani R. Penetration of cephalosporins into bone. Infection. 1984;12:80–84. doi: 10.1007/BF01641676. [DOI] [PubMed] [Google Scholar]
- 30.Barberan J. Management of infections of osteoarticular prosthesis. Clin Microbiol Infect. 2006;12:93–101. doi: 10.1111/j.1469-0691.2006.01400.x. [DOI] [PubMed] [Google Scholar]
- 31.Zeller V, Desplaces N. Antibiotherapy of bone and joint infections. Rev Rhum. 2006;73:129–135. [Google Scholar]
- 32.Rayner C, Munckhof WJ. Antibiotics currently used in the treatment of infections caused by Staphylococcus aureus. Intern Med J. 2005;36:142–143. doi: 10.1111/j.1444-0903.2005.00976.x. [DOI] [PubMed] [Google Scholar]
- 33.Mantripragada VP, Jayasuriya AC. Injectable chitosan microparticles incorporating bone morphogenetic protein-7 for bone tissue regeneration: in vitro study. J Biomed Mat Res A. 2014;102:4276–4289. doi: 10.1002/jbm.a.35100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mantripragada VP, Jayasuriya AC. IGF-1 release kinetics from chitosan microparticles fabricated using environmentally benign conditions. Mater Sci Eng C. 2014;42:506–516. doi: 10.1016/j.msec.2014.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim K, Luu YK, Chang C, Fang D, Hsiao BS, Chu B, Hadjiargyrou M. Incorporation and controlled release of a hydrophilic antibiotic using poly (lactide-co-glycolide)-based electrospun nanofibrous scaffolds. J Control Release. 2004;98:47–56. doi: 10.1016/j.jconrel.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 36.Gupta M, Saini T. Preformulation parameters characterization to design, development and formulation of vancomycin hydrochloride tablets for pseudomembranous colitis. Int J Pharm Res Dev. 2009;1:1–7. [Google Scholar]
- 37.Loveymi BD, Jelvehgari M, Milani PZ, Valizadeh H. Design of vancomycin RS-100 nanoparticles in order to increase the intestinal permeability. Adv Pharm. 2012;2:43–56. doi: 10.5681/apb.2012.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu W, Sun S, Cao Z, Zhang X, Yao K, Lu WW. An investigation on the physicochemical properties of chitosan/DNA polyelectrolyte complexes. Biomaterials. 2005;26:2705–2711. doi: 10.1016/j.biomaterials.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 39.Jayasuriya AC, Bhat A. Optimization of scaled-up chitosan microparticles for bone regeneration. Biomed Mater. 2009;4:1–8. doi: 10.1088/1748-6041/4/5/055006. [DOI] [PubMed] [Google Scholar]
- 40.Yin J, Noda Y, Yotsuyanagi T. Properties of poly(lactic-co-glycolic acid) nanospheres containing protease inhibitors: camostat mesilate and nafamostat mesilate. Int J Pharm. 2006;314:46–55. doi: 10.1016/j.ijpharm.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 41.Feng K, Sun H, Bradley MA, Dupler EJ, Giannobile WV, Ma PX. Novel antibacterial nanofibrous PLLA scaffolds. J Control Release. 2010;146:363–369. doi: 10.1016/j.jconrel.2010.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duewelhenke N, Krut O, Eysel P. Influence on mitochondria and cytotoxicity of different antibiotics administered in high concentrations on primary human osteoblasts and cell lines. Antimicrob Agents Chemother. 2007;51:54–63. doi: 10.1128/AAC.00729-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li H, Ogle H, Jiang B. Cefazolin embedded biodegradable polypeptide nanofilms promising for infection prevention: a preliminary study on cell responses. J Orthop Res. 2010;28:992–999. doi: 10.1002/jor.21115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rathbone CR, Cross JD, Brown KV, Murray CK, Wenke JC. Effect of various concentrations of antibiotics on osteogenic cell viability and activity. J Orthop Res. 2011;29:1070–1074. doi: 10.1002/jor.21343. [DOI] [PubMed] [Google Scholar]
- 45.Li B, Brown KV, Wenke JC, Guelcher SA. Sustained release of vancomycin from polyurethane scaffolds inhibits infection of bone wounds in a rat femoral segmental defect model. J Control Release. 2010;145:221–230. doi: 10.1016/j.jconrel.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 46.Salzmann GM, Naal FD, von Knoch F. Effects of cefuroxime on human osteoblasts in vitro. J Biomed Mat Res A. 2007;82:462–468. doi: 10.1002/jbm.a.31158. [DOI] [PubMed] [Google Scholar]
- 47.Guelcher SA, Brown KV, Li B, Guda T, Lee BH, Wenke JC. Dual-purpose bone grafts improve healing and reduce infection. J Orthop Trauma. 2011;25:477–482. doi: 10.1097/BOT.0b013e31821f624c. [DOI] [PubMed] [Google Scholar]
- 48.Liu SJ, Chi PS, Lin SS, Ueng SW, Chan EC, Chen JK. Novel solvent-free fabrication of biodegradable polylactic-glycolic acid (PLGA) capsules for antibiotics and rhBMP-2 delivery. Int J Pharm. 2007;330:45–53. doi: 10.1016/j.ijpharm.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y, Wang X, Li H, Xue D, Shi Z, Qi Y, Ma Q, Pan Z. Assessing the character of the rhBMP-2 and vancomycin-loaded calcium sulphate composites in vitro and in vivo. Arch Orthop Trauma Surg. 2011;131:991–1001. doi: 10.1007/s00402-011-1269-6. [DOI] [PubMed] [Google Scholar]
- 50.Sayin B, Alis SC, Illa BLA, Marangoz SI, Hincal AL. Implantation of vancomycin microspheres in blend with human/rabbit bone grafts to infected bone defects. J Microencapsul. 2006;23:553–556. doi: 10.1080/02652040600775632. [DOI] [PubMed] [Google Scholar]