Abstract
The aim of this study is to determine the nano and micro mechanical properties for uncross-linked and cross-linked chitosan films. Specifically, we looked at nanoindentation hardness, microhardness, and elastic modulus. It is important to study the nano and microscale mechanical properties of chitosan since chitosan has been widely used for biomedical applications. Using the solvent-cast method, the chitosan films were prepared at room temperature on the cleaned glass plates. The chitosan solution was prepared by dissolving chitosan in acetic acid 1% (v/v). Tripolyphosphate (TPP) was used to create the cross-links between amine groups in chitosan and phosphate groups in TPP. In this study, atomic force microscopy was used to measure the nanoindentation hardness and surface topography of the uncross-linked and cross-linked chitosan films. Elastic modulus was then calculated from the nanoindentation results. The effective elastic modulus was determined by microhardness with some modifications to previous theories. The microhardness of the chitosan films were measured using Vicker’s hardness meter under three different loads. Our results show that the microhardness and elastic modulus for cross-linked chitosan films are higher than the uncross-linked films. However, the cross-linked chitosan films show increased brittleness when compared to uncross-linked films. By increasing the load magnitude, the microhardness increases for both uncross-linked and cross-linked chitosan films.
Keywords: Chitosan films, Cross-linked, Nanoindentation, Microhardness, Vicker’s hardness, Atomic force microscope, Elastic modulus
1. Introduction
For several decades, chitosan has been extensively investigated for diverse applications: molecular separation, food packaging film, artificial skin, bone substitutes, and water engineering. Chitosan has become an important material due to its relatively good mechanical properties, biocompatibility, biodegradability, multi functional groups, and its solubility in aqueous medium (Liu and Webster, 2010; Goosen, 1997). Recently, chitosan research for biomedical applications has increased dramatically, specifically in the areas of bone and cartilage tissue engineering (Alexandre and Dubois, 2000; Ray and Okamoto, 2003; Ruiz-Hitzky, 2003; Nettles et al., 2002; Martino et al., 2005; Khor and Lim, 2003). Tissue engineering applications have explored chitosan’s many solid forms: flakes, powder, films, fibers, spongers and microparticles (Dumitriu, 2002).
In order to successfully use chitosan in the bone tissue regeneration applications, the mechanical properties of chitosan must be improved (Kim et al., 2003). Recently, we have fabricated the chitosan based microparticles to apply in bone tissue regeneration (Jayasuriya and Bhat, 2009). The structural integrity of the chitosan microparticles was improved by forming cross-links between the amine groups of the chitosan and phosphate groups of the sodium tripolyphosphate (TPP). TPP was selected to make the cross-links in the microparticles because it is a non-toxic compound. This is preferable to other toxic chemical cross-linkers such as glutaraldehyde (Anal and Stevens, 2005). It was difficult to determine the mechanical properties of chitosan based microparticles due to their particle form. To overcome this issue, we fabricated chitosan films using the same chitosan solvents and cross-linking chemistry as the chitosan microparticles prepared previously (Jayasuriya and Bhat, 2009, 2010). As a film, we were able to obtain the nano and micro mechanical properties of cross-linked chitosan films and uncross-linked chitosan films.
Introducing different types of additives into chitosan films will change the mechanical properties of those films. Wang et al. (2005) used montmorillonite nanoparticles to improve the mechanical properties of chitosan including the elastic modulus. Caner et al. (1998) ran a comparative study to determine the effects of different acids and plasticizers on the mechanical properties of chitosan films.
Creating cross-links between an external material and chitosan can improve the chemical and biological properties of chitosan (Desai and Park, 2006; Stulzer et al., 2009; Yi et al., 2005; Co et al., 2005; Jayasuriya and Bhat, 2009). However, there are a few studies that show the mechanical properties of cross-linked chitosan films (Ludovic et al., 2004). To the best of authors knowledge, there is no comparative study that shows the effects of creating cross-link on micro and nano mechanical properties such as hardness and elastic modulus of chitosan films. Based on this understanding, hardness and elastic modulus of uncross-linked and cross-linked chitosan films were investigated by nanoindentation and Vicker’s hardness meter. First, the nano-hardness of uncross-linked and cross-linked chitosan films was measured. Next, based on the load–tip’s deflection graph, elastic modulus was calculated (Oliver and Pharr, 1992). Finally, after determining the microhardness for both cross-linked and uncross-linked chitosan films, a modified method was used to find the elastic modulus of chitosan films.
2. Materials and methods
2.1. Materials
Chitosan (85% deacetylated, medium molecular weight), acetic acid and TPP were used to fabricate the thin films purchased from Sigma-Aldrich. Thin glass plates (1 mm thick, Fisher Brand) were used to make the chitosan thin films.
2.2. Chitosan film fabrication
2.2.1. Uncross-linked chitosan films
Chitosan (2% w/v) solution was prepared by adding 200 mg of chitosan to 10 ml of acetic acid 1% (v/v). The solution was stirred for 1 h to get a homogeneous mixture and cast at room temperature into the clean glass plates purified with acetone. The films were dried over night to evaporate any trace of solvents, allowing us to obtain the uncross-linked thin films.
2.2.2. Cross-linked chitosan films
To obtain the cross-linked chitosan films, 80% (w/w) TPP solution was prepared by dissolving 16 g of TPP in 100 ml of deionized water. The glass plate cast with the chitosan thin films were then placed in a container filled with 80% (w/w) TPP solution immediately after adding chitosan solution to the glass plate and covered. After stirring the mixture for 30 min, the glass plate was dried over night at room temperature.
2.3. Nanoindentation, nanohardness measurement and theory
Atomic Force Microscope (AFM) (Veeco multimode with nano scope V controller) was used to find nanohardness and material behavior under loading and unloading conditions during the nanoindentation test. To obtain the best results, a J scanner was installed on AFM. Nano-indentation measurement was performed by using a diamond tip with spring constant of 216.8 N/m. Applied load was approximately 20,000 nN. To ensure accurate results, the test was performed 30 times for each case.
Currently, there is a common method used to find elastic modulus from nanoindentation (Nowicki et al., 2003; Donnelly et al., 2005; Van Meerbeek et al., 1993; Rho et al., 1997). The key quantities to determine the mechanical properties from loading–unloading curve are defined as follows (Oliver and Pharr, 1992). The maximum indentation depth hmax includes elastic and plastic deformation. The depth at which the applied force becomes zero on unloading is called hf, the depth hc is the contact depth at which the cross section area Ac is taken to calculate hardness and indentation modulus. The nanohardness of the sample (HN) is determined using the formula:
| (1) |
where Fmax is the maximum applied load and Ac is the cross sectional area corresponding to the depth hc. The determination of the contact depth hc is given by:
| (2) |
where S is the contact stiffness:
| (3) |
with being the slope of the unloading curve at the initial point of unloading. The reduced Young’s indentation modulus Er is a measure of the elastic properties of the tip sample system and can be calculated from the load-depth curves according to the formula:
| (4) |
For elastically deformable indenters, the reduced modulus Er can be generalized and is defined as:
| (5) |
where Es and υs are the indentation modulus and Poisson ratio of the sample, Et and υt are the indentation modulus and Poisson ratio of the indenter tip. Since Et is much higher than Es the value of Er will hardly differ from Es.
For the indents at nano scale, the remaining area is difficult to be measured with the traditional optical microscopy because of a too low resolution. The area function for a perfect indenter can be obtained as:
| (6) |
2.4. Microhardness measurement and theory
According to Vicker’s indenter geometry (Fig. 1), the indent’s diagonal length can be obtained as (Chandler, 1999):
| (7) |
where F, HV are applied load and Vicker’s hardness number, respectively.
Fig. 1.
Geometry of Vicker’s indent on surface.
Similarly, the projected area (A) and final depth (hf) can be simply found as:
| (8) |
| (9) |
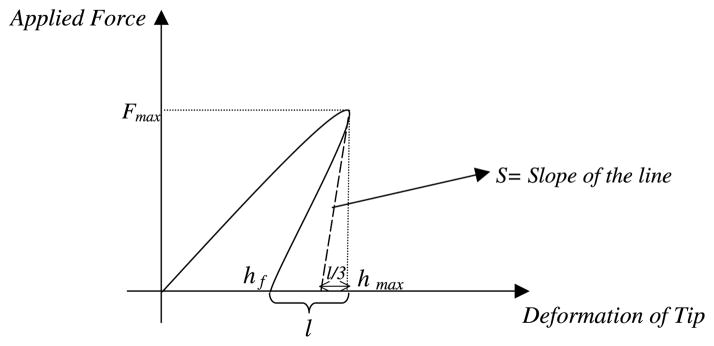
Unfortunately, there is not enough information from microhardness test to find elastic modulus by using approximately similar theory like nanoindentation hardness test. As a result, there should be some assumptions based on the material behavior. First of all, it is necessary to determine S and hmax. To find S, we need to find hmax and hmax can be obtained by the ratio of elastic part of deformation to plastic part of deformation which was obtained from AFM. Based on data from nanoindentation results, which will be presented later, (hmax − hf)/hf (the ratio of elastic elongation to plastic elongation) was determined to calculate the microhardness. According to the materials deformation theory, this assumption is applicable because after removing a load from the surface only permanent or plastic depth is measured and hmax is the maximum depth (plastic and elastic depth). According to Fig. 2 and using loading–unloading curves from AFM, S can be approximated as follows:
| (10) |
Fig. 2.
Schematic representation of material behavior during loading and unloading which was extended to microhardness experiment.
Fmax where Fmax is the applied load for the microhardness test. To determine the elastic modulus from microhardness, the same procedure was applied to nanoindentation.
3. Results
3.1. Nanoindentation hardness and elastic modulus
Capturing image from different parts of the samples reveals more information about the effect of cross-links on chitosan films. An interesting finding shown in Fig. 3 is the different surface roughness of the chitosan films. Fig. 3(A) demonstrates the top view of surface for uncross-linked chitosan thin film. Fig. 3(A) shows the height range to be about 23 nm which shows the flat surface. Conversely, based on Fig. 3(B), the height range for cross-linked chitosan is approximately 3 μm which is significantly larger in comparison to the uncross-linked chitosan thin film. Fig. 3(C) and (D) exhibit a 3D plot of the surface of each type of chitosan films. These topography images show depressions and elevations on the surface of chitosan film which is also reported by other researchers (El-hefian et al., 2009; Modrzejewska et al., 2006). The imaged surfaces show that chitosan film is composed of irregular, bulbous structural elements. However, when dipped in TPP solution, the film becomes homogeneous, smoother and the bumps disappear.
Fig. 3.
AFM image from the surface of samples: uncross-linked chitosan top view (A), cross-linked chitosan top view (B), 3D uncross-linked chitosan image (C), 3D cross-linked chitosan image (D).
The nanoindentation test was repeated 30 times for each sample in order to obtain accurate results. Fig. 4(A) and (B) show a top view of the indent effect on uncross-linked chitosan and cross-linked chitosan films, respectively. The distance between each indentation is 450 nm. Additionally, Fig. 4(C) and (D) show topological image of the indent on uncross-linked chitosan and cross-linked chitosan films, respectively.
Fig. 4.
AFM image from the surface of samples after nanoindentation test: uncross-linked chitosan top view (A), cross-linked chitosan top view (B), 3D uncross-linked chitosan image (C), 3D cross-linked chitosan image (D).
Data obtained from AFM was analyzed by SPIP (Scanning Probe Image Processor) commercial software to generate the specified parameters which were described in the theory section. Fig. 5(A) demonstrates the average hardness number for each case. As expected, the hardness increases for cross-linked chitosan films compared to the uncross-linked chitosan films. Hardness is 1200 MPa and 1550 MPa for uncross-linked chitosan films and cross-linked chitosan films, respectively. Similarly, by calculating the required parameters from the graph obtained from SPIP, average elastic modulus was obtained for both samples, as displayed in Fig. 5(B). Elastic modulus is about 3.1 MPa and 6.3 MPa for uncross-linked chitosan and cross-linked chitosan, respectively.
Fig. 5.
Comparison for nano-hardness (A) and elastic modulus (B) between uncross-linked chitosan and cross-linked chitosan films.
3.2. Microhardness and elastic modulus
The modified theory explained previously was used to determine the deformation of the chitosan films. The deformation of material during the loading was derived for uncross-linked (Fig. 6(A)) and cross-linked chitosan thin films (Fig. 6(B)). Fig. 6 is obtained by determining the ratio of elastic deformation to plastic deformation for uncross-linked chitosan and cross-linked chitosan films. According to nanoindentation results, (hmax − hf)/hf for uncross-linked and cross-linked chitosan films is around 2.1 and 1.3, respectively. As stated before, these fractions approximate the ratio of plastic deformation to total deformation. Fig. 6(A) and (B) show cross-linked chitosan films decrease in plastic deformation and increase in brittleness, as was observed from the nanoindentation test.
Fig. 6.
Suggested behavior during loading and unloading in microhardness test, uncross-linked chitosan film (A) and cross-linked chitosan film (B).
Fig. 7(A) shows the results for Vicker’s microhardness test under three different loads for both types of chitosan films. Elastic modulus was determined for both types of chitosan films by applying the method of nanoindentation into microhardness. Fig. 7(B) demonstrates the average value of elastic modulus for both types of chitosan films under three different loads. Average elastic moduli of uncross-linked and cross-linked chitosan films are approximately 1.5 GPa and 4.7 GPa, respectively. The elastic modulus found using microhardness measurements were less in value than those from the nanoindentation test. Similarly, creating cross-links in chitosan films at least doubled the elastic modulus as illustrated in Fig. 8. Fig. 8 summarizes the elastic moduli results from the different types of techniques used. One observation from this data is that by increasing the load in microhardness test, the elastic modulus also increases for both chitosan samples. Furthermore, the nanoindentation test yields a much higher elastic modulus when compared to any of the microhardness test results.
Fig. 7.
Variation of microhardness (A) and elastic modulus (B) versus applied load for uncross-linked and cross-linked chitosan films.
Fig. 8.
Comparison between the elastic modulus which was obtained in uncross-linked and cross-linked chitosan films using two different methods.
4. Discussion
In this study, nano and micro mechanical properties of uncross-linked and cross-linked chitosan films were measured using experimental and theoretical modifications. The nanomechanical properties were measured using AFM and microhardness was measured using Vicker’s microhardness test for chitosan thin films. AFM is a commonly used to obtain the nanomechanical properties of polymer thin films (Raynaud et al., 2000; Tao et al., 1992). AFM cannot only be used for imaging the topography of different surfaces but it is applicable for measuring the forces in nano-scale (Florin et al., 1994).
Elastic modulus was measured using the nanoindentation method and the theoretical modification based on the ratio of plastic and elastic elongations of the chitosan films with the data of the microhardness test. The elastic modulus values measured by nanoindentation were in agreement with the elastic modulus obtained using the microhardness and theoretical modifications for chitosan films (Fig. 8).
Chitosan has recently been used for a wide variety of biomedical applications including tissue engineering scaffolds (Arpornmaeklong et al., 2007). In order to apply chitosan based materials for bone tissue engineering it is necessary to improve the mechanical properties of these materials. We fabricated the micro-scale particles in our laboratory to research their role in bone regeneration (Jayasuriya and Bhat, 2009, 2010). As stated earlier, the microparticles size and spherical shape made determining their mechanical properties difficult. Therefore, by making chitosan films, this study provided us with insight about the mechanical properties of chitosan materials.
Our results from nanoindentation and microhardness tests have shown that cross-linked chitosan films significantly improve the elastic modulus compared to uncross-linked chitosan films. The measured elastic modulus values for chitosan films are close to the reported values for human bone. Different numbers for elastic modulus of human bone were reported based on gender, age and bone diseases (Rho et al., 1997). The reported elastic modulus number varies between 8.2 GPa and 26.6 GPa. Also, an average Vicker’s micro-hardness of 100 μm-thick sections from bone sample at 25 g applied load is 50 kg/mm2 (490 MPa) (Boivin et al., 2008). This value is relatively closer to our results of microhardness for cross-linked chitosan films (528 MPa) than microhardness of uncross-linked chitosan films (239 MPa). This result suggests that creating cross-links in the chitosan films is beneficial because it improves the hardness and elastic modulus.
The average number for nanoindentation hardness is larger than microhardness for different material films (Qian et al., 2005; Swadener et al., 2002) which is in agreement with our results. This may be due to the micro dislocations which soften the sample and therefore affect the microhardness test. However, because of the micro order of dislocations, they do not affect the nanoindentation hardness.
By changing the load in microhardness, materials films show different characteristics (Low, 1998). According to Fig. 7, microhardness and elastic modulus increase as the applied load is increased. This usually happens for polymer thin films (Domke and Radmacher, 1998). By increasing the load, the effect of the substrate will be more obvious. By increasing the load, the indenter tip can feel the substrate and because the substrate is usually a hard material, the total value for hardness increases with increasing of the load.
Creating cross-links in the chitosan films show the surface brittleness and smoothness (Fig. 3(B) and (D)) which is larger in height compared to the uncross-linked chitosan films. In contrast, the uncross-linked chitosan film surface is rough and flat (Fig. 3(A) and (C)). This shows that creating cross-links in chitosan film affects the surface roughness. Prior to adding TPP to chitosan, the maximum difference in altitude is about 300 times smaller than cross-linked chitosan sample. Exposing the chitosan sample to the TPP solution even for a short time can change the surface properties of chitosan film.
Brittleness of cross-linked chitosan was evident from the microhardness test. During the microhardness test, the chitosan with TPP treatment showed some cracks that initiated from the corners of indents. This behavior is common for brittle materials (Qian et al., 2005). In contrast, chitosan without the TPP treatment has a more ductile surface and no cracks were observed during the tests, as seen in Fig. 6. The chitosan sample without TPP has a larger material deflection at the same loads of the cross-linked chitosan. This shows the ductility of chitosan. For instance, Fig. 6 demonstrates the loading condition of uncross-linked chitosan film for maximum applied load of 100 N, when load gradually increases to 80 N, the deflection of uncross-linked chitosan film below the indenter is about 35 μm, but for cross-linked chitosan film the deflection is about 15 μm in the same loading condition.
5. Conclusion
In this study, nano and micro mechanical properties of uncross-linked and cross-linked chitosan films were successfully obtained. We measured nanohardness, microhardness and elastic modulus of two types of chitosan films. A new modified method was applied to determine the elastic modulus from microhardness. Results show that cross-linked chi-tosan films using TPP improve the hardness and mechanical properties of chitosan films. Furthermore, the results show that cross-linked chitosan films are more brittle than uncross-linked chitosan films. Additionally, the new modified method is in agreement with common methods for determining the elastic modulus from hardness.
Acknowledgments
We would like to thank National Science Foundation (NSF) for providing financial support to accomplish this work with NSF grant number 0652024 and National Institute of Health (NIH) with grant number DE019508.
References
- Alexandre M, Dubois P. Polymer-layered silicate nanocomposites: preparation, properties and uses of a new class of materials. Math Sci Eng. 2000;28:1–63. [Google Scholar]
- Anal KA, Stevens WF. Chitosan–alginate multilayer beads for controlled release of ampicillin Chitosan. Int J Pharm. 2005;290:45–54. doi: 10.1016/j.ijpharm.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Arpornmaeklong P, Suwatwirote N, Pripatnanont P, Oungbho K. Growth and differentiation of mouse osteoblasts on Chitosan–Collagen sponges. Int J Oral Maxillofac Surg. 2007;36:328–337. doi: 10.1016/j.ijom.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Boivin G, Bala Y, Doublier A, Farlay D, Ste-Marie LG, Meunier PJ, Delmas PD. The role of mineralization and organic matrix in the microhardness of bone tissue from controls and osteoporotic patients. Bone. 2008;43:532–538. doi: 10.1016/j.bone.2008.05.024. [DOI] [PubMed] [Google Scholar]
- Caner C, Vergano PJ, Wiles JL. Chitosan film mechanical and permeation properties as affected by acid, plasticizer, and storage. J Food Sci. 1998;63:1049–1053. [Google Scholar]
- Chandler H. Hardness Testing. 2. ASM International; 1999. [Google Scholar]
- Co CC, Wang YC, Ho CC. Biocompatible micropatterning of two different cell types. J Am Chem Soc. 2005;127:1598–1599. doi: 10.1021/ja044382a. [DOI] [PubMed] [Google Scholar]
- Desai KG, Park HJ. Effect of manufacturing paprameters on the characteristics of vitamin C encapsulated tripolyphosphate-chitosan microspheres prepared by spray-drying. J Microencapsul. 2006;23:91–103. doi: 10.1080/02652040500435436. [DOI] [PubMed] [Google Scholar]
- Domke J, Radmacher M. Measuring the elastic properties of thin polymer films with the atomic force microscope. Langmuir. 1998;14:3320–3325. [Google Scholar]
- Donnelly E, Baker SP, Boskey SL, van der Meulen SCH. effects of surface roughness and maximum load on mechanical properties of cancellous bone measured by nanoindentation. J Biomed Mater Res Part A. 2005;77:426–435. doi: 10.1002/jbm.a.30633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitriu S. Polymeric Biomaterials. 2. Marcel Dekker; New York: 2002. pp. 193–198. [Google Scholar]
- El-hefian EA, Misran M, Yahaya AH. Surface investigation of chitosan film with fatty acid monolayers. Maejo Int J Sci Technol. 2009;3:277–286. [Google Scholar]
- Florin EL, Moy VT, Gaub HE. Adhesion forces between individual ligand-receptor pairs. Science. 1994;264:415–417. doi: 10.1126/science.8153628. [DOI] [PubMed] [Google Scholar]
- Goosen MFA. Application of Chitin and Chitosan. Technomic; Pennsylvania: 1997. [Google Scholar]
- Jayasuriya AC, Bhat A. Optimization of scaled-up chitosan microparticles for bone regeneration. Biomed Mater. 2009;4:1–8. doi: 10.1088/1748-6041/4/5/055006. [DOI] [PubMed] [Google Scholar]
- Jayasuriya CA, Bhat A. Fabrication and characterization of novel hybrid organic/inorganic microparticles to apply in bone regeneration. J Biomed Mater Res Part A. 2010;92:1280–1288. doi: 10.1002/jbm.a.32623. [DOI] [PubMed] [Google Scholar]
- Khor E, Lim LY. Implantable applications of chitin and chitosan. Biomaterials. 2003;24:2339–2349. doi: 10.1016/s0142-9612(03)00026-7. [DOI] [PubMed] [Google Scholar]
- Kim SE, Park JH, Cho YW, Chung H, Jeong SY, Lee EB, Kwon IC. J Control Release. 2003;91:365–374. doi: 10.1016/s0168-3659(03)00274-8. [DOI] [PubMed] [Google Scholar]
- Liu H, Webster TJ. Mechanical properties of dispersed ceramic nanoparticles in polymer composites for orthopedic applications. Int J Nanomed. 2010;5:299–313. doi: 10.2147/ijn.s9882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low IM. Effects of load and time on the hardness of a viscoelastic polymer. Mater Res Bull. 1998;33:1753–1758. [Google Scholar]
- Ludovic R, Engler AJ, Discher DE, Picart C. Elasticity of native and cross-linked polyelectrolyte multilayer films. Biomacromolecules. 2004;5:1908–1916. doi: 10.1021/bm0498023. [DOI] [PubMed] [Google Scholar]
- Martino AD, Sittingerc M, Risbud MV. A versatile biopolymer for orthopaedic tissue—engineering. Biomaterials. 2005;26:5983–5990. doi: 10.1016/j.biomaterials.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Modrzejewska Z, Stawczyk J, Matyka K, Matyka M, Mroz I, Ciszewski A. Surface microstructure of Chitosan membranes—AFM investigations. Pol J Environ Stud. 2006;4:84–87. [Google Scholar]
- Nettles DN, Elder SH, Gilbert JA. Potential use of chitosan as a cell scaffold material for cartilage tissue engineering. Tissue Eng. 2002;8:1009–1016. doi: 10.1089/107632702320934100. [DOI] [PubMed] [Google Scholar]
- Nowicki M, Richter A, Wolf B, Kaczmarek H. Nanoscale mechanical properties of polymers irradiated by UV. Int J Sci Technol Polym. 2003;44:6599–6606. [Google Scholar]
- Oliver WC, Pharr GM. An improved technique for determining hardness and elastic modulus using load and displacement sensing indemtation experiment. J Mater Res. 1992;7:1564–1583. [Google Scholar]
- Qian L, Li M, Zhou Z, Yang H, Shi X. Comparison of nano-indentation hardness to microhardness. Surf Coat Technol. 2005;195:264–271. [Google Scholar]
- Ray SS, Okamoto M. Polymer/layered silicate nanocomposites: a review from preparation to processing. Prog Polym Sci. 2003;28:1539–1641. [Google Scholar]
- Raynaud C, Sommer F, Quet C, El Bounia N, Duc TM. Quantitative determination of young’s modulus on a biphase polymer system using atomic force microscopy. Surf Interface Anal. 2000;30:185–189. [Google Scholar]
- Rho JY, Tsui TY, Pharr GM. Elastic properties of human cortical and trabecular lamellar bone measured by nanoindentation. J Biomater. 1997;97:1325–1330. doi: 10.1016/s0142-9612(97)00073-2. [DOI] [PubMed] [Google Scholar]
- Ruiz-Hitzky E. Functionalizing inorganic solids: towards organic–inorganic nanostructured materials for intelligent and bio-inspired systems. Chem Rec. 2003;3:88–100. doi: 10.1002/tcr.10054. [DOI] [PubMed] [Google Scholar]
- Stulzer HK, Tagliari MP, Parize AL, Silva MAS, Laranjeira MCM. Evaluation of cross-linked chitosan microparticles containing acyclovir obtained by spray-drying. Mater Sci Eng C. 2009;29:387–392. [Google Scholar]
- Swadener JG, George EP, Pharr GM. The correlation of the indentation size effect measured with indenters of various shapes. J Mech Phys Solids. 2002;50:681–694. [Google Scholar]
- Tao NJ, Lindsay NM, Lees S. Measuring the microelastic properties of biological material. Biophys J. 1992;63:1165–1169. doi: 10.1016/S0006-3495(92)81692-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Meerbeek B, Willems G, Celis JP, Roos JR, Braem M, Lambrechts P, Vanherle G. Assessment by nano-indentation of the hardness and elasticity of the resin–dentin bonding area. J Dent Res. 1993;72:1434–1442. doi: 10.1177/00220345930720101401. [DOI] [PubMed] [Google Scholar]
- Wang SF, Shen L, Tong YJ, Chen L, Phang IY, Lim PQ, Liu TX. Biopolymer chitosan/montmorillonite nanocomposites: preparation and characterization. Polym Degrad Stab. 2005;90:123–131. [Google Scholar]
- Yi HM, Wu LQ, Bentley WE, Ghodssi R, Rubloff GW, Culver JN, Payne GF. Biofabrication with Chitosan. Biomacromolecules. 2005;6:2881–2894. doi: 10.1021/bm050410l. [DOI] [PubMed] [Google Scholar]