Abstract
Objective
Our objective is to determine in patients with preclinical diastolic dysfunction (PDD), if there is development of tachyphylaxis to enhancement of cardiorenal response to acute volume loading (AVL) with B-type natriuretic peptide (BNP) after 12-week, twice-daily subcutaneous BNP administration.
Background
PDD is characterized by normal systolic function, moderate or severe diastolic dysfunction but no symptoms of heart failure (HF). Impairment in cardiorenal endocrine response to stress by AVL exists in PDD and is corrected by acute administration of subcutaneous BNP.
Methods
Double-blinded, placebo controlled proof-of-concept study to compare 12 weeks of twice daily subcutaneous BNP 10 ug/kg (n=24) versus placebo (n=12) in PDD. Subjects underwent two study visits: baseline and after 12 weeks. At each study visit, echocardiography, renal, and neurohumoral assessments were performed before and after intravascular AVL.
Results
Among those with PDD, there was a statistically significant improvement in diastolic function after 12 weeks of BNP as measured by a decrease in Doppler E/e’ ratio (p = 0.004) and improvement of diastolic dysfunction grade (p = 0.008). After 12 weeks there was a statistically significantly greater sodium excretion, urine flow, and urinary cGMP excretion to AVL (all p < 0.001), as well as a trend towards greater glomerular filtration rate (p = 0.050) in the BNP group as compared to the placebo group.
Conclusion
In subjects with PDD, chronic BNP administration resulted in sustained improvement in diastolic function without development of tachyphylaxis to the enhancement of cardiorenal response to volume expansion with BNP.
Keywords: B-type natriuretic peptide, Heart failure with preserved ejection fraction, Diastolic dysfunction, Cardio-renal syndrome
INTRODUCTION
Heart failure (HF) is a prevalent condition affecting over 5 million Americans, and is projected to increase as the population of the United States continues to age.(1) Among those with heart failure, approximately half have preserved ejection fraction.(2) Despite significant advances in management of heart failure with reduced ejection fraction over the past decade, evidence-based therapeutic options in heart failure with preserved ejection fraction remain limited.
Preclinical Diastolic Dysfunction (PDD) is defined as subjects with normal systolic function, moderate or severe diastolic dysfunction determined by Doppler criteria but no symptoms of heart failure (HF).(3) PDD falls within stage B HF as defined by the American College of Cardiology / American Heart Association criteria of those without heart failure symptoms yet have structural heart disease. PDD is very prevalent, estimated to afflict 20-30% of the general adult population.(3-5) There is known progression of PDD to symptomatic heart failure, with an estimated annual incidence of 2%.(3) Furthermore, the three-year cardiac hospitalization and mortality for those with PDD exceeds 17% and 10%, respectively.(6)
Natriuretic peptides are secreted by the heart in response to volume expansion and cardiac distension, and have multiple important cardiac and renal properties. B-type natriuretic peptide (BNP)’s beneficial cardiac effects include anti-hypertrophic, anti-fibrotic, and lusitropic effects.(7,8) Natriuretic peptides are important in cardiorenal neurohormonal regulation and homeostasis.(9)
We have previously demonstrated that in those with PDD, there is impairment in cardiorenal neurohormonal response to volume expansion, and a subsequent lack in natriuretic response.(10) The pathophysiology is due to impairment in renal cyclic GMP activation following AVL, which is rescued with acute subcutaneous (SQ) BNP administration.(10) The burden of PDD coupled with the lack of therapeutic interventions available for the prevention of heart failure leads to the search for novel therapies that may rescue the impaired cardiorenal physiology which may contribute to the progression to symptomatic heart failure. Previous demonstration that acute SQ BNP administration improves cardiorenal response to AVL suggests a potential therapeutic option among those with PDD, but chronic peptide administration may lead to the development of pharmacologic tolerance or tachyphylaxis.(11,12)
The objective of the current study is to determine in patients with PDD, if there is development of tachyphylaxis to enhancement of cardiorenal response to AVL with BNP after 12-week, twice-daily subcutaneous administration of BNP. We hypothesize that chronic subcutaneous BNP administration for 12 weeks will lead to a sustained improvement of ventricular diastolic function without evidence of development of tachyphylaxis to the enhanced cardiorenal response with BNP following acute volume expansion in subjects with PDD.
METHODS
Study Design
This proof of concept study was designed to determine safety, efficacy, and assess if there is development of tachyphylaxis to long term BNP therapy in PDD. The study is a randomized, double-blinded and placebo controlled study designed to compare, in subjects with PDD, cardiorenal response to AVL (0.9% normal saline, 0.25 mL/kg/min for 1 hour) between those that have received 12 weeks of twice daily SQ BNP versus placebo. The study was approved by the Mayo Foundation Institutional Review Board and performed at the Center for Clinical and Translational Science. ClinicalTrials.gov Identifier: NCT00405548
Participants
Subjects with PDD (n=49) were recruited between March 2008 and August 2012 at Mayo Clinic, Rochester, MN, identified based on an echocardiographic database. Preclinical diastolic dysfunction was defined as subjects with normal systolic function (EF > 50%), moderate or severe diastolic dysfunction determined by Doppler criteria, and no symptoms and diagnosis of heart failure. Inclusion criteria included adults aged 18 years or greater with an ejection fraction greater than 50% and moderate or severe diastolic dysfunction as identified by Doppler echocardiography. The subjects must also not have any signs, symptoms, prior diagnosis of or hospitalization for congestive heart failure. The subjects’ medical records were extensively reviewed for any prior diagnosis of HF, previous heart failure hospitalization, or symptomatic volume overload. Subjects must have a 6-minute walk distance of greater than 450 meters. Subjects who had a 6-minute walk distance < 450 m due to an orthopedic limitation but no dyspnea or fatigue as assessed by the investigators were included in the study. All subjects who were on cardiovascular medications must be on stable doses for at least two weeks. Informed consent was obtained and documented for all participants.
Exclusion criteria include a myocardial infarction within 3 months, unstable angina within 14 days, or any evidence of myocardial ischemia. Additional exclusion criteria included significant valvular stenosis, hypertrophic, restrictive or obstructive cardiomyopathy, constrictive pericarditis, primary pulmonary hypertension, biopsy proven active myocarditis, severe congenital heart diseases, sustained ventricular tachycardia or ventricular fibrillation within 14 days, second or third degree heart block without a permanent cardiac pacemaker, stroke within 3 months of screening, or evidence of significantly compromised CNS perfusion. Laboratory exclusion criteria included total bilirubin of > 1.5 mg/dL, other liver enzymes > 1.5 times the upper limit of normal, serum creatinine of > 3.0 mg/dL, serum sodium < 125 mEq/dL or > 160 mEq/dL, serum potassium < 3.5 mEq/dL or > 5.0/dL, serum digoxin level > 2.0 ng/ml, systolic pressure < 85 mm Hg, or hemoglobin < 10 gm/dl.
Echocardiographic Assessment
Echocardiography was performed according to the guidelines of the American Society of Echocardiography.(13) Diastolic dysfunction was classified as grade 1 (impaired relaxation, E/A≤0.75, E/e’<10), grade 1a (impaired relaxation, E/A ≤ 0.75, E/e’≥10), grade 2 (pseudonormal, 0.75<E/A<1.5, DT>140ms and PV S/D≥1 or E/e’≥10), or grade 3/4 (restrictive, E/A>1.5, DT<140ms and PV S/D≥1 or E/e’≥10).(4) E is early mitral inflow velocity, A is atrial component of mitral inflow velocity, e’ is mitral annulus early diastolic motion, DT is deceleration time, PV S/D is pulmonic vein systolic flow to diastolic flow ratio. Right ventricular systolic pressure (RVSP) was also collected.
Study Protocol
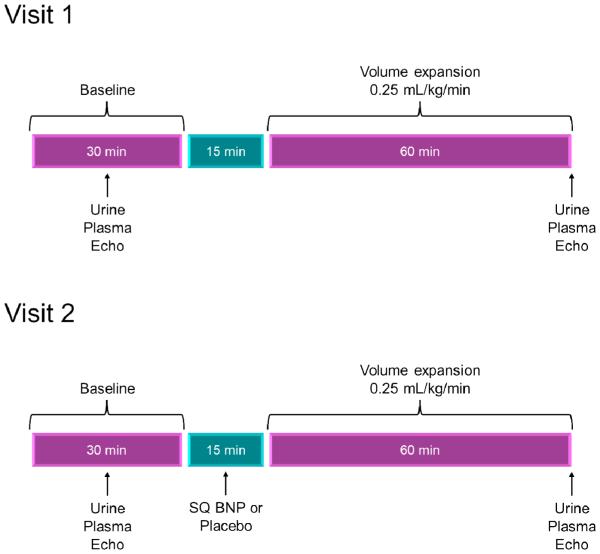
Visit 1
Each subject had two study visits: visit 1 at the beginning of the study, and visit 2 after 12 weeks of subcutaneous BNP or placebo. At visit 1, subjects would report to the Center for Clinical and Translational Science at 7:00 am after one week of salt restricted (120 mEq/day) diet. Subjects were given their usual morning medications; they were subsequently placed in a supine position for 60 minutes, with spontaneous bladder emptying every 30 minutes. Subjects who were unable to spontaneously void every 30 minutes were given a urinary catheter, and bladder emptying was determined by bladder ultrasound. Subjects drank water equivalent to the sum of blood and urine losses every 30 minutes. Baseline Doppler echocardiography was performed, followed by a 30 minute clearance period which included neurohormonal measurements, renal studies and urinary collection, including glomerular filtration rate, urine flow, urinary sodium excretion, and urinary cGMP excretion. These measurements were followed by 60 minutes of volume expansion (normal saline, 0.25 mL/kg/min) and a subsequent repeat in echocardiographic measurements, urine collection and blood sampling.
Subjects were randomized in a 2:1 fashion to the subcutaneous (SQ) BNP group or the placebo group. Randomization was provided by the Mayo Clinic Division of Biomedical Statistics and Informatics in conjunction with the Mayo Clinic Pharmacy, and the investigators were blinded to which therapy the subjects received. The subjects, caregivers, and investigators were blinded during the trial and had no knowledge of whether the contents of the vial used for subcutaneous injections, as dispensed by pharmacy, contained placebo or BNP. Those in the SQ BNP group self-administered subcutaneous BNP at 10 micrograms per kilogram twice daily for 12 weeks (Scios Inc., Mountain View, CA). Those in the control group self-administered subcutaneous placebo twice daily for 12 weeks. Subjects were given instructions for the proper abdominal wall subcutaneous administration technique. The first and second doses were self-administered but under supervision, and monitored for 12 hours. If hypotension occurred (systolic BP < 85 mmHg) after the first dose, the second dose was decreased by half. Subjects were discharged from the clinical research unit 12 hours after the second dose with 12 weeks of supplies. Subjects had to be stable on their medication therapy at least 3 weeks prior to enrollment, and during the trial, subjects were highly encouraged not to change their other medications. There were no significant changes in the background medical therapy during the 12 weeks of the trial in both the BNP and placebo groups.
Visit 2
Subjects returned for study visit 2 after 12 weeks. In a protocol identical to study visit 1, the subjects had repeat echocardiographic, plasma, and renal clearance studies. They received their last subcutaneous BNP or placebo dose, then received further measurements after volume expansion (Figure 2).
Figure 2.
Methods and Visit Flowchart
Safety data were collected, including identification of development of heart failure symptoms, gastrointestinal side effects, hypersensitivity reaction at the injection site, joint pains, dyslipidemia, and hypotension with blood pressure monitoring.
Neurohormonal, Electrolyte, and Renal Assessment
Plasma and urine cyclic GMP (cGMP) were measured by radioimmunoassay. Plasma BNP was measured by fluorescence immunoassay.
For measurement of renal parameters, intravenous catheters were placed in each arm of the subjects. Continuous iothalamate was infused to achieve a plasma concentration of 15 to 20 mg/L. After 60 minutes of equilibrium, urine and blood was collected for determination of GFR. Measurements were made by the Mayo Clinic Core Renal laboratory.
Statistical Methods
Sample size calculation: Based on our previous study on the acute cardiorenal and humoral effects of acute SQ BNP in PDD, the sample size was calculated by construction of the magnitude of difference that could be detected for sodium excretion and urine flow in response to volumes expansion.(10) It was assumed that the standard deviation in the untreated group would be only ½ of that in the treated group, since the untreated group response data is largely due to biological and measurement variability between 2 occasions, with little or no actual signal. Hence, subjects were randomized in a 2 to 1 fashion to treatment versus placebo to optimize the precision of the group comparison. Power calculations were done with these assumptions. With a total of 40 subjects, the below values give the detectable change (with 85% power) between group mean changes, both as a raw number and as a percent of baseline mean.
For sodium excretion, the detectable change is 131 μEq/min and the detectable percent change is +100%. For urine flow, the detectable change is 1.89 ml/min and the detectable percent change is +54%. Continuous variables are presented as mean ± standard deviation and categorical variables as percentage. Comparisons between the two treatment groups (SQ BNP and SQ placebo) were made by t-test for normally distributed continuous variables, the rank-sum test for continuous variables with a skewed distribution, and the Chi-square test for independence for categorical variables. Comparisons within groups (between visit 1 and visit 2) were made using a paired t-test. For all analyses, statistical significance was accepted as P<0.05.
RESULTS
Patient population
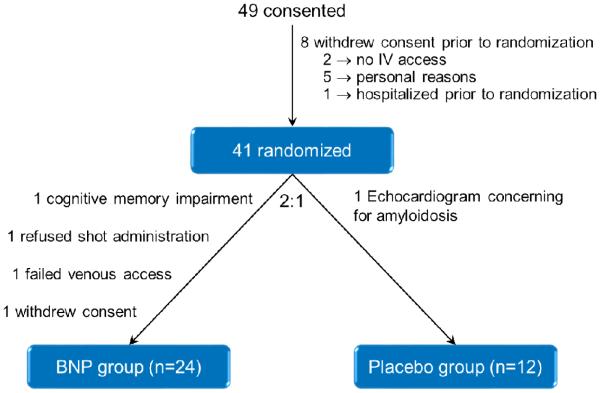
There were 49 subjects who consented to the study (Figure 1). 8 subjects withdrew consent prior to randomization due to lack of IV access, hospitalization prior to randomization, or for personal reasons. 41 subjects were randomized in a 2 to 1 fashion to the BNP group and the placebo group. 4 subjects withdrew from the BNP group due to existing cognitive impairment, failed venous access, refusal of subcutaneous shot administration, and withdrawal of consent. 1 subject was withdrawn from the placebo group due to echocardiographic findings concerning for amyloidosis. Hence in the final analysis there were n = 24 subjects in the BNP group and n = 12 subjects in the placebo group.
Figure 1.
Consort Diagram
The baseline characteristics shown in Table 1 demonstrate that age, gender, and comorbidities were similar between the BNP and placebo groups. Echocardiographic and neurohormonal measurements including left atrial volume index and left ventricular filling pressures were also similar between the two groups. The mean left atrial volume indices were 35.9 ± 7.4 mL/m2 and 33.2 ± 8.5 mL/m2 in the BNP and placebo groups respectively. The mean E/e’ ratios, representing left ventricular filling pressure, were 14.9 ± 4.1 and 14.9 ± 4.5 in the BNP and placebo groups respectively.
Table 1.
Baseline Characteristics
| Variable | BNP (n=24) | Placebo (n=12) |
|---|---|---|
| Age in years, mean ±SD | 68.8±8.1 | 69.6±10.9 |
| Female, no. (%) | 9 (38%) | 7 (58%) |
| BMI (kg/m2), mean±SD | 33.5±6.6 | 30.8±7.3 |
| Heart rate (BPM), mean±SD | 61.7±9.5 | 63.1±13.0 |
| Blood pressure SBP (mmHg), mean±SD DBP (mmHg), mean±SD |
134.0±14.5 75.9±9.0 |
132.5±14.3 76.0±7.5 |
| Hypertension, no. (%) | 18 (75%) | 8 (67%) |
| Creatinine (mg/dL), mean±SD | 1.0±0.2 | 0.9±0.3 |
| BUN (mg/dL), mean±SD | 20.2±5.2 | 20.0±7.5 |
| Hyperlipidemia, no. (%) | 18 (75%) | 10 (91%) |
| Diabetes mellitus, no. (%) | 9 (38%) | 3 (27%) |
| Coronary artery disease, no. (%) | 12 (52%) | 9 (75%) |
| Myocardial infarction, no. (%) | 5 (22%) | 3 (25%) |
| Cardiovascular medications ACEI or ARB, no. (%) Beta blocker, no. (%) Thiazide diuretic, no. (%) Loop diuretic, no. (%) Statin, no. (%) |
16 (67%) 10 (42%) 6 (25%) 3 (13%) 18 (75%) |
7 (58%) 6 (50%) 2 (17%) 0 (0%) 9 (75%) |
| LVEF (%), mean±SD | 62.6±4.9 | 62.3±4.4 |
| LA volume index (mL/m2), mean±SD |
35.9±7.4 | 33.2±8.5 |
| E/e′, mean±SD | 14.9±4.1 | 14.9±4.5 |
| RV pressure (mm Hg), mean±SD | 32.4±6.0 | 30.1±12.1 |
There were no statistically significant differences between the 2 groups. Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure; BUN, blood urea nitrogen; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; LVEF, left ventricular ejection fraction; LA, left atrium; RV, right ventricle
Effects of Chronic SQ BNP on LV Diastolic Function
After 12 weeks, there was a statistically significant reduction in E/e’ in the BNP group (14.9±4.1 to 12.6±3.5, p = 0.004) while it remained unchanged in the placebo group (14.9±4.5 to 14.0±5.1, p = 0.43). Of the 24 subjects in the BNP group, 17 had reduction in E/e’, 2 had no change, and 5 had increases in E/e’ over the 12 week period. The average change in E/e’ was −2.4 with standard deviation 3.7. Of the 12 subjects in the placebo group, 6 had reduction in E/e’, 3 had no change, and 3 had increases in E/e’ over the 12 week period. The average change in E/e’ was −0.9 with standard deviation 3.8. Furthermore, after 12 weeks, there was also improvement in the diastolic grade in the BNP group (38% with improvement in diastolic grade as assessed by Doppler echocardiography, p = 0.008) while it remained unchanged in the placebo group (8% with improvement, p = 1.0), as shown in Figure 3.
Figure 3.
E/e’ (a) and Diastolic Grade (b) at study entry and after 12 weeks
a. E/e’ From Visit 1 to Visit 2
b. Diastolic Grade From Visit 1 to Visit 2
Cardiorenal Response to Acute Volume Expansion
Data was collected on sodium excretion, urine flow, glomerular filtration rate, and urinary cGMP excretion both before and after volume expansion at both visit 1 and visit 2, as shown in Figure 4.
Figure 4.
Sodium excretion (a), urine flow (b), glomerular filtration rate (c), and urinary cGMP excretion (d)
a. Sodium Excretion Response
b. Urine Flow Response
c. Glomerular Filtration Rate Response
d. Urinary cGMP Excretion Response
There was a statistically significantly greater sodium excretion response to volume expansion at visit 2 in the BNP group as compared to the placebo group (381.6±450.8 mEq/min versus −10.5±90.1 mEq/min, p < 0.001), whereas there was no difference in the two groups at visit 1 (p = 0.95).
There was a statistically significantly greater urine flow response to volume expansion at visit 2 in the BNP group as compared to the placebo group (4.2±5.4 mL/min versus −1.0±2.1 mL/min, p < 0.001), whereas there was no difference in the two groups at visit 1 (p = 0.92).
There was a trend towards greater glomerular filtration rate response to volume expansion at visit 2 in the BNP group as compared to the placebo group (6.8±19.3 mL/min/1.73m2 versus 4.4±27.3 mL/min/1.73m2, p = 0.050), whereas there was no difference in the two groups at visit 1 (p = 0.46).
Importantly, there was no evidence of development of tachyphylaxis to BNP as demonstrated by significant increases in both plasma cGMP and urinary cGMP excretion response following SQ BNP administration in the BNP cohort at visit 2. There was a statistically significantly greater urinary cGMP excretion response to volume expansion at visit 2 in the BNP group as compared to the placebo group (1810.1±2195.6 pmol/min versus −148.0±174.5 pmol/min, p < 0.001), whereas there was no difference in the two groups at visit 1 (p = 0.15). Similarly, plasma cGMP response to volume expansion at visit 2 in the BNP group was significantly greater as compared to the placebo group (7.3±6.5 pmol versus 0.0±0.5 pmol, p < 0.001), whereas there was no difference in the two groups at visit 1 (−0.2±1.0 pmol versus 0.2±0.7 pmol, p = 0.27).
Upon sensitivity analysis, assuming the four dropped patients in the BNP group had no changes, the results remain statistically significant for cardiorenal response and diastolic grade.
Furthermore, right ventricular systolic pressure (RVSP) was measured in both groups before and after volume expansion both at visit 1 and visit 2. Whereas there was no change in RVSP response in the placebo group from visit 1 to visit 2 (4.4±4.5 to 4.7±8.9 mmHg, p = 0.36), there was a statistically significant reduction in RVSP response in the BNP group from visit 1 to visit 2 (1.0±3.9 to −3.7±4.9 mmHg, p = 0.002).
Blood Pressure
The systolic blood pressures, diastolic blood pressures, and heart rates were similar between the BNP and placebo groups throughout the study, both at visit 1 and visit 2, and both before and after volume expansion (p > 0.05). At visit 1 before AVL, the systolic blood pressure was 133±14.9 mmHg for the BNP group and 123.5±11.9 mmHg for the placebo group. At visit 1 after AVL, the systolic blood pressure was 130.6±14.9 mmHg for the BNP group and 127.5±20.0 mmHg for the placebo group. At visit 2 before AVL, the systolic blood pressure was 127.4±11.5 mmHg for the BNP group and 128.6±17.9 mmHg for the placebo group. At visit 2 after AVL, the systolic blood pressure was 126.5±14.4 mmHg for the BNP group and 130.8±19.9 mmHg for the placebo group. (Table 2)
Table 2.
Systolic and Diastolic Blood Pressures and Heart Rate
| 2a: Systolic Blood Pressure (SBP) |
| BNP Group SBP, mean, mmHg (n=24) |
Placebo Group SBP, mean, mmHg (n=12) |
P | |
|---|---|---|---|
| Visit 1 Before AVL | 133±14.9 | 123.5±11.9 | 0.06 |
| Visit 1 After AVL | 130.6±14.9 | 127.5±20.0 | 0.60 |
| Visit 2 Before AVL | 127.4±11.5 | 128.6±17.9 | 0.81 |
| Visit 2 After AVL | 126.5±14.4 | 130.8±19.9 | 0.49 |
| 2b: Diastolic Blood Pressure (DBP) |
| BNP Group DBP, mean, mmHg (n=24) |
Placebo Group DBP, mean, mmHg (n=12) |
P | |
|---|---|---|---|
| Visit 1 Before AVL | 67.1±9.9 | 62.2±10.4 | 0.18 |
| Visit 1 After AVL | 65.3±10.4 | 62.3±9.1 | 0.41 |
| Visit 2 Before AVL | 65.4±9.0 | 62.4±10.6 | 0.39 |
| Visit 2 After AVL | 64.6±12.5 | 66.7±10.3 | 0.63 |
| 2c: Heart Rate (HR) |
| BNP Group HR, mean, beats/min (n=24) |
Placebo Group HR, mean, beats/min (n=12) |
P | |
|---|---|---|---|
| Visit 1 Before AVL | 58.9±8.9 | 62.8±14.7 | 0.33 |
| Visit 1 After AVL | 61.9±12.3 | 59.5±14.5 | 0.62 |
| Visit 2 Before AVL | 60.5±9.5 | 58.1±11.4 | 0.51 |
| Visit 2 After AVL | 60.8±12.7 | 59.3±6.7 | 0.74 |
Adverse Events
Adverse events, which included hypotension, diarrhea, symptoms of heart failure, hyperglycemia, joint pains, and rash, were similar between the two groups, as shown in Table 3. There were 2 subjects in the BNP group and 2 subjects in the placebo group that developed hypotension, requiring reduction in subsequent subcutaneous injections (p = 0.45).
Table 3.
Adverse Events
| Variable | Overall (n=36) |
BNP (n=24) |
Placebo (n=12) |
P |
|---|---|---|---|---|
| Decrease dose of SQ injection |
4 (11%) | 2 (8%) | 2 (17%) | 0.45 |
| Diarrhea | 1 (3%) | 1 (4%) | 0 | 0.47 |
| Heart failure symptoms |
1 (3%) | 0 | 1 (8%) | 0.15 |
| Hyperglycemia | 1 (3%) | 1 (4%) | 0 | 0.47 |
| Hypotension | 4 (11%) | 2 (8%) | 2 (17%) | 0.45 |
| Joint pains | 2 (6%) | 2 (8%) | 0 | 0.30 |
| Rash | 1 (3%) | 1 (4%) | 0 | 0.47 |
DISCUSSION
This proof-of-concept study is the first investigation of the benefits of chronic administration of SQ BNP among those with preclinical diastolic dysfunction. The results suggest 12 weeks of chronic SQ BNP administration resulted in significant improvement in both left ventricular filling pressures and diastolic function as evident in the change in Doppler E/e’ ratio and diastolic dysfunction grade without tachyphylaxis or development of tolerance to the enhancement of cardiorenal response to acute volume loading with BNP administration. Further studies are warranted to determine if these physiologic observations with chronic natriuretic peptide system augmentation can be translated into a delay in the progression of PDD (stage B HF) to symptomatic HF with preserved EF (stage C HF).
The beneficial effects of chronic BNP on diastolic function, as demonstrated by reduction in diastolic filling pressures and improvement in diastolic grade, are a result of the pro-lusitropic properties of the cGMP system. In-vitro studies have shown that cardiomyocytes in response to exposure to BNP have greater relaxation and cell length.(14) In canine studies, BNP has been demonstrated to enhance left ventricular relaxation.(7) When infused in humans with HFpEF, BNP, with its action on natriuretic peptide receptor A (NPR-A) and cyclic GMP system, has hemodynamic enhancement and beneficial neurohormonal effects in response to exercise.(15) The left atrial filling pressures, as measured by the pulmonary capillary wedge pressure, demonstrated an attenuated increase during exercise among those with diastolic heart failure who received BNP compared to placebo.
The important role of the cGMP system is also highlighted by the recent studies with neprilysin inhibition, which results in reduction of natriuretic peptide degradation. The PARAMOUNT study showed that LCZ696, an angiotensin receptor neprilysin inhibitor, when administered over 12 weeks to subjects with HFpEF, was well tolerated and resulted in reduction in NT-proBNP levels as well as reduction in left atrial volumes.(16) Furthermore, there was also renal preservation with chronic LCZ696 use when compared to angiotensin receptor blocker alone in those with HFpEF.(17) The PARAGON-HF study, a phase 3 HFpEF trial designed to evaluate the efficacy and safety of LCZ696, is currently ongoing with estimated enrollment of over 4000 subjects (NCT01920711).
While we have previously shown that the acute administration of BNP results in short term benefits on cardiorenal response to acute volume loading in PDD, the development of tachyphylaxis or tolerance over time to chronic BNP administration remains a major concern.(10) Sustained administration without evidence of pharmacologic tolerance is necessary for novel peptide therapy for chronic heart failure prevention. Receptor downregulation after continued exposure to a peptide is one potential mechanism of tolerance induction. Tachyphylaxis remains a concern for peptide therapy, such as with the incretin hormone glucagon-like peptide 1 (GLP-1).(11,12) The current study suggests that there is not a pharmacologic tolerance as there is a sustained activation of its second messenger cGMP and improvement in cardiorenal response to acute volume loading with BNP after 12 weeks of chronic administration.
The natural history of PDD suggests progression to symptomatic heart failure at an annual incidence of 2 percent, which increases to 12 percent at 3 years.(6) Importantly, renal dysfunction was independently associated with increased risk of progression to symptomatic heart failure, as those with GFR<60 mL/min/1.73m2 had a hazard ratio of 2 for heart failure development, compared to those with normal renal function.(6) Furthermore, PDD impairment of renal response to AVL may contribute to the progression of HF.(10) Therefore, these observations suggest that preserving renal function might attenuate the development and progression of symptomatic heart failure.
In addition, targeting diastolic dysfunction is also essential in prevention of heart failure progression, as worsening diastolic dysfunction has been shown to be associated with increased HF development.(18) Kane et al demonstrated that over a six-year period, those with normal, mild, and moderate to severe diastolic function had incidence of progression to symptomatic HF of 3%, 8%, and 12% respectively.(18) Preclinical studies have demonstrated that improvement in cardiorenal parameters is important in prevention of cardiac apoptosis, fibrosis and diastolic dysfunction progression.(19) In a rodent model of renal insufficiency, Martin et al showed that impaired renal function results in neurohormonal alterations that results in cardiac remodeling and hypertrophy with clinical outcome implications, by induction of transforming growth factor beta, apoptotic, inflammatory, and fibrotic pathways.(19) The findings of the current study, which include improvement of LV diastolic function and the preservation of the enhanced response to AVL with chronic BNP therapy, suggest the potential of chronic subcutaneous BNP as a novel therapeutic agent for improvement in diastolic function and cardiorenal parameters, and subsequent prevention of heart failure development.
Study Limitations
While the study protocol randomized subjects in a two to one fashion to the BNP and placebo groups to maximize power, one limitation of the current study is the small study population. After randomization, there were four subjects that dropped out of the BNP group prior to study protocol initiation. Additionally, there are limitations regarding multiple testing, so these factors remain a limitation in this small study population. While there was no significant reduction in blood pressure from visit 1 to visit 2, the lack of invasive vital sign measurements remains a limitation of this study, as minor changes in systolic blood pressure could have contributed to the observed changes in E/e’.
While the 12-week duration of this study is the first of its kind in analyzing the chronic effects and possibility of pharmacologic tolerance or lack thereof from BNP administration, even longer study durations are necessary to assess for the effects of chronic BNP administration. Further studies are also necessary to characterize whether or not the observed beneficial echocardiographic and neurohormonal improvements in the BNP treated group can translate to actual delay in progression to the development of symptomatic heart failure in this population of preclinical diastolic dysfunction.
CONCLUSIONS
In subjects with PDD, 12 weeks of chronic BNP administration resulted in sustained improvement in diastolic function without development of tachyphylaxis to the enhancement of cardiorenal response to volume expansion with BNP. Further studies are warranted to determine if these physiologic observations with chronic natriuretic peptide system augmentation can be translated into a delay in the progression of PDD (stage B HF) to symptomatic HF with preserved EF (stage C HF).
CLINICAL PERSPECTIVES.
Competency in Medical Knowledge
In patients with PDD, chronic BNP administration results in improvement in diastolic function and enhancement of cardiorenal response to volume expansion. Further studies are necessary to determine if there is correlation to disease progression, morbidity, and mortality outcomes.
Translational Outlook
Further large, prospective, randomized, and long-term studies are needed to investigate the utility of chronic low dose BNP for the prevention of symptomatic heart failure development.
Acknowledgments
Financial disclosures:
This research was supported by grants from the National Institutes of Health: PO1 HL 76611, R01 HL 84155, and NIH/NCRR CTSA Grant Number UL1 RR024150. Scios Inc supplied the study drug. Mayo Clinic, Drs. Burnett and Chen have patented and licensed designer natriuretic peptides. Drs. Burnett and Chen are the Co-founders of Zumbro Discovery.
Grant support:
This research was supported by grants from the National Institutes of Health: PO1 HL 76611, R01 HL 84155, and NIH/NCRR CTSA Grant Number UL1 RR024150. This research supported by grants from the National Institutes of Health PO1 HL-76611, R01 HL-84155 and Mayo Foundation.
ABBREVIATIONS
- AVL
Acute volume loading
- BNP
B-type natriuretic peptide
- cGMP
Cyclic guanosine monophosphate
- CNS
Central nervous system
- DT
Deceleration time
- E
Early mitral inflow velocity
- e'
Mitral annulus early diastolic motion
- EF
Ejection fraction
- GFR
Glomerular filtration rate
- GLP
Glucagon-like peptide
- HF
Heart failure
- HFpEF
Heart failure with preserved ejection fraction
- NPR
Natriuretic peptide receptor
- PDD
Preclinical diastolic dysfunction
- RVSP
Right ventricular systolic pressure
- SQ
Subcutaneous
Footnotes
Clinical trial identifier: ClinicalTrials.gov NCT00405548
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bursi F, Weston SA, Redfield MM, et al. Systolic and diastolic heart failure in the community. JAMA. 2006;296:2209–16. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 3.Wan SH, Vogel MW, Chen HH. Pre-clinical diastolic dysfunction. Journal of the American College of Cardiology. 2014;63:407–16. doi: 10.1016/j.jacc.2013.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Redfield MM, Jacobsen SJ, Burnett JC, Jr., Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 5.Abhayaratna WP, Marwick TH, Smith WT, Becker NG. Characteristics of left ventricular diastolic dysfunction in the community: an echocardiographic survey. Heart. 2006;92:1259–64. doi: 10.1136/hrt.2005.080150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vogel MW, Slusser JP, Hodge DO, Chen HH. The natural history of preclinical diastolic dysfunction: a population-based study. Circ Heart Fail. 2012;5:144–51. doi: 10.1161/CIRCHEARTFAILURE.110.959668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lainchbury JG, Burnett JC, Jr., Meyer D, Redfield MM. Effects of natriuretic peptides on load and myocardial function in normal and heart failure dogs. American journal of physiology. Heart and circulatory physiology. 2000;278:H33–40. doi: 10.1152/ajpheart.2000.278.1.H33. [DOI] [PubMed] [Google Scholar]
- 8.Huntley BK, Sandberg SM, Noser JA, et al. BNP-induced activation of cGMP in human cardiac fibroblasts: interactions with fibronectin and natriuretic peptide receptors. Journal of cellular physiology. 2006;209:943–9. doi: 10.1002/jcp.20793. [DOI] [PubMed] [Google Scholar]
- 9.Stevens TL, Burnett JC, Jr., Kinoshita M, Matsuda Y, Redfield MM. A functional role for endogenous atrial natriuretic peptide in a canine model of early left ventricular dysfunction. The Journal of clinical investigation. 1995;95:1101–8. doi: 10.1172/JCI117757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKie PM, Schirger JA, Costello-Boerrigter LC, et al. Impaired natriuretic and renal endocrine response to acute volume expansion in pre-clinical systolic and diastolic dysfunction. J Am Coll Cardiol. 2011;58:2095–103. doi: 10.1016/j.jacc.2011.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meguro S, Kawai T, Matsuhashi T, et al. Basal-Supported Oral Therapy with Sitagliptin Counteracts Rebound Hyperglycemia Caused by GLP-1 Tachyphylaxis. International journal of endocrinology. 2014;2014:927317. doi: 10.1155/2014/927317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nauck MA, Kemmeries G, Holst JJ, Meier JJ. Rapid tachyphylaxis of the glucagon-like peptide 1-induced deceleration of gastric emptying in humans. Diabetes. 2011;60:1561–5. doi: 10.2337/db10-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Picard MH, Adams D, Bierig SM, et al. American Society of Echocardiography recommendations for quality echocardiography laboratory operations. J Am Soc Echocardiogr. 2011;24:1–10. doi: 10.1016/j.echo.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Shah AM, Spurgeon HA, Sollott SJ, Talo A, Lakatta EG. 8-bromo-cGMP reduces the myofilament response to Ca2+ in intact cardiac myocytes. Circulation research. 1994;74:970–8. doi: 10.1161/01.res.74.5.970. [DOI] [PubMed] [Google Scholar]
- 15.Clarkson PB, Wheeldon NM, MacFadyen RJ, Pringle SD, MacDonald TM. Effects of brain natriuretic peptide on exercise hemodynamics and neurohormones in isolated diastolic heart failure. Circulation. 1996;93:2037–42. doi: 10.1161/01.cir.93.11.2037. [DOI] [PubMed] [Google Scholar]
- 16.Solomon SD, Zile M, Pieske B, et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet. 2012 doi: 10.1016/S0140-6736(12)61227-6. [DOI] [PubMed] [Google Scholar]
- 17.Voors AA, Gori M, Liu LC, et al. Renal effects of the angiotensin receptor neprilysin inhibitor LCZ696 in patients with heart failure and preserved ejection fraction. Eur J Heart Fail. 2015;17:510–7. doi: 10.1002/ejhf.232. [DOI] [PubMed] [Google Scholar]
- 18.Kane GC, Karon BL, Mahoney DW, et al. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA. 2011;306:856–63. doi: 10.1001/jama.2011.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin FL, McKie PM, Cataliotti A, et al. Experimental mild renal insufficiency mediates early cardiac apoptosis, fibrosis, and diastolic dysfunction: a kidney-heart connection. Am J Physiol Regul Integr Comp Physiol. 2012;302:R292–9. doi: 10.1152/ajpregu.00194.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]