Abstract
Excessive accumulation of reactive oxygen species (ROS) and chronic activation of mechanistic target of rapamycin (mTOR) complex 1 (mTORC1) are well-characterized promoters of aging and age-associated degenerative pathologies. Sestrins, a family of highly conserved stress-inducible proteins, are important negative regulators of both ROS and mTORC1 signaling pathways; however, the mechanistic basis of how Sestrins suppress these pathways remains elusive. In the past couple of years, breakthrough discoveries about Sestrin signaling and its molecular nature have deeply heightened our biochemical understanding of Sestrin function. These discoveries have also uncovered new potential therapeutic strategies that may eventually enable us to attenuate aging and age-associated diseases.
Keywords: Sestrin, aging, structure, ROS, mTOR, leucine
Sestrin, a versatile anti-aging molecule
One theory for the cause of aging is the accumulation of cellular damage, facilitated by environmental stressors that induce genotoxic or oxidative stress [1]. Although appropriate levels of reactive oxygen species (ROS) (see Glossary) are necessary for physiological homeostasis [2], excessive ROS accumulation can cause damage to DNA and proteins, thereby facilitating the aging process [1, 2]. Nutritional overabundance and obesity are also well-established promoters of aging, while appropriate dietary restriction extends longevity and healthspan in virtually all model organisms [1]. One of the major signaling components that can mediate the nutritional effect on aging is mechanistic target of rapamycin complex 1 (mTORC1). Genetic or pharmacological inhibition of mTORC1 extends longevity and healthspan in most organisms including mammals [3–5], suggesting a central role of mTORC1 in the acceleration of aging.
Sestrins are a family of highly conserved proteins that are induced upon various conditions of stress, including DNA damage and oxidative stress. Invertebrates have a single orthologue, while vertebrates have three paralogues, Sestrin1-3 (Table 1) [6]. In cultured cells, Sestrin1 and Sestrin2 reduced ROS [7] and suppressed mTORC1 activity [8], suggesting that this family of proteins may perform anti-aging functions through the inhibition of these two well-characterized aging facilitators. Indeed, genetic depletion of Sestrin in many animal models, including worms [9], flies [10] and mice [11–16], has led to the accelerated progression of diverse age- and obesity-associated pathological disorders including fat accumulation, insulin resistance, muscle degeneration, cardiac dysfunction, mitochondrial pathologies and tumorigenesis, which could be relieved by treatments that suppressed ROS or mTORC1 signaling. However, the biochemical mechanisms underlying how this small, 55kDa protein performs such versatile physiological functions and prevents the progression of diverse age-associated pathologies have remained elusive. Recently, our understanding of Sestrin protein biochemistry has dramatically improved. Major recent findings include identifying GAP activity towards Rags 2 (GATOR2) as a direct physical target of Sestrin [17–19], acquiring an in-depth understanding of the antioxidant activity of Sestrin [13, 20, 21], identifying Sestrin as an amino acid sensor [22–25], and determining the three-dimensional molecular structure of Sestrin [21, 22]. The Sestrin structure revealed three functional sites for each of its identified activities: mTOR regulation, ROS suppression and leucine binding (Figure 1, Key Figure) [21, 22]. In this review, we discuss the current findings related to Sestrin function and signaling while highlighting questions elicited by these discoveries.
Table 1.
Identified functions of mammalian Sestrin isoforms.
| Functions | Inhibits ROS in cells | In vitro a Ahp activity | Binds to Keap1 | Inhibits mTORC1 in cells | Binds to GATOR2 | Binds to mTORC2 | Activates Akt in cells | Activates AMPK in cells | In vitro leucine binding |
|---|---|---|---|---|---|---|---|---|---|
| Sesn1 | O | - | O | O | O | - | O | O | O |
| Sesn2 | O | O | O | O | O | O | O | O | O |
| Sesn3 | O | - | - | O | O | O | O | O | ▲ |
| Ref | [7,27] | [21] | [13] | [8,62] | [17–19] | [15] | [11,15] | [8,62] | [23] |
alkylhydroperoxidase
O = Functional
▲ = Weak
- = Undetermined
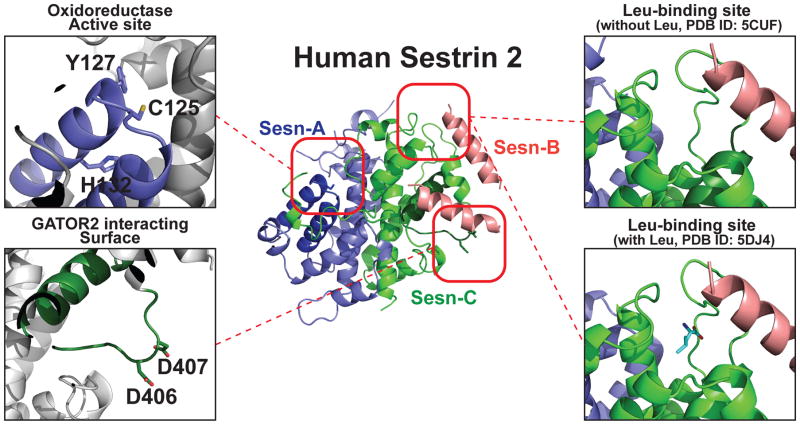
Figure 1. Structure of hSesn2 with highlighted functional domains.
Ribbon diagram of full-length hSesn2 (PDB ID: 5CUF [21]). Sesn-A, Sesn-B and Sesn-C domains, which were originally predicted through a phylogenetic analysis [73], are represented in blue, pink and green, respectively. The oxidoreductase active site of Sesn-A is magnified in the top left, and the catalytic cysteine (C125) and conserved residues of the proton relay system (Y127 and H132) are indicated. These residues were found to be critical for the antioxidant function of hSesn2. The GATOR2-interacting surface of the Sesn-C domain is magnified on the bottom left with the critical residues (D406 and D407; the ‘DD motif’) labeled. This DD motif is required for the interaction between hSesn2 and GATOR2, and for subsequent control of AMPK and mTORC1 signaling. Finally, the leucine-binding site of the Sesn-C domain is magnified on the right. The top representation is the crystallized structure without added leucine (PDB ID: 5CUF [21]), and the bottom representation is the structure with leucine (PDB ID: 5DJ4 [22]). No significant conformational change was observed between these two structures. Illustrations of the protein structure were generated with PYMOL (Delano Scientific, LLC).
Sestrin suppresses oxidative damage
Sestrins are transcriptionally induced upon oxidative damage through diverse transcription factors including p53, Nrf2, AP-1 and FoxOs [6, 26]. Cells overexpressing Sestrin are protected from oxidative damage [7, 26, 27], while Sestrin deficiency renders cells and tissues hypersensitive to oxidative stress [7, 10, 13, 28]. Therefore, Sestrin is considered an important component of antioxidant defense, and many different theories have been proposed to explain its antioxidant activity.
Sestrin as a Prx reductase
Sestrin exhibits a remote sequence similarity to AhpD, an antioxidant protein in Mycobacterium tuberculosis [7]. AhpD has two known redox functions: detoxification of alkylhydroperoxides (a group of hydrophobic ROS) [29], and reduction of the cysteine disulfides (Cys-S-S-Cys) of AhpC [30], a peroxiredoxin (Prx)-family peroxidase in M. tuberculosis.
Mutation of Cys125 in Sestrin (SestrinCS), which corresponds to one of the two catalytic cysteines in AhpD, abolished the ability of Sestrin to protect cells from oxidative stress [7]. Based on this functional similarity, it was originally proposed that Sestrin acts as a reductase for Prx. However, unlike AhpD, which can reduce the cysteine disulfides of AhpC [30], Sestrin2 was suggested to function as a reductase for cysteine sulfinic acids (Cys-SO2H), an overoxidized form of Prx [7]. Supporting this argument, induction of Sestrin expression significantly reduced the level of overoxidized Prx in two independent studies [7, 31].
Although the role of Sestrin as a Prx reductase can explain its antioxidant function, another study rebuked this hypothesis; purified Sestrin did not exhibit any intrinsic cysteine sulfinic acid reductase activity toward Prx, while sulfiredoxin (Srx) did [32]. A genetic study in Caenorhabditis elegans further demonstrated that Sestrin is not genetically required for recycling overoxidized Prx [33]. Therefore, it is now widely agreed that Sestrin is not a stand-alone sulfinic acid reductase for Prx [6, 20]. Nevertheless, it seems clear that the induction of Sestrin can promote recycling of overoxidized Prx [7, 31] and reduce oxidative damage in cells [7, 26, 27]. These effects likely occur through an indirect transcriptional upregulation of Srx or direct detoxification of ROS, mechanisms that will be discussed in more detail later in this Review.
Sestrin as an autophagy regulator
Autophagic degradation of dysfunctional mitochondria, known as mitophagy, is critical for redox homeostasis because the accumulation of damaged mitochondria results in excessive ROS production that can lead to diverse degenerative pathologies [34]. Sestrin can upregulate autophagy through AMP-activated Protein Kinase (AMPK) activation and mTORC1 inhibition (see the section ‘Sestrin regulates the mTOR signaling network’). Correspondingly, Sestrin was found to induce autophagy during diverse environmental stresses that provoke mitochondrial dysfunction [13, 35–38].
A genetic study in Drosophila also supported the importance of autophagy in mediating the antioxidant activity of Sestrin because the loss of Sestrin led to autophagy impairment, resulting in accumulation of damaged mitochondria and subsequent elevation of ROS in skeletal muscle [10]. Similar muscle phenotypes were also observed in Sestrin-deficient C. elegans [9]. These phenotypes were dependent on AMPK/mTORC1 misregulation and autophagy abrogation because (i) pharmacological activation of AMPK or inhibition of mTORC1 restored mitochondrial homeostasis in Sestrin-deficient flies, (ii) SestrinCS, a redox-inactive Sestrin that can still regulate AMPK/mTORC1 signaling [8], also restored mitochondrial homeostasis, and (iii) RNAi-mediated downregulation of Ampk or Atg1, the fly homolog of ULK1, resulted in mitochondrial phenotypes like those seen in Sestrin-deficient flies [10].
Even though the mitophagy-regulating function of Sestrin is important for redox homeostasis, Sestrin also contributes to redox biology by additional mechanisms. First, in several non-muscle cell types, SestrinCS was less effective than SestrinWT in suppressing ROS accumulation [7, 39]. Second, Sestrin suppressed ROS in physiological contexts that minimally involve mitochondrial damage, including neuronal synaptic stimulation [40], replicative senescence [28], macrophage peroxide signaling [31], brown adipocyte thermogenesis [39] and renal dopamine signaling [41]. Thus, Sestrin-mediated redox regulation is not narrowly confined to the control of mitophagy and autophagy but must involve additional mechanisms.
Sestrin as an Nrf2 regulator
Nrf2 is a transcription factor that upregulates the expression of a wide range of antioxidant genes including Prx, Srx, glutathione-S-transferase (GST) and thioredoxin (Trx) [42]. In normal environments, kelch-like ECH-associated protein 1 (Keap1) binds to Nrf2 and targets it for proteasomal degradation (Figure 2A). Keap1 inactivation occurs through oxidation of its several cysteine residues [42] or by autophagic elimination mediated by an autophagy adapter p62/sequestosome-1 (SQSTM1) [43, 44].
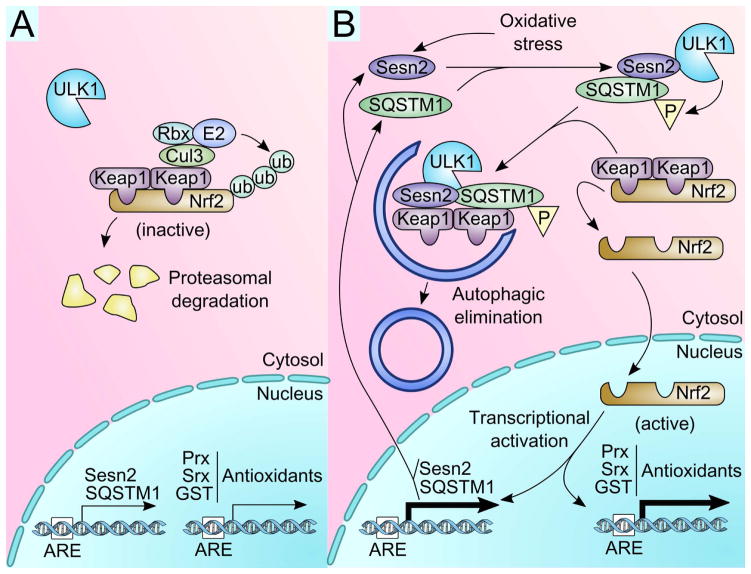
Figure 2. Sestrin-mediated regulation of Nrf2.
(A) Under normal environmental conditions, Nrf2 is inactivated when Keap1 binds and targets it for proteasomal degradation through the Cul3-based E3 ligase (Cul3) and associated RING box protein (Rbx), which recruits an E2 ligase to tag Nrf2 with ubiquitin (ub). (B) Under conditions of oxidative stress, Sestrin2 (Sesn2) and SQSTM1 are expressed. Sesn2 binds ULK1, an initiator of autophagy, and SQSTM1, an autophagy adaptor protein. This interaction between the three proteins promotes ULK1-dependent phosphorylation of SQSTM1, which then specifically targets Keap1 for autophagic elimination. Now, Nrf2 is freed from Keap1, translocates to the nucleus, and promotes transcriptional activation of genes controlled by the antioxidant response element (ARE), including Sesn2, SQSTM1 and antioxidant enzymes such as Prx, Srx, and GST.
Sestrin2 was found to associate with both Keap1 and SQSTM1 [13], as well as autophagy-initiator ULK1 [45]. Sestrin2 promotes ULK1-induced phosphorylation of SQSTM1 [45], which facilitates Keap1 degradation and Nrf2 activation [46]. This pathway can explain how Sestrin2 functions as an antioxidant through upregulation of Nrf2 and its antioxidant targets (Figure 2B) [20]. Indeed, the Sestrin2-Keap1-Nrf2 pathway was shown to be physiologically important for the antioxidant defense of hepatocytes against nutritional [13] and chemical [47] stress.
Another intriguing point is that both Sestrin2 and SQSTM1, which contribute to the degradation of Keap1, are direct transcriptional targets of Nrf2 [48, 49]. Therefore, Nrf2-dependent induction of Sestrin2 and SQSTM1 can generate a positive feedback loop that guarantees the maximal activation of the antioxidant activity of Nrf2 upon oxidative stress.
Although this Nrf2-dependent mechanism provides a convincing explanation of the antioxidant role of Sestrin2, several questions still remain: (i) is the physical interaction between Sestrin2, Keap1, SQSTM1 and ULK1 supported by structural studies, (ii) can Sestrin2, in conjunction with Keap1, regulate Nrf2 activity in a redox-sensitive manner, and (iii) does Sestrin regulation of AMPK and mTORC1 signaling also contribute to the autophagic regulation of Keap1 and Nrf2. Assessing the effect of specific mutations that block the peroxidase activity of Sestrin or mTORC1-regulating function (Table 2) may provide some clues about its regulation of the Keap1-Nrf2 pathway.
Table 2.
Point mutations of hSesn2 that significantly affect its molecular function.
| Point Mutation | Inhibits ROS in cells | In vitro a Ahp activity | Binds to Keap1 | Inhibits mTORC1 in cells | Binds to GATOR2 | Activates AMPK in cells | Binds to leucine | References |
|---|---|---|---|---|---|---|---|---|
| WT | O | O | O | O | O | O | O | [7, 8, 13, 17–19, 21–24] |
| Mutations in Sesn-A domain | ||||||||
| H86A | - | - | - | - | O | - | X | [22] |
| C125S | X | X | O | O | O | - | - | [7, 8, 13, 21] |
| Y127F | - | X | - | O | - | - | - | [21] |
| H132A | - | X | - | O | - | - | - | [21] |
| S190W | - | - | - | X | X | - | O | [23] |
| Mutations in Sesn-B domain | ||||||||
| L261A | - | - | - | O | O | - | X | [23] |
| Mutations in Sesn-C domain | ||||||||
| T374A | - | - | - | - | - | - | X | [22] |
| Y375F | - | - | - | - | O | - | X | [22] |
| T386A | - | - | - | - | O | - | X | [22] |
| R390A | - | - | - | - | O | - | X | [22] |
| R404A/D406A/D407A | - | - | - | X | X | - | - | [21] |
| D406A | - | O | - | X | X | X | - | [21] |
| D407A | - | O | - | X | X | X | - | [21] |
| D406A/D407A | - | - | - | - | X | - | O | [22] |
| R419A | - | - | - | OX | - | - | - | [21, 24] |
| R419A/K422A/K426A | - | - | - | OX | - | - | - | [21, 24] |
| W444E | - | - | - | - | O | - | X | [22] |
| W444L | - | - | - | - | O | - | ▲ | [22] |
| E451A | - | - | - | O | O | - | X | [23] |
| E451Q | - | - | - | - | O | - | X | [22] |
alkylhydroperoxidase
O = Functional
▲ = Partially functional
OX = Conflicting results
X = Non-functional
- = Undetermined
Sestrin as a peroxidase
Recent determination of the human Sestrin2 (hSesn2) structure by X-ray crystallography allowed us to gain novel insights into its antioxidant function. The crystal structure revealed that hSesn2 contains two structurally similar subdomains, Sesn-A and Sesn-C. Both subdomains share significant structural homology with the Ralstonia eutropha protein YP_296737.1, which belongs to a family of alkylhydroperoxidases, including the M. tuberculosis AhpD [21, 22]. Moreover, the Sesn-A domain of hSesn2 contains the helix–turn–helix oxidoreductase motif (Figure 1) that has an intact proton relay system with the catalytic cysteine (Cys125) and other key residues (Tyr127 and His132) conserved in AhpD and YP_296737.1 [21].
Subsequent biochemical studies demonstrated that hSesn2 is indeed an active alkylhydroperoxidase [21]. This conclusion was based on the observations that (i) recombinant hSesn2 catalyzed the reduction of cumene hydroperoxide, an alkylhydroperoxide, at an efficiency comparable to M. tuberculosis AhpC and AhpD; (ii) substitutions of the key catalytic residues of Sesn-A, including Cys125, Tyr127 and His132 (Figure 1), abolished the alkylhydroperoxidase activity of hSesn2; and (iii) the proposed reaction intermediate form—cysteine sulfenic acid (Cys-SOH)—of hSesn2 was detectable in wild-type hSesn2 but not Cys125-substituted hSesn2 (hSesn2CS).
Although the structural and biochemical evidence demonstrates that hSesn2 has intrinsic peroxidase activity, the physiological ROS substrate of hSesn2 still needs to be identified. Hydrogen peroxide, which is among the most abundant and biologically significant ROS in cells, is not efficiently reduced by hSesn2 [21]. The only known substrate for hSesn2 is cumene hydroperoxide [21], which is not a physiological ROS in any known cell type. Because hSesn2 is expected to be specific for hydrophobic alkylhydroperoxides [21], lipid hydroperoxides may be a physiologically relevant substrate for Sestrin as they are implicated in diverse age- and obesity-associated diseases [50–52]. It is also possible that hSesn2 acts as a redox sensor, if the reversible oxidation of Cys125 in hSesn2 is found to affect its function in promoting autophagy or regulating Nrf2.
Sestrin regulates the mTOR signaling network
Independent of redox regulation, Sestrin is also involved in stress-dependent mTOR regulation. mTOR is present in two different protein kinase complexes, mTORC1 and mTORC2. Sestrin specifically inhibits mTORC1 by inhibiting Rheb and RagA/B, the two GTPases essential for mTORC1 activation. The AMPK-TSC2 pathway mediates the effects of Sestrin on Rheb, while the GATOR1-GATOR2 complexes are responsible for the effects of Sestrin on RagA/B (Figure 3). Interestingly, although Sestrin strongly inhibits mTORC1, it activates mTORC2 through several independent mechanisms. In the following sections, we discuss the different mechanisms by which Sestrin regulates the mTOR-associated signaling network.
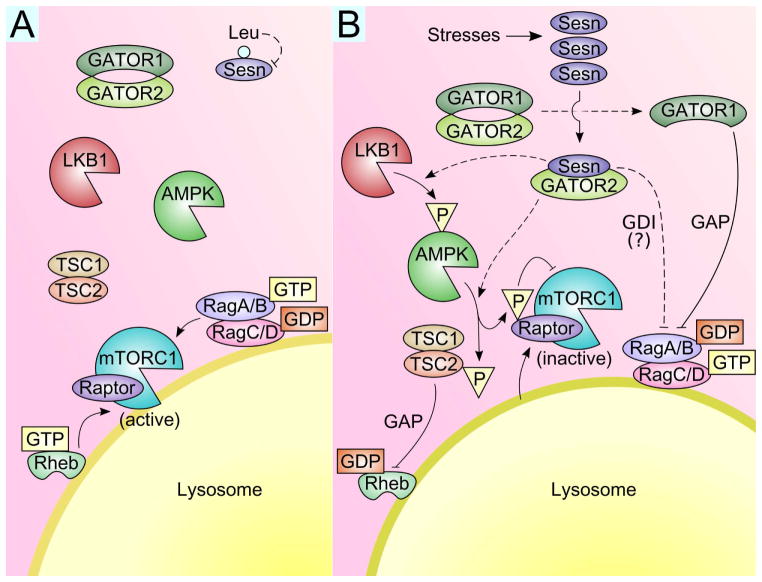
Figure 3. Sestrin-mediated regulation of mTORC1.
(A) Under unstressed conditions, Sestrin expression and activity is low, and GATOR1 and GATOR2 form a supercomplex. mTORC1 is fully activated; activated RagA/B:RagC/D localizes mTORC1 to the lysosomal membrane, and GTP-loaded Rheb activates mTORC1’s kinase activity. (B) Under conditions of stress, Sestrin is upregulated and binds GATOR2. This then frees GATOR1 from GATOR2 inhibition, and GATOR1 subsequently acts as a GAP for RagA/B. Inactivated RagA/B:RagC/D is then unable to localize mTORC1 to the lysosome, and mTORC1 disperses to the cytosol. In addition, the Sestrin-GATOR2 interaction also promotes AMPK activation through unclear biochemical mechanisms possibly involving LKB1, the major kinase upstream of AMPK. Activated AMPK then phosphorylates TSC2, a GAP for Rheb, leaving Rheb now in its inactive GDP-loaded form. AMPK also phosphorylates Raptor, a regulatory subunit of mTORC1, and this phosphorylation inhibits mTORC1 activity [74]. Sestrin may also regulate RagA/B proteins as a GDI. Solid lines indicate established mechanisms, while dashed lines indicate models that require further biochemical clarification.
Sestrin as an AMPK regulator
The study that first described Sestrin-mediated mTORC1 control concluded that this regulation was dependent on AMPK [8]. In this study, Sestrin was shown to associate with the TSC1:TSC2 complex and promote the activation of TSC2 by AMPK-mediated phosphorylation. Sestrin also increased AMPK phosphorylation at Thr172 [8], a marker of AMPK activation. As TSC2 is a GTPase-activating protein (GAP) for Rheb, Sestrin-dependent TSC2 activation inactivates Rheb and mTORC1 (Figure 3B). Supporting this model, pharmacological (via compound C) or shRNA-mediated inhibition of AMPK and TSC2 blunted the inhibition of mTORC1 by Sestrin [8]. Importantly, Sestrin was necessary for DNA damage-induced inhibition of mTORC1 [8], which was also dependent on the activation of AMPK and TSC2 [53].
The AMPK-activating role of Sestrin was widely observed and shown to be important for mTORC1 control in diverse cellular contexts [12, 14, 38, 54–56]. Genetic studies in Drosophila also supported this model because the AMPK-TSC2 axis was critical for the effect of Sestrin in controlling tissue growth and attenuating age-associated pathologies [10, 57]. Metabolic phenotypes of Sestrin2-deficient mice, including insulin resistance and steatohepatitis, were also strongly suppressed by pharmacological (via AICAR) or viral (via Ad-AMPKCA) activation of AMPK [11, 12], further supporting the idea that AMPK is the critical downstream target of Sestrin controlling metabolic homeostasis.
Although this evidence indicates that AMPK is physiologically important for mediating Sestrin activity, Sestrin was recently found to inhibit mTORC1 in AMPK-null mouse embryonic fibroblasts (MEF) [17, 19, 24], suggesting that AMPK is not the only target of Sestrin in the mTORC1 pathway. In addition, the molecular mechanism of Sestrin-induced AMPK activation is still unknown. Some studies suggested that Sestrin functions as a signaling scaffold between AMPK and LKB1 [14, 58] and that Sestrin induces the expression of AMPK regulatory subunits to achieve maximal AMPK activation [58]. Although these models do explain a mechanism for Sestrin-induced AMPK activation, more robust experimental evidence is needed to clarify the exact biochemical role of Sestrin in AMPK signaling.
Sestrin as a Rag GDI
In search of alternative mechanisms to account for Sestrin regulation of mTORC1 in an AMPK-independent manner, Sestrin was suggested to act as a GDP dissociation inhibitor (GDI) to inhibit RagA/B [24]. This model was based on the following observations: (i) Sestrin could not inhibit mTORC1 when constitutively active RagB was expressed, (ii) Sestrin exhibited physical interaction with the RagA/B:RagC/D complex, (iii) Sestrin inhibited GTP loading on the RagA/B proteins in an in vitro assay, and (iv) Sestrin shows limited sequence homology to human and mouse GDI1 proteins [24].
Although this model could explain the observations that Sestrin-mediated mTORC1 inhibition is dependent on RagA/B [17–19, 24], several findings contradict this model. Crystal structures of hSesn2 show no structural homology between Sestrin and GDI proteins [21, 22] and actually indicate that the proposed GDI motif in Sestrin has a very different conformation from GDI1. Indeed, mutations of the putative GDI motif did not prevent Sestrin from inhibiting mTORC1 [21]. In addition, although the initial study reported that hSesn2 immunostaining overlaps with the Rag complex on the lysosomal membrane [24], subsequent studies with higher immunofluorescence resolution demonstrated that hSesn2 is actually excluded from the lysosomal surface [17, 19], indicating that hSesn2 does not directly control RagA/B activity on the lysosomal membrane. Finally, the direct physical interaction between Sestrin and RagA/B was undetectable in several subsequent experiments [17, 21]. These observations suggest that Sestrin-mediated control of RagA/B could occur through indirect mechanisms involving additional signaling components.
Sestrin as a GATOR modulator
In several independent labs, proteomic studies identified a strong physical interaction between Sestrin and GATOR2 [17–19], a RagA/B-regulating hetero-pentameric protein complex [59]. GATOR2 is known to suppress GATOR1, a heterotrimeric complex that functions as a GAP for RagA/B [60]. Therefore, Sestrin was postulated to bind GATOR2, liberating GATOR1 from GATOR2-mediated inhibition, and thereby promote the RagA/B-inhibiting activity of GATOR1 (Figure 3) [17–19]. Supporting this model, silencing or knocking out GATOR1 components abolished inhibition of mTORC1 by Sestrin [17–19]. The genetic relationship between Sestrin, GATOR1 and GATOR2 was also conserved in Drosophila; silencing GATOR1 inhibited suppression of wing growth by Sestrin, and mutations of GATOR2 restored autophagy defects in Sestrin-null flies [17]. These results suggest that Sestrin inhibits RagA/B-dependent mTORC1 activation through modulation of the GATOR complexes.
Even though these results implicate a functional relationship between Sestrin, GATOR2, GATOR1 and RagA/B, the biochemical mechanisms of how Sestrin modulates the GATOR complexes are still elusive with noticeable inconsistencies between the reports. Two papers indicated that Sestrin does not affect the association between GATOR1 and GATOR2 [18, 19], while another study demonstrated that high levels of Sestrin expression destabilized the GATOR1:GATOR2 supercomplex [17]. One paper indicated that Sestrin does not affect the GTP loading status of RagB [19], while the other two papers showed that GTP loading of RagB was altered by Sestrin overexpression [17, 24]. This confusion is in part because the molecular natures of the GATOR1 and GATOR2 complexes are not yet biochemically defined. For example, although GATOR1 was suggested to act as a GAP for RagA/B [60], it is unknown how its three individual subcomponents contribute to the GAP activity of the holocomplex [59]. Furthermore, the biochemical mechanism of how GATOR2 suppresses GATOR1 is also unknown.
Despite these unanswered questions, the recently determined crystal structure of hSesn2 led to the discovery of a structural motif that mediates the hSesn2-GATOR2 interaction. As previously mentioned, hSesn2 has two-fold pseudosymmetrical subdomains, Sesn-A and Sesn-C (Figure 1), each of which is structurally homologous to an alkylhydroperoxidase [21, 22]. Whereas the helix–turn–helix oxidoreductase motif was found to be well-conserved and functional in Sesn-A, the corresponding structural motif was absent in Sesn-C and replaced with a long loop structure containing two surface-exposed aspartates (Asp406 and Asp407, the DD motif) (Figure 1) [21]. Mutations in the DD motif almost completely abolished the binding between hSesn2 and GATOR2 [21, 22], suggesting that the DD motif constitutes a key protein-protein interaction site. Importantly, mutations in the DD motif abolished both AMPK-activating and mTORC1-inhibiting functions of hSesn2 [21], signifying the importance of the DD motif and GATOR2 in Sestrin signaling.
Sestrin as an amino acid sensor
Sestrin-null MEF or HEK293 cells, generated by triple knockout or knockdown of Sestrin1-3 genes, failed to downregulate mTORC1 activity after amino acid starvation [18, 24]. These observations implicated Sestrin in the amino acid-dependent control of mTORC1. Recently, two papers further suggested that hSesn2 acts as a direct sensor of leucine, based on the following observations: (i) hSesn2 physically binds to leucine at a Kd of 20μM [23], which is lower than the KM value of leucyl tRNA synthetase for leucine (45μM) [61], (ii) leucine binding to hSesn2 changes its melting temperature and prevents its binding to GATOR2 [23], (iii) crystal structure of hSesn2 was determined in the presence of added leucine, and leucine was found in a binding pocket in the crystal structure of hSesn2 [22], and (iv) wild-type hSesn2, but not leucine-binding defective hSesn2 mutants, restored the leucine sensitivity of mTORC1 signaling in Sestrin-null HEK293 cells [22, 23]. According to this model, hSesn2 cannot inhibit mTORC1 in the presence of leucine because leucine binding prohibits hSesn2 from interacting with its effector, GATOR2.
In addition to leucine, hSesn2 can also bind to other hydrophobic amino acids such as methionine, isoleucine and valine, although with a lower binding affinity [23]. hSesn1 can also strongly bind to leucine while the binding between hSesn3 and leucine was found to be weak (Table 1) [23]. On the other hand, all three Sestrin isoforms, hSesn1-3, can strongly bind to GATOR2 and inhibit mTORC1 [17–19]. Although leucine was reported to disrupt the hSesn1-GATOR2 and hSesn2-GATOR2 interactions, the hSesn3-GATOR2 interaction was not disrupted by the in vitro addition of leucine [23].
Although these are potentially important findings in the field of amino acid signaling, there are several caveats that need to be addressed before considering hSesn2 as an established leucine sensor. First, in contrast to the prediction that Sestrin2 is inactivated by leucine above 20μM, numerous studies [8, 10, 12, 17–19, 21, 24, 58, 62, 63] indicated that hSesn2 is still able to suppress mTORC1 signaling in conditions rich with leucine. These conditions include conventional cell culture media such as RPMI and DMEM that contains 380μM and 800μM of leucine, respectively, and the intracellular amino acid concentration can be higher due to the presence of active transporters [64, 65]. Therefore, the effect of leucine on Sestrin2 function could be variable depending on the biological context. Second, in contrast to the theory that the hSesn2-GATOR2 interaction is completely disrupted by leucine [22, 23], the physical interaction between hSesn2 and GATOR2 was detectable in various cell lines cultured in leucine-rich conditions [17–19]. Finally, although the leucine sensor model predicted that leucine binding and dissociation would induce large conformational changes in hSesn2 [22, 23], the crystal structures determined in the presence or absence of leucine were actually quite similar (Figure 1) [21, 22]. Thus, whether hSesn2 is indeed inactivated by leucine binding under physiological conditions needs further clarification.
More recently, several additional mechanisms were suggested to explain the role of Sestrin2 in amino acid sensing. First, GCN2, an established sensor of amino acids [66], was shown to induce hSesn2 transcription through upregulation of the ATF4 transcription factor [25]. The GCN2-dependent hSesn2 induction was important for mTORC1 regulation in response to amino acid availability, including leucine and glutamine [25]. Second, it was also shown that Sestrin2 is phosphorylated by ULK1 in response to leucine starvation [67]. Because phosphorylation may affect the leucine-binding or mTORC1-regulating activities of Sestrin2, further studies may clarify why different studies contradict each other in regards to whether physiological leucine concentrations can inhibit Sestrin2 function and/or Sestrin2-GATOR2 binding. It is also worth noting that, in addition to being phosphorylated by ULK1, Sestrin2 can potentiate ULK1-dependent phosphorylation [45] and degradation [13] of SQSTM1. Considering that SQSTM1 was recently characterized as an important mTORC1 regulator that is involved in amino acid sensing [68–70], future studies are necessary to understand how Sestrin2, ULK1, SQSTM1 and GATOR2 are functionally connected with each other in the context of amino acid sensing.
Sestrin as an mTORC2 regulator
Even though Sestrin strongly suppresses mTORC1 signaling, Sestrin upregulated mTORC2-dependent AKT phosphorylation in cultured cells as well as in mouse and Drosophila tissues [11, 15]. Because chronic activation of mTORC1 and S6K signaling is known to cause insulin resistance [71], Sestrin-mediated mTORC2-AKT activation could be dependent on the mTORC1-suppressive activity of Sestrin. However, Sestrin-induced activation of the TSC1:TSC2 complex can also contribute to mTORC2 upregulation through an mTORC1-independent mechanism [72]. Recently, Sestrin2 and Sestrin3 were shown to physically bind to mTORC2 through Rictor, one of its regulatory subunits, and directly promote the catalytic activity of mTORC2 [15]. Thus, it seems that Sestrin can upregulate mTORC2-AKT signaling through multiple mechanisms. The AKT-upregulating activities of Sestrin could be critical for its protection against insulin resistance and diabetic progression [11, 15]. Further investigation should be targeted towards defining a clear molecular mechanism for Sestrin-mediated AKT upregulation and defining the context-dependent contributions of the multiple signaling pathways.
Concluding Remarks
In recent years, significant progress has been made towards understanding the biochemical mechanisms behind the actions of Sestrin; however, many questions still remain (see Outstanding Questions Box). Sestrin is a versatile protein that can single-handedly control a variety of anti-aging functions, which can be roughly classified into its ROS-reducing and mTOR-regulating functions. The recently determined crystal structure of hSesn2 supported this dual role by revealing two subdomains responsible for each of these functions. In addition, structure-guided mutagenesis studies generated a list of point mutations that specifically ablate the redox-controlling or mTORC1-modulating functions of Sestrin (Table 2), which constitutes an essential toolkit for the molecular dissection of Sestrin function in diverse physiological contexts. Considering the important physiological functions of Sestrin in regulating metabolic homeostasis and attenuating age-associated pathologies, further investigation into the biochemical functions of Sestrin may generate novel insight into developing a new class of anti-aging therapeutics that harness the beneficial activities of Sestrin.
Outstanding Questions Box.
Is the physical interaction between Sestrin2, Keap1, SQSTM1, and ULK1 supported by the recently obtained hSesn2 structural information? For example, which specific residues and surfaces of Sestrin2 are required for its regulation of the Keap1-Nrf2 pathway?
What are the physiological peroxide substrates of Sestrin2? How can the intrinsic peroxidase activity of Sestrin2 be reconciled with its Nrf2-regulating activity under conditions of oxidative stress? Can the redox status of Sestrin2 affect its activity in regulating Keap1 and Nrf2?
What is the biochemical mechanism responsible for Sestrin-mediated AMPK control? How are the DD motif and GATOR2 complex involved in AMPK regulation?
What are the similarities and differences between the biochemical functions of Sestrins1-3?
What are the biochemical mechanisms behind regulation of the GATOR complexes by Sestrin? How does GATOR2 suppress GATOR1, and how does Sestrin liberate GATOR1 from GATOR2-mediated suppression?
Is the binding between Sestrin and leucine indeed important for physiological leucine sensing? If so, how does leucine binding regulate Sestrin activity? If leucine disrupts association between Sestrin2 and GATOR2, how can Sestrin regulate the GATOR complexes and mTORC1 signaling in the presence of physiological levels of leucine? Are there any molecular determinants that can regulate the sensitivity of Sestrin to leucine levels?
As summarized in the review, Sestrin interacts with many different protein partners, including GATOR2, p62, ULK1, Keap1, AMPK, TSC2 and mTORC2. Are these proteins altogether in the same complex? If they are in separate complexes, do they compete for the limiting amount of Sestrin? If so, can this be a mechanism for how Sestrin switches between functions in response to different stresses?
Trends Box.
Sestrin suppresses oxidative damage by activation of the antioxidant transcription factor Nrf2, autophagic elimination of damaged mitochondria, and direct detoxification of ROS through its intrinsic peroxidase activity.
The hetero-pentameric GATOR2 complex is a direct physical target of Sestrin that mediates its mTORC1-regulating functions.
Sestrin activity is regulated by amino acids and other nutrients at multiple levels, including both transcriptional and post-transcriptional mechanisms.
A hSesn2 crystal structure revealed three structural motifs, each responsible for one of the identified functions of hSesn2: ROS-detoxification, GATOR2-binding (and subsequently regulating AMPK and mTORC1 signaling), and amino acid (leucine)-binding.
Acknowledgments
The authors thank Dr. Judith Connett for critical reading of the manuscript and Daniel B. Kowalsky for preparing figures. The work was supported by NIH (5T32GM008322 to A.H., 1R21AG050903 to U.-S.C. and J.H.L., R21AG045432 and 1R01DK102850 to J.H.L.), American Diabetes Association (1-13-BS-106 to J.H.L. and 1-16-JDF-017 to U.-S.C), Ellison Medical Foundation (AG-NS-0932-12 to J.H.L.) and MCubed Research Initiatives from the University of Michigan (to U.-S.C. and J.H.L.).
Glossary
- Alkylhydroperoxidase
a family of oxidoreductases that converts alkylhydroperoxides to their corresponding alcohols
- Alkylhydroperoxide
an organic hydroperoxide (R-O-O-H) where R is a hydrophobic alkyl group
- AhpC
Alkylhydroperoxidase C, a peroxiredoxin-family peroxidase in Mycobacterium tuberculosis
- AhpD
Alkylhydroperoxidase D, an antioxidant protein of M. tuberculosis that can detoxify alkylhydroperoxides and reduce cysteine disulfides of AhpC. In M. tuberculosis, AhpC and AhpD are controlled under the same promoter
- Cumene hydroperoxide
an alkylhydroperoxide that has been identified as an in vitro substrate for AhpC, AhpD and Sestrin2
- GTPase activating protein (GAP)
a protein that can bind to GTPase proteins and stimulate their GTP-hydrolyzing activity, resulting in the termination of the signaling event
- GAP activity towards Rags 1 (GATOR1)
a heterotrimeric protein complex that consists of DEPDC5, Nprl2, and Nprl3; has GAP activity towards RagA/B
- GAP activity towards Rags 2 (GATOR2)
a hetero-pentameric protein complex that consists of WDR59, WDR24, Mios, Seh1L, and Sec13; has GATOR1-inhibitory function
- General control nonderepressible 2 (GCN2)
a serine/threonine-protein kinase that is activated upon amino acid deprivation from binding to uncharged tRNA
- GDP dissociation inhibitor (GDI)
a protein that can bind to GTPase proteins and inhibit the GDP-GTP exchange reaction, resulting in inhibition of the signaling event
- Leucyl tRNA synthetase
an enzyme that catalyzes the ligation of leucine to tRNA
- Liver kinase B1 (LKB1)
a protein kinase that phosphorylates AMPK at Thr172. LKB1 forms a complex with STRAD and MO25, and is one of the major activators of AMPK
- Mechanistic target of rapamycin complex 1 (mTORC1)
a nutrient-responsive serine/threonine kinase consisting of several proteins, including mTOR, Raptor, mLST8, PRAS40, and DEPTOR. mTORC1 upregulates anabolic activities such as protein and lipid synthesis and downregulates autophagic catabolism
- Mechanistic target of rapamycin complex 2 (mTORC2)
a complex composed of: mTOR, Rictor, mLST8, and mSIN1. mTORC2 directly phosphorylates Akt at Ser473
- Nuclear factor (erythroid-derived 2)-like 2 (Nrf2)
a transcription factor that upregulates the expression of diverse antioxidant proteins
- Oxidoreductase
an enzyme that catalyzes oxidation or reduction reactions, where electrons are transferred from one molecule, the reductant, to another molecule, the oxidant
- Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α)
a transcriptional coactivator that regulates genes involved in energy metabolism
- Peroxiredoxin (Prx)
a ubiquitous family of antioxidant enzymes that reduce hydrogen peroxide and organic hydroperoxides in mammalian cells
- Reactive oxygen species (ROS)
chemically reactive molecules containing oxygen, natural byproducts of metabolism that can damage cellular macromolecules, depending on localization and quantity, but also important for cell signaling and homeostasis
- Tuberous sclerosis complex 2 (TSC2)
also known as tuberin; a tumor suppressor that associates with TSC1 and functions as a GAP for Rheb GTPase
- Unc-51 like autophagy activating kinase 1 (ULK1)
a protein kinase that is responsible for the initiation of autophagy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lopez-Otin C, et al. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hekimi S, et al. Taking a “good” look at free radicals in the aging process. Trends in cell biology. 2011;21:569–576. doi: 10.1016/j.tcb.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson SC, et al. mTOR is a key modulator of ageing and age-related disease. Nature. 2013;493:338–345. doi: 10.1038/nature11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu JJ, et al. Increased mammalian lifespan and a segmental and tissue-specific slowing of aging after genetic reduction of mTOR expression. Cell Rep. 2013;4:913–920. doi: 10.1016/j.celrep.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrison DE, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee JH, et al. Sestrins orchestrate cellular metabolism to attenuate aging. Cell metabolism. 2013;18:792–801. doi: 10.1016/j.cmet.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Budanov AV, et al. Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science. 2004;304:596–600. doi: 10.1126/science.1095569. [DOI] [PubMed] [Google Scholar]
- 8.Budanov AV, Karin M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell. 2008;134:451–460. doi: 10.1016/j.cell.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang YL, et al. SESN-1 is a positive regulator of lifespan in Caenorhabditis elegans. Experimental gerontology. 2013;48:371–379. doi: 10.1016/j.exger.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 10.Lee JH, et al. Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies. Science. 2010;327:1223–1228. doi: 10.1126/science.1182228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JH, et al. Maintenance of metabolic homeostasis by Sestrin2 and Sestrin3. Cell metabolism. 2012;16:311–321. doi: 10.1016/j.cmet.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park HW, et al. Hepatoprotective role of Sestrin2 against chronic ER stress. Nat Commun. 2014;5:4233. doi: 10.1038/ncomms5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bae SH, et al. Sestrins activate Nrf2 by promoting p62-dependent autophagic degradation of Keap1 and prevent oxidative liver damage. Cell Metab. 2013;17:73–84. doi: 10.1016/j.cmet.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Morrison A, et al. Sestrin2 promotes LKB1-mediated AMPK activation in the ischemic heart. FASEB J. 2015;29:408–417. doi: 10.1096/fj.14-258814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tao R, et al. Sestrin 3 protein enhances hepatic insulin sensitivity by direct activation of the mTORC2-Akt signaling. Diabetes. 2015;64:1211–1223. doi: 10.2337/db14-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ro SH, et al. Tumor suppressive role of sestrin2 during colitis and colon carcinogenesis. eLife. 2016;5:12204. doi: 10.7554/eLife.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JS, et al. Sestrin2 inhibits mTORC1 through modulation of GATOR complexes. Scientific reports. 2015;5:9502. doi: 10.1038/srep09502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chantranupong L, et al. The Sestrins interact with GATOR2 to negatively regulate the amino-acid-sensing pathway upstream of mTORC1. Cell reports. 2014;9:1–8. doi: 10.1016/j.celrep.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parmigiani A, et al. Sestrins inhibit mTORC1 kinase activation through the GATOR complex. Cell Rep. 2014;9:1281–1291. doi: 10.1016/j.celrep.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rhee SG, Bae SH. The antioxidant function of sestrins is mediated by promotion of autophagic degradation of Keap1 and Nrf2 activation and by inhibition of mTORC1. Free radical biology & medicine. 2015;88:205–211. doi: 10.1016/j.freeradbiomed.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Kim H, et al. Janus-faced Sestrin2 controls ROS and mTOR signalling through two separate functional domains. Nature communications. 2015;6:10025. doi: 10.1038/ncomms10025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saxton RA, et al. Structural basis for leucine sensing by the Sestrin2-mTORC1 pathway. Science. 2016;351:53–58. doi: 10.1126/science.aad2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolfson RL, et al. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science. 2016;351:43–48. doi: 10.1126/science.aab2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peng M, et al. Sestrins Function as Guanine Nucleotide Dissociation Inhibitors for Rag GTPases to Control mTORC1 Signaling. Cell. 2014;159:122–133. doi: 10.1016/j.cell.2014.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ye J, et al. GCN2 sustains mTORC1 suppression upon amino acid deprivation by inducing Sestrin2. Genes Dev. 2015;29:2331–2336. doi: 10.1101/gad.269324.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Budanov AV, et al. Identification of a novel stress-responsive gene Hi95 involved in regulation of cell viability. Oncogene. 2002;21:6017–6031. doi: 10.1038/sj.onc.1205877. [DOI] [PubMed] [Google Scholar]
- 27.Kopnin PB, et al. Repression of sestrin family genes contributes to oncogenic Ras-induced reactive oxygen species up-regulation and genetic instability. Cancer Res. 2007;67:4671–4678. doi: 10.1158/0008-5472.CAN-06-2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nogueira V, et al. Akt determines replicative senescence and oxidative or oncogenic premature senescence and sensitizes cells to oxidative apoptosis. Cancer cell. 2008;14:458–470. doi: 10.1016/j.ccr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hillas PJ, et al. The AhpC and AhpD antioxidant defense system of Mycobacterium tuberculosis. The Journal of biological chemistry. 2000;275:18801–18809. doi: 10.1074/jbc.M001001200. [DOI] [PubMed] [Google Scholar]
- 30.Bryk R, et al. Metabolic enzymes of mycobacteria linked to antioxidant defense by a thioredoxin-like protein. Science. 2002;295:1073–1077. doi: 10.1126/science.1067798. [DOI] [PubMed] [Google Scholar]
- 31.Essler S, et al. Role of sestrin2 in peroxide signaling in macrophages. FEBS letters. 2009;583:3531–3535. doi: 10.1016/j.febslet.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 32.Woo HA, et al. Sestrin 2 is not a reductase for cysteine sulfinic acid of peroxiredoxins. Antioxidants & redox signaling. 2009;11:739–745. doi: 10.1089/ars.2008.2360. [DOI] [PubMed] [Google Scholar]
- 33.Thamsen M, et al. Is overoxidation of peroxiredoxin physiologically significant? Antioxid Redox Signal. 2011;14:725–730. doi: 10.1089/ars.2010.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J. Teaching the basics of autophagy and mitophagy to redox biologists--mechanisms and experimental approaches. Redox biology. 2015;4:242–259. doi: 10.1016/j.redox.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maiuri MC, et al. Stimulation of autophagy by the p53 target gene Sestrin2. Cell Cycle. 2009;8:1571–1576. doi: 10.4161/cc.8.10.8498. [DOI] [PubMed] [Google Scholar]
- 36.Ishihara M, et al. Sestrin-2 and BNIP3 regulate autophagy and mitophagy in renal tubular cells in acute kidney injury. American journal of physiology. Renal physiology. 2013;305:F495–509. doi: 10.1152/ajprenal.00642.2012. [DOI] [PubMed] [Google Scholar]
- 37.Chen YS, et al. Induction of sestrin2 as an endogenous protective mechanism against amyloid beta-peptide neurotoxicity in primary cortical culture. Experimental neurology. 2014;253:63–71. doi: 10.1016/j.expneurol.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 38.Hou YS, et al. Sestrin2 Protects Dopaminergic Cells against Rotenone Toxicity through AMPK-Dependent Autophagy Activation. Mol Cell Biol. 2015;35:2740–2751. doi: 10.1128/MCB.00285-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ro SH, et al. Sestrin2 inhibits uncoupling protein 1 expression through suppressing reactive oxygen species. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:7849–7854. doi: 10.1073/pnas.1401787111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Papadia S, et al. Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nature neuroscience. 2008;11:476–487. doi: 10.1038/nn2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang Y, et al. Sestrin2 decreases renal oxidative stress, lowers blood pressure, and mediates dopamine D2 receptor-induced inhibition of reactive oxygen species production. Hypertension. 2014;64:825–832. doi: 10.1161/HYPERTENSIONAHA.114.03840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suzuki T, et al. Toward clinical application of the Keap1-Nrf2 pathway. Trends in pharmacological sciences. 2013;34:340–346. doi: 10.1016/j.tips.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 43.Komatsu M, et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol. 2010;12:213–223. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- 44.Lau A, et al. A noncanonical mechanism of Nrf2 activation by autophagy deficiency: direct interaction between Keap1 and p62. Molecular and cellular biology. 2010;30:3275–3285. doi: 10.1128/MCB.00248-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ro SH, et al. Sestrin2 promotes Unc-51-like kinase 1 mediated phosphorylation of p62/sequestosome-1. The FEBS journal. 2014;281:3816–3827. doi: 10.1111/febs.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ichimura Y, et al. Phosphorylation of p62 activates the Keap1-Nrf2 pathway during selective autophagy. Mol Cell. 2013;51:618–631. doi: 10.1016/j.molcel.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 47.Buitrago-Molina LE, et al. The degree of liver injury determines the role of p21 in liver regeneration and hepatocarcinogenesis in mice. Hepatology. 2013;58:1143–1152. doi: 10.1002/hep.26412. [DOI] [PubMed] [Google Scholar]
- 48.Jain A, et al. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. The Journal of biological chemistry. 2010;285:22576–22591. doi: 10.1074/jbc.M110.118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shin BY, et al. Nrf2-ARE pathway regulates induction of Sestrin-2 expression. Free radical biology & medicine. 2012;53:834–841. doi: 10.1016/j.freeradbiomed.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 50.Reed TT. Lipid peroxidation and neurodegenerative disease. Free radical biology & medicine. 2011;51:1302–1319. doi: 10.1016/j.freeradbiomed.2011.06.027. [DOI] [PubMed] [Google Scholar]
- 51.Davi G, et al. Lipid peroxidation in diabetes mellitus. Antioxidants & redox signaling. 2005;7:256–268. doi: 10.1089/ars.2005.7.256. [DOI] [PubMed] [Google Scholar]
- 52.Zhang PY, et al. Cardiovascular diseases: oxidative damage and antioxidant protection. European review for medical and pharmacological sciences. 2014;18:3091–3096. [PubMed] [Google Scholar]
- 53.Feng Z, et al. The coordinate regulation of the p53 and mTOR pathways in cells. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:8204–8209. doi: 10.1073/pnas.0502857102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ben-Sahra I, et al. Sestrin2 integrates Akt and mTOR signaling to protect cells against energetic stress-induced death. Cell Death Differ. 2013;20:611–619. doi: 10.1038/cdd.2012.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hong-Brown LQ, et al. Adamts1 mediates ethanol-induced alterations in collagen and elastin via a FoxO1-sestrin3-AMPK signaling cascade in myocytes. Journal of cellular biochemistry. 2015;116:91–101. doi: 10.1002/jcb.24945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eid AA, et al. Sestrin 2 and AMPK connect hyperglycemia to Nox4-dependent endothelial nitric oxide synthase uncoupling and matrix protein expression. Molecular and cellular biology. 2013;33:3439–3460. doi: 10.1128/MCB.00217-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim M, Lee JH. Identification of an AMPK phosphorylation site in Drosophila TSC2 (gigas) that regulate cell growth. International journal of molecular sciences. 2015;16:7015–7026. doi: 10.3390/ijms16047015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sanli T, et al. Sestrin2 modulates AMPK subunit expression and its response to ionizing radiation in breast cancer cells. PloS one. 2012;7:e32035. doi: 10.1371/journal.pone.0032035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dokudovskaya S, Rout MP. SEA you later alli-GATOR--a dynamic regulator of the TORC1 stress response pathway. J Cell Sci. 2015;128:2219–2228. doi: 10.1242/jcs.168922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bar-Peled L, et al. A Tumor suppressor complex with GAP activity for the Rag GTPases that signal amino acid sufficiency to mTORC1. Science. 2013;340:1100–1106. doi: 10.1126/science.1232044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen X, et al. Modular pathways for editing non-cognate amino acids by human cytoplasmic leucyl-tRNA synthetase. Nucleic Acids Res. 2011;39:235–247. doi: 10.1093/nar/gkq763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen CC, et al. FoxOs inhibit mTORC1 and activate Akt by inducing the expression of Sestrin3 and Rictor. Dev Cell. 2010;18:592–604. doi: 10.1016/j.devcel.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bruning A, et al. Nelfinavir and bortezomib inhibit mTOR activity via ATF4-mediated sestrin-2 regulation. Mol Oncol. 2013;7:1012–1018. doi: 10.1016/j.molonc.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eagle H, et al. The intracellular amino acid concentrations required for protein synthesis in cultured human cells. The Journal of biological chemistry. 1961;236:2039–2042. [PubMed] [Google Scholar]
- 65.Baydoun AR, et al. Substrate-dependent regulation of intracellular amino acid concentrations in cultured bovine aortic endothelial cells. Biochemical and biophysical research communications. 1990;173:940–948. doi: 10.1016/s0006-291x(05)80876-9. [DOI] [PubMed] [Google Scholar]
- 66.Gallinetti J, et al. Amino acid sensing in dietary-restriction-mediated longevity: roles of signal-transducing kinases GCN2 and TOR. Biochem J. 2013;449:1–10. doi: 10.1042/BJ20121098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kimball SR, et al. Leucine induced dephosphorylation of Sestrin2 promotes mTORC1 activation. Cellular signalling. 2016 doi: 10.1016/j.cellsig.2016.03.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Linares JF, et al. Amino Acid Activation of mTORC1 by a PB1-Domain-Driven Kinase Complex Cascade. Cell reports. 2015;12:1339–1352. doi: 10.1016/j.celrep.2015.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moscat J, Diaz-Meco MT. Feedback on fat: p62-mTORC1-autophagy connections. Cell. 2011;147:724–727. doi: 10.1016/j.cell.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Duran A, et al. p62 is a key regulator of nutrient sensing in the mTORC1 pathway. Molecular cell. 2011;44:134–146. doi: 10.1016/j.molcel.2011.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dann SG, et al. mTOR Complex1-S6K1 signaling: at the crossroads of obesity, diabetes and cancer. Trends Mol Med. 2007;13:252–259. doi: 10.1016/j.molmed.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 72.Huang J, et al. The TSC1-TSC2 complex is required for proper activation of mTOR complex 2. Mol Cell Biol. 2008;28:4104–4115. doi: 10.1128/MCB.00289-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Budanov AV, et al. Stressin’ Sestrins take an aging fight. EMBO molecular medicine. 2010;2:388–400. doi: 10.1002/emmm.201000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gwinn DM, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]