Abstract
Patients infected or colonized with carbapenem-resistant Klebsiella pneumoniae (CRKp) are often chronically and acutely ill, which results in substantial mortality unrelated to infection. Therefore, estimating excess mortality due to CRKp infections is challenging. The Consortium on Resistance against Carbapenems in K. pneumoniae (CRACKLE) is a prospective multicenter study. Here, patients in CRACKLE were evaluated at the time of their first CRKp bloodstream infection (BSI), pneumonia, or urinary tract infection (UTI). A control cohort of patients with CRKp urinary colonization without CRKp infection was constructed. Excess hospital mortality was defined as mortality in cases after subtracting mortality in controls. In addition, the adjusted hazard ratios (aHR) for time-to-hospital-mortality censored at 30 days associated with infection as compared to colonization were calculated in Cox proportional hazard models. In the study period, 260 patients with CRKp infections were included in the BSI (90), pneumonia (49), and UTI (121) groups, who were compared to 223 controls. All-cause hospital mortality in controls was 12%. Excess hospital mortality was 27% and 27% in patients with BSI and pneumonia, respectively. Excess hospital mortality was not observed in patients with UTI. In multivariable analyses, BSI and pneumonia as compared to controls was associated with an aHR of 2.59 (95% CI 1.52–4.50, p<0.001) and 3.44 (95% CI 1.80–6.48, p<0.001), respectively. In conclusion, in patients with CRKp infection, pneumonia is associated with the highest excess hospital mortality. Patients with BSI have slightly lower excess hospital mortality rates, whereas excess hospital mortality was not observed in hospitalized patients with UTI.
Keywords: carbapenem resistant Enterobacteriaceae, Klebsiella pneumoniae, mortality, epidemiology, pneumonia
Introduction
Infections with carbapenem-resistant Enterobacteriaceae (CRE) are an important and growing threat to vulnerable hospitalized patients [1]. Patients who are at risk for colonization and/or infection with CRE during hospitalization tend to be chronically and acutely ill [2–4]. Carbapenem-resistant Klebsiella pneumoniae (CRKp) are the most common CRE in the United States. Reported estimates of all-cause hospital mortality rates of CRKp infection depend on the type of infection [2]. Most data are available for CRKp bloodstream infections for which estimates range from ~40 to ~70% [5–11]. For non-bacteremic CRKp infections, fewer studies specifically report mortality rates. CRKp pneumonia was associated with 24% all-cause hospital mortality in a recent US-based report and 40% all-cause 14-day mortality in a larger Italian study [11, 12]. Available data suggest that CRKp urinary tract infections are associated with only limited mortality [2, 11, 13].
Determining the impact of CRKp infection on mortality in hospitalized patients is challenging given the extensive chronic comorbidities which these patients tend to have. Moreover, infections with CRKp tend to be associated with various invasive procedures and devices during hospitalization, as well as with exposure to intensive care settings. These risk factors are independently associated with mortality, even in the absence of infection. Therefore, estimating the hospital mortality associated with infection with CRKp is not straightforward. The overall mortality of patients who die in the hospital following CRKp infection is the sum of infection-related mortality and non-infection-related mortality. This non-infection-associated contribution to overall mortality is relatively larger in patients colonized with CRKp as compared to patients colonized with more susceptible organisms, since risk factors for mortality such as chronic and acute illness, overlap with risk factors for CRKp colonization. An estimate of this non-infection-related mortality may be approximated in patients who are colonized, but not infected with CRKp. Estimating the infection-related mortality by subtracting this non-infection-related mortality is important, given the expectation that any intervention geared towards improved treatment of infection will only impact that infection-related mortality. Therefore, even a “perfect” antibiotic – i.e. a treatment that would reduce infection-related mortality in patients infected with CRKp to 0% – is expected to only decrease overall hospital mortality to the rate of CRKp colonized controls.
In this study, prospectively collected data from the multicenter Consortium on Resistance against Carbapenems in Klebsiella pneumoniae (CRACKLE) were analyzed to approximate the impact on hospital mortality of specific CRKp infections by comparing the hospital mortality rates associated with these infections to those observed in patients colonized but not infected with CRKp.
Methods
Design
The Consortium on Resistance against Carbapenems in Klebsiella pneumoniae (CRACKLE) was previously described [14, 15]. Briefly, CRACKLE is a multicenter, prospective, longitudinal, observational study of hospitalized patients with positive cultures for CRKp. In the study period from 12/24/2011 to 10/1/2014, 3 nested case cohorts were constructed from patients enrolled in CRACKLE; patients with CRKp bloodstream infection (BSI), CRKp pneumonia, and CRKp urinary tract infection (UTI), respectively. A control cohort consisting of patients with CRKp urinary colonization was also constructed. In the control cohort, patients who previously or subsequently developed CRKp BSI, CRKp pneumonia, and/or CRKp UTI were excluded. In all cohorts, patients were included only once at the time of the first occurrence of infection or colonization. Routine screening of asymptomatic patients for CRKp carriage was not performed at any of the study sites during the study period. The Institutional Review Boards of all sites involved approved the study.
Definitions
Time-to-hospital-mortality was the primary outcome of study. The day of the first positive culture for CRKp was used as day 0 for time-to-event analyses. For analysis purposes, patients discharged to hospice were considered deceased at the time of discharge. Critical illness was defined as a Pitt bacteremia score greater or equal to 4 points, on the day of the index culture [16]. Standardized definitions of infections were used, as previously described [2, 17]. Briefly, a patient with a blood culture positive for CRKp was deemed to have a CRKp BSI. For patients with positive respiratory cultures the criteria outlined by the American Thoracic Society (ATS) and the Infectious Diseases Society of America (IDSA) were used [18, 19]. Criteria as outlined by Centers for Disease Control/National Healthcare Safety Network (CDC/NHSN) were used to define UTI and asymptomatic bacteremic UTI (ABUTI); these two categories were grouped together as UTI for analysis purposes [20]. Briefly, patients with a positive urine culture for CRKp, who had at least one of the following signs or symptoms: fever (>38.0°C), suprapubic tenderness, costovertebral angle pain or tenderness, urinary urgency, urinary frequency, or dysuria were considered to have a UTI [20]. In addition, ABUTI was defined as having a positive blood and urine culture positive for CRKp without urinary symptoms [20]. Patients who had positive urine cultures for CRKp, who did not meet criteria for infection per CDC/NHSN were considered to have CRKp urinary colonization.
Microbiology
Any K. pneumoniae isolate with non-susceptibility (MIC > 1 μg/ml) to any tested carbapenem was considered a CRKp as per guidelines from the Clinical and Laboratory Standards Institute [21]. Bacterial identification and routine antimicrobial susceptibility testing was performed with MicroScan (Siemens Healthcare Diagnostics) or Vitek2 (BioMerieux), supplemented by GN4F Sensititre tray (Thermo Fisher) or Etest (bioMerieux), as indicated. In more than 90% of tested isolates, carbapenem resistance was mediated through blaKPC-2 or blaKPC-3, as previously described [14].
Statistical Analysis
Differences between groups were analyzed using Wilcoxon Rank Sum for continuous variables. Modified Wald method was used to calculate 95% confidence intervals for proportions. Fisher’s Exact, and Pearson testing were used for categorical variables where appropriate. Kaplan-Meier curves were used to compare time-to-hospital-mortality in the various cohorts. Cox proportional hazards models on time-to-hospital-mortality was used to calculate adjusted hazard ratios (aHR). For time-to-mortality analyses, patients were censored at the time of hospital discharge, or if still admitted and alive at 30 days after first positive culture, patient data were censored at 30 days after the date of the first positive culture. Forward selection was used for inclusion of variables in multivariable Cox proportional hazards models; any variable that was associated with time-to-hospital-mortality at p<0.2 was included. However, if the Charlson comorbidity index was included in the multivariable model, components of this score (such as renal failure) were not separately included. P values of ≤0.05 were considered statistically significant. JMP 10.0.1 software (SAS, Inc, Cary, NC) was used for all analyses.
Results
Patients
During the study period, 90, 49, and 121 patients had BSI, pneumonia, and UTI, respectively. In the control cohort 223 patients with CRKp urinary colonization were included (Table 1). Patients with urinary colonization were more likely to be female, and tended to be slightly older. No differences were observed in the distribution of comorbid conditions, such as diabetes, heart disease and chronic kidney disease.
Table 1. Demographics.
All data expressed as n (%), unless otherwise indicated. IQR, interquartile range.
| Controls | BSI | p# | pneumonia | p# | UTI | p# | |
|---|---|---|---|---|---|---|---|
| n | 223 | 90 | - | 49 | - | 121 | - |
| Age, median (IQR) | 71 (63–81) | 63 (54–78) | <0.01 | 68 (58–81) | 0.36 | 69 (57–82) | 0.20 |
| Female | 148 (66) | 44 (48) | <0.01 | 24 (49) | 0.03 | 65 (54) | 0.03 |
| Race/Ethnicity | 0.06 | 0.53 | 0.05 | ||||
| White | 132 (59) | 45 (50) | 27 (55) | 58 (48) | |||
| Black | 78 (35) | 44 (49) | 17 (35) | 58 (48) | |||
| Hispanic | 5 (2) | 0 | 3 (6) | 4 (3) | |||
| Other | 8 (4) | 1 (1) | 2 (4) | 1 (1) | |||
| Charlson comorbidity index, median (IQR) | 3 (2–5) | 3 (2–5) | 0.51 | 3 (2–5) | 0.18 | 3 (2–5) | 0.43 |
| Diabetes mellitus | 117 (52) | 40 (44) | 0.21 | 24 (49) | 0.75 | 60 (50) | 0.65 |
| Renal failure‡ | 55 (25) | 31 (34) | 0.09 | 9 (18) | 0.45 | 30 (25) | 1.0 |
| Heart disease | 125 (56) | 41 (46) | 0.13 | 31 (63) | 0.43 | 57 (47) | 0.12 |
| COPD | 63 (29) | 19 (21) | 0.20 | 18 (37) | 0.30 | 34 (28) | 1.0 |
| Malignancy | 42 (19) | 21 (23) | 0.44 | 4 (8) | 0.09 | 18 (15) | 0.38 |
comparison with controls.
renal failure defined as creatinine >2 mg/dL upon admission.
Excess mortality
In the BSI cohort, 35/90 (39%) patients died or were discharged to hospice, as compared to 26/223 (12%) in controls (Table 2). This results in an excess hospital-mortality of CRKp BSI of 27% (95% CI 16%–38%). In patients with pneumonia, 19/49 (39%) patients died or were discharged to hospice, resulting in excess hospital mortality of CRKp pneumonia of 27% (95% CI 14%–43%). In contrast, CRKp UTI was not associated with excess mortality (estimate −3%, 95% CI −9%–3%).
Table 2. Outcomes.
All data expressed as n (%), unless otherwise indicated. IQR, interquartile range.
| controls | BSI | p# | pneumonia | p# | UTI | p# | |
|---|---|---|---|---|---|---|---|
| n | 223 | 90 | 49 | 121 | |||
| Length of stay, days, median (IQR) | 9 (5–16) | 14 (9–24) | <0.01 | 19 (10–30) | <0.0001 | 10 (6–17) | 0.76 |
| Post-culture length of stay, days, median (IQR) | 7 (4–12) | 9 (4–16) | 0.03 | 10 (6–17) | <0.01 | 7 (4–12) | 0.30 |
| Critical illness§ | 49 (22) | 50 (56) | <0.0001 | 42 (86) | <0.0001 | 33 (27) | 0.29 |
| Disposition* | <0.0001 | <0.0001 | 0.59 | ||||
| Death | 21 (9) | 34 (38) | 17 (34) | 9 (7) | |||
| Hospice | 5 (2) | 1 (1) | 2 (4) | 2 (2) | |||
| Alive | 197 (88) | 55 (61) | 30 (61) | 110 (91) |
time from admission to first positive culture in days.
comparison with controls.
critical illness defined as Pitt bacteremia score ≥4 at the time of index culture.
comparison between death/hospice and alive.
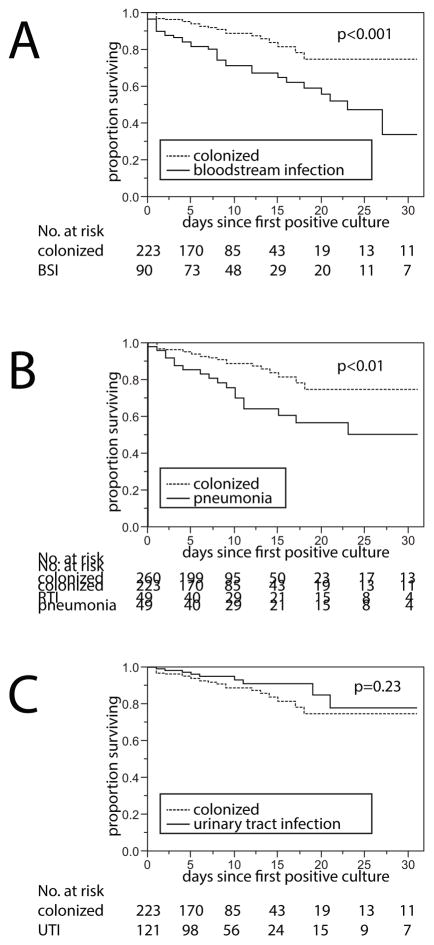
The time-to-hospital-mortality was compared between each infection cohort and the control cohort (Figure 1). BSI and pneumonia were associated with increased hazards of mortality (see Tables 3 and 4). In multivariable Cox proportional hazard modeling, the adjusted HR (aHR) of BSI and pneumonia were 2.59 (95% CI 1.52–4.50, p<0.001) and 3.44 (95% CI 1.80–6.48, p<0.001), respectively. UTI was not associated with an increased hazard (aHR 0.68, 95% CI 0.30–1.45, Table 5). In the models comparing patients with pneumonia and controls, as well as the model that compared patients with UTI and their controls, the Charlson comorbidity index was associated with time-to-hospital-mortality with an aHR of 1.19 (95% CI 1.06–1.34, p<0.01), and 1.20 (95% CI 1.06–1.36, p<0.01) per point increase, respectively. When comparing BSI and controls, no association between the Charlson comorbidity index and mortality was observed (Table 3). To determine the impact of grouping patients who were discharged to hospice together with those who died, the Cox models were repeated and patients discharged to hospice were considered “alive”. In these models, BSI vs. colonization and pneumonia vs. colonization were associated with slight increases in the point estimates of the aHR of 3.23 (95% CI 1.82–5.89, p<0.0001), and 3.64 (95% CI 1.80–7.27, P<0.001). The aHR for the association between UTI vs. colonization remained similar at 0.63 (95% CI 0.24–1.46, p=0.28).
Figure 1. Time-to-hospital-mortality compared between patients infected vs. colonized with carbapenem-resistant K. pneumoniae (CRKp).
Kaplan-Meier curves are shown and groups were compared using log-rank testing. Data were censored at the time of hospital discharge. A. Time-to-hospital-mortality in patients with CRKp bloodstream infection. B. Time-to-hospital-mortality in patients with CRKp pneumonia. C. Time-to-hospital-mortality in patients with CRKp urinary tract infection.
Table 3. Cox proportional hazards model for time-to-hospital-mortality in cohorts of patients with bloodstream infection (n=90) and their controls (n=223).
BSI: bloodstream infection. IQR, interquartile range.
| Univariable analysis | Multivariable model | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | aHR | 95% CI | p | |
| BSI vs. colonization | 2.71 | 1.60–4.65 | <0.001 | 2.59 | 1.52–4.50 | <0.001 |
| Age, median (IQR)* | 1.00 | 0.99–1.02 | 0.72 | |||
| Female | 1.18 | 0.69–2.06 | 0.55 | |||
| Race/Ethnicity | 0.02 | 0.19 | ||||
| White (ref.) | - | - | - | - | ||
| Black | 2.02 | 1.19–3.44 | 1.65 | 0.95–2.87 | ||
| Hispanic or Other# | 0.62 | 0.03–2.92 | 0.82 | 0.05–3.98 | ||
| Charlson comorbidity index, median (IQR) | 1.10 | 1.00–1.21 | 0.05 | 1.09 | 0.99–1.20 | 0.09 |
| Diabetes mellitus | 0.77 | 0.45–1.30 | 0.33 | |||
| Renal failure‡ | 1.95 | 1.14–3.29 | 0.02 | |||
| Heart disease | 1.04 | 0.61–1.76 | 0.90 | |||
| COPD | 1.09 | 0.58–1.93 | 0.78 | |||
| Malignancy | 1.54 | 0.84–2.69 | 0.16 | |||
renal failure defined as creatinine >2 mg/dL upon admission.
Grouped given small numbers in the “Other” and “Hispanic” race categories.
Analyzed per year increase.
Table 4. Cox proportional hazards model for time-to-hospital-mortality in cohorts of patients with pneumonia (n=49) and their controls (n=260).
RTI: respiratory tract infection. IQR, interquartile range.
| Univariable analysis | Multivariable model | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | aHR | 95% CI | p | |
| pneumonia vs. colonization | 2.48 | 1.31–4.58 | <0.01 | 3.44 | 1.80–6.48 | <0.001 |
| Age, median (IQR)* | 1.02 | 1.00–1.05 | 0.07 | 1.02 | 0.99–1.04 | 0.12 |
| Female | 1.34 | 0.71–2.67 | 0.37 | |||
| Race/Ethnicity | 0.03 | 0.04 | ||||
| White (ref.) | - | - | - | - | ||
| Black | 1.97 | 1.05–3.65 | 1.65 | 0.87–3.10 | ||
| Hispanic or Other# | 0.39 | 0.02–1.87 | 0.25 | 0.01–1.23 | ||
| Charlson comorbidity index, median (IQR) | 1.18 | 1.05–1.31 | <0.01 | 1.19 | 1.06–1.34 | <0.01 |
| Diabetes mellitus | 1.41 | 0.76–2.69 | 0.27 | |||
| Renal failure‡ | 2.21 | 1.16–4.09 | 0.02 | |||
| Heart disease | 1.36 | 0.73–2.68 | 0.33 | |||
| COPD | 1.17 | 0.60–2.20 | 0.63 | |||
| Malignancy | 1.10 | 0.47–2.25 | 0.82 | |||
renal failure defined as creatinine >2 mg/dL upon admission.
Grouped given small numbers in the “Other” and “Hispanic” race categories.
Analyzed per year increase.
Table 5. Cox proportional hazards model for time-to-hospital-mortality in cohorts of patients with urinary tract infections (n=121) and their controls (n=232).
UTI: urinary tract infection. IQR, interquartile range.
| Univariable analysis | Multivariable model | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | aHR | 95% CI | p | |
| UTI vs. colonization | 0.63 | 0.28–1.31 | 0.22 | 0.68 | 0.30–1.45 | 0.33 |
| Age, median (IQR)* | 1.03 | 1.01–1.06 | 0.01 | 1.03 | 1.00–1.06 | 0.07 |
| Female | 1.20 | 0.60–2.58 | 0.61 | |||
| Race/Ethnicity | <0.01 | 0.03 | ||||
| White (ref.) | - | - | - | - | ||
| Black | 2.66 | 1.33–5.48 | 2.06 | 0.99–4.38 | ||
| Hispanic or Other# | 0.00 | 0.00–1.79 | 0.00 | 0.00–1.71 | ||
| Charlson comorbidity index, median (IQR) | 1.27 | 1.12–1.44 | <0.001 | 1.20 | 1.06–1.36 | <0.01 |
| Diabetes mellitus | 1.39 | 0.70–2.87 | 0.35 | |||
| Renal failure‡ | 2.53 | 1.26–5.02 | 0.01 | |||
| Heart disease | 2.12 | 1.04–4.67 | 0.04 | |||
| COPD | 0.99 | 0.43–2.06 | 0.97 | |||
| Malignancy | 1.56 | 0.66–3.33 | 0.29 | |||
renal failure defined as creatinine >2 mg/dL upon admission.
Grouped given small numbers in the “Other” and “Hispanic” race categories.
Analyzed per year increase.
Other outcomes
Patients with BSI and pneumonia had significantly longer durations of hospitalization when compared to colonized controls (Table 2); BSI and pneumonia were associated with an increased median total length of stay of 5 days (p<0.01) and 10 days (p<0.0001) respectively. The median length of stay after first positive culture was also significantly prolonged in patients with BSI or pneumonia; by 2 days (p=0.03) and 3 days (p<0.01), respectively. In contrast, the length of stay in patients with UTI vs. colonization was similar; 10 days vs. 9 days for median total length of stay and 7 days vs. 7 days for the median post-culture length of stay (Table 2). Patients with BSI as well as those patients with pneumonia were more likely to be critically ill at the time of first positive culture when compared to controls. In the BSI cohort, 56% of patients as compared to 22% in controls were critically ill (p<0.0001). In the pneumonia cohort, this difference was even more pronounced; 86% of patients with CRKp pneumonia were critically ill (p<0.0001). No significant difference in critical illness rates were observed between patients with UTI and colonization.
Discussion
A novel method of estimating excess mortality in patients with infections caused by MDR-O is described here. Using this method and after adjustment for confounding variables, patients with CRKp pneumonia died at more than 3 times the rate of similar patients with CRKp colonization. This effect size is consistent with the known severity of pneumonia in hospitalized patients caused by multidrug-resistant organisms (MDR-O) [22–24]. Patients with CRKp BSI died at more than twice the rate compared to those with CRKp colonization. This is also consistent with previous studies on all-cause mortality in patients with CRKp BSI [5–9]. In addition to mortality risk, CRKp pneumonia and BSI were also associated with increases in both total and post-culture length of hospitalization. Increased length of stay is important for several reasons; it is associated with increased health care costs, increased risk for other hospital-acquired infections and complications, and increased spread of CRE to other vulnerable patients.
In this study, the excess hospital mortality rates in three cohorts of patients with CRKp infections were estimated by comparing them to patients with urinary CRKp colonization. This is a novel way of evaluating mortality that takes into account measured and unmeasured risk factors that are associated with CRKp colonization in hospitalized patients. Risk factors for CRKp colonization include exposure to acute care and long-term care settings, antibiotics, indwelling devices, as well as certain comorbid conditions [25–27]. Reported risk factors for subsequent CRKp infection in colonized patients included ICU admission, central venous catheters, receipt of antibiotics, diabetes mellitus, abdominal invasive procedures, and chemotherapy or radiation therapy [28, 29]. As outlined by Safdar and Maki in 2002, risk factors for colonization with MDR-O tend to overlap and therefore co-colonization of CRKp with other MDR-O is often found [30, 31]. To some extent these risk factors can be measured and controlled for, but a cohort of patients without MDR-O will always be different in a variety of ways from a cohort with MDR-O. Therefore, the current approach was chosen to estimate as closely as possible the impact of getting a specific infection caused by CRKp in a relatively homogeneous population. Patients with enteral CRKp colonization form the background population from whom it is postulated that both cases and controls arose in this study.
In contrast to patients with pneumonia and BSI, patients with CRKp UTI had no excess mortality when compared to colonized patients. This is a good example that simply examining crude mortality rates may be misleading. An important limitation in this context is that the diagnosis of UTI is notoriously difficult. We used standardized definitions which nonetheless may misclassify patients. This misclassification may have contributed to the lack of difference in mortality between patients with UTI and urinary colonization. However, the data presented here are biologically plausible and consistent with previous reports [11, 13]. Another limitation of the study is that patients were not followed after their discharge from the hospital. This could potentially result in informative censoring which could have biased the results of the Cox models. In addition, longer term outcomes are of great interest in this population and deserve further study.
In summary, CRKp pneumonia and CRKp BSI are associated with large mortality risks in hospitalized patients. CRKp UTI, on the other hand, is not associated with excess mortality. The quantification of these risks may help guide future interventions and hopefully motivate interventional studies to decrease both the risk of CRE colonization and infection.
Acknowledgments
The authors would like to thank Dr. Anthony D. Harris for his thoughtful comments.
Funding:
This work was supported by the National Institute Of Allergy And Infectious Diseases of the National Institutes of Health under Award Number UM1AI104681, and by funding to DVD and FP from the Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research. VGF was supported by Mid-Career Mentoring Award K24-AI093969 from NIH. In addition, this work was supported in part by the Veterans Affairs Merit Review Program (RAB), the National Institutes of Health (Grant AI072219-05 and AI063517-07 to RAB), and the Geriatric Research Education and Clinical Center VISN 10 (RAB), the Research Program Committees of the Cleveland Clinic (DVD), an unrestricted research grant from the STERIS Corporation (DVD). YD was supported by research awards R01AI104895 and R21AI107302 from the NIH. KSK is supported by the National Institute of Allergy and Infectious Diseases (Division of Microbiology and Infectious Diseases protocol 10-0065 and RO1 1R01AI119446-01)
Footnotes
Preliminary results from this study were presented at ICAAC; September 17–21, 2015, San Diego, California, USA.
Disclaimer. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest:
No potential conflicts: J.A.M, E.C., F.P., R.A.S., R.C.K., R.R.W., N.M.S., and S.E.
Potential conflicts of interest: S.S.R: Research support from bioMerieux, BD Diagnostics, BioFire, OpGen, Forest Laboratories, Achaogen, Nanosphere and Pocared. Honorarium from bioMerieux. Y.D.: Grant support: Merck, NIH. Consulting fee: Melinta. Advisory board: Shionogi. K.K: Forest Laboratories, Inc., Consultant, Grant Investigator and Speaker’s Bureau, Consulting fee, Grant recipient and Speaker honorarium. R.A.B.: AstraZeneca: Grant Investigator, Grant recipient, Merck: Grant Investigator, Grant recipient, Melinta: Grant Investigator, Grant recipient, Steris: Grant Investigator, Grant recipient, NIH: Grant Investigator, Grant recipient, VA Merit Review: Grant Investigator, Grant recipient. V.G.F.: Grant/Research Support: Advanced Liquid Logic, Cubist, Cerexa, MedImmune, Merck, NIH, Novartis, Pfizer, Theravance. Paid Consultant: Affinium, Baxter, Cerexa, Cubist, Debiopharm, Durata, Merck, Novartis, NovaDigm, The Medicines Company, MedImmune, Pfizer, Theravance, Trius. Honoraria: Arpida, Astellas, Cubist, Inhibitex, Merck, Pfizer, Targanta, Theravance, Wyeth, Ortho-McNeil, Novartis, Vertex Pharmaceuticals. Membership: Merck Co-Chair V710 Vaccine.
D.v.D.: Actavis, Tetraphase, Sanofi-Pasteur, Advisory Board. Steris Inc., Research funding. Scynexis Research funding
All other authors: no conflicts reported
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Perez F, van Duin D. Carbapenem-resistant enterobacteriaceae: A menace to our most vulnerable patients. Cleve Clin J Med. 2013;80:225–33. doi: 10.3949/ccjm.80a.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Duin D, Perez F, Rudin SD, et al. Surveillance of carbapenem-resistant klebsiella pneumoniae: Tracking molecular epidemiology and outcomes through a regional network. Antimicrob Agents Chemother. 2014;58:4035–41. doi: 10.1128/AAC.02636-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nouvenne A, Ticinesi A, Lauretani F, et al. Comorbidities and disease severity as risk factors for carbapenem-resistant klebsiella pneumoniae colonization: Report of an experience in an internal medicine unit. PLoS One. 2014;9:e110001. doi: 10.1371/journal.pone.0110001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin ET, Tansek R, Collins V, et al. The carbapenem-resistant enterobacteriaceae score: A bedside score to rule out infection with carbapenem-resistant enterobacteriaceae among hospitalized patients. Am J Infect Control. 2013;41:180–2. doi: 10.1016/j.ajic.2012.02.036. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen M, Eschenauer GA, Bryan M, et al. Carbapenem-resistant klebsiella pneumoniae bacteremia: Factors correlated with clinical and microbiologic outcomes. Diagn Microbiol Infect Dis. 67:180–4. doi: 10.1016/j.diagmicrobio.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Tumbarello M, Viale P, Viscoli C, et al. Predictors of mortality in bloodstream infections caused by klebsiella pneumoniae carbapenemase-producing k. Pneumoniae: Importance of combination therapy. Clin Infect Dis. 2012;55:943–50. doi: 10.1093/cid/cis588. [DOI] [PubMed] [Google Scholar]
- 7.Qureshi ZA, Paterson DL, Potoski BA, et al. Treatment outcome of bacteremia due to kpc-producing klebsiella pneumoniae: Superiority of combination antimicrobial regimens. Antimicrob Agents Chemother. 2012;56:2108–13. doi: 10.1128/AAC.06268-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neuner EA, Yeh JY, Hall GS, et al. Treatment and outcomes in carbapenem-resistant klebsiella pneumoniae bloodstream infections. Diagn Microbiol Infect Dis. 2011;69:357–62. doi: 10.1016/j.diagmicrobio.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ben-David D, Kordevani R, Keller N, et al. Outcome of carbapenem resistant klebsiella pneumoniae bloodstream infections. Clin Microbiol Infec. 18:54–60. doi: 10.1111/j.1469-0691.2011.03478.x. [DOI] [PubMed] [Google Scholar]
- 10.Tzouvelekis LS, Markogiannakis A, Piperaki E, Souli M, Daikos GL. Treating infections caused by carbapenemase-producing enterobacteriaceae. Clin Microbiol Infect. 2014;20:862–72. doi: 10.1111/1469-0691.12697. [DOI] [PubMed] [Google Scholar]
- 11.Tumbarello M, Trecarichi EM, De Rosa FG, et al. Infections caused by kpc-producing klebsiella pneumoniae: Differences in therapy and mortality in a multicentre study. J Antimicrob Chemother. 2015;70:2133–43. doi: 10.1093/jac/dkv086. [DOI] [PubMed] [Google Scholar]
- 12.Ny P, Nieberg P, Wong-Beringer A. Impact of carbapenem resistance on epidemiology and outcomes of nonbacteremic klebsiella pneumoniae infections. Am J Infect Control. 2015 doi: 10.1016/j.ajic.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Qureshi ZA, Syed A, Clarke LG, Doi Y, Shields RK. Epidemiology and clinical outcomes of patients with carbapenem-resistant klebsiella pneumoniae bacteriuria. Antimicrob Agents Chemother. 2014;58:3100–4. doi: 10.1128/AAC.02445-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Duin D, Perez F, Rudin SD, et al. Surveillance of carbapenem-resistant klebsiella pneumoniae: Tracking molecular epidemiology and outcomes through a regional network. Antimicrob Agents Chemother. 2014;58:4035–41. doi: 10.1128/AAC.02636-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Duin D, Cober E, Richter S, et al. Tigecycline therapy for carbapenem-resistant klebsiella pneumoniae (crkp) bacteriuria leads to tigecycline resistance. Clin Microbiol Infect. 2014 doi: 10.1111/1469-0691.12714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chow JW, Yu VL. Combination antibiotic therapy versus monotherapy for gram-negative bacteraemia: A commentary. Int J Antimicrob Agents. 1999;11:7–12. doi: 10.1016/s0924-8579(98)00060-0. [DOI] [PubMed] [Google Scholar]
- 17.van Duin D, Cober E, Richter SS, et al. Impact of therapy and strain type on outcomes in urinary tract infections caused by carbapenem-resistant klebsiella pneumoniae. J Antimicrob Chemother. 2015;70:1203–11. doi: 10.1093/jac/dku495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mandell LA, Wunderink RG, Anzueto A, et al. Infectious diseases society of america/american thoracic society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Thoracic Society and Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Cdc/nhsn surveillance definitions for specific types of infections. 2014 www.cdc.gov/nhsn.
- 21.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; twenty-fourth informational supplement. 2014. p. 34. CLSI document M100-S24. [Google Scholar]
- 22.Tedja R, Nowacki A, Fraser T, et al. The impact of multidrug resistance on outcomes in ventilator-associated pneumonia. Am J Infect Control. 2014;42:542–5. doi: 10.1016/j.ajic.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 23.Parker CM, Kutsogiannis J, Muscedere J, et al. Ventilator-associated pneumonia caused by multidrug-resistant organisms or pseudomonas aeruginosa: Prevalence, incidence, risk factors, and outcomes. J Crit Care. 2008;23:18–26. doi: 10.1016/j.jcrc.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Caffrey AR, Morrill HJ, Puzniak LA, Laplante KL. Comparative effectiveness of linezolid and vancomycin among a national veterans affairs cohort with methicillin-resistant staphylococcus aureus pneumonia. Pharmacotherapy. 2014;34:473–80. doi: 10.1002/phar.1390. [DOI] [PubMed] [Google Scholar]
- 25.Prabaker K, Lin MY, McNally M, et al. Transfer from high-acuity long-term care facilities is associated with carriage of klebsiella pneumoniae carbapenemase-producing enterobacteriaceae: A multihospital study. Infect Control Hosp Epidemiol. 2012;33:1193–9. doi: 10.1086/668435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ben-David D, Masarwa S, Navon-Venezia S, et al. Carbapenem-resistant klebsiella pneumoniae in post-acute-care facilities in israel. Infect Control Hosp Epidemiol. 2011;32:845–53. doi: 10.1086/661279. [DOI] [PubMed] [Google Scholar]
- 27.Papadimitriou-Olivgeris M, Marangos M, Fligou F, et al. Risk factors for kpc-producing klebsiella pneumoniae enteric colonization upon icu admission. J Antimicrob Chemother. 2012;67:2976–81. doi: 10.1093/jac/dks316. [DOI] [PubMed] [Google Scholar]
- 28.Giannella M, Trecarichi EM, De Rosa FG, et al. Risk factors for carbapenem-resistant klebsiella pneumoniae bloodstream infection among rectal carriers: A prospective observational multicentre study. Clin Microbiol Infect. 2014;20:1357–62. doi: 10.1111/1469-0691.12747. [DOI] [PubMed] [Google Scholar]
- 29.Schechner V, Kotlovsky T, Kazma M, et al. Asymptomatic rectal carriage of bla(kpc) producing carbapenem-resistant enterobacteriaceae: Who is prone to become clinically infected? Clin Microbiol Infect. 2013;19:451–6. doi: 10.1111/j.1469-0691.2012.03888.x. [DOI] [PubMed] [Google Scholar]
- 30.Safdar N, Maki DG. The commonality of risk factors for nosocomial colonization and infection with antimicrobial-resistant staphylococcus aureus, enterococcus, gram-negative bacilli, clostridium difficile, and candida. Ann Intern Med. 2002;136:834–44. doi: 10.7326/0003-4819-136-11-200206040-00013. [DOI] [PubMed] [Google Scholar]
- 31.Marchaim D, Perez F, Lee J, et al. “Swimming in resistance”: Co-colonization with carbapenem-resistant enterobacteriaceae and acinetobacter baumannii or pseudomonas aeruginosa. Am J Infect Control. 2012;40:830–5. doi: 10.1016/j.ajic.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]