Abstract
The risk for respiratory diseases increases in adults >65 years of age, which may be partially due to ageing-related weakening and atrophy (i.e., sarcopenia) of the diaphragm muscle (DIAm). However, mechanisms underlying DIAm sarcopenia remain unknown. Based on existing evidence, we hypothesized that sarcopenia is most evident in type IIx and/or IIb DIAm fibres comprising more fatigable motor units. Currently, the USA National Institute on Aging supports Fischer 344 (F344) and Brown Norway (BN) rat strains for ageing related research, yet DIAm sarcopenia has not been comprehensively evaluated in either strain. Thus, the current study examined DIAm sarcopenia in older adult (24 month, 50% survival) F344 and (32 month, 50% survival) BN rats, compared to young adult (6 month) F344 and BN rats. Measurements of contractility, contractile protein concentration, fibre type distribution and fibre cross-sectional area were obtained from midcostal DIAm strips. Maximal specific force was reduced by ∼24% and ∼13% in older F344 and BN rats, respectively. Additionally, although cross-sectional area of type I and IIa DIAm fibres was unchanged in both F344 and BN rats, cross-sectional area of type IIx and/or IIb DIAm fibres was reduced by ∼20% and ∼15% in F344 and BN rats, respectively. Thus, although there was ageing-related DIAm weakness and atrophy, selective to type IIx and/or IIb DIAm fibres in both F344 and BN rats, the sarcopenic phenotype was more pronounced in F344 rats.
Keywords: atrophy, ageing, respiratory muscle
Introduction
The ongoing global demographic shift toward an increasing population of adults >65 years of age is undisputed and a widely acknowledged future socioeconomic challenge, particularly for the healthcare industry. In the USA alone, the population of adults >65 years of age will more than double by 2030, exceeding 70 million people (2012). Respiratory diseases associated with advancing age, are significant contributors to morbidity and mortality in adults >65 years of age (Xu et al., 2010; Heron, 2011). For example, compared to younger adults, there is a 3-fold higher incidence for pneumonia in otherwise healthy adults >65 years of age, and a 10-fold higher incidence for pneumonia for patients >65 years of age in chronic care institutions (Janssens & Krause, 2004; Chong & Street, 2008). This ageing-related increased incidence of respiratory diseases may be partially due to an ageing-related progressive weakening of the respiratory muscles (Tolep et al., 1995; Polkey et al., 1997), resulting in less productive coughing in adults >65 years of age. Indeed, previous work from our laboratory demonstrated an ageing-related weakening (reduced force per cross-sectional area - specific force) of the diaphragm muscle (DIAm) in Fischer 344 (F344) rats (Gosselin et al., 1994a) and C57BL/6 × 129 mice (Greising et al., 2013; Greising et al., 2015a), and a decrease in type IIx and/or IIb muscle fibre cross-sectional area in C57BL/6 × 129 mice (Greising et al., 2013). Together, reduced specific force and muscle fibre atrophy are hallmarks of sarcopenia (Cruz-Jentoft et al., 2010; Fielding et al., 2011). We also found that sarcopenia of the mouse DIAm specifically impairs higher force, non-ventilatory motor behaviours (e.g., coughing, sneezing), while not impacting normal ventilatory behaviours(Greising et al., 2015b). Thus, based on existing evidence, we hypothesized that sarcopenia in the rat DIAm is most evident in type IIx and/or IIb fibres comprising more fatigable motor units.
Rodent models of ageing are commonly used to study ageing-related changes in muscle physiology (Gosselin et al., 1994a; Alway et al., 2005), primarily due to the relatively short live span of rodents. In an effort to standardize rat models used in ageing-related research, the USA National Institute on Ageing (NIA) currently recommends the use of F344 and Brown Norway (BN) inbred rat strains and provides these animals freely for relevant ageing-related research.
Our previous observation of an ageing-related reduction in DIAm specific force in F344 rats (Gosselin et al., 1994a) was confirmed in subsequent studies (Powers et al., 1996; Criswell et al., 1997; Smith et al., 2002). An ageing-related reduction in DIAm specific force has not been explored in BN rats per se. There is very little information regarding the second major component of sarcopenia, ageing-related DIAm atrophy in these rat models. Using F344 rats, Kavazis and colleagues reported that ageing was not associated with DIAm fibre atrophy (Kavazis et al., 2007; Kavazis et al., 2012), an observation that is inconsistent with sarcopenia. To date, there are no reports on the impact of ageing on DIAm fibre cross-sectional area in BN rats. Accordingly, the purpose of this study was to comprehensively examine whether DIAm sarcopenia exists in both F344 and BN rat strains provided by the NIA.
Methods
All experiments were designed according to the guidelines for animal use in gerontological research (Miller & Nadon, 2000). All protocols were approved by the Institutional Animal Care and Use Committee (#A57714) at the Mayo Clinic, and were in compliance with the Physiological Society's policy on animal experimentation (Drummond, 2009), and the National Institutes of Health Guide for Care and Use of Laboratory Animals.
Animals
Young (6 month, 100% survival, n = 7) and old (24 month, 50% survival, n = 12) F344 and young (6 month, 100% survival, n = 7) and old (32 month, 50% survival, n = 12) BN male, pathogen free, rats were obtained from the NIA rodent colony. Male rats were chosen to avoid potential confounding effects of ageing-related changes in estrogen. In a previous study in C57BL/6 × 129 mice, we found that ageing-related changes in DIAm were similar between males and females (Greising et al., 2015b). The age of animal strains were matched based on survival information provided by the NIA. Previous studies have provided justification for age selection based on such survival information (Miller & Nadon, 2000).
Animals were maintained on an alternating 12 h light-dark cycle and provided with Purina rat chow and fresh water ad libitum. Following the arrival of each animal, a 48 h acclimatization period was provided prior to establishing a baseline measure of body mass or conducting any experimental procedures. Animals were then weighed every 3-4 days until the terminal experiment (∼7-14 days). Animals displaying an apparent rapid deterioration, evidenced by a progressive reduction in body weight (>10%) were excluded (n = 3, 24 month F344; n = 3, 32 month BN). In addition, some animals died prior to the terminal experiment (n = 2, 24 month F344; n = 2, 32 month BN). This attrition rate in older animals generally matched the 50% survival rate. A final of n = 7 old F344 and n = 7 old BN rats were studied, with a matching group of n = 7 young rats for each strain.
Diaphragm muscle excision and tissue preparation
At the terminal experiment, rats were anaesthetized with an intramuscular (hindlimb) injection of ketamine (100 mg · kg−1) and xylazine (10 mg · kg−1). Following induction of anaesthesia, animals were killed by exsanguination via the rapid excision of the entire DIAm leaving the ribs intact. The DIAm was then laid on a dissecting table, cleaned, excessive rib tissue trimmed, and cut in half coronally with the central tendon intact on both right and left sides. Muscle strips (∼3 mm wide) from the right midcostal DIAm were cut, and prepared for subsequent measures of contractility (at 26°C), contractile protein concentration, fibre type distribution and cross-sectional areas.
Diaphragm muscle contractility
DIAm strips were positioned in a vertical glass tissue chamber (30 ml volume) containing Reese-Simpson buffer (in mM: 135 Na+, 5 K+, 2 Ca2+, 1 Mg2+, 120 Cl−, 25 HCO3−; pH 7.4) maintained at 26°C and aerated with hyperoxic gas (95% O2 and 5% CO2). The muscle segment was suspended in the tissue chamber by clamping the rib to a micro-positioner and attaching the central tendon to a force transducer (Model 6350, Cambridge Technology, Cambridge, MA, USA). Platinum plate electrodes were placed on either side of the muscle strip and 1.0 ms duration monophasic rectangular pulses were delivered (Grass S88; SIU5D stimulus isolation unit; Grass Telefactor, Warwick, RI, USA) to induce isometric force. Stimulation intensity was gradually increased until a maximal twitch force response was achieved. Thereafter, stimulus intensity was set at 125% of this level (supramaximal) and muscle segment optimal length (Lo) was determined. Determination of Lo was accomplished by carefully adjusting the muscle strip length, and simultaneously eliciting isometric twitch responses until peak isometric twitch force (Pt) was reached. Following the conclusion of contractile measurements Lo was measured using digital callipers. Contractile measurements consisted of determination of Pt, as well as electrical stimulation across a range of frequencies; 5, 10, 20, 30, 40, 50, 75 and 100 Hz (corresponding to maximum tetanic force; Po) with train durations of 1 s allowing 2 min rest between each stimulus train. Both Pt and Po were normalized for muscle cross-sectional area, estimated by:
Where muscle weight (MW) was determined by weighing the muscle strip after blotting dry and carefully trimming and discarding rib tissue and central tendon, and 1.056 is the density (g/cm3) of muscle.
Myosin heavy chain concentration
The procedures for electrophoretic separation of MyHC isoforms in the rat DIAm have been previously described (Gosselin et al., 1994a; Geiger et al., 1999). Briefly, sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) was used to separate DIAm MyHC isoforms, from a segment of midcostal DIAm that had been rapidly frozen in liquid nitrogen. Samples were prepared according to Butler-Browne and Whalen (Butler-Browne & Whalen, 1984) with minor modifications (Gosselin et al., 1994a). Muscle samples were weighed and combined with 4× vol/wt of high salt myosin extraction buffer (in mM: 300 NaCl, 100 NaH2PO4, 50 Na2HPO4, 1 MgCl2, 10 Na4P2O7, and 10 EDTA; pH 6.50) with 0.1% 2-mercaptoethanol and scissor minced on ice for ∼5-10 min. Extracts were then centrifuged for 30 min at 12,800 g at 4°C (Model 367121 microfuge; Beckman Instruments, Palo Alto, CA, USA). The supernatant was recovered and 50 μl was diluted into 450 μl of low salt buffer (1 mM EDTA and 0.1% 2-mercaptoethanol), which was vortexed and allowed to sit overnight at 4°C. The sample was then centrifuged again for 30 min at 12,800 g at 4°C, the supernatant removed, and the resulting myosin pellet was re-suspended, boiled for 2 min, and dissolved directly in SDS sample buffer (62.5 mMTris/HCl, 2% wt/vol SDS, 10% vol/vol glycerol, 5% 2-mercaptoethanol, and 0.001% wt/vol bromophenol blue at a pH 6.8 and a final dilution of 1:200). Myosin extract was stored at -80°C until undergoing gel electrophoresis.
Gel preparation was based on a modification (Gosselin et al., 1994a; Geiger et al., 1999) of the procedure first described by Sugiura and Murakami (Sugiura & Murakami, 1990). Briefly, the stacking gel contained a 3.5% acrylamide concentration (30% total acrylamide, 37.5:1 acrylamide:bis-acrylamide ratio, pH 6.80) with 20% vol/vol glycerol, and the separating gel (16 × 18 cm in size, 0.75 mm thick; Hoefer SE600, Holliston, MA, USA) consisted of a 5-8% linear gradient acrylamide concentration (30% total acrylamide, 37.5:1 acrylamide:bis-acrylamide ratio, pH 8.80) with 40% vol/vol glycerol. Sample volumes of 10 μl were loaded into each well. Gels were run at a constant current of 20 mA/gel for ∼18 h, after which gels were removed from the plates and silver stained according to the procedure of Oakley and colleagues (Oakley et al., 1980).
To determine MyHC concentration, gels were imaged and analysed by densitometry, using a procedure first described by Geiger et al. (Geiger, 1999 #8193) for single muscle fibres. Briefly, three myosin standards of known concentrations (0.006, 0.044 and 0.089 μg), verified using the Bradford method (Bradford, 1976), were run on each gel. Following subtraction of local background, the cumulative brightness area product (BAP) comprising all four MyHC isoform bands (MyHCSlow, MyHC2A, MyHC2X, MyHC2B) of each sample was compared to the cumulative BAP of myosin standards (fit by linear regression) to determine total MyHC concentration.
Diaphragm muscle fibre type proportions and cross-sectional area
In determining fibre cross-sectional area, it is important to control for sarcomere length. In previous studies, we verified that when the midcostal DIAm is stretched to 150% of resting length this approximates measured Lo (Prakash et al., 1993) and provides a consistent sarcomere length of ∼2.5 μm. Accordingly, midcostal DIAm segments were stretched to 150% resting length, pinned to cork using minutien pins (Fine Science Tools, #26002-15), and frozen in melting isopentane (cooled to its freezing point by liquid nitrogen). Serial 10 μm thick cross-sections were cut using a Reichert-Jung Frigocut cryostat (Reichert Microscope Services, model 2800E, Depew, NY, USA) maintained at -20°C. Three serial cross-sections were placed on each slide and stored at -80°C prior to immunohistochemical analysis. Cross-sections were fixed in acetone kept at 4°C for 10 min, rinsed with 0.1 M phosphate buffer and incubated for 30 min in 10% Goat Serum (∼50 μl/cross-section). Excess Goat Serum was drained and cross-sections were then incubated overnight at 4°C in primary antibodies (∼50 μl/cross-section) for MyHC isoforms: anti-MyHCSlow (BA-F8 obtained from Hybridoma Bank, Iowa City, IA, USA), anti-MyHC2A (SC-71 obtained from Hybridoma Bank, Iowa City, IA) and laminin (Sigma L9393) to visualize the sarcolemma. DIAm sections were then treated with appropriate fluorescently-conjugated secondary antibodies (∼50 μl/cross-section): Alexa 488 nm (Reference #: A21141; Life Technologies, Eugene, OR, USA) for MyHCSlow, Alexa Fluor 568 nm (Reference #: A21124; Life Technologies, Eugene, OR, USA) for MyHC2A, and Alexa Fluor 405 nm (Reference #: A31556; Invitrogen, Molecular Probes, Eugene, OR, USA) for laminin. Based on the pattern of staining for MyHC isoforms muscle fibre types were classified as type I, type IIa, and type IIx and/or IIb (Greising et al., 2013).
DIAm cross-sections were imaged using a confocal microscope (Nikon Eclipse C1, Nikon Instruments Inc., Melville, NY, USA) with Argon (488 nm) and solid-state (405 and 564 nm) lasers capable of simultaneous multi-label fluorescence imaging. Digital images (12-bit, 512 × 512 pixel array) were obtained using a 20× oil-immersion objective (calibrated at 1.24 μm2/pixel) and saved as a grey scale-TIFF file with each fluorescence channel separated. Images were then pseudo-coloured and merged using NIS-Elements (Nikon Instruments Inc. Version 4.10). Fibre type proportion and cross-sectional areas were determined using the integrated morphometric analysis tool in NIS-Elements. In assessing ageing-related changes in DIAm fibre type proportions and cross-sectional areas, ∼150 fibres were evaluated from each muscle cross-section per animal. Thus, a total of ∼1,050 fibres (roughly evenly spread across fibre types) were analysed for each age group and each rat strain.
Images were exported into Photoshop (Adobe Systems Inc., San Jose, CA, USA) as TIFF files, and representative areas were cropped to be of equal size, avoiding the pleural or peritoneal surface, blood vessels and any sectioning artefacts. Image fluorescence was pseudo-coloured and optimized by changing the RGB colour gamut while preserving whole image original contrast and brightness.
Additionally, separate DIAm cross-sections were labelled with wheat germ agglutinin (WGA) to assess the percentage of total muscle cross-sectional area comprising interstitial space, as previously described (Gosselin et al., 1993; Greising et al., 2013). Briefly, freshly cut DIAm cross-sections were incubated in 1 μg/ml of WGA conjugated to Alexa Fluor 488 nm (Reference #: W11261; Invitrogen, Molecular Probes, Eugene, OR, USA). Images were obtained for each cross-section using confocal microscopy (as above) and were analysed using the morphometric analysis tools in Metamorph (Universal Imaging Corp.). An inclusive threshold was applied to images to clearly delineate muscle fibres, the image was then binarized, and total muscle cross-sectional area and the cross-sectional area comprising interstitial space were measured.
Statistical Analysis
Comparisons between younger and older animals within species for data in Tables 1 and 2 were made using a Student's unpaired two-tail t test. Data in Figures 1, 2, 4, 5, and 6 were analysed using a two-way repeated measures ANOVA, and Bonferroni post-hoc test if warranted based on a significant interaction. All comparisons were determined a priori and significance was set to P <0.05. Data are presented as mean ± SE unless otherwise specified. The description of P values has been grouped into those that are <0.05 and those that are <0.01.
Table 1. Fischer 344 animal and DIAm contractile characteristics.
| Young (6 month) | Old (24 month) | P value | |
|---|---|---|---|
| Body weight, g | 393 ± 9 | 376 ± 11 | 0.25 |
| Lo, cm | 2.11 ± 0.06 | 2.20 ± 0.04 | 0.21 |
| Pt, N · cm2 | 8.0 ± 0.3 | 6.7 ± 0.4* | <0.05 |
| Po, N · cm2 | 23.4 ± 0.9 | 17.7 ± 0.6* | <0.01 |
| Pt/Po | 0.35 ± 0.02 | 0.38 ± 0.02 | 0.30 |
| CT, ms | 40 ± 2 | 46 ± 2 | 0.06 |
| 1/2RT, ms | 40 ± 2 | 44 ± 5 | 0.38 |
Values are mean ± SE. Lo, optimal diaphragm muscle segment length; Pt, peak twitch force; Po, maximal tetanic tension; Pt/Po, peak twitch force to maximal tetanic tension ratio; CT, time to peak tension; 1/2RT, half-relaxation time.
Significantly different from young (Student's unpaired two-tailed t test).
Table 2. Brown Norway animal and DIAm contractile characteristics.
| Young (6 month) | Old (32 month) | P value | |
|---|---|---|---|
| Body weight, g | 319 ± 11 | 383 ± 14* | <0.01 |
| Lo, cm | 1.72 ± 0.03 | 2.20 ± 0.07* | <0.01 |
| Pt, N · cm2 | 8.9 ± 0.3 | 7.5 ± 0.5* | <0.05 |
| Po, N · cm2 | 19.9 ± 0.3 | 17.0 ± 0.6* | <0.01 |
| Pt/Po | 0.45 ± 0.01 | 0.44 ± 0.03 | 0.86 |
| CT, ms | 50 ± 1 | 50 ± 2 | 0.95 |
| 1/2RT, ms | 48 ± 3 | 59 ± 5 | 0.08 |
Values are mean ± SE. See Table 1 for definitions of abbreviations and acronyms.
Figure 1.
Diaphragm muscle force-frequency curve from young and old F344 (A) and BN (B) rats, and normalized to the maximum force from young and old F344 (C) and BN (D) rats. Open circles denote young F344 rats, and closed circles denote old F344 and BN rats. Open squares denote young F344 rats, and closed squares denote old F344 and BN rats. *Young compared to old F344 and BN rats, P <0.05, two-way repeated measures ANOVA with Bonferroni post-hoc test. Data are mean ± SE.
Figure 2.
Representative diaphragm muscle cross-sections from young and old F344 and BN rats.Fibre types were classified according to myosin heavy chain (MyHC) expression based on immunoreactivity to isoforms MyHCSlow (green) and MyHC2A (red). The absence of staining (black) was classified as MyHC2X and/or MyHC2B. Anti-laminin (blue) was used to define the muscle fibre sarcolemma. Scale bar represents 50 μm.
Figure 4.
Summary of diaphragm muscle cross-sectional area data from young and old F344 (A) and BN (B) rats. Open bars denote young F344 and BN rats, and closed bars denote old F344 and BN rats. *Young compared to old F344 and BN rats, P <0.05, two-way repeated measures ANOVA with Bonferroni post-hoc test. Data are mean ± SE.
Figure 5.
Summary of the relative contribution of diaphragm muscle fibre types to total diaphragm muscle cross-sectional area from young and old F344 (A) and BN (B) rats. Open bars denote young F344 and BN rats, and closed bars denote old F344 and BN rats. *Young compared to old F344 and BN rats, P <0.05, two-way repeated measures ANOVA with Bonferroni post-hoc test. Data are mean ± SE
Results
Animal characteristics
Changes in body weights for F344 rats and BN rats are presented in Table 1 and Table 2, respectively. In F344 rats, body weights were ∼5% lower in 24 month compared to 6 month animals. In contrast, body weights were ∼20% greater in 32 month BN rats compared to 6 month animals. All older rats from both strains demonstrated an old age phenotype characterized by poor grooming and mild kyphosis.
Diaphragm muscle force
Peak twitch specific force (Pt) was reduced by ∼17% and ∼14% in the aged F344 and BN rats, respectively (Tables 1 & 2). No ageing-related differences were observed in time to peak twitch force (CT), or the half-relaxation time (1/2RT) in either F344 or BN rats (Tables 1 & 2). Maximal specific force (Po) was reduced by ∼24% and ∼13% in the aged F344 and BN rats, respectively (Tables 1 & 2; Figure 1A & B). The ratio of Pt/Po was unchanged with ageing in both rat strains (Tables 1 & 2). In both rat strains, there was main effect of ageing on the force/frequency response. In F344 rats, significant differences were observed across all frequencies of tetanic stimulation ≥30 Hz (Figure 1A). In BN rats, only a main effect of ageing was significant (Figure 1B). When the force/frequency response curves were normalized to maximum force, there were no ageing-related changes in both F344 and BN rats (Figure 1C & D). Thus, although both rat strains demonstrated evidence of significant ageing-related DIAm weakness, there were larger reductions in both Pt and Po in old F344 compared to BN rats.
Myosin heavy chain concentration
In both F344 and BN rat DIAm, all four adult MyHC isoforms were expressed. Total MyHC concentration in the DIAm was reduced by ∼15% in old (0.59 ± 0.13 μg/μl) compared to young (0.70 ± 0.07 μg/μl) F344 rats. In contrast, BN rats demonstrated a less pronounced ageing-related reduction (∼5%) in MyHC concentration in DIAm (old: 0.69 ± 0.11 vs. young: 0.73 ± 0.08 μg/μl).
Diaphragm muscle fibre type proportions and cross-sectional areas
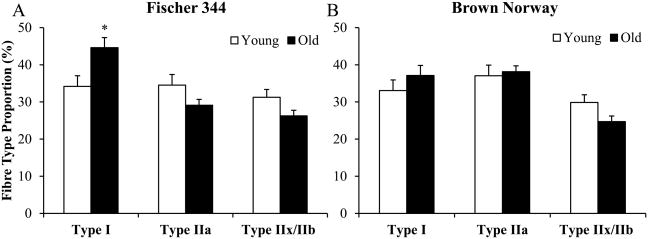
In both F344 and BN rat DIAm, three fibre types could be clearly distinguished immunohistochemically (Figure 2). Based on previous analyses of MyHC isoform expression in single rat DIAm fibres, it is likely that the MyHC2X isoform is either singularly expressed (type IIx fibres) or co-expressed with the MyHC2B isoform (hybrid IIx and/or IIb fibres) (Sieck, 1995; Geiger et al., 2001). The distinction between type IIx and IIx/IIb hybrid DIAm fibre is difficult based on immunohistochemistry, so type IIx and type IIx/IIb hybrid fibres were combined into a single IIx and/or IIb group for analysis. In F344 rats, there was no main effect of ageing on DIAm fibre type proportions (Figure 3A), however there was a significant interaction with an increase in the proportion of type I fibres in old (45 ± 3%) animals compared to young (35 ± 3%; P <0.05) rats, with a concomitant decrease in the relative proportions of both type IIa fibres and type IIx and/or IIb fibres. No significant interactions between age and fibre type proportions were observed in BN rats (Figure 3B).
Figure 3.
Summary of diaphragm muscle proportions from young and old F344 (A) and BN (B) rats. Open bars denote young F344 and BN rats, and closed bars denote old F344 and BN rats. *Young compared to old F344 and BN rats, P <0.05, two-way repeated measures ANOVA with Bonferroni post-hoc test. Data are mean ± SE.
In F344 rats, cross-sectional areas of type IIx and/or IIb DIAm fibres were significantly reduced by ∼20% in old (2,266 ± 91 μm2) compared to young (2,843 ± 93 μm2) animals (Figure 4A; P <0.01). In contrast, the cross-sectional areas of type I (old: 766 ± 21 μm2 vs. young: 742 ± 43 μm2) and IIa (old: 895 ± 27 μm2 vs. young: 871 ± 26 μm2) DIAm fibres were unchanged between old and young F344 rats (Figure 4A). Similarly, the cross-sectional areas of type IIx and/or IIb DIAm fibres were significantly reduced by ∼15% in old (2,163 ± 44 μm2) compared to young (2,522 ± 75 μm2) BN rats (P <0.01; Figure 4B) whereas the cross-sectional areas of type I (old: 735 ± 36 μm2 vs. young: 779 ± 40 μm2) and IIa (old: 851 ± 22 μm2 vs. young: 834 ± 34 μm2) DIAm fibres were unchanged between old and young BN rats (Figure 4B).
In both F344 and BN rats, there were no ageing-related differences in the percentage of total muscle cross-sectional area comprising interstitial space between young (24.5 ± 1.0%) and old (23.9 ± 2.1%) F344 rats, as well as between young (21.5 ± 1.5%) and old (22.6 ± 1.6%) BN rats.
Combining the ageing related changes in fibre type proportions and cross-sectional areas, the relative contribution of each fibre type to the total DIAm CSA was estimated (Figure 5). First, the average CSA of each fibre type is multiplied by the average relative proportion of the corresponding fibre type, and this value for each fibre type is summed to obtain grand total, e.g., (CSA type I * rel prop. type I)+(CSA type n * rel. prop. type n)). Then, the product of a given fibre types CSA and relative proportion is divided by the grand total value, to determine that fibre types relative contribution to overall DIAM CSA. In F344 rats, there was a significant ageing-related increase (∼54%;P <0.05; Figure 5A) in the relative contribution of type I fibres to total DIAm CSA with a concomitant decrease in the relative contribution of type IIx and/or IIb fibres (∼15%; P <0.05; Figure 5A). The relative contribution of type IIa fibres to total DIAm CSA was unaffected by ageing in the F344 rats. In BN rats, there was also an ageing related decrease in the relative contribution of type IIx and/or IIb fibres (∼19%; P <0.05; Figure 5B) to total DIAm CSA but no ageing-related changes in the relative contribution of type I and type IIa fibres.
Discussion
The present study comprehensively examined the presence of DIAm sarcopenia in F344 and BN rat strains provided through the USA NIA aged rodent colony. Although both the F344 and BN inbred rat strains demonstrated the presence of DIAm sarcopenia, this ageing-related reduction in maximal DIAm specific force and cross-sectional areas were more pronounced in the F344 strain. Indeed, old F344 rats demonstrated an ∼24% reduction in maximal DIAm specific force, corresponding to an ∼15% reduction in MyHC concentration and a selective ∼20% reduction in cross-sectional areas of type IIx and/or IIb DIAm fibres. In contrast, old BN rats demonstrated an ∼13% reduction in maximal DIAm specific force, corresponding to an ∼5% reduction in MyHC concentration and a selective ∼15% reduction in cross-sectional areas of type IIx and/or IIb DIAm fibres. No ageing- related changes in the cross-sectional areas of type I and IIa DIAm fibres was observed in either F344 or BN rats. Thus, although DIAm sarcopenia is evident in both rat strains, it is more pronounced in F344 rats, and appears to be driven by the selective atrophy of type IIx and/or IIb DIAm fibres.
Sarcopenia and rat models
Sarcopenia is a term first proposed by Irwin Rosenberg in 1989 (Rosenberg, 1989) to describe the progressive ageing-related decrease in muscle mass experienced by adults >65 years of age. Later, Evans (Evans & Campbell, 1993) revised the definition of sarcopenia to include an ageing-related loss in skeletal muscle strength; a description that aligns with current working consensus definitions (Cruz-Jentoft et al., 2010; Fielding et al., 2011). In humans, the progressive ageing-related decline in skeletal muscle mass and strength begins at ∼30 years of age (similar to other physiologic systems; (Sehl & Yates, 2001)), where thereafter, 0.5-1.0% of muscle mass is lost per year with a more rapid decline in humans >65 years of age (Lexell et al., 1988). The presence of sarcopenia is well characterized in limb muscles in both humans (Doherty, 2003) and rodent models of ageing (Brooks & Faulkner, 1988). However, the presence of sarcopenia specific to the DIAm remains less well characterized and comprehensively studied. Previous work in humans has established an ageing-related decline in DIAm strength of ∼20% based on measurements of maximal transdiaphragmatic pressure following a combined expulsive-Mueller manoeuvre (Tolep et al., 1995) and maximal sniff test (Polkey et al., 1997). Taken together these data suggest DIAm sarcopenia to be present in ageing humans. However, ageing related atrophy of DIAm fibres has not been examined in humans.
The use of animal models to explore the cellular and molecular mechanisms underlying ageing related physiological changes has proven invaluable in advancing the science of ageing. Unfortunately, there is no single ideal animal model for human ageing, and instead, the specific research question dictates what animal model is best suited to address it. In the context of skeletal muscle sarcopenia, rodents are commonly used because of their relatively short life-spans enabling longitudinal studies, the ability to perform invasive and terminal experiments, and the ability to tightly control their environmental conditions. The value of rodent models of ageing was recognized in the early 1970's by the USA NIA, prompting the establishment of rodent colonies in order to standardize specific strains available to researchers (Sprott, 1991). In the early 1980's, the NIA stopped supporting the use of Sprague-Dawley rats for ageing-related research and moved forward by supporting only the inbred F344 and BN rat strains (Sprott, 1991). Since then, these two inbred rat strains, along with the F344 × BN F1 hybrid have been consistently supported by the NIA, and thus, are the dominant rat strains chosen for ageing research.
In the context of DIAm physiology, rats differ from humans in that all four MyHC isoforms are expressed, although the MyHC2B isoform is rarely expressed alone, instead co-expressed with the MyHC2X isoform (Sieck, 1995; Geiger et al., 2001). In contrast the MyHC2B isoform is not expressed in the human diaphragm (Levine et al., 1997). Specifically, the human DIAm comprises ∼40% type I, ∼40% type IIa, and ∼20% type IIx fibres (Levine et al., 1997). In contrast, the rat DIAm comprises ∼35% type I, ∼35% type IIa, and ∼30% type IIx and/or IIb fibers (expressing MyHC2x alone or in combination with MyHC2B) (Sieck, 1995; Geiger et al., 2001). Thus, the fibre type composition of the rat DIAm is similar to humans and it is likely that the mechanical and fatigue properties of the motor units these fibres comprise is equally diverse (Sieck & Fournier, 1989; Elliott et al., 2015). In contrast, the mouse DIAm comprises ∼10% type I, ∼50% type IIa, and ∼40% type IIx and/or IIb fibres(Greising et al., 2013). Although neither the rat nor the mouse provide a perfect physiological match in terms of the human DIAm fibre type composition, both of these rodent models have been used extensively in ageing-related research specific to skeletal muscle sarcopenia. In fact, both rats and mice have unique strengths and weaknesses that investigators should consider when planning ageing-related studies. For example, the ventilatory frequency and heart rate of rats is much slower (∼50 bpm and ∼400 bpm, respectively) than mice (∼200 bpm and ∼800 bpm, respectively). In mice, genetic modification is more readily available and allows specific exploration of protein interactions and possible underlying mechanisms.
Regardless of the species used in aging studies, the selection of age is often based on survival information, either as a % of survival or a % of mean life span, both of which control for differences in life-spans across species/strains. In the current study, we chose to match rat strains based on % survival, as this is more commonly used in the literature. Both 24 month old F344 and 32 month old BN rats are at their respective mean life spans, while 6 month old F344 and 6 month old BN rats are at 25% and ∼19% of their respective mean life spans. However, this difference between young animals is unlikely to impact the primary outcome variables in the current study. In the present study, we chose to evaluate DIAm sarcopenia in older rats at 50% survival for both F344 and BN strains. From an ageing perspective, this is similar to previous humans studies, where DIAm weakness was reported in subjects ∼68-73 years of age (an age range that corresponds to ∼50% survival in humans (WHO, 2015)) compared to younger (∼24-29 years of age) adults (Tolep et al., 1995; Polkey et al., 1997).
Diaphragm muscle weakness
Ageing-related muscle weakness is a major component of sarcopenia. Muscle weakness may result from a decrease in specific force (force per muscle cross-sectional area) and/or muscle fibre atrophy. The present study demonstrates a significant ageing-related decrease in both DIAm specific force as well as muscle fibre atrophy in both the F344 and BN rat strains, indicating DIAm sarcopenia. However, ageing-related reductions in both Pt and Po were more pronounced in F344 rats. Indeed, maximal specific force was reduced by ∼24% in F344 rats, compared the ∼13% reduction observed in BN rats. The results of the present study are consistent with a previous study from our laboratory that showed an ∼20% reduction in maximal DIAm specific force in 24 vs. 6 month old F344 rats (Gosselin et al., 1994a). Subsequently, several studies using F344 rats in similar age groups (young, 4-9 month old; old, 23-26 month old) found similar ageing-related reductions in specific force (Powers et al., 1996; Criswell et al., 1997; Smith et al., 2002). To our knowledge, no previous studies have reported ageing-related changes in DIAm strength in BN rats.
Potential mechanisms explaining the reduction in DIAm specific force in older animals include a reduction in, 1) the fraction of cross-bridges in the strongly bound force generating state (αfs), 2) the average force produced per cross-bridge (F), and/or 3) the number of myosin heads in parallel per half-sarcomere (n). Indeed, force production by single muscle fibres is a product of αfs, F, and n (Sieck et al., 2013). To fully elucidate the contribution of each factor will require studies in single type-identified DIAm fibres, which is beyond the scope of this study. However, the present study demonstrates that MyHC concentration from a segment of DIAm was reduced by ∼15% in old F344 rats, and ∼5% in old BN rats. Additionally, we also found a selective atrophy of type IIx and/or IIb DIAm fibres, and a preservation of CSA in type I and IIa DIAm fibres in old age. Taken together, it is likely that the reduction in MyHC concentration occurred primarily in type IIx and/or IIb DIAm fibres. Previous work from our laboratory has shown that when the force generated by a single DIAm fibre was normalized for MyHC content per half sarcomere (n), all type II DIAm fibres produced greater forces compared to type I fibres, but there were no differences across type II fibre types (Geiger et al., 2000). Thus, the reduction in DIAm specific force in older animals is likely due to a reduction in MyHC concentration in type IIx and/or IIb DIAm fibres. We also found that the selective atrophy of type IIx and/or IIb DIAm fibres was associated with a decrease MyHC content per half-sarcomere and a reduction in specific force of these fibres following denervation (Geiger et al., 2003) and hypothyroidism (Geiger et al., 2002). The selective atrophy of type IIx and/or IIb DIAm fibres is also associated with a reduction in specific force following corticosteroid treatment (Lewis et al., 1992) and nutritional deprivation (Lewis & Sieck, 1990). Additionally, in previous studies (Geiger et al., 2000; Geiger et al., 2002; Han et al., 2003) we found no apparent differences in αfs across DIAm fibre types during Ca2+ activation.
Diaphragm muscle fibre atrophy
The second major component of sarcopenia is muscle fibre atrophy. The present study demonstrates an ageing-related atrophy of type IIx and/or IIb DIAm fibres in both F344 and BN rat strains. Similar to the more pronounced reduction in DIAm strength observed in F344 rats, the reduction in type IIx and/or IIb DIAm fibre cross-sectional areas was also more pronounced in F344 rats. The selective atrophy of type IIx and/or IIb fibres in the ageing DIAm of F344 and BN rats that we observed is consistent with previous work from our laboratory for DIAm fibre atrophy in ageing C57BL/6 × 129 mice (Greising et al., 2013). Unfortunately, in our previous study examining ageing-related changes in DIAm force in F344 rats (Gosselin et al., 1994a), we did not investigate ageing-related changes in DIAm fibre cross-sectional area. The relatively few studies that have evaluated ageing-related changes in DIAm fibre cross-sectional areas report somewhat conflicting results. For example, Kavazis et al.(Kavazis et al., 2012) reported no change in DIAm cross-sectional areas in 24-26 vs. 6 month old male F344 rats; however, this study did not evaluate cross-sectional area in type-identified DIAm fibres. In contrast, in another study by Kavazis et al. (Kavazis et al., 2007) comparing 24-26 vs. 6 month old male F344 rats, they reported an ageing-related hypertrophy of type IIa DIAm fibres with no change in the cross-sectional areas of other fibre types. To our knowledge, no previous study has reported ageing-related changes in DIAm fibre cross-sectional area in BN rats.
The selective ageing-related atrophy of type IIx and/or IIb DIAm fibres is consistent with observations in limb muscles (Larsson et al., 1978; Grimby et al., 1982). As in the present study, these previous studies found that type I and IIa limb muscle fibres appear to be relatively protected from ageing-related atrophy.
Functional impact of diaphragm muscle sarcopenia
The selective atrophy of type IIx and/or IIb DIAm fibres with attendant reductions in maximal specific force would impact the relative contributions of more fatigable motor units in DIAm force generation (c.f., (Sieck & Fournier, 1989; Elliott et al., 2015)). These more fatigable motor units in the DIAm are involved in higher force, non-ventilatory motor behaviours (i.e., coughing, sneezing), whereas lower force, ventilatory behaviours (i.e., eupnoea) are accomplished by the recruitment of fatigue resistant motor units comprising type I and IIa fibres. Accordingly, a selective sarcopenic effect on type IIx and/or IIb fibres would impact higher force, non-ventilatory motor behaviours rather than quiet breathing. Indeed, previous work by our laboratory demonstrated a functional impairment only in higher force, non-ventilatory motor behaviours resulting from DIAm sarcopenia in C57BL/6 × 129 mice (Greising et al., 2015b).
Fischer 344 vs. Brown Norway rat models of diaphragm muscle sarcopenia
The presence of DIAm sarcopenia was evident in both F344 and BN rat strains, although the reduction in specific force and selective atrophy of type IIx and/or IIb DIAm fibres was more pronounced in the F344 rat strain. However, this does not discount the potential utility of BN rats for future work investigating DIAm sarcopenia. Interestingly, the F344 rat strain demonstrated a reduction in body weight with age, while the BN rats continued to gain body weight. In both strains there is an ageing-related increase in adiposity, however the magnitude of this increase is greater in BN rats (5 months of age ∼10% adipose tissue; 29 months of age ∼20% adipose tissue; (Wolden-Hanson et al., 1999)), compared to F344 rats (6 months of age, ∼11% adipose tissue; 24 months of age ∼15% adipose tissue; (Delp et al., 1998)). Thus, F344 rats may provide a useful model of DIAm sarcopenia for humans that experience a reduction in lean body mass with relatively no change in overall adiposity. In contrast, BN rats may provide a useful model of DIAm sarcopenia for humans that experience a reduction in lean body mass with a coincident increase in overall adiposity (i.e., sarcopenic obesity). Ultimately, the human population of interest may dictate the utility of either the F344 or BN rat models for future work investigating DIAm sarcopenia.
Despite the differences observed between rat strains, one notable similarity is the lack of an ageing-related change in the percentage of total muscle CSA comprising interstitial space, as determined via WGA staining. Previous work from our lab (Gosselin et al., 1994b) showed that total collagen content as well as the extent of collagen cross linking increased with ageing in F344 rats, corresponding to an increase in passive stiffness of the DIAm. How such a change in passive stiffness could affect active contractile properties is not well established. However, recent work by Ramaswamy et al. (Ramaswamy et al., 2011) has shown that the lateral transmission of force is impaired in old rats, which is correlated to an ageing-related disruption in the dystrophin-associated glycoprotein complex in the extracellular matrix.
An important consideration relevant to the current study is the potential contribution of ageing-related pulmonary structure/function changes that may precipitate ageing-related DIAm adaptations. Although it is established that many mammalian species demonstrate an ageing-related increase in lung compliance and breakdown of lung parenchyma leading to an enlargement of alveolar airspaces, it is currently unclear whether DIAm sarcopenia can be attributed to these changes. Interestingly, previous work has shown F344 rats at 24 months of age demonstrate a statistically insignificant (30%) ageing-related enlargement of air spaces (Kerr et al., 1990). These data may suggest, at least in F344 rats, that DIAm sarcopenia occurs independently of potential ageing-related changes in lung structure. However, additional work is needed, in both F344 and BN rats, to more completely characterize these ageing-related changes in lung structure as they relate to potential changes in lung mechanics.
Summary
The present study reports a comprehensive evaluation of DIAm sarcopenia, defined by the presence of ageing-related DIAm weakness and atrophy, in F344 and BN rat strains. These inbred rat strains have been supported by the USA NIA for roughly 30 years and are the predominant rat strains used for ageing-related research. Accordingly, the present study provides important data characterizing the presence and extent of sarcopenia in these two rat strains to help guide future work investigating DIAm sarcopenia. Both maximal specific force and DIAm atrophy, selective to type IIx and/or IIb fibres were more pronounced in the F344 rat strain. Future work utilizing single type-identified DIAm fibres is needed to elucidate the mechanism underlying this ageing-related decrease in maximal specific force. However, it is likely that the selective atrophy of type IIx and/or IIb DIAm fibres corresponds to a reduction in MyHC content per half-sarcomere in these fibres. Likewise, the maintenance of fibre cross-sectional areas in type I fibres and type IIa fibres, suggests maintenance of MyHC content per half-sarcomere in these fibres that are essential for maintaining adequate ventilation. Future research aimed at understanding the underlying mechanism responsible for driving the ageing-related selective atrophy of type IIx and/or IIb DIAm fibres is clearly warranted.
What is the central question of this study?
Several rat models are commonly used to study the physiology of ageing (e.g., Fischer 344 and Brown Norway rats are recommended by the USA National Institute of Ageing). Diaphragm muscle sarcopenia (ageing related muscle weakness and atrophy) remains incompletely described in these rat models.
What is the main finding and its importance?
Diaphragm muscle sarcopenia is present in both the Fischer 344 and Brown Norway rat strain, but appears more pronounced in Fischer 344 rats.
Acknowledgments
The authors would like to thank Rebecca Macken, Yun-Hua Fang, and Wen-Zhi Zhan, MD, for their technical assistance in the completion of this project.
Funding: This research was supported by grants from National Institute of Health R01-AG044615 (C.B.M. & G.C.S.) and T32-HL105355 (J.E.E.).
Footnotes
Competing Interests: The authors have no conflicts or competing interests to disclose.
Author contributions: Conception and design of the experiments: J.E.E., T.S.O., C.B.M., G.C.S.; Collection, analysis and interpretation of data: J.E.E., T.S.O., C.B.M., G.C.S.; Drafting the article or revising it critically for important intellectual content: J.E.E., T.S.O., C.B.M., G.C.S. All authors approved the final version for publication, agree to be accountable for all aspects of the work and qualify for authorship.
References
- Federal Interagency Forum on Aging-Related Statistics. Older Americans 2012: Key Indicators of Well-Being. U.S. Government Printing Office; Washington, DC: [Google Scholar]
- Alway SE, Siu PM, Murlasits Z, Butler DC. Muscle hypertrophy models: applications for research on aging. Canadian J Appl Physiol. 2005;30:591–624. doi: 10.1139/h05-143. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brooks SV, Faulkner JA. Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol. 1988;404:71–82. doi: 10.1113/jphysiol.1988.sp017279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler-Browne GS, Whalen RG. Myosin isozyme transitions occurring during the postnatal development of the rat soleus muscle. Dev Biol. 1984;102:324–334. doi: 10.1016/0012-1606(84)90197-0. [DOI] [PubMed] [Google Scholar]
- Chong CP, Street PR. Pneumonia in the elderly: a review of the epidemiology, pathogenesis, microbiology, and clinical features. South Med J. 2008;101:1141–1145. doi: 10.1097/SMJ.0b013e318181d5b5. [DOI] [PubMed] [Google Scholar]
- Criswell DS, Powers SK, Herb RA, Dodd SL. Mechanism of specific force deficit in the senescent rat diaphragm. Respir Physiol. 1997;107:149–155. doi: 10.1016/s0034-5687(96)02509-1. [DOI] [PubMed] [Google Scholar]
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinkova E, Vandewoude M, Zamboni M. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age and ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delp MD, Evans MV, Duan C. Effects of aging on cardiac output, regional blood flow, and body composition in Fischer-344 rats. J Appl Physiol. 1998;85:1813–1822. doi: 10.1152/jappl.1998.85.5.1813. [DOI] [PubMed] [Google Scholar]
- Doherty TJ. Invited review: Aging and sarcopenia. J Appl Physiol. 2003;95:1717–1727. doi: 10.1152/japplphysiol.00347.2003. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in the Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott JE, Greising SM, Mantilla CB, Sieck GC. Functional impact of sarcopenia in respiratory muscles. Resp Physiol Neuro. 2015 doi: 10.1016/j.resp.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans WJ, Campbell WW. Sarcopenia and age-related changes in body composition and functional capacity. J Nutr. 1993;123:465–468. doi: 10.1093/jn/123.suppl_2.465. [DOI] [PubMed] [Google Scholar]
- Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, Cederholm T, Chandler J, De Meynard C, Donini L, Harris T, Kannt A, Keime Guibert F, Onder G, Papanicolaou D, Rolland Y, Rooks D, Sieber C, Souhami E, Verlaan S, Zamboni M. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. Journal of the American Medical Directors Association. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger PC, Bailey JP, Zhan WZ, Mantilla CB, Sieck GC. Denervation-induced changes in myosin heavy chain expression in the rat diaphragm muscle. J Appl Physiol. 2003;95:611–619. doi: 10.1152/japplphysiol.00862.2002. [DOI] [PubMed] [Google Scholar]
- Geiger PC, Cody MJ, Han YS, Hunter LW, Zhan WZ, Sieck GC. Effects of hypothyroidism on maximum specific force in rat diaphragm muscle fibers. J Appl Physiol. 2002;92:1506–1514. doi: 10.1152/japplphysiol.00095.2001. [DOI] [PubMed] [Google Scholar]
- Geiger PC, Cody MJ, Macken RL, Bayrd ME, Sieck GC. Mechanisms underlying increased force generation by rat diaphragm muscle fibers during development. J Appl Physiol. 2001;90:380–388. doi: 10.1152/jappl.2001.90.1.380. [DOI] [PubMed] [Google Scholar]
- Geiger PC, Cody MJ, Macken RL, Sieck GC. Maximum specific force depends on myosin heavy chain content in rat diaphragm muscle fibers. J Appl Physiol. 2000;89:695–703. doi: 10.1152/jappl.2000.89.2.695. [DOI] [PubMed] [Google Scholar]
- Geiger PC, Cody MJ, Sieck GC. Force-calcium relationship depends on myosin heavy chain and troponin isoforms in rat diaphragm muscle fibers. J Appl Physiol. 1999;87:1894–1900. doi: 10.1152/jappl.1999.87.5.1894. [DOI] [PubMed] [Google Scholar]
- Gosselin LE, Johnson BD, Sieck GC. Age-related changes in diaphragm muscle contractile properties and myosin heavy chain isoforms. Am J Respir Crit Care Med. 1994a;150:174–178. doi: 10.1164/ajrccm.150.1.8025746. [DOI] [PubMed] [Google Scholar]
- Gosselin LE, Martinez DA, Vailas AC, Sieck GC. Interstitial space and collagen alterations of the developing rat diaphragm. J Appl Physiol. 1993;74:2450–2455. doi: 10.1152/jappl.1993.74.5.2450. [DOI] [PubMed] [Google Scholar]
- Gosselin LE, Martinez DA, Vailas AC, Sieck GC. Passive length-force properties of senescent diaphragm: Relationship with collagen characteristics. J Appl Physiol. 1994b;76:2680–2685. doi: 10.1152/jappl.1994.76.6.2680. [DOI] [PubMed] [Google Scholar]
- Greising SM, Ermilov LG, Sieck GC, Mantilla CB. Ageing and neurotrophic signalling effects on diaphragm neuromuscular function. J Physiol. 2015a;593:431–440. doi: 10.1113/jphysiol.2014.282244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greising SM, Mantilla CB, Gorman BA, Ermilov LG, Sieck GC. Diaphragm muscle sarcopenia in aging mice. Exp Gerontol. 2013;48:881–887. doi: 10.1016/j.exger.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greising SM, Mantilla CB, Medina-Martinez JS, Stowe JM, Sieck GC. Functional Impact of Diaphragm Muscle Sarcopenia in both Male and Female Mice. Am J Physiol Lung Cell Mol Physiol. 2015b;309:L46–52. doi: 10.1152/ajplung.00064.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimby G, Danneskiold-samsoe B, Hvid K, Saltin B. Morphology and enzymatic capacity in arm and leg muscles in 78-81 year old men and women. Acta Physiol Scand. 1982;115:125–134. doi: 10.1111/j.1748-1716.1982.tb07054.x. [DOI] [PubMed] [Google Scholar]
- Han YS, Geiger PC, Cody MJ, Macken RL, Sieck GC. ATP consumption rate per cross bridge depends on myosin heavy chain isoform. J Appl Physiol. 2003;94:2188–2196. doi: 10.1152/japplphysiol.00618.2002. [DOI] [PubMed] [Google Scholar]
- Heron M. Deaths: leading causes for 2007. Natl Vital Stat Rep. 2011;59:1–95. [PubMed] [Google Scholar]
- Janssens JP, Krause KH. Pneumonia in the very old. Lancet Infect Dis. 2004;4:112–124. doi: 10.1016/S1473-3099(04)00931-4. [DOI] [PubMed] [Google Scholar]
- Kavazis AN, Deruisseau KC, Gordon DM. The senescent rat diaphragm does not exhibit age-related changes in caspase activities, DNA fragmentation, or myonuclear domain. Eur J Appl Physiol. 2012;112:3983–3990. doi: 10.1007/s00421-012-2380-2. [DOI] [PubMed] [Google Scholar]
- Kavazis AN, DeRuisseau KC, McClung JM, Whidden MA, Falk DJ, Smuder AJ, Sugiura T, Powers SK. Diaphragmatic proteasome function is maintained in the ageing Fisher 344 rat. Exp Physiol. 2007;92:895–901. doi: 10.1113/expphysiol.2007.038307. [DOI] [PubMed] [Google Scholar]
- Kerr JS, Yu SY, Riley DJ. Strain specific respiratory air space enlargement in aged rats. Exp Gerontol. 1990;25:563–574. doi: 10.1016/0531-5565(90)90022-t. [DOI] [PubMed] [Google Scholar]
- Larsson L, Sjodin B, Karlsson J. Histochemical and biochemical changes in human skeletal muscle with age in sedentary males, age 22-65 years. Acta Physiol Scand. 1978;103:31–39. doi: 10.1111/j.1748-1716.1978.tb06187.x. [DOI] [PubMed] [Google Scholar]
- Levine S, Kaiser L, Leferovich J, Tikunov B. Cellular adaptations in the diaphragm in chronic obstructive pulmonary disease. N Engl J Med. 1997;337:1799–1806. doi: 10.1056/NEJM199712183372503. [DOI] [PubMed] [Google Scholar]
- Lewis MI, Monn SA, Sieck GC. Effect of corticosteroids on diaphragm fatigue, SDH activity, and muscle fiber size. J Appl Physiol. 1992;72:293–301. doi: 10.1152/jappl.1992.72.1.293. [DOI] [PubMed] [Google Scholar]
- Lewis MI, Sieck GC. Effect of acute nutritional deprivation on diaphragm structure and function. J Appl Physiol. 1990;68:1938–1944. doi: 10.1152/jappl.1990.68.5.1938. [DOI] [PubMed] [Google Scholar]
- Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988;84:275–294. doi: 10.1016/0022-510x(88)90132-3. [DOI] [PubMed] [Google Scholar]
- Miller RA, Nadon NL. Principles of animal use for gerontological research. J Gerontol A Biol Sci Med Sci. 2000;55:B117–123. doi: 10.1093/gerona/55.3.B117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley BR, Kirsch DR, Morris NR. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980;105:361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Polkey MI, Harris ML, Hughes PD, Hamnegard CH, Lyons D, Green M, Moxham J. The contractile properties of the elderly human diaphragm. Am J Respir Crit Care Med. 1997;155:1560–1564. doi: 10.1164/ajrccm.155.5.9154857. [DOI] [PubMed] [Google Scholar]
- Powers SK, Criswell D, Herb RA, Demirel H, Dodd S. Age-related increases in diaphragmatic maximal shortening velocity. J Appl Physiol. 1996;80:445–451. doi: 10.1152/jappl.1996.80.2.445. [DOI] [PubMed] [Google Scholar]
- Prakash YS, Fournier M, Sieck GC. Effects of prenatal undernutrition on developing rat diaphragm. J Appl Physiol. 1993;75:1044–1052. doi: 10.1152/jappl.1993.75.3.1044. [DOI] [PubMed] [Google Scholar]
- Ramaswamy KS, Palmer ML, van der Meulen JH, Renoux A, Kostrominova TY, Michele DE, Faulkner JA. Lateral transmission of force is impaired in skeletal muscles of dystrophic mice and very old rats. J Physiol. 2011;589:1195–1208. doi: 10.1113/jphysiol.2010.201921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg IH. Summary comments: epidemiological and methodological problems in determining nutritional status of older persons. Am J Clin Nutr. 1989;50:1231–1233. [Google Scholar]
- Sehl ME, Yates FE. Kinetics of human aging: I. Rates of senescence between ages 30 and 70 years in healthy people. J Gerontol A Biol Sci Med Sci. 2001;56:B198–208. doi: 10.1093/gerona/56.5.b198. [DOI] [PubMed] [Google Scholar]
- Sieck GC. Organization and recruitment of diaphragm motor units. In: Roussos C, editor. The Thorax. Marcel Dekker; New York, NY: 1995. pp. 783–820. [Google Scholar]
- Sieck GC, Ferreira LF, Reid MB, Mantilla CB. Mechanical properties of respiratory muscles. Comprehensive Physiology. 2013;3:1553–1567. doi: 10.1002/cphy.c130003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieck GC, Fournier M. Diaphragm motor unit recruitment during ventilatory and nonventilatory behaviors. J Appl Physiol. 1989;66:2539–2545. doi: 10.1152/jappl.1989.66.6.2539. [DOI] [PubMed] [Google Scholar]
- Smith WN, Dirks A, Sugiura T, Muller S, Scarpace P, Powers SK. Alteration of contractile force and mass in the senescent diaphragm with β2-agonist treatment. J Appl Physiol. 2002;92:941–948. doi: 10.1152/japplphysiol.00576.2001. [DOI] [PubMed] [Google Scholar]
- Sprott RL. Development of animal models of aging at the National Institute of Aging. Neurobiology of Aging. 1991;12:635–638. doi: 10.1016/0197-4580(91)90113-x. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Murakami N. Separation of myosin heavy chain isoforms in rat skeletal muscles by gradient sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Biomed Res. 1990;11:87–91. [Google Scholar]
- Tolep K, Higgins N, Muza S, Criner G, Kelsen SG. Comparison of diaphragm strength between healthy adult elderly and young men. Am J Respir Crit Care Med. 1995;152:677–682. doi: 10.1164/ajrccm.152.2.7633725. [DOI] [PubMed] [Google Scholar]
- WHO. World Health Statistics. World Health Organization; 2015. [Google Scholar]
- Wolden-Hanson T, Marck BT, Smith L, Matsumoto AM. Cross-sectional and longitudinal analysis of age-associated changes in body composition of male Brown Norway rats: association of serum leptin levels with peripheral adiposity. J Gerontol A Biol Sci Med Sci. 1999;54:B99–107. doi: 10.1093/gerona/54.3.b99. [DOI] [PubMed] [Google Scholar]
- Xu J, Kochanek KD, Murphy SL, Tejada-Vera B. Deaths: Final Data for 2007. Natl Vital Stat Rep. 2010;58 [PubMed] [Google Scholar]