Abstract
As the most common cancer in women, one in eight will develop invasive breast cancer over their lifetime making it the second most common cause of cancer-related death among women. Of the many known risk factors for developing breast cancer, obesity stands out as prominent and modifiable. Interestingly, elevated cholesterol is highly associated with obesity and has emerged as an independent risk factor for breast cancer onset and recurrence. This indicates that cholesterol also contributes to the breast cancer pathogenicity of obesity. This review highlights our current understanding of the mechanisms by which cholesterol impacts breast cancer. Key preclinical studies have been highlighted, including the discussion of homeostatic control of cholesterol levels, signaling by cholesterol metabolites through the estrogen receptors, cholesterol formation of lipid rafts and subsequent signaling, and the potential roles of cholesterol in creating a pro-inflammatory tumor microenvironment. Future directions and avenues for therapeutic exploitation are also considered.
Keywords: Breast Cancer, Cholesterol, Ezetimibe, Elevated Cholesterol, Intracellular Cholesterol
Introduction
Breast cancer is the most prevalent form of cancer in women and remains the second most common cause of cancer-related mortality, with an estimated 40,000 deaths every year [99]. Therefore, there is a pressing need for new therapeutic or lifestyle strategies to complement currently available approaches. Using histological markers, breast cancer is subdivided based on the presence of the estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), or those that lack the expression of any of these receptors, termed triple negative. Hormone receptor positive classes respond to mitogenic signaling from hormone stimulation. Therefore, there has been large success in the targeting of their respective endocrine axes either at the level of hormone synthesis, as in the case of aromatase inhibitors, or receptor inhibition using small molecule antagonists such as tamoxifen against the ER, or lapatinib and monoclonal antibody therapy such as trastuzumab against HER2 signaling. Of the different histological subtypes of breast cancers, the treatment of triple negative breast cancer is the most challenging, as there are currently no targeted therapies available. Regardless of the type of cancer, the overwhelming majority of deaths due to breast cancer (greater than 90 %) are attributed to metastatic relapse. Although targeted therapies in receptor-positive disease are initially very successful, many patients relapse with endocrine-therapy-resistant disease. Currently, there are no targeted therapy options for this stage of breast cancer, forcing patients and clinicians to rely on cytotoxic chemotherapy and/or radiation. Thus, there remains an urgent clinical need for a greater understanding of the underlying mechanisms that govern tumor progression, coupled with novel therapies targeting these processes.
The risks of developing breast cancer include genetics [18, 47], age of menarche, age of menopause, parity, age of first child, previous occurrence of cancer, and lifestyle [25, 26, 34, 65, 69, 87, 100]. Since the identification of mutations within BRCA1 and BRCA2 genes as potent predictors of breast cancer development, other genes have been identified as being linked to breast cancer, such as ATM, CHEK2, and PALB2 [70]. While the identification of the genes associated with heritable breast cancer was critical to the understanding of breast cancer and invaluable for providing women with the choice of preventative resection surgery, these genetic mutations only account for a relatively small percentage (5–10 %) of breast cancers [97]. On the other hand, lifestyle is proving to be an increasingly important component in the etiology of breast cancer. For example, obesity, the metabolic syndrome, diabetes type II, and hypercholesterolemia have all been established as risk factors, while regular exercise appears to be protective [12, 19, 45, 49, 53, 56, 71, 94]. Additionally, these disorders have also been shown to be prognostic indicators for breast cancer [10, 35, 63, 84], thus further emphasizing the importance of investigating the mechanisms underlying the contribution of obesity and hypercholesterolemia to breast cancer development and progression. Below, we discuss evidence implicating elevated cholesterol as a mediator of the effects of obesity on breast cancer risk and prognosis and highlight currently proposed mechanisms by which cholesterol influences breast cancer pathophysiology.
Obesity and Breast Cancer
The percentage of the obese population has doubled since 1980. In the USA, >68 % of adults were either overweight or obese in 2012, and the prevalence of obesity in women over the age of 60 has also increased since 2004 [82]. Importantly, several epidemiological studies have implicated obesity as a risk factor for the onset of breast cancer [12]. A recent analysis of the Women’s Health Initiative clinical trial sampling 67,142 post-menopausal women between the ages of 50 and 79 concluded that women who were overweight or obese (body mass index of ≥25 or ≥30 kg/m2, respectively) were at greater risk for developing invasive breast cancer compared to women who were not overweight [5, 30, 77]. In addition to onset, obesity, as defined by body mass index of greater than >30 kg/m2, has also been associated with a decreased recurrence-free survival among breast cancer patients [52]. Interestingly, the risk of onset is most clearly defined in women who present post-menopause, or those cases that are predominantly ER positive. It is unclear whether the obesity-driven risk of recurrence is subtype specific or not. Although the effects of obesity on breast cancer risk or prognosis are likely multifactorial, several likely mechanisms have been proposed and are supported by preclinical evidence. These include obesity-induced hyperinsulinemia, increased insulin-like growth factors, adipokines, infiltration of inflammatory immune cells, and increased inflammatory cytokines [60, 92]. In the case of ER-positive breast cancers, adipose tissue is known to express aromatase, potentially providing a local source of estrogens [112]. Intriguingly, recent evidence indicates that elevated cholesterol, a common comorbidity of obesity, is also a risk factor for breast cancer onset and recurrence. Due to recent advancements in our understanding the mechanisms by which cholesterol impacts breast cancer progression, the remainder of this paper will review our current understanding of how cholesterol contributes to breast cancer pathophysiology.
Cholesterol Metabolism
In terms of cellular physiology, cholesterol is involved in maintaining cell membrane fluidity and the formation of cellular microdomains such as caveolae and lipid rafts, which are important for the signaling and function of integral membrane proteins [68]. Dysregulated cholesterol homeostasis is a characteristic of many diseases, including atherosclerosis [72], metabolic disorders, and as increasing evidence shows, numerous cancer types [3, 76, 85].
Circulating cholesterol levels are regulated by biosynthesis within hepatic cells, by dietary absorption, and a highly orchestrated set of cholesterol transport molecules that absorb and release cholesterol. Indeed, statin-class drugs, which inhibit 3-hydroxy-3methyl-glutaryl-coenzyme A (HMG-CoA reductase), the rate-limiting step in cholesterol biosynthesis, have proven very effective in lowering plasma cholesterol and preventing cardiovascular events [16, 41]. Although circulating cholesterol levels may change under normal physiological conditions, intracellular cholesterol levels remain tightly controlled in the majority of cells.
Intracellular cholesterol homeostasis is maintained by intricate and highly regulated pathways that rely on both short-negative feedback loops and longer-loop, feed-forward mechanisms [48, 102]. Although the majority of studies have been carried out in hepatic cells, it is generally understood that both homeostatic mechanisms are present to some degree in all cell types. Short-loop negative feedback is governed by sterol regulatory element-binding proteins (SREBPs), which are part of the basic helix-loop-helix leucine zipper family of transcription factors. SREBP transcription factors are expressed as three main isoforms, SREBP 1a, SREBP 1c, and SREBP 2, with SREBP 2 being the major regulator of cholesterol homeostasis. In a normo-cholesterol state, they remain bound to the endoplasmic reticulum, in a complex promoted by the presence of sterols. Under conditions of low cholesterol, all three isoforms dissociate and are escorted to the Golgi apparatus by SREBP cleavage activation protein (SCAP), where they undergo proteolytic processing, leading to their activation and nuclear translocation of the amino terminal domain of SREBP. Once translocated, they act as transcription factors inducing cholesterol synthesis-promoting genes such as HMG-CoA reductase, fatty acid synthase and squalene synthase, and genes associated with cholesterol import such as low-density lipoprotein receptor (LDLR), thus resulting in the restoration of intracellular cholesterol [4, 8, 50].
Longer loop regulation is mediated by the liver X receptors (LXRs) which are intracellular receptors of the nuclear receptor superfamily, and which work to eliminate cholesterol through a feed-forward mechanism [74]. Specifically, certain precursors and metabolites of cholesterol such as oxysterols bind to and activate the LXRs, ultimately leading to decreased cellular uptake and increased efflux of cholesterol. LXR receptors have two isoforms, LXRα and LXRβ. LXRα is predominately expressed in hepatocytes, whereas the expression of LXRβ is more ubiquitous. Both isoforms are basally inactive, primarily bound with their heterodimeric partners the retinoid X receptors (RXRs) along with co-repressor proteins. Endogenous LXR agonists include the cholesterol precursor desmosterol, and metabolites 24S-, 25-, and 27-hydroxycholesterol [39]. On the other hand, certain unsaturated fatty acids such as arachidonate have been identified as endogenous antagonists of the LXR [83]. Synthetic LXR ligands include the pan-LXR-activating compounds GW3965 and T0901217 and the intestinal tissue-specific ligand GW6340 [48]. Conversely, inverse agonists such as the recently described SR9243 inhibit LXR-mediated gene expression [37]. Such inverse agonists reduce receptor function by inducing co-repressor interaction. In the presence of LXR agonists, the heterodimeric complex changes conformation such that co-activators are able to bind and drive the transcription of sterol regulatory genes in a ligand-dependent manner. These include apolipoprotein E (ApoE), an apolipoprotein that serves as a ligand for LDL receptor and mediates cholesterol reuptake by the liver [11, 58, 86], ATP-binding cassette transporters A1 and G1 (ABCA1 and ABCG1) which both mediate cholesterol efflux [11], and IDOL, an E3 ligase responsible for the degradation of LDLR [118, 120]. The net effects of LXR activation are decreased cholesterol uptake and increased efflux.
Cholesterol as a Breast Cancer Risk and Prognostic Factor
Elevated cholesterol is a strongly associated comorbidity of obesity, indirectly implicating cholesterol as a mediator of the risk associated with obesity and breast cancer [22, 42, 73, 78, 88]. In terms of breast cancer onset, epidemiological studies investigating the links between circulating cholesterol and risk have yielded conflicting results [reviewed in 27]. Likewise, pre-diagnostic use of statins has been associated with lowered risk of breast cancer onset, while other studies report an increased risk or no significant associations with statin use [7, 14, 15, 20, 21, 29, 38, 43, 55]. The conflicting nature of these reports was supported by a large meta-analyses of retrospective data which found no significant relationship with statin therapy and onset [109]. Some of this variability may be due to access to primary care, potential confounding effects of BMI, whether total, LDL, or HDL cholesterol was investigated, or the possibility that different breast cancer subtypes have differing susceptibilities to cholesterol. Indeed, a recent study found that when adjusted for BMI, elevated cholesterol in the diet was a significant risk factor for breast cancer onset in post-menopausal but not in premenopausal women [49]. Dietary cholesterol was also found to be a risk factor in a separate and distinct cohort of women [94]. Furthermore, prospective trials in a Korean cohort have also implicated circulating cholesterol as a risk factor for breast cancer onset [45, 56].
On the other hand, cholesterol may not be tumorigenic in and of itself, but may promote tumor progression. In support of this notion are data indicating that circulating cholesterol levels correlate with recurrence [6]. Furthermore, large studies have now shown that patients taking statins demonstrate a significantly increased time to breast cancer recurrence [1, 57, 81], as has been supported by a recent meta-analysis [122]. Thus, the most recent epidemiologic evidence is strongly suggestive of a distinct role for cholesterol in breast cancer progression.
Cholesterol and Breast Cancer Pathophysiology
A relationship between cholesterol and tumors has long been known. In 1909, White et al. first described waxy crystals within tumor, a substance that turned out to be cholesterol [114]. In 1953, Waxler et al. reported that tumor incidence in murine models is increased with obesity and elevated cholesterol [113]. Since then, several preclinical studies have found that standard “Western” diets (those high in both fat and cholesterol) decrease tumor latency and increase the growth and metastasis of mammary cancers in preclinical models [2, 62, 63]. It is important to note that all of these studies used a combination of a high-fat and high-cholesterol diet, and that two studies utilized mice on a transgenic background (ApoE−/− or adiponectin-deficient mice), making it difficult to ascribe specific dietary effects to cholesterol. However, a recent study investigated the specific effects of elevating dietary cholesterol on tumor growth in the mouse mammary tumor virus-polyomavirus middle T antigen (MMTV-PyMT) mouse model. As expected, cholesterol decreased latency and increased both tumor multiplicity and growth rate [76]. On the other hand, inhibiting de novo cholesterol synthesis by oral treatment with a statin inhibited the increased tumor growth rate observed in mice on a high-fat (normal cholesterol) diet [76]. It is important to note that these studies utilized transgenic mice where the murine Apoe gene had been replaced with the human APOE3 allele, generating mice that better mimic human cholesterol biology [105]. Furthermore, blocking cholesterol uptake with ezetimibe was sufficient to attenuate the effects of a Western diet on the growth of breast cancer xenografts [85]. Therefore, preclinical studies strongly indicate that cholesterol can impact tumor pathophysiology, and it is a significant mediator of the effects of obesity.
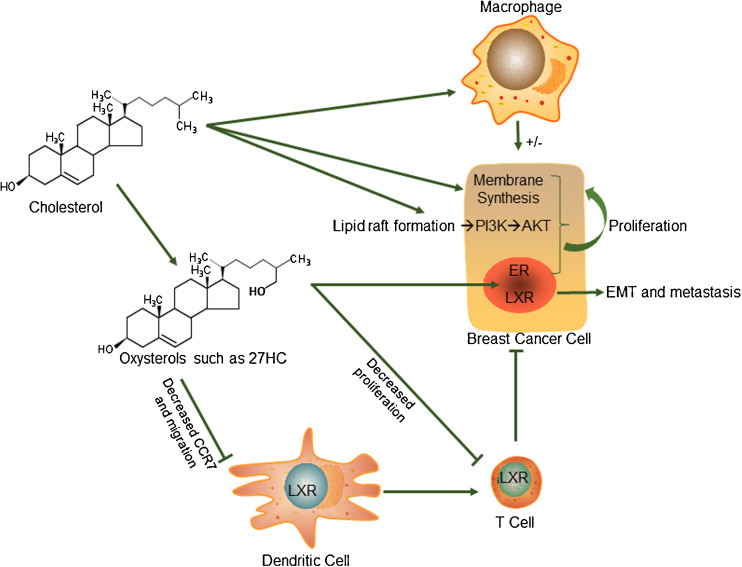
However, what is less clear are the mechanisms by which cholesterol influences breast cancer progression, especially given the fact that intracellular cholesterol concentration is tightly regulated (see section above on Cholesterol Metabolism). As with obesity, the effects of cholesterol elevation are likely to be multifactorial. We explore the most accepted paradigms below (Fig. 1).
Fig. 1.
Proposed mechanisms by which cholesterol influences breast cancer pathophysiology. Cholesterol may have direct actions on the cancer cells by being a limiting factor in membrane synthesis or an integral part of lipid raft formation and subsequent PI3K/AKT signaling. It may also act on macrophages to enhance the inflammatory tumor-favoring microenvironment. On the other hand, loss of ABCG1 and subsequent increased in intracellular cholesterol can polarize macrophages into an anti-cancer M1 phenotype such as in the case of bladder cancer and melanoma. Furthermore, metabolites of cholesterol such as oxysterols like 27HC can act as ligands for the ERs and LXRs. ER activation induces cellular proliferation of cancer cells. While LXR activation decreases cellular proliferation, it induces epithelial to mesenchymal transition (EMT) and subsequent metastasis. Furthermore, in dendritic cells, LXR activation decreases CCR7, reducing their migration and subsequent antigen presentation to T cells. LXR activation also inhibits T cell proliferation, further creating an immune-suppressive environment for tumors
Cholesterol Metabolites as Active Signaling Molecules in Breast Cancer
In addition to the potential direct effects of cholesterol on tumor progression described below, recent work has identified that certain oxysterols can behave as selective estrogen receptor modulators (SERMs). The most abundant circulating oxysterol, 27-hydroxycholesterol (27HC), is a primary metabolite of cholesterol, being synthesized by the cytochrome P450 oxidase, sterol 27-hydroxylase (CYP27A1). 27HC can bind to and modulate the activity of both ERα and ERβ. In models of the cardiovascular system, 27HC behaves as an ER antagonist, while in models of osteoblasts and ER-positive breast cancers, it behaves as an ER partial agonist [32, 33, 76, 107, 108, 115]. 25-hydroxycholesterol has also been shown to activate the ER in breast cancer cells, although this oxysterol circulates at levels far lower than its EC50 for ER [59]. However, it is important to consider that local concentrations of less abundant oxysterols might reach levels that can contribute to pathophysiology.
Importantly, by activating the ERs, 27HC can increase breast cancer cellular proliferation and tumor growth [33, 76, 115]. CYP27A1 is highly expressed in myeloid cells such as macrophages, potentially providing another mechanism by which myeloid cells contribute to tumor pathogenesis. Furthermore, it was shown that CYP27A1 can be expressed in cancer cells themselves, the extent to which is correlated with tumor grade [76]. Interestingly in this regard, 27HC was found to be at higher concentrations in breast tumors compared to adjacent tissue or tissue from normal volunteers, indicating that in addition to systemic 27HC from the blood, tumors can provide important local sources of 27HC [115].
Key experiments using the MMTV-PyMT model found that the effects of a high-cholesterol diet were dependent on the expression of CYP27A1 [76]. Thus, the majority of cholesterol’s pro-tumorigenic properties are mediated by the actions of 27HC. Furthermore, the effects of a high-fat diet on ER-positive tumor growth were attenuated by treatment with a small molecule inhibitor of CYP27A1, indicating that some of the effects of obesity are mediated by 27HC [76].
As mentioned above, oxysterols such as 27HC also activate the LXRs to promote cholesterol efflux thereby inhibiting cellular proliferation [76, 110]. It appears that the ER and LXR activities of 27HC are at opposition to one another. Indeed, in both breast cancer and osteoblast cells, siRNA knockdown of LXRs increases the ER activity of 27HC and vice versa [75, 76]. Thus, the relative abundance of these receptors and or respective co-factor milieu may be important determining factors in the pro-tumorigenic properties of 27HC. Intriguing, however, were observations that 27HC can also promote breast cancer metastasis. In contrast to its proliferative properties, these effects were in part mediated by the LXRs [76]. Mechanistically, LXR activation could induce properties of the epithelial to mesenchymal transition (EMT). However, the effects of LXR activation are unlikely to fully explain the robust metastases observed in 27HC-treated mice. In this regard, oxysterols including 27HC have been demonstrated to promote the migration of myeloid cells in a CXCR2-dependent manner [91], indicating that in addition to its effects on cancer cells, 27HC may also exert its influences on the host to promote metastasis.
Cholesterol and Membrane Signaling
In addition to merely being a membrane component required for fluidity, cholesterol is also an integral component of lipid rafts and subsequent membrane associated signaling events. Thus, it is possible that excess cholesterol might increase signaling events thereby promoting breast cancer progression. In this regard, tumors grown in hyperlipidemic (ApoE−/−) mice on a high-fat, high-cholesterol diet displayed increased PI3K activation and downstream AKT phosphorylation [2]. Importantly, treatment of these mice with a small molecule inhibitor of PI3K decreased mammary tumor growth, indicating that this pathway is active in the presence of elevated cholesterol. It is, however, important to note that the mice in this study had circulating cholesterol levels far exceeding those that would be observed in hypercholesterolemic humans. Thus, although excess cholesterol has the capacity to promote lipid-raft signaling, it remains to be determined whether this pathway is active under clinically relevant conditions.
Cholesterol as a Limiting Factor in Membrane Biogenesis
A rapidly dividing population of cells such as cancerous tumors requires large amounts of cholesterol for membrane synthesis. Thus, it would be logical to hypothesize that cholesterol is a limiting factor and exogenous cholesterol would be consumed rapidly. However, as detailed above, intracellular cholesterol levels are tightly controlled [28]. Hence, it is unclear whether increased extracellular cholesterol could be utilized by the cancer cells.
Interestingly, upon antigen stimulation, T cells begin to divide rapidly, placing an increased demand on available cholesterol, required in order to complete membrane biogenesis. To meet this demand upon activation, T cells increase the expression of SULT2B1, an enzyme that adds an inactivating sulfating moiety on the oxysterol ligands of the LXRs, ultimately leading to decreased LXR activity and increased intracellular cholesterol [9]. It remains unclear whether cancer cells employ similar strategies to accommodate the increased need for cholesterol as they proliferate.
Regardless of whether excess cholesterol can promote cellular proliferation of cancer cells, inhibiting the cellular capacity to synthesize cholesterol decreases proliferation. For example, several in vitro studies have shown that statin-mediated inhibition of HMG-CoA reductase or inhibition of oxidosqualene cyclase can limit proliferation [17, 67]. The interpretation of these results is difficult as HMG-CoA reductase not only produces the precursors for cholesterol synthesis but also those required for protein prenylation and farnesylation. In support of the role of cholesterol, synthetic LXR agonists have been found to inhibit the proliferation of several cancer models including breast, prostate, and melanoma, due to their ability to inhibit cholesterol synthesis and promote cholesterol efflux [61, 79, 86, 89]. Interestingly, the anti-proliferative effects of LXR agonists on breast cancer appear to be isolated to ER-positive models [76, 110]. However, chronic treatment of LXR agonists results in hepatic steatosis and elevated circulating triglycerides, tempering enthusiasm for their development as cancer therapeutics [96]. A recently reported inverse agonist of LXR continues to exhibit significant anti-tumor growth effects but has no apparent effects on circulating triglycerides [37]. This unique class of LXR modulators may help circumvent the historic problems with this class of drugs.
Cholesterol and Inflammation
It is well established that inflammation plays a strong role in tumor promotion across many different types of cancer [44]. For most solid tumors, infiltration by myeloid cells such as macrophages, myeloid-derived suppressor cells, or polymorphoneutrophils is associated with poor prognosis [24, 31, 40, 51, 90, 98, 117, 121]. Tumor-associated macrophages (TAMs) respond to tumor-derived factors such as VCAM1 and CSF1 and are alternatively activated, representing an M2-like polarization state [23, 93]. They have many pro-tumorigenic properties including the release of cytokines, thus stimulating angiogenesis, suppressing acquired immunity, and aiding in the invasion and intravasation of cancer cells [46, 66, 101, 104].
Cholesterol itself is known to have a strong stimulating effect on innate and adaptive immunity across many pathophysiological conditions [reviewed by 36]. Elevated serum cholesterol strongly activates macrophages and promotes the formation of arterial plaques and the subendothelial accumulation of foam cells in the development of atherosclerosis. Specifically, high levels of LDL cholesterol activate macrophages through toll-like receptor (TLR) activation [103]. TLR activation, in turn, increases the release of pro-inflammatory cytokines, thereby promoting further inflammation. TLR activation further exacerbates inflammation by suppressing LXR signaling [64]. Although not formally tested in breast cancer, many aspects of this inflammatory circuit such as cytokines have been shown to exacerbate tumor progression. On the other hand, perturbed cholesterol homeostasis itself can also support the growth of tumors by modulating the activity of tumor-associated leukocytes. For example, when ABCG1, a transporter responsible for regulating macrophage intracellular cholesterol, is ablated, the growth of bladder and melanoma cancers is suppressed. This is presumably due to the altered cholesterol homeostasis promoting the polarization of macrophages into the anti-tumorigenic M1 state [95].
Disordered cholesterol metabolism also affects the function of adaptive immunity. Importantly, it has been shown that sterol metabolism and LXR signaling are able to modulate T cell responses. Activation of LXR was able to suppress T cell proliferation, whereas genetic ablation of LXRβ restored proliferative function of T cells. The suppressive effect of LXR signaling was mediated in part by ABCG1 expression [9]. LXR activation has also been reported to inhibit CCR7 expression on dendritic cells and thus limit their chemotactic capacity to migrate to the lymphoid organs. Since dendritic cells are critical for antigen presentation to T and B cells, their impairment can result in the escape of tumors from immune attack [111].
Cholesterol and Response to Endocrine Therapy
Standard of care for women with ER-positive breast cancer includes long-term use of either tamoxifen or an aromatase inhibitor. Both of these therapies work very well and have significantly extended the long-term survival of patients. However, many patients eventually develop recurrent disease which is endocrine therapy resistant. Interestingly, obesity has been associated with an increased likelihood for the development of tamoxifen resistance [116]. Furthermore, tumors from tamoxifen-resistant patients had an increased infiltrate of tumor-associated macrophages, cells known to highly express the enzyme responsible for 27HC synthesis (CYP27A1). In terms of resistance to aromatase inhibitors, a recent report has found that the expression of many genes involved in cholesterol metabolism is altered due to epigenetic reprogramming [80]. These studies modeled aromatase inhibitor resistance by long-term deprivation of MCF7 cells from estrogens. Importantly, one of the transcripts that were upregulated in this model was CYP27A1. Thus, altered cholesterol homeostasis, especially elevations in 27HC, may promote resistance to endocrine therapies, an important clinical problem.
Concluding Remarks and Future Perspectives
There is a strong epidemiological evidence supporting obesity as a risk factor for breast cancer, and in particular, ER-positive breast cancer. This is particularly concerning given the current obesity rates. While cholesterol can exert direct effects on the immune system and thus the tumor microenvironment, certain cholesterol metabolites such as 27HC can modulate the activity of the ERs and LXRs directly impacting tumor growth and metastasis.
Fortunately, cholesterol is a highly amenable risk factor, either by lifestyle, dietary, or pharmacological interventions. For example, statin therapy is fairly well tolerated and has proven to be very effective at managing cholesterol levels. Other cholesterol-lowering therapies include PCSK9 inhibitors and niacin. Importantly, circulating 27HC levels are correlated to those of cholesterol, and men taking pravastatin exhibit both decreased cholesterol and 27HC levels [54, 106]. Retrospective trials suggest that statin therapy increases recurrence-free survival. This provides rationale for prospective trials evaluating the efficacy of statins in combination with standard of care therapy in preventing and treating metastatic disease.
The development of specific CYP27A1 inhibitors for the treatment of breast cancer should also be considered. This is especially relevant in light of a recent “window of opportunity” trial indicating that the tumoral expression of HMG-CoA reductase is increased post-statin therapy [13]. Similar observations have been observed in macrophages, which exhibit elevated cholesterol even when serum cholesterol is lowered [119]. Due to the limited systemic exposure of oral statins, this induction likely represents a mechanism by which cancer cells can escape cholesterol-limiting strategies. Thus, a more targeted approach that can be systemically delivered such as CYP27A1 inhibitors may prove to have clinical utility.
Acknowledgments
This work in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number R00CA172357 (E.R.N.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Compliance with Ethical Standards
Conflict of Interest
Amy E. Baek declares no conflict of interest.
Erik R. Nelson is the co-inventor of patent WO2015065505 A1, “Use of CYP27A1 inhibitors, statin, or LXR antagonists alone or in combination with conventional therapy for the treatment of breast cancer.”
References
- 1.Ahern TP, Pedersen L, Tarp M, Cronin-Fenton DP, Garne JP, Silliman RA, Sorensen HT, Lash TL. Statin prescriptions and breast cancer recurrence risk: a Danish nationwide prospective cohort study. J Natl Cancer Inst. 2011;103(19):1461–1468. doi: 10.1093/jnci/djr291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alikhani N, Ferguson RD, Novosyadlyy R, Gallagher EJ, Scheinman EJ, Yakar S, LeRoith D. Mammary tumor growth and pulmonary metastasis are enhanced in a hyperlipidemic mouse model. Oncogene. 2013;32(8):961–967. doi: 10.1038/onc.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allott EH, Howard LE, Cooperberg MR, Kane CJ, Aronson WJ, Terris MK, Amling CL, Freedland SJ. Serum lipid profile and risk of prostate cancer recurrence: results from the SEARCH database. Cancer Epidemiol Biomarkers Prev. 2014;23(11):2349–2356. doi: 10.1158/1055-9965.EPI-14-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amemiya-Kudo M, Shimano H, Hasty AH, Yahagi N, Yoshikawa T, Matsuzaka T, Okazaki H, et al. Transcriptional activities of nuclear SREBP-1a, −1c, and −2 to different target promoters of lipogenic and cholesterogenic genes. J Lipid Res. 2002;43(8):1220–1235. [PubMed] [Google Scholar]
- 5.Anderson GL, Manson J, Wallace R, Lund B, Hall D, Davis S, Shumaker S, Wang CY, Stein E, Prentice RL. Implementation of the Women’s Health Initiative study design. Ann Epidemiol. 2003;13(9 Suppl):S5–S17. doi: 10.1016/S1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 6.Bahl M, Ennis M, Tannock IF, Hux JE, Pritchard KI, Koo J, Goodwin PJ. Serum lipids and outcome of early-stage breast cancer: results of a prospective cohort study. Breast Cancer Res Treat. 2005;94(2):135–144. doi: 10.1007/s10549-005-6654-9. [DOI] [PubMed] [Google Scholar]
- 7.Beck P, Wysowski DK, Downey W, Butler-Jones D. Statin use and the risk of breast cancer. J Clin Epidemiol. 2003;56(3):280–285. doi: 10.1016/S0895-4356(02)00614-5. [DOI] [PubMed] [Google Scholar]
- 8.Bennett MK, Seo YK, Datta S, Shin DJ, Osborne TF. Selective binding of sterol regulatory element-binding protein isoforms and co-regulatory proteins to promoters for lipid metabolic genes in liver. J Biol Chem. 2008;283(23):15628–15637. doi: 10.1074/jbc.M800391200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bensinger SJ, Bradley MN, Joseph SB, Zelcer N, Janssen EM, Hausner MA, Shih R, et al. LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell. 2008;134(1):97–111. doi: 10.1016/j.cell.2008.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berrino F, Villarini A, Traina A, Bonanni B, Panico S, Mano MP, Mercandino A, et al. Metabolic syndrome and breast cancer prognosis. Breast Cancer Res Treat. 2014;147(1):159–165. doi: 10.1007/s10549-014-3076-6. [DOI] [PubMed] [Google Scholar]
- 11.Beyea MM, Heslop CL, Sawyez CG, Edwards JY, Markle JG, Hegele RA, Huff MW. Selective up-regulation of LXR-regulated genes ABCA1, ABCG1, and APOE in macrophages through increased endogenous synthesis of 24(S), 25-epoxycholesterol. J Biol Chem. 2007;282(8):5207–5216. doi: 10.1074/jbc.M611063200. [DOI] [PubMed] [Google Scholar]
- 12.Bianchini F, Kaaks R, Vainio H. Overweight, obesity, and cancer risk. Lancet Oncol. 2002;3(9):565–574. doi: 10.1016/S1470-2045(02)00849-5. [DOI] [PubMed] [Google Scholar]
- 13.Bjarnadottir O, Romero Q, Bendahl PO, Jirstrom K, Ryden L, Loman N, Uhlen M, et al. Targeting HMG-CoA reductase with statins in a window-of-opportunity breast cancer trial. Breast Cancer Res Treat. 2013;138(2):499–508. doi: 10.1007/s10549-013-2473-6. [DOI] [PubMed] [Google Scholar]
- 14.Blais L, Desgagne A, LeLorier J. 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors and the risk of cancer: a nested case-control study. Arch Intern Med. 2000;160(15):2363–2368. doi: 10.1001/archinte.160.15.2363. [DOI] [PubMed] [Google Scholar]
- 15.Boudreau DM, Gardner JS, Malone KE, Heckbert SR, Blough DK, Daling JR. The association between 3-hydroxy-3-methylglutaryl conenzyme A inhibitor use and breast carcinoma risk among postmenopausal women: a case-control study. Cancer. 2004;100(11):2308–2316. doi: 10.1002/cncr.20271. [DOI] [PubMed] [Google Scholar]
- 16.Brown MS, Dana SE, Goldstein JL. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in human fibroblasts by lipoproteins. Proc Natl Acad Sci U S A. 1973;70(7):2162–2166. doi: 10.1073/pnas.70.7.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campbell MJ, Esserman LJ, Zhou Y, Shoemaker M, Lobo M, Borman E, Baehner F, et al. Breast cancer growth prevention by statins. Cancer Res. 2006;66(17):8707–8714. doi: 10.1158/0008-5472.CAN-05-4061. [DOI] [PubMed] [Google Scholar]
- 18.Cancer Genome Atlas Network Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Capasso I, Esposito E, Pentimalli F, Crispo A, Montella M, Grimaldi M, De Marco M, et al. Metabolic syndrome affects breast cancer risk in postmenopausal women: National Cancer Institute of Naples experience. Cancer Biol Ther. 2011;10(12):1240–1243. doi: 10.4161/cbt.10.12.13473. [DOI] [PubMed] [Google Scholar]
- 20.Cauley JA, McTiernan A, Rodabough RJ, LaCroix A, Bauer DC, Margolis KL, Paskett ED, et al. Statin use and breast cancer: prospective results from the Women’s Health Initiative. J Natl Cancer Inst. 2006;98(10):700–707. doi: 10.1093/jnci/djj188. [DOI] [PubMed] [Google Scholar]
- 21.Cauley JA, Zmuda JM, Lui LY, Hillier TA, Ness RB, Stone KL, Cummings SR, Bauer DC. Lipid-lowering drug use and breast cancer in older women: a prospective study. J Womens Health (Larchmt) 2003;12(8):749–756. doi: 10.1089/154099903322447710. [DOI] [PubMed] [Google Scholar]
- 22.Centers fo Disease Control and Prevention Prevalence of abnormal lipid levels among youths—United States, 1999–2006. MMWR. 2010;59(2):29–33. [PubMed] [Google Scholar]
- 23.Chen Q, Zhang XH, Massague J. Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs. Cancer Cell. 2011;20(4):538–549. doi: 10.1016/j.ccr.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chittezhath M, Dhillon MK, Lim JY, Laoui D, Shalova IN, Teo YL, Chen J, et al. Molecular profiling reveals a tumor-promoting phenotype of monocytes and macrophages in human cancer progression. Immunity. 2014;41(5):815–829. doi: 10.1016/j.immuni.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 25.Colditz GA, Rosner B. Cumulative risk of breast cancer to age 70 years according to risk factor status: data from the Nurses’ Health Study. Am J Epidemiol. 2000;152(10):950–964. doi: 10.1093/aje/152.10.950. [DOI] [PubMed] [Google Scholar]
- 26.Collaborative Group on Hormonal Factors in Breast, Cancer Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 2012;13(11):1141–1151. doi: 10.1016/S1470-2045(12)70425-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Danilo C, Frank PG. Cholesterol and breast cancer development. Curr Opin Pharmacol. 2012;12(6):677–682. doi: 10.1016/j.coph.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 28.Das A, Brown MS, Anderson DD, Goldstein JL, Radhakrishnan A (2014) Three pools of plasma membrane cholesterol and their relation to cholesterol homeostasis. Elife (Cambridge) e02882. doi:10.7554/eLife.02882. [DOI] [PMC free article] [PubMed]
- 29.Desai P, Chlebowski R, Cauley JA, Manson JE, Wu C, Martin LW, Jay A, et al. Prospective analysis of association between statin use and breast cancer risk in the women’s health initiative. Cancer Epidemiol Biomarkers Prev. 2013;22(10):1868–1876. doi: 10.1158/1055-9965.EPI-13-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.(1998) Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Control Clin Trials 19(1):61–109. [DOI] [PubMed]
- 31.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58(1):49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DuSell CD, Nelson ER, Wang X, Abdo J, Modder UI, Umetani M, Gesty-Palmer D, Javitt NB, Khosla S, McDonnell DP. The endogenous selective estrogen receptor modulator 27-hydroxycholesterol is a negative regulator of bone homeostasis. Endocrinology. 2010;151(8):3675–3685. doi: 10.1210/en.2010-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DuSell CD, Umetani M, Shaul PW, Mangelsdorf DJ, McDonnell DP. 27-hydroxycholesterol is an endogenous selective estrogen receptor modulator. Mol Endocrinol. 2008;22(1):65–77. doi: 10.1210/me.2007-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Endogenous, Hormones, Group Breast Cancer Collaborative. Key TJ, Appleby PN, Reeves GK, Travis RC, Alberg AJ, et al. Sex hormones and risk of breast cancer in premenopausal women: a collaborative reanalysis of individual participant data from seven prospective studies. Lancet Oncol. 2013;14(10):1009–1019. doi: 10.1016/S1470-2045(13)70301-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ewertz M, Jensen MB, Gunnarsdottir KA, Hojris I, Jakobsen EH, Nielsen D, Stenbygaard LE, Tange UB, Cold S. Effect of obesity on prognosis after early-stage breast cancer. J Clin Oncol. 2011;29(1):25–31. doi: 10.1200/JCO.2010.29.7614. [DOI] [PubMed] [Google Scholar]
- 36.Fessler MB. Regulation of adaptive immunity in health and disease by cholesterol metabolism. Curr Allergy Asthma Rep. 2015;15(8):48. doi: 10.1007/s11882-015-0548-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flaveny CA, Griffett K, El-Gendy Bel D, Kazantzis M, Sengupta M, Amelio AL, Chatterjee A, et al. Broad anti-tumor activity of a small molecule that selectively targets the Warburg effect and lipogenesis. Cancer Cell. 2015;28(1):42–56. doi: 10.1016/j.ccell.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Friis S, Poulsen AH, Johnsen SP, McLaughlin JK, Fryzek JP, Dalton SO, Sorensen HT, Olsen JH. Cancer risk among statin users: a population-based cohort study. Int J Cancer. 2005;114(4):643–647. doi: 10.1002/ijc.20758. [DOI] [PubMed] [Google Scholar]
- 39.Fu X, Menke JG, Chen Y, Zhou G, MacNaul KL, Wright SD, Sparrow CP, Lund EG. 27-hydroxycholesterol is an endogenous ligand for liver X receptor in cholesterol-loaded cells. J Biol Chem. 2001;276(42):38378–38387. doi: 10.1074/jbc.M105805200. [DOI] [PubMed] [Google Scholar]
- 40.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goldstein JL, Brown MS. Familial hypercholesterolemia: identification of a defect in the regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity associated with overproduction of cholesterol. Proc Natl Acad Sci U S A. 1973;70(10):2804–2808. doi: 10.1073/pnas.70.10.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gostynski M, Gutzwiller F, Kuulasmaa K, Doring A, Ferrario M, Grafnetter D, Pajak A, Who Monica Project Analysis of the relationship between total cholesterol, age, body mass index among males and females in the WHO MONICA Project. Int J Obes Relat Metab Disord. 2004;28(8):1082–1090. doi: 10.1038/sj.ijo.0802714. [DOI] [PubMed] [Google Scholar]
- 43.Graaf MR, Beiderbeck AB, Egberts AC, Richel DJ, Guchelaar HJ. The risk of cancer in users of statins. J Clin Oncol. 2004;22(12):2388–2394. doi: 10.1200/JCO.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 44.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ha M, Sung J, Song YM. Serum total cholesterol and the risk of breast cancer in postmenopausal Korean women. Cancer Causes Control. 2009;20(7):1055–1060. doi: 10.1007/s10552-009-9301-7. [DOI] [PubMed] [Google Scholar]
- 46.Haile LA, Gamrekelashvili J, Manns MP, Korangy F, Greten TF. CD49d is a new marker for distinct myeloid-derived suppressor cell subpopulations in mice. J Immunol. 2010;185(1):203–210. doi: 10.4049/jimmunol.0903573. [DOI] [PubMed] [Google Scholar]
- 47.Hall JM, Lee MK, Newman B, Morrow JE, Anderson LA, Huey B, King MC. Linkage of early-onset familial breast cancer to chromosome 17q21. Science. 1990;250(4988):1684–1689. doi: 10.1126/science.2270482. [DOI] [PubMed] [Google Scholar]
- 48.Hong C, Tontonoz P. Liver X receptors in lipid metabolism: opportunities for drug discovery. Nat Rev Drug Discov. 2014;13(6):433–444. doi: 10.1038/nrd4280. [DOI] [PubMed] [Google Scholar]
- 49.Hu J, La Vecchia C, de Groh M, Negri E, Morrison H, Mery L, Group Canadian Cancer Registries Epidemiology Research Dietary cholesterol intake and cancer. Ann Oncol. 2012;23(2):491–500. doi: 10.1093/annonc/mdr155. [DOI] [PubMed] [Google Scholar]
- 50.Hua X, Nohturfft A, Goldstein JL, Brown MS. Sterol resistance in CHO cells traced to point mutation in SREBP cleavage-activating protein. Cell. 1996;87(3):415–426. doi: 10.1016/S0092-8674(00)81362-8. [DOI] [PubMed] [Google Scholar]
- 51.Huang SH, Waldron JN, Milosevic M, Shen X, Ringash J, Su J, Tong L, et al. Prognostic value of pretreatment circulating neutrophils, monocytes, and lymphocytes in oropharyngeal cancer stratified by human papillomavirus status. Cancer. 2015;121(4):545–555. doi: 10.1002/cncr.29100. [DOI] [PubMed] [Google Scholar]
- 52.Jiralerspong S, Kim ES, Dong W, Feng L, Hortobagyi GN, Giordano SH. Obesity, diabetes, and survival outcomes in a large cohort of early-stage breast cancer patients. Ann Oncol. 2013;24(10):2506–2514. doi: 10.1093/annonc/mdt224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jones LW, Alfano CM. Exercise-oncology research: past, present, and future. Acta Oncol. 2013;52(2):195–215. doi: 10.3109/0284186X.2012.742564. [DOI] [PubMed] [Google Scholar]
- 54.Karuna R, Holleboom AG, Motazacker MM, Kuivenhoven JA, Frikke-Schmidt R, Tybjaerg-Hansen A, Georgopoulos S, et al. Plasma levels of 27-hydroxycholesterol in humans and mice with monogenic disturbances of high density lipoprotein metabolism. Atherosclerosis. 2011;214(2):448–455. doi: 10.1016/j.atherosclerosis.2010.10.042. [DOI] [PubMed] [Google Scholar]
- 55.Kaye JA, Jick H. Statin use and cancer risk in the General Practice Research Database. Br J Cancer. 2004;90(3):635–637. doi: 10.1038/sj.bjc.6601566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kitahara CM, Berrington de Gonzalez A, Freedman ND, Huxley R, Mok Y, Jee SH, Samet JM. Total cholesterol and cancer risk in a large prospective study in Korea. J Clin Oncol. 2011;29(12):1592–1598. doi: 10.1200/JCO.2010.31.5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kwan ML, Habel LA, Flick ED, Quesenberry CP, Caan B. Post-diagnosis statin use and breast cancer recurrence in a prospective cohort study of early stage breast cancer survivors. Breast Cancer Res Treat. 2008;109(3):573–579. doi: 10.1007/s10549-007-9683-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Laffitte BA, Repa JJ, Joseph SB, Wilpitz DC, Kast HR, Mangelsdorf DJ, Tontonoz P. LXRs control lipid-inducible expression of the apolipoprotein E gene in macrophages and adipocytes. Proc Natl Acad Sci U S A. 2001;98(2):507–512. doi: 10.1073/pnas.98.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lappano R, Recchia AG, De Francesco EM, Angelone T, Cerra MC, Picard D, Maggiolini M. The cholesterol metabolite 25-hydroxycholesterol activates estrogen receptor alpha-mediated signaling in cancer cells and in cardiomyocytes. PLoS One. 2011;6(1):e16631. doi: 10.1371/journal.pone.0016631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Law JH, Habibi G, Hu K, Masoudi H, Wang MY, Stratford AL, Park E, et al. Phosphorylated insulin-like growth factor-i/insulin receptor is present in all breast cancer subtypes and is related to poor survival. Cancer Res. 2008;68(24):10238–10246. doi: 10.1158/0008-5472.CAN-08-2755. [DOI] [PubMed] [Google Scholar]
- 61.Lee JH, Gong H, Khadem S, Lu Y, Gao X, Li S, Zhang J, Xie W. Androgen deprivation by activating the liver X receptor. Endocrinology. 2008;149(8):3778–3788. doi: 10.1210/en.2008-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu J, Xu A, Lam KS, Wong NS, Chen J, Shepherd PR, Wang Y. Cholesterol-induced mammary tumorigenesis is enhanced by adiponectin deficiency: role of LDL receptor upregulation. Oncotarget. 2013;4(10):1804–1818. doi: 10.18632/oncotarget.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Llaverias G, Danilo C, Mercier I, Daumer K, Capozza F, Williams TM, Sotgia F, Lisanti MP, Frank PG. Role of cholesterol in the development and progression of breast cancer. Am J Pathol. 2011;178(1):402–412. doi: 10.1016/j.ajpath.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lo Sasso G, Murzilli S, Salvatore L, D’Errico I, Petruzzelli M, Conca P, Jiang ZY, Calabresi L, Parini P, Moschetta A. Intestinal specific LXR activation stimulates reverse cholesterol transport and protects from atherosclerosis. Cell Metab. 2010;12(2):187–193. doi: 10.1016/j.cmet.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 65.Lord SJ, Bernstein L, Johnson KA, Malone KE, McDonald JA, Marchbanks PA, Simon MS, et al. Breast cancer risk and hormone receptor status in older women by parity, age of first birth, and breastfeeding: a case-control study. Cancer Epidemiol Biomarkers Prev. 2008;17(7):1723–1730. doi: 10.1158/1055-9965.EPI-07-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Luo Y, Zhou H, Krueger J, Kaplan C, Lee SH, Dolman C, Markowitz D, et al. Targeting tumor-associated macrophages as a novel strategy against breast cancer. J Clin Invest. 2006;116(8):2132–2141. doi: 10.1172/JCI27648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mafuvadze B, Liang Y, Hyder SM. Cholesterol synthesis inhibitor RO 48-8071 suppresses transcriptional activity of human estrogen and androgen receptor. Oncol Rep. 2014;32(4):1727–1733. doi: 10.3892/or.2014.3332. [DOI] [PubMed] [Google Scholar]
- 68.Martinez-Outschoorn UE, Sotgia F, Lisanti MP. Caveolae and signalling in cancer. Nat Rev Cancer. 2015;15(4):225–237. doi: 10.1038/nrc3915. [DOI] [PubMed] [Google Scholar]
- 69.McPherson K, Steel CM, Dixon JM. ABC of breast diseases. Breast cancer—epidemiology, risk factors, and genetics. BMJ. 2000;321(7261):624–628. doi: 10.1136/bmj.321.7261.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Michailidou K, Hall P, Gonzalez-Neira A, Ghoussaini M, Dennis J, Milne RL, Schmidt MK, et al. Large-scale genotyping identifies 41 new loci associated with breast cancer risk. Nat Genet. 2013;45(4):353–361. doi: 10.1038/ng.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Michels KB, Solomon CG, Hu FB, Rosner BA, Hankinson SE, Colditz GA, Manson JE, Study Nurses’ Health Type 2 diabetes and subsequent incidence of breast cancer in the Nurses’ Health Study. Diabetes Care. 2003;26(6):1752–1758. doi: 10.2337/diacare.26.6.1752. [DOI] [PubMed] [Google Scholar]
- 72.Mundal L, Sarancic M, Ose L, Iversen PO, Borgan JK, Veierod MB, Leren TP, Retterstol K. Mortality among patients with familial hypercholesterolemia: a registry-based study in Norway, 1992–2010. J Am Heart Assoc. 2014;3(6):e001236. doi: 10.1161/JAHA.114.001236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282(16):1523–1529. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 74.Nelson ER, Chang CY, McDonnell DP. Cholesterol and breast cancer pathophysiology. Trends Endocrinol Metab. 2014;25(12):649–655. doi: 10.1016/j.tem.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nelson ER, DuSell CD, Wang X, Howe MK, Evans G, Michalek RD, Umetani M, et al. The oxysterol, 27-hydroxycholesterol, links cholesterol metabolism to bone homeostasis through its actions on the estrogen and liver X receptors. Endocrinology. 2011;152(12):4691–4705. doi: 10.1210/en.2011-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nelson ER, Wardell SE, Jasper JS, Park S, Suchindran S, Howe MK, Carver NJ, et al. 27-Hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science. 2013;342(6162):1094–1098. doi: 10.1126/science.1241908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Neuhouser ML, Aragaki AK, Prentice RL, Manson JE, Chlebowski R, Carty CL, Ochs-Balcom HM. Overweight, obesity, and postmenopausal invasive breast cancer risk: a secondary analysis of the Women’s Health Initiative randomized clinical trials. JAMA Oncol. 2015 doi: 10.1001/jamaoncol.2015.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Neuhouser ML, Aragaki AK, Prentice RL, Manson JE, Chlebowski R, Carty CL, Ochs-Balcom HM, et al. Overweight, obesity, and postmenopausal invasive breast cancer risk: a secondary analysis of the Women’s Health Initiative randomized clinical trials. JAMA Onco. 2015;1(5):611–621. doi: 10.1001/jamaoncol.2015.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nguyen-Vu T, Vedin LL, Liu K, Jonsson P, Lin JZ, Candelaria NR, Candelaria LP, et al. Liver X receptor ligands disrupt breast cancer cell proliferation through an E2F-mediated mechanism. Breast Cancer Res. 2013;15(3):R51. doi: 10.1186/bcr3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nguyen VT, Barozzi I, Faronato M, Lombardo Y, Steel JH, Patel N, Darbre P, et al. Differential epigenetic reprogramming in response to specific endocrine therapies promotes cholesterol biosynthesis and cellular invasion. Nat Commun. 2015;6:10044. doi: 10.1038/ncomms10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. N Engl J Med. 2012;367(19):1792–1802. doi: 10.1056/NEJMoa1201735. [DOI] [PubMed] [Google Scholar]
- 82.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ou J, Tu H, Shan B, Luk A, DeBose-Boyd RA, Bashmakov Y, Goldstein JL, Brown MS. Unsaturated fatty acids inhibit transcription of the sterol regulatory element-binding protein-1c (SREBP-1c) gene by antagonizing ligand-dependent activation of the LXR. Proc Natl Acad Sci U S A. 2001;98(11):6027–6032. doi: 10.1073/pnas.111138698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pasanisi P, Berrino F, De Petris M, Venturelli E, Mastroianni A, Panico S. Metabolic syndrome as a prognostic factor for breast cancer recurrences. Int J Cancer. 2006;119(1):236–238. doi: 10.1002/ijc.21812. [DOI] [PubMed] [Google Scholar]
- 85.Pelton K, Coticchia CM, Curatolo AS, Schaffner CP, Zurakowski D, Solomon KR, Moses MA. Hypercholesterolemia induces angiogenesis and accelerates growth of breast tumors in vivo. Am J Pathol. 2014;184(7):2099–2110. doi: 10.1016/j.ajpath.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pencheva N, Buss CG, Posada J, Merghoub T, Tavazoie SF. Broad-spectrum therapeutic suppression of metastatic melanoma through nuclear hormone receptor activation. Cell. 2014;156(5):986–1001. doi: 10.1016/j.cell.2014.01.038. [DOI] [PubMed] [Google Scholar]
- 87.Petracci E, Decarli A, Schairer C, Pfeiffer RM, Pee D, Masala G, Palli D, Gail MH. Risk factor modification and projections of absolute breast cancer risk. J Natl Cancer Inst. 2011;103(13):1037–1048. doi: 10.1093/jnci/djr172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pierobon M, Frankenfeld CL. Obesity as a risk factor for triple-negative breast cancers: a systematic review and meta-analysis. Breast Cancer Res Treat. 2013;137(1):307–314. doi: 10.1007/s10549-012-2339-3. [DOI] [PubMed] [Google Scholar]
- 89.Pommier AJ, Alves G, Viennois E, Bernard S, Communal Y, Sion B, Marceau G, et al. Liver X Receptor activation downregulates AKT survival signaling in lipid rafts and induces apoptosis of prostate cancer cells. Oncogene. 2010;29(18):2712–2723. doi: 10.1038/onc.2010.30. [DOI] [PubMed] [Google Scholar]
- 90.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Raccosta L, Fontana R, Maggioni D, Lanterna C, Villablanca EJ, Paniccia A, Musumeci A, et al. The oxysterol–CXCR2 axis plays a key role in the recruitment of tumor-promoting neutrophils. J Exp Med. 2013;210(9):1711–1728. doi: 10.1084/jem.20130440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Renehan AG, Roberts DL, Dive C. Obesity and cancer: pathophysiological and biological mechanisms. Arch Physiol Biochem. 2008;114(1):71–83. doi: 10.1080/13813450801954303. [DOI] [PubMed] [Google Scholar]
- 93.Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, Rey-Giraud F, et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 2014;25(6):846–859. doi: 10.1016/j.ccr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 94.Ronco AL, De Stefani E, Stoll M. Hormonal and metabolic modulation through nutrition: towards a primary prevention of breast cancer. Breast. 2010;19(5):322–332. doi: 10.1016/j.breast.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 95.Sag D, Cekic C, Wu R, Linden J, Hedrick CC. The cholesterol transporter ABCG1 links cholesterol homeostasis and tumour immunity. Nat Commun. 2015;6:6354. doi: 10.1038/ncomms7354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schultz JR, Tu H, Luk A, Repa JJ, Medina JC, Li L, Schwendner S, et al. Role of LXRs in control of lipogenesis. Genes Dev. 2000;14(22):2831–2838. doi: 10.1101/gad.850400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shah R, Rosso K, Nathanson SD. Pathogenesis, prevention, diagnosis and treatment of breast cancer. World J Clin Oncol. 2014;5(3):283–298. doi: 10.5306/wjco.v5.i3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shen M, Hu P, Donskov F, Wang G, Liu Q, Du J. Tumor-associated neutrophils as a new prognostic factor in cancer: a systematic review and meta-analysis. PLoS One. 2014;9(6):e98259. doi: 10.1371/journal.pone.0098259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 100.Singletary SE. Rating the risk factors for breast cancer. Ann Surg. 2003;237(4):474–482. doi: 10.1097/01.SLA.0000059969.64262.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Solito S, Falisi E, Diaz-Montero CM, Doni A, Pinton L, Rosato A, Francescato S, et al. A human promyelocytic-like population is responsible for the immune suppression mediated by myeloid-derived suppressor cells. Blood. 2011;118(8):2254–2265. doi: 10.1182/blood-2010-12-325753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Spann NJ, Glass CK. Sterols and oxysterols in immune cell function. Nat Immunol. 2013;14(9):893–900. doi: 10.1038/ni.2681. [DOI] [PubMed] [Google Scholar]
- 103.Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11(2):155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Strachan DC, Ruffell B, Oei Y, Bissell MJ, Coussens LM, Pryer N, Daniel D. CSF1R inhibition delays cervical and mammary tumor growth in murine models by attenuating the turnover of tumor-associated macrophages and enhancing infiltration by CD8 T cells. Oncoimmunology. 2013;2(12):e26968. doi: 10.4161/onci.26968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sullivan PM, Mezdour H, Aratani Y, Knouff C, Najib J, Reddick RL, Quarfordt SH, Maeda N. Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis. J Biol Chem. 1997;272(29):17972–17980. doi: 10.1074/jbc.272.29.17972. [DOI] [PubMed] [Google Scholar]
- 106.Thelen KM, Lutjohann D, Vesalainen R, Janatuinen T, Knuuti J, von Bergmann K, Lehtimaki T, Laaksonen R. Effect of pravastatin on plasma sterols and oxysterols in men. Eur J Clin Pharmacol. 2006;62(1):9–14. doi: 10.1007/s00228-005-0068-9. [DOI] [PubMed] [Google Scholar]
- 107.Umetani M, Domoto H, Gormley AK, Yuhanna IS, Cummins CL, Javitt NB, Korach KS, Shaul PW, Mangelsdorf DJ. 27-Hydroxycholesterol is an endogenous SERM that inhibits the cardiovascular effects of estrogen. Nat Med. 2007;13(10):1185–1192. doi: 10.1038/nm1641. [DOI] [PubMed] [Google Scholar]
- 108.Umetani M, Ghosh P, Ishikawa T, Umetani J, Ahmed M, Mineo C, Shaul PW. The cholesterol metabolite 27-hydroxycholesterol promotes atherosclerosis via proinflammatory processes mediated by estrogen receptor alpha. Cell Metab. 2014;20(1):172–182. doi: 10.1016/j.cmet.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Undela K, Srikanth V, Bansal D. Statin use and risk of breast cancer: a meta-analysis of observational studies. Breast Cancer Res Treat. 2012;135(1):261–269. doi: 10.1007/s10549-012-2154-x. [DOI] [PubMed] [Google Scholar]
- 110.Vedin LL, Lewandowski SA, Parini P, Gustafsson JA, Steffensen KR. The oxysterol receptor LXR inhibits proliferation of human breast cancer cells. Carcinogenesis. 2009;30(4):575–579. doi: 10.1093/carcin/bgp029. [DOI] [PubMed] [Google Scholar]
- 111.Villablanca EJ, Raccosta L, Zhou D, Fontana R, Maggioni D, Negro A, Sanvito F, et al. Tumor-mediated liver X receptor-alpha activation inhibits CC chemokine receptor-7 expression on dendritic cells and dampens antitumor responses. Nat Med. 2010;16(1):98–105. doi: 10.1038/nm.2074. [DOI] [PubMed] [Google Scholar]
- 112.Wang X, Simpson ER, Brown KA. Aromatase overexpression in dysfunctional adipose tissue links obesity to postmenopausal breast cancer. J Steroid Biochem Mol Biol. 2015;153:35–44. doi: 10.1016/j.jsbmb.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 113.Waxler SH, Tabar P, Melcher LR. Obesity and the time of appearance of spontaneous mammary carcinoma in C3H mice. Cancer Res. 1953;13(3):276–278. [PubMed] [Google Scholar]
- 114.White CP. On the occurrence of crystals in tumours. J Pathol Bacteriol. 1909;13(1):3–10. doi: 10.1002/path.1700130103. [DOI] [Google Scholar]
- 115.Wu Q, Ishikawa T, Sirianni R, Tang H, McDonald JG, Yuhanna IS, Thompson B, et al. 27-Hydroxycholesterol promotes cell-autonomous, ER-positive breast cancer growth. Cell Rep. 2013;5(3):637–645. doi: 10.1016/j.celrep.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xuan QJ, Wang JX, Nanding A, Wang ZP, Liu H, Lian X, Zhang QY. Tumor-associated macrophages are correlated with tamoxifen resistance in the postmenopausal breast cancer patients. Pathol Oncol Res. 2014;20(3):619–624. doi: 10.1007/s12253-013-9740-z. [DOI] [PubMed] [Google Scholar]
- 117.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181(8):5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zelcer N, Hong C, Boyadjian R, Tontonoz P. LXR regulates cholesterol uptake through Idol-dependent ubiquitination of the LDL receptor. Science. 2009;325(5936):100–104. doi: 10.1126/science.1168974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang JR, Coleman T, Langmade SJ, Scherrer DE, Lane L, Lanier MH, Feng C, et al. Niemann-Pick C1 protects against atherosclerosis in mice via regulation of macrophage intracellular cholesterol trafficking. J Clin Invest. 2008;118(6):2281–2290. doi: 10.1172/JCI32561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang L, Reue K, Fong LG, Young SG, Tontonoz P. Feedback regulation of cholesterol uptake by the LXR–IDOL–LDLR axis. Arterioscler Thromb Vasc Biol. 2012;32(11):2541–2546. doi: 10.1161/ATVBAHA.112.250571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH, Wang XZ, Zhao YW, Wei YQ. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS One. 2012;7(12):e50946. doi: 10.1371/journal.pone.0050946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhong S, Zhang X, Chen L, Ma T, Tang J, Zhao J. Statin use and mortality in cancer patients: systematic review and meta-analysis of observational studies. Cancer Treat Rev. 2015;41(6):554–567. doi: 10.1016/j.ctrv.2015.04.005. [DOI] [PubMed] [Google Scholar]