Abstract
Background
Autologous stem cell transplantation (SCT) is a common management strategy for select patients with immunoglobulin light chain (AL) amyloidosis, but no trials have documented improved overall survival.
Methods
Eighty-nine patients with biopsy-proven AL amyloidosis were allowed to select treatment with melphalan plus dexamethasone (n=34) or SCT (n=55) (all patients were transplant eligible). Treatment preference resulted in imbalanced study arms. Patients who selected SCT were younger, more frequently had an Eastern Cooperative Oncology Group performance status score less than 2, had lower-stage amyloidosis, and a lower incidence of cardiac amyloidosis.
Results
Patients receiving melphalan plus dexamethasone had a 3-year progression-free survival rate of 29.1% and an overall survival rate of 58.8%. Patients receiving SCT had a 3-year progression-free survival rate of 51.7% and an overall survival rate of 83.6%. An attempt to match patients between the 2 arms, in terms of risk, produced 24 matched triplet sets (2 SCT patients for each melphalan plus dexamethasone patient) and showed no difference in hematologic response but better survival after autologous SCT. A propensity score−matched analysis of cohorts (melphalan plus dexamethasone vs SCT) showed an overall mortality hazard ratio of 2.56 (P<.01).
Conclusion
Acknowledging the limitations of our study, we observed similar hematologic response and improved survival after SCT compared with melphalan plus dexamethasone. (Trial registration: Clinicaltrials.gov, NCT00477971)
Keywords: dexamethasone, immunoglobulin light chains, light chain amyloidosis, melphalan, stem cell transplantation
Introduction
Primary systemic amyloidosis has an incidence rate of 8 patients per million per year (1), and virtually all patients die of this disease (median survival, 12-18 months; 10-year survival rate, <5%) (2). Reports of the use of oral melphalan for the management of amyloidosis first appeared 40 years ago (3). Subsequently, a case series reported that oral melphalan and prednisone prolonged survival (4). The survival benefit of oral melphalan and prednisone with or without colchicine vs colchicine alone was demonstrated in prospective randomized studies (5). However, the median survival with this combination was only 17 months overall, and in the subset of patients with cardiac amyloidosis, it was only 6 months. The combination of alkylating agents vincristine, carmustine, melphalan, cyclophosphamide, and prednisone did not improve outcomes compared with melphalan and prednisone (6).
The combination of melphalan and dexamethasone, building on single-arm studies with high-dose dexamethasone, produced hematologic responses and organ regression (7). Melphalan plus dexamethasone was shown to be an effective option for patients with amyloidosis. The benefits of melphalan plus prednisone or melphalan plus dexamethasone are that they are oral combinations and can be administered to patients with severe cardiac failure, renal insufficiency, and hepatic involvement with amyloid (7).
Since the original report describing 5 patients benefitting from autologous stem cell transplantation (SCT) (8), well over 1,000 patients have been treated with this technique (9). SCT has been associated with regression of predominant amyloid-related organ involvement, including cardiac, renal, hepatic, and soft tissue (10). Hematologic and organ response rates as high as 60% have been reported, with a relatively low relapse rate, presumably because of the kinetics of the clone in amyloidosis (eg, small in number, low proliferative rate, and favorable genetics) (11). However, these original reports also described high treatment-related mortality rates. This led to the development of a risk-adapted approach that stratified patients by the extent of cardiac involvement: non– transplant candidates, candidates for reduced-intensity conditioning, or candidates for full-dose conditioning (12).
Because patient selection heavily influences outcomes, the superiority of SCT over oral therapy has not been definitively shown (13). In a cohort-matched control study, SCT appeared to improve survival (14), but a prospective randomized study completed by the Intergroupe Francophone du Myelome (15) and a subsequent meta-analysis did not show a survival advantage for SCT over chemotherapy in patients with amyloidosis (16). Nevertheless, the ability to reduce SCT-related mortality through appropriate patient selection has substantially reduced day-100 all-cause mortality rates and substantially increased 10-year survivorship. In one trial, median survival of all patients was 96 months from registration; for patients undergoing SCT, median survival was 10 years from the date of transplantation. The 30-day mortality rate after SCT was 4% (17). To address the confusion regarding the optimal therapy for amyloidosis, this trial was initiated to compare high-dose melphalan with stem cell reconstitution to oral melphalan plus dexamethasone.
Patients and Methods
The study was approved by the Mayo Clinic Institutional Review Board. Informed consent was obtained from all patients before study entry. The trial was registered at Clinicaltrials.gov (NCT00477971).
This trial was designed to enroll patients with biopsy-proven light chain amyloidosis who were deemed eligible for transplantation. The trial enrolled individuals at least 18 years old with biopsy-proven light chain amyloidosis. To be eligible, patients also had to have an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0, 1, or 2, a baseline platelet count >100,000/µL, a direct bilirubin <2 mg/dL, an alkaline phosphatase ≤6 times the upper limit of normal, a serum creatinine of ≤3 mg/dL, and a New York Heart Association class from I to III. All women of childbearing potential had a negative pregnancy test within 7 days of registration.
Exclusions to enrollment included overt multiple myeloma, a cardiac or pulmonary condition that contraindicated transplantation, or active infection. Patients whose only manifestation of light chain amyloidosis was carpal tunnel syndrome or purpura were excluded. Pregnant and lactating women were ineligible.
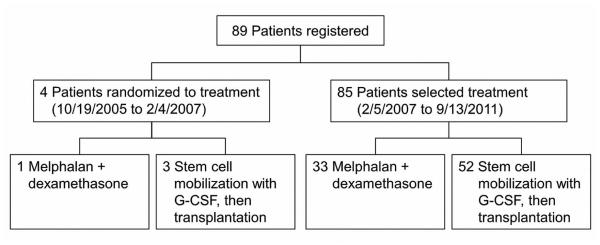
Within 4 weeks of study entry, patients underwent a medical examination that included blood and cardiac chemistry, serum and urine protein electrophoresis, a bone survey, a bone marrow biopsy, and measurement of immunoglobulin, immunoglobulin free light chain, β2 microglobulin, and C-reactive protein. All patients underwent subcutaneous fat pad aspiration, chest radiography, echocardiography, and electrocardiography. The CONSORT diagram of patient registration is shown in Figure 1. From October 19, 2005, through February 4, 2007, patients were randomized to receive either melphalan plus dexamethasone orally (arm A) or melphalan with SCT (arm B). A dynamic allocation procedure was used to randomize patients to treatment so that the marginal distribution of stratification factors, risk group (high vs low), and ECOG performance score (0-1 vs 2) were balanced between treatment arms. The low-risk group consisted of those with at most 2 organs involved, cardiac ejection fraction of 50% or more, age 65 years or younger, New York Heart Association class I or II, alkaline phosphatase ≤4 times the upper limit of normal, cardiac involvement fully compensated or asymptomatic, and serum creatinine <2 mg/dL.
Figure 1.
CONSORT Diagram. G-CSF indicates granulocyte-colony stimulating factor (filgrastim).
Because of slow accrual and the unwillingness of patients to be randomized to SCT or all-oral therapy, the protocol was amended in February 2007 so that patients who met all eligibility criteria, agreed to all protocol requirements, and provided written consent were allowed to choose the treatment that they would receive. On September 13, 2011, the study closed to enrollment because of poor accrual and a lack of funding.
Therapy Specifics
Melphalan Plus Dexamethasone (Study Arm A)
Before May 28, 2008, melphalan was administered parenterally at 20 mg/m2 on day 1; afterward, it was administered orally at 0.12 mg/kg on days 1 through 7. Parenteral melphalan was initially chosen to avoid response issues attributable to absorption of melphalan in patients with AL amyloidosis, but extensive insurance issues regarding reimbursement for parenteral melphalan for AL amyloidosis required us to abandon the parenteral approach. Dexamethasone was administered at 40 mg on days 1 through 4 and 22 through 25 in a 42-day cycle. Monitoring evaluations included blood and cardiac chemistry, serum and urine protein electrophoresis, bone survey, bone marrow biopsy, immunoglobulin and immunoglobulin free light chain testing, electrocardiography, and echocardiography at the end of cycles 3, 7, and 10 and every 6 months thereafter until disease progression or a maximum of 10 years after registration. During therapy, β2 microglobulin measurement, C-reactive protein testing, chest radiography, and peripheral blood measurement of circulating plasma cells were also performed.
Stem Cell Transplantation (Study Arm B)
Filgrastim was given at 10 µg/kg per day to mobilize blood stem cells, with a minimum requirement of 2×106 CD34 cells/kg. After stem cell collection was completed, melphalan was administered intravenously on the 2 days preceding stem cell infusion. The total dose of melphalan administered was 140 mg/m2 for patients not considered low risk and 200 mg/m2 for those considered low risk. Evaluation included blood and cardiac chemistry, serum and urine protein electrophoresis, bone survey, bone marrow biopsy, immunoglobulin and immunoglobulin free light chain testing, electrocardiography, and echocardiography at day 100, 9 months, and 12 months after transplantation and every 6 months thereafter until progression or a maximum of 10 years after transplantation.
Statistical Design
This study was originally designed as a randomized phase III clinical trial to detect an improvement in the confirmed hematologic response rate during the first 12 months after registration, from 30% with melphalan to 60% or more with SCT. From October 2005 through February 2007, only 4 patients consented to be randomized to treatment. The protocol was amended to allow patients who met all aspects of eligibility criteria, agreed to all protocol requirements, and provided written consent to choose the treatment that they would receive.
To account for potential biases attributable to nonrandomized treatment, a Mantel-Haenszel test was planned for the primary comparison of outcomes between arms, using cohorts of patients matched by risk group and ECOG performance score. We also performed a sensitivity analysis by using an unconditional and conditional regression model to adjust for imbalanced factors in the matched cohorts. An additional sensitivity analysis, using propensity-score matching to reduce the effect of treatment selection bias, was conducted to confirm the primary analysis results. For each patient, we calculated their propensity score (that is, probability of choosing SCT, given their covariates) by using a logistic regression model with patient and disease characteristics that differed between those who chose melphalan plus dexamethasone and those who chose SCT. Patients who chose SCT were matched to those who chose melphalan plus dexamethasone on the basis of these propensity scores. The greedy matching procedure proposed by Rosenbaum (18) was used for covariate matching in the primary analysis, as well as for propensity-score matching in the sensitivity analysis. Matching was done while blinded to patient outcomes.
Secondary end points in this trial included progression-free and overall survival. Progression-free survival was defined as time from registration to documentation of progression, either hematologic or organ, and patients who died without documentation of disease progression were considered to have progression on the date of death. Overall survival was defined as time from registration to death due to any cause. Estimates of time-to-event distributions were constructed using the Kaplan-Meier method. Overall survival and progression-free survival were compared between arms using the marginal Cox model of Lee et al (19) to account for potential correlation within the matched sets.
Results
Study Cohort
From October 19, 2005, through September 13, 2011, 89 individuals enrolled in the trial (34 in arm A [melphalan plus dexamethasone] and 55 in arm B [autologous SCT]). Four patients were randomized to treatment (arm A, n=1; arm B, n=3) before the trial was amended to allow patients their choice of treatment. Patient and disease characteristics of the 89 patients are presented in Table 1.
Table 1.
Patient and Disease Characteristics (N=89)
| Characteristic |
Melphalan + Dexamethasone (n=34) |
Autologous Stem Cell Transplantation (n=55) |
|---|---|---|
| Age, median (range), y | 62 (48-74) | 57 (44-73) |
| Male, No. (%) | 17 (50.0) | 39 (70.9) |
| Eastern Cooperative Oncology Group performance status, No. (%) |
||
| 0-1 | 25 (73.5) | 50 (90.9) |
| 2 | 9 (26.5) | 5 (9.1) |
| High-risk group, No. (%) | 14 (41.2) | 17 (30.9) |
| Immunoglobulin light chain amyloidosis stage, No. (%) |
||
| I | 4 (11.8) | 26 (47.3) |
| II | 20 (58.8) | 22 (40.0) |
| III | 10 (29.4) | 7 (12.7) |
| Dominant disease site, No. (%) | ||
| Kidney | 18 (52.9) | 36 (65.5) |
| Heart | 14 (41.2) | 8 (14.6) |
| Gastrointestinal tract or small bowel | 1 (2.9) | 3 (5.5) |
| Macroglossia | 0 (0) | 3 (5.5) |
| Peripheral nerve | 1 (2.9) | 1 (1.8) |
| Liver | 0 (0) | 1 (1.8) |
| Lung | 0 (0) | 1 (1.8) |
| Muscle | 0 (0) | 1 (1.8) |
| Stomach | 0 (0) | 1 (1.8) |
| New York Heart Association class, No. (%) | … | |
| I | 17 (50.0) | |
| II | 16 (47.1) | |
| III | 1 (2.9) | |
| Grade 2 or 3 symptoms present at study entry, No. (%) |
||
| Fatigue | 7 (20.6) | 9 (16.4) |
| Weight loss | 5 (14.7) | 3 (5.5) |
| Edema | 3 (8.8) | 4 (7.3) |
| Neurosensory difficulties | 2 (5.9) | 4 (7.3) |
| Left ventricular ejection fraction, No. (%) | ||
| <50% | 2 (5.9) | 1 (1.8) |
| ≥50% | 29 (94.1) | 42 (98.2) |
| M spike present, No. (%) | 10 (29.4) | 23 (41.8) |
| Free light chain assay, median (IQR), mg/dL | ||
| κ free light chain | 1.42 (0.99-1.98) | 1.28 (0.90-2.72) |
| λ free light chain | 28.25 (8.79-44.8) | 8.41 (2.32-18.6) |
| κ/λ ratio | 0.07 (0.02-0.15) | 0.15 (0.04-0.96) |
Abbreviation: IQR, interquartile range.
Patients who chose SCT differed from those who chose melphalan plus dexamethasone. Patients undergoing transplantation were younger, had a better PS, a lower amyloid stage, and did not have the heart as their dominant site of disease (Table 2).
Table 2.
Patient and Disease Characteristics for Comparison Cohorts Matched on ECOG Performance Status and Risk Category
| Characteristic | Melphalan + Dexamethasone (n=24) |
Stem Cell Transplantation (n=48) |
|---|---|---|
| Age, median (IQR), y | 60 (57-63) | 57 (53-61) |
| Male, No. (%) | 14 (58.3) | 36 (75.0) |
| Eastern Cooperative Oncology Group performance status 0-1, No. (%) |
24 (100) | 48 (100) |
| High-risk group, No. (%) | 18 (75.0) | 36 (75.0) |
| Immunoglobulin light chain amyloidosis stage, No. (%) |
||
| I | 3 (12.5) | 23 (47.9) |
| II | 16 (66.7) | 20 (41.7) |
| III | 5 (20.8) | 5 (10.4) |
| Heart as a disease site, No. (%) | 15 (62.5) | 15 (31.3) |
Abbreviation: IQR, interquartile range.
Outcomes of Melphalan Plus Dexamethasone
Of the 34 patients who were assigned to or chose melphalan plus dexamethasone, 9 received melphalan parenterally and 25 received melphalan orally. None of the 9 receiving parenteral melphalan was switched to oral administration when oral melphalan was offered as an option. The median number of treatment cycles was 8. Seventeen patients completed all 10 treatment cycles; 17 patients completed a maximum of 6 cycles. Nine patients (26.5%) required at least 1 dose reduction because of neutropenia or thrombocytopenia. All reductions occurred after the fourth cycle of treatment. Thirteen patients (38.2%) had stem cells collected during cycles 2 or 3 for banking. The reasons for treatment discontinuation included completion of the planned cycles (n=16), disease progression (n=5), refusal to continue therapy (n=1), persistent thrombocytopenia (n=2), desire to withdraw from the study and change to a nonprotocol therapy (n=3), massive stroke (n=1), diagnosis of esophageal cancer (n=1), and death due to sudden cardiac arrest (n=2), neutropenic sepsis (n=1), or disease progression (n=2).
Nine patients (26.5%; 95% confidence interval [CI], 12.8%−44.0%) had an organ response at 12 months after entry (kidney, n=6; heart, n=3). At 24 months after registration, 6 patients (17.6%; 95% CI, 6.8%−34.5%) had an organ response (kidney, n=4; heart, n=1; or both, n=1). The overall organ response rate was 11/34 (32.4%; 95% CI, 17.2%−50.5%). Confirmed hematologic responses were documented in 19 patients (55.9%; 95% CI, 37.9%−72.8%); this included 3 complete responses, 13 partial responses, and 3 very good partial responses. Two patients (5.9%) died within 60 days of study entry because of cardiac arrest (day 14) or advanced cardiac amyloidosis with end-stage cardiac function (day 39).
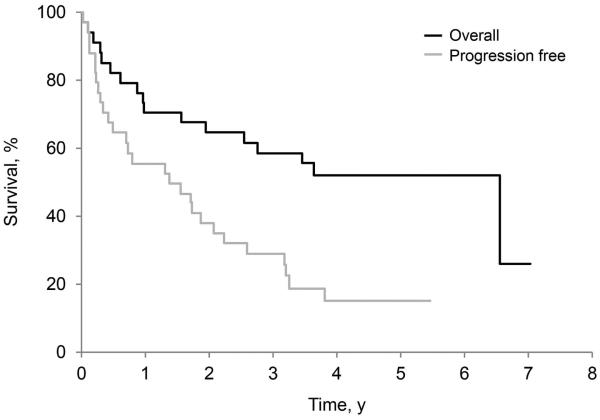
As of October 15, 2014, 17 patients in this study arm were alive: 6 without progression and 11 with progression. Among these patients, the minimum follow-up was 3.2 years (median, 5.1 years). The 3-year progression-free survival rate was 29.1% (95% CI, 17.2%−49.4%). The 3-year overall survival rate was 58.8% (95% CI, 44.4%−77.9%) (Figure 2A).
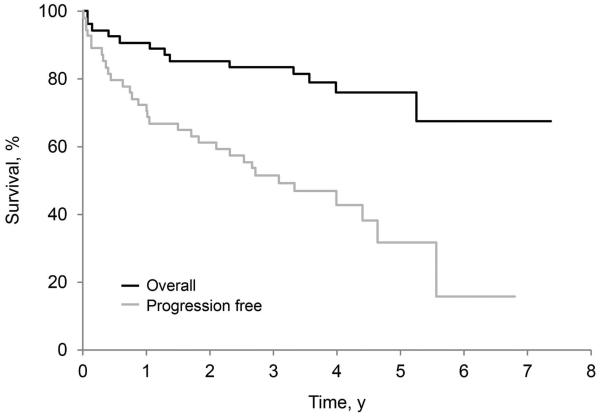
Figure 2.
Overall and Progression-Free Survival. A, Patients received melphalan plus dexamethasone. B, Patients received high-dose melphalan with autologous stem cell transplantation.
Outcomes of SCT
One patient, a 67-year-old man with macroglossia-dominant high-risk disease, did not begin stem cell mobilization because of disease progression. Of the remaining 54 patients who underwent stem cell mobilization, 7 (13.0%) had severe toxicity, including grade 4 neutropenia (n=1) or thrombocytopenia (n=1) and grade 3 sepsis (n=1), atrial fibrillation (n=1), hemorrhage (n=1), thrombocytopenia (n=1), alkaline phosphatase increase (n=1), and serum potassium reduction (n=1). The goal of infusing 4×106 CD34 cells/kg was met by 43 patients (79.6%). The median number of CD34 cells/kg infused was 4.47×106 (range, 2.7-10.1×106 cells/kg). Within 30 days of SCT, 49 patients (90.7%) had hematologic engraftment (neutrophil count, >0.5×109/L; platelet count, >50×109/L). The median time to neutrophil engraftment was 14 days (range, 11-43 days). One patient died 21 days after SCT because of cardiopulmonary arrest, without documentation of platelet engraftment. The median time to platelet engraftment among the other 53 patients was 16 days (range, 10-51 days). Two additional patients died within 60 days of SCT because of pneumonia from respiratory syncytial virus (n=1) and cardiac arrest (n=1). The all-cause SCT mortality rate was 3 of 54 (5.6%).
Sixteen of the 54 patients who began treatment (29.6%; 95% CI, 18.0%−43.6%) had an organ response at 12 months after entry into the study (kidney, n=12; heart, n=2; liver, n=1; heart and kidney both, n=1). Twenty-four months after registration, 18 patients (33.3%) had an organ response (kidney, n=14; heart, n=2; or both heart and kidney, n=2). The overall organ response rate was 19 out of 54 (35.2%; 95% CI, 22.7%−49.4%).
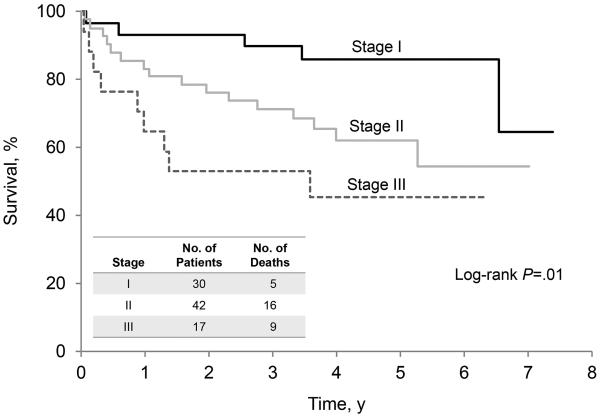
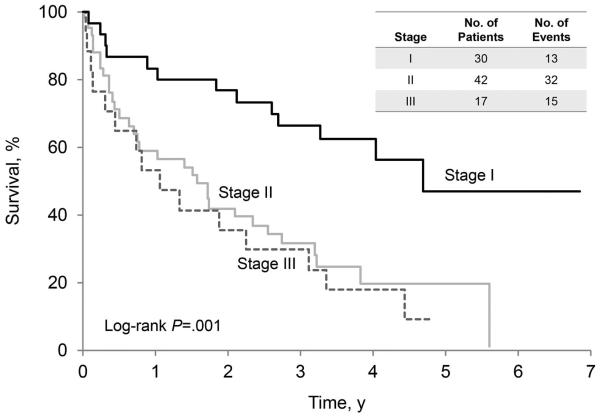
Hematologic responses were confirmed in 38 of the 54 patients who began treatment (70.4%; 95% CI, 56.4%−82.0%), including 20 complete responses, 13 partial responses, and 5 very good partial responses. As of October 15, 2014, 42 patients in this study arm were alive: 23 without progression and 19 patients with disease progression. Among the surviving patients, the minimum follow-up was 2.4 years (median, 4.1 years). The 3-year progression-free survival rate was 51.7%, and the 3-year overall survival rate was 83.6% (95% CI, 74.7%−94.0%). An analysis of progression-free survival (Figure 3A) and overall survival (Figure 3B) was conducted for all 89 patients and illustrates the impact of cardiac stage on outcome. Patients were stratified by Mayo cardiac stage: stage I was defined as N-terminal pro-brain natriuretic peptide <332 ng/L and cardiac troponin T <0.035 mcg/L; stage II was defined as only 1 marker being elevated; stage III was defined as both markers being elevated (20).
Figure 3.
Survival of Patients, Stratified by Mayo Amyloid Stage Before Treatment. A, Overall survival. B, Progression-free survival.
Comparison of the Hematologic Response Rate Between Treatment Arms
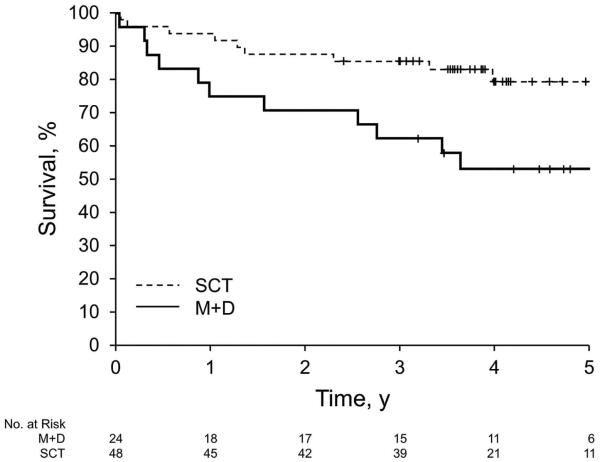
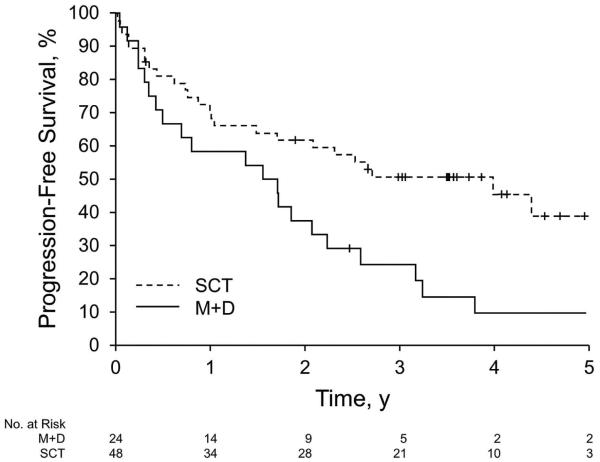
Twenty-four triplet sets, matched on risk category and ECOG PS, were identified for the primary analysis. Patients with ECOG PS 2 were not included in this analysis because we identified only poor matches (or no matches) for these patients (9 in arm A and 4 in arm B). In addition to being balanced in terms of risk category and ECOG PS, the matched cohorts were also well-balanced for age (Table 2). Differences between arms remained with regard to amyloid stage (P<.01) and heart as the dominant disease site (P=.02). The likelihood of hematologic response did not differ with respect to treatment (matched odds ratio, 1.86; 95% CI, 0.72-4.82; P=.20). However, the likelihood of a confirmed complete hematologic response at 12 months was greater among those who chose SCT vs those who chose melphalan plus dexamethasone (matched odds ratio, 4.84; 95% CI, 1.05-22.39; P=.04). A sensitivity analysis using conditional and unconditional logistic regression models on the matched cohorts, after adjusting for amyloid stage and heart as the dominant disease site, showed similar results. The risk of overall mortality (inverse of overall survival) was higher among those patients who chose melphalan plus dexamethasone vs those who chose SCT (hazard ratio, 2.62; 95% CI, 1.14-6.04; P=.02) (Figure 4A). The risk of treatment failure (inverse of progression-free survival) was also higher among those who chose melphalan plus dexamethasone vs those who chose SCT (hazard ratio, 2.21; 95% CI, 1.23-3.95; P<.01) (Figure 4B).
Figure 4.
Survival of Matched Patients, Stratified by Treatment. A, Overall survival. Median (95% CI) survival was 6.53 (1.56-NA) for M+D and NA (5.26-NA) for SCT (P=.02). B, Progression-free survival. Median (95% CI) survival was 1.63 (0.42-2.23) for M+D and 4 (1.49-NA) for SCT (P=.008). M+D indicates melphalan plus dexamethasone; NA, not applicable; SCT, stem cell transplantation.
In another sensitivity analysis, propensity scores were computed from ECOG PS, amyloid stage, number of dominant disease sites, and whether the patient was older than 60 years. We identified 28 matched sets (15 with 2 matches and 13 with 1 match). Propensity score−matched cohorts were well balanced in these characteristics. Results from this analysis confirmed the conclusion from the primary analysis. The matched odds ratio for hematologic response at 12 months was 1.69 (95% CI, 0.55-5.21; P=.36); for a confirmed complete hematologic response, the odds ratio was 6.32 (95% CI, 1.37-29.21; P=.02). The hazard ratio of overall mortality (inverse of overall survival) for those who chose melphalan plus dexamethasone vs those who chose SCT was 2.56 (95% CI, 1.41-4.64; P<.01); the hazard ratio of treatment failure (inverse of progression-free survival) was 1.99 (95% CI, 1.18-3.35; P<.01).
Discussion
Patient enrollment in this study terminated before accrual targets were met; therefore, this trial was underpowered to ascertain definitive differences in outcomes after melphalan plus dexamethasone vs SCT for patients with amyloidosis. The 2 study arms showed 3-year progression-free survival rates of 29.1% and 51.7% for melphalan plus dexamethasone and SCT, respectively, and 3-year overall survival rates were 58.8% vs 83.6%, respectively. However, patients undergoing SCT were younger, had a better PS, had lower-stage amyloidosis, and a lower incidence of cardiac involvement. This suggests significant bias in terms of patients with more advanced disease selecting less-intense therapy. Moreover, the patients’ greater preference for SCT suggests an inherent bias in the referral process. Possibly, patients who strongly favored oral chemotherapy did not come to Mayo Clinic because such therapy could readily be administered at home; in such a scenario, patients referred to our institution by local oncologists may have been biased to favor SCT. Melphalan is not without risks. The current study has too short a follow-up period to reliably report therapy-related myelodysplasia rates; however, we have had 1 patient with myelodysplastic syndrome 17 months after the first exposure to melphalan and dexamethasone. Our transplant database identifies 5 of 662 patients with myelodysplastic syndrome or acute nonlymphoblastic leukemia, and we previously reported 10 of 101 melphalan-treated patients with myelodysplastic syndrome or acute nonlymphoblastic leukemia (21).
In the matched cohort of 24 melphalan-dexamethasone patients and 48 transplantation patients, no response differences were noted, but the CIs were very broad. However, the likelihood of a confirmed complete hematologic response at 12 months was greater among those who chose SCT. Better progression-free and overall survival were observed among those who chose SCT. Sensitivity analysis using propensity-score matching to improve balance in baseline characteristics between treatment arms showed similar conclusions.
SCT is not universally accepted as a first-line therapy for managing amyloidosis, as evidenced by significant geographic differences in its application (13). In Great Britain, only 90 patients underwent transplantation in 117 months (2001-2010). Data published from a European collaborative suggests that SCT is uncommon (22). However, treatment centers around the world apply high-dose therapy and SCT for patients with amyloidosis (23-26). In our study, the differences in progression-free survival rates suggest that high-dose melphalan may produce a more durable response. However, the role of SCT is difficult to interpret because our patients who chose that therapy were younger, had less cardiac amyloidosis, and had a lower stage. Although we believe that these results are compelling, they are not a substitute for a phase III prospective randomized trial using cardiac biomarkers to determine eligibility and risk stratification. A multicenter collaboration is required to fulfill this unmet need.
Conclusion
Acknowledging the limitations of our study, we observed similar hematologic response and improved survival after SCT over melphalan plus dexamethasone. However, because of enrollment closure before meeting our accrual targets and because treatment assignment based on patient choice resulted in an imbalance in key factors (including the extent of cardiac involvement and age), our study was unable to show evidence of superiority of SCT over melphalan plus dexamethasone.
Acknowledgments
Funding: This study was supported by NIH R01 CA111345-04 (M.A.G.).
Abbreviations
- CI
confidence interval
- ECOG
Eastern Cooperative Oncology Group
- PS
performance status
- SCT
stem cell transplantation
Footnotes
Conflict of interest: None.
Author Contributions
Conception and design: Gertz, Lacy, Dispenzieri, Buadi, Leung, Lust, and Russell.
Collection and assembly of data: Suman, Le-Rademacher, Dingli, Hayman, Kumar, Rajkumar, and Hogan.
Data analysis and interpretation: Gertz, Suman, and Le-Rademacher. Manuscript writing: All authors.
Final approval of manuscript: All authors.
References
- 1.Wechalekar AD, Gillmore JD, Hawkins PN. Systemic amyloidosis. Lancet. 2015 Dec;:21. doi: 10.1016/S0140-6736(15)01274-X. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Kastritis E, Dimopoulos MA. Recent advances in the management of AL Amyloidosis. Br J Haematol. 2016 Jan;172(2):170–86. doi: 10.1111/bjh.13805. Epub 2015 Oct 22. [DOI] [PubMed] [Google Scholar]
- 3.Jones NF, Hilton PJ, Tighe JR, Hobbs JR. Treatment of “primary” renal amyloidosis with melphalan. Lancet. 1972 Sep 23;2(7778):616–9. doi: 10.1016/s0140-6736(72)93014-0. [DOI] [PubMed] [Google Scholar]
- 4.Kyle RA, Greipp PR, Garton JP, Gertz MA. Primary systemic amyloidosis: comparison of melphalan/prednisone versus colchicine. Am J Med. 1985 Dec;79(6):708–16. doi: 10.1016/0002-9343(85)90521-2. [DOI] [PubMed] [Google Scholar]
- 5.Skinner M, Anderson J, Simms R, Falk R, Wang M, Libbey C, et al. Treatment of 100 patients with primary amyloidosis: a randomized trial of melphalan, prednisone, and colchicine versus colchicine only. Am J Med. 1996 Mar;100(3):290–8. doi: 10.1016/s0002-9343(97)89487-9. [DOI] [PubMed] [Google Scholar]
- 6.Gertz MA, Lacy MQ, Lust JA, Greipp PR, Witzig TE, Kyle RA. Prospective randomized trial of melphalan and prednisone versus vincristine, carmustine, melphalan, cyclophosphamide, and prednisone in the treatment of primary systemic amyloidosis. J Clin Oncol. 1999 Jan;17(1):262–7. doi: 10.1200/JCO.1999.17.1.262. [DOI] [PubMed] [Google Scholar]
- 7.Palladini G, Milani P, Foli A, Vidus Rosin M, Basset M, Lavatelli F, et al. Melphalan and dexamethasone with or without bortezomib in newly diagnosed AL amyloidosis: a matched case-control study on 174 patients. Leukemia. 2014 Dec;28(12):2311–6. doi: 10.1038/leu.2014.227. Epub 2014 Jul 25. [DOI] [PubMed] [Google Scholar]
- 8.Comenzo RL, Vosburgh E, Simms RW, Bergethon P, Sarnacki D, Finn K, et al. Dose-intensive melphalan with blood stem cell support for the treatment of AL amyloidosis: one-year follow-up in five patients. Blood. 1996 Oct 1;88(7):2801–6. [PubMed] [Google Scholar]
- 9.Sanchorawala V. High dose melphalan and autologous peripheral blood stem cell transplantation in AL amyloidosis. Hematol Oncol Clin North Am. 2014 Dec;28(6):1131–44. doi: 10.1016/j.hoc.2014.08.013. Epub 2014 Sep 30. [DOI] [PubMed] [Google Scholar]
- 10.Merlini G, Comenzo RL, Seldin DC, Wechalekar A, Gertz MA. Immunoglobulin light chain amyloidosis. Expert Rev Hematol. 2014 Feb;7(1):143–56. doi: 10.1586/17474086.2014.858594. Epub 2013 Dec 18. [DOI] [PubMed] [Google Scholar]
- 11.Sanchorawala V, Sun F, Quillen K, Sloan JM, Berk JL, Seldin DC. Long-term outcome of patients with AL amyloidosis treated with high-dose melphalan and stem cell transplantation: 20-year experience. Blood. 2015 Nov 12;126(20):2345–7. doi: 10.1182/blood-2015-08-662726. Epub 2015 Oct 6. [DOI] [PubMed] [Google Scholar]
- 12.Cohen AD, Zhou P, Chou J, Teruya-Feldstein J, Reich L, Hassoun H, et al. Risk-adapted autologous stem cell transplantation with adjuvant dexamethasone +/− thalidomide for systemic light-chain amyloidosis: results of a phase II trial. Br J Haematol. 2007 Oct;139(2):224–33. doi: 10.1111/j.1365-2141.2007.06783.x. [DOI] [PubMed] [Google Scholar]
- 13.Venner CP, Gillmore JD, Sachchithanantham S, Mahmood S, Lane T, Foard D, et al. Stringent patient selection improves outcomes in systemic light-chain amyloidosis after autologous stem cell transplantation in the upfront and relapsed setting. Haematologica. 2014 Dec;99(12):e260–3. doi: 10.3324/haematol.2014.108191. Epub 2014 Sep 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dispenzieri A, Kyle RA, Lacy MQ, Therneau TM, Larson DR, Plevak MF, et al. Superior survival in primary systemic amyloidosis patients undergoing peripheral blood stem cell transplantation: a case-control study. Blood. 2004 May 15;103(10):3960–3. doi: 10.1182/blood-2003-12-4192. Epub 2004 Jan 22. [DOI] [PubMed] [Google Scholar]
- 15.Jaccard A, Moreau P, Leblond V, Leleu X, Benboubker L, Hermine O, et al. Myélome Autogreffe (MAG) and Intergroupe Francophone du Myélome (IFM) Intergroup. High-dose melphalan versus melphalan plus dexamethasone for AL amyloidosis. N Engl J Med. 2007 Sep 13;357(11):1083–93. doi: 10.1056/NEJMoa070484. [DOI] [PubMed] [Google Scholar]
- 16.Mhaskar R, Kumar A, Behera M, Kharfan-Dabaja MA, Djulbegovic B. Role of high-dose chemotherapy and autologous hematopoietic cell transplantation in primary systemic amyloidosis: a systematic review. Biol Blood Marrow Transplant. 2009 Aug;15(8):893–902. doi: 10.1016/j.bbmt.2009.01.022. Epub 2009 Apr 2. [DOI] [PubMed] [Google Scholar]
- 17.Hazenberg BP, Croockewit A, van der Holt B, Zweegman S, Bos GM, Delforge M, et al. Dutch-Belgian Cooperative Trial Group for Hematology Oncology. Extended follow up of high-dose melphalan and autologous stem cell transplantation after vincristine, doxorubicin, dexamethasone induction in amyloid light chain amyloidosis of the prospective phase II HOVON-41 study by the Dutch-Belgian Co-operative Trial Group for Hematology Oncology. Haematologica. 2015 May;100(5):677–82. doi: 10.3324/haematol.2014.119198. Epub 2015 Feb 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenbaum PR. Optimal matching for observational studies. J Am Stat Assoc. 1989 Dec;84(408):1024–32. [Google Scholar]
- 19.Lee EW, Wei LJ, Amato DA, Leurgans S. Cox-type regression analysis for large numbers of small groups of correlated failure time observations. In: Klein JP, Goel PK, editors. Survival analysis: state of the art. Vol. 211. Kluwer Academic Publishers; Dordrect: pp. 237–47. c1992. [Google Scholar]
- 20.Dispenzieri A, Gertz MA, Kyle RA, Lacy MQ, Burritt MF, Therneau TM, et al. Serum cardiac troponins and N-terminal pro-brain natriuretic peptide: a staging system for primary systemic amyloidosis. J Clin Oncol. 2004 Sep 15;22(18):3751–7. doi: 10.1200/JCO.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 21.Gertz MA, Lacy MQ, Lust JA, Greipp PR, Witzig TE, Kyle RA. Long-term risk of myelodysplasia in melphalan-treated patients with immunoglobulin light-chain amyloidosis. Haematologica. 2008 Sep;93(9):1402–6. doi: 10.3324/haematol.12982. Epub 2008 Jul 18. [DOI] [PubMed] [Google Scholar]
- 22.Wechalekar AD, Schonland SO, Kastritis E, Gillmore JD, Dimopoulos MA, Lane T, et al. A European collaborative study of treatment outcomes in 346 patients with cardiac stage III AL amyloidosis. Blood. 2013 Apr 25;121(17):3420–7. doi: 10.1182/blood-2012-12-473066. Epub 2013 Mar 11. [DOI] [PubMed] [Google Scholar]
- 23.Charlinski G, Ziarkiewicz M, Boguradzki P, Wiater E, Torosian T, Dwilewicz-Trojaczek J, et al. High-dose melphalan and autologous hematopoietic stem cell transplantation in primary amyloidosis: single-center results. Transplant Proc. 2014 Oct;46(8):2877–81. doi: 10.1016/j.transproceed.2014.09.053. [DOI] [PubMed] [Google Scholar]
- 24.Hayashi T, Ikeda H, Igarashi T, Maruyama Y, Aoki Y, Nojima M, et al. Autologous stem cell transplantation for AL amyloidosis: adjustment of melphalan dose by factors including BNP. Int J Hematol. 2014 Dec;100(6):554–8. doi: 10.1007/s12185-014-1680-1. Epub 2014 Oct 4. [DOI] [PubMed] [Google Scholar]
- 25.Huang B, Li J, Xu X, Zheng D, Zhou Z, Liu J. Successful treatment of renal light chain (AL) amyloidosis with bortezomib and dexamethasone (VD) Pathol Biol (Paris) 2015 Feb;63(1):17–20. doi: 10.1016/j.patbio.2014.10.001. Epub 2014 Nov 6. [DOI] [PubMed] [Google Scholar]
- 26.Kikukawa Y, Yuki H, Hirata S, Ide K, Nakata H, Miyakawa T, et al. Combined use of bortezomib, cyclophosphamide, and dexamethasone induces favorable hematological and organ responses in Japanese patients with amyloid light-chain amyloidosis: a single-institution retrospective study. Int J Hematol. 2015 Feb;101(2):133–9. doi: 10.1007/s12185-014-1705-9. Epub 2014 Nov 28. [DOI] [PubMed] [Google Scholar]