Abstract
Background
This three arm study was designed to make CRC screening with FOBTs more accessible, understandable and actionable for patients cared for in predominantly rural Federally Qualified Health Centers (FQHCs). Patients in an enhanced version of usual care received an annual CRC recommendation and FOBT kit; those in the education arm additionally received brief literacy and culturally appropriate education and those in the nurse arm received the education by a nurse manager who followed up by telephone. Baseline FOBT rates in this population were 3%. We evaluated if FOBT rates could be sustained over three years.
Methods
A three-arm, quasi-experimental evaluation was conducted among 8 clinics in Louisiana. Screening efforts included: 1) enhanced usual care, 2) literacy-informed education of patients, and 3) education plus nurse support. Overall, 961 average-risk patients, ages 50-85, eligible for routine CRC screenings were recruited. The primary outcome was completing three annual FOBT tests.
Results
Of 961 patients enrolled, 381 (39.6%) participants did not complete a single FOBT, 60.4% completed at least one FOBT of which 318 (33.1%) completed only one, 162 (16.9%) completed two and 100 (10.4%) completed three FOBTs over the three-year period (the primary study outcome). The primary outcome, return of three FOBT kits over the three-year period, was achieved by 4.7% in Enhanced Care, 11.4% in Education and 13.6% in the Nurse arm (p=0.005).
Conclusions
Overall three-year FOBT screening rates were not sustained with any of the three interventions, despite reports of promising interim results at years 1 and 2. New strategies for sustaining FOBT screening over several years must be developed.
Keywords: Health Literacy, Colon Cancer Screening, Annual screening, Federally Qualified Health Centers
Introduction
Colorectal cancer screening (CRC) with Fecal Occult Blood Tests (FOBTs) reduces mortality rates [1-5]. U.S. clinical guidelines recommend annual FOBTs [6, 7] yet sustaining yearly screening is challenging [8-10]. Innovative, evidence-based strategies are needed to promote continued longitudinal adherence to annual FOBT screening particularly among vulnerable populations cared for in resource limited safety-net clinics [9-14]. The National Colorectal Cancer Roundtable recently recommended focusing initiatives on Federally Qualified Health Centers (FQHCs) to address national screening challenges [15]. FQHCs provide primary care to over 22 million individuals regardless of insurance status [16] and have recently added CRC screening as a quality indicator.
Strategies to improve first-time rates of CRC screening included patient-directed interventions (written materials, DVDs, mailed FOBTs and reminders, telephone counseling), and physician-directed interventions (chart stickers, electronic reminders, academic detailing) [9-22]. Few studies promoting multi-year screening (focusing on FOBTs in particular) have been conducted in the US; most of these were with insured patients in large health systems [23]. The most effective strategies involve giving educational materials and FOBT kits during a clinic visit the first year with telephone follow up if needed and mailing the kits the second year, yet less than one in four patients were adherent with two rounds of screening [23].
Population based screening programs abroad where FOBTs are mailed by centralized health service to eligible individuals have higher repeat FOBT screening rates (39% to 55%) [24-27]. Interestingly, in these programs which eliminate geographic and cost barriers completion rates were lower in individuals from rural areas and those of lower socioeconomic status [28]. These programs also found barriers to completing one FOBT appeared to differ from those in sustaining FOBT screening over multiple years [24].
Our team designed, implemented and evaluated a multifaceted health literacy informed intervention to promote FOBT completion annually over three years in a vulnerable population served by predominately rural FQHCs in Louisiana. Our three arm study was designed to make annual CRC screening with FOBTs more accessible, understandable and actionable. Patients in an enhanced version of usual care received an annual recommendation and FOBT kit; those in the education arm additionally received brief literacy and culturally appropriate education, and simplified FOBT instructions; those in the nurse arm received the education materials by a nurse manager who followed up by telephone. The interim results, measured at the end of years 1 and 2, were promising, as reported previously [29, 30]. Baseline FOBT rates in this population were 3%. After year 1 of the intervention, initial FOBT rates improved to 39% with enhanced care, 57% with educational support, and 61% with nurse support. After year 2, repeat FOBT rates were 38% with enhanced care, 33% with educational support, and 59% with nurse support. We now report on the overall results of all 961 patients who were enrolled in this three-arm intervention- FOBT completion study for three years.
Methods
Theoretical Framework
The Health Belief Model and Social Cognitive theory guided the framing of the intervention to highlight the benefit of FOBT screening and the need to take action annually [31-33]. Health literacy best practices were employed to help ensure the information was easy to understand and act on [34,35].
Study Design
A three-arm intervention was conducted among three FQHC networks in predominately rural areas of Louisiana between May 2008 and August 2011. Of the five FQHC networks in the area, three participated and were assigned to one of three arms by simple randomization (the other two network FQHCs were involved in cancer screening programs at the time). Each participating clinic was assigned to the same study arm as their parent FQHC network. This resulted in two clinics in enhanced care, two in the educational intervention, and three in nurse support arm. After the first study year, one additional clinic was enrolled in the enhanced care arm due to limited patient recruitment in this arm during the first year. The eight clinics were located in eight towns. Baseline CRC screening rates at each clinic ranged from 1% to 3%.
Patients aged 76-85 were included per the request of clinic directors. Further eligibility included: 1) English-speaking, 2) not requiring screening at an earlier age according to American Cancer Society guidelines [6], 3) not up-to-date with United States Preventive Services Task Force [7] CRC screening recommendations (i.e., an FOBT annually, flexible sigmoidoscopy every 5 years or colonoscopy every 10 years), 4) medical staff believing patients too ill to be interviewed. All participants were consented prior to data collection.
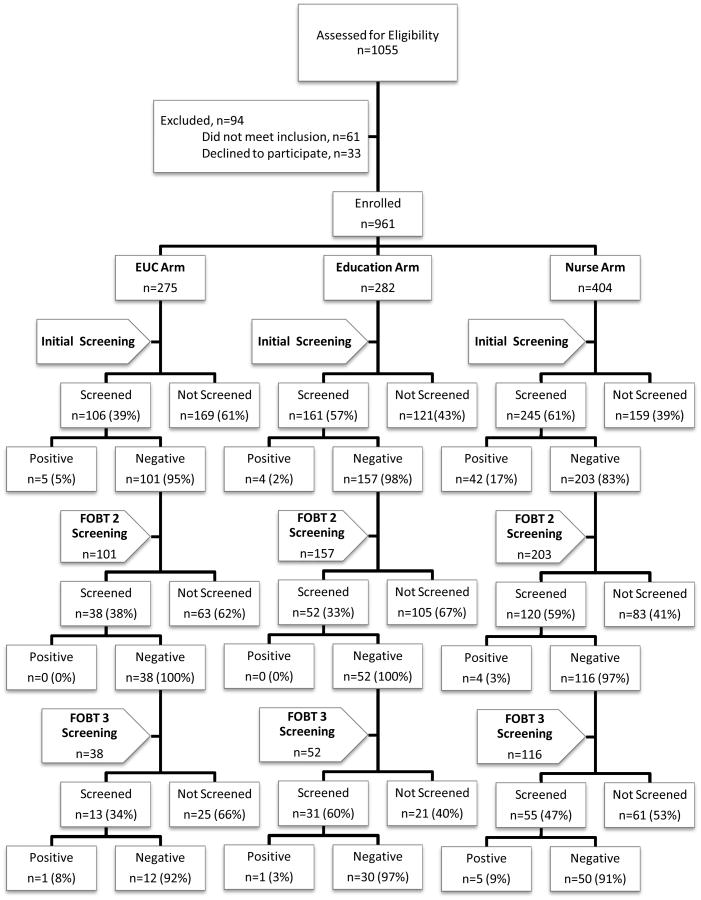
At enrollment (fig. 1), 1,055 patients were identified as meeting age criteria, of these 33 (3.1%) refused to participate and 61 (5.8%) were ineligible because they were up to date on CRC screening. A total of 961 patients were consented and enrolled, with a determined cooperation rate of 91.1%. A total of 512 patients completed a FOBT within three months, 51 had a positive test and received a provider referral for a colonoscopy and were therefore not eligible for a 2nd annual FOBT. In year 2, 461 patients were eligible for a repeat annual FOBT; of these, 210 (46%) completed screening and four had a positive test. In year 3, 206 patients were eligible for a 3rd annual screening test; of these, 99 (48%) completed screening and seven had a positive test.
Figure 1. Flowchart of Initial and Repeat Screening (those who completed initial screening).
The Louisiana State University Health Sciences Center – Shreveport Institutional Review Board approved the study. Each participant received $10 for their participation in the baseline survey.
Study Instruments
A structured survey, including patient demographics, CRC screening items from validated questionnaires used previously by the authors, and a literacy assessment were administered at enrollment. A detailed description of the survey, which was administered orally, has been reported previously [36]. Literacy was assessed using the Rapid Estimate of Adult Literacy in Medicine (REALM) [37].
Interventions
Enhanced Care
An enhanced version of usual care where patients waiting for a scheduled appointment with their provider received a recommendation for CRC screening by a clinic based research assistant (RA) and an FOBT kit with a stamped envelope addressed to the clinic. In year 2 and 3, twelve months after a patient's previous FOBT was returned or twelve months after the enrollment date if the FOBT hadn't been completed, a letter was sent by a central RA as a reminder that it was time for their annual FOBT and to inform them that a kit would be mailed the following week. The central RA mailed the FOBT with a stamped envelope addressed to the clinic. Patients returned FOBTs to the clinic by mail. Regular clinic protocol was followed for positive test results and referral for diagnostic testing.
Literacy-Informed Education
Patients in this arm additionally received brief education by a clinic based RA using literacy and culturally appropriate material (pamphlet, simplified FOBT instructions and video). The RA demonstrated how to perform the test and used ‘teach back’ to confirm patient understanding [34]. In years two and three, a central RA mailed a reminder letter and FOBT kit that also included simplified FOBT instructions and a pamphlet. Tracking and follow-up were the same as in the enhanced care arm.
Nurse Support
In this arm a designated clinic nurse provided the education and FOBT kit. If the patient did not mail the FOBT to the clinic within two weeks the nurse followed up by telephone within two weeks and again in one month to problem-solve barriers and motivate them to complete the test [28]. At the same specified dates for follow-up used in the other arms, the nurse mailed patients the simplified materials. If patients did not return their FOBT, the nurse followed up by telephone. Patients returned FOBTs to the clinic by mail. The nurse recorded and tracked results. If results were positive, the nurse called patients to discuss results, facilitate appointments with their primary care provider, and if indicated, schedule patients for a diagnostic colonoscopy.
Outcomes for the Intervention
The primary study outcome was completion of three FOBTs annually within the time frame of the study or having a positive FOBT that was followed up with colonoscopy. Secondary outcomes included completion of 0, 1, or 2 FOBTs during the three-year intervention period. Screening results were documented by the clinic nurse (enhanced care and education arms) or study nurse (nurse support arm).
Analyses
The denominator for analyses is the number of patients in each arm, eligible for the first year study (Enhanced Care n = 275, Education n = 282, Nurse n = 404). To examine whether patients in study arms differed on baseline characteristics, generalized estimating equations (GEE) accounting for clustering by clinic was used. Both global and pairwise tests for FOBT completion (0, 1, 2, 3 FOBTs) were calculated using GEE. Multivariate analyses adjusted for age, race, gender, and literacy level.
Results
Baseline characteristics are compared among groups in Table 1. Participants ranged in age from 50-89; 77% were female. The majority (67%) were African American; over half (56%) had low literacy (i.e. read ≤ 9th grade level). Almost all wanted to know if they had cancer (90%) and believed that an FOBT would find CRC early (96%). Although no patient was up-to-date with screening, 28% reported completing a FOBT sometime in the past. There were significant differences across groups for age, race/ethnicity, marital status, literacy, prior recommendation, previous FOBT, wanting to know if they had CRC and positive beliefs concerning FOBT efficacy.
Table 1. Characteristics of Study Sample at Baseline, Stratified by Study Arm.
| Characteristic | All Patients (n=961) | Study Arm | p-value | ||
|---|---|---|---|---|---|
|
| |||||
| Enhanced Usual Care (n=275) | Education (n=282) | Nurse (n=404) | |||
| Age, Mean (sd) | 58.4 (7.3) | 57.7 (7.5) | 57.8 (6.5) | 59.2 (7.5) | 0.014 |
| N (%) | N (%) | N (%) | N (%) | ||
| Age Categories | |||||
| 50-59 | 611 (64) | 190 (69) | 181 (64) | 240 (59) | 0.11 |
| 60-69 | 265 (28) | 63 (23) | 80 (28) | 122 (30) | |
| 70-85 | 85 (9) | 22 (8) | 21 (7) | 42 (10) | |
| Female | 740 (77) | 207 (75) | 224 (79) | 309 (77) | 0.50 |
| Years of Education | |||||
| Less than high school | 313 (33) | 98 (36) | 92 (33) | 123 (31) | 0.26 |
| High school grad | 435 (45) | 109 (40) | 139 (49) | 187 (47) | |
| Some College | 157 (16) | 50 (18) | 40 (14) | 67 (17) | |
| ≥ College Graduate | 53 (6) | 18 (7) | 11 (4) | 24 (6) | |
| Race | |||||
| African-American | 645 (67) | 199 (72) | 114 (40) | 332 (83) | <0.0001 |
| Caucasian/Hispanic | 313 (33) | 76 (28) | 168 (60) | 69 (17) | |
| Marital Status | |||||
| Single | 276 (29) | 62 (23) | 55 (20) | 159 (40) | <0.0001 |
| Married | 330 (34) | 101 (37) | 142 (50) | 87 (22) | |
| Separated | 66 (7) | 22 (8) | 14 (5) | 30 (7) | |
| Divorced | 155 (16) | 47 (17) | 38 (13) | 70 (17) | |
| Widowed | 131 (14) | 43 (16) | 33 (12) | 55 (14) | |
| Literacy Level | |||||
| Limited (0-60) | 537 (56) | 188 (68) | 98 (35) | 251 (62) | <0.0001 |
| Adequate (61-66) | 424 (44) | 87 (32) | 184 (65) | 153 (38) | |
| Seen Doctor in Past 12 months | 849 (89) | 236 (86) | 258 (91) | 355 (89) | 0.09 |
| Prior Recommendation | 357 (39) | 96 (35) | 83 (29) | 178 (48) | <0.0001 |
| Ever Completed an FOBT | 262 (28) | 62 (23) | 26 (9) | 174 (47) | <0.0001 |
| Would want to know if have CRC? | |||||
| Yes | 837 (90) | 242 (90) | 261 (93) | 334 (89) | 0.03 |
| No | 56 (6) | 12 (4) | 15 (5) | 29 (8) | |
| Don't know | 34 (4) | 17 (6) | 6 (2) | 11 (3) | |
| FOBT finds CRC early | |||||
| Strongly Agree/Agree | 889 (96) | 255 (94) | 276 (98) | 358 (96) | 0.24 |
| Disagree/Strongly Disagree | 9 (1) | 3 (1) | 2 (1) | 4 (1) | |
| Don't know | 29 (3) | 13 (5) | 4 (1) | 12 (3) | |
| FOBT decreases chances of dying from CRC | |||||
| Strongly Agree/Agree | 742 (80) | 194 (72) | 243 (86) | 305 (82) | 0.0001 |
| Disagree/Strongly Disagree | 109 (12) | 43 (16) | 29 (10) | 37 (10) | |
| Don't know | 76 (8) | 34 (13) | 10 (4) | 32 (9) | |
| Barrier Index, Mean (sd) | 8.98 (2.29) | 9.42 (2.24) | 8.04 (2.11) | 9.38 (2.26) | <0.0001 |
Overall 62 (6.5%) of patients had a positive FOBT result. Most of these (82.3%) were found on the first FOBT, 6.5% were found on the second test, and 11.3% on the 3rd. Of the 51 patients that had a diagnostic colonoscopy, 4 had polyps, and 2 were diagnosed with colon cancer.
Discussion
While the interim results for this three-arm intervention focusing on improving FOBT rates among medically underserved persons in Louisiana who receive care at FQHCs were promising with FOBT rates that increased from a baseline of 5% to over two years as high as 59% (in the nurse support arm), the overall results at the end of three years are disappointing. After removing participants who had positive FOBT tests such that they became ineligible for the subsequent FOBTs from the total sample, FOBT completion rates at the end of the three years were 13% for the nurse intervention, 11% for the educational intervention, and 4% for the enhanced usual care arms.
The low rate of patients' completing FOBTs annually for three years demonstrates the challenge of sustaining annual FOBT completion among vulnerable populations. The pattern of FOBT compliance suggests that more intensive strategies, particularly after two years of FOBT screening, are needed to promote sustained annual FOBT screening or whether support for every ten-year colonoscopy needs to be considered. Whether the FOBT completion rate at fourth year would continue for the small numbers of persons who completed three FOBTs with or without continued interventions past the 3-year duration of the study is also unknown.
Limitations include differences between arms in sociodemographic characteristics (adjustments were made in the statistical analyses), and generalizability from a predominantly African American and female population receiving care from FQHCs in one state. However, this is representative of FQHC populations in the southern United States. In addition, half of the sample had low literacy, which is more common in older, lower-income populations.
Conclusion
This study's findings illustrate the challenge in sustaining annual CRC screening over a three-year period by use of FOBTs. For FOBT screening to be effective it must be done annually – and hence strategies for improving repeat screening must be developed in resource challenged settings or strategies that support colonoscopy for persons in these settings should be developed. Compliance to repeat FOBT screening over time was improved three-fold by either education or nurse support, but overall rates were disappointing low (< 14%) in each of the three study arms. Helping disadvantaged populations with limited literacy will likely require more personal outreach and ongoing support if sustaining annual FOBT screening is to occur or strategies that allow for colonoscopy as a screening test should be developed.
Table 2. Return of no, 1, 2 or 3 FOBTs over a three-year period.
| Study Arm | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| All Patients (n=961) | Enhanced care (n=275) | Education (n=282) | Nurse (n=404) | p-value | ||
| N (%) | N (%) | N (%) | N (%) | |||
| Return of no FOBTs | 381 (40) | 157 (57.1) | 107 (37.9) | 117 (29.0) | 0.006 | |
| Screening Ratio | 1.00 | 0.83 | 0.58 | |||
| 95% Confidence Interval | (0.57 – 1.20) | (0.41 – 0.81) | ||||
| p-value | 0.33 | 0.001 | ||||
| Screening Ratio | 1.00 | 0.70 | ||||
| 95% Confidence Interval | (0.47 – 1.03) | |||||
| p-value | 0.07 | |||||
| Return of 1 FOBT | 318 (33) | 74 (26.9) | 112 (39.7) | 132 (32.7) | 0.25 | |
| Screening Ratio | 1.00 | 1.37 | 1.25 | |||
| 95% Confidence Interval | (0.94 – 1.99) | (0.95 – 1.65) | ||||
| p-value | 0.10 | 0.11 | ||||
| Screening Ratio | 1.00 | 0.92 | ||||
| 95% Confidence Interval | (0.75 – 1.11) | |||||
| p-value | 0.37 | |||||
| Return of 2 FOBTs | 162 (17) | 31 (11.3) | 31 (11.0) | 100 (24.8) | <0.0001 | |
| Screening Ratio | 1.00 | 0.96 | 2.10 | |||
| 95% Confidence Interval | (0.55 – 1.70) | (1.20 – 3.69) | ||||
| p-value | 0.89 | 0.01 | ||||
| Screening Ratio | 1.00 | 2.18 | ||||
| 95% Confidence Interval | (1.65 – 2.89) | |||||
| p-value | <0.0001 | |||||
| Return of 3 FOBTs | 100 (10) | 13 (4.7) | 32 (11.4) | 55 (13.6) | 0.005 | |
| Screening Ratio | 1.00 | 2.39 | 2.65 | |||
| 95% Confidence Interval | (1.21 – 4.72) | (1.47 – 4.77) | ||||
| p-value | 0.01 | 0.001 | ||||
| Screening Ratio | 1.00 | 1.11 | ||||
| 95% Confidence Interval | (0.76 – 1.62) | |||||
| p-value | 0.59 | |||||
Screening ratios and p-values control for age (in years), race (African American vs Caucasian and Hispanic), gender and literacy (limited vs adequate).
Footnotes
Funded by the National Cancer Institute (R01 CA115869) and supported in part by 1 U54 GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health which funds the Louisiana Clinical and Translational Science Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
No conflicts are noted by the authors related to the work described.
References
- 1.Zauber AG, Lansdorp-Vogelaar I, Knudsen AB, Wilschut J, van Ballegooijen M, Kuntz KM. Evaluating test strategies for colorectal cancer screening: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149(9):659–669. doi: 10.7326/0003-4819-149-9-200811040-00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Towler BP, Irwig L, Glasziou P, Weller D, Kewenter J. Screening for colorectal cancer using the faecal occult blood test, hemoccult. Cochrane Database Syst Rev. 2000;(2):Cd001216. doi: 10.1002/14651858.CD001216. [DOI] [PubMed] [Google Scholar]
- 3.Hewitson P, Glasziou P, Watson E, Towler B, Irwig L. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): an update. Am J Gastroenterol. 2008;103(6):1541–1549. doi: 10.1111/j.1572-0241.2008.01875.x. [DOI] [PubMed] [Google Scholar]
- 4.Mandel JS, Church TR, Bond JH, et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med. 2000;343(22):1603–1607. doi: 10.1056/NEJM200011303432203. [DOI] [PubMed] [Google Scholar]
- 5.Luo D, Cambon AC, Wu D. Evaluating the long-term effect of FOBT in colorectal cancer screening. Cancer Epidemiol. 2012;36(1):e54–60. doi: 10.1016/j.canep.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 6.American Cancer Society. Cancer Facts & Figures. 2011 Retrieved from: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-029771.pdf.
- 7.Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149(9):627–637. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 8.Niv Y, Lev-El M, Fraser G, Abuksis G, Tamir A. Protective effect of faecal occult blood test screening for colorectal cancer: worse prognosis for screening refusers. Gut. 2002;50(1):33–37. doi: 10.1136/gut.50.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker DW, Brown T, Buchanan DR, et al. Design of a randomized controlled trial to assess the comparative effectiveness of a multifaceted intervention to improve adherence to colorectal cancer screening among patients cared for in a community health center. BMC Health Serv Res. 2013;13:153. doi: 10.1186/1472-6963-13-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baker DW, Brown T, Buchanan DR, et al. Comparative effectiveness of a multifaceted intervention to improve adherence to annual colorectal cancer screening in community health centers: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1235–1241. doi: 10.1001/jamainternmed.2014.2352. [DOI] [PubMed] [Google Scholar]
- 11.Baker DW, Brown T, Goldman SN, et al. Two-year follow-up of the effectiveness of a multifaceted intervention to improve adherence to annual colorectal cancer screening in community health centers. Cancer Causes Control. 2015;26(11):1685–1690. doi: 10.1007/s10552-015-0650-0. [DOI] [PubMed] [Google Scholar]
- 12.Fenton JJ, Elmore JG, Buist DS, Reid RJ, Tancredi DJ, Baldwin LM. Longitudinal adherence with fecal occult blood test screening in community practice. Ann Fam Med. 2010;8(5):397–401. doi: 10.1370/afm.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooper GS, Doug Kou T. Underuse of colorectal cancer screening in a cohort of Medicare beneficiaries. Cancer. 2008;112(2):293–299. doi: 10.1002/cncr.23176. [DOI] [PubMed] [Google Scholar]
- 14.Gellad ZF, Stechuchak KM, Fisher DA, Olsen MK, McDuffie JR, Ostbye T, Yancy WS., Jr Longitudinal adherence to fecal occult blood testing impacts colorectal cancer screening quality. Am J Gastroenterol. 2011;106(6):1125–1134. doi: 10.1038/ajg.2011.11. [DOI] [PubMed] [Google Scholar]
- 15.Walsh JM, Salazar R, Nguyen TT, et al. Healthy colon, healthy life: a novel colorectal cancer screening intervention. Am J Prev Med. 2010;39(1):1–14. doi: 10.1016/j.amepre.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roetzheim RG, Christman LK, Jacobsen PB, et al. A randomized controlled trial to increase cancer screening among attendees of community health centers. Ann Fam Med. 2004;2(4):294–300. doi: 10.1370/afm.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Potter MB, Walsh JM, Yu TM, Gildengorin G, Green LW, McPhee SJ. The effectiveness of the FLU-FOBT program in primary care a randomized trial. Am J Prev Med. 2011;41(1):9–16. doi: 10.1016/j.amepre.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Sarfaty M, Doroshenk M, Hotz J, et al. Strategies for expanding colorectal cancer screening at community health centers. CA Cancer J Clin. 2013;63(4):221–231. doi: 10.3322/caac.21191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levy BT, Daly JM, Xu Y, Ely JW. Mailed fecal immunochemical tests plus educational materials to improve colon cancer screening rates in Iowa Research Network (IRENE) practices. J Am Board Fam Med. 2012;25(1):73–82. doi: 10.3122/jabfm.2012.01.110055. [DOI] [PubMed] [Google Scholar]
- 20.Tu SP, Taylor V, Yasui Y, et al. Promoting culturally appropriate colorectal cancer screening through a health educator: a randomized controlled trial. Cancer. 2006;107(5):959–966. doi: 10.1002/cncr.22091. [DOI] [PubMed] [Google Scholar]
- 21.Sequist TD, Zaslavsky AM, Marshall R, Fletcher RH, Ayanian JZ. Patient and physician reminders to promote colorectal cancer screening: a randomized controlled trial. Arch Intern Med. 2009;169(4):364–371. doi: 10.1001/archinternmed.2008.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dietrich AJ, Tobin JN, Cassells A, et al. Telephone care management to improve cancer screening among low-income women: a randomized, controlled trial. Ann Intern Med. 2006;144(8):563–571. doi: 10.7326/0003-4819-144-8-200604180-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Myers RE, Balshem AM, Wolf TA, Ross EA, Millner L. Adherence to continuous screening for colorectal neoplasia. Med Care. 1993;31(6):508–519. doi: 10.1097/00005650-199306000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Duncan A, Turnbull D, Wilson C, Osborne JM, Cole SR, Flight I, Young GP. Behavioural and demographic predictors of adherence to three consecutive faecal occult blood test screening opportunities: a population study. BMC Public Health. 2014;14:238. doi: 10.1186/1471-2458-14-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steele RJ, McClements PL, Libby G, Carey FA, Fraser CG. Patterns of uptake in a biennial faecal occult blood test screening programme for colorectal cancer. Colorectal Dis. 2014;16(1):28–32. doi: 10.1111/codi.12393. [DOI] [PubMed] [Google Scholar]
- 26.Janda M, Hughes KL, Auster JF, Leggett BA, Newman BM. Repeat participation in colorectal cancer screening utilizing fecal occult blood testing: a community-based project in a rural setting. J Gastroenterol Hepatol. 2010;25(10):1661–1667. doi: 10.1111/j.1440-1746.2010.06405.x. [DOI] [PubMed] [Google Scholar]
- 27.Cole SR, Gregory T, Whibley A, et al. Predictors of re-participation in faecal occult blood test- based screening for colorectal cancer. Asian Pac J Cancer Prev. 2012;13(12):5989–5994. doi: 10.7314/apjcp.2012.13.12.5989. [DOI] [PubMed] [Google Scholar]
- 28.Hecht J, Borrelli B, Breger RK, Defrancesco C, Ernst D, Resnicow K. Motivational interviewing in community-based research: experiences from the field. Ann Behav Med. 2005;29(Suppl):29–34. doi: 10.1207/s15324796abm2902s_6. [DOI] [PubMed] [Google Scholar]
- 29.Davis T, Arnold C, Rademaker A, et al. Improving colon cancer screening in community clinics. Cancer. 2013;119(21):3879–3886. doi: 10.1002/cncr.28272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis TC, Arnold CL, Bennett CL, Wolf MS, Reynolds C, Liu D, Rademaker A. Strategies to improve repeat fecal occult blood testing cancer screening. Cancer Epidemiol Biomarkers Prev. 2014;23(1):134–143. doi: 10.1158/1055-9965.EPI-13-0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bandura A. Health promotion by social cognitive means. Health Educ Behav. 2004;31(2):143–164. doi: 10.1177/1090198104263660. [DOI] [PubMed] [Google Scholar]
- 32.Rosenstock IM, Strecher VJ, Becker MH. Social learning theory and the Health Belief Model. Health Educ Q. 1988;15(2):175–183. doi: 10.1177/109019818801500203. [DOI] [PubMed] [Google Scholar]
- 33.Glanz K, Rimer BK, Viswanath K. Health behavior and health education : theory, research, and practice. 4th. San Francisco, CA: Jossey-Bass; 2008. [Google Scholar]
- 34.Weiss B, Schwartzberg J, Davis T, Parker R, Sokol P, Williams M. Health literacy and patient safety: help patients understand: manual for clinicians 2007 [Google Scholar]
- 35.U.S. Department of Health and Human Services, & Office of Disease Prevention and Health Promotion. National Action Plan to Improve Health Literacy. Washington, D.C.: 2010. Retrieved from. http://health.gov/communication/hlactionplan/pdf/Health_Literacy_Action_Plan.pdf. [Google Scholar]
- 36.Davis TC, Arnold CL, Rademaker AW, et al. FOBT completion in FQHCs: impact of physician recommendation, FOBT information, or receipt of the FOBT kit. J Rural Health. 2012;28(3):306–311. doi: 10.1111/j.1748-0361.2011.00402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davis TC, Long SW, Jackson RH, et al. Rapid estimate of adult literacy in medicine: a shortened screening instrument. Fam Med. 1993;25(6):391–395. [PubMed] [Google Scholar]