Abstract
Objectives
Axon tracers provide crucial insight into the development, connectivity, and function of neural pathways. A tracer can be characterized as a substance that allows for the visualization of a neuronal pathway. Axon tracers have previously been used exclusively with in vivo studies; however, newer methods of axon tracing can be applied to ex vivo studies. Ex vivo studies involve the examination of cells or tissues retrieved from an organism. These post mortem methods of axon tracing offer several advantages, such as reaching inaccessible tissues and avoiding survival surgeries.
Methods
In order to evaluate the quality of the ex vivo tracing methods, we performed a systematic review of various experimental and comparison studies to discern the optimal method of axon tracing.
Results
The most prominent methods for ex vivo tracing involve enzymatic techniques or various dyes. It appears that there are a variety of techniques and conditions that tend to give better fluorescent character, clarity, and distance traveled in the neuronal pathway. We found direct comparison studies that looked at variables such as the type of tracer, time required, effect of temperature, and presence of calcium, however, there are other variables that have not been compared directly.
Discussion
We conclude there are a variety of promising tracing methods available depending on the experimental goals of the researcher, however, more direct comparison studies are needed to affirm the optimal method.
Keywords: Carbocyanine, Axon tracing, Axon labeling, DiI, NeuroVue, Ex vivo, Postmortem, Fixed tissue
Introduction
Axon tracing methods are invaluable for studying the anatomy of neuronal pathways in normal and pathological states, but the most commonly used method, in vivo application of a tracer, requires survival surgery which causes a risk of research animal morbidity or discomfort.1,2,3 Chen (2006) suggests that the risk for animal loss associated with an additional surgery, and thereby valuable data, is 10–20 percent.4 Other drawbacks include the fact that the target pathway is often difficult to reach or inaccessible.2 Fortunately, within the last few decades, substantial advancements have been established regarding techniques to study neuronal pathways ex vivo by removing organs, or parts of organs, and subsequently applying a tracer. Ex vivo studies have many characteristics that make them advantageous in studying neuroanatomy. For instance, ex vivo application is beneficial because it eliminates the need to perform a survival surgical procedure for tracer application. Additionally, it is possible to trace tissues that would otherwise be inaccessible in vivo.
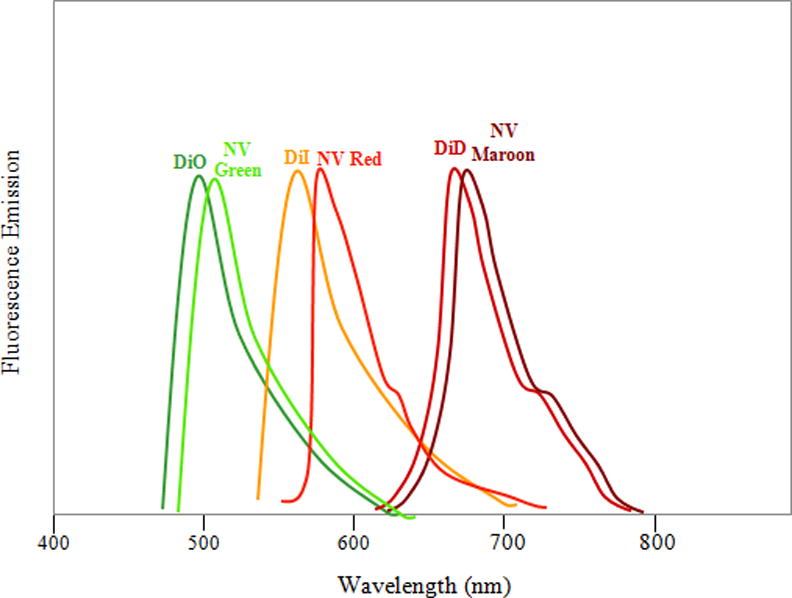
The most commonly used methods for axon tracing ex vivo use various carbocyanines dyes. These dyes are capable of being use in ex vivo studies due to their lipophilic nature. Upon application to fixed tissues, the dyes possess the ability to diffuse through the fixed plasma membranes of neurons.4 Axon tracers exhibit the potential to trace neuronal pathways anterograde (away from the cell body), retrograde (towards the cell body), or bidirectionally.4 Illustrations demonstrating the directionality of axon tracers are shown in figure 1 and 2. Once in the plasma membrane, carbocyanines have been shown to move bidirectionally and even to adjacent cells through the aqueous medium.2,3,4,5 Carbocyanine dyes are fluorescent, making visualizing morphology and connectivity along a desired axonal pathway feasible.4 Figure 3 illustrates the various colors emitted at the corresponding wavelength by different carbocyanines. Due to the different colors emitted, multiple carbocyanines can be used for two-color or three-color tracing. The most frequently used carbocyanines are 1,1′-dioctadecyl-3,3,3′3′-tetramethylindocarbocyanine perchlorate (DiI), 4-4-dihexadecylaminostyryl (DiA), and 3,3 9-dioctadecyloxacarbocyanine perchlorate (DiO).2,4 Other viable methods for axon tracing include other lipophilic dyes, dextrans, and multiple pigments, including, Horseradish Peroxidase (HRP), Lucifer Yellow, Mini Ruby, and NeuroVue Dyes.1,3,6,7,8,9 We sought to compare the available methods and refinements for axon tracing in order to determine which histological method is the most effective for ex vivo axon tracing.
Figure 1.
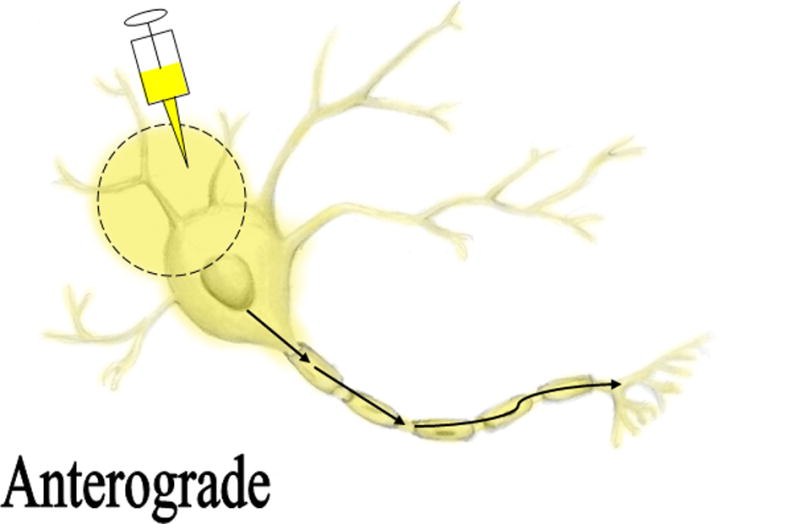
Figure 2.
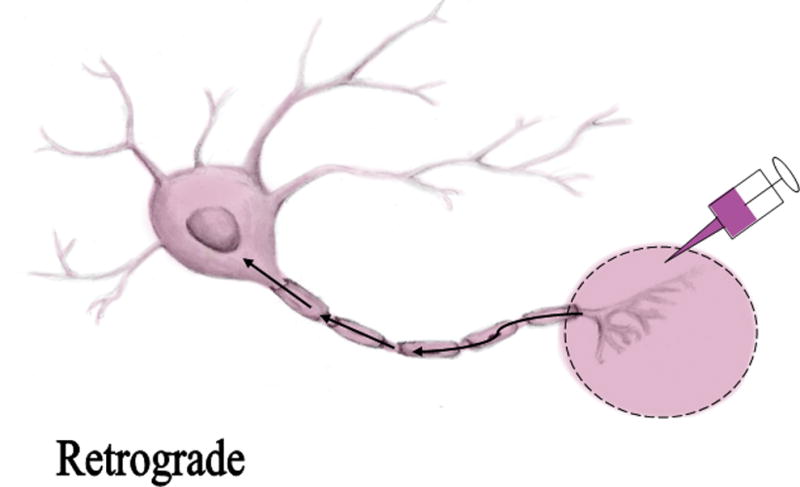
Figure 3.
Methods
We searched Pubmed in December 2015 using the search terms: (fixed OR postmortem OR ex vivo) AND ((neur* OR axon*) AND trac*). We included full-text articles in English published prior to December 2015 that contained unique experimental data for ex vivo placement of an axon tracer. We also reviewed the references of our selected articles for additional studies that matched our selection criteria.
Results
Our search resulted in 275 articles, of which 14 matched our inclusion criteria. We found twelve other matching studies by reviewing references in our selected articles, making a total of 26 articles. These studies are summarized in Table 1. Table 2 contains the major conclusions we drew based on the studies that incorporated comparative data. Although all the studies indicated the usage of a tracer, there was variability among likely key variables of the method. Aspects of the methods that may be of importance for precise and clear tracing include the species evaluated, location of tracer insertion, fixation method, incubation solution, temperature, duration, tissue sectioning method, histology method, visualization method, and method for quantification.
Table 1.1. ex vivo Axon Tracing Studies.
This table summarizes the studies obtained from our search. Aspects such as tracer used, tissue used, fixative, location, temperature, incubation solution, incubation duration, sectioning method, distance, and microscope used were all assessed from the studies in order to assist in comparing them.
| Author/year | Tracer | Animal | Fixative | Location of Tracer/Tools |
Incubation Temperature |
Incubation Solution |
Incubation Duration |
Sectioning Method |
Length Traced |
Visualization |
|---|---|---|---|---|---|---|---|---|---|---|
| Auclair et al. 1993 | DiI and DiA Crystals | 13/14 day albino rat embryos | 4% PFA | 5–10 Crystals placed at C3 level of spinal cord to study brain stem. Method of insertion not specified. | 37°C | 4% PFA | 2–4 months | Cryostat | Unclear | Epifluorescence Microscope |
| Baier et al. 1996 | DiI and DiO | Zebrafish larvae | 4% PFA | DiI and DiO injected using an apparatus to visualize retinotectal projection. DiI injected into temporal-ventral region and DiO injected into nasal-dorsal region | Room temperature | Unclea | Overnight | Unclear | Unclear | Fluorescence Microscope |
| Buhl 1989 | Lucifer Yellow | Cats | 4% PFA | Visual cortex | 4°C | Unclear | Unclear | Vibratome | Unclear | Electron Microscope |
| Ceranik et al. 1999 | DiI | Pre-natal and post-natal rats | 4% PFA | Crystals placed on entorhinal cortex | Room temperature | 4% PFA | 3–6 weeks | Coronal sections 80–100 μm with vibratome | Unclear | Epifluorescence microscope/Confocal Laser Scanning Microscope |
| Chen et al. 2006 | DiI and DiO | Adult Female Sprague Dawley Rats | 4% PFA | Developed own method for insertion using 2mm thick and 4 mm diameter disc with 10 pins in it and placed these discs on either side of the spinal cord section and coated with wax to prevent leakage of dye | 37°C and Room Temperature | 1% and 4% PFA | 2–24 weeks | Longitudinal vibratome sections | Distance varied depending on conditions. 37°C traveled up to 8.89±.23 mm over 24 wks And DiO traveled up to 1.67±0.11 mm in 4 weeks |
Laser Scanning Confocal microscope |
| Collinge et al. 1991 | DiI | Hamster | 4%PFA | Cochlea. Placed crystals with micropipette. | 40°C | Unclear | 18–42 days | Transversely cut sections either 75 or 100 μm | Unclear | Epifluorescence |
| Eide et al. 1997 | DiI | Chicken Embryo | 4% PFA | Dorsal Root Ganglion | 37°C | 4% PFA | 4–400 days | Vibratome | Unclear | Fluorescence Microscope |
| Friedland 1996 | Fast DiI | Rat | 4% PFA 0.1 M PB | Upper Respiratory Tract | 37°C | 4% PFA and 0.1 M PB | 1 week | Vibratome | 5–10mm | Epifluorescence |
| Fritzsch 1990 | DiI | Chicks | Unclear | Optic Nerve | 21–25°C | 2.5% formalin in 0.1M PB | 4–28 weeks | Unclear | 3 mm | Epifluorescence |
| Fritzsch et al. 2005 | DiI, DiA, DiO, DiD, NeuroVue Maroon, NeuroVue Red, NeuroVue Green | Mice | 4% PFA | Auditory Cortex, Inferior Colliculus, Saccule, Posterior Crista, Cerebellum, Brain Stem. NV has dye loaded filters | 37°C | 4% | 24–96 hrs | Vibratome | Unclear | Epifluorescence |
| Haber. 1988 | Horseradish peroxidase | Human | Unclear | Basal ganglia. Injected with a glass pipette | 37°C and 4°C | Low calcium phosphate buffer or 10% glucose, 10% fetal bovine serum and 10% penicillin streptomycin | 24 hrs | Microtome and vibratome | 10mm from center of injection site | Unclear |
| Hofmann and Bleckmann 1999 | DiI | Goldfish | 4% PFA | Optic Nerve | 6, 20, 30, 35, 40°C | 4% PFA, or 1–4% glutaraldehyde with 1% CaCl2 and 3% potassium dichromate, or 1–4% glutaraldyhyde in PB with 0.1% EDTA | 1 week to 2 months | Vibratome | Unclear | Fluorescence |
| Jensen-Smith | NeuroVue Emerald, NeuroVue Jade, NeuroVue Red, NeuroVue Maroon | Mice embryos | Neutral-buffered PF (NBPF). | Spinal Cord Nylon filter | 37–60°C | 4% NBPF | 24, 48, and 96 hours | Vibratome | Unclear | Confocal |
| Kayeyama et al. 1987 | HRP | Cats/goldfish | 2.5% depolymerized PF and 1.5% glutaraldehyde | Central Nervous System. Inserted with a glass micropipette | 4°C | 0.1 M PB for the goldfish sections | 1–2 weeks | Vibratome | 1.5 mm | Electron microscope |
| Kikkawa 2007 | DiI, DiO, DiD, NV Red, and Golgi-Cox | Rat/mouse/guinea pig | Unclear | Nylon filters for NV Red. Looked at cochlea | Unclear | Unclear | NV Red 7–10 days. Golgi-Cox 3 weeks. Carbocyanine dye 4 weeks | Vibratome | Unclear | Confocal or light or Fluorescence |
| Lukas 1998 | DiI, DiO, DiA | Human cadavers and guinea pigs | 4% PFA | Placed crystal with glass micropipette | 37°C or 40°C | 4% PFA | 3 weeks to 1 year | Vibratome | ≈29.2 mm in guinea pigs and 28.5mm in human fixed tissue for DiI | Epifluorescence |
| Linke 1995 | DiI | Rat | 4% PFA | Hippocamposeptal Projection | Unclear | 4% PFA | 4–12 weeks | Vibratome | Unclear | Fluorescent |
| Magoul 1994 | DiI | Rat | 4% PFA | Arcuate nucleus | 37°C | 4% PFA | 5 months | Vibratome and microtome | Unclear | Epifluorescence |
| Makarenko 2005 | DiI | Rat | 4% PFA in 0.1M sodium phosphate buffer | Pituitary intermediate lobe | 20°C | 4% PFA | 6–24 Months | Vibratome | Unclear | Fluorescent |
| Mastick 1996 | DiI and DiO | Mouse | 4% PFA 0.1M PB | Forebrain and Midbrain | 37°C | 4% PFA 0.1M PB | 4–24 hour | Unclear | Unclear | Dissecting Microscope |
| Mufson 1990 | DiI | Human | 4% PFA | Hippocampal complex | 20°C | 4% PFA | 6 months | Vibratome | 8mm | Epifluorescence |
| Murphy et al. 2007 | DiI oil and crystal form | Mice | 4% PFA | Vagal innervations | Unclear | 4% PFA | 4–7 weeks | Unclear | 2–3.5mm | Fluorescent |
| Richards et al. 1997 | DiI dissolved in DMF | Rats | 2% PFA and post fixed with 10% buffered formalin | Neocortex | RT | Unclear | >3 weeks | Vibratome | Unclear | Fluorescence |
| Soukup et al. 2009 | NeuroVue Dyes | Mice | 4% PFA | Inner ear | – | – | – | – | – | Confocal |
| Swift et al 2004 | DiI, DiO, DiA, DiR crystals dissolved in ethanol | Human cadavers | 10% formalin | Peripheral nerve tissue | RT | Unclear | Up to 48 hours | Vibratome | 12 hrs-15.4mm 24 hrs-29mm 48 hrs-53.7 |
Unclear |
| Zec 2001 | DiI | Human | 4% PFA in 0.1 M PB with .05% Sodium Azide and 4% sucrose | Nucleus paragigantocelluaris lateralis | 20°C | Same as fixative | 8.5–15.5 months | Unclear | Unclear | Fluorescent |
Table 2.
Author’s Conclusions for Comparison Studies
| Author/Year | Comparison | Summarized Author’s Conclusions |
|---|---|---|
| Haber 1998 | Performed a tracing study with Wheat Germ Agglutinin Horseradish Peroxidase. Two incubation solutions and temperatures were used. The tissue was incubated in a culture medium containing 10% glucose, 10% fetal bovine serum, and 10% penicillin streptomycin or a calcium-low phosphate buffer at either 4°C or 37°C |
|
| Kikkawa et al. 2007 | This study compared three techniques to visualize the cochlear nerve fibers, namely DiI, the modified Golgi-Cox method and NeuroVue dyes. |
|
| Chen et al. 2005 | Comparison study between DiI/DiO for the tracer, 1%/4% PFA for the preferred concentration, and RT vs. 37°C for the optimal temperature. |
|
| Lukas et al 1998 | This study sought to optimize the axon tracing procedure in human and guinea pig tissue. |
|
| Murphy and Fox 2007 | This study compared DiI crystals to oil 0.1%, EDTA vs no EDTA, and PBS mounting medium vs glycerol and n-propyl gallate |
|
| Hofmann et al 1999 | The researchers aimed to improve the staining and limit transneuronal labeling. They investigated the effect of increased temperatures and calcium. |
|
| Fritzsch et al 2005 | Compared NeuroVue Red, Green, and Maroon with DiI, DiO, DiA and DiD. |
|
From analyzing Table 2, it is apparent that the most frequently used tracer is DiI. Twenty out of the 23 studies referenced in Table 2 use some form of DiI as the preferred axon tracer. This can be attributed to the fact that there are a limited number of studies that use a new tracer. For example, since NeuroVue dyes are more recent relative to the carbocyanine dyes, there are fewer studies to assess the performance of this tracer. However, it is consistently seen that among the carbocyanine dyes, DiI is the preferred tracer. In regards to the form of the tracer, DiI is available in a crystallized form as well as an oil-based form. The Murphy and Fox (2007) study suggests that DiI in the crystallized form obtains slightly faster rates of diffusion as well as increased staining quality.3 This is reflected in the fact that 20 out of 21 studies in Table 1 that used DiI used it in its crystallized form. NeuroVue dyes appear to work by similar mechanisms to the carbocyanine dyes. Similarly, these NeuroVue dyes are lipophilic – they are capable of traveling through the aldehyde fixed membranes of cells by lateral diffusion. Additionally, both DiI and NeuroVue dyes are compatible with counterstaining and immunohistochemical techniques, therefore making these carbocyanines advantageous. However, it is crucial to be wary of the detergents used during immunohistochemical methods.8 Some stronger detergents such as Triton X may be too harsh to use with DiI and will promote dye leakage out of the cells; therefore, Tween 20 may be compatible with axon tracing methods.2
An apparent advantage to the carbocyanine tracers is that they appear to work consistently in a variety of tissue types. Chen et al (2006) suggests that the carbocyanine dyes do not discriminate between human and guinea pig tissue or between peripheral and central nervous system tissue.4 Therefore, this method seems to work well with a variety of species. On the contrary, data suggests that although dextran methods are excellent for labeling adult neural connections, they may have limited usefulness in embryos and afferent cells.8,10
There is a diversity of tracer application methods among different studies. The DiI crystals are most frequently dissolved in organic solvents and then dried onto a micropipette tip. Once the pipette tip is dry, the crystals can be dabbed at the site of interest. However, other plausible methods exist for tracer insertion, such as using pins, injections, and dye-impregnated filters.
Four percent paraformaldehyde (PFA) tends to be the standard fixation solution in 19 out of the 26 studies. Additionally, four percent PFA also was a prominent choice for the incubation solution. Chen et al (2007) compares two different PFA concentrations (one percent and four percent).4 The study results suggest that there is no significant difference between the two; therefore, it appears that the investigator can choose the concentration of the PFA based on preference. Occasionally, studies such as Hoffman (1998) integrated 0.1 percent ethylenediaminetetraacetic acid (EDTA) into the incubation solution to aid in decalcification of the tissue.11
Substantial variability exists in the incubation temperatures; these temperatures ranged from 6° C to 40° C. Colder temperatures limit diffusion, while higher temperatures promote diffusion. However, as a consequence of higher temperatures, the amount of non-specific staining may increase as a result of dye leakage out of the membranes.
The use of a vibratome appears to be the preferred method of sectioning, found in 19 of 27 studies. However, the studies did not always explicitly state the sectioning method. One study announced that there was a potential for the cryostat to promote dye leakage by destabilizing the dye.2
Discussion
Successful histological methods for axon tracing in ex vivo tissue have the potential to aid in a better understanding of neuron behavior, connectivity, and treatments for various pathological states.2,3,4,12 After review of the selected articles, it is apparent that there are both advantages and disadvantages associated with each method. Thus, the best method varies depending on the research goals of the user. Each researcher may have different interpretations as to what constitutes the best method or axon traced tissues. For example, these differences in interpretation may be in regards to ease of use, cost, distance, time, and sharpness. Although direct comparison studies are the most helpful for providing valuable information regarding factors that affect the effectiveness of a tracer, the majority of our included studies only used a single method. Consequently, our ability to draw conclusions about superiority over other methods was limited. Table 2 summarizes the major findings and conclusions drawn from the seven comparison studies we obtained from our search. Trends among studies were apparent and will be outlined in the subsequent paragraphs. From all of the selected articles, it is evident that ex vivo tracing methods originally used Lucifer Yellow and HRP in the 1980s.1,6,13,14 Since then, tracers started to deviate from HRP and Lucifer Yellow to using the long distance, lipophilic dyes such as the carbocyanines. Carbocyanines are the most frequent method used. NeuroVue dyes are suggested to be promising according to the two direct comparison studies.7,10 We will outline this progression of tracers and provide potential explanations for the observed trends.
Lucifer Yellow is common in studies from the 1980s and 1990s. Although still used, its prevalence has dwindled due to the development of other ex vivo tracer counterparts. While this tracer has the potential for labeling a single neuron structure, it lacks the capability to trace long distances. This method requires injecting the individual neuron iontophoretically in four percent PFA fixed tissues. Therefore, injecting many neurons can be time consuming (>50 min) and require precision.6 Consequently, this technique does not require lengthy incubation periods compared to other tracers such as the carbocyanines. This method also works with storing the tissue in the cold, whereas other lipophilic tracers require room temperature or above for diffusion. Many of the studies had the sections cut with a vibratome after being treated with gelatin for support during the sectioning process. Another feature that emerged while analyzing studies is that Lucifer Yellow’s characteristic fluorescent color diminishes due to exposure of illumination after a couple of weeks.14 Applying the DAB method allows for the fluorescent neuron to convert to a dark color, which aids in a longer stabilization of the labeling. DAB processed tissue can be viewed under an electron microscope. While DAB aids in overcoming the issue with the stability of the fluorescent neuron, it also has the potential to hinder visualization of precise neuronal processes.6
Similar to Lucifer Yellow, HRP was established in the mid-1980s. This technique requires the tracer to be injected with a micropipette under the visual guidance of a stereo microscope. The duration of injection was recorded to be five to 15 minutes (Haber 1988 and Kageyama 1987). This technique also requires precision for insertion. Incubation solutions in different studies showed variability. Haber et al reported longer distances achieved using a low calcium phosphate buffer; however, it is difficult to discern what exactly is responsible for these differences in distance.13 Variation was also noted in regards to incubation duration, making it difficult to form a conclusion discerning the best tracing method. In both studies, HRP methods use a DAB procedure in order to view the neurons with an electron microscope. Kageyama et al indicates that disadvantages of this tracing method include “the process of injection or the mere presence of the marker itself may cause tissue damage or may alter the structure, activity or behavior of cells.”1 Different studies reported a range of distances. For instance, Kageyama reported that the tracer diffused only 1.5 mm from the injection site, while Haber 1988 reported 10 mm.1,13 The differences may be due to Haber et al using Wheat Germ Agglutinin -HRP while Kageyama used generic HRP.1,13 Without a direct comparison study to assess these differences in methods, the variables responsible for these differences cannot be precisely determined.
DiI is suggested to be the most frequently used method for axon tracing among the carbocyanines. Due to other comparison studies, Chen et al (2006), Lukas et al, (1998) and Fritzsch (2005) reiterated that DiI advances through the tissue more quickly than the other carbocyanines.2,4,7 The rate of diffusion for DiI seemed to be similar among studies conducted independently. Lukas et al (1998) identified a diffusion coefficient to be 1.14×10−2 mm/hr and similarly, Swift et al (2004) found the control to have a rate of diffusion of 1.20+−0.08 ×10−2 mm/h.2,15 DiI travels not only farther and faster, but it also continues to travel even after several weeks, whereas DiO ceases to diffuse after a couple of weeks. Fritzsch et al (2005) stated that “DiO is impractical as a long range tracer” and has “limited usefulness”.7 Additionally, DiI is more favorable because it exhibits more fluorescent character than other dyes. An aspect that is found to increase the efficiency of the DiI tracer is applying DiI in crystallized form.3 Murphy et al (2007) stated that DiI in oil form takes a week longer than crystallized DiI to diffuse.3
Despite these differences, there are certain trends towards agreement between authors of the included articles. First, it is common for 0.1 percent EDTA to be added to assist in binding to the excess calcium in the tissue, reducing the occurrence of transneuronal labeling.3,11 Second, temperature assists in increasing the rate of diffusion of the dye; however, it can also facilitate the tracer to diffuse out of the neuron.2,4,11 We found that it is the most common to allow the tissue to incubate in four percent PFA in 0.1M phosphate buffer (PB). A vibratome is suggested to be the best option for sectioning tissue embedded in gelatin.1,2,4,6,7,11,12,15 Cryoprotected tissues sectioned on a microtome may promote dye leakage.2
Discrepancies arise with the method of insertion and incubation period for many of the studies. Most frequently, the tool used to insert the dye was a glass micropipette. However, many other techniques were successful, including pins and dye-covered filters. There are too many changing variables between studies to discern which method for inserting the dye achieves the most desirable results. Even studies using the same tool may insert the dye in a different way. For example, Richards et al (1997) inserted the tracer via micropipette through the length of the tissue and dispensed the tracer every few millimeters.12 Other methods that also used a micropipette involved placing DiI in the tissue superficially. Both techniques seem to be feasible. In regards to incubation period, the studies all came to a consensus that at some point, the tracer would cease to diffuse. However, the time (measured in weeks) among the studies differed substantially. Some studies such as Lukas et al (1998) indicated that the diffusion of the dye plateaued at 12 to 15 weeks.2 However, other studies like Chen et al (2006) specify that the dye continued to diffuse from nine to 24 weeks at a decreased rate.4 Other studies maintained the tracer diffusion for a couple of days to a few weeks. In some cases, it was even reported that 24 hours was sufficient for dye incubation.15 Temperature, varying concentrations, and varying types of tissues may also account for differences in data. Future research should assess ways to speed up the rate of diffusion without causing dye leakage.
NeuroVue dyes are lipophilic dyes similar to carbocyanines. Compared to these carbocyanines, however, Fritzsch et al (2005) suggests in a direct comparison study that Neuro Vue Red is “superior to DiI with respect to visibility over longer diffusion distances”.7 The NeuroVue Red and Maroon double labeling is purportedly better due to the fact that they both have similar tracing distances/speeds, whereas double labeling with DiI and DiA “gets complicated because of different diffusion times for each”.7 NeuroVue dyes have a reduced duration necessary for diffusion. These studies involve similar tissue preparation methods to carbocyanines. For fixation and incubation solution, researchers most commonly used four percent PFA and 0.1 M PB. These dyes also need to be exposed to RT-37 °C to facilitate dye diffusion. We suggest more comparison studies by multiple independent labs to determine if NeuroVue dyes are superior to DiI.
The strengths of this review are that it is comprehensive in regards to a variety of variables that may impact the quality of axon tracing. A limitation of this review is the lack of recent published articles concerning axon tracing ex vivo. The lack of recently published research on new tracers may be due to the emergence of new imaging techniques such as dMRI tractography; however, this technique relies on the availability of specialized equipment and experimental software. With that said, we chose to focus our review on the more widely available histological methods of axon tracing. Additionally, due to limitations of systematic review methodology, perhaps the search terms missed potentially applicable studies as well as recently published studies. However, because of the use of ancestral searching, we are confident that we have identified the majority of the important studies.
Conclusion
We conclude multiple methods are feasible for ex vivo axon tracing and the best histological method is dependent on the goals of the researcher. To each tracer and application method, there are advantages and disadvantages in regards to cost, time, distance, specificity of tracing, single-cell labeling, ability to perform multiple labeling, and ease of use. Thus, what may be good for one purpose may not be good for another. According to the articles that were selected, DiI was reported to perform well in many aspects such as tracing length, ease of use, resolution, and price, as it is fairly inexpensive to use. A major disadvantage of DiI is that diffusion is slow, the order of weeks to months, which delays analysis. With the emergence of NeuroVue Dyes, some comparison studies have suggested superiority over the carbocyanine dyes; however, the majority of researchers still use DiI, which suggests more evaluations should be done to confirm superiority. Moreover, we suggest multiple independent labs conduct more comparison studies in order to definitively identify if this method yields better axon traced tissues.
Acknowledgments
Supported by NIH grant 1K08NS079622
Literature Cited
- 1.Kageyama GH, Meyer RL. Dense HRP filling in prefixed brain tissue for light and electron microscopy. J Histochem Cytochem. 1987;35(10):1127–36. doi: 10.1177/35.10.3624853. [DOI] [PubMed] [Google Scholar]
- 2.Lukas JR, Aigner M, Denk M, Heinzl H, Burian M, Mayr R. Carbocyanine postmortem neuronal tracing. Influence of different parameters on tracing distance and combination with immunocytochemistry. J Histochem Cytochem. 1998;46(8):901–10. doi: 10.1177/002215549804600805. [DOI] [PubMed] [Google Scholar]
- 3.Murphy MC, Fox EA. Anterograde tracing method using DiI to label vagal innervations of the embryonic and early postnatal mouse gastrointestinal tract. J Neurosci Methods. 2007;163:213–25. doi: 10.1016/j.jneumeth.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen BK, Miller SM, Mantilla CB, Gross L, Yaszemski MJ, Windebank AJ. Optimizing conditions and avoiding pitfalls for prolonged axonal tracing with carbocyanine dyes in fixed rat spinal cords. J Neurosci Methods. 2006;154(1):256–63. doi: 10.1016/j.jneumeth.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 5.Collinge C, Schweitzer L. Details of the central projections of the cochlear nerve in in the hamster revealed by the fluorescent tracer DiI. Hear Res. 1991;53(2):159–72. doi: 10.1016/0378-5955(91)90051-a. [DOI] [PubMed] [Google Scholar]
- 6.Ohm T, Dieckmann S. The use of Lucifer Yellow and Mini Ruby for intracellular staining in fixed brain tissue. Methodological considerations evaluated in rat and humanautopsy brains. J Neurosci Methods. 1994;55(1):105–10. doi: 10.1016/0165-0270(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 7.Fritzsch B, Muirhead KA, Feng F, Gray BD, Ohlsson-Wilhelm BM. Diffusion and imaging properties of three new lipophilic tracers, NeuroVue Maroon, NeuroVue Red and NeuroVue Green and their use for double and triple labeling of neuronal profile. Brain Res Bull. 2005;66(3):249–58. doi: 10.1016/j.brainresbull.2005.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jensen-Smith H, Gray B, Muirhead K, Ohlsson-Wilhelm B, Fritzsch B. Long-distance three-color neuronal tracing in fixed tissue using NeuroVue dyes. Immunol Invest. 2007;36(5):763–78. doi: 10.1080/08820130701706711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soukup GA, Fritzsch B, Pierce ML, Weston MD, Jahan I, McManus MT, et al. Residual microRNA expression dictates the extent of inner ear development in conditional Dicer knockout mice. Dev Biol. 2009;328(2):328–41. doi: 10.1016/j.ydbio.2009.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kikkawa YS, Pawlowski KS. Cochlear neuronal tracing for frequency mapping with DiI, NeuroVue, and Golgi methods. Acta Otolaryngol Suppl. 2007;559(S559):19–23. doi: 10.1080/03655230701595311. [DOI] [PubMed] [Google Scholar]
- 11.Hofmann MH, Bleckmann H. Effect of temperature and calcium on transneuronal diffusion of DiI in fixed brain preparations. J Neurosci Methods. 1999;88(1):27–31. doi: 10.1016/s0165-0270(99)00007-2. [DOI] [PubMed] [Google Scholar]
- 12.Richards LJ, Koester SE, Tuttle R, O’Leary DD. Directed growth of early cortical axons is influenced by a chemoattractant released from an intermediate target. J Neurosci. 1997;17(7):2445–58. doi: 10.1523/JNEUROSCI.17-07-02445.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haber S. Tracing intrinsic fiber-connections in postmortem human-brain with WGA-Hrp. J Neurosci Methods. 1988;23(1):15–22. doi: 10.1016/0165-0270(88)90017-9. [DOI] [PubMed] [Google Scholar]
- 14.Buhl EH, Lobke J. Intracellular lucifer yellow injection in fixed brain slices combined with retrograde tracing, light and electron microscopy. Neuroscience. 1989;28(1):3–16. doi: 10.1016/0306-4522(89)90227-3. [DOI] [PubMed] [Google Scholar]
- 15.Swift MJ, Crago PE, Grill WM. Applied electric fields accelerate the diffusion rate and increase the diffusion distance of DiI in fixed tissue. J Neurosci Methods. 2005;141(1):155–63. doi: 10.1016/j.jneumeth.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 16.Calabrese E, Badea A, Cofer G, Qi Y, Johnson GA. A diffusion MRI tractography connectome of the mouse brain and comparison with neuronal tracer data. Cere cortex. 2015;25(11):4628–4637. doi: 10.1093/cercor/bhv121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reveley C, Seth AK, Pierpaoli C, Silvia AC, Yu D, Saunders RC, et al. Superficial white matter fiber system impede detection of long-range cortical connections in diffusion MR tractography. Proc Natl Acad Sci. 2015;112(21):E2820–8. doi: 10.1073/pnas.1418198112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mastick GS, Easter SS., Jr Initial organization of neurons and tracts in the embryonic mouse fore- and midbrain. Dev Biol. 1996;173(1):79–94. doi: 10.1006/dbio.1996.0008. [DOI] [PubMed] [Google Scholar]
- 19.Auclair F, Bélanger MC, Marchand R. Ontogenetic study of early brain stem projections to the spinal cord in the rat. Brain Res Bull. 1993;30(3):281–9. doi: 10.1016/0361-9230(93)90256-b. [DOI] [PubMed] [Google Scholar]
- 20.Baier H, Klostermann S, Trowe T, Karlstrom R, Nüsslein-Volhard C, Bonhoeffer F. Genetic dissection of the retinotectal projection. Development. 1996;123:415–25. doi: 10.1242/dev.123.1.415. [DOI] [PubMed] [Google Scholar]
- 21.Ceranik K, Deng J, Heimrich B, Lübke J, Zhao S, Förster E, et al. Hippocampal Cajal-Retzius cells project to the entorhinal cortex: retrograde tracing and intracellular labeling studies. Eur J Neurosci. 1999;11(12):4278–90. doi: 10.1046/j.1460-9568.1999.00860.x. [DOI] [PubMed] [Google Scholar]
- 22.Eide AL, Glover JC. Developmental dynamics of functionally specific primary sensory afferent projections in the chicken embryo. Anat Embryol. 1997;195(3):237–50. doi: 10.1007/s004290050043. [DOI] [PubMed] [Google Scholar]
- 23.Friedland DR, Eden AR, Laitman JT. Use of the novel carbocyanine tracer fast-DiI for investigating upper respiratory tract cranial nerves in prenatal rats. Lab Animal Sci. 1996;46(2):220–5. [PubMed] [Google Scholar]
- 24.Linke R, Pabst T, Frotscher M. Development of the hippocamposeptal projection in the rat. JComp Neural. 1995;351(4):602–16. doi: 10.1002/cne.903510409. [DOI] [PubMed] [Google Scholar]
- 25.Magoul R, Ciofi P, Tramu G. Visualization of an efferent projection route of the hypothalamic ratarcuate nucleus through the stria terminalis after labeling with carbocyanine dye DiI or proopiomelanocortin-immunohistochemistry. Neurosci Lett. 1994;172(1–2):134–8. doi: 10.1016/0304-3940(94)90680-7. [DOI] [PubMed] [Google Scholar]
- 26.Makarenko IG, Ugrumov MV, Calas A. Axonal projections from the hypothalamus to the pituitary intermediate lobe in rats during ontogenesis: DiI tracing study. Brain Res. 2005;155(2):117–26. doi: 10.1016/j.devbrainres.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Mufson EJ, Brady DR, Kordower JH. Tracing neuronal connections in postmortem human hippocampal complex with the carbocyanine dye DiI. Neurobiol Aging. 1990;11(6):649–53. doi: 10.1016/0197-4580(90)90031-t. [DOI] [PubMed] [Google Scholar]
- 28.Zec N, Kinney HC. Anatomic relationships of the human nucleus paragigantocellularis lateralis: A DiI labeling study. Auton Neurosci. 2001;89:110–24. doi: 10.1016/S1566-0702(01)00258-2. [DOI] [PubMed] [Google Scholar]


