Abstract
Background
A deficit in the ability to inhibit fear has been proposed as a biomarker of post-traumatic stress disorder (PTSD). Previous research indicates that individuals with PTSD show reduced inhibition-related activation in rostral anterior cingulate cortex (rACC). The goal of the current study was to investigate differential influences of an early environmental risk factor for PTSD – childhood maltreatment – on inhibition-related brain function in individuals with PTSD versus trauma-exposed controls.
Methods
Individuals with PTSD (n=37) and trauma-exposed controls (n=53) were recruited from the primary care waiting rooms of an urban public hospital in Atlanta, GA. Participants completed an inhibition task during fMRI, and reported childhood and adult traumatic experiences. The groups were matched for adult and child trauma load.
Results
We observed an interaction between childhood maltreatment severity and PTSD status in the rACC (p<.05, corrected), such that maltreatment was negatively associated with inhibition-related rACC activation in the PTSD group, but did not influence rACC activation in the TC group. Rostral ACC activation was associated with inhibition-related task performance in the TC group but not the PTSD group, suggesting a possible contribution to stress resilience.
Conclusions
Findings highlight individual differences in neural function following childhood trauma, and point to inhibition-related activation in rostral ACC as a risk factor for PTSD.
Keywords: Stress, development, fMRI, neuroimaging, prefrontal cortex
Post-traumatic stress disorder (PTSD) is a pervasive disorder that affects some people after a traumatic experience. Difficulties with fear inhibition appear to be central to many PTSD symptoms, and the inhibition deficit may serve as a specific biomarker that distinguishes PTSD from mood disorders [1]. Research on the brain systems involved in PTSD support this theory, showing changes in the function of a broad network of regions involved in fear inhibition. These include greater reactivity in threat-processing regions such as the amygdala, dorsal anterior cingulate cortex (dACC), and insula [2, 3], and decreased engagement of regions involved in inhibition such as the rostral ACC (rACC), ventromedial prefrontal cortex (vmPFC), and hippocampus [3–7].
One critical outstanding question is whether impaired prefrontal inhibition can account for the effects of early risk factors for PTSD such as early life stress and genetic risk. This question is highly relevant to identifying individuals at risk for PTSD and physiological targets for treatment. Prefrontal function appears causal to both fear inhibition and fear extinction deficits in PTSD [8, 9] and PTSD-related deficits in prefrontal inhibition-related activity have been observed not only in the context of fear, but also in tasks probing cognitive and response inhibition [5, 10–13]. It has been proposed that the prefrontal inhibition deficit represents a “core process” that maintains traumatic stress [14], contributing to multiple PTSD symptom categories and other negative sequelae of trauma including executive and social functioning deficits. Here, we investigated links between early life stress—specifically childhood maltreatment—prefrontal inhibition, and adult PTSD.
Childhood maltreatment is one of the primary risk factors for PTSD and a number of other psychiatric and medical issues [15]. It has been linked to changes in function of the hypothalamic-pituitary-adrenal (HPA) axis [16, 17] as well as physiological reactivity [18], and mediates genetic risk for PTSD [17, 19]. Its effects on brain development are long lasting, and result in widespread changes to the function and structure of fear-processing networks of the brain [20–30]. Maltreatment has been shown to impact prefrontal activation in cognitive inhibition tasks in children and youth [31–33]. Little research has addressed whether these changes persist into adulthood, although initial evidence suggests that inhibition performance [34] and prefrontal inhibition-related activity [35] are impaired in adults with a history of maltreatment. Here we investigated associations between childhood trauma severity and prefrontal function in adults, and potential contributions to PTSD. Although childhood maltreatment is a strong predictor of PTSD following adult trauma, it is notable that a proportion of childhood trauma survivors do not develop PTSD [36, 37]. The neurobiological features that differentiate these individuals from those who do develop PTSD remain unclear.
We compared the effects of childhood maltreatment in adults with PTSD and in a resilient control group matched for childhood and adult trauma levels, but with significantly fewer PTSD and depression symptoms. Participants were selected from a high-risk urban primary care population experiencing trauma and PTSD comparable to those of post-deployment veteran samples [38, 39]. Using functional magnetic resonance imaging (fMRI), we investigated brain activation during a response inhibition task shown to engage multiple aspects of the prefrontal cortex [40], in which we previously observed a PTSD-related deficit in rACC activation [5]. We tested the hypothesis that the effects of childhood trauma on inhibition-related brain activation might differ among those with current PTSD, relative to trauma-exposed controls. We predicted that maltreatment would be negatively associated with rACC activation in the PTSD group, but not the traumatized control (TC) group.
Methods
Participants
One hundred and thirty-three African-American women ages 18 to 62 were recruited from a larger study of risk factors for PTSD. Participants were approached in the general medical clinics of Grady Memorial Hospital, a large publicly-funded hospital in inner-city Atlanta, Georgia. This study focused on women, because of their greater burden of PTSD relative to men [41]. For data homogeneity, we included only African-American individuals. Participants experienced at least one criterion A trauma according to the Diagnostic and Statistical Manual of Mental Disorders[42], as reported on the Traumatic Events Inventory (TEI), and had normal or corrected-to-normal vision. Exclusion criteria included a history of bipolar disorder or schizophrenia or current psychotic symptoms, current psychotropic medication, and contraindications for MRI scanning including neurological disorder or other known brain abnormality, head injury with loss of consciousness, implanted metal objects. Urine tests for pregnancy and drug use (cocaine, marijuana, opiates, amphetamines, methamphetamines) were conducted 24 to 48 hours before the MRI scan, and individuals with positive results were excluded.
Testing took place at Grady Memorial Hospital and the Biomedical Imaging Technology Center of Emory University. All participants provided written informed consent. The Institutional Review Boards of Emory University and Grady Memorial Hospital approved the study procedures. FMRI data from a subset of these participants (20 PTSD, 21 TC) have been reported elsewhere [5].
Psychological assessment
Adult trauma was assessed using the Traumatic Events Inventory (TEI), which has been validated in this population [38]. Childhood maltreatment was assessed using the Childhood Trauma Questionnaire [CTQ, 43, 44].
The Modified PTSD Symptom Scale [PSS, 45] is a 17-item self-report measure that was used to assess PTSD symptoms. DSM-IV-TR criteria were used to classify participants into PTSD and TC groups, including endorsement of at least 1 re-experiencing symptom, 3 avoidance symptoms, and 2 hyper-arousal symptoms. The measure was administered by asking participants to consider all of their traumas, with the goal of capturing current symptom severity in a sample in which multiple lifetime traumas are common.
Depressive symptoms were assessed using the Beck Depression Inventory [BDI, 46]. Alcohol and drug abuse were assessed using the Alcohol Use Disorders Identification Test [AUDIT, 47] and 10-item version of the Drug Abuse Screening Test [DAST, 48], respectively. Trait-level resilience was assessed using the Connor-Davidson Resilience scale [CD-RISC, 49]. These measures have been used in our previous studies with this population [5, 38].
FMRI Task
The task followed previous work by Leibenluft et al. [40], and a detailed description can be found elsewhere [5]. Trials consisted of either an X or an O displayed for 1000ms, and participants responded with a 1 for X and 2 for O. On “No Go” trials, a red rectangle appeared behind the X or O, and participants were asked to withhold all responses. The stimulus event was followed by a jittered inter-trial interval ranging from 1250 to 2500ms, and 500ms fixation cross. There were 4 runs of 26 Go trials, 13 No-Go trials, and 14 blank trials (black background rather than X or O), randomly ordered.
Brain imaging acquisition and analysis
Brain imaging data were acquired on a 3.0-Tesla MRI scanner (Siemens Magnetom Trio TIM, Malvern, PA) using a 12-channel head coil. Statistical parametric mapping software was used to preprocess and analyze fMRI data [SPM5, Wellcome Trust Centre for Neuroimaging, 50]. See Supplemental Methods for additional information on MRI acquisition parameters, preprocessing, and first-level models.
Inhibition-related activation was modeled using subject-level statistical maps for No-Go relative to Go trials. These were entered into group-level regression analyses conducted for an a priori rACC ROI, based on previous findings showing an inhibition deficit in PTSD[3, 5]. An exploratory analysis was also conducted voxel-wise across the whole brain. The rACC ROI was constructed using a mask of Brodmann’s Areas 24, 25, and 32. To limit these regions to the rostral and subgenual aspects of the ACC, the ROI included only voxels ventral to z=0. To test for regions showing different effects of child maltreatment in the PTSD versus control groups, group-level models included an interaction term for CTQ total score x PTSD, main effects of CTQ score and PTSD status, and an age covariate. Secondary analyses controlling for adult trauma load and depression symptom severity were conducted using this interaction model, adding number of adult traumas (TEI total) and BDI total as covariates. To investigate whether findings were accounted for by particular type of abuse, the same interaction model was used to examine the abuse subscales (physical, sexual, and emotional abuse) separately. The models were implemented in SPSS 22.0 for the ROI analyses, and in SPM5 for whole-brain analyses.
For whole-brain analyses, within-groups analyses of task-related activation were corrected for multiple comparisons using a combined height-extent threshold implemented using 3dclustsim within AFNI, version 16.0.6, for all voxels within the group mask, with 10,000 iterations. A cluster-forming threshold of p < 0.001 was used and when combined with a cluster size of k = 31 resulted in a corrected probability of p < 0.05. For the primary moderation analyses of childhood trauma and PTSD diagnosis, no clusters survived this threshold. In order to determine whether brain regions outside the ROI might show similar effect sizes, and to facilitate direct comparison with previous studies, exploratory whole-brain analyses were thresholded with an uncorrected p<.001, and k=5 cluster extent.
Results
Group characteristics
Of the 133 participants recruited, 11 were excluded for anatomical abnormalities such as falx calcification, 6 for technical problems with the scanner or stimulus presentation, 16 for head motion greater than 3 mm over the entire task, and 10 for low performance on the Go No-Go task (more than 80% of trials incorrect in the No-Go condition; commission errors). The excluded participants had lower CTQ total scores (included: M(SD)=43.1(18.2), excluded: M(SD)=36.3(11.0), p=.01), and greater lifetime drug use endorsed on the DAST (included: M(SD)=1.8(1.8), excluded: M(SD)=3.3(2.6), p=.006), but did not differ from the included participants on any of the other clinical or demographic measures. Demographic and clinical characteristics of the final sample of 90 participants are shown in Table 1. PTSD and TC groups did not differ in task performance, or adult or childhood trauma severity. Participants in the PTSD group were younger than in the control group; all neuroimaging analyses included age as a covariate. PTSD and depression severity were greater in the PTSD group than in the TC group. Given the high comorbidity of PTSD and depression, the primary neuroimaging analyses did not include depression severity as a covariate, and secondary analyses were conducted to control for depression. The TC group also showed greater trait-level resilience. Both groups reported very low levels of alcohol and drug abuse, particularly relative to other traumatized samples [51, 52].
Table 1.
Clinical and demographic characteristics of the PTSD and control groups
| PTSD (N = 37) | Trauma Control (N = 53) | Group difference t | |||
|---|---|---|---|---|---|
| M | (SD) | M | (SD) | ||
| Age | 35.4 | (12.5) | 41.1 | (12.2) | −2.1* |
| % correct: No-Go condition | 99.2 | (1.6) | 98.1 | (4.3) | 1.4 |
| % correct: Go condition | 98.2 | (2.3) | 97.8 | (6.0) | 0.4 |
| % Hits-False alarms | 97.4 | (3.0) | 95.9 | (8.8) | 0.9 |
| Adult trauma load (TEI) | 4.7 | (2.5) | 3.9 | (2.1) | 1.6 |
| Childhood trauma severity (CTQ) | 46.7 | (18.6) | 40.5 | (17.7) | 1.6 |
| Sexual abuse | 10.2 | (5.8) | 9.1 | (5.8) | 0.9 |
| Physical abuse | 9.0 | (4.1) | 7.8 | (3.5) | 1.5 |
| Emotional abuse | 9.9 | (4.9) | 8.3 | (4.0) | 1.8 |
| Physical neglect | 7.1 | (3.1) | 6.2 | (2.9) | 1.5 |
| Emotional neglect | 10.0 | (4.4) | 9.1 | (5.1) | 0.9 |
| Depression severity (BDI) | 19.4 | (8.9) | 6.8 | (5.4) | 7.7*** |
| PTSD symptom severity (MPSS) | 25.8 | (7.8) | 5.0 | (3.9) | 14.9*** |
| Intrusive | 6.4 | (2.9) | 0.9 | (1.3) | 10.8*** |
| Avoidance/Numbing | 10.8 | (4.4) | 1.7 | (2.0) | 11.7*** |
| Hyperarousal | 8.5 | (2.8) | 2.3 | (2.3) | 11.4*** |
| Resilience (CD-RISC) | 31.1 | (6.2) | 34.0 | (5.4) | −2.2* |
| Alcohol abuse (AUDIT): Past year | 3.9 | (6.8) | 2.7 | (3.3) | 1.1 |
| Alcohol abuse: Lifetime | 6.5 | (7.2) | 6.3 | (7.8) | 0.1 |
| Drug abuse (DAST): Past year | 0.8 | (1.4) | 0.4 | (0.8) | 1.8 |
| Drug abuse: Lifetime | 1.7 | (1.9) | 1.9 | (1.8) | −0.5 |
p<.05,
p<.01,
p<.001
Brain activation in the inhibition task
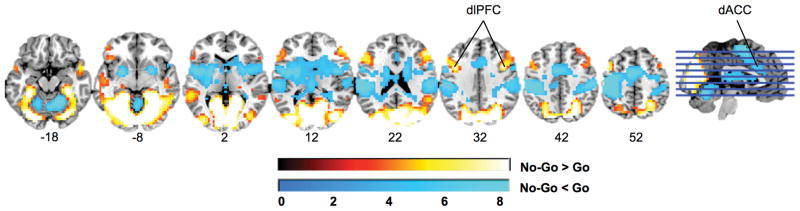
Brain regions engaged by the task, for all participants irrespective of PTSD diagnosis, are shown in Figure 1, and Table S1. Activation for No-Go relative to Go trials was consistent with previous studies [53]. Participants showed increased activation of regions associated with visual attention and top-down inhibition including bilateral dorsolateral prefrontal cortex (dlPFC), and occipital cortex. Participants showed decreased activation of regions associated with environmental and internal salience [54, 55] including dACC, bilateral insula, striatum, and inferior parietal cortex.
Figure 1.
Brain regions significantly activated in the inhibition task, for all participants irrespective of PTSD status (p<.05, corrected). Results are displayed in neurological orientation on a representative single-subject template brain in MNI space.
Influence of childhood maltreatment on inhibition in individuals with and without PTSD
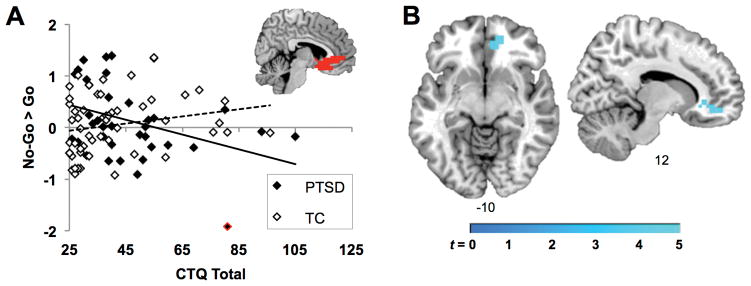
The interaction of CTQ score and PTSD diagnosis significantly influenced inhibition-related activity in the rACC ROI, above and beyond the main effects of age, CTQ total, and PTSD diagnosis, R2Δ = .09, p<.005 (Table 2A, Figure 2A). There was no main effect of CTQ score on rACC activation, but there was a significant main effect of PTSD diagnosis. The removal of a potential outlier in the PTSD group, highlighted red in Figure 2A, reduced the effect size of the interaction, although the effect remained significant, R2Δ = .05, p=.03. The interaction effect also remained significant after controlling for adult trauma load and depression symptom severity, R2Δ = .08, p<.01 (Table 2B), as well as inhibition-related performance on the Go No-Go task, R2Δ = .08, p<.01 (proportion hits-false alarms; Table 2C). Follow-up analyses separately examining the PTSD and TC groups showed that CTQ score was negatively correlated with rACC activation in the PTSD group (r(34) = −.36, p = .03), but not in the TC group (r(50) = .22, p = .13).
Table 2.
Results of moderated regression analyses for the rostral anterior cingulate
| Predictor | R2 change | Beta | t | |
|---|---|---|---|---|
| A. Initial model | ||||
| Step 1 | Age | .002 | .04 | 0.37 |
| Step 2 | CTQ total | .01 | −.11 | −1.02 |
| PTSD diagnosis | .80 | 2.95** | ||
| Step 3 | CTQ* PTSD | .09** | −.77 | −2.86** |
| B. Covarying for adult trauma and depression symptoms | ||||
| Step 1 | Age | .01 | .03 | 0.30 |
| TEI total # traumas | .12 | 1.08 | ||
| BDI total | −.11 | −0.77 | ||
| Step 2 | CTQ total | .03 | −.12 | −1.01 |
| PTSD diagnosis | .86 | 3.11** | ||
| Step 3 | CTQ* PTSD | .08* | −.78 | −2.75* |
| C. Covarying for task performance | ||||
| Step 1 | Age | .02 | .05 | 0.54 |
| Go No-Go: hits-false alarms | .13 | 1.20 | ||
| Step 2 | CTQ total | .01 | −.12 | −1.13 |
| PTSD diagnosis | .78 | 2.85** | ||
| Step 3 | CTQ* PTSD | .08* | −.76 | −2.81* |
p<.01;
p<.005
Figure 2.
Influence of childhood maltreatment on inhibition in the PTSD and TC groups. A: Within the rACC ROI, there was a significant interaction between childhood maltreatment and PTSD status. Scatterplot shows the association between individuals’ CTQ total scores and the mean contrast estimate for No-Go > Go across voxels in the anatomically-defined ROI, which is illustrated in red on a coronal slice from a representative template brain in MNI space. The regression line for the PTSD group is shown in solid black, and for the TC group in dotted black. The potential outlier from the PTSD group is highlighted in red. B: Whole-brain analysis conducted at a low threshold of p<.001, k = 5, confirmed that the largest child maltreatment * PTSD interaction was observed in the rostral ACC, and was not observed in other regions.
In exploratory whole brain analysis conducted at an uncorrected threshold, only a single cluster showed an interaction between CTQ score and PTSD diagnosis, overlapping right orbitofrontal cortex, rACC, and subgenual ACC (Table 3, Figure 2B). In the PTSD group, but not the TC group, CTQ score was negatively correlated with activation in the same rACC cluster from the interaction analysis. In both the PTSD and TC groups, CTQ score correlated positively with activation in the precentral gyrus.
Table 3.
Differential effects of childhood maltreatment in the PTSD versus TC groups
| Region | HEMa | BA | MNI Coordinates | Z | Cluster size (mm3) | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Interaction of CTQ and PTSD diagnosis | |||||||
| Positive relationship | |||||||
| No significant clusters | |||||||
| Negative relationship | |||||||
| Orbitofrontal cortex | R | 11 | 12 | 44 | −12 | 3.83 | 960 |
| Ant. Cingulate G. | R | 32 | 4 | 24 | −8 | 3.47 | (LM) |
| Ant. Cingulate G. | R | 11 | 12 | 32 | −4 | 3.18 | (LM) |
| CTQ effects: PTSD group | |||||||
| Positive correlation | |||||||
| Precentral G. | L | 6 | −28 | 0 | 40 | 3.59 | 768 |
| Negative correlation | |||||||
| Ant. Cingulate G. | R | 11 | 12 | 44 | −16 | 4.40 | 576 |
| CTQ effects: TC group | |||||||
| Positive correlation | |||||||
| Precentral G. | R | 6 | 48 | −8 | 48 | 3.57 | 448 |
| Negative correlation | |||||||
| No significant clusters | |||||||
Note. Clusters exceeding an uncorrected voxel-wise threshold of p<.001, k=5.
HEM = hemisphere. L = left, R = right. LM = local maximum. WM = white matter. BA = Brodmann’s area.
Relationship between rostral ACC activation and inhibition performance and symptoms
To test for relationships between rACC activation and inhibition and/or symptoms in each group, we extracted the mean contrast value for No-Go > Go from voxels within the cluster from the interaction analysis, and examined correlations with task performance and symptom severity. Inhibition performance on the Go No-Go task was quantified by subtracting the proportion of false alarms to No-Go trials from the proportion of hits to Go trials, PTSD symptom severity using MPSS total score, depression symptom severity using BDI total score, and trait-level resilience using CD-RISC total score. Inhibition performance was not significantly associated with rACC activation in the PTSD group [r(37)=−.09, p=.59], but was positively associated with rACC activation in the TC group [r(53)=.31, p=.03]. In the PTSD group, rACC activation was negatively associated with depression symptom severity [r(37)=−.34, p=.04] but was not correlated with PTSD symptom severity [r(37)=−.10, p=.57] or resilience [r(37)=.32, p=.08]. In the TC group, rACC activation was not related to depression symptoms, PTSD symptoms, or resilience, ps>.30.
Discussion
In the current study we investigated the effects of childhood maltreatment on inhibition-related brain activity in individuals with and without current PTSD. The findings supported the hypothesis that childhood maltreatment would be associated with a deficit in inhibition-related activity in the rACC among individuals with current PTSD, but not among trauma-exposed individuals without PTSD. Reduced activation in this region has previously been associated with PTSD and other anxiety disorders [3, 4, 6] and fear inhibition deficits [5]. Examination of different types of child abuse showed that only physical abuse was negatively associated with rACC activation in the PTSD but not TC group. However, this finding was observed only in whole-brain analyses with a low threshold, and must be interpreted carefully.
Studies examining the neural correlates of childhood maltreatment have focused primarily on negative emotion reactivity and regulation, observing that maltreatment predicted increased activation of the amygdala and other limbic areas [20–23, 56–58]. These studies suggest that childhood maltreatment may influence inhibitory brain activity: maltreatment predicts changes in functional connectivity between the amygdala and prefrontal regions that are typically involved in inhibiting amygdala activation [24, 25, 59]. Furthermore, recent evidence indicates that maltreatment impacts prefrontal activation during inhibition, showing decreased activation of middle frontal gyrus [31], and increased activation of dorsomedial regions including dACC and dmPFC [31–33]. The current findings indicate a long lasting reduction in inhibitory prefrontal activation associated with early maltreatment, in individuals who develop PTSD in adulthood. A deficit in inhibition has been proposed as a biomarker for PTSD [1]; in combination with previous research, the findings suggest that an inhibition deficit may also be a risk factor that pre-dates adult index trauma and a neural mechanism by which early adverse experiences confer such risk. Interestingly, decreased rACC activation in the PTSD group was associated with more severe symptoms of depression, but not PTSD, suggesting that reduced rACC activation may moderate the effects of early life stress on risk for the depressive aspects of adult post-traumatic stress. Further research is needed to address this hypothesis.
In contrast, maltreatment did not predict reduced prefrontal activation among traumatized individuals without PTSD. Despite comparable levels of childhood and adult trauma between the PTSD and TC groups, the TC group showed no significant association between maltreatment and inhibition-related activity in the rACC. This raised the intriguing possibility that preserved inhibition-related activation in the rACC may protect against PTSD after childhood maltreatment. Alternatively, plasticity in the inhibitory activity of the rACC may be a substrate for recovery in individuals who experienced high levels of early life stress. This interpretation is consistent with previous studies showing increased activation in this region associated with spontaneous recovery from PTSD [60] and treatment response [61–64]. In addition, a twin study showed rACC gray-matter loss in combat-exposed twins with current PTSD, relative to their combat unexposed co-twins, as well as combat-exposed individuals without PTSD [65], suggesting that plasticity in this region may reflect the PTSD state. The structure and function of this region may thus reflect the combined environmental influences of childhood and adult trauma. Further research will be needed in order to distinguish a plasticity-related hypothesis from the possibility that rACC activity reflects a stable individual difference predicting whether childhood trauma will lead to PTSD or resilience.
The interaction of total childhood maltreatment and PTSD was highly region-specific, restricted to the rACC (and overlapping nearby mPFC and OFC) in an analysis of the whole brain. Activation in this region may index a direct contribution to response inhibition, as this region has previously been associated with top-down regulation of emotional responses [66–68], and fear inhibition [69, 70]. A second alternative is that the rACC may only be engaged in some individuals as a prefrontal “helper,” which facilitates inhibition in the face of early life stress among controls but not in individuals with PTSD. The findings were consistent with this interpretation: behavioral performance on the task was positively correlated with rACC activation in the TC group but not the PTSD group. A third possibility, given that the rACC has been associated with emotion regulation and the regulation and monitoring of autonomic physiology [54, 68], is that activation of this region may not be directly related to inhibition but may instead reflect efforts to down-regulate distracting afferent signals from baseline autonomic activity or default-mode central activity, in order to complete the inhibition task.
Response inhibition tasks in healthy individuals more commonly involve other prefrontal regions such as the dorsal ACC and dlPFC [53]. We observed dlPFC activation in the analysis of task-related activity, but it did not correlate with child maltreatment. In contrast, dACC was less activated for No Go than Go trials. This may be related to population differences. The current study investigated a different population than most previous studies of the effects of trauma and PTSD on inhibition. We recruited trauma-exposed, adult female participants, who were sampled from a high-risk epidemiological sample rather than psychiatric sample. Only one other study of inhibition has examined adults exposed to childhood maltreatment [35], and this study did not report regional activation for No Go > Go, but instead focused on functional connectivity analyses. Other studies of inhibition in trauma-exposed adults focused on male veteran samples [10–12, 71]. In addition, dACC has been linked with conflict processing and error detection [33, 72], whereas the task used in the current study was very simple and resulted in few errors. A previous study of PTSD with a similar, simple Go No-Go task showed no dACC activation in trauma-exposed participants [13]. The current task may recruit neural correlates of response inhibition but not error-related processing. An analysis controlling for task performance supported this idea, as the covariate reflecting the number of errors did not impact the results.
The current study is limited in its use of retrospective reports of childhood trauma to examine long-standing effects on brain function. The CTQ shows high convergence with other measures of childhood maltreatment [44], and longitudinal studies of early life stress across development would provide a complementary perspective, possibly revealing additional factors that determine why rACC function decreases with childhood maltreatment in some individuals but not others. Furthermore, the current study did not include non-trauma-exposed controls, but rather examined the effects of childhood maltreatment along a spectrum of low to high exposure. Further study is needed to compare prefrontal inhibition in exposed versus unexposed individuals. In addition, further research is needed in order to determine whether the findings generalize to men.
Conclusions
Childhood maltreatment is an early factor that contributes to PTSD risk. The current findings suggest that activation of the anterior cingulate cortex moderates the effect of early maltreatment experiences on PTSD. Further research is needed in order to determine whether inhibition-related activity in this region may be an individual difference that promotes resilience to PTSD in the face of risk factors such as childhood maltreatment.
Supplementary Material
Acknowledgments
We would like to thank Ebony Glover, Renuka Reddy, and Ye Ji Kim for their assistance with MRI data collection, and Allan Graham, Angelo Brown, and the Grady Trauma Project staff for their work in recruiting participants.
Financial support
This work was primarily supported by the National Institutes of Mental Health (MH098212 to T.J., F32MH101976 to J.S.S., MH071537 to K.J.R.). Support was also received from Emory and Grady Memorial Hospital General Clinical Research Center, NIH National Centers for Research Resources (M01RR00039), National Center for Advancing Translational Sciences of the NIH (UL1TR000454), and Howard Hughes Medical Institute (K.J.R.).
Footnotes
Conflict of interest
The authors declare no conflict of interest, financial or otherwise. Jennifer Stevens had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Jovanovic T, Norrholm SD, Blanding NQ, et al. Impaired fear inhibition is a biomarker of PTSD but not depression. Depress Anxiety. 2010;27(3):244–51. doi: 10.1002/da.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fonzo GA, Simmons AN, Thorp SR, et al. Exaggerated and disconnected insular-amygdalar blood oxygenation level-dependent response to threat-related emotional faces in women with intimate-partner violence posttraumatic stress disorder. Biol Psychiatry. 2010;68(5):433–441. doi: 10.1016/j.biopsych.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Milad MR, Pitman RK, Ellis CB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66(12):1075–82. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lanius RA, Williamson PC, Hopper J, et al. Recall of emotional states in posttraumatic stress disorder: an fMRI investigation. Biol Psychiatry. 2003;53(3):204–210. doi: 10.1016/s0006-3223(02)01466-x. [DOI] [PubMed] [Google Scholar]
- 5.Jovanovic T, Ely T, Fani N, et al. Reduced neural activation during an inhibition task is associated with impaired fear inhibition in a traumatized civilian sample. Cortex. 2013;49(7):1884–91. doi: 10.1016/j.cortex.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shin LM, Whalen PJ, Pitman RK, et al. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biol Psychiatry. 2001;50(12):932–42. doi: 10.1016/s0006-3223(01)01215-x. [DOI] [PubMed] [Google Scholar]
- 7.Etkin A, Wager TD. Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, Social Anxiety Disorder, and Specific Phobia. Am J Psychiatry. 2007;164(10):1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annual review of psychology. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jovanovic T, Kazama A, Bachevalier J, Davis M. Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology. 2012;62(2):695–704. doi: 10.1016/j.neuropharm.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shin LM, Bush G, Milad MR, et al. Exaggerated activation of dorsal anterior cingulate cortex during cognitive interference: a monozygotic twin study of posttraumatic stress disorder. Am J Psychiatry. 2011;168(9):979–85. doi: 10.1176/appi.ajp.2011.09121812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shin LM, Bush G, Whalen PJ, et al. Dorsal anterior cingulate function in posttraumatic stress disorder. J Trauma Stress. 2007;20(5):701–12. doi: 10.1002/jts.20231. [DOI] [PubMed] [Google Scholar]
- 12.van Rooij SJ, Geuze E, Kennis M, et al. Neural correlates of inhibition and contextual cue processing related to treatment response in PTSD. Neuropsychopharmacology. 2015;40(3):667–75. doi: 10.1038/npp.2014.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falconer E, Bryant R, Felmingham KL, et al. The neural networks of inhibitory control in posttraumatic stress disorder. J Psychiatry Neurosci. 2008;33(5):413–22. [PMC free article] [PubMed] [Google Scholar]
- 14.Liberzon I, Sripada CS. The functional neuroanatomy of PTSD: A critical review. Stress Hormones and Post Traumatic Stress Disorder: Basic Studies and Clinical Perspectives. 2007;167:151–169. doi: 10.1016/S0079-6123(07)67011-3. [DOI] [PubMed] [Google Scholar]
- 15.Heim C, Shugart M, Craighead WE, Nemeroff CB. Neurobiological and psychiatric consequences of child abuse and neglect. Developmental psychobiology. 2010;52(7):671–690. doi: 10.1002/dev.20494. [DOI] [PubMed] [Google Scholar]
- 16.Dackis MN, Rogosch FA, Oshri A, Cicchetti D. The role of limbic system irritability in linking history of childhood maltreatment and psychiatric outcomes in low-income, high-risk women: Moderation by FK506 binding protein 5 haplotype. Dev Psychopathol. 2012;24(Special Issue 04):1237–1252. doi: 10.1017/S0954579412000673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Binder EB, Bradley RG, Liu W, et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299(11):1291–305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jovanovic T, Blanding NQ, Norrholm SD, et al. Childhood abuse is associated with increased startle reactivity in adulthood. Depress Anxiety. 2009;26(11):1018–26. doi: 10.1002/da.20599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Almli LM, Mercer KB, Kerley K, et al. ADCYAP1R1 genotype associates with post-traumatic stress symptoms in highly traumatized African-American females. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2013;162(3):262–272. doi: 10.1002/ajmg.b.32145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dannlowski U, Kugel H, Huber F, et al. Childhood maltreatment is associated with an automatic negative emotion processing bias in the amygdala. Human Brain Mapping. 2012 doi: 10.1002/hbm.22112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dannlowski U, Stuhrmann A, Beutelmann V, et al. Limbic scars: Long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry. 2012;71(4):286–293. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 22.Grant MM, Cannistraci C, Hollon SD, et al. Childhood trauma history differentiates amygdala response to sad faces within MDD. Journal of Psychiatric Research. 2011;45(7):886–895. doi: 10.1016/j.jpsychires.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herringa RJ, Phillips ML, Fournier JC, et al. Childhood and adult trauma both correlate with dorsal anterior cingulate activation to threat in combat veterans. Psychol Med. 2013;43(07):1533–1542. doi: 10.1017/S0033291712002310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Birn RM, Patriat R, Phillips ML, et al. Childhood maltreatment and combat posttraumatic stress differentially predict fear-related fronto-subcortical connectivity. Depress Anxiety. 2014;31(10):880–92. doi: 10.1002/da.22291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grant MM, Wood K, Sreenivasan K, et al. Influence of Early Life Stress on Intra- and Extra-Amygdaloid Causal Connectivity. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teicher MH, Anderson CM, Polcari A. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proceedings of the National Academy of Sciences. 2012;109(9):E563–E572. doi: 10.1073/pnas.1115396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bremner JD, Randall P, Vermetten E, et al. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse—a preliminary report. Biological Psychiatry. 1997;41(1):23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bremner JD, Vythilingam M, Vermetten E, et al. MRI and PET Study of Deficits in Hippocampal Structure and Function in Women With Childhood Sexual Abuse and Posttraumatic Stress Disorder. American Journal of Psychiatry. 2003;160(5):924–932. doi: 10.1176/appi.ajp.160.5.924. [DOI] [PubMed] [Google Scholar]
- 29.Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behav. 2012;106(1):29–39. doi: 10.1016/j.physbeh.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 30.Tomoda A, Suzuki H, Rabi K, et al. Reduced prefrontal cortical gray matter volume in young adults exposed to harsh corporal punishment. Neuroimage. 2009;47:T66–T71. doi: 10.1016/j.neuroimage.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carrion VG, Garrett A, Menon V, et al. Posttraumatic stress symptoms and brain function during a response-inhibition task: an fMRI study in youth. Depression and Anxiety. 2008;25(6):514–526. doi: 10.1002/da.20346. [DOI] [PubMed] [Google Scholar]
- 32.Mueller SC, Maheu FS, Dozier M, et al. Early-life stress is associated with impairment in cognitive control in adolescence: an fMRI study. Neuropsychologia. 2010;48(10):3037–3044. doi: 10.1016/j.neuropsychologia.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lim L, Hart H, Mehta MA, et al. Neural Correlates of Error Processing in Young People With a History of Severe Childhood Abuse: An fMRI Study. American Journal of Psychiatry. 2015 doi: 10.1176/appi.ajp.2015.14081042. appi. ajp. 2015.14081042. [DOI] [PubMed] [Google Scholar]
- 34.Navalta CP, Polcari A, Webster DM, et al. Effects of childhood sexual abuse on neuropsychological and cognitive function in college women. J Neuropsychiatry Clin Neurosci. 2006;18(1):45–53. doi: 10.1176/jnp.18.1.45. [DOI] [PubMed] [Google Scholar]
- 35.Elton A, Tripathi SP, Mletzko T, et al. Childhood maltreatment is associated with a sex-dependent functional reorganization of a brain inhibitory control network. Human brain mapping. 2014;35(4):1654–1667. doi: 10.1002/hbm.22280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez N, Ryan SW, Rowan AB, Foy DW. Posttraumatic stress disorder in a clinical sample of adult survivors of childhood Sexual Abuse. Child Abuse & Neglect. 1996;20(10):943–952. doi: 10.1016/0145-2134(96)00083-x. [DOI] [PubMed] [Google Scholar]
- 37.Ullman SE, Brecklin LR. Sexual assault history, PTSD, and mental health service seeking in a national sample of women. Journal of Community Psychology. 2002;30(3):261–279. [Google Scholar]
- 38.Gillespie CF, Bradley B, Mercer K, et al. Trauma exposure and stress-related disorders in inner city primary care patients. Gen Hosp Psychiatry. 2009;31(6):505–14. doi: 10.1016/j.genhosppsych.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hines LA, Sundin J, Rona RJ, et al. Posttraumatic Stress Disorder Post Iraq and Afghanistan: Prevalence Among Military Subgroups. Canadian journal of psychiatry Revue canadienne de psychiatrie. 2014;59(9):468–479. doi: 10.1177/070674371405900903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leibenluft E, Rich B, Vinton D, et al. Neural circuitry engaged during unsuccessful motor inhibition in pediatric bipolar disorder. American Journal of Psychiatry. 2007;164(1):52–60. doi: 10.1176/ajp.2007.164.1.A52. [DOI] [PubMed] [Google Scholar]
- 41.Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 42.American Psychiatric Association. Diagnostic and statistical manual of mental disorders, (DSM-5®) American Psychiatric Pub; 2013. [Google Scholar]
- 43.Scher CD, Stein MB, Asmundson GJG, et al. The Childhood Trauma Questionnaire in a community sample: Psychometric properties and normative data. J Trauma Stress. 2001;14(4):843–857. doi: 10.1023/A:1013058625719. [DOI] [PubMed] [Google Scholar]
- 44.Bernstein DP, Fink L, Handelsman L, et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. American Journal of Psychiatry. 1994;151(8):1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- 45.Foa EB, Tolin DF. Comparison of the PTSD Symptom Scale-Interview Version and the Clinician-Administered PTSD scale. J Trauma Stress. 2000;13(2):181–91. doi: 10.1023/A:1007781909213. [DOI] [PubMed] [Google Scholar]
- 46.Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin Psychol Rev. 1988;8(1):77–100. [Google Scholar]
- 47.Saunders JB, Aasland OG, Babor TF, et al. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction. 1993;88(6):791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 48.Yudko E, Lozhkina O, Fouts A. A comprehensive review of the psychometric properties of the Drug Abuse Screening Test. Journal of substance abuse treatment. 2007;32(2):189–198. doi: 10.1016/j.jsat.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 49.Connor KM, Davidson JR. Development of a new resilience scale: The Connor-Davidson resilience scale (CD-RISC) Depression and anxiety. 2003;18(2):76–82. doi: 10.1002/da.10113. [DOI] [PubMed] [Google Scholar]
- 50.Holmes AP, Friston KJ. Generalisability, random effects and population inference. Neuroimage. 1998;7:S754. [Google Scholar]
- 51.Conigrave KM, Hall WD, Saunders JB. The AUDIT questionnaire: choosing a cut-off score. Addiction. 1995;90(10):1349–1356. doi: 10.1046/j.1360-0443.1995.901013496.x. [DOI] [PubMed] [Google Scholar]
- 52.Cross D, Crow T, Powers A, Bradley B. Childhood trauma, PTSD, and problematic alcohol and substance use in low-income, African-American men and women. Child abuse & neglect. 2015 doi: 10.1016/j.chiabu.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46(1):224–232. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Medford N, Critchley H. Conjoint activity of anterior insular and anterior cingulate cortex: awareness and response. Brain Structure and Function. 2010;214(5–6):535–549. doi: 10.1007/s00429-010-0265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function. 2010;214(5–6):655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.White MG, Bogdan R, Fisher PM, et al. FKBP5 and emotional neglect interact to predict individual differences in amygdala reactivity. Genes Brain Behav. 2012;11(7):869–78. doi: 10.1111/j.1601-183X.2012.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tottenham N, Hare TA, Millner A, et al. Elevated amygdala response to faces following early deprivation. Developmental Science. 2011;14(2):190–204. doi: 10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maheu F, Dozier M, Guyer A, et al. A preliminary study of medial temporal lobe function in youths with a history of caregiver deprivation and emotional neglect. Cognitive, Affective, & Behavioral Neuroscience. 2010;10(1):34–49. doi: 10.3758/CABN.10.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gee DG, Gabard-Durnam LJ, Flannery J, et al. Early developmental emergence of human amygdala–prefrontal connectivity after maternal deprivation. Proceedings of the National Academy of Sciences. 2013;110(39):15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dickie EW, Brunet A, Akerib V, Armony JL. Neural correlates of recovery from post-traumatic stress disorder: A longitudinal fMRI investigation of memory encoding. Neuropsychologia. 2011;49(7):1771–1778. doi: 10.1016/j.neuropsychologia.2011.02.055. [DOI] [PubMed] [Google Scholar]
- 61.Aupperle RL, Allard CB, Simmons AN, et al. Neural responses during emotional processing before and after cognitive trauma therapy for battered women. Psychiatry Res. 2013;214(1):48–55. doi: 10.1016/j.pscychresns.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 62.Felmingham K, Kemp A, Williams L, et al. Changes in anterior cingulate and amygdala after cognitive behavior therapy of posttraumatic stress disorder. Psychological Science. 2007;18(2):127–129. doi: 10.1111/j.1467-9280.2007.01860.x. [DOI] [PubMed] [Google Scholar]
- 63.Seedat S, Warwick J, van Heerden B, et al. Single photon emission computed tomography in posttraumatic stress disorder before and after treatment with a selective serotonin reuptake inhibitor. J Affect Disord. 2004;80(1):45–53. doi: 10.1016/S0165-0327(03)00047-8. [DOI] [PubMed] [Google Scholar]
- 64.Roy MJ, Francis J, Friedlander J, et al. Improvement in cerebral function with treatment of posttraumatic stress disordera. Annals of the New York Academy of Sciences. 2010;1208(1):142–149. doi: 10.1111/j.1749-6632.2010.05689.x. [DOI] [PubMed] [Google Scholar]
- 65.Kasai K, Yamasue H, Gilbertson MW, et al. Evidence for acquired pregenual anterior cingulate gray matter loss from a twin study of combat-related posttraumatic stress disorder. Biol Psychiatry. 2008;63(6):550–6. doi: 10.1016/j.biopsych.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. The Journal of Neuroscience. 2001;21(18):165RC. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: Reappraisal and suppression of negative emotion. Biological Psychiatry. 2008;63(6):577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43(6):897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 70.Jovanovic T, Ressler KJ. How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. Am J Psychiatry. 2010;167(6):648–62. doi: 10.1176/appi.ajp.2009.09071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Rooij SJ, Rademaker AR, Kennis M, et al. Impaired right inferior frontal gyrus response to contextual cues in male veterans with PTSD during response inhibition. Journal of psychiatry & neuroscience: JPN. 2014;39(5):330. doi: 10.1503/jpn.130223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Critchley HD, Tang J, Glaser D, et al. Anterior cingulate activity during error and autonomic response. Neuroimage. 2005;27(4):885–895. doi: 10.1016/j.neuroimage.2005.05.047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.