Abstract
Background
Although many studies have analyzed the association of 3′-untranslated region (UTR) variable number tandem repeat (VNTR) polymorphism in SLC6A3 with alcohol dependence (AD), the results remain controversial. This study aimed to determine whether this variant indeed has any genetic effect on AD by integrating 17 reported studies with 5,929 participants included.
Methods
The A9-dominant genetic model that considers A9-repeat and non-A9 repeat as two genotypes and compared their frequencies in alcoholics with that in controls was adopted. Considering the potential influence of ethnicity, differences in diagnostic criteria of AD, and alcoholic subgroups, stratified meta-analyses were conducted. There existed no evidence for the presence of heterogeneity among the studied samples, indicating the results under the fixed-effects model are acceptable.
Results
We found a significant association of VNTR A9 genotypes with AD in all ethnic populations (pooled odds ratio [OR] 1.12; 95% confidence interval [CI] 1.00, 1.25; P=0.045) and the Caucasian population (pooled OR 1.15; 95% CI 1.01, 1.31; P=0.036). We also found VNTR A9 genotypes to be significantly associated with alcoholism as defined by the DSM-IV criteria (pooled OR 1.18; 95% CI 1.03, 1.36; P=0.02). Further, we found a significant association between VNTR A9 genotypes and alcoholism associated with alcohol withdrawal seizure or delirium tremens (pooled OR 1.55; 95% CI 1.24, 1.92; P=1.0×10−4). In all these meta-analyses, no evidence of publication bias was detected.
Conclusions
We concluded that the VNTR polymorphism has an important role in the etiology of AD, and individuals with at least one A9 allele are more likely to be dependent on alcohol than persons carrying the non-A9 allele.
Keywords: 3′-UTR VNTR, SLC6A3, meta-analysis, alcoholism, alcohol dependence, polymorphism
Introduction
Alcoholism is one of the most prevalent psychiatric disorders. Data from the World Health Organization (WHO) show that there were approximately 2 billion alcohol abusers worldwide in 2004 (WHO, 2008). Alcohol dependence (AD) is considered a “reward deficiency syndrome” that severely influences public health (Comings and Blum, 2000, Parry et al., 2011). The heritability of alcoholism is estimated to be moderate to high, ranging from 50% to 60% (Heath et al., 1997, Goldman et al., 2005)
The dopaminergic reward circuit plays an important role in drug abuse and subsequent dependence. Particularly, the dopaminergic mesocorticolimbic reward pathways have been linked to the pathogenesis of addictions, including alcoholism. Consequently, genes with a dopaminergic function have received much attention in relation to the etiology of AD and other addictions (Muramatsu and Higuchi, 1995, van der Zwaluw et al., 2009, Ma et al., 2014, Ma et al., 2015a).
There are three functional parts in this reward system: dopamine transporters, receptors, and enzyme targets. The dopamine transporters and pre-synaptic and post-synaptic receptors collectively modulate the concentration of dopamine in the synaptic cleft. Alcohol could take advantage of this system to increase synaptic dopamine to a higher concentration than is stimulated by natural rewards, such as sexual intercourse and food. There has been increasing evidence of an association of dysfunction of the dopaminergic neurotransmission with craving for ethanol (Wise, 1988) and alcohol withdrawal symptoms (Heinz et al., 1996). Thus, variants in those genes of the dopaminergic system likely convey vulnerability to alcoholism.
A membrane-spanning protein of DAT, encoded by SLC6A3 (also called DAT1) on chromosome 5p15.3, is a Na+- and Cl−- dependent dopamine transporter. By making use of its free energy from the transmembrane concentration gradients of Na+ and Cl− generated by Na+/K+ ATPase and potassium chloride symport (Pramod et al., 2013), the dopamine transporter can take up extracellular dopamine into the dopaminergic presynaptic neurons, an important approach to terminating the signal of dopamine neurotransmission. According to neuroimaging studies, the extent of expression of SLC6A3 is influenced by chronic alcohol use (Laine et al., 1999), although the results of these studies have been somewhat inconsistent (Volkow et al., 1996).
Of the variants in SLC6A3, a 40-basepair variable-number tandem repeat (VNTR) in the 3′-UTR of the gene has been investigated extensively. For the VNTR, a number ranging from 3 to 16 has been documented in various ethnic populations, with the A9 and A10 alleles being the most common. Functional in vitro and in vivo studies have shown that the VNTR genotypes are highly correlated with decreased SLC6A3 availability (Heinz et al., 2000, Mill et al., 2002), which may slack the biological function of DAT reuptake with increasing the concentrations of dopamine in synaptic cleft. Further, functional magnetic resonance imaging (fMRI) studies on healthy subjects without any diagnosed mental disorders demonstrated that A9 homozygote or heterozygote carriers had a greater brain ventral striatal response to both anticipation and receipt of reward than that of A10 homozygote carriers (Dreher et al., 2009, Forbes et al., 2009). In addition, several meta-analyses, including the one recently reported by our group, have shown that the SLC6A3 VNTR is significantly associated with smoking cessation (Ma et al., 2015b) and various psychiatric disorders (Yang et al., 2007, Kambeitz et al., 2014), which are all correlated with AD (Ma et al., 2014, Li and Burmeister, 2009). Therefore, it is plausible to infer that the VNTR genotypes confer susceptibility to alcoholism.
Since Muramatsu et al. (1995) first examined the association between SLC6A3 VNTR polymorphism and AD, many studies have been carried out (Sander et al., 1997, Ueno et al., 1999, Wernicke et al., 2002, Gorwood et al., 2003, Heinz et al., 2000, Kohnke et al., 2005, Le Strat et al., 2008, Bau et al., 2001, Chen et al., 2001, Dobashi et al., 1997, Ivashchenko et al., 2015, Mignini et al., 2012, Nedic Erjavec et al., 2014, Vasconcelos et al., 2015, Bhaskar et al., 2012, Sery et al., 2015, Foley et al., 2004, Wu et al., 2009), unfortunately with inconclusive results (van der Zwaluw et al., 2009). So far, there have been two meta-analyses (Xu and Lin, 2011, Du et al., 2011) regarding the association between SLC6A3 VNTR genotypes and alcoholism. After combining 13 studies with 2,483 cases and 1,753 controls, Xu and Lin (2011) found no significant association of this polymorphism with AD. Similarly, Du et al. (2011) could not find any significant difference between alcoholics and controls after meta-analyzing 13 reported studies with 2,353 cases and 1,776 controls. However, when considering the influence of alcoholic subtypes, Du et al. (2011) found VNTR A9 to be significantly associated with alcohol withdrawal seizures (AWS) or delirium tremens (DT) at both the genotypic and allelic levels based on the samples with all ethnic ancestries or only Caucasian ancestry.
Thus, it seems that these conflicting results can be attributed to the definition and selection of cases with AD, sampling variation, sample size, and other potentially confounding factors. Further, with several more corresponding studies published recently, we carried out this study with the primary goal of determining the effect of this polymorphism on the pathogenesis of AD by considering the influence of ethnicity, the diagnostic criteria of AD, and the subtype of alcoholism.
Materials and Methods
Search strategy and inclusion criteria
We searched published papers on the association between SLC6A3 3′-UTR VNTR and alcoholism by interrogating the database of PubMed (http://www.ncbi.nlm.nih.gov/pubmed) from the first date available up to 13 October 2015. The following key words were used: “dopamine transporter,” “DAT,” “DAT1,” and “SLC6A3” combined with “alcohol dependence,” “AD,” and “alcoholism.” All electronic abstracts of the recovered papers were examined in compliance with the standard inclusion and exclusion criteria suggested by Moher et al. (2009), and the references were hand-checked to identify additional studies not indexed by PubMed.
Study selection and data synthesis
According to the “Quality of Reporting of Meta-Analysis and PRISMA guidelines” (Moher et al., 2009), 24 studies were retrieved for possible inclusion after a stringently systematic review (Supplementary Table S1). Only those studies that meet the following five criteria were included: (1) a peer-reviewed publication; (2) a case-control design (i.e., family-based studies were excluded) (3) original genotype or allele frequency available; (4) independent from the others (i.e., duplications were discarded, and studies with previously published data were excluded); (5) sufficient information to compute an odds ratio (OR) and 95% confidence interval (CI). For each study, by using a standardized spreadsheet, two authors (Y. Ma and R. Fan) independently performed extraction of the following information: authors and year of publication, language (English or other), population ancestry origin, ethnicity/race, type of study, sample size, sex ratio in both cases and controls, state of Hardy-Weinberg equilibrium, diagnostic criteria for AD, and AD status categorized by the VNTR genotypes (see Table 1 and Supplementary Table S1).
Table 1.
Specific number of 3′-UTR VNTR genotypes/alleles in controls and alcoholics for this meta-analysis
| Study names | Alcoholics/Controls (Genotypes) | Alcoholics/Controls (Alleles) | |||
|---|---|---|---|---|---|
| A9/A9 | A9/A* | A*/A* | A9 | A* | |
| Sander et al. (1997) | 19/5 | 107/25 | 167/63 | 145/35 | 441/151 |
| Ueno et al. (1999) | 0/0 | 18/8 | 106/99 | 18/8 | 230/206 |
| Heinz et al. (2000) | 0/0 | 8/4 | 9/8 | 8/4 | 26/20 |
| Bau et al. (2001) | 9/5 | 31/45 | 74/62 | 49/55 | 179/169 |
| Chen et al. (2001) (Atayal) | 0/1 | 6/3 | 35/27 | 6/5 | 76/57 |
| Chen et al. (2001) (Ami) | 0/0 | 1/5 | 25/18 | 1/5 | 51/41 |
| Chen et al. (2001) (Bunun) | 0/1 | 21/15 | 35/40 | 21/17 | 91/95 |
| Chen et al. (2001) (Paiwan) | 0/0 | 1/3 | 35/31 | 1/3 | 71/65 |
| Chen et al. (2001) (Han) | 1/1 | 11/7 | 56/51 | 13/9 | 123/109 |
| Wernicke et al. (2002) | 21/25 | 147/125 | 183/186 | 189/175 | 513/497 |
| Gorwood et al. (2003) | 10/9 | 52/26 | 58/30 | 72/44 | 168/86 |
| Foley et al. (2004) | 4/6 | 22/14 | 35/23 | 30/26 | 92/60 |
| Kohnke et al. (2005) | 104/33 | 112/69 | NR/NR | NR/NR | |
| Samochowiec et al. (2008) | 6/10 | 35/51 | 81/89 | 47/71 | 197/229 |
| Le Strat et al. (2008) | 16/15 | 86/47 | 130/59 | 118/77 | 346/165 |
| Du et al. (2011) | 13/8 | 107/87 | 238/230 | 133/103 | 583/547 |
| Bhashar et al. (2012) (Badaga) | 16/5 | 20/18 | 45/38 | 52/28 | 110/94 |
| Bhashar et al. (2012) (Kota) | 6/6 | 54/50 | 51/39 | 66/62 | 156/128 |
| Mignini et al. (2012) | 36/28 | 114/111 | 128/141 | 186/167 | 370/393 |
| Anton et al. (2012) | 12/18 | 12/41 | NR/NR | NR/NR | |
| Vasconcelos et al. (2015) | 10/2 | 39/32 | 64/80 | 59/36 | 167/192 |
| Sery et al. (2015) | 22/17 | 158/141 | 252/235 | 202/178 | 662/605 |
Note: There were five ethnic populations in the study of Chen et al. (2001) including Atayal, Ami, Bunun, Paiwan and Han, and two ethnic populations in study of Bhashar et al. (2012) including Badaga and Kota, which were independently analyzed in current meta-analysis.
Classification of phenotypes and genotypes
For all studies, several criteria were adopted for diagnosing the syndrome of AD, which include those of the Diagnostic and Statistical Manual of Mental Disorders, Revised Third Edition (DSM-III-R), the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV), the International Classification of Diseases 10th revision (ICD-10), and the Modified Feighner Criteria (MFC). Because SLC6A3 VNTR A9 is believed to exert its effect through a genetic mode of dominance (Heinz et al., 2000), VNTR genotypes were mainly analyzed under a dominant model (Dreher et al., 2009, Forbes et al., 2009, Ma et al., 2015b). In all our data, SLC6A3 VNTR genotypes were divided into two categories: A9/A9 and A9/A* genotypes vs. A*/A* genotypes, where A* refers to the A10 or other alleles (except for A9). Thus, an A9-dominant model, which assumes that carriers are more susceptible to AD than are non-carriers, was adopted for this meta-analysis, where we compared the frequencies of A9/A9 and A9/A* genotypes in alcoholism groups with that in control subjects. In addition, several studies (Le Strat et al., 2008, Wernicke et al., 2002, Gorwood et al., 2003, Kohnke et al., 2005, Sander et al., 1997, Du et al., 2011) were performed to reveal the relations between SLC6A3 VNTR genotypes and alcoholic subtypes such as alcohol withdrawal seizure (AWS) and delirium tremens (DT); both groups are considered more homogeneous or genetically determined (Du et al., 2011, Walters, 2002, Schuckit et al., 2003).
Statistical analysis
We first incorporated all the data from the chosen studies to implement an overall analysis. Then we conducted stratified meta-analyses considering (1) the influence of ethnicity: Caucasian, Asian, and mixed ancestry (including Brazilian and Mexican); (2) the difference in the diagnostic criteria for AD: DSM-IV, DSM-III-R, and ICD-10; and (3) the influence of alcoholic subtypes: persons with AWS or DT vs. controls. All reported meta-analyses were carried out with the Comprehensive Meta-Analysis statistical software package (v. 2.0; Biostat Inc., Englewood, NJ, USA).
Both fixed-effects and random-effects models were applied. For the fixed-effects analyses, the effect size of each individual study was integrated using inverse variance methods (Laird and Mosteller, 1990) to generate a pooled OR and 95% confidence interval (CI) by assuming that the effect of genotype is constant across studies and the detected variation is attributable to random variation. For the random-effects model, the effect sizes of individual studies were pooled using the DerSimonian and Larid method (1986), which assumes that the heterogeneity among studies is attributable to both between- and within-study variation. Compared with the fixed-effects model, the random-effects model is more conservative and produces a broad CI. Thus, when there exists no significant heterogeneity among studies, the fixed-effects model tends to be more appropriate; otherwise, the random-effects model should be used. The significance of a pooled OR is determined by a Z statistical test, and P < 0.05 is considered significant.
Both Cochran’s Q and I2 statistical tests were used to evaluate the potent heterogeneity among studies. The heterogeneity for all studies is considered statistically significant when PQ < 0.05 and can be quantified by the metric of I2 (I2 = Q − d.f./Q), which is used to determine the percentage of variation across studies that is caused by heterogeneity rather than by chance. For example, if the I2 equals to 50%, the results from meta-analysis can inflate the percentage of variation across studies not explained by genotype to 50%. We used the method introduced by Hedges et al. (Hedges and Pigott, 2001, Hedges and Pigott, 2004) to calculate the statistical power for current meta-analysis.
Publication bias was graphically and statistically assessed using funnel plots and the Egger regression test (Egger et al., 1997). The Egger’s funnel plot utilizes a linear regression approach to measure the funnel plot asymmetry on the natural logarithm of the OR, where the deviation of each study larger than the funnel-shaped distribution, the funnel plot is predisposed to be more prominently asymmetric, indicating a possibility of publication bias.
Results
Basic information on the included studies
A total of 24 studies were published between 1995 and 2015 regarding the association of 3′-UTR VNTR genotypes with AD (Supplementary Table S1). After excluding the nonapplicable data, we included 17 studies with 22 cohorts and 5,929 participants (3,280 alcoholics and 2,649 controls) under the A9-dominant model. The sample size in the studies differed greatly, ranging from 25 to 841.
Among the studies, 12 were based predominantly on persons of Caucasian ancestry (2,265 alcoholics and 1,762 controls), 3 studies with 8 cohorts were based on Asian ancestry (543 alcoholics and 450 controls), and 2 studies included mixed ancestry groups, including Brazilian and Mexican (472 alcoholics and 437 controls). In the Caucasian samples, the frequency of A9 genotypes was 43.9%, which is remarkably higher than that in the Asian population (28.0%) and the mixed population (33.6%). Genotype distributions and other basic information of the included studies are shown in Tables 1 and 2.
Table 2.
Specific number of 3′-UTR VNTR genotypes/alleles in controls and alcoholics with withdrawal seizure or delirium tremens
| Study names | Cases/Controls (Genotypes) | Cases/Controls (Alleles) | |||
|---|---|---|---|---|---|
| A9/A9 | A9/A* | A*/A* | A9 | A* | |
| Sander et al. (1997) | 10/5 | 40/25 | 43/63 | 60/35 | 126/151 |
| Wernicke et al. (2002) | 75/150 | 59/186 | NR/NR | NR/NR | |
| Gorwood et al. (2003) | 4/9 | 19/26 | 11/30 | 27/44 | 41/86 |
| Kohnke et al. (2005) | 56/33 | 63/69 | NR/NR | NR/NR | |
| Le Strat et al. (2008) | 8/15 | 18/47 | 33/59 | 34/77 | 113/165 |
| Du et al. (2011) | 3/8 | 38/87 | 69/230 | 44/103 | 176/547 |
Association between VNTR A9 genotypes and AD based on ethnicity
We first conducted a meta-analysis to determine the association between the VNTR A9 genotypes and AD by combining all the studies. The datasets from the studies reported by Chen et al. (2001) and Bhashar et al. (2012) were divided into several groups according to ancestral origin (Table 1). The results from the Cochran’s Q and I2 statistical test showed that there was marginal evidence of heterogeneity among studies (I2 = 32.8%; PQ = 0.07). The pooled OR was 1.12 (P = 0.045) with the 95% CI from 1.00 to 1.25 under the fixed-effects model (Fig. 1) and 1.12 (P = 0.13) with 95% CI from 0.97 to 1.30 under the random-effects model (Table 3), suggesting that the VNTR A9 genotypes confer a higher risk of AD.
Figure 1.
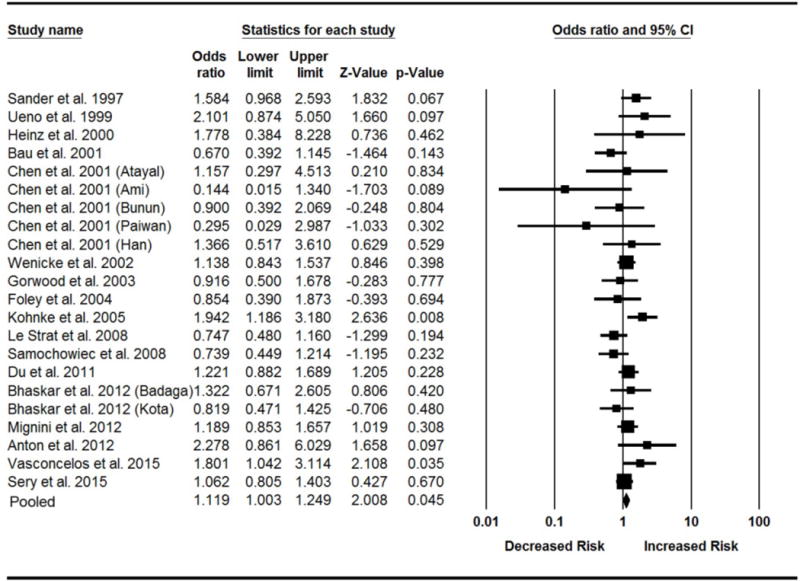
Forest plot of the meta-analysis results for the association of 3′-UTR VNTR A9 genotypes with AD in all ethnic populations. The Z value and P value of each eligible study are displayed by rows. The central vertical solid line stands for ORs equal to 1 for the null hypothesis. The OR and 95% CI of each study are represented by the square and horizontal bar, respectively. The pooled OR, which is represented by the diamond symbol underneath the forest plot, was calculated under the fixed-effects model.
Table 3.
Results from the meta-analysis under an A9-dominant model for alcohol dependence stratified by different datasets
| Datasets of meta-analysis (A9/A9+A9/A* vs. A*/A*) | Cohorts (n) | Sample size (N) | Estimate of heterogeneity | Fixed-effects model | Random-effects model | Egger (j) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| I2 (%) | PQ | Pooled OR | 95% CI | PZ | Pooled OR | 95% CI | PZ | PE | |||
| Data based on all ethnic population(a) | 22 | 5,929 | 32.8 | 0.07 | 1.12 | 1.00–1.25 | 0.045** | 1.12 | 0.97–1.30 | 0.13 | 0.74 |
| Data based on Caucasian population(b) | 12 | 4,027 | 41.9 | 0.06 | 1.15 | 1.01–1.31 | 0.036** | 1.17 | 0.97–1.41 | 0.097 | 0.45 |
| Data based on Asian population(c) | 8 | 993 | 15.2 | 0.31 | 1.06 | 0.77–1.45 | 0.73 | 1.06 | 0.74–1.52 | 0.74 | 0.33 |
| Data based on other population(d) | 2 | 909 | 71.6 | 0.06 | 1.04 | 0.79–1.37 | 0.79 | 0.94 | 0.53–1.69 | 0.84 | NA |
| Data based on DSM-IV(e) | 10 | 3,457 | 39.0 | 0.10 | 1.18 | 1.03–1.36 | 0.02** | 1.21 | 1.00–1.47 | 0.05** | 0.28 |
| Data based DSM-III-R(f) | 8 | 1,082 | 22.9 | 0.25 | 0.91 | 0.67–1.23 | 0.54 | 0.93 | 0.64–1.35 | 0.70 | 0.66 |
| Data based ICD-10(g) | 3 | 1,345 | 56.6 | 0.10 | 1.12 | 0.89–1.40 | 0.35 | 1.11 | 0.76–1.60 | 0.59 | 0.93 |
| Data based on alcoholic subgroups(h) | 6 | 1,591 | 37.9 | 0.15 | 1.55 | 1.24–1.92 | 1.0×10−4** | 1.55 | 1.16–2.06 | 0.0028** | 0.996 |
| Data based on alcoholic subgroups(i) | 5 | 1,411 | 0.0 | 0.70 | 1.71 | 1.36–2.16 | 1.0×10−5** | 1.71 | 1.36–2.16 | 1.0×10−5** | 0.34 |
Note:
Statistically significant value. 95% CI indicates the 95% confidence interval. NA: Not Applicable.
Analysis of the data combined all ethnic population from included studies.
Analysis of the data only based on Caucasian population.
Analysis of the data only based on Asian population.
Analysis of the data only based on Brazilian and Mexican population.
Analysis of the data that the criteria of the alcohol dependence syndrome according to DSM-IV.
Analysis of the data that the criteria of the alcohol dependence syndrome according to DSM-III-R.
Analysis of the data that the criteria of the alcohol dependence syndrome according to ICD-10.
Analysis of the data that contain the subgroups of alcoholics with withdrawal seizure or delirium tremens and controls.
Analysis of the data that contain the subgroups of alcoholics with withdrawal seizure or delirium tremens and controls (excluded the study of Le Strat et al., 2008)
Egger regression test for publication bias.
By considering the genotype frequency differences in ethnicity, we performed three stratified meta-analyses, combining the data based on Caucasian, Asian, and other mixed populations. With regard to the Caucasian population, the pooled OR was 1.15 (95% CI 1.01, 1.31; P = 0.036) under the fixed-effects model (Fig. 2). Similar to all ethnic samples, tests for heterogeneity also showed marginal evidence in the Caucasian population (I2 = 41.9%; PQ= 0.06). However, when the meta-analysis was carried out under the random-effects model, the pooled OR was 1.17 with a 95% CI from 0.97 to 1.41 (P = 0.097). There was no significant association in other ethnic populations (Table 3).
Figure 2.

Forest plot of the meta-analysis results for the association of 3′-UTR VNTR A9 genotypes with alcoholism in Caucasian populations. The Z value and P value of each eligible study are displayed by rows. The central vertical solid line stands for ORs equal to 1 for the null hypothesis. The OR and 95% CI of each study are represented by the square and horizontal bar, respectively. The pooled OR, which is represented by the diamond symbol underneath the forest plot, was calculated under the fixed-effects model.
Association between VNTR A9 genotypes and AD based on different diagnostic criteria
In view of the differences in the published criteria for alcoholism, we performed three stratified meta-analyses, separately analyzing the studies based on the DSM-IV, DSM-III-R, and ICD-10. For the studies based on DSM-IV criteria, we found a significant association between VNTR A9 genotypes and AD under the fixed-effects model (pooled OR 1.18; 95% CI 1.03, 1.36; P = 0.02; see Fig. 3). The P value of the Q statistical test was 0.10, indicating that there was no significant between-study heterogeneity; however, there still was some variability across studies (I2 = 39.0%). When we performed the meta-analysis again under the random-effects model, we found a significant association of the variant with AD (pooled OR 1.21; 95% CI 1.00, 1.47; P = 0.05). No significant association was detected in the subgroups according to the diagnostic criteria of DSM-III-R and ICD-10 (Table 3).
Figure 3.

Forest plot of the meta-analysis results for the association of 3′-UTR VNTR A9 genotypes with alcoholism based on the diagnostic criteria of DSM-IV. The Z value and P value of each eligible study are displayed by rows. The central vertical solid line stands for ORs equal to 1 for the null hypothesis. The OR and 95% CI of each study are represented by the square and horizontal bar, respectively. The pooled OR, which is represented by the diamond symbol underneath the forest plot, was calculated under the fixed-effects model.
Association between VNTR A9 genotypes and AD within the alcoholic subgroups
By taking into account the influence of severity of AD, we carried out a meta-analysis for determining the effect of VNTR A9 genotypes within a subgroup of alcoholics with AWS or DT. A total of six studies with 549 alcoholics had the recorded information of severe withdrawal symptoms (Table 2). With a meta-analysis of all the data from these six studies, we found a significant association between VNTR A9 genotypes and alcoholics with AWS or DT under both the fixed-effects model (pooled OR 1.55; 95% CI 1.24, 1.92; P = 1.0 × 10−4) and the random-effects model (pooled OR 1.55; 95% CI 1.16, 2.06; P = 0.0028). There was no significant evidence of between-study heterogeneity (I2 = 37.9%; PQ = 0.15). The forest plot of this meta-analysis under the fixed-effects model is shown in Figure 4.
Figure 4.

Forest plot of the meta-analysis results for the association of 3′-UTR VNTR A9 genotypes with alcoholism based on alcoholism subtype. The Z value and P value of each eligible study are displayed by rows. The central vertical solid line stands for ORs equal to 1 for the null hypothesis. The OR and 95% CI of each study are represented by the square and horizontal bar, respectively. The pooled OR, which is represented by the diamond symbol underneath the forest plot, was calculated under the fixed-effects model.
Assessment of publication bias
The methods of Egger’s linear regression test and funnel plot were applied to assess the possible publication bias for all the meta-analyses. As presented in Table 3, all the P values from the Egger regression test were > 0.05, indicating that there was no significant publication bias in our meta-analyses. The funnel plots for these meta-analyses based on all ethnic populations, Caucasian populations, DSM-IV, and alcoholic subgroups were symmetrical (Supplementary Figure S1a–S1d), providing further evidence that no publication bias exists for the meta-analyses.
Results from sensitivity and retrospective analyses
We also performed a sensitivity analysis for the overall population under the fixed-effects model to determine whether the significant association detected between VNTR A9 and alcoholism was prominently influenced by any specific study. Each study was removed in turn from the total, and the remaining studies were re-analyzed, the method being used to determine whether any individual study contributed primarily to the pooled effect size. As displayed in Supplementary Figure S2, the pooled ORs ranged from 1.10 to 1.15, indicating that the results of our meta-analyses were not changed substantially by removal of any of the studies. The accumulated results from the retrospective analysis for the overall populations under a fixed-effects model showed that the pooled OR was prone to stability as the publication year increased (Fig. 5).
Figure 5.

Plot of retrospective analysis results. The pooled effect size of the 3′-UTR VNTR polymorphism for the possibility of alcoholism was plotted against the publication year across all studies. The Y axis stands for the pooled OR and the X axis for the publication year relative to the pooled effect size. The diamond symbols indicate the pooled effect size, and each vertical line with horizontal bars represents the 95% CI.
Discussion
The present study advances our understanding of the effect of the SLC6A3 VNTR polymorphism on AD using a more comprehensive and detailed meta-analysis of all reported studies. Although there was no significant association observed in previous two meta-analyses (Xu and Lin, 2011, Du et al., 2011), which probably was attributable to the insufficient sample sizes or the unclear classification of AD cases, we found a significant association of VNTR A9 genotypes with AD with a larger sample by considering the influence of ethnicity and diagnostic criteria for AD. We detected an even more significant association between VNTR A9 genotypes and AWS or DT, which is consistent with the results of Du et al. (2011). We thus concluded that the VNTR A9 allele plays an important role in the etiology of AD, indicating that carriers of these genotypes have a higher likelihood of being alcoholics than those who carry A*/A* genotypes.
The dopamine transporter is a well-documented molecular target of potent psychoactive compounds, including cocaine, nicotine, ethanol, and amphetamine derivatives, indicating that modulation of the concentration of extracellular dopamine is important in various physiological processes (Pramod et al., 2013, Iversen, 2006). Very recently, Hirth et al. (2016) showed a strong down-regulation of the D1 receptor- and dopamine transporter-binding sites in postmortem brain samples from human alcoholics. Furthermore, many genetic studies have demonstrated an association of variants in SLC6A3 with addiction-related behaviors (Ma et al., 2015b, van der Zwaluw et al., 2009) and neuropsychiatric disorders (Gill et al., 1997, Cornish et al., 2005). In particular, the VNTR polymorphism has been studied extensively for AD-related phenotypes. However, the results remain ambiguous, which probably is attributable to sampling bias, different diagnostic criteria of alcoholism, low statistical power, the variability of the selection of AD cases, or some combination thereof. Significantly, accumulating evidence has indicated that association studies based on small samples have insufficient statistical power, causing pronouncedly contradictory findings (Yang et al., 2007). A meta-analysis-based approach, which is an effective way to enhance the statistical power and examine the diversity of studies, should be applied to incorporate the data extracted from various studies on the same topic.
In this study, the A9-dominant genetic model was adopted for all the meta-analyses. We also tried other genetic models, such as recessive, additive, and allelic, for our meta-analyses, but no significant evidence for the association was observed (data not shown). In view of previous reports (Dreher et al., 2009, Forbes et al., 2009, Ma et al., 2015b, Heinz et al., 2000) and current findings, it is reasonable to speculate that the A9 allele of 3′-UTR VNTR has a dominant genetic effect on alcoholism. However, it is worthy of mention that low-to-moderate heterogeneity was detected among these studies (I2 value: 32.8%–41.9%), indicating that there might exist other underlying factors such as sampling variation or different definition of AD among these included studies.
We observed no evidence of publication bias in any of the reported stratified meta-analyses in this study. Further, we found no study that deviated markedly from the funnel-shaped plot in each of the meta-analysis, except for the meta-analysis for alcoholics with AWT or DT, where the study reported by Le Strat et al. (2008) was out of the distribution (Supplementary Figure S1d). After application of the Duval and Tweedie trim and fill method by adding missing negative studies, the results of the meta-analysis remain significant, and the adjusted pooled OR was 1.44 (95% CI 1.17, 1.77) under the fixed-effects model and 1.43 (95% CI 1.06, 1.91) under the random-effects model. When we excluded the Le Strat study (Le Strat et al., 2008) and repeated the meta-analysis, the genotypes of VNTR A9 showed a more significant association with alcoholism subtypes under both the fixed- and random-effects models (pooled OR 1.71; 95% CI 1.36, 2.16; P = 1.0 × 10−5; see Supplementary Figure S3). No evidence for between-study heterogeneity was detected, with the P value of the Q test increasing from 0.15 to 0.70 and the I2 metric reduced from 37.9% to 0 (Table 3), providing supportive evidence for the aforementioned claim that underlying heterogeneous factors still exist among the chosen studies. In addition, from sensitivity and retrospective analysis, the results showed that the pooled OR of this meta-analysis was not substantially susceptible to any individual study and gradually predisposed to be stable as publication year increased, lending support to the robustness of the finding that the VNTR A9 genotypes confer vulnerability risk for AD. Based on the sample size used in this study, our power analysis indicated that we have at least 94.1% power to detect the effect of VNTR polymorphism on alcohol dependence (Supplementary Table S2).
There are potential limitations to the current study. First, AD is a complex addictive disorder with a multifactorial pathogenesis and often comorbid with other substance abuse or neuropsychiatric disorders, which likely share common genetic risk factors in the dopaminergic reward system. Also, many of the studies did not provide information allowing us to exclude or include patients with comorbid disorders in the reported sample (van der Zwaluw et al., 2009). Thus, inclusion of alcoholics or controls with these comorbidities might cofound the findings of this study. Second, in this meta-analysis, we included only case-control studies, which are more susceptible to sampling bias resulting from the potential differences between alcoholics and control group than are family-based studies (Gamma et al., 2005). Third, we did not calculate the inter-rater-reliability for selecting reported studies, which might lead to some biases. However, if such bias existed, it would be minimal as two raters reviewed all these reported studies independently of each other. Finally, because of the insufficient number of studies on other variants, we could not examine the gene-by-gene or SNP-by-SNP interaction, which contribute significantly to vulnerability for complex addictive phenotypes. For example, the polymorphism of Taq1A of DRD2 has been suggested to interact with the polymorphism of VNTR of SLC6A3, both of which may have a role in smoking cessation (Ma et al., 2015b, Ma et al., 2015a). To date, multiple lines of evidence (Wang et al., 2013) have shown that DRD2 Taq1A polymorphism is significantly associated with AD, suggesting an interactive function of these two variants for AD as well.
In sum, we first provide strong evidence for the effect of SLC6A3 3′-UTR VNTR genotypes on AD. Individuals with one or more A9 alleles have a greater likelihood of dependence on alcohol than those who carry non-A9 alleles. This study underscores the view that the definition and selection of AD cases highly influences the identification of underlying associations. More studies are warranted to reveal the biological function of the variant on AD and investigate interactions with other genes. Advancing our understanding of heritable factors underlying alcoholism would help develop effective strategies for prevention and novel medications for addictive therapy.
Supplementary Material
Acknowledgments
We thank Dr. David L. Bronson for excellent editing of this manuscript. This study was supported in part by the Research Center for Air Pollution and Health of Zhejiang University, Ministry of Science and Technology of China (2012AA020405), National Natural Science Foundation of China grant 81273223, and NIH grant DA012844.
References
- Bau CH, Almeida S, Costa FT, Garcia CE, Elias EP, Ponso AC, Spode A, Hutz MH. DRD4 and DAT1 as modifying genes in alcoholism: interaction with novelty seeking on level of alcohol consumption. Molecular psychiatry. 2001;6:7–9. doi: 10.1038/sj.mp.4000819. [DOI] [PubMed] [Google Scholar]
- Bhaskar LV, Thangaraj K, Wasnik S, Singh L, Raghavendra Rao V. Dopamine transporter (DAT1) VNTR polymorphism and alcoholism in two culturally different populations of south India. The American journal on addictions/American Academy of Psychiatrists in Alcoholism and Addictions. 2012;21:343–347. doi: 10.1111/j.1521-0391.2012.00244.x. [DOI] [PubMed] [Google Scholar]
- Chen WJ, Chen CH, Huang J, Hsu YP, Seow SV, Chen CC, Cheng AT. Genetic polymorphisms of the promoter region of dopamine D2 receptor and dopamine transporter genes and alcoholism among four aboriginal groups and Han Chinese in Taiwan. Psychiatric genetics. 2001;11:187–195. doi: 10.1097/00041444-200112000-00002. [DOI] [PubMed] [Google Scholar]
- Comings DE, Blum K. Reward deficiency syndrome: genetic aspects of behavioral disorders. Progress in brain research. 2000;126:325–341. doi: 10.1016/S0079-6123(00)26022-6. [DOI] [PubMed] [Google Scholar]
- Cornish KM, Manly T, Savage R, Swanson J, Morisano D, Butler N, Grant C, Cross G, Bentley L, Hollis CP. Association of the dopamine transporter (DAT1) 10/10-repeat genotype with ADHD symptoms and response inhibition in a general population sample. Molecular psychiatry. 2005;10:686–698. doi: 10.1038/sj.mp.4001641. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled clinical trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Dobashi I, Inada T, Hadano K. Alcoholism and gene polymorphisms related to central dopaminergic transmission in the Japanese population. Psychiatric genetics. 1997;7:87–91. doi: 10.1097/00041444-199722000-00006. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Kohn P, Kolachana B, Weinberger DR, Berman KF. Variation in dopamine genes influences responsivity of the human reward system. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:617–622. doi: 10.1073/pnas.0805517106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Nie Y, Li Y, Wan YJ. The association between the SLC6A3 VNTR 9-repeat allele and alcoholism-a meta-analysis. Alcoholism, clinical and experimental research. 2011;35:1625–1634. doi: 10.1111/j.1530-0277.2011.01509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley PF, Loh EW, Innes DJ, Williams SM, Tannenberg AE, Harper CG, Dodd PR. Association studies of neurotransmitter gene polymorphisms in alcoholic Caucasians. Annals of the New York Academy of Sciences. 2004;1025:39–46. doi: 10.1196/annals.1316.005. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Brown SM, Kimak M, Ferrell RE, Manuck SB, Hariri AR. Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Molecular psychiatry. 2009;14:60–70. doi: 10.1038/sj.mp.4002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamma F, Faraone SV, Glatt SJ, Yeh YC, Tsuang MT. Meta-analysis shows schizophrenia is not associated with the 40-base-pair repeat polymorphism of the dopamine transporter gene. Schizophrenia research. 2005;73:55–58. doi: 10.1016/j.schres.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Gill M, Daly G, Heron S, Hawi Z, Fitzgerald M. Confirmation of association between attention deficit hyperactivity disorder and a dopamine transporter polymorphism. Molecular psychiatry. 1997;2:311–313. doi: 10.1038/sj.mp.4000290. [DOI] [PubMed] [Google Scholar]
- Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nature reviews. Genetics. 2005;6:521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- Gorwood P, Limosin F, Batel P, Hamon M, Ades J, Boni C. The A9 allele of the dopamine transporter gene is associated with delirium tremens and alcohol-withdrawal seizure. Biological psychiatry. 2003;53:85–92. doi: 10.1016/s0006-3223(02)01440-3. [DOI] [PubMed] [Google Scholar]
- Heath AC, Bucholz KK, Madden PA, Dinwiddie SH, Slutske WS, Bierut LJ, Statham DJ, Dunne MP, Whitfield JB, Martin NG. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: consistency of findings in women and men. Psychol Med. 1997;27:1381–1396. doi: 10.1017/s0033291797005643. [DOI] [PubMed] [Google Scholar]
- Hedges LV, Pigott TD. The power of statistical tests in meta-analysis. Psychological methods. 2001;6:203–217. [PubMed] [Google Scholar]
- Hedges LV, Pigott TD. The power of statistical tests for moderators in meta-analysis. Psychological methods. 2004;9:426–445. doi: 10.1037/1082-989X.9.4.426. [DOI] [PubMed] [Google Scholar]
- Heinz A, Goldman D, Jones DW, Palmour R, Hommer D, Gorey JG, Lee KS, Linnoila M, Weinberger DR. Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2000;22:133–139. doi: 10.1016/S0893-133X(99)00099-8. [DOI] [PubMed] [Google Scholar]
- Heinz A, Schmidt K, Baum SS, Kuhn S, Dufeu P, Schmidt LG, Rommelspacher H. Influence of dopaminergic transmission on severity of withdrawal syndrome in alcoholism. Journal of studies on alcohol. 1996;57:471–474. doi: 10.15288/jsa.1996.57.471. [DOI] [PubMed] [Google Scholar]
- Hirth N, Meinhardt MW, Noori HR, Salgado H, Torres-Ramirez O, Uhrig S, Broccoli L, Vengeliene V, Rossmanith M, Perreau-Lenz S, Kohr G, Sommer WH, Spanagel R, Hansson AC. Proceedings of the National Academy of Sciences of the United States of America. 2016. Convergent evidence from alcohol-dependent humans and rats for a hyperdopaminergic state in protracted abstinence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivashchenko DV, Shuvalov SA, Chuprova NA, Kibitov AO. Psychiatric genetics. 2015. The association of polymorphisms in DAT (40 bp VNTR, C>T 3′UTR) and DBH (−1021 C/T) genes with the severe complications of alcohol withdrawal state. [DOI] [PubMed] [Google Scholar]
- Iversen L. Neurotransmitter transporters and their impact on the development of psychopharmacology. British journal of pharmacology. 2006;147(Suppl 1):S82–88. doi: 10.1038/sj.bjp.0706428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambeitz J, Romanos M, Ettinger U. Meta-analysis of the association between dopamine transporter genotype and response to methylphenidate treatment in ADHD. The pharmacogenomics journal. 2014;14:77–84. doi: 10.1038/tpj.2013.9. [DOI] [PubMed] [Google Scholar]
- Kohnke MD, Batra A, Kolb W, Kohnke AM, Lutz U, Schick S, Gaertner I. Association of the dopamine transporter gene with alcoholism. Alcohol and alcoholism. 2005;40:339–342. doi: 10.1093/alcalc/agh179. [DOI] [PubMed] [Google Scholar]
- Laine TP, Ahonen A, Torniainen P, Heikkila J, Pyhtinen J, Rasanen P, Niemela O, Hillbom M. Dopamine transporters increase in human brain after alcohol withdrawal. Molecular psychiatry. 1999;4:189–191. 104–185. doi: 10.1038/sj.mp.4000514. [DOI] [PubMed] [Google Scholar]
- Laird NM, Mosteller F. Some statistical methods for combining experimental results. International journal of technology assessment in health care. 1990;6:5–30. doi: 10.1017/s0266462300008916. [DOI] [PubMed] [Google Scholar]
- Le Strat Y, Ramoz N, Pickering P, Burger V, Boni C, Aubin HJ, Ades J, Batel P, Gorwood P. The 3′ part of the dopamine transporter gene DAT1/SLC6A3 is associated with withdrawal seizures in patients with alcohol dependence. Alcoholism, clinical and experimental research. 2008;32:27–35. doi: 10.1111/j.1530-0277.2007.00552.x. [DOI] [PubMed] [Google Scholar]
- Li MD, Burmeister M. New insights into the genetics of addiction. Nature reviews. Genetics. 2009;10:225–231. doi: 10.1038/nrg2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Wang M, Yuan W, Su K, Li MD. The significant association of Taq1A genotypes in DRD2/ANKK1 with smoking cessation in a large-scale meta-analysis of Caucasian populations. Transl Psychiatry. 2015a;5:e686. doi: 10.1038/tp.2015.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Yuan W, Jiang X, Cui WY, Li MD. Molecular neurobiology. 2014. Updated Findings of the Association and Functional Studies of DRD2/ANKK1 Variants with Addictions. [DOI] [PubMed] [Google Scholar]
- Ma YL, Yuan WJ, Cui WY, Li MD. Meta-analysis reveals significant association of 3′-UTR VNTR in SLC6A3 with smoking cessation in Caucasian populations. The pharmacogenomics journal. 2015b doi: 10.1038/tpj.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignini F, Napolioni V, Codazzo C, Carpi FM, Vitali M, Romeo M, Ceccanti M. DRD2/ANKK1 TaqIA and SLC6A3 VNTR polymorphisms in alcohol dependence: association and gene-gene interaction study in a population of Central Italy. Neuroscience letters. 2012;522:103–107. doi: 10.1016/j.neulet.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Mill J, Asherson P, Browes C, D’Souza U, Craig I. Expression of the dopamine transporter gene is regulated by the 3′ UTR VNTR: Evidence from brain and lymphocytes using quantitative RT-PCR. American journal of medical genetics. 2002;114:975–979. doi: 10.1002/ajmg.b.10948. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of clinical epidemiology. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Muramatsu T, Higuchi S. Dopamine transporter gene polymorphism and alcoholism. Biochemical and biophysical research communications. 1995;211:28–32. doi: 10.1006/bbrc.1995.1773. [DOI] [PubMed] [Google Scholar]
- Nedic Erjavec G, Nenadic Sviglin K, Nikolac Perkovic M, Muck-Seler D, Jovanovic T, Pivac N. Association of gene polymorphisms encoding dopaminergic system components and platelet MAO-B activity with alcohol dependence and alcohol dependence-related phenotypes. Progress in neuro-psychopharmacology & biological psychiatry. 2014;54:321–327. doi: 10.1016/j.pnpbp.2014.07.002. [DOI] [PubMed] [Google Scholar]
- Parry CD, Patra J, Rehm J. Alcohol consumption and non-communicable diseases: epidemiology and policy implications. Addiction. 2011;106:1718–1724. doi: 10.1111/j.1360-0443.2011.03605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pramod AB, Foster J, Carvelli L, Henry LK. SLC6 transporters: structure, function, regulation, disease association and therapeutics. Molecular aspects of medicine. 2013;34:197–219. doi: 10.1016/j.mam.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander T, Harms H, Podschus J, Finckh U, Nickel B, Rolfs A, Rommelspacher H, Schmidt LG. Allelic association of a dopamine transporter gene polymorphism in alcohol dependence with withdrawal seizures or delirium. Biological psychiatry. 1997;41:299–304. doi: 10.1016/s0006-3223(96)00044-3. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Danko GP, Smith TL, Hesselbrock V, Kramer J, Bucholz K. A 5-year prospective evaluation of DSM-IV alcohol dependence with and without a physiological component. Alcoholism, clinical and experimental research. 2003;27:818–825. doi: 10.1097/01.ALC.0000067980.18461.33. [DOI] [PubMed] [Google Scholar]
- Sery O, Paclt I, Drtilkova I, Theiner P, Kopeckova M, Zvolsky P, Balcar VJ. A 40-bp VNTR polymorphism in the 3′-untranslated region of DAT1/SLC6A3 is associated with ADHD but not with alcoholism. Behavioral and brain functions: BBF. 2015;11:21. doi: 10.1186/s12993-015-0066-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno S, Nakamura M, Mikami M, Kondoh K, Ishiguro H, Arinami T, Komiyama T, Mitsushio H, Sano A, Tanabe H. Identification of a novel polymorphism of the human dopamine transporter (DAT1) gene and the significant association with alcoholism. Molecular psychiatry. 1999;4:552–557. doi: 10.1038/sj.mp.4000562. [DOI] [PubMed] [Google Scholar]
- van der Zwaluw CS, Engels RC, Buitelaar J, Verkes RJ, Franke B, Scholte RH. Polymorphisms in the dopamine transporter gene (SLC6A3/DAT1) and alcohol dependence in humans: a systematic review. Pharmacogenomics. 2009;10:853–866. doi: 10.2217/pgs.09.24. [DOI] [PubMed] [Google Scholar]
- Vasconcelos AC, Neto Ede S, Pinto GR, Yoshioka FK, Motta FJ, Vasconcelos DF, Canalle R. Association study of the SLC6A3 VNTR (DAT) and DRD2/ANKK1 Taq1A polymorphisms with alcohol dependence in a population from northeastern Brazil. Alcoholism, clinical and experimental research. 2015;39:205–211. doi: 10.1111/acer.12625. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS, Pappas N, Shea C, Piscani K. Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcoholism, clinical and experimental research. 1996;20:1594–1598. doi: 10.1111/j.1530-0277.1996.tb05936.x. [DOI] [PubMed] [Google Scholar]
- Walters GD. The heritability of alcohol abuse and dependence: a meta-analysis of behavior genetic research. The American journal of drug and alcohol abuse. 2002;28:557–584. doi: 10.1081/ada-120006742. [DOI] [PubMed] [Google Scholar]
- Wang F, Simen A, Arias A, Lu QW, Zhang H. A large-scale meta-analysis of the association between the ANKK1/DRD2 Taq1A polymorphism and alcohol dependence. Human genetics. 2013;132:347–358. doi: 10.1007/s00439-012-1251-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernicke C, Smolka M, Gallinat J, Winterer G, Schmidt LG, Rommelspacher H. Evidence for the importance of the human dopamine transporter gene for withdrawal symptomatology of alcoholics in a German population. Neuroscience letters. 2002;333:45–48. doi: 10.1016/s0304-3940(02)00985-0. [DOI] [PubMed] [Google Scholar]
- WHO. Report on the Global Tobacco Epidemic, 2008: The MPOWER package. World Health Organization; Geneva: 2008. [Google Scholar]
- Wise RA. The neurobiology of craving: implications for the understanding and treatment of addiction. Journal of abnormal psychology. 1988;97:118–132. doi: 10.1037//0021-843x.97.2.118. [DOI] [PubMed] [Google Scholar]
- Wu XH, Zhong SR, Gao CQ, Bao JJ, Hu LP, Ruan Y, Jiang Q. Association analysis of dopamine D2 (DRD2), dopamine D4 receptor (DRD4) and dopamine transporter (DAT) gene polymorphisms with alcohol dependence. Prog Mod Biomed 1140–1142 Chinese 2009 [Google Scholar]
- Xu M, Lin Z. Genetic influences of dopamine transport gene on alcohol dependence: a pooled analysis of 13 studies with 2483 cases and 1753 controls. Progress in neuro-psychopharmacology & biological psychiatry. 2011;35:1255–1260. doi: 10.1016/j.pnpbp.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Chan RC, Jing J, Li T, Sham P, Chen RY. A meta-analysis of association studies between the 10-repeat allele of a VNTR polymorphism in the 3′-UTR of dopamine transporter gene and attention deficit hyperactivity disorder. American journal of medical genetics. Part B, Neuropsychiatric genetics: the official publication of the International Society of Psychiatric Genetics. 2007;144B:541–550. doi: 10.1002/ajmg.b.30453. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


