Abstract
For more than 25 years, advances have been made in developing medications to treat alcohol use disorder (AUD), highlighted by the U.S. Food and Drug Administration’s (FDA’s) approval of naltrexone (oral and long-acting) and acamprosate. Despite this progress, more work remains to be done in this area because these medications, though effective for some people, do not work for everyone. A high priority for the National Institute on Alcohol Abuse and Alcohol (NIAAA) is to put into place a solid infrastructure to aid in the development of medications that are more effective than those currently available, and with few side effects. Medication development, especially for a disorder as complex as AUD, is challenging and involves multiple phases, including discovery of “druggable” targets, preclinical studies, human clinical trials, and the adoption and implementation of the new medication into mainstream medicine. A successful medications development program requires clearly established goals for each phase to ensure that a candidate compound is not trapped in one particular phase, a condition known as “the valley of death.” In this article, the phases of medication development are described as they apply to AUD, and specific goals of each phase are identified for the next decade. In addition, several important crosscutting themes are outlined for each phase, all of which are essential for advancing medications development. These include identifying and validating screening models and druggable targets, making use of precision medicine, and establishing partnerships among key stakeholders. Our goal in writing this article is to provide a guide on medications development that will aid the alcohol research community in planning, testing, and developing medications for AUD.
Keywords: medications development, alcohol use disorder, phases of drug development, personalized medicine, drug development pipeline
Alcohol Use Disorder (AUD) is a complex and devastating disease, affecting 13.9 percent of Americans in a 1-year period and resulting in a range of medical, psychological, social, economic, and personal problems (Grant et al., 2015). Problem drinking costs the U.S. society more than $249 billion annually (Sacks et al., 2015) and causes nearly 88,000 deaths each year (Centers for Disease Control and Prevention, 2013). Encouragingly, advances have been made in developing effective treatments for AUD, especially medications. Four medications are approved for alcohol dependence by the U.S. Food and Drug Administration (FDA): disulfiram, oral naltrexone, long-acting injectable naltrexone, and acamprosate. In addition, nalmefene was approved in Europe by the European Medicines Agency for the treatment of alcohol dependence. However, AUD consists of multiple neurobiological mechanisms and through complex genetic and environmental interactions, exhibits a variety of phenotypes. Because of this heterogeneity, no medication works for everyone and in every situation. Thus, efforts continue to discover and develop new, more effective and well-tolerated medications.
Obtaining regulatory approval of novel medications is challenging, particularly for central nervous system (CNS) compounds. For example, in CNS trials, treatment endpoints are difficult to measure, behavioral changes can occur during treatment that are independent of the effects of the medication (placebo effect), and long-term treatment periods are often required for regulatory evaluation (Litten et al., 2012). A CNS candidate compound takes approximately 18 years from discovery to marketplace, 4.5 years longer than non-CNS compounds, and costs more than $1.8 billion (Kaitin and Milne, 2011). The slow pace and high costs are caused, in part, by the failure of many compounds, particularly in human studies. Only 8 percent of new CNS compounds entering a human Phase I study will reach the market. Moreover, only 46 percent of new CNS compounds succeed in pivotal clinical trials, compared with 66 percent for non-CNS compounds (Kaitin and Milne, 2011).
To meet these challenges of medication development, the National Institute on Alcohol Abuse and Alcoholism (NIAAA) recently identified three long-term goals: 1) develop approaches to make the medication development pipeline faster, more predictable, and less expensive; 2) develop strategies to increase the effect size of candidate medications; and 3) facilitate the use of alcohol treatment medications in real-world clinical practice (Litten et al., 2012a). To achieve these goals, strategies are needed for each phase of medication development. Delays in any of the phases of medications development will hamper the progress of candidate compounds through the medication development pipeline. In this article, we review the current state of each phase as it pertains to medications to treat AUD, and identify goals to make each phase more efficient. The different phases of medications development include discovery of “druggable” targets (that is, biological targets that will bind the compound to produce a therapeutic effect), identification of lead compounds, preclinical efficacy testing, fulfillment of Investigational New Drug (IND) requirements, human safety and tolerability (Phase I) studies, proof-of-concept (Phase II) clinical studies, pivotal (Phase III) clinical trials, and the adoption of alcohol medications in real-world settings (Phase IV). In addition to reviewing each phase, several cross-cutting themes of medications development phases are discussed, including validation of druggable targets and medication screening models, precision medicine, accounting for comorbidity, and collaborations and partnerships among key stakeholders from a variety of disciplines.
Phases of Medications Development
Discovering Druggable Targets
One of the challenges in discovering effective targets and, subsequently, developing efficacious medications is the heterogeneity of AUD itself. By heterogeneity, we are referring to certain aspects, or domains, underlying alcohol addiction that include, but are not limited to: incentive salience/reward, stress and negative affect (negative emotionality), cognitive control, habituation, and social processes (Litten et al., 2015). These domains, in turn, can be linked to three stages of the alcohol addiction cycle: the preoccupation-anticipation stage, binge-intoxication stage, and withdrawal-negative affect stage (Koob, 2008). A long-range goal of NIAAA is to further understand the heterogeneity underlying AUD by developing an Alcohol Addiction Research Domain Criteria (AARDoC), similar to the Research Domain Criteria (RDoC) that was developed by the National Institute of Mental Health (NIMH) (Litten et al., 2015). To advance this goal, NIAAA plans to initiate a program called the Addictions Neuroclinical Assessment (ANA), which will use a neuroscience-based approach to measure constructs for three domains: reward/incentive salience, negative emotionality, and cognitive control (Kwako et al., 2015). Other domains, such as social processes, could be addressed in the future. Ultimately, our hope is that the AARDoC will provide a better roadmap for identifying targets and developing specific treatments tailored to individual patients.
Over the past two decades, significant progress has been made in understanding the biological mechanisms underlying AUD (Noronha et al., 2014). Currently, NIAAA is exploring more than 30 targets that may lead to new candidate medications. To manage these targets, we must organize and prioritize them, determine their role in causing and/or maintaining problematic drinking, and verify whether they are related or act independently of one another. For example, we know that several of the targets currently under investigation are important in the body’s response to stress, which corresponds to the withdrawal-negative affect stage of addiction. The following neurotransmitter systems have a role in the stress response: corticotropin-releasing factor (CRF), dynorphin, norepinephrine, vasopressin, hypocretin (orexin), and substance P, as well as those that are anti-stress, including neuropeptide Y, nociceptin (orphanin FQ), and endocannabinoids (Koob, 2008; Koob and Mason, 2016). Other physiological systems, such as the immune system, have also been shown to influence alcohol seeking and drinking behavior and may be targets for medications development (Blednow et al., 2012; Cui et al., 2011).
Initiatives also are underway that may help identify more effective targets. The National Institutes of Health (NIH) Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Initiative, launched in 2013, supports research on how the human brain works by advancing the development and application of innovative technologies, investigating how individual cells and complex neural circuits interact to produce specific types of behaviors, and determining mechanisms underlying brain disorders that disrupt normal brain function (Insel et al., 2013; http://braininitiative.nih.gov/). The NIH Illuminating the Druggable Genome program was recently initiated to advance our understanding of properties and functions of proteins that lie within the four most commonly drug-targeted protein families: G-protein coupled receptors, nuclear receptors, ion channels, and protein kinases (http://commonfund.nih.gov/idg/index). Another program, the NIH Human Connectome Project, is helping scientists to map the neural pathways for human brain function (http://neuroscienceblueprint.nih.gov/connectome/). Finally, BrainSeq, a human brain genomics consortium consisting of seven pharmaceutical companies and a just-for-profit medical research institute, is consolidating publicly available archival brain genomic data related to neuropsychiatric illness (BrainSeq, 2015). It is hoped that understanding the underlying molecular mechanisms of genetic associations among psychiatric disorders will help to identify novel therapeutic targets.
New imaging techniques, undoubtedly, will have an impact in this area as well. In particular, it is important to identify circuits underlying the different domains of AUD and how they are altered during the development of AUD (Luthi and Luscher, 2014). Methods include high angular resolution diffusion imaging (HARDI) with magnetic resonance, resting state functional magnetic resonance imaging (R-fMRI), electrophysiological or magnetoencephalographical recording combined with fMRI (E/M-fMRI), optogenetics, and CLARITY, a method that makes brain tissue more transparent, enabling us to observe real-time activity of specific neurons and their projections (Acheson et al., 2014; Chung and Deisseroth, 2013; Deisseroth et al., 2015; Zhu et al., 2015;). Each technique provides different information about the brain’s connectivity, so combining techniques offers significant additional information concerning the structure and function of individual circuits. Progress is also being made to develop a wearable PET scanner to monitor brain activity. This would make it possible to measure brain activity in deep brain structures as a person moves, talks, and performs a task (http://home.hsc.wvu.edu/news/story?headline=brain-research-at-wvu-awarded--1-5-million).
Finally, to advance our understanding of individual neurons involved in mental and addictive disorders, a conductive polymer mesh is being developed that can be implanted into the brain to study the in-vivo action of each cell. This approach could prove to be an important tool for discovering new targets for medications development (Fletcher, 2015).
In summary, advances are being made in characterizing and measuring the domains of AUD and how they vary across individuals and during disease progression. Progress is also being made in developing tools to elucidate the mechanisms underlying brain function. This, ultimately, will lead to the identification of more effective drug targets, especially if these tools are used in combination to understand the complex nature of AUD. (See the Supplemental Section for more information.)
Goals for identifying targets include:
Characterizing promising targets by prioritizing their importance, determining their contribution to AUD, and establishing their relationship to other targets
Identifying different domains of AUD (AARDoC) and developing constructs to measure each domain
Identifying novel approaches to discover additional therapeutic target(s)
Developing tools/technologies to explore brain functions
Identifying Lead Compounds
Once a promising target has been selected, it is necessary to identify compounds that bind or modulate the active site on that selected target. Compounds can be both repurposed medications and novel compounds. Novel compounds are obtained from various sources, including the NIH Molecular Libraries Program (http://mli.nih.gov/mli/). Historically, academic researchers have had difficulty in obtaining novel compounds from pharmaceutical companies. However, in recent years, alcohol researchers have made progress in partnering directly with the pharmaceutical industry to gain access to novel compounds. For example, ongoing NIAAA-supported human studies now include a number of novel compounds that academic researchers have obtained from pharmaceutical companies, including a vasopressin 1b antagonist, CRF antagonist, ghrelin antagonist, neurokinin antagonist, phosphodiesterase inhibitor, and glucocorticoid antagonist (see ClinicalTrials.Gov). In addition, the National Center for Advancing Translational Sciences (NCATS) initiated a New Therapeutic Uses Program to give researchers access to partially developed novel compounds provided by pharmaceutical companies (http://ncats.nih.gov/ntu/assets/current). Finally, NIAAA has supported studies to discover and develop novel compounds to treat AUD. For example, Barron et al (2012) employed high-throughput screening to isolate a potent and selective aryliminoguanidene, JR 220, which acts as an inhibitory modulator of the glutamate NMDA receptor. This compound reduced drinking in animal models and appears to be non-toxic in preclinical studies. Whether working through NIH-led programs, NIAAA, or in direct collaboration with the pharmaceutical industry, alcohol researchers now have more opportunities to access the compounds necessary to develop more effective medications.
Goals for identifying lead compounds:
Developing partnerships with the pharmaceutical industry to obtain novel compounds
Exploring NIH programs, such as NCATS and Molecular Libraries Program, for access to novel compounds
Supporting studies to synthesize promising novel compounds using rational drug design techniques
Preclinical Efficacy Testing
The alcohol field is fortunate to have a variety of animal models for studying different aspects of AUD. Many of these have been used to test the preclinical efficacy of various medications (Egli, 2005). Animal paradigms also can be linked to the three stages of the addiction cycle: preoccupation-anticipation, binge-intoxication, and withdrawal-negative affect (Table 1). In a new initiative, NIAAA has created a standardized screening model using a network of sites to test promising candidate compounds using designated animal paradigms. Currently, the models include a limited access two-bottle choice in alcohol-preferring and high alcohol drinking rats and in mice that are dependent on alcohol (through the use of chronic-intermittent alcohol vapor exposure). Other models likely will be added in the future. The standardization of models is essential to decrease variability across academic sites, both in the methods and in the use of animal species and strains. To validate those models, reference medications (compounds whose efficacy has already been established via human clinical evaluation) are tested for specific patterns of efficacy. Sufficient validation of the models using additional reference medications will enable them to be used to screen novel compounds (with unknown efficacy), leading to better Go/No Go decision making prior to the initiation of time-consuming and costly human studies. Even if this screening step proves to be only 50% accurate in predicting clinical success in human studies, it would still result in significant savings in both time and resources. So far, pharmaceutical companies have successfully tested novel compounds in this program, which has yielded useful information on several compounds: amtifadine, a triple reuptake inhibitor (Marshall et al., 2013); ibudilast, a phosphodiesterase inhibitor (Bell et al., 2015); and AC3174, a glucagon-like petide-1 agonist (Suchankova et al. 2015).
Table 1.
Examples of Animal and Human Laboratory Models for Different Stages of the Alcohol Addiction Cycle
| Stage of Addiction Cycle | Animal Models | Human Laboratory Models |
|---|---|---|
| Binge/intoxication | Alcohol self-administration Conditioned place preference Brain stimulation reward thresholds Increased motivation for self-administration in dependent animal Responding in the face of punishment |
Alcohol self-administration Impulsivity: Delay discounting and Stop Task Alcohol administration (clamping technique: stimulant and sedative responses Progressive-ratio operant conditioning |
| Withdrawal/negative affect | Anxiety-like responses Conditioned place aversion Withdrawal-induced alcohol self- administration |
Self-medication Response bias to negative cues |
| Preoccupation/anticipation | Alcohol-induced reinstatement Cue-induced reinstatement Stress-induced reinstatement |
Alcohol-induced craving Cue-induced craving Stress-induced craving Cue-Induced brain imaging |
See reviews of Plebani et al. (2012), de Wit (2009), and Koob and Mason (2016).
Other preclinical models, particularly in-vitro models, are also being explored, including the use of induced pluripotent stem cells (iPSCs) for drug discovery. This technology may well be a “game changer” for future medications development, enabling the rapid identification of compounds that are specifically targeted to a disease or disorder, with limited side effects (Inoue et al., 2014; Ko and Gelb, 2014). Using this approach, adult cells are genetically reprogrammed to behave as embryonic stem cells. Differentiation of these cells then can be precisely controlled so that novel compounds can be tested on a specific cell type. This technology is being explored in drug testing for a variety of diseases, including cardiovascular diseases and brain disorders such as amyotrophic lateral sclerosis, Alzheimer dementia, familial dysautonomia, Rett syndrome, schizophrenia, and spinal muscular atrophy (Ko and Gelb, 2014). New tools also are being developed to help identify the actions of novel compounds within these cells.
Goals for preclinical efficacy screening models:
Standardizing and validating animal models for screening candidate compounds that may predict clinical response
Exploring the development and validation of in-vitro models for preclinical screening of novel compounds
IND-Enabling Development of Novel Compounds
After testing positive for efficacy in preclinical models, a series of rigorous studies must be conducted on a novel compound before it can be administered to humans. These include tests of bioactivity, stability, manufacturability, pharmacokinetics, target engagement, and toxicology. A human clinical protocol also must be developed at this time. This ensures that every level of exposure to the compound is addressed, prior to beginning clinical testing in humans.
Unfortunately, this stage—moving promising novel compounds that have been tested positively for efficacy in animal models through IND requirements into human studies—has been a “valley of death” for alcohol medications development, impeding the development of several promising compounds (Barron et al., 2012; Wang et al., 2002). Because of difficulty in obtaining funding, most researchers need to partner with pharmaceutical companies to carry out the required IND studies. Recently, however, NIH and NIAAA initiated two programs that provide an opportunity for alcohol researchers and small companies to advance their promising compounds without the aid of large pharmaceutical companies. The first program, the NIH blueprint Neurotherapeutics Network (BPN) (http://grants.nih.gov/grants/guide/pa-files/PAR-14-293.html and http://grants.nih.gov/grants/guide/pa-files/PAR-14-292.html) supports the advancement of promising small molecule drug discovery and development projects. Participants receive funding for activities conducted in their laboratories and are given the opportunity to collaborate with NIH-funded consultants and contract research organizations (CROs) specializing in IND-enabling components, including: medicinal chemistry, pharmacokinetics, toxicology, formulations development, chemical synthesis under Good Manufacturing Practices, and even eventual Phase I clinical testing. NIAAA has also issued funding opportunity announcements directed to Small Business Innovation Research (SBIR) and Small Business Technology Transfer (STTR) applicants for the IND-enabling development of promising compounds (http://grants.nih.gov/grants/guide/pa-files/PAR-15-154.html, http://grants.nih.gov/grants/guide/pa-files/PAR-15-153.html, http://grants.nih.gov/grants/guide/pa-files/PAR-16-026.html, and http://grants.nih.gov/grants/guide/pa-files/PAR-16-027.html). These research opportunities are designed to help advance promising compounds through the IND process and into clinical trials for humans to further test their efficacy and safety.
Goals for IND-enabling development of novel compounds include the following:
Developing partnerships among NIAAA, academics, and the pharmaceutical and biotechnology industries during preclinical development of candidate compounds
Stimulating IND-enabling development of promising compounds through NIH BPN and NIAAA SBIR/STTR funding opportunities
Phase I Human Studies
Before clinical trials can be conducted with AUD patients, a variety of tests, usually conducted in healthy volunteers, are required to ensure safety, tolerability, and proper dosing. These studies evaluate the pharmacokinetics, single and multiple ascending dose effects, target engagement, alcohol interactions, and, if needed, abuse liability of the compounds. Similar to IND-enabling development, investigators may partner with pharmaceutical companies or apply for NIH BPN and NIAAA SBIR/STTR funding to support this stage of development. In addition, the NIAAA Clinical Investigation Group (NCIG) (http://www.niaaa.nih.gov/NCIG-NIAAA-Clinical-Investigations-Group) was created as a contract mechanism for conducting multisite human laboratory and clinical trials as well as Phase I studies on promising compounds. These options should help to advance candidate compounds from preclinical studies to human studies of AUD.
Goals for Phase I human studies include:
Developing partnerships among NIAAA, academics, and the pharmaceutical and biotechnology industries to conduct various Phase I studies
Stimulating IND-enabling development of promising compounds through funding opportunities, such as the NIH BPN and NIAAA SBIR/STTR
Conducting Phase I studies using the NCIG program
Phase II Proof-of-Concept Studies
Proof-of-concept studies are an essential part of the medications development pipeline, providing valuable information on whether an experimental medication appears to be safe and efficacious and warrants subsequent, time-consuming and expensive, pivotal trials. Proof-of-concept studies consist of both human laboratory studies and early Phase II clinical trials.
Many alcohol human laboratory paradigms have been developed to study the mechanisms underlying AUD. In addition, these paradigms can provide information on the efficacy of experimental medications and whether the compound should be moved into formal clinical trials (i.e., answering the Go/No Go decision). Currently, several human laboratory paradigms exist that can be used as screening models for evaluating leading compounds (Plebani et al., 2012; Table 1).
NIAAA uses both contracts and grants (http://grants.nih.gov/grants/guide/pa-files/PA-15-254.html) to fund human laboratory studies. As with the NIAAA animal model program, a newly established contract mechanism called the NIAAA Human Laboratory Program (HLAB) is helping standardize human laboratory paradigms to serve as screening models for promising medications. Similar to the NIAAA animal screening program, the HLAB paradigms will be standardized to reduce variability in the methods used across academic sites. Moreover, a limited number of paradigms, addressing various domains of AUD, will be selected to ensure that experimental medications are tested across the various mechanisms, with the possibility of adding new paradigms over time. The HLAB paradigms will be validated by comparing the results with those of clinical trials.
Although human laboratory trials are able to provide preliminary evidence for a medication’s efficacy in humans, randomized placebo-controlled Phase II clinical trials are the next level of rigor and generalizability required to validate a medication’s efficacy and safety. Over the past 25 years, NIAAA has supported state-of-the-art early Phase II clinical trials for promising medications (Zindel and Kranzler, 2014). Currently, NIAAA supports Phase II trials examining a variety of medications, acting on a variety of targets, both in AUD patients and in those with comorbid conditions (i.e., both psychiatric [e.g., depression, anxiety disorders, bipolar disorder, and schizophrenia] and medical [e.g., HIV/AIDs, Hepatitis C]) (https://clinicaltrials.gov/ct2/results?term=NIAAA&Search=Search).
NIAAA funds most clinical trials via grant mechanisms. The larger, more definitive of these NIAAA grant-funded clinical trials take 5 to 6 years to complete. As an alternative, to shorten this time, NIAAA has introduced the new NCIG contract-mediated program that uses a network of sites to conduct alcohol clinical trials in approximately 2 years (Litten et al., 2012a). This quick turnaround is important in forming partnerships with pharmaceutical companies, because their candidate compounds have a limited patent life. Initiated in January 2008, four NCIG trials have now been completed, with a fifth trial now underway. Results of the first three trials were published in peer-reviewed journals (Falk et al., 2015; Fertig et al., 2012; Litten et al., 2012b and 2013a), and a manuscript describing the fourth trial is in preparation. In three of the five trials, a clinical trial agreement or Cooperative Research and Development Agreement (CRADA) was signed by a pharmaceutical company that collaborated on the trial. Because pharmaceutical companies have the expertise and resources to obtain FDA approval, partnering with them helps to ensure regulatory approval of a candidate compound.
Conducting CNS trials is more challenging than non-CNS clinical trials (Kaitin and Milne, 2011). For example, in CNS trials, the treatment endpoints can be difficult to measure and behavioral changes that occur during treatment independent of the effects of the medication (e.g., the placebo effect) can complicate their interpretation. Methodological issues are particularly complex in alcohol clinical trials and include those related to study design, population selection, recruitment, adherence, treatment retention, behavioral platform, outcome endpoints, and safety (Witkiewitz et al., 2015a and 2015b). During the past two decades, alcohol researchers and NIAAA have sought to improve and standardize the methodology of alcohol clinical trials. Those efforts include holding workshops on methodology and publishing a dozen manuals, mostly from large NIAAA-funded alcohol treatment trials (e.g., Project MATCH and COMBINE), on various aspects of conducting alcohol trials (http://pubs.niaaa.nih.gov/publications/ProjectMatch/matchIntro.htm; http://pubs.niaaa.nih.gov/publications/ProjectMatch/matchIntro.htm). A joint effort is underway to advance the methodology of alcohol clinical trials through the Alcohol Clinical Trials Initiative (ACTIVE; Anton et al., 2012). The group includes members from pharmaceutical companies, the FDA, EMA, academic researchers (American and international), NIAAA, and the National Institute on Drug Abuse (NIDA). Secondary analyses have been conducted on existing datasets from several multisite alcohol trials to explore various endpoints, different methods of handling missing data, and to gain a better understanding of the placebo response (Falk et al., 2010, 2014, and 2015; Litten et al., 2013b; Witkiewitz et al., 2014).
Efforts also are underway to develop a wearable alcohol sensor that can provide objective measures of alcohol consumption by patients in real time for use in clinical trials (Litten et al., 2010). One such device, the Secure Continuous Remote Alcohol Monitor (or SCRAM), developed by Alcohol Monitoring Systems Inc. (AMS), measures alcohol intake in real time and is currently being used in the criminal justice system (Litten et al., 2010). This device has the ability to detect low-level drinking (2 to 3 drinks) (Roache et al., 2015). NIAAA is currently supporting several projects to improve wearable alcohol sensors, making them more precise in measuring alcohol intake, less expensive, and more comfortable to wear.
Goals for Phase II proof-of-concept studies include:
Evaluating promising alcohol treatment medications in proof-of-concept studies
Developing and validating standardized human laboratory paradigms as screening models for testing candidate compounds
Improving methodology for conducting alcohol clinical trials
Pivotal Trials (Phase III)
Pivotal trials are Phase III clinical trials necessary for FDA approval of experimental medications. Typically, the FDA requires that a medication be efficacious and safe in two pivotal trials before granting approval for the medication. Recently, the FDA released draft guidance for the pharmaceutical industry when conducting pivotal trials for the development of drugs for AUD treatment (http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm433618.pdf). According to this document, the recommended endpoints are either abstinence or percent of subjects with no heavy drinking days (PSNHHD), both of which are dichotomous outcomes (i.e., success/failure). To date, AUD remains the only substance use disorder with a primary endpoint other than total abstinence. The FDA included PSNHHD as a result of NIAAA-supported studies. Trials are expected to be 6 months in duration and may include a grace period—the time during the treatment phase which the outcome is not considered in the analysis because the measured treatment effect may not represent the full potential of the drug. The EMA, the regulatory agency in Europe, also has issued guidelines, in which the endpoints are abstinence or reductions in alcohol consumption or heavy drinking days (dichotomous and continuous measures), and the required trial duration is 6 to 12 months (http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/03/WC500074898.pdf). Thus, in contrast to the FDA, the EMA allows continuous measures (drinking/day and heavy drinking days) as primary endpoints, whereas the FDA allows only dichotomous measures (PSNHDD and abstinence).
Efforts are underway to explore how the currently recommended endpoints designated by the FDA can be expanded to include alternative measures focused on drinking reduction. For example, current analyses using NIAAA-supported epidemiological and multisite clinical trial data examine the clinical relevance of different levels of drinking by correlating them with acute and long-term alcohol-related consequences. Hopefully, analyses of the “validated” drinking endpoints would consistently lead to a meaningful reduction in consequences. Secondary analyses of NIAAA-supported clinical trials are being conducted, comparing the sensitivity of these alternative endpoints to more traditional ones in terms of their ability to detect the effect of medication relative to placebo. Ultimately, the adoption of a new endpoint will require that it is both clinically meaningful and sensitive to the effect of medication.
Because pivotal trials are costly (over $20 million), it is important to elicit support from the pharmaceutical industry at this stage of medications development. Such private-public partnerships are vital in conducting pivotal trials and obtaining regulatory approval of medications for AUD.
Goals for pivotal trials include:
Developing interest and partnerships with pharmaceutical industry to conduct pivotal trials
Conducting analyses and providing results to regulatory agencies to develop evidence–based guidelines for the pharmaceutical industry
Use of Medications in Real World Settings (Phase IV)
Despite its heterogeneity, a number of medications and behavioral therapies have proven effective for treating AUD. NIAAA’s goal is to provide clinicians with a menu of evidence-based treatments. Then, if one therapy does not work, clinicians can select another from the menu, a strategy common in treating psychiatric disorders, such as depression.
Unfortunately, treatment settings in the United States rarely make use of all the evidence-based alcohol interventions available for patients. Medications use is particularly infrequent. Only 658,000 prescriptions were written for alcohol medications in 2010, despite 17 million US adults having AUD (http://pubs.niaaa.nih.gov/publications/AlcoholFacts&Stats/AlcoholFacts&Stats.htm; IMH Health Report 2012; Mark et al., 2009). To put this in perspective, the prevalence of adults with major depression is similar to that of AUD, yet sales of the antidepressant escitalopram were $1.7 billion in 2004 compared with $77 million in total sales for all FDA-approved alcohol medications in 2010 (Mark et al., 2009; IMH Health Report 2012).
Several barriers prevent the adoption of alcohol pharmacotherapy into mainstream medicine. Clinicians and consumers may not be aware that medications exist or that they are effective, there may be a lack of payer/provider/organizational support in promoting medications for alcohol treatment, or there may be financial constraints (Ducharme et al., 2006; Knudsen and Roman, 2014; Mark et al., 2003; Thomas et al., 2003 and 2008). In addition, addiction medicine needs to be included into medical education curriculum.
During the next decade, NIAAA has several goals to improve alcohol medication use in the United States. First is to educate key stakeholders, including the pharmaceutical industry, health care providers, public and private insurers, program and systems administrators, and, of course, patients by emphasizing the benefits of alcohol pharmacotherapy and de-stigmatizing AUD. Pharmaceutical companies will have a significant role in promoting the use of medications, both to clinicians and consumers, which can lead to greater awareness of the high prevalence of AUD and the clinical utility of using medications to treat the disorder. A quick and easy screening method for use in routine practice could facilitate medication use in real-world practice settings. For example, NIAAA’s Clinician’s Guide: Helping Patients Who Drink Too Much offers guidance on screening for heavy drinking, provides information on brief interventions and medications, offers instructions for follow-up, and, if necessary, includes referrals to specialty treatments (http://pubs.niaaa.nih.gov/publications/Practitioner/CliniciansGuide2005/clinicians_guide.htm). Recently, NIAAA partnered with SAMHSA to produce an important resource for health care professionals on the use of medications to treat AUD (http://store.samhsa.gov/shin/content/SMA15-4907/SMA15-4907.pdf).
Ensuring parity for insurance coverage, including pharmaceutical coverage for alcohol medications, also can help to broaden their use. The 2010 Patient Protection and Affordable Care Act should make treatment more accessible and affordable to AUD patients (Beronio et al., 2014). Studies on the effectiveness of a particular alcohol treatment medication in real-world settings will be important in determining its priority for adoption and implementation into mainstream medicine. Overall, research in health services should focus on the attractiveness, affordability, accessibility, efficacy, and safety of medications for both short- and long-term use. Ultimately, the main motivation to use medications may come from patients themselves. As medications become more effective, and more people learn of them from their doctors and through Internet sites, advertising, and social media, the consumer demand for their use will increase.
Goals for the use of medications in real-world settings include:
Developing strategies for the dissemination, adoption, implementation, and sustainability of alcohol treatment medications in various settings, including specialty addiction, primary care, and mental health care settings
Encouraging pharmaceutical companies to participate in medication development efforts aimed at obtaining FDA approval of medications for AUD, culminating in major marketing efforts to both providers and consumers
Conducting research on the effectiveness and use of alcohol treatment medications in real-world settings
Themes that Cut Across the Different Phases of Medications Development
Several themes exist that cut across the various phases of medications development and are essential for the establishment of a strong development program. These include identifying and validating screening models and druggable targets, making use of opportunities for precision medicine, taking into account comorbidity (for more information, see online supplemental material), and establishing collaborations among key stakeholders.
Bidirectional Validation of Screening Models and Druggable Targets
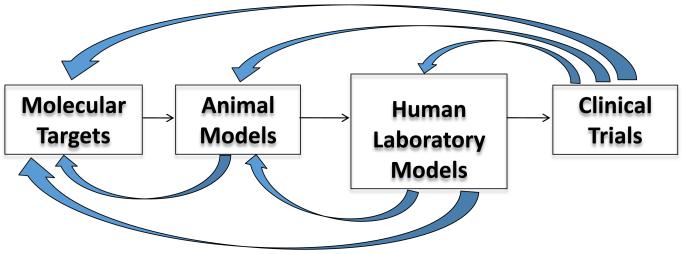
As previously discussed, animal and human laboratory paradigms can be used to screen for promising medications for AUD. These models will help to determine if the experimental compound is likely to be successful, before investing in costly clinical trials. Nearly half of the cost of drug development is spent in conducting clinical trials (Paul et al, 2010). Since not even half (46%) of all new compounds in pivotal trials actually make it to market (Kaitin and Milne, 2011), it is important to develop screening models with some predictive value. Models can be validated by comparing the results from clinical trials to those from animal models and human laboratory studies (Figure 1). This bidirectional validation has been used successfully by the National Institute of Neurological Disorders and Stroke (NINDS) in developing an effective preclinical screening model for anticonvulsants (http://www.ninds.nih.gov/research/asp/asp_working_group_report_052915.htm). It remains to be seen how valuable this approach will be in predicting efficacy for AUD medications.
Figure 1.
Bidirectional Validation of Screening Models and Druggable Targets
A similar approach can be used to validate druggable targets by comparing the results of clinical trials with animal and human laboratory studies to the specific target(s) of the experimental medication (Koob et al., 2009).
Goals for bidirectional validation include:
Validating animal and human laboratory screening models by comparing their results to those from clinical trials
Validating druggable targets by comparing the results of clinical trials and animal and human laboratory paradigms using medications that bind to specific target(s)
Precision Medicine
Research on personalized medicine is a major focus for all complex diseases, including AUD. A new initiative on precision medicine (also called personalized medicine) recently was unveiled by President Obama for implementation by NIH (Collins and Varmus, 2015). This includes launching a Precision Medicine Initiative Cohort Program where a 1 million person cohort will be recruited by 2019 (http://www.nih.gov/about-nih/who-we-are/nih-director/statements/preparing-launch-precision-medicine-initiative-cohort-program). Advancing precision medicine has an important role in medications development for AUD, including: 1) helping us to understand complex mechanisms underlying AUD; 2) increasing the effect size of an experimental medication in alcohol clinical trials by identifying subpopulations for whom the medication works best; and 3) delivering medications in a way that is efficient, effective, and safe.
The complexity of AUD will, most likely, mean that multiple factors must be considered when developing an algorithm to determine who will respond best to a specific medication (Garbutt et al., 2014; Hou et al., 2015). This algorithm would include factors such as a profile and integration of “–omics” (e.g., genomics, epigenomics, transcriptomics, proteomics, and metabolomics), particularly as they relate to the different circuits underlying AUD, physiological indicators (e.g., results from brain imaging), and the patient’s characteristics (e.g., demographics, family history, and disease severity).
Progress has been made in making medications more precise through pharmacogenetics (Jones et al., 2015; Sun et al., 2015). Oslin et al. (2003) first found that an Asn40Asp single nucleotide polymorphism (SNP) (rs1799971) in OPRM1, which encodes the mu-opioid receptor, could be used to predict which patients might respond more favorably naltrexone. However, after many other retrospective studies (Jones et al., 2015) and one prospective study (Oslin et al., 2015), it appears that this SNP may have only a minor influence (Kranzler, 2014). A study by Karpyak et al. (2014) found that alcohol dependent patients with a genetic variant (the rs2058878*A allele of the GRIN2B gene) had a longer period of abstinence when treated with acamprosate than those without this variant. Kiefer et al. (2010) reported that variation in the GATA4 gene influenced the response to acamprosate. In another finding, Johnson et al. (2011 and 2013) found that alcohol dependent patients were more likely to reduce their drinking when treated with ondansetron if they had one or more genetic variants of the serotonin transporter (SLC6A4) gene and 5-HT3 receptors. Using a combination of five genetic variants increased the effect size for ondansetron, identifying 34% of European Americans (EAs) likely to benefit from the medication. Finally, Kranzler et al. (2014) reported that the efficacy of topiramate is moderated by a single nucleotide polymorphism (rs2832407) in GRIK1, a gene that encodes the kainate GluK1 receptor subunit. The topiramate-responsive genotype is present in about 42% of EAs. In addition to pharmacogenetics, progress is also being made in using imaging techniques to predict patient response to a medication (Mann et al., 2014).
The goal for precision medicine includes:
Developing an algorithm that considers a variety of factors, including a genomic profile, physiological indicators (e.g., brain imaging), and individual patient characteristics (such as demographics, family history, and disease severity) to identify those patients who may respond best to specific experimental medications
Collaborations among Key Stakeholders
Developing collaborative networks and partnerships will provide more opportunities and choices for advancing alcohol medications development. Because medications development covers such a wide range of disciplines, one institute, company, or organization cannot do everything. There are many opportunities to strengthen the relationships among the various stakeholders, including researchers, clinicians, patients, regulatory agencies, the pharmaceutical industry, and third-party payers (Litten et al., 2014). These associations will be important in executing the specific tasks of drug development. For example, NIAAA and academia are essential in discovering druggable targets, validating screening models, and conducting proof-of-concept clinical trials; pharmaceutical companies are needed to provide novel compounds, conduct pivotal trials, and implement major marketing efforts; and health care organizations and advocacy groups play important roles in promoting the appropriate use of medications in mainstream medicine. A final, key element is to establish data sharing among private-public organizations.
Making pharmaceutical companies aware of the importance of AUD and its potential as a therapeutic area has been challenging. There are three main goals that need to be met before pharmaceutical companies will take a major interest in drug development for AUD. First, AUD must be seen as an unmet medical need. Most companies now agree that effective treatments are needed to treat this population. Second, the regulatory path for approval must be clear and achievable. Currently, no consensus exists among companies on how feasible it is to do this. Ongoing efforts are being made to analyze data from completed alcohol clinical trials to inform the FDA’s development of a final, evidence-based guidance document for the pharmaceutical industry. Lastly, the pharmaceutical industry needs to see a return on their investment. As mentioned earlier, sales of FDA-approved alcohol treatment medications have historically been modest, though to date no substantial marketing efforts have been made for these medications.
As mentioned earlier, the ACTIVE group, consisting of experts and stakeholders from various organizations (pharmaceutical companies, regulatory agencies, academia, NIAAA, and NIDA), was founded to improve the methodology of alcohol clinical trials (Anton et al., 2012). This group has successfully fostered collaboration among stakeholders to collectively identify, prioritize, and address the key methodological opportunities and impediments in alcohol pharmacotherapy trials. By analyzing data from completed clinical trials, the ACTIVE group has taken an empirical approach towards resolving these issues.
Finally, because of the complexity of drug development, it will be necessary for research scientists from a variety of disciplines, including genetics, chemistry, basic behavioral and clinical sciences, biostatistics, and health service research, to cooperate to advance candidate compounds through the medication development pipeline.
Goals for stakeholders working together include:
Developing collaborative networks and partnerships among key stakeholders
Encouraging scientists from different disciplines to work together to discover and advance novel compounds through the development pipeline
Summary
Although advances have been made in medications development, more work needs to be done to develop a wider variety of effective and safe medications to treat AUD. NIAAA is building a solid infrastructure to accelerate the development of promising medications. To do this, it is important to address the requirements of each phase of drug development and establish goals to ensure that candidate compounds do not perish as they progress through each of the phases (Table 2). In addition, it is important to address cross-cutting themes of medication development that include bidirectional validation of screening models and druggable targets, advancement of precision medicine, and networking and partnerships among key stakeholders. Achieving the goals outlined in this article are essential to advancing medications development, leading to more effective treatments, providing relief for patients and their families, and improving public health.
Table 2.
Goals for Phases of Medications Development and Cross-Cutting Themes
| Phases of Medications Development & Cross-Cutting Themes |
Goals |
|---|---|
| Discovery of druggable targets |
|
| Identifying lead compounds |
|
| Preclinical efficacy screening models |
|
| IND-enabling development of novel compounds |
|
| Phase I Human studies |
|
| Phase II Proof-of-concept studies |
|
| Phase III Pivotal trials |
|
| Phase IV Use of medications in real-world settings |
|
| Bidirectional Validation of Screening Models and Druggable Targets |
|
| Precision medicine |
|
| Collaborations among Key Stakeholders |
|
Supplementary Material
Acknowledgements
The authors thank Barbara Vann of CSR, Incorporated for her review and thoughtful comments.
References
- Acheson A, Wijtenburg SA, Rowland LM, Bray BC, Gaston F, Mathias CW, Fox PT, Lovallo WR, Wright SN, Hong LE, McGuire S, Kochunov P, Dougherty DM. Combining diffusion tensor imaging and magnetic resonance spectroscopy to study reduced frontal white matter integrity in youths with family histories of substance use disorders. Hum Brain Mapp. 2014;35:5877–5887. doi: 10.1002/hbm.22591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton RF, Litten RZ, Falk DE, Palumbo JM, Bartus RT, Robinson RL, Kranzler HR, Kosten TR, Meyer RE, O’Brien CP, Mann K, Meulien D. The Alcohol Clinical Trials Initiative (ACTIVE): purpose and goals for assessing important and salient issues for medications development in alcohol use disorders. Neuropsychopharmacology. 2012;37:402–411. doi: 10.1038/npp.2011.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron S, Lewis B, Wellmann K, Carter M, Farook J, Ring J, Rogers DT, Holley R, Crooks P, Littleton J. Polyamine modulation of NMDARs as a mechanism to reduce effects of alcohol dependence. Recent Pat CNS Drug Discover. 2012;7:129–144. doi: 10.2174/157488912800673128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Lopez MF, Cui C, Egli M, Johnson KW, Franklin KM, Becker HC. Ibudilast reduces alcohol drinking in multiple animal models of alcohol dependence. Addict Biol. 2013;20:38–42. doi: 10.1111/adb.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beronio K, Glied S, Frank R. How the Affordable Care Act and Mental Health Parity and Addiction Equity Act greatly expand coverage of behavioral health care. J Behav Health Serv Res. 2014;41:410–428. doi: 10.1007/s11414-014-9412-0. [DOI] [PubMed] [Google Scholar]
- Blednow YA, Ponomarev I, Geil C, Bergeson S, Koob GF, Harris RA. Neuroimmune regulation of alcohol consumption: behavioral validation of genes obtained from genomic studies. Addict Biol. 2012;17:108–120. doi: 10.1111/j.1369-1600.2010.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BrainSeq: A Human Brain Genomics Consortium BrainSeq: neurogenomics to drive novel target discovery for neuropsychiatric disorders. Neuron. 2015;88:1078–1083. doi: 10.1016/j.neuron.2015.10.047. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Alcohol and public health: Alcohol-related disease impact (ARDI) 2013 Available at: http://nccd.cdc.gov/DPH_ARDI/default/default.aspx.
- Chung K, Deisseroth CLARITY for mapping the nervous system. Nature Methods. 2013;10:508–513. doi: 10.1038/nmeth.2481. [DOI] [PubMed] [Google Scholar]
- Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372:793–795. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui C, Grandison L, Noronha A. Neuroimmune mechanisms of brain function and alcohol related disorders. Brain Behav Immun. 2011;25:S1–S3. doi: 10.1016/j.bbi.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Etkin A, Malenka RC. Optogenetics and the circuit dynamics of psychiatric disease. JAMA. 2015;313:2019–2020. doi: 10.1001/jama.2015.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wit H. Impulsivity as a determinant and consequence of drug use: A review of underlying processes. Addict Biol. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducharme LJ, Knudsen HK, Roman PM. Trends in the adoption of medications for alcohol dependence. J Clin Psychopharmacol. 2006;26:S13–S19. doi: 10.1097/01.jcp.0000246209.18777.14. [DOI] [PubMed] [Google Scholar]
- Egli M. Can experimental paradigms and animal models be used to discover clinically effective medications for alcoholism? Addict Biol. 2005;10:309–319. doi: 10.1080/13556210500314550. [DOI] [PubMed] [Google Scholar]
- Falk DE, Wang X-Q, Liu L, Fertig F, Mattson M, Ryan M, Johnson B, Stout R, Litten RZ. Percentage of subjects with no heavy drinking days: Evaluation as an efficacy endpoint for alcohol clinical trials. Alcohol Clin Exp Res. 2010;34:2022–2034. doi: 10.1111/j.1530-0277.2010.01290.x. [DOI] [PubMed] [Google Scholar]
- Falk DE, Litten RZ, Anton RF, Kranzler HR, Johnson BA, the ACTIVE Group Cumulative proportion of responders analysis (CRPA) as a tool to assess treatment outcome in alcohol clinical trials. J Stud Alcohol Drugs. 2014;75:335–346. doi: 10.15288/jsad.2014.75.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk DE, Castle IJP, Ryan M, Fertig J, Litten RZ. Moderators of varenicline treatment effects in a double-blind, placebo-controlled trial for alcohol dependence: An exploratory analysis. J Addict Med. 2015;9(4):296–303. doi: 10.1097/ADM.0000000000000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fertig JB, Ryan ML, Falk DE, Litten RZ, Mattson ME, Ransom J, Rickman WJ, Scott C, Ciraulo D, Green AI, Tiouririne NA, Johnson B, Pettinati H, Strain EC, Devine E, Brunette MF, Kampman K, Tompkins DA, Stout R. A double-blind, placebo-controlled trial assessing the efficacy of levetiracetam extended-release in very heavy drinking alcohol-dependent patients. Alcohol Clin Exp Res. 2012;36(8):1421–1430. doi: 10.1111/j.1530-0277.2011.01716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher S. Soft, injectable electronic probes for the brain. Sci Am. 2015;313:34. [Google Scholar]
- Garbutt JC, Greenblatt AM, West SL, Morgan LC, Kampov-Polevoy A, Jordan HS, Bobashev GV. Clinical and biological moderators of response to naltrexone in alcohol dependence: A systematic review of the evidence. Addiction. 2014;109:1274–1284. doi: 10.1111/add.12557. [DOI] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, Hasin DS. Epidemiology of DSM-5 alcohol use disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72(8):757–766. doi: 10.1001/jamapsychiatry.2015.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou J, Seneviratne C, Su X, Taylor J, Johnson B, Wang XQ, Zhang H, Kranzler HR, Kang J, Liu L. Subgroup identification in personalized treatment of alcohol dependence. Alcohol Clin Exp Res. 2015;39:1253–1259. doi: 10.1111/acer.12759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H, Nagata N, Kurokawa H, Yamanaka S. iPS cells: a game changer for future medicine. EMBO J. 2014;33:409–417. doi: 10.1002/embj.201387098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Landis SC, Collins FS. The NIH BRAIN initiative. Science. 2013;340:687–688. doi: 10.1126/science.1239276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Seneviratne C, Wang XQ, Ait-Daoud N, Li MD. Determination of genotype combinations that can predict the outcome of the treatment of alcohol dependence using the 5-HT3 antagonist ondansetron. Am J Psychiatry. 2013;170:1020–1031. doi: 10.1176/appi.ajp.2013.12091163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Seneviratne C, Roache JD, Javors MA, Wang XQ, Liu L, Penberthy JK, DiClemente CC, Li MD. Pharmacogenetic approach at the serotonin transporter gene as a method of reducing the severity of alcohol drinking. Am J Psychiatry. 2011;168:265–275. doi: 10.1176/appi.ajp.2010.10050755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Comer SD, Kranzler HR. The pharmacogenetics of alcohol use disorder. Alcohol Clin Exp Res. 2015;39:391–402. doi: 10.1111/acer.12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaitin KI, Milne CP. A dearth of new meds. Sci Am. 2011;305:16. doi: 10.1038/scientificamerican0811-16. [DOI] [PubMed] [Google Scholar]
- Karpyak VM, Biernacka JM, Geske JR, Jenkins GD, Cunningham JM, Ruegg J, Kononenko O, Leontovich AA, Abulseoud OA, Hall-Flavin DK, Loukianova LL, Schneekloth TD, Skime MK, Frank J, Nothen MM, Rietschel M, Kiefer F, Mann KF, Weinshilboum RM, Frye MA, Choi DS. Genetic markers associated with abstinence length in alcohol-dependent subjects treated with acamprosate. Transl Psychiatry. 2014;4:e462. doi: 10.1038/tp.2014.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer F, Witt SH, Frank J, Richter A, Treutlein J, Lemenager T, Nothen MM, Cichon S, Batra A, Berner M, Wodarz N, Zimmermann US, Spanagel R, Wiedemann K, Smolka MN, Heinz A, Rietschel M, Mann K. Involvement of the atrial natriuretic peptide transcription factor GATA4 in alcohol dependence, relapse risk and treatment response to acamprosate. Pharmacogenomics. 2010;11(5):368–374. doi: 10.1038/tpj.2010.51. [DOI] [PubMed] [Google Scholar]
- Ko H, Gelb B. Concise review: drug discovery in the age of the induced pluripotent stem cell. Stem Cells Translational Med. 2014;3:500–509. doi: 10.5966/sctm.2013-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Lloyd GK, Mason BJ. Development of pharmacotherapies for drug addiction: a Rosetta Stone approach. Nature Rev Drug Discov. 2009;8:500–515. doi: 10.1038/nrd2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Mason BJ. Existing and future drugs for the treatment of the dark side of addiction. Annu Rev Pharmacol Toxicol. 2016;56:299–322. doi: 10.1146/annurev-pharmtox-010715-103143. [DOI] [PubMed] [Google Scholar]
- Knudsen HK, Roman PM. Dissemination, adoption, and implementation of acamprosate for treating alcohol use disorders. J Stud Alcohol Drugs. 2014;75:467–475. doi: 10.15288/jsad.2014.75.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR. Can we predict who benefits from naltrexone in the treatment of alcohol dependence? Addiction. 2014;109:1285–1286. doi: 10.1111/add.12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Covault J, Feinn R, Armeli S, Tennen H, Arias AJ, Gelernter J, Pond T, Oncken C, Kampman KM. Topiramate treatment for heavy drinkers: Moderation by a GRIK1 polymorphism. Am J Psychiatry. 2014;171:445–452. doi: 10.1176/appi.ajp.2013.13081014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwako LE, Litten RZ, Momenan R, Koob GF, Holdman D. Addictions Neuroclinical Assessment: A neuroscience-based framework for addictive disorders. Biol Psychiatry. 2015 Nov 17; doi: 10.1016/j.biopsych.2015.10.024. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litten RZ, Bradley AM, Moss HB. Alcohol biomarkers in applied settings: Recent advances and future research opportunities. Alcohol Clin Exp Res. 2010;34:955–967. doi: 10.1111/j.1530-0277.2010.01170.x. [DOI] [PubMed] [Google Scholar]
- Litten RZ, Egli M, Heilig M, Cui C, Fertig JB, Ryan ML, Falk DE, Moss H, Huebner R, Noronha A. Medications development to treat alcohol dependence: A vision for the next decade. Addict Biol. 2012a;17:513–527. doi: 10.1111/j.1369-1600.2012.00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litten RZ, Fertig JB, Falk DE, Ryan ML, Mattson ME, Collins JF, Murtaugh C, Ciraulo D, Green AI, Johnson BA, Pettinati H, Swift R, Afshar M, Brunette MF, Tiouririne NAD, Kampman K, Stout R, Study Group A double-blind, placebo-controlled trial to assess the efficacy of quetiapine fumarate XR in very heavy-drinking alcohol-dependent patients. Alcohol Clin Exp Res. 2012b;36(3):406–416. doi: 10.1111/j.1530-0277.2011.01649.x. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litten RZ, Ryan ML, Fertig JB, Falk DE, Johnson B, Dunn KE, Green AI, Pettinati HM, Ciraulo DA, Sarid-Segal O, Kampman K, Brunette MF, Strain EC, Tiouririne NA, Ransom J, Scott C, Stout R. A double-blind, placebo-controlled trial assessing the efficacy of varenicline tartrate for alcohol dependence. J Addict Med. 2013;7:277–286. doi: 10.1097/ADM.0b013e31829623f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litten RZ, Pan I-J, Falk D, Ryan M, Fertig J, Chen CM, Yi H. The placebo effect in clinical trials for alcohol dependence: An analysis of 51 naltrexone and acamprosate studies. Alcohol Clin Exp Res. 2013b;37:2128–2137. doi: 10.1111/acer.12197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litten RZ, Falk D, Ryan M, Fertig J. Research opportunities for medications to treat alcohol dependence: addressing stakeholders’ needs. Alcohol Clin Exp Res. 2014;38:27–32. doi: 10.1111/acer.12193. [DOI] [PubMed] [Google Scholar]
- Litten RZ, Ryan ML, Falk DE, Reilly M, Fertig JB, Koob GF. Heterogeneity of alcohol use disorder: Understanding mechanisms to advance personalized treatment. Alcohol Clin Exp Res. 2015;39:579–584. doi: 10.1111/acer.12669. [DOI] [PubMed] [Google Scholar]
- Luthi A, Luscher C. Pathological circuit function underlying addiction and anxiety disorders. Nature Neurosci. 2014;17:1635–1643. doi: 10.1038/nn.3849. [DOI] [PubMed] [Google Scholar]
- Mann K, Vollstadt-Klein S, Reinhard I, Lemenager T, Fauth-Buhler M, Hermann D, Hoffmann S, Zimmermann US, Kiefer F, Heinz A, Smolka MN. Predicting naltrexone response in alcohol-dependent patients: the contribution of functional magnetic resonance imaging. Alcohol Clin Exp Res. 2014;38:2754–2762. doi: 10.1111/acer.12546. [DOI] [PubMed] [Google Scholar]
- Mark TL, Kassed CA, Wandivort-Warren R, Levit KR, Kranzler HR. Alcohol and opioid dependence medications: Prescription trends, overall and by physician specialty. Drug Alcohol Depend. 2009;99:345–349. doi: 10.1016/j.drugalcdep.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark TL, Kranzler HR, Poole VH, Hagen CA, McLeod C, Crosse S. Barriers to the use of medications to treat alcoholism. Am J Addict. 2003;12:281–294. [PubMed] [Google Scholar]
- Marshall RD, Tran P, Becker HC, Lopez MF, Egli M, Bell RL, Franklin KM, Grahame NJ, Bymaster FP. The serotonin-preferring triple reuptake inhibitor amitifadine decreases alcohol intake and impulsivity in rodents. Presented at NCDEU Meeting; Hollywood, Florida. 2013. [Google Scholar]
- Noronha ABC, Cui C, Harris RA, Crabbe JC. Neurobiology of Alcohol Dependence. Elsevier Academic Press; Amsterdam: 2014. [Google Scholar]
- Oslin DW, Berrettini W, Kranzler HR, Pettinati H, Gelernter J, Volpicelli JR, O’Brien CP. A functional polymorphism of the μ-opioid receptor gene is associated with naltrexone response in alcohol-dependent patients. Neuropsychopharmacology. 2003;28:1546–1552. doi: 10.1038/sj.npp.1300219. [DOI] [PubMed] [Google Scholar]
- Oslin DW, Leon SH, Lynch KG, Berrettini W, O’Brien CP, Gordon AJ, Rukstalis M. Naltrexone vs placebo for the treatment of alcohol dependence: A randomized clinical trial. JAMA Psychiatry. 2015;72:430–437. doi: 10.1001/jamapsychiatry.2014.3053. [DOI] [PubMed] [Google Scholar]
- Paul SM, Mytelka DS, Dunwiddie CT, Persinger CC, Munos BH, Lindborg ST, Schacht AL. How to improve R&D productivity: The pharmaceutical industry’s grand challenge. Nature Rev/Drug Discov. 2010;9:203–214. doi: 10.1038/nrd3078. [DOI] [PubMed] [Google Scholar]
- Plebani JG, Ray LA, Morean ME, Corbin WR, MacKillop J, Amlung M, King AC. Human laboratory paradigms in alcohol research. Alcohol Clin Exp Res. 2012;36:972–983. doi: 10.1111/j.1530-0277.2011.01704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roache JD, Karns TE, Hill-Kapturczak N, Mullen J, Liang Y, Lamb RJ, Dougherty DM. Using transdermal alcohol monitoring to detect low-level drinking. Alcohol Clin Exp Res. 2015;39:1120–1127. doi: 10.1111/acer.12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks JJ, Gonzales KR, Bouchery EE, et al. 2010 national and state costs of excessive alcohol consumption. American Journal of Preventive Medicine. 2015;49(5):e73–e79. doi: 10.1016/j.amepre.2015.05.031. PMID: 26477807. [DOI] [PubMed] [Google Scholar]
- Sun Y, Zhang Y, Wang F, Sun Y, Shi J, Lu L. From genetic studies to precision medicine in alcohol dependence. Behav Pharmacol. 2015 Nov 17; doi: 10.1097/FBP.0000000000000202. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Suchankova P, Yan J, Schwandt ML, Stangl BL, Caparelli EC, Momenan R, Jerlhag E, Engel JA, Hodgkinson CA, Egli M, Lopez MF, Becker H, Goldman D, Heilig M, Ramchandani VA, Leggio L. The glucagon-like peptide-1 receptor as a potential treatment target in alcohol use disorder: Evidence from human genetic association studies and a mouse model of alcohol dependence. Transl Psychiatry. 2015;16(5):e583. doi: 10.1038/tp.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CP, Wallack SS, Lee S, McCarty D, Swift R. Research to practice: Adoption of naltrexone in alcoholism treatment. J Subst Abuse Treat. 2003;24:1–11. [PubMed] [Google Scholar]
- Thomas SE, Miller PM, Randall PK, Book SW. Improving acceptance of naltrexone in community addiction treatment centers: A pilot study. J Subst Abuse Treat. 2008;35:260–268. doi: 10.1016/j.jsat.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z-J, Snell LD, Tabakoff B, Levinson SR. Inhibition of neuronal Na+ channels by the novel antieplileptic compound DCUKA: identification of the diphenylureido moiety as an inactivation modifier. Exp Neurol. 2002;178:129–138. doi: 10.1006/exnr.2002.8029. [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, Falk DE, Kranzler HR, Litten RZ, Hallgren KA, O’Malley SS, Anton RF. Methods to analyze treatment effects in the presence of missing data for a continuous heavy ddrinking outcome measure when participants drdop out from treatment in alcohol clinical trials. Alcohol Clin Exp Res. 2014;38:2826–2834. doi: 10.1111/acer.12543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Finney JW, Harris AHS, Kivlahan DR, Kranzler HR. Recommendations for the design and analysis of treatment trials for alcohol use disorders. Alcohol Clin Exp Res. 2015a;39:1157–1170. doi: 10.1111/acer.12800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Finney JW, Harris AHS, Kivlahan DR, Kranzler HR. Guidelines for the reporting of treatment trials for alcohol use disorders. Alcohol Clin Exp Res. 2015b;39:1571–1581. doi: 10.1111/acer.12797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Cortes CR, Mathur K, Tomasi D, Momenan R. Model-free functional connectivity and ipulsivity correlates of alcohol dependence: A resting-state study. Addict Biol. 2015 Jun 3; doi: 10.1111/adb.12272. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zindel LR, Kranzler HR. Pharmacotherapy of alcohol use disorders: Seventy-five years of progress. J Stud Alcohol Drugs. 2014;75((Suppl)17):79–88. doi: 10.15288/jsads.2014.s17.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.