Abstract
Background
Drug and alcohol abusers develop strong memories for drug-related stimuli. Preclinical studies suggest that such memories are a result of drug actions on reward pathways, which facilitate learning about drug-related stimuli. However, few controlled studies have investigated how drugs affect memory for drug-related stimuli in humans.
Methods
The current study examined the direct effect of alcohol on memory for images of alcohol-related or neutral beverages. Participants received alcohol (0.8 g/kg) either before viewing visual images (Encoding condition; n=20) or immediately after viewing them (Consolidation condition; n=20). A third group received placebo both before and after viewing the images (Control condition; n=19). Memory retrieval was tested exactly 48 hours later, in a drug-free state.
Results
Alcohol impaired memory in the Encoding condition and enhanced memory in the Consolidation condition, but these effects did not differ for alcohol-related and neutral beverage stimuli. However, in the Encoding condition, participants who experienced greater alcohol-induced stimulation exhibited better memory for alcohol-related, but not neutral-beverage stimuli.
Conclusions
These findings suggest that individual differences in sensitivity to the positive, rewarding effects of alcohol are associated with greater propensity to remember alcohol-related stimuli encountered while intoxicated. As such, stimulant responders may form stronger memory associations with alcohol-related stimuli, which might then influence their drinking behavior.
Keywords: memory, encoding, consolidation, alcohol stimuli, stimulation
Introduction
Drug-related stimuli exert powerful influences over individuals with drug and alcohol use disorders, often leading to relapse in those attempting to remain abstinent. For this reason, researchers have long been interested in characterizing reactions to drug cues, and in understanding how drug-cue associations are formed, how they are stored in memory, and how they influence drug use. The ultimate goal of this research is to reduce drug abuse and dependence by finding ways to decrease the control that drug-associated cues exert over drug-taking behavior.
To date, most of our knowledge regarding the formation of drug-cue associations has come from animal models. These models show that drugs of abuse increase striatal glutamate and dopamine levels, both of which produce powerful reward learning signals (Gipson et al., 2014; Kalivas, 2004; Torregrossa et al., 2011). As a result, drug-paired stimuli become rapidly and strongly associated with the rewarding effects of the drug, causing the stimuli to take on heightened incentive properties (Robinson and Berridge, 2001). The ability of drugs of abuse to create these robust learning signals is thought to underlie the pronounced memory activations elicited by drug cues (Everitt et al., 2001; Hyman et al., 2006).
In humans, researchers have assessed the impact of cues on psychophysiological measures and craving, and the degree to which responses to cues predict drug use and relapse. Among heavy users, drug and alcohol cues elicit physiological responses (e.g., increased heart rate and skin conductance), increase attention, and increase reports of drug craving (Drummond, 2000; Field and Cox, 2008; Field et al., 2009). Cues also increase activation in mesocortolimbic brain regions involved in reward, motivation, and associative learning (Jasinska et al., 2014; Schacht et al., 2012), and in some but not all studies, are associated with relapse (Courtney et al., 2016; Drummond, 2000; Niaura et al., 1988).
Surprisingly, few studies have examined the direct effects of drugs on learning or memory for drug-associated stimuli in humans. Based on the preclinical evidence, drugs of abuse, especially those with actions on dopamine systems, would be expected to facilitate learning about environmental stimuli associated with their use (Di Chiara et al., 2004; Torregrossa et al., 2011). In the case of alcohol, the effects of the drug on memory are complex. On one hand, alcohol has well-known memory impairing effects when it is present during encoding (Zorumski et al., 2014), but on the other hand alcohol is known to increase synaptic levels of dopamine, and it enhances learning and memory for salient stimuli when it is present during consolidation (Bruce and Pihl, 1997; Bruce et al., 1999a; Knowles and Duka, 2004; Mann et al., 1984; Parker et al., 1980). We hypothesize that the effects of alcohol on memory may depend on the nature of the to-be-remembered material. Specifically, alcohol impairment of memory during encoding may be reduced for alcohol-related stimuli, and alcohol facilitation of memory during consolidation may be enhanced for alcohol-related stimuli.
Another consideration in studying the effects of alcohol on memory is the presence of individual differences in sensitivity to alcohol reward. Individuals differ markedly in subjective and rewarding response to alcohol: some are more sensitive to the positive, rewarding effects of alcohol, experiencing euphoria, arousal and increased talkativeness, whereas others are more sensitive to the negative effects and report feeling down, sluggish, and tired (Holdstock and de Wit, 1998; Newlin and Thomson, 1990; Quinn and Fromme, 2011). Greater sensitivity to alcohol-induced stimulation is associated with greater alcohol consumption, and longitudinal evidence suggests that ‘stimulant responders’ are at greater risk for developing alcohol use disorder symptoms (King et al., 2011; King et al., 2014; King et al., 2016; Newlin and Thomson, 1990; Quinn and Fromme, 2011). Given that stimulant responders are more sensitive to the positive rewarding effects of alcohol, we propose that they may also be more likely to form memory associations between contextual stimuli and the rewarding effects of the drug.
The current study examined the effects of alcohol on encoding and consolidation of memory for alcohol-related pictures (e.g., beer bottles, liquor glasses) and neutral beverage pictures (e.g., water bottles, soda cans) in regular social drinkers. Subjects were assigned to one of three conditions: 1) encoding, in which alcohol was administered prior to stimulus viewing; 2) consolidation, in which alcohol was administered post-viewing; and 3) control, in which placebo was administered both pre- and post-viewing. Two days later subjects performed a surprise memory test in a drug-free state. We hypothesized that alcohol would impair memory in the encoding condition, specifically for neutral beverage stimuli. By contrast, we hypothesized that alcohol would enhance memory in the consolidation condition, especially for alcohol-related stimuli. Additionally, we examined the degree to which individual differences in subjective response influenced memory for alcohol-related stimuli. We hypothesized that greater sensitivity to the positive, stimulant effects of alcohol relative to the negative, sedative effects would be associated with greater memory for alcohol-related stimuli in both the encoding and consolidation conditions.
Materials and Methods
Participants
Healthy volunteers (n=59) were recruited from the community through online and printed advertisements. Volunteers were eligible for participation if they consumed an average of 10–30 standard drinks per week (e.g., 12oz beer or 1.5oz liquor), with at least one heavy-drinking episode (i.e., four or five drinks/occasion for women and men, respectively) in the past month. These minimum drinking criteria were included to ensure that participants could tolerate the alcohol dose. Additional inclusion criteria were age 21–30, BMI between 19 and 26, at least a high school education, fluency in English, no current or past year DSM-IV diagnosis (including substance dependence), no lifetime history of substance dependence, and no serious medical conditions. Participants were excluded if they reported smoking more than 5 cigarettes/day or daily use of any medication other than birth control, or if they were pregnant, lactating, or planning to become pregnant in the next three months. Females who were not on hormonal contraception were tested only in the follicular phase of their menstrual cycle. The Institutional Review Board of the University of Chicago approved the study and it was carried out in accordance with the Declaration of Helsinki. All participants provided written informed consent for participation.
Design
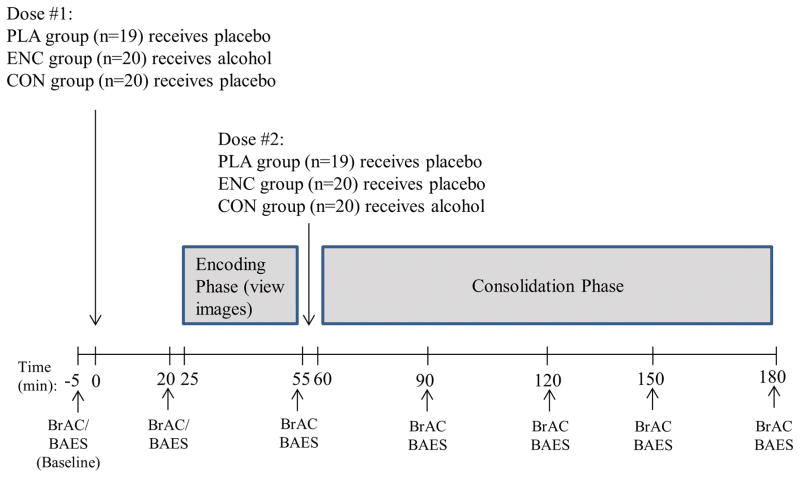
This study utilized a two-session, double-blind, between-subjects design in which subjects attended a viewing session, followed by a retrieval session exactly 48 hours later. Equal numbers of men and women were randomly assigned to the encoding (ENC; n=20), consolidation (CON; n=20) or placebo control (PLA; n=19) condition. On the viewing session (see Figure 1 for a schematic of the viewing session protocol), subjects consumed a beverage containing alcohol (ENC condition) or placebo (CON and PLA conditions), before viewing and rating a series of labels and pictures with both alcohol-related and non-alcohol related content. Immediately after viewing the stimuli, subjects consumed a second beverage containing alcohol (CON condition) or placebo (ENC and PLA conditions). Participants completed the Biphasic Alcohol Effects Scale (BAES; Martin et al., 1993) to assess the subjective stimulant and sedative effects of alcohol every 30 minutes throughout the session. In the retrieval session, subjects completed a surprise memory test for the stimuli, without any administration of alcohol.
Figure 1.
Schematic of the timeline of events for a viewing session. PLA = Placebo; ENC = Encoding; CON = Consolidation; BrAC = breath alcohol concentration; BAES = Biphasic Alcohol Effects Scale.
Procedure
Participants first attended an orientation session in which they provided informed consent and were familiarized with laboratory procedures and study protocol. Participants were told that the study was investigating the effects of drugs and pictures on mood, and to minimize drug expectancies, they were told they could receive one of the following: stimulant, sedative, alcohol, or placebo. They were instructed to consume their normal amounts of caffeine and nicotine, but to abstain from drugs, including alcohol, for 24 hours prior to each session, and to not consume any food after 9am.
Participants attended two experimental sessions, exactly 48 hours apart. They were tested individually in comfortably furnished rooms. Upon arrival, compliance with drug abstinence was verified by both self-report and breath (alcohol) and urine screens (testing for amphetamine, cocaine, methamphetamine, opiates, and tetrahydrocannabinol). Baseline (pre-drug) BAES measures were assessed.
Session 1: Viewing session
The viewing session took place from 1pm to 6pm. After baseline measures were obtained, participants consumed a dose of alcohol (0.8 g/kg; ENC condition; see below) or placebo (CON and PLA conditions) in five minutes. Twenty minutes later, participants completed the BAES and provided breath samples to assess breath alcohol concentration (BrAC; Alco-sensor III; Intoximeters, St. Louis, MO). They then completed the viewing phase of the memory task (see below). The picture viewing required approximately 30 minutes to complete, with completion coinciding with the ascending limb peak of the BrAC curve in the ENC condition. Immediately following the picture viewing, BrAC was assessed and subjects again completed the BAES. All subjects then consumed a second dose, which contained alcohol for the CON condition and placebo for the ENC and PLA conditions. BrAC and BAES responses were collected 30 and 60 minutes after consuming the second dose (i.e., over the ascending limb of the blood alcohol concentration curve for the CON condition). Participants remained in the lab for 3.5 hours after picture viewing. During the first two hours (i.e., the early consolidation period) they listened to non-verbal music (classical or jazz), but were not allowed to watch movies, use the internet, read, or sleep. This was done to control for potential retrograde (post-learning) interference, as one potential mechanism through which alcohol may exert its effect on memory consolidation is through reduction of retrograde interference (Bruce and Pihl, 1997; Bruce et al., 1999a; Bruce et al., 1999b). After two hours they were given a snack and allowed to read or watch movies for the remainder of the session. Those who received alcohol were only allowed to leave after their BrAC had fallen below 40 mg/100 ml.
Session 2: Retrieval session
Retrieval sessions took place from 1:00 pm to 3:30 pm, exactly two days after the viewing session, and involved no alcohol or placebo. After compliance testing, participants performed the surprise cued recollection and recognition tests (see below). Subjects were then debriefed and compensated for their time.
Alcohol administration
Alcohol and placebo were administered in individual servings of black cherry sugar-free jello. The alcohol dose was 0.8 g/kg for men, and 0.7 g/kg for women to achieve equivalent BrACs across sex (Fillmore, 2001; Mulvihill et al., 1997). This dose was chosen to produce peak BrACs of 80 mg/100 ml, and to be consistent with previous studies that have examined alcohol effects on memory formation (Bruce and Pihl, 1997; Bruce et al., 1999a; Bruce et al., 1999b). Alcohol was administered in jello to mask the taste (Ralevski et al., 2006) and for fast consumption. The fast administration was chosen in order to produce a rapid rise in BrAC so that in the Encoding condition, BrAC would peak at the conclusion of the viewing period, and in the Consolidation condition, BrAC would rise immediately following stimulus viewing. The alcohol jello was prepared with 3 parts 95% alcohol and 5 parts water, mixed together with the jello powder and then refrigerated overnight. Placebo jello was prepared with 8 parts water. Participants were served individual jello servings (5 g alcohol each) in black opaque 2 oz cups. The number of servings required to reach a dose of 0.8 g/kg alcohol (men) and 0.7 g/kg (women) was determined for each participant based on body weight, and ranged form 10–14 for men and 7–10 for women. This number was calculated for placebo doses as well, and subjects received an equivalent number of placebo jello servings.
Measures
Memory tasks (Cued Recollection and Recognition: Gallo et al., 2009; Weafer et al., 2014)
Viewing session
During the viewing phase, participants were instructed to rate labels and pictures on measures of liking, valence, and arousal, but they were not informed that their memory for the stimuli would be tested. The stimuli were 96 alcohol and neutral-beverage images, matched on various characteristics including the portion of the image occupied by the beverage and the presence, number, and sex of faces1. For both alcohol-related and neutral stimuli, 66% of the images included people, and 33% did not. In order to make the images variable and distinct, we included a range of indoor and outdoor scenes, daylight and night-time scenes, images of men and women, and images including both groups and individuals. Two or three word descriptive labels were created for each picture (e.g., ‘girls at oktoberfest’ and ‘ladies having tea’). Additional pictures drawn from the International Affective Picture System (IAPS; Lang et al., 1999), which consists of standardized positive, neutral, and negative pictures, were also presented. These other stimuli depicted objects and scenes that were unrelated to the beverage stimuli of interest here, and data from these stimuli are reported elsewhere (Weafer et al., 2016).
During the viewing phase, participants viewed each of the labels (48 describing alcohol-related images and 48 describing neutral-beverage images) in random order. Before viewing each label, subjects focused on a fixation point on the screen, and when they pressed the space bar a label was presented for 1500 ms. Participants rated how much they thought they would like a picture associated with that label, on a scale from 1 ‘not at all’ to 5 ‘very much’. Then, for half of the labels, the picture described by the label was presented on the screen for 2000 ms, and participants rated the perceived valence and arousal of the picture. Valence was defined as how positive and how negative the picture made the subjects feel, and was measured using the evaluative space grid (Larsen et al., 2009), a two-dimensional grid allowing independent ratings of positivity and negativity (from 1 to 5). Greater values indicated more positive valence. Arousal was defined as how stimulated, excited, or awake subjects felt in response to the picture (Lang et al., 1993), and was measured using a Likert scale from 1 to 5. The other half of the labels were not followed by a picture.
Retrieval session
Exactly 48 hours later, participants returned to the laboratory for the retrieval session and performed two surprise tests of their memory for the labels and pictures presented during the viewing session. The cued recollection test assessed the ability to remember whether or not a label was associated with a picture, and the recognition test assessed the ability to remember whether or not a picture was previously viewed.
Cued recollection test
For the first test, participants viewed the labels from the viewing phase in random order, half of which had been associated with a picture during study (targets) and half had not (lures). For each label they were asked whether they remembered seeing a picture that was associated with the label or not (yes/no). Because participants viewed all of the test labels during encoding, all of the labels should have been familiar to the participants. As such, the ability to discriminate between targets and lures heavily relied on accurate recollection of the studied pictures associated with the targets (and not lures).
Recognition test
Immediately following the cued recollection task, participants performed the recognition task, in which they viewed the studied pictures (targets) randomly intermixed with pictures that were not seen at study (but whose labels had been presented, lures). For each picture they were asked whether they remembered seeing the picture or not (yes/no).
Biphasic Alcohol Effects Scale (BAES; Martin et al., 1993)
The BAES is a measure of subjective stimulant (e.g., talkative, elated) and sedative (i.e., sedated, sluggish) responses to alcohol. Responses for individual items are reported on a Likert scale, and stimulation and sedation item scores are summed separately to provide a total subscale score for each (score range 0 – 70),
Timeline Follow-Back (TLFB; Sobell and Sobell, 1992)
At the beginning of the study, participants completed a retrospective time line calendar of their alcohol consumption for the past month to assess daily patterns of drinking, including number of heavy drinking episodes. For each day, participants estimated the number of standard drinks they consumed. Any day in which participants consumed 5 (men) or 4 (women) or more drinks was considered a binge episode. From the TLFB, we calculated subjects’ average number of drinks per week and total number of binge drinking days over the past month.
Data analysis
Memory performance
Both cued recollection and recognition accuracy were analyzed by computing signal detection estimates (d′), using the correction described in Snodgrass and Corwin (1988) to avoid ceiling and floor effects. We calculated d′ by subtracting the z score of the hit rate (correct responses to targets) from the z score of the false alarm rate (incorrect responses to lures). The acute effects of alcohol on d′ estimates for cued recollection and recognition of alcohol-related and neutral beverage stimuli were tested by separate (condition: ENC, CON, PLA) × (image: alcohol-related vs. neutral beverage) mixed design analyses of variance (ANOVA), with condition as the between groups factor and image type as the within-subjects factor. We hypothesized that alcohol would impair memory during encoding, especially for neutral-beverage images, and enhance memory during consolidation, especially for alcohol-related images.
Associations with stimulation and sedation
We conducted correlational analyses to test the degree to which individual differences in BAES Stimulation and Sedation predicted memory accuracy for alcohol-related and neutral beverage stimuli. We calculated change from baseline scores for BAES scores over the ascending limb of the BAC curve for both the encoding and early consolidation periods. We then subtracted peak change from baseline Sedation scores from peak change from baseline Stimulation scores to provide a composite measure of Stimulation minus Sedation (STIM-SED) during encoding and early consolidation. We hypothesized that greater STIM-SED scores would be associated with greater memory accuracy for alcohol-related stimuli in both the ENC and CON conditions.
Results
Sample characteristics
The three conditions did not differ in mean age, education, or drinking habits (ps > 0.21). The sample consisted of young adult (mean age = 24.1, SD = 2.3) moderate to heavy drinkers. Participants consumed an average of 15.0 (SD = 7.1) drinks per week, with an average of 5.7 (SD = 3.5) binge episodes over the previous 30 days. Seven participants (ENC n=2; CON n=2, PLA, n=3) met DSM-IV (APA, 2000) criteria for alcohol abuse in the past year, and four participants (ENC n= 2, CON n=1, PLA n=1) met DSM-IV (APA, 2000) criteria for marijuana abuse.
Breath alcohol concentrations
In the ENC condition, BrAC was rising throughout stimulus viewing, and peaked at 82.5 mg/100 ml (SD = 21.3) 60 min post-beverage administration, coinciding with the end of the stimulus viewing. In the CON condition, BrAC was rising for 60 min post-stimulus viewing (i.e., during the early consolidation period) and peaked at 84.8 mg/100 ml (SD = 19.4). No detectable BrACs were observed in the PLA condition.
Stimulus ratings
Across conditions, participants rated alcohol stimuli as higher in valence, arousal, and liking than neutral beverage stimuli [Table 1; main effects of image: Fs(1, 56) > 5.9, ps < 0.018]. Alcohol did not affect ratings of either image type (ENC condition compared to combined PLA and CON conditions; ps > 0.10).
Table 1.
Mean (SD) stimulus ratings during the viewing session by condition.
| Alcohol-related Stimuli | Neutral Beverage Stimuli | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Like | Valence | Arousal | Like | Valence | Arousal | |
| ENC | 2.6 (0.9) | 1.2 (0.9) | 2.3 (0.8) | 2.3 (0.6) | 1.0 (0.6) | 1.9 (0.6) |
| CON | 2.4 (0.7) | 1.2 (0.6) | 2.1 (0.5) | 2.3 (0.6) | 1.0 (0.5) | 1.7 (0.4) |
| PLA | 2.3 (0.6) | 0.9 (0.2) | 2.0 (0.5) | 1.9 (0.5) | 0.6 (0.8) | 1.8 (0.5) |
Note. ENC = Alcohol prior to encoding; CON = Alcohol prior to consolidation; PLA = Placebo prior to encoding and consolidation
Cued recollection test
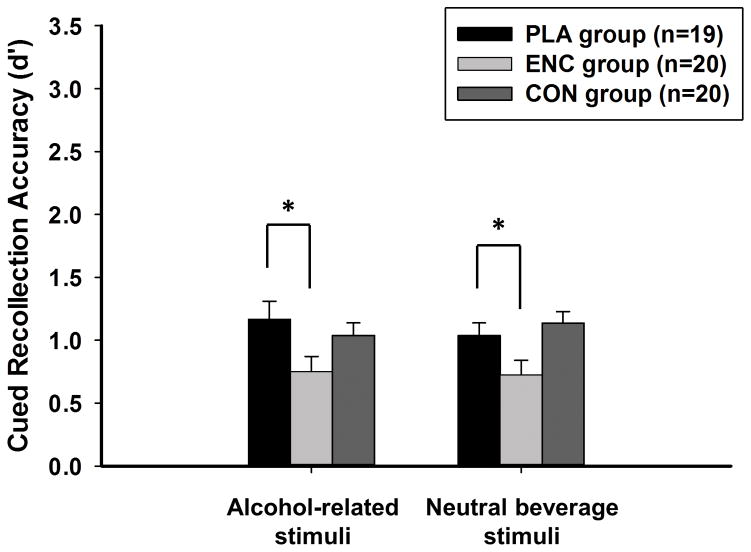
One outlier was removed from the CON condition due to a d′ score greater than 3.5 standard deviations above the mean. Figure 2 presents mean cued recollection accuracy (d′) by condition for alcohol-related and neutral beverage stimuli. Contrary to hypothesis, alcohol did not differentially affect cued recollection for alcohol-related and neutral beverage stimuli (condition × image: p = 0.487). Alcohol effects were examined collapsing across valence [main effect of condition, F(2, 55) = 5.2, p = 0.009, partial η2 = 0.16]. Alcohol during encoding impaired cued recollection (Dunnett t test comparing ENC to PLA: p = 0.012) whereas alcohol during consolidation did not affect cued recollection (Dunnett t test comparing CON to PLA: p = 0.992). Mean cued recollection hit rate and false alarm rate are presented individually for alcohol-related and neutral beverage stimuli in Table 2.
Figure 2.
Mean (SE) cued recollection accuracy (d′) during the drug-free retrieval session for alcohol-related and neutral beverage stimuli in the three conditions. PLA = Placebo; ENC = Encoding; CON = Consolidation. * indicates p < 0.05.
Table 2.
Mean (SD) hit rate and false alarm rate in the retrieval session by condition
| Cued Recollection | ||||
|---|---|---|---|---|
| Hit rate | False alarm rate | |||
| Condition | Alcohol-related | Neutral beverage | Alcohol-related | Neutral beverage |
| ENC | 0.63 (0.19) | 0.42 (0.21) | 0.35 (0.16) | 0.18 (0.11) |
| CON | 0.73 (0.16) | 0.62 (0.18) | 0.36 (0.17) | 0.22 (0.12) |
| PLA | 0.77 (0.15) | 0.60 (0.16) | 0.36 (0.12) | 0.23 (0.13) |
| Recognition | ||||
|---|---|---|---|---|
| Hit rate | False alarm rate | |||
| Condition | Alcohol-related | Neutral beverage | Alcohol-related | Neutral beverage |
| ENC | 0.79 (0.13) | 0.76 (0.19) | 0.12 (0.13) | 0.06 (0.08) |
| CON | 0.93 (0.17) | 0.93 (0.07) | 0.07 (0.07) | 0.04 (0.05) |
| PLA | 0.86 (0.11) | 0.87 (0.12) | 0.12 (0.12) | 0.07 (0.07) |
Note. ENC = Alcohol prior to encoding; CON = Alcohol prior to consolidation; PLA = Placebo prior to encoding and consolidation
Recognition test
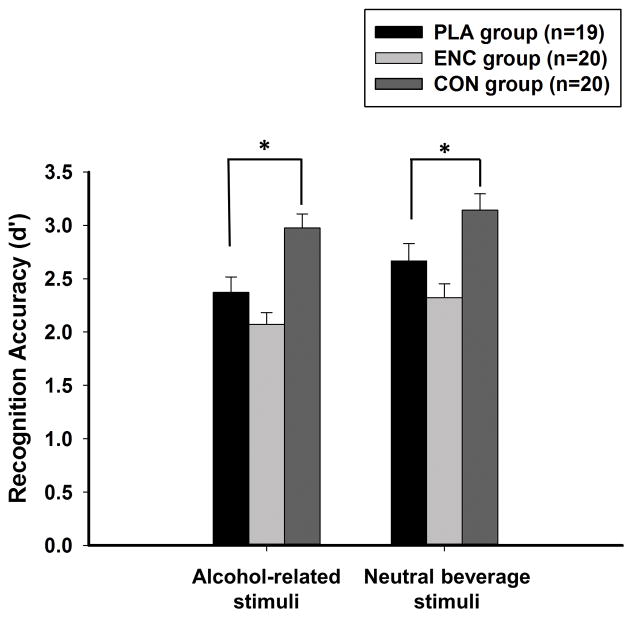
Figure 3 presents mean recognition accuracy (d′) by condition for alcohol-related and neutral beverage stimuli. As with cued recollection, alcohol did not differentially affect recognition for alcohol-related and neutral beverage stimuli (condition × image: p = 0.854), so alcohol effects were again examined collapsing across valence [main effect of condition, F(2, 56) = 15.2, p < 0.001, partial η2 = 0.35]. Alcohol during encoding did not affect recognition (Dunnett t test comparing ENC to PLA: p = 0.09), whereas alcohol during consolidation significantly improved recognition accuracy (Dunnett t test comparing CON to PLA: p = 0.003). Across conditions, recognition accuracy was greater for neutral beverage stimuli compared to alcohol-related stimuli [main effect of valence, F(1, 56) = 6.4, p = 0.014, partial η2 = 0.10]. Mean recognition hit rate and false alarm rate are presented individually for alcohol-related and neutral beverage stimuli in Table 2.
Figure 3.
Mean (SE) recognition accuracy (d′) during the drug-free retrieval session for alcohol-related and neutral beverage stimuli in the three conditions. PLA = Placebo; ENC = Encoding; CON = Consolidation. * indicates p < 0.05.
Stimulation and Sedation
The conditions did not differ in baseline measures of Stimulation or Sedation (ps > 0.17). Alcohol did not significantly affect peak change STIM-SED scores during the encoding phase [ENC = −8.4 (SD =28.1) vs. PLA = −20.6 (SD=28.8); p = 0.19], or during the early consolidation phase [CON = 8.8 (SD=33.4) vs. PLA = 12.7 (SD =31.2); p = 0.72]. The lack of alcohol effect in the sample overall is likely due to the marked variability in scores within both the ENC and CON conditions, with some participants reporting positive STIM-SED values, indicating greater stimulation relative to sedation, and others reporting more negative STIM-SED values, indicating greater sedation relative to stimulation (ENC STIM-SED range = −58 – 44; CON STIM-SED range = −40 – 87). Within the Encoding group specifically, half of the participants (n=9) had positive STIM-SED scores (range = 5 to 44; ‘Stimulant responders’), and half (n=11) had negative STIM-SED scores (range = −58 to −5; ‘Sedative responders’). Alcohol increased stimulation relative to placebo in the Stimulant responders only, whereas alcohol increased sedation relative to placebo in the Sedative responders only. Supplementary Figure 1 presents raw Stimulation and Sedation scores separately for the Encoding (Stimulant and Sedative responders) and Placebo Conditions during the viewing period.
Associations between STIM-SED and memory accuracy
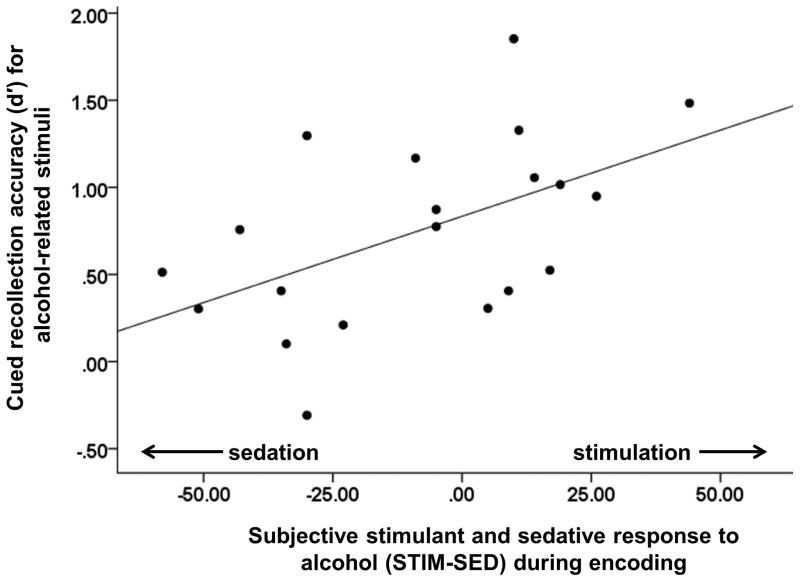
Correlational analyses showed that individual differences in STIM-SED scores during stimulus viewing in the ENC condition predicted cued recollection of alcohol-related (r = 0.52, p = 0.018), but not neutral beverage (r = 0.29, p = 0.219), stimuli. Figure 4 shows a scatter plot illustrating that greater alcohol-induced stimulation relative to sedation was associated with greater cued recollection accuracy for alcohol-related stimuli. By contrast, individual differences in alcohol effects on STIM-SED scores during the early consolidation period were not related to cued recollection of alcohol-related or neutral stimuli (ps > 0.14) in the CON condition, and STIM-SED scores did not predict cued recollection accuracy during encoding or early consolidation in the PLA condition (ps > 0.34). Finally, no associations were observed between STIM-SED scores and recognition accuracy in any condition (ps >= 0.05).
Figure 4.
Association between subjective stimulant and sedative responses to alcohol (STIM-SED) during stimulus viewing in the ENC condition (alcohol prior to encoding) and cued recollection accuracy for alcohol-related stimuli (d′) during the drug-free retrieval session.
Associations between STIM-SED and drinking habits
No associations were observed between STIM-SED scores and measures of alcohol consumption as reported on the TLFB (average drinks per week and number of binge episodes) in either the Encoding or the Consolidation group (ps > 0.10).
Associations between stimulus ratings and memory accuracy
No consistent associations were observed between stimulus ratings (i.e., liking, valence, arousal) and cued recollection, recognition, or STIM-SED scores (ps > 0.05).
Discussion
This study examined the effects of alcohol during encoding and early consolidation on memory accuracy for alcohol-related and neutral beverage stimuli, when subjects were tested two days later. Alcohol during encoding impaired cued recollection accuracy, whereas alcohol during early consolidation enhanced recognition accuracy. Contrary to hypothesis, alcohol did not differentially affect memory for alcohol-related and neutral beverage stimuli when administered prior to encoding or early consolidation. However, individual differences in sensitivity to alcohol’s rewarding effects influenced memory for alcohol-related stimuli. Specifically, for individuals who consumed alcohol prior to encoding, those who experienced greater stimulant effects had greater memory for alcohol-related, but not neutral-beverage, stimuli.
Our findings that alcohol impaired encoding and enhanced early consolidation of memory for beverage-related stimuli are consistent with previous studies that have examined alcohol effects on memory across a range of stimulus categories. First, these results are largely consistent with those observed for emotional and neutral stimuli in this same sample (Weafer et al., 2016). Specifically, alcohol also impaired encoding and enhanced consolidation for emotional and neutral stimuli, and the magnitude of alcohol effects differed according to the specific memory tests employed (i.e., cued recollection vs. recognition) for beverage-related, emotional, and neutral stimuli (see Weafer et al., 2016 for a discussion). Further, in previous studies from other laboratories, alcohol prior to encoding impaired memory for both neutral and emotionally-valenced stimuli, whether the stimuli were presented as images, word pairs, or in narrative form (Bisby et al., 2010; Brown et al., 2010; Knowles and Duka, 2004; Mintzer and Griffiths, 2002; Ray et al., 2012). Similarly, alcohol prior to early consolidation improved recall and recognition of affective and neutral words, statements, and pictures (Bruce and Pihl, 1997; Bruce et al., 1999a; Knowles and Duka, 2004; Mann et al., 1984; Parker et al., 1981). Taken together, these findings suggest that alcohol effects on memory for the appetitive (i.e., alcohol-related and neutral beverage) stimuli tested here follow the same general pattern observed for other types of material, including affective and neutral stimuli.
Contrary to hypothesis, alcohol did not enhance memory for alcohol-related over neutral beverage stimuli. This hypothesis was based on addiction theories suggesting that drug effects on reward pathways create learning signals to facilitate learning about stimuli in the environment associated with the drug (Everitt et al., 2001; Hyman et al., 2006; Torregrossa et al., 2011). As such, facilitation of memory for drug-specific stimuli would only be expected in the context of positive, rewarding drug effects. It is important to note, however, that we did not observe a main effect of alcohol on STIM-SED scores in either the encoding or consolidation condition relative to the placebo control condition, suggesting that, on average within the sample as a whole, alcohol did not produce significant stimulant rewarding effects. It is possible that the lack of an overall increase in alcohol-induced stimulation contributed to the failure to observe enhanced memory for alcohol-related stimuli.
Although alcohol did not increase stimulation in the sample as a whole, there were marked individual differences in STIM-SED scores. Some participants showed a clear increase in stimulation relative to sedation, while others showed the opposite response (i.e., increase in sedation relative to stimulation). Moreover, individual difference analyses supported the hypothesis that alcohol’s rewarding effects are associated with enhanced memory for alcohol-related images. Specifically, individuals who reported greater alcohol-induced stimulation had better memory accuracy for alcohol-related, but not neutral beverage, stimuli. Although speculative at this point, these findings suggest that, despite an overall impairing effect of alcohol on encoding, the rewarding stimulant effects of the drug might serve to increase learning about environmental stimuli specifically associated with its use.
This study had several limitations. First, the between-subjects design did not allow us to determine if stimulant responders would also be more likely to remember alcohol-related stimuli when viewed in a sober state. However, the lack of an association between stimulation and alcohol-related memory in the consolidation condition (when stimuli were viewed following placebo) suggests that this might not be the case. Instead, it seems that the association between alcohol reward and memory for alcohol stimuli is contingent on simultaneous presentation of stimuli and experience of alcohol reward, but future studies will be needed to directly test this hypothesis. A second limitation concerns our use of images of alcohol beverages for the alcohol-related stimuli. As all participants were regular drinkers, they would have necessarily formed associations between the presented stimuli and alcohol consumption based on previous learning outside of the laboratory environment. It will be important for future studies to examine associations between stimulant responses and memory for novel stimuli paired with alcohol consumption to gain a better understanding of alcohol effects on memory formation. Third, specific characteristics of the alcohol-related and neutral beverage stimuli may have limited our ability to observe enhanced memory for alcohol compared to neutral beverage stimuli. Specifically, the similarity of content was greater for alcohol-related stimuli (consisting of beer, wine, or liquor) compared to neutral beverage stimuli (consisting of a range of beverages, including water, milk, coffee, tea, soda, and juice). Additionally, the alcohol-related stimuli were not personalized (i.e., they did not necessarily represent the alcoholic beverages subjects usually drink). Personalized cues are more likely to have developed incentive properties (Christiansen et al., 2015), and thus a memory bias for alcohol stimuli may be more readily detectable with personalized rather than generic stimuli. Finally, the administration of a single dose of alcohol did not allow us to determine whether similar effects would have been observed at other doses. However, alcohol effects on memory consolidation for neutral stimuli are dose-dependent (Parker et al., 1981), and so we expect the same to be true here.
These findings could have important implications for the increased risk for alcohol abuse in individuals who are especially sensitive to the stimulant effects of alcohol. That is, in addition to experiencing stronger rewarding effects from drinking, stimulant responders may also be more likely to form associations with alcohol-related stimuli in memory, and in turn more likely to associate those stimuli with alcohol’s rewarding effects. Over time and with repeated associations between alcohol stimuli and alcohol reward, such associations will become stronger, and the stimuli may become increasingly likely to evoke positive memories of previous drinking episodes in these individuals. Such memory activations may induce craving, followed by alcohol-seeking and consumption (Everitt et al., 2001; Robinson and Berridge, 2001). Taken together, this suggests that stimulant responders may be at increased risk for developing these strong associations in memory, and that alcohol cues might have a stronger influence on drinking behavior in these individuals.
Supplementary Material
Acknowledgments
Funding: This research was supported by NIDA DA02812 (HdW) and DA031796 (HdW).
Footnotes
The complete picture set is available upon request from the Corresponding Author.
The authors have no conflict of interest to declare.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: Author; 2000. test rev. [Google Scholar]
- Bisby JA, Leitz JR, Morgan CJ, Curran HV. Decreases in recollective experience following acute alcohol: a dose-response study. Psychopharmacology (Berl) 2010;208(1):67–74. doi: 10.1007/s00213-009-1709-y. [DOI] [PubMed] [Google Scholar]
- Brown J, Brignell CM, Dhiman SK, Curran HV, Kamboj SK. Acute effects of alcohol on memory: impact of emotional context and serial position. Neurobiol Learn Mem. 2010;93(3):428–34. doi: 10.1016/j.nlm.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Bruce KR, Pihl RO. Forget “drinking to forget”: enhanced consolidation of emotionally charged memory by alcohol. Exp Clin Psychopharmacol. 1997;5(3):242–50. doi: 10.1037//1064-1297.5.3.242. [DOI] [PubMed] [Google Scholar]
- Bruce KR, Pihl RO, Mayerovitch JI, Shestowsky JS. Alcohol and retrograde memory effects: role of individual differences. J Stud Alcohol. 1999a;60(1):130–6. doi: 10.15288/jsa.1999.60.130. [DOI] [PubMed] [Google Scholar]
- Bruce KR, Shestowsky JS, Mayerovitch JI, Pihl RO. Motivational effects of alcohol on memory consolidation and heart rate in social drinkers. Alcohol Clin Exp Res. 1999b;23:693–701. [PubMed] [Google Scholar]
- Christiansen P, Mansfield R, Duckworth J, Field M, Jones A. Internal reliability of the alcohol-related visual probe task is increased by utilizing personalized stimuli and eye-tracking. Drug Alcohol Depend. 2015;155:170–174. doi: 10.1016/j.drugalcdep.2015.07.672. [DOI] [PubMed] [Google Scholar]
- Courtney KE, Schacht JP, Hutchison K, Roche DJ, Ray LA. Neural substrates of cue reactivity: association with treatment outcomes and relapse. Addict Biol. 2016;21:3–22. doi: 10.1111/adb.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, Acquas E, Carboni E, Valentini V, Lecca D. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47(Suppl 1):227–41. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Drummond DC. What does cue-reactivity have to offer clinical research? Addiction. 2000;95(Suppl 2):S129–44. doi: 10.1080/09652140050111708. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Res Brain Res Rev. 2001;36(2–3):129–38. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- Field M, Cox WM. Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug Alcohol Depend. 2008;97(1–2):1–20. doi: 10.1016/j.drugalcdep.2008.03.030. [DOI] [PubMed] [Google Scholar]
- Field M, Munafo MR, Franken IH. A meta-analytic investigation of the relationship between attentional bias and subjective craving in substance abuse. Psychol Bull. 2009;135(4):589–607. doi: 10.1037/a0015843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore MT. Cognitive preoccupation with alcohol and binge drinking in college students: alcohol-induced priming of the motivation to drink. Psychol Addict Behav. 2001;15(4):325–32. [PubMed] [Google Scholar]
- Gallo DA, Foster KT, Johnson EL. Elevated false recollection of emotional pictures in young and older adults. Psychol Aging. 2009;24(4):981–8. doi: 10.1037/a0017545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, Kupchik YM, Kalivas PW. Rapid, transient synaptic plasticity in addiction. Neuropharmacology. 2014;76(Pt B):276–86. doi: 10.1016/j.neuropharm.2013.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdstock L, de Wit H. Individual differences in the biphasic effects of ethanol. Alcohol Clin Exp Res. 1998;22(9):1903–11. [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–98. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Jasinska AJ, Stein EA, Kaiser J, Naumer MJ, Yalachkov Y. Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies. Neurosci Biobehav Rev. 2014;38:1–16. doi: 10.1016/j.neubiorev.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. Recent understanding in the mechanisms of addiction. Curr Psychiatry Rep. 2004;6(5):347–51. doi: 10.1007/s11920-004-0021-0. [DOI] [PubMed] [Google Scholar]
- King AC, de Wit H, McNamara PJ, Cao D. Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch Gen Psychiatry. 2011;68(4):389–99. doi: 10.1001/archgenpsychiatry.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Hasin D, O'Connor SJ, McNamara PJ, Cao D. A prospective 5-year reexamination of alcohol response in heavy drinkers progressing in alcohol use disorder. Biol Psychiatry. 2016;79:489–98. doi: 10.1016/j.biopsych.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, McNamara PJ, Hasin DS, Cao D. Alcohol challenge responses predict future alcohol use disorder symptoms: a 6-year prospective study. Biol Psychiatry. 2014;75(10):798–806. doi: 10.1016/j.biopsych.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles SK, Duka T. Does alcohol affect memory for emotional and non-emotional experiences in different ways? Behav Pharmacol. 2004;15(2):111–21. doi: 10.1097/00008877-200403000-00003. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. NIMH Center for the study of emotion and attention. University of Florida; Gainesville, FL: 1999. International Affective Picture System (IAPS): Technical manual and affective ratings. [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30(3):261–73. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Larsen JT, Norris CJ, McGraw AP, Hawkley LC, Cacioppo JT. The evaluative space grid: A single-item measure of positivity and negativity. Cogn Emot. 2009;23:453–480. [Google Scholar]
- Mann RE, Cho-Young J, Vogel-Sprott M. Retrograde enhancement by alcohol of delayed free recall performance. Pharmacol Biochem Behav. 1984;20(4):639–42. doi: 10.1016/0091-3057(84)90317-4. [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res. 1993;17(1):140–6. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Griffiths RR. Alcohol and triazolam: differential effects on memory, psychomotor performance and subjective ratings of effects. Behav Pharmacol. 2002;13(8):653–8. doi: 10.1097/00008877-200212000-00007. [DOI] [PubMed] [Google Scholar]
- Mulvihill LE, Skilling TA, Vogel-Sprott M. Alcohol and the ability to inhibit behavior in men and women. J Stud Alcohol. 1997;58(6):600–5. doi: 10.15288/jsa.1997.58.600. [DOI] [PubMed] [Google Scholar]
- Newlin DB, Thomson JB. Alcohol challenge with sons of alcoholics: a critical review and analysis. Psychol Bull. 1990;108(3):383–402. doi: 10.1037/0033-2909.108.3.383. [DOI] [PubMed] [Google Scholar]
- Niaura RS, Rohsenow DJ, Binkoff JA, Monti PM, Pedraza M, Abrams DB. Relevance of cue reactivity to understanding alcohol and smoking relapse. J Abnorm Psychol. 1988;97(2):133–52. doi: 10.1037//0021-843x.97.2.133. [DOI] [PubMed] [Google Scholar]
- Parker ES, Birnbaum IM, Weingartner H, Hartley JT, Stillman RC, Wyatt RJ. Retrograde enhancement of human memory with alcohol. Psychopharmacology (Berl) 1980;69(2):219–22. doi: 10.1007/BF00427653. [DOI] [PubMed] [Google Scholar]
- Parker ES, Morihisa JM, Wyatt RJ, Schwartz BL, Weingartner H, Stillman RC. The alcohol facilitation effect on memory: a dose-response study. Psychopharmacology (Berl) 1981;74(1):88–92. doi: 10.1007/BF00431763. [DOI] [PubMed] [Google Scholar]
- Quinn PD, Fromme K. Subjective response to alcohol challenge: a quantitative review. Alcohol Clin Exp Res. 2011;35(10):1759–70. doi: 10.1111/j.1530-0277.2011.01521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevski E, Gueorguieva R, Limoncelli DD, Husain R, Jane JS, Petrakis I. Gelatin “shots” as a new method for alcohol administration in a laboratory setting. Alcohol Clin Exp Res. 2006;30:473–479. doi: 10.1111/j.1530-0277.2006.00064.x. [DOI] [PubMed] [Google Scholar]
- Ray S, Mun EY, Buckman JF, Udo T, Bates ME. Memory for emotional picture cues during acute alcohol intoxication. J Stud Alcohol Drugs. 2012;73(5):718–25. doi: 10.15288/jsad.2012.73.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96(1):103–14. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Myrick H. Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addict Biol. 2012;18(1):121–33. doi: 10.1111/j.1369-1600.2012.00464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: applications to dementia and amnesia. J Exp Psychol Gen. 1988;117(1):34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Humana Press; Totowa, NJ: 1992. pp. 41–72. [Google Scholar]
- Torregrossa MM, Corlett PR, Taylor JR. Aberrant learning and memory in addiction. Neurobiol Learn Mem. 2011;96(4):609–23. doi: 10.1016/j.nlm.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weafer J, Gallo DA, de Wit H. Amphetamine fails to alter cued recollection of emotional images: study of encoding, retrieval, and state-dependency. PLoS One. 2014;9(2):e90423. doi: 10.1371/journal.pone.0090423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weafer J, Gallo DA, de Wit H. Acute effects of alcohol on encoding and consolidation of memory for emotional stimuli. J Stud Alcohol Drugs. 2016;77:86–94. doi: 10.15288/jsad.2016.77.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorumski CF, Mennerick S, Izumi Y. Acute and chronic effects of ethanol on learning-related synaptic plasticity. Alcohol. 2014;48(1):1–17. doi: 10.1016/j.alcohol.2013.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.