Abstract
Steroid receptor coactivator (SRC) family members (SRC-1, SRC-2, SRC-3) interact with nuclear receptors (NRs) and many transcription factors to enhance target gene transcription. Deregulation of SRCs is widely implicated in NR mediated diseases, especially hormone dependent cancers. By integrating steroid hormone signaling and growth factor pathways, SRC proteins exert multiple modes of oncogenic regulation in cancers and represent emerging targets for cancer therapeutics. Recent work has identified SRC-targeting agents that show promise in blocking tumor growth in vitro and in vivo, and have the potential to function as powerful and broadly encompassing treatments for different cancers.
Keywords: Androgen Receptor, Focal Adhesion Kinase, Breast Cancer Cell Proliferation, Steroid Receptor Coactivator, Steroid Hormone Signaling
Introduction
The nuclear receptor (NR) superfamily of transcription factor is composed of ligand-dependent members and orphan receptors whose ligands have not yet been identified or do not have a cognate ligand. The former respond to and transduce hormonal signals into transcriptional programs that drive physiological functions. Decades of research has gained tremendous insight into the actions of NRs on target gene promoters, revealing that they work in close collaboration with coactivators and corepressors to enhance and repress gene transcription, respectively.
The first NR coactivator, steroid receptor coactivator 1 (SRC-1/NCOA1) was identified two decades ago by our laboratory in a yeast two-hybrid screen as a protein that interacts with the ligand binding domain of the progesterone receptor (PR) and it was found to strongly potentiate the transcriptional activities of PR, the estrogen receptor α (ERα) and many other NRs in a ligand-dependent manner [1]. SRC-2 (NCOA2/TIF2/GRIP1) and SRC-3 (NCOA3/AIB1/RAC3/ACTR/pCIP) were cloned and characterized shortly afterwards [2–9]. These three proteins comprise the most in-depth studied coactivator family. SRC family members share ∼60 % sequence similarity and contain several conserved structural domains: an N-terminal basic helix-loop-helix-Per/ARNT/Sim (bHLH-PAS) domain, a central NR interaction domain (RID) with three LXXLL motifs, and two activation domains (AD1 and AD2) at the C-terminus [10, 11]. Once recruited to target gene promoters by NRs, SRC proteins can assemble a multi-protein coactivator complex by further recruiting secondary coactivators and histone modifying enzymes such as CBP/p300, coactivator associated arginine methyltransferase 1 (CARM1) and protein arginine methyltransferase 1 (PRMT1) to increase chromatin accessibility for active transcription [6, 12–14]. From knock-out mouse models, we have learned that although highly homologous to one another, SRC proteins are functionally distinct and that each controls distinct physiological processes, including growth and development, reproduction, and energy homeostasis [10, 15]. As our knowledge of the biology of SRC proteins improves, it is clear now that these proteins undergo heavy post-translational modifications which dictate their preference for transcription factors and alter their protein stability and transcriptional activity [16, 17]. This has revealed multiple layers of complexity to the transcriptional programs controlled by SRC proteins leading to the realization that SRCs are key integrators of extracellular environmental cues and upstream signaling pathways with cellular transcriptional programs.
Due to their strong association with NRs, SRC proteins have been found to be intricately involved in the physiological and pathological processes in which these receptors are implicated. For instance, SRC proteins are fundamental in the proper functions of steroid hormones; abnormal changes that lead to altered SRC expression or activity can be a driving force in the initiation and progression of metabolic diseases, reproductive diseases, and especially cancers in hormone-sensitive tissues. This review will focus on the roles of SRC proteins in hormone dependent cancers and their therapeutic potentials.
Breast Cancer
Ever since its initial identification, SRC-3 has been recognized as a very prominent player in breast cancer. The gene encoding SRC-3 is amplified at a frequency of approximately 5–10 % in breast cancers [5, 18], while SRC-3 mRNA or protein has been found to be overexpressed in up to 60 % of cases in different breast cancer cohorts [5, 19, 20]. Clinically, SRC-3 overexpression in breast cancer correlates with larger tumor size [18], higher tumor grade [21] and poor survival rates [22]. Both in vitro and in vivo breast cancer models have provided valuable information to help us understand SRC-3’s role in breast cancer. SRC-3 has been shown to mediate estrogen-dependent breast cancer cell proliferation and survival [23, 24]. In vivo, loss of SRC-3 significantly reduces breast tumor incidence, and delays tumor growth and development in breast cancer mouse models driven by oncogenes (i.e., MMTV-v-ras, MMTV-Erbb2) [25, 26] or induced by chemical carcinogens [27]. In these models, insulin-like growth factor 1 (IGF1) and ERBB2 signaling pathways are impaired in the absence of SRC-3. Direct evidence supporting the oncogenic role of SRC-3 comes from the MMTV-SRC-3 transgenic mouse model, which shows that overexpressing SRC-3 is sufficient to cause spontaneous development of malignant mammary tumors as well as tumors in other organs including the pituitary and uterus [28]. Furthermore, genetic ablation of SRC-3 in the MMTV-PyMT mice dramatically reduces breast cancer metastasis to the lung [29]. Molecular analysis reveals that SRC-3 coactivates the Ets transcription factor polyoma enhancer activator 3 (PEA3) to promote the expression of matrix metalloproteinase 2 (MMP2) and MMP9. Interestingly, the expression of SRC-3 in human breast tumors positively correlates with that of PEA3, MMP2, and MMP9. A similar report from a study using MDA-MB-231 breast cancer cells shows that SRC-3 regulates activating protein −1 (AP-1)-driven MMP7 and MMP10 expression [30]. These findings demonstrate that SRC-3 is a critical pro-metastatic factor in breast cancer that also upregulates MMP expression to promote cancer cell invasion. Clinical data from breast cancer patients with or without tamoxifen treatment indicate that high SRC-3 expression is associated with worse disease free survival, implicating SRC-3 in therapy resistance [31].
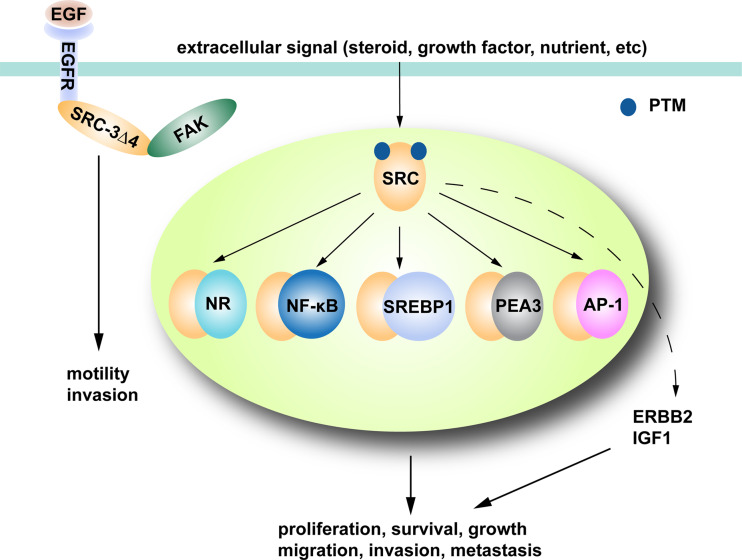
Intriguingly, a splice variant of SRC-3 (SRC-3Δ4) was recently demonstrated as a bridging adaptor between the epidermal growth factor receptor (EGFR) and focal adhesion kinase (FAK) that potentiates cancer cell migration and invasion in a non-genomic manner [32]. Lacking the bHLH-PAS domain which contains the nuclear localization sequence, SRC-3Δ4 is mainly localized in the cytosol. Upon cancer cell exposure to EGF, SRC-3Δ4 is phosphorylated by p21-activated kinase 1 (PAK1), which triggers its translocation to the plasma membrane where it binds to both EGFR and FAK, allowing signal transduction and full activation of this signaling cascade essential for cancer cell invasion and metastasis.
Expression of SRC-1 also is significantly increased in about 20 % of breast cancers, and is positively correlated with ERBB2 expression, disease recurrence, and poor survival [33–35]. SRC-1 is overexpressed in aromatase inhibitor-resistant breast tumors [36]. Although SRC-1 promotes estrogen-dependent breast cancer cell growth and proliferation in vitro [37, 38], in an MMTV-PyMT breast cancer mouse model, SRC-1 deficiency does not affect tumor initiation and growth, but drastically inhibits lung metastasis [39]. Mechanistic studies have shown that SRC-1 promotes metastasis by regulating the expression of colony stimulating factor 1 (CSF1) which recruits tumor associated macrophages [39], and by coactivating PEA3-mediated expression of TWIST1, a master regulator of epithelial-mesenchymal-transition (EMT) [40].
Despite being reported to mediate estrogen-induced breast cancer cell proliferation and target gene expression in vitro [41], compared with SRC-3 and SRC-1, the role of SRC-2 in breast cancer has been less well characterized.
Prostate Cancer
A comprehensive sequencing analysis of human prostate tumors, prostate cancer cell lines, and xenografts has shown that 8 % of primary and 37 % of metastatic tumors have gain-of-expression (overexpression and amplification) of the SRC-2 gene [42]. Primary tumors with SRC-2 amplification display increased androgen receptor (AR) signaling based on their AR target gene signature, consistent with the known role of SRC-2 as an AR coactivator. Moreover, castrate patients with primary tumors harboring SRC-2 mutations, overexpression, or amplification have higher rates of recurrence [42]. These findings are in agreement with previous reports about the positive correlation between SRC-2 expression and high tumor grade and poor survival [43–45]. Hence, SRC-2 has been proposed as a driver oncogene in primary prostate tumors. Since SRC-2 has long been recognized as a master regulator of energy homeostasis [46–49], a recent study from our laboratory probed the functional significance of SRC-2 upregulation in prostate cancer metabolic programming and found that SRC-2 can drive glutamine-dependent and sterol regulatory element binding protein 1 (SREBP1)-mediated de novo lipogenesis, supporting prostate cancer cell survival and metastasis [50].
SRC-3 has been reported to be overexpressed in prostate cancers and its expression level is positively correlated with tumor grade and disease recurrence [51]. SRC-3 not only is required for prostate cancer cell proliferation and survival [52, 53], but also promotes invasion and metastasis by activating FAK and focal adhesion turnover as well as by increasing the expression of MMP2 and MMP13 [54]. A high level of SRC-1 also is found in recurrent prostate cancer [45], and it has been shown in cell culture to promote AR function [55].
Endometrial Cancer and Ovarian Cancer
As coactivators of ERα and PR, roles for SRC family proteins have been investigated in malignancies in the endometrium and ovary. It has been reported that the mRNA levels of all three SRCs are significantly increased in endometrial carcinoma [56]. In particular, SRC-3 expression is correlated with clinical stage, depth of myometrial invasion and differentiation, as well as poor prognosis [57, 58].
Even at the time of its discovery, the SRC-3 gene was found to be amplified in ovarian cancer [5, 18]. Additionally, upregulation of SRC-3 has been observed in 64 % of high-grade ovarian cancers, and its level is positively associated with disease severity [59]. Interestingly, independent of ERα status, SRC-3 is required for the correct cellular localization of FAK in ovarian cancer cells, and experimental disruption of SRC-3 expression blocks ovarian cancer cell motility and migration [59].
SRC Family Proteins as Therapeutic Targets
As coactivators of steroid NRs, aberrant overactivation or overexpression of SRC family proteins greatly enhances steroid hormone signaling, giving a competitive edge for cancer to develop in hormone-sensitive organs. Moreover, the ability of SRC proteins to coactivate many other transcription factors such as NF-κB [60], SREBP1 [50], PEA3 [29, 40] and AP-1 [30, 54] extends their impact to more pathways pertinent to cancer cells. Importantly, SRC proteins can be phosphorylated and activated by various kinases so that they can receive extracellular signals and convert them into distinct and enhanced transcriptional outputs crucial for cancer cell proliferation, survival, and metastasis (Fig. 1). For instance, seven Ser/Thr (Thr24, Ser505, Ser543, Ser601, Ser857, Ser860, and Ser867) and one Tyr (Tyr1357) phosphorylation sites on SRC-3 have been identified, which are targeted by different kinases such as MAPK, IKK, GSK3, PKA, casein kinase 1 isoform δ (CK1δ) and ABL, under the stimulation of steroid hormones or growth factors, resulting in increased SRC-3 coactivator function and gene transcription [61–63]. Similarly, phosphorylation of Thr1179, and Ser1185 on SRC-1 by IL-6 signaling or MAPK augments its affinity for NRs and leads to stronger NR-dependent transcription [64, 65]. SRC-2 also has been reported to be activated by phosphorylations on Ser469, Ser487, Ser493, Ser499, Ser699, or Ser736 mediated by casein kinase (CK), cyclin-dependent kinase 9 (CDK9), MAPK, or mTOR [50, 66–69]. Therefore, SRC proteins are central integrators and promoters of multiple signaling pathways, which makes them key targets for future cancer drug development. Because inhibiting SRCs can simultaneously interfere with many pathways, the chance of cancer cells to develop resistance and evade therapy to these agents is expected to be significantly reduced.
Fig. 1.
SRC family proteins are integrators of multiple signaling pathways crucial for tumor progression. SRC proteins partner with different NRs and transcription factors in the nucleus to activate target gene transcription important for cancer cell proliferation, growth, survival, and metastasis. SRCs also promote the oncogenic ERBB2 and IGF1 pathways. SRCs can be regulated by extracellular signals through post-translational modification (PTM), so that they can generate transcriptional outputs in a signal-dependent manner. SRC-3Δ4, an SRC-3 splice variant, can mediate signal transduction from EGFR to FAK in the cytosol and enhance the migratory potential of cancer cells
Lacking a structurally defined enzymatic activation domain or a high affinity ligand binding domain, SRC proteins have not been the focus of current drug development which has largely been restricted to a small number of proteins. However, given the key roles of SRCs in tumorigenesis, the difficulty in their targeting is outweighed by the potential therapeutic benefits. Efforts have been invested to identify compounds which can disrupt the binding of SRC family members to NRs such as ERα, ERβ, and PPARγ [70–72]. In a proof-of-principle study, our laboratory initially identified gossypol as a small molecule inhibitor (SMI) of SRC-1 and SRC-3 which can target these coactivators for degradation and cause cell death in various cancer cell lines, for the first time establishing that SRC proteins can be targeted by small molecule compounds [73].
A subsequent high-throughput screening of a chemical library containing compounds from the large NIH-Molecular Libraries Probe Production Centers Network (MLPCN) was carried out by our laboratory to identify inhibitors of SRCs’ transcriptional activity. This study led to the identification of improved SRC SMIs, including bufalin and verrucarin A [74, 75]. Bufalin promotes the degradation of SRC-3 and SRC-1 in a proteasome-dependent manner and efficiently blocks cancer cell growth in vitro and in vivo. A water soluble analog, 3-phospho bufalin, was developed to overcome the solubility problem of bufalin, and it was proved to be equally efficient in inhibiting tumor growth in an orthotopic breast cancer model [76]. Verrucarin A also selectively degrades SRC-3, while affecting SRC-1/-2 to a lesser extent, and blocks cancer cell proliferation and migration. Compared with gossypol, bufalin and verrucarin A are more potent SRC SMIs, exerting strong effects at low nanomolar concentrations. Unlike gossypol and bufalin, which directly bind to the RID of SRC-3, verrucarin A does not physically interact with SRCs. Gossypol, bufalin, and verrucarin A are structurally unrelated compounds originally identified for different purposes: gossypol is a natural polyphenol found in cotton seeds which once was pursued as a male contraceptive agent; bufalin is a cardiac glycoside; verrucarin A belongs to a group of sesquiterpene found in toxins of pathogenic fungus, and yet they all can unexpectedly induce SRC protein degradation, implying that ample sources and opportunities exist to identify additional SRC SMIs.
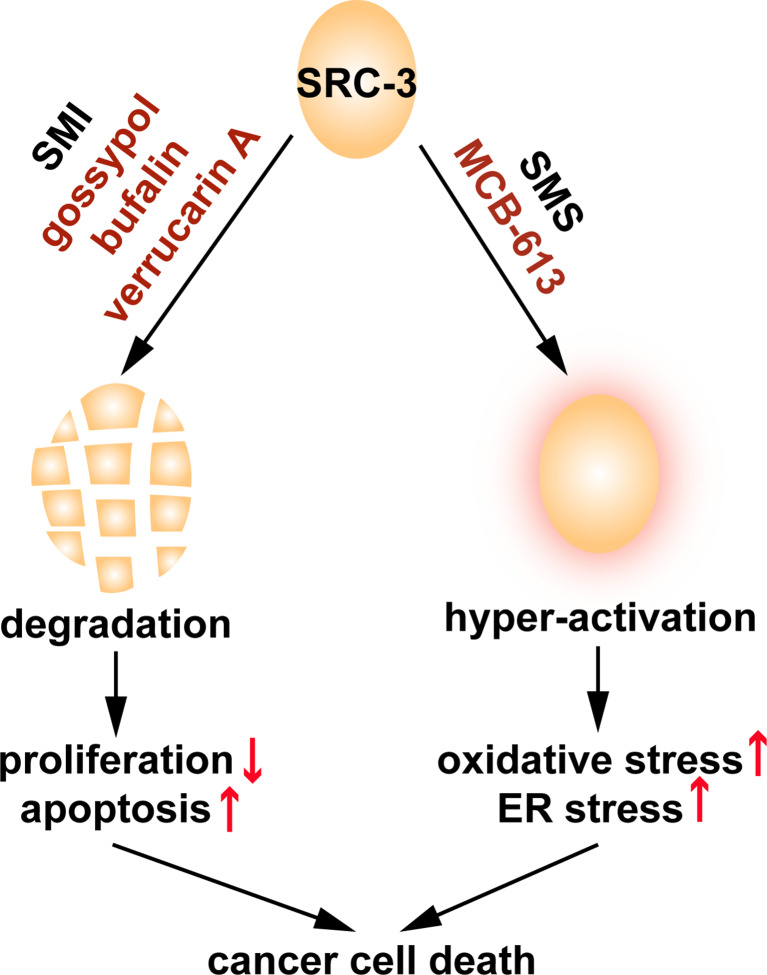
Interestingly, a small molecule stimulator (SMS) of SRCs named MCB-613 also was indentified in the MLPCN high-throughput screen [77]. MCB-613 hyper-stimulates SRCs’ transcriptional activity, efficiently kills cancer cells, and inhibits tumor growth in a breast cancer xenograft mouse model. Molecular analysis revealed that MCB-613 increases SRCs’ interaction with other coactivators such as CBP and CARM1, and markedly elevates the intracellular levels of reactive oxygen species (ROS) which is coupled to the strong induction of endoplasmic reticulum stress. Multiple lines of evidence indicate that phosphorylation plays an important role in the activation of SRC-3 by MCB-613: (1) MCB-613 treatment induces phosphorylation on SRC-3; (2) MCB-613 promotes the interaction of SRC-3 with ABL kinase; and (3) ABL kinase inhibitors significantly impair the activation of SRC-3 by the compound [77]. However, whether ABL is the only kinase involved and which sites on SRCs are phosphorylated still remains an open question. The identification of MCB-613 has opened up a new avenue to target SRCs in cancer treatment. Considering the oncogenic roles of SRCs, it appears to be counterintuitive to employ SRC SMS in treating cancer. However, in comparison with normal cells, cancer cells are under high levels of stress from increased protein synthesis/folding and metabolism due to their highly proliferative nature. It is of utmost importance for them to fully engage their stress response pathways in order to maintain homeostasis, making them more vulnerable than normal cells to stressors such as MCB-613. By acutely over-stimulating SRC family proteins, MCB-613 overloads the stress response system of cancer cells and selectively kills them. Thus, targeting the SRC coactivators, either by inhibition or stimulation, represents a novel and promising approach in cancer therapeutic development (Fig. 2).
Fig. 2.
SRC proteins can be targeted by small molecule compounds. The identified SRC SMIs (gossypol, bufalin, and verrucarin A) can degrade SRC proteins, leading to decreased proliferation and increased apoptosis. MCB-613, an SRC SMS, can hyper-activate the transcriptional activities of SRC proteins and cause cancer cell-specific cytotoxic stress. Although mechanistically different, both of these two classes of chemicals can efficiently kill cancer cells and block tumor growth
Conclusion
Transcriptional coactivators are indispensible for NRs and transcription factors to regulate target gene expression and carry out important physiological functions. Aberrant activity or expression of SRC proteins are found in pathological conditions, and most prominently, in cancers. By integrating and promoting growth signaling pathways upon which cancer cells rely, SRC proteins represent emerging targets for cancer therapeutics and several SRC SMIs or SMS have been identified that show considerable promise in breast cancer mouse models. Although this review focuses on hormone dependent cancers, SRC proteins have been found to be overexpressed in many other cancer types [10]. Therefore, one can foresee that these SRC-targeting drugs could be proved to be widely useful for cancer treatment. Future efforts should be directed not only to broadening our understanding of this group of proteins but also to delivering effective and safe coactivator-targeting agents for clinical use.
Acknowledgments
This work was supported by funding from the Susan G. Komen Foundation (PG12221410), the Prostate Cancer Foundation, the Department of Defense Breast Cancer Research Program (BC120894), the Cancer Prevention and Research Institute of Texas (RP100348 and RP101251), from the National Institutes of Health (DK059820) to BWO; and from the National Institutes of Health (HD076596) to DML. High-throughput screening was supported through the National Institutes of Health Molecular Libraries Program.
Compliance with Ethical Standards
Conflict of Interest
LW, DML, and BWO are co-founders and hold stock in Coregon, Inc., which is developing steroid receptor coactivator stimulators for clinical use.
References
- 1.Onate SA, Tsai SY, Tsai MJ, O’Malley BW. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science. 1995;270:1354–1357. doi: 10.1126/science.270.5240.1354. [DOI] [PubMed] [Google Scholar]
- 2.Hong H, Kohli K, Trivedi A, Johnson DL, Stallcup MR. GRIP1, a novel mouse protein that serves as a transcriptional coactivator in yeast for the hormone binding domains of steroid receptors. Proc Natl Acad Sci U S A. 1996;93:4948–4952. doi: 10.1073/pnas.93.10.4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hong H, Kohli K, Garabedian MJ, Stallcup MR. GRIP1, a transcriptional coactivator for the AF-2 transactivation domain of steroid, thyroid, retinoid, and vitamin D receptors. Mol Cell Biol. 1997;17:2735–2744. doi: 10.1128/MCB.17.5.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voegel JJ, Heine MJ, Zechel C, Chambon P, Gronemeyer H. TIF2, a 160 kDa transcriptional mediator for the ligand-dependent activation function AF-2 of nuclear receptors. EMBO J. 1996;15:3667–3675. [PMC free article] [PubMed] [Google Scholar]
- 5.Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan XY, Sauter G, Kallioniemi OP, Trent JM, Meltzer PS. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science. 1997;277:965–968. doi: 10.1126/science.277.5328.965. [DOI] [PubMed] [Google Scholar]
- 6.Chen H, Lin RJ, Schiltz RL, Chakravarti D, Nash A, Nagy L, Privalsky ML, Nakatani Y, Evans RM. Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell. 1997;90:569–580. doi: 10.1016/S0092-8674(00)80516-4. [DOI] [PubMed] [Google Scholar]
- 7.Li H, Gomes PJ, Chen JD. RAC3, a steroid/nuclear receptor-associated coactivator that is related to SRC-1 and TIF2. Proc Natl Acad Sci U S A. 1997;94:8479–8484. doi: 10.1073/pnas.94.16.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takeshita A, Cardona GR, Koibuchi N, Suen CS, Chin WW. TRAM-1, a novel 160-kDa thyroid hormone receptor activator molecule, exhibits distinct properties from steroid receptor coactivator-1. J Biol Chem. 1997;272:27629–27634. doi: 10.1074/jbc.272.44.27629. [DOI] [PubMed] [Google Scholar]
- 9.Torchia J, Rose DW, Inostroza J, Kamei Y, Westin S, Glass CK, Rosenfeld MG. The transcriptional co-activator p/CIP binds CBP and mediates nuclear-receptor function. Nature. 1997;387:677–684. doi: 10.1038/42652. [DOI] [PubMed] [Google Scholar]
- 10.Xu J, Wu RC, O’Malley BW. Normal and cancer-related functions of the p160 steroid receptor co-activator (SRC) family. Nat Rev Cancer. 2009;9:615–630. doi: 10.1038/nrc2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson AB, O’Malley BW. Steroid receptor coactivators 1, 2, and 3: critical regulators of nuclear receptor activity and steroid receptor modulator (SRM)-based cancer therapy. Mol Cell Endocrinol. 2012;348:430–439. doi: 10.1016/j.mce.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen D, Ma H, Hong H, Koh SS, Huang SM, Schurter BT, Aswad DW, Stallcup MR. Regulation of transcription by a protein methyltransferase. Science. 1999;284:2174–2177. doi: 10.1126/science.284.5423.2174. [DOI] [PubMed] [Google Scholar]
- 13.Koh SS, Chen D, Lee YH, Stallcup MR. Synergistic enhancement of nuclear receptor function by p160 coactivators and two coactivators with protein methyltransferase activities. J Biol Chem. 2001;276:1089–1098. doi: 10.1074/jbc.M004228200. [DOI] [PubMed] [Google Scholar]
- 14.Foulds CE, Feng Q, Ding C, Bailey S, Hunsaker TL, Malovannaya A, Hamilton RA, Gates LA, Zhang Z, Li C, Chan D, Bajaj A, Callaway CG, Edwards DP, Lonard DM, Tsai SY, Tsai MJ, Qin J, O’Malley BW. Proteomic analysis of coregulators bound to ERalpha on DNA and nucleosomes reveals coregulator dynamics. Mol Cell. 2013;51:185–199. doi: 10.1016/j.molcel.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.York B, O’Malley BW. Steroid receptor coactivator (SRC) family: masters of systems biology. J Biol Chem. 2010;285:38743–38750. doi: 10.1074/jbc.R110.193367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han SJ, Lonard DM, O’Malley BW. Multi-modulation of nuclear receptor coactivators through posttranslational modifications. Trends Endocrinol Metab. 2009;20:8–15. doi: 10.1016/j.tem.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lonard DM, O’Malley BW. Nuclear receptor coregulators: judges, juries, and executioners of cellular regulation. Mol Cell. 2007;27:691–700. doi: 10.1016/j.molcel.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 18.Bautista S, Valles H, Walker RL, Anzick S, Zeillinger R, Meltzer P, Theillet C. In breast cancer, amplification of the steroid receptor coactivator gene AIB1 is correlated with estrogen and progesterone receptor positivity. Clin Cancer Res. 1998;4:2925–2929. [PubMed] [Google Scholar]
- 19.Bouras T, Southey MC, Venter DJ. Overexpression of the steroid receptor coactivator AIB1 in breast cancer correlates with the absence of estrogen and progesterone receptors and positivity for p53 and HER2/neu. Cancer Res. 2001;61:903–907. [PubMed] [Google Scholar]
- 20.Glaeser M, Floetotto T, Hanstein B, Beckmann MW, Niederacher D. Gene amplification and expression of the steroid receptor coactivator SRC3 (AIB1) in sporadic breast and endometrial carcinomas. Horm Metab Res. 2001;33:121–126. doi: 10.1055/s-2001-14938. [DOI] [PubMed] [Google Scholar]
- 21.Hudelist G, Czerwenka K, Kubista E, Marton E, Pischinger K, Singer CF. Expression of sex steroid receptors and their co-factors in normal and malignant breast tissue: AIB1 is a carcinoma-specific co-activator. Breast Cancer Res Treat. 2003;78:193–204. doi: 10.1023/A:1022930710850. [DOI] [PubMed] [Google Scholar]
- 22.Zhao C, Yasui K, Lee CJ, Kurioka H, Hosokawa Y, Oka T, Inazawa J. Elevated expression levels of NCOA3, TOP1, and TFAP2C in breast tumors as predictors of poor prognosis. Cancer. 2003;98:18–23. doi: 10.1002/cncr.11482. [DOI] [PubMed] [Google Scholar]
- 23.Planas-Silva MD, Shang Y, Donaher JL, Brown M, Weinberg RA. AIB1 enhances estrogen-dependent induction of cyclin D1 expression. Cancer Res. 2001;61:3858–3862. [PubMed] [Google Scholar]
- 24.List HJ, Lauritsen KJ, Reiter R, Powers C, Wellstein A, Riegel AT. Ribozyme targeting demonstrates that the nuclear receptor coactivator AIB1 is a rate-limiting factor for estrogen-dependent growth of human MCF-7 breast cancer cells. J Biol Chem. 2001;276:23763–23768. doi: 10.1074/jbc.M102397200. [DOI] [PubMed] [Google Scholar]
- 25.Kuang SQ, Liao L, Zhang H, Lee AV, O’Malley BW, Xu J. AIB1/SRC-3 deficiency affects insulin-like growth factor I signaling pathway and suppresses v-Ha-ras-induced breast cancer initiation and progression in mice. Cancer Res. 2004;64:1875–1885. doi: 10.1158/0008-5472.CAN-03-3745. [DOI] [PubMed] [Google Scholar]
- 26.Fereshteh MP, Tilli MT, Kim SE, Xu J, O’Malley BW, Wellstein A, Furth PA, Riegel AT. The nuclear receptor coactivator amplified in breast cancer-1 is required for Neu (ErbB2/HER2) activation, signaling, and mammary tumorigenesis in mice. Cancer Res. 2008;68:3697–3706. doi: 10.1158/0008-5472.CAN-07-6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuang SQ, Liao L, Wang S, Medina D, O’Malley BW, Xu J. Mice lacking the amplified in breast cancer 1/steroid receptor coactivator-3 are resistant to chemical carcinogen-induced mammary tumorigenesis. Cancer Res. 2005;65:7993–8002. doi: 10.1158/0008-5472.CAN-05-1179. [DOI] [PubMed] [Google Scholar]
- 28.Torres-Arzayus MI, Font de Mora J, Yuan J, Vazquez F, Bronson R, Rue M, Sellers WR, Brown M. High tumor incidence and activation of the PI3K/AKT pathway in transgenic mice define AIB1 as an oncogene. Cancer Cell. 2004;6:263–274. doi: 10.1016/j.ccr.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 29.Qin L, Liao L, Redmond A, Young L, Yuan Y, Chen H, O’Malley BW, Xu J. The AIB1 oncogene promotes breast cancer metastasis by activation of PEA3-mediated matrix metalloproteinase 2 (MMP2) and MMP9 expression. Mol Cell Biol. 2008;28:5937–5950. doi: 10.1128/MCB.00579-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li LB, Louie MC, Chen HW, Zou JX. Proto-oncogene ACTR/AIB1 promotes cancer cell invasion by up-regulating specific matrix metalloproteinase expression. Cancer Lett. 2008;261:64–73. doi: 10.1016/j.canlet.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 31.Osborne CK, Bardou V, Hopp TA, Chamness GC, Hilsenbeck SG, Fuqua SA, Wong J, Allred DC, Clark GM, Schiff R. Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J Natl Cancer Inst. 2003;95:353–361. doi: 10.1093/jnci/95.5.353. [DOI] [PubMed] [Google Scholar]
- 32.Long W, Yi P, Amazit L, LaMarca HL, Ashcroft F, Kumar R, Mancini MA, Tsai SY, Tsai MJ, O’Malley BW. SRC-3Delta4 mediates the interaction of EGFR with FAK to promote cell migration. Mol Cell. 2010;37:321–332. doi: 10.1016/j.molcel.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fleming FJ, Myers E, Kelly G, Crotty TB, McDermott EW, O’Higgins NJ, Hill AD, Young LS. Expression of SRC-1, AIB1, and PEA3 in HER2 mediated endocrine resistant breast cancer; a predictive role for SRC-1. J Clin Pathol. 2004;57:1069–1074. doi: 10.1136/jcp.2004.016733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Myers E, Fleming FJ, Crotty TB, Kelly G, McDermott EW, O’Higgins NJ, Hill AD, Young LS. Inverse relationship between ER-beta and SRC-1 predicts outcome in endocrine-resistant breast cancer. Br J Cancer. 2004;91:1687–1693. doi: 10.1038/sj.bjc.6602156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fleming FJ, Hill AD, McDermott EW, O’Higgins NJ, Young LS. Differential recruitment of coregulator proteins steroid receptor coactivator-1 and silencing mediator for retinoid and thyroid receptors to the estrogen receptor-estrogen response element by beta-estradiol and 4-hydroxytamoxifen in human breast cancer. J Clin Endocrinol Metab. 2004;89:375–383. doi: 10.1210/jc.2003-031048. [DOI] [PubMed] [Google Scholar]
- 36.McBryan J, Theissen SM, Byrne C, Hughes E, Cocchiglia S, Sande S, O’Hara J, Tibbitts P, Hill AD, Young LS. Metastatic progression with resistance to aromatase inhibitors is driven by the steroid receptor coactivator SRC-1. Cancer Res. 2012;72:548–559. doi: 10.1158/0008-5472.CAN-11-2073. [DOI] [PubMed] [Google Scholar]
- 37.Tai H, Kubota N, Kato S. Involvement of nuclear receptor coactivator SRC-1 in estrogen-dependent cell growth of MCF-7 cells. Biochem Biophys Res Commun. 2000;267:311–316. doi: 10.1006/bbrc.1999.1954. [DOI] [PubMed] [Google Scholar]
- 38.Kishimoto H, Wang Z, Bhat-Nakshatri P, Chang D, Clarke R, Nakshatri H. The p160 family coactivators regulate breast cancer cell proliferation and invasion through autocrine/paracrine activity of SDF-1alpha/CXCL12. Carcinogenesis. 2005;26:1706–1715. doi: 10.1093/carcin/bgi137. [DOI] [PubMed] [Google Scholar]
- 39.Wang S, Yuan Y, Liao L, Kuang SQ, Tien JC, O’Malley BW, Xu J. Disruption of the SRC-1 gene in mice suppresses breast cancer metastasis without affecting primary tumor formation. Proc Natl Acad Sci U S A. 2009;106:151–156. doi: 10.1073/pnas.0808703105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qin L, Liu Z, Chen H, Xu J. The steroid receptor coactivator-1 regulates twist expression and promotes breast cancer metastasis. Cancer Res. 2009;69:3819–3827. doi: 10.1158/0008-5472.CAN-08-4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cavarretta IT, Mukopadhyay R, Lonard DM, Cowsert LM, Bennett CF, O’Malley BW, Smith CL. Reduction of coactivator expression by antisense oligodeoxynucleotides inhibits ERalpha transcriptional activity and MCF-7 proliferation. Mol Endocrinol. 2002;16:253–270. doi: 10.1210/mend.16.2.0770. [DOI] [PubMed] [Google Scholar]
- 42.Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva B, Antipin Y, Mitsiades N, Landers T, Dolgalev I, Major JE, Wilson M, Socci ND, Lash AE, Heguy A, Eastham JA, Scher HI, Reuter VE, Scardino PT, Sander C, Sawyers CL, Gerald WL. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18:11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Agoulnik IU, Vaid A, Nakka M, Alvarado M, Bingman WE, 3rd, Erdem H, Frolov A, Smith CL, Ayala GE, Ittmann MM, Weigel NL. Androgens modulate expression of transcription intermediary factor 2, an androgen receptor coactivator whose expression level correlates with early biochemical recurrence in prostate cancer. Cancer Res. 2006;66:10594–10602. doi: 10.1158/0008-5472.CAN-06-1023. [DOI] [PubMed] [Google Scholar]
- 44.Agoulnik IU, Weigel NL. Androgen receptor coactivators and prostate cancer. Adv Exp Med Biol. 2008;617:245–255. doi: 10.1007/978-0-387-69080-3_23. [DOI] [PubMed] [Google Scholar]
- 45.Gregory CW, He B, Johnson RT, Ford OH, Mohler JL, French FS, Wilson EM. A mechanism for androgen receptor-mediated prostate cancer recurrence after androgen deprivation therapy. Cancer Res. 2001;61:4315–4319. [PubMed] [Google Scholar]
- 46.Chopra AR, Louet JF, Saha P, An J, Demayo F, Xu J, York B, Karpen S, Finegold M, Moore D, Chan L, Newgard CB, O’Malley BW. Absence of the SRC-2 coactivator results in a glycogenopathy resembling Von Gierke’s disease. Science. 2008;322:1395–1399. doi: 10.1126/science.1164847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chopra AR, Kommagani R, Saha P, Louet JF, Salazar C, Song J, Jeong J, Finegold M, Viollet B, DeMayo F, Chan L, Moore DD, O’Malley BW. Cellular energy depletion resets whole-body energy by promoting coactivator-mediated dietary fuel absorption. Cell Metab. 2011;13:35–43. doi: 10.1016/j.cmet.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Picard F, Gehin M, Annicotte J, Rocchi S, Champy MF, O’Malley BW, Chambon P, Auwerx J. SRC-1 and TIF2 control energy balance between white and brown adipose tissues. Cell. 2002;111:931–941. doi: 10.1016/S0092-8674(02)01169-8. [DOI] [PubMed] [Google Scholar]
- 49.Stashi E, Lanz RB, Mao J, Michailidis G, Zhu B, Kettner NM, Putluri N, Reineke EL, Reineke LC, Dasgupta S, Dean A, Stevenson CR, Sivasubramanian N, Sreekumar A, Demayo F, York B, Fu L, O’Malley BW. SRC-2 is an essential coactivator for orchestrating metabolism and circadian rhythm. Cell Rep. 2014;6:633–645. doi: 10.1016/j.celrep.2014.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dasgupta S, Putluri N, Long W, Zhang B, Wang J, Kaushik AK, Arnold JM, Bhowmik SK, Stashi E, Brennan CA, Rajapakshe K, Coarfa C, Mitsiades N, Ittmann MM, Chinnaiyan AM, Sreekumar A, O’Malley BW. Coactivator SRC-2-dependent metabolic reprogramming mediates prostate cancer survival and metastasis. J Clin Invest. 2015;125:1174–1188. doi: 10.1172/JCI76029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gnanapragasam VJ, Leung HY, Pulimood AS, Neal DE, Robson CN. Expression of RAC 3, a steroid hormone receptor co-activator in prostate cancer. Br J Cancer. 2001;85:1928–1936. doi: 10.1054/bjoc.2001.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou HJ, Yan J, Luo W, Ayala G, Lin SH, Erdem H, Ittmann M, Tsai SY, Tsai MJ. SRC-3 is required for prostate cancer cell proliferation and survival. Cancer Res. 2005;65:7976–7983. doi: 10.1158/0008-5472.CAN-04-4076. [DOI] [PubMed] [Google Scholar]
- 53.Zhou G, Hashimoto Y, Kwak I, Tsai SY, Tsai MJ. Role of the steroid receptor coactivator SRC-3 in cell growth. Mol Cell Biol. 2003;23:7742–7755. doi: 10.1128/MCB.23.21.7742-7755.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yan J, Erdem H, Li R, Cai Y, Ayala G, Ittmann M, Yu-Lee LY, Tsai SY, Tsai MJ. Steroid receptor coactivator-3/AIB1 promotes cell migration and invasiveness through focal adhesion turnover and matrix metalloproteinase expression. Cancer Res. 2008;68:5460–5468. doi: 10.1158/0008-5472.CAN-08-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Agoulnik IU, Vaid A, Bingman WE, 3rd, Erdeme H, Frolov A, Smith CL, Ayala G, Ittmann MM, Weigel NL. Role of SRC-1 in the promotion of prostate cancer cell growth and tumor progression. Cancer Res. 2005;65:7959–7967. doi: 10.1158/0008-5472.CAN-04-3541. [DOI] [PubMed] [Google Scholar]
- 56.Kershah SM, Desouki MM, Koterba KL, Rowan BG. Expression of estrogen receptor coregulators in normal and malignant human endometrium. Gynecol Oncol. 2004;92:304–313. doi: 10.1016/j.ygyno.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 57.Sakaguchi H, Fujimoto J, Sun WS, Tamaya T. Clinical implications of steroid receptor coactivator (SRC)-3 in uterine endometrial cancers. J Steroid Biochem Mol Biol. 2007;104:237–240. doi: 10.1016/j.jsbmb.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 58.Balmer NN, Richer JK, Spoelstra NS, Torkko KC, Lyle PL, Singh M. Steroid receptor coactivator AIB1 in endometrial carcinoma, hyperplasia and normal endometrium: correlation with clinicopathologic parameters and biomarkers. Mod Pathol. 2006;19:1593–1605. doi: 10.1038/modpathol.3800696. [DOI] [PubMed] [Google Scholar]
- 59.Yoshida H, Liu J, Samuel S, Cheng W, Rosen D, Naora H. Steroid receptor coactivator-3, a homolog of Taiman that controls cell migration in the Drosophila ovary, regulates migration of human ovarian cancer cells. Mol Cell Endocrinol. 2005;245:77–85. doi: 10.1016/j.mce.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 60.Wu RC, Qin J, Hashimoto Y, Wong J, Xu J, Tsai SY, Tsai MJ, O’Malley BW. Regulation of SRC-3 (pCIP/ACTR/AIB-1/RAC-3/TRAM-1) Coactivator activity by I kappa B kinase. Mol Cell Biol. 2002;22:3549–3561. doi: 10.1128/MCB.22.10.3549-3561.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu RC, Qin J, Yi P, Wong J, Tsai SY, Tsai MJ, O’Malley BW. Selective phosphorylations of the SRC-3/AIB1 coactivator integrate genomic reponses to multiple cellular signaling pathways. Mol Cell. 2004;15:937–949. doi: 10.1016/j.molcel.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 62.Giamas G, Castellano L, Feng Q, Knippschild U, Jacob J, Thomas RS, Coombes RC, Smith CL, Jiao LR, Stebbing J. CK1delta modulates the transcriptional activity of ERalpha via AIB1 in an estrogen-dependent manner and regulates ERalpha-AIB1 interactions. Nucleic Acids Res. 2009;37:3110–3123. doi: 10.1093/nar/gkp136. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 63.Oh AS, Lahusen JT, Chien CD, Fereshteh MP, Zhang X, Dakshanamurthy S, Xu J, Kagan BL, Wellstein A, Riegel AT. Tyrosine phosphorylation of the nuclear receptor coactivator AIB1/SRC-3 is enhanced by Abl kinase and is required for its activity in cancer cells. Mol Cell Biol. 2008;28:6580–6593. doi: 10.1128/MCB.00118-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rowan BG, Weigel NL, O’Malley BW. Phosphorylation of steroid receptor coactivator-1. Identification of the phosphorylation sites and phosphorylation through the mitogen-activated protein kinase pathway. J Biol Chem. 2000;275:4475–4483. doi: 10.1074/jbc.275.6.4475. [DOI] [PubMed] [Google Scholar]
- 65.Ueda T, Mawji NR, Bruchovsky N, Sadar MD. Ligand-independent activation of the androgen receptor by interleukin-6 and the role of steroid receptor coactivator-1 in prostate cancer cells. J Biol Chem. 2002;277:38087–38094. doi: 10.1074/jbc.M203313200. [DOI] [PubMed] [Google Scholar]
- 66.Dobrovolna J, Chinenov Y, Kennedy MA, Liu B, Rogatsky I. Glucocorticoid-dependent phosphorylation of the transcriptional coregulator GRIP1. Mol Cell Biol. 2012;32:730–739. doi: 10.1128/MCB.06473-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lopez GN, Turck CW, Schaufele F, Stallcup MR, Kushner PJ. Growth factors signal to steroid receptors through mitogen-activated protein kinase regulation of p160 coactivator activity. J Biol Chem. 2001;276:22177–22182. doi: 10.1074/jbc.M010718200. [DOI] [PubMed] [Google Scholar]
- 68.Gregory CW, Fei X, Ponguta LA, He B, Bill HM, French FS, Wilson EM. Epidermal growth factor increases coactivation of the androgen receptor in recurrent prostate cancer. J Biol Chem. 2004;279:7119–7130. doi: 10.1074/jbc.M307649200. [DOI] [PubMed] [Google Scholar]
- 69.Frigo DE, Basu A, Nierth-Simpson EN, Weldon CB, Dugan CM, Elliott S, Collins-Burow BM, Salvo VA, Zhu Y, Melnik LI, Lopez GN, Kushner PJ, Curiel TJ, Rowan BG, McLachlan JA, Burow ME. p38 mitogen-activated protein kinase stimulates estrogen-mediated transcription and proliferation through the phosphorylation and potentiation of the p160 coactivator glucocorticoid receptor-interacting protein 1. Mol Endocrinol. 2006;20:971–983. doi: 10.1210/me.2004-0075. [DOI] [PubMed] [Google Scholar]
- 70.Rodriguez AL, Tamrazi A, Collins ML, Katzenellenbogen JA. Design, synthesis, and in vitro biological evaluation of small molecule inhibitors of estrogen receptor alpha coactivator binding. J Med Chem. 2004;47:600–611. doi: 10.1021/jm030404c. [DOI] [PubMed] [Google Scholar]
- 71.Williams AB, Weiser PT, Hanson RN, Gunther JR, Katzenellenbogen JA. Synthesis of biphenyl proteomimetics as estrogen receptor-alpha coactivator binding inhibitors. Org Lett. 2009;11:5370–5373. doi: 10.1021/ol901999f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mettu NB, Stanley TB, Dwyer MA, Jansen MS, Allen JE, Hall JM, McDonnell DP. The nuclear receptor-coactivator interaction surface as a target for peptide antagonists of the peroxisome proliferator-activated receptors. Mol Endocrinol. 2007;21:2361–2377. doi: 10.1210/me.2007-0201. [DOI] [PubMed] [Google Scholar]
- 73.Wang Y, Lonard DM, Yu Y, Chow DC, Palzkill TG, O’Malley BW. Small molecule inhibition of the steroid receptor coactivators, SRC-3 and SRC-1. Mol Endocrinol. 2011;25:2041–2053. doi: 10.1210/me.2011-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Y, Lonard DM, Yu Y, Chow DC, Palzkill TG, Wang J, Qi R, Matzuk AJ, Song X, Madoux F, Hodder P, Chase P, Griffin PR, Zhou S, Liao L, Xu J, O’Malley BW. Bufalin is a potent small-molecule inhibitor of the steroid receptor coactivators SRC-3 and SRC-1. Cancer Res. 2014;74:1506–1517. doi: 10.1158/0008-5472.CAN-13-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yan F, Yu Y, Chow DC, Palzkill T, Madoux F, Hodder P, Chase P, Griffin PR, O’Malley BW, Lonard DM. Identification of verrucarin a as a potent and selective steroid receptor coactivator-3 small molecule inhibitor. PLoS One. 2014;9:e95243. doi: 10.1371/journal.pone.0095243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Song X, Zhang C, Zhao M, Chen H, Liu X, Chen J, Lonard DM, Qin L, Xu J, Wang X, Li F, O’Malley BW, Wang J. Steroid receptor coactivator-3 (SRC-3/AIB1) as a novel therapeutic target in triple negative breast cancer and its inhibition with a phospho-bufalin prodrug. PLoS One. 2015;10:e0140011. doi: 10.1371/journal.pone.0140011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang L, Yu Y, Chow DC, Yan F, Hsu CC, Stossi F, Mancini MA, Palzkill T, Liao L, Zhou S, Xu J, Lonard DM, O’Malley BW. Characterization of a steroid receptor coactivator small molecule stimulator that overstimulates cancer cells and leads to cell stress and death. Cancer Cell. 2015;28:240–252. doi: 10.1016/j.ccell.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]