Summary
Problem
The role of plasma estradiol in the accumulation of intra-abdominal fat (IAF) in men is uncertain. Cross-sectional studies using imaging of IAF have shown either a positive or no association. In contrast, a randomised controlled trial using an aromatase inhibitor to suppress estradiol production found an association between oestrogen deficiency and short-term IAF accumulation. No longitudinal study has been conducted to examine the relationship between plasma estradiol concentration and the change in IAF area measured using direct imaging.
Methods
This is a longitudinal observational study in community-dwelling Japanese-American men (n = 215, mean age 52 years, BMI 25.4 kg/m2). IAF and subcutaneous fat areas were assessed using computerized tomography (CT) at baseline, 5 and 10 years. Baseline plasma estradiol concentrations were measured using liquid chromatography-tandem mass spectrometry.
Results
Univariate analysis found no association between baseline estradiol concentration and baseline IAF, or 5- or 10-year changes in IAF area (r = −0.05 for both time points, p = 0.45 and p = 0.43, respectively). Multivariate linear regression analysis of the change in IAF area by baseline estradiol concentration adjusted for age, baseline IAF area, and weight change found no association with either the 5- or 10-year IAF area change (p = 0.52 and p = 0.55, respectively).
Conclusions
Plasma estradiol concentration was not associated with baseline IAF nor with change in IAF area over 5 or 10 years based on serial CT scans in community-dwelling Japanese-American men. These results do not support a role for oestrogen deficiency in IAF accumulation in men.
Keywords: Estradiol, Intra-abdominal fat, Adiposity, Japanese Americans
Introduction
Obesity is a well-established risk factor for many chronic diseases, including cardiovascular disease and diabetes mellitus. In addition to overall excess adiposity, the pattern of body fat deposition has been shown to affect risk for adiposity-related complications. Both cross-sectional and longitudinal data have demonstrated a higher risk of diabetes and cardiovascular disease in association with increased intra-abdominal compared to subcutaneous fat [1—3].
Men and premenopausal women typically have different patterns of fat deposition, with the former having more intra-abdominal fat compared to women, whose adipose tissue is distributed more commonly in the thighs and buttocks [4]. Observational data suggest that endogenous sex steroids play a role in these different patterns of fat deposition. Women experience a redistribution of adipose tissue towards a more central pattern of adiposity after menopause [4]. Adipose tissue contains both oestrogen and androgen receptors, and expresses aromatase, the enzyme that converts testosterone to estradiol [4,5]. These proteins likely facilitate local sex steroid effects which could contribute to the regulation of regional adipose tissue deposition [5].
A recent large interventional study examined the effects of oestrogen deficiency of fat accumulation in men by pharmacologically lowering estradiol concentration with an aromatase inhibitor to suppress the conversion of testosterone to estradiol. This study included 202 men who received anastrozole for 16 weeks (in combination with a GnRH antagonist and testosterone transdermal gel) and demonstrated an inverse relationship between estradiol concentrations and changes in intra-abdominal fat area [6]. The authors concluded that endogenous estradiol likely plays an important role in the regulation of adiposity in men, and in regulating intra-abdominal fat mass in particular.
No observational research has been conducted with adequate rigour to determine whether physiologic concentrations of oestrogen in men predict fat accumulation in the important intra-abdominal depot. Several cross-sectional studies have examined the association between estradiol concentrations and regional adiposity in men, but this study design cannot establish whether estradiol concentration preceded change in the intra-abdominal fat depot. Of 13 cross-sectional studies examining this association, 5 found a positive association and 8 found no association between circulating estradiol concentrations and overall or regional adiposity [7—19]. These studies used various methods to assess adiposity, including clinical measures such as weight and waist-to-hip ratio and radiographic measures including dual-energy X-ray absorptiometry (DXA), magnetic resonance imaging (MRI), or computerized tomography (CT). Only four of these studies specifically examined the association between estradiol concentration and intra-abdominal fat, with one demonstrating a positive association and three finding no statistically significant association [7,9,12,18]. Additionally, most of these studies used radioimmunoassay (RIA) techniques to measure estradiol, which is less accurate than liquid chromatography—tandem mass spectrometry (LCMS) in quantifying the relatively low concentrations of circulating estradiol in men [20].
Longitudinal observational research on the association between estradiol concentration and intra-abdominal fat accumulation is also limited. The two longitudinal studies that examined this relationship did not directly image the intra-abdominal fat depot but instead used body surface measurements, with differing results [8,15].
Since estradiol may play an important role in regulating fat mass in men based on one well-conducted interventional study, we wondered whether physiologic serum estradiol concentrations might predict the accumulation of fat mass over time. Therefore, we examined the relationship between plasma estradiol concentrations and IAF accumulation measured serially over time by abdominal CT in a well-characterised cohort of middle-aged to older community-dwelling Japanese-American men who have been followed longitudinally for changes in body composition.
Subjects, methods, and materials
Study subjects
Our study population included both second- and third-generation Japanese-American men (Nisei and Sansei, respectively) from the Japanese-American Community Diabetes Study (JACDS). The selection and recruitment process for this cohort has been described previously [21]. Briefly, study subjects were healthy volunteers who were representative of the Japanese-American population of King County, WA in age distribution, residence, and parental immigration patterns. Subjects were enrolled starting in 1983. Subjects with diabetes at baseline, as defined below, were excluded. Baseline, 5, and 10 year follow-up evaluations were performed at the General Clinical Research Center at the University of Washington (Seattle, WA). The research protocol was approved by the institutional review board and signed informed consent was obtained from all participants.
Measurement of body fat distribution
Computerized tomography (CT) measurements of body fat distribution were performed at baseline and at 5 and 10 year follow-up visits. Subcutaneous and intra-abdominal fat areas were measured at the level of the umbilicus (approximately L4-5) using 10 mm slice thickness as previously described [22]. Density contour software was used for analysis of the CT scans, with areas in the attenuation range of −250 to −50 Hounsfield units classified as adipose tissue. Intra-abdominal fat was considered that within the confines of the transversalis fascia [22]. All scans were read by the same person. Changes in fat areas were calculated as the difference between the baseline value and both the 5- and 10-year measurements respectively.
Laboratory measurement
Estradiol was quantified from fresh frozen plasma stored at −70 °C by liquid chromatography tandem mass spectrometry (LCMS), using a modification of the dansyl chloride derivatization method on an AB Sciex 5500 QTRAP tandem quadrupole mass spectrometer (MS) with positive electrospray ionization (ESI+) (AB Sciex; Foster City, CA) [23]. The lower limit of quantitation was 7.34 pmol/L and the intra-assay co-efficient of variation was 2.6%. The normal range for serum estradiol is 51.39—172.54 pmol/L defined as the 2.5—97.5th percentile for healthy men ages 18—50. Plasma was originally collected during a 75 g oral glucose tolerance test (OGTT) at the baseline study visit from the 60- and 90-minute samples. Subjects were classified as having diabetes if treated with a hypoglycemic medication or insulin at baseline, if the fasting plasma glucose was ≥6.99 mmol/L, or if the 120-minute plasma glucose during the OGTT was ≥11.10 mmol/L.
Statistical analysis
We assessed the associations in univariate analyses between estradiol concentration in tertiles and body composition and related metabolic measurements. Univariate linear regression analysis was performed to examine the association between the change in intra-abdominal fat area over 5 and 10 years and estradiol concentration and other measures of body composition. Multivariate linear regression was used to estimate the association between the change in the intra-abdominal fat area over 5 and 10 years and the baseline estradiol concentration with adjustment for covariates relating to body composition and fasting plasma glucose. Backwards stepwise regression was used to develop the final models, with p < 0.05 used to determine which variables remained in the model. A variance inflation factor value of 4 was used to assess the presence of multi-collinearity. We assessed the presence of non-linear associations between estradiol and change in intra-abdominal fat area using fractional polynomials [24]. A p-value of <0.05 was considered statistically significant. Stata version 13 (StataCorp, College Station, TX) was used for all statistical analyses.
Results
A total of 264 men from the Japanese-American Community Diabetes Study had a baseline CT scan with 216 men returning for a CT scan 10 years later, resulting in an 81% rate of follow-up. Of the 216 men, one had missing laboratory data giving a final sample size of 215 for the 10 year analysis. A total of 206 men also had a CT scan 5 years after study entry, and this group was used for the 5 year analysis.
Among the 215 study subjects, 113 were second generation (Nisei) and 112 were third generation Japanese Americans (Sansei). The mean baseline estradiol concentration was 81.20 pmol/L (standard deviation 25.95 pmol/L, range 31.06—182.45 pmol/L). As a group, the subjects were middle-aged to older (range 34—74 years), slightly overweight, and had mean fasting plasma glucose concentrations in the non-diabetic range (Table 1). There was no correlation between baseline estradiol concentration and baseline intra-abdominal fat area (r = 0.05, p = 0.49) or baseline subcutaneous abdominal fat area (r = −0.10, p = 0.15). During the 10 years of follow-up, men gained weight and had an increase in both intra-abdominal and subcutaneous fat on average. These increases were observed at both the 5 and 10 year time points (Table 1).
Table 1.
Characteristics of subjects, overall and by baseline estradiol concentration tertiles.
| Variable | Overall (SD) | Estradiol tertiles (pmol/L) | p-Value | ||
|---|---|---|---|---|---|
| Lowest | Middle | Highest | |||
| Estradiol (pmol/L) | 81.20 (25.95) | 31.06—66.37 | 66.38—90.01 | 90.02—182.45 | |
| Age (years) | 51.85 (11.50) | 47.97 | 52.24 | 55.40 | <0.001 |
| Intra-abdominal fat area at baseline (cm2) | 96 (51.88) | 97.70 | 91.51 | 98.83 | 0.66 |
| 5 year change in intra-abdominal fat area (cm2) | 11.44 (35.46) | 11.40 | 11.99 | 10.91 | 0.99 |
| 10 year change in intra-abdominal fat area (cm2) | 19.41 (39.77) | 20.69 | 16.61 | 20.96 | 0.77 |
| Subcutaneous fat area at baseline (cm2) | 140.53 (68.27) | 149.62 | 135.63 | 136.29 | 0.38 |
| 5 year change in subcutaneous fat area (cm2) | 14.63 (29.98) | 20.61 | 10.23 | 12.69 | 0.10 |
| 10 year change in subcutaneous fat area (cm2) | 22.24 (40.43) | 30.58 | 16.80 | 19.30 | 0.09 |
| Weight at baseline (kg) | 71.31 (10.09) | 73.62 | 69.62 | 70.68 | 0.05 |
| 5 year change in weight (kg) | 0.89 (3.34) | 1.47 | 0.89 | 0.27 | 0.10 |
| 10 year change in weight (kg) | 1.89 (4.71) | 2.63 | 1.44 | 1.61 | 0.26 |
| BMI at baseline (kg/m2) | 25.37 (3.10) | 25.92 | 24.88 | 25.30 | 0.13 |
| Fasting plasma glucose at baseline (mmol/L) | 5.33 (0.64) | 5.22 | 5.33 | 5.43 | 0.12 |
SD: standard deviation; BMI: body mass index.
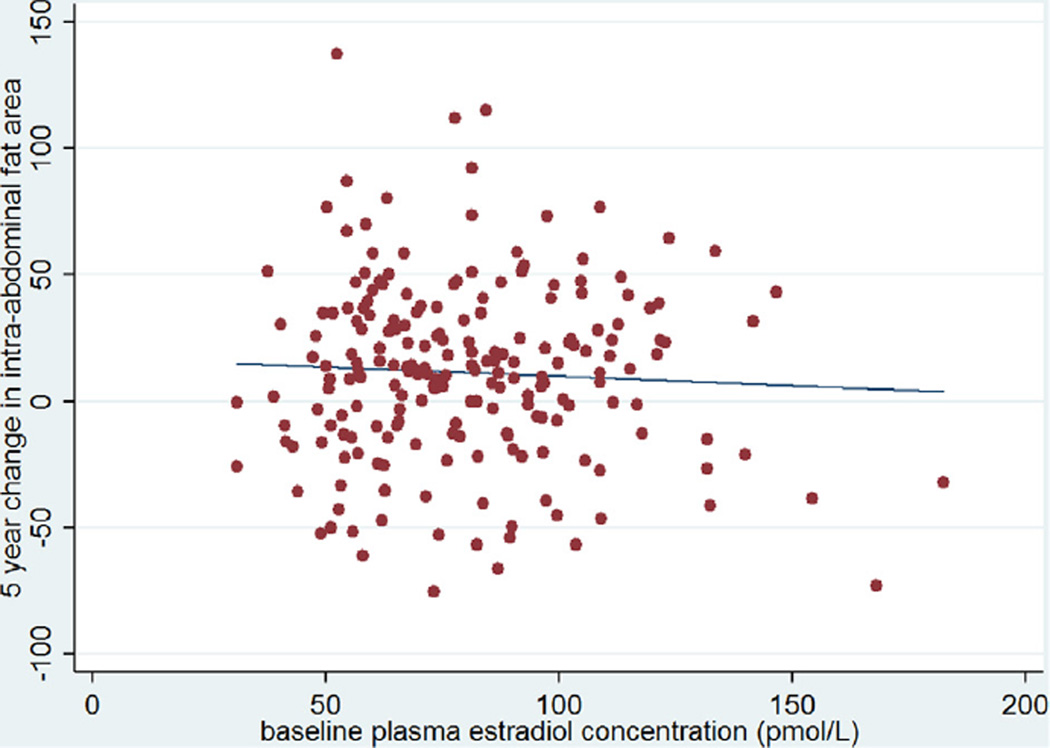
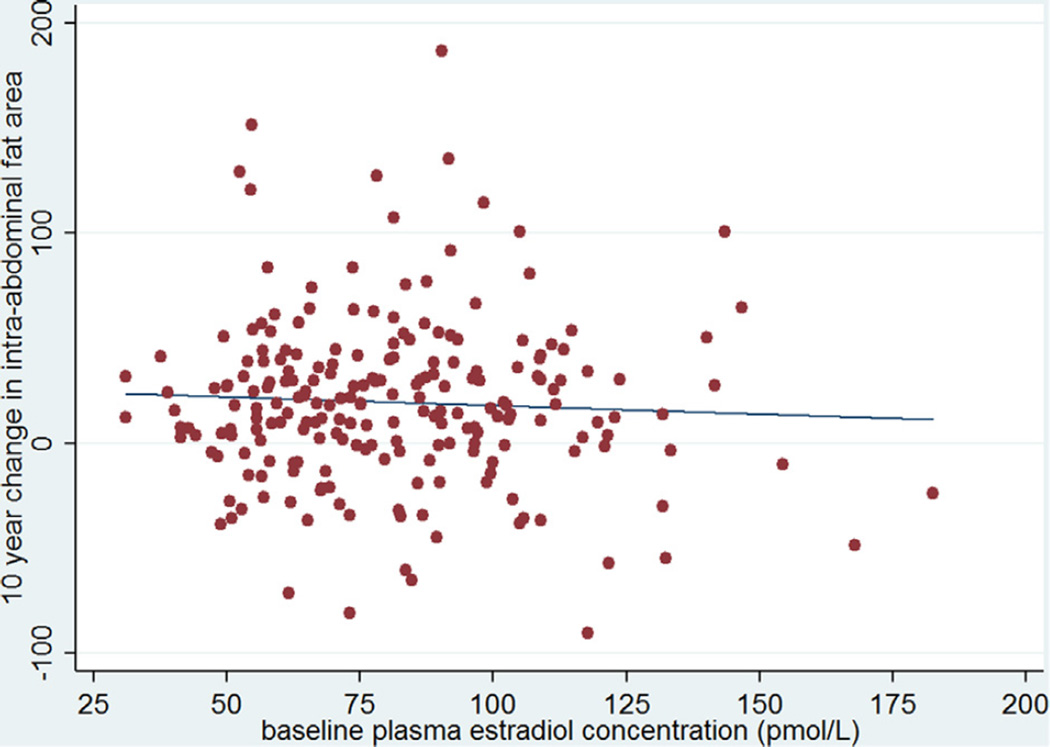
We explored associations between the change in intra-abdominal fat over 5 and 10 years and several possible predictors of this change (Table 2). Plasma estradiol concentrations at baseline were not significantly associated with the change in intra-abdominal fat over either 5 or 10 years of follow-up in univariate linear regression analysis (r = −0.05 for both time points, p = 0.45 for 5 years of follow-up and p = 0.43 for 10 years of follow-up) (Figs. 1 and 2). Baseline intra-abdominal fat area was significantly and inversely correlated with both 5 and 10 year changes in the intra-abdominal fat area (r = −0.30 and −0.24 respectively, p < 0.01 for both values). Age was inversely and significantly correlated with the change in intra-abdominal fat at 10 years of follow-up (r = −0.17, p = 0.01), but not at 5 years (r = −0.05, p = 0.46). A significant association was seen between the change in intra-abdominal fat and the change in subcutaneous fat over both 5 and 10 years (r = 0.27 and 0.40, respectively, p < 0.01 for both values). The change in weight was also associated with the change in intra-abdominal fat over both time periods (r = 0.44 and r = 0.55 for 5 and 10 years, respectively, p < 0.01 for both values). There was no significant relationship between the change in intra-abdominal fat over either time period and the baseline fasting plasma glucose. Baseline BMI, weight, total subcutaneous fat, and fasting glucose were unrelated to changes in intra-abdominal fat over either 5 or 10 years of follow-up (Table 2).
Table 2.
Univariate analysis of the change in intra-abdominal fat area (cm2) over (a) 5 and (b) 10 years.
| β coefficient | 95% confidence interval | p-Value | |
|---|---|---|---|
| (a) Change in intra-abdominal fat area over 5 years | |||
| Age (years) | −0.16 | (−0.58, 0.26) | 0.46 |
| Intra-abdominal fat area at baseline (cm2) | −0.20 | (−0.29, −0.11) | <0.01 |
| Subcutaneous abdominal fat area at baseline (cm2) | −0.03 | (−0.11, 0.04) | 0.35 |
| 5 year change in subcutaneous fat (cm2) | 0.31 | (0.16, 0.47) | <0.01 |
| Weight at baseline (kg) | −0.42 | (−0.90, 0.06) | 0.09 |
| 5 year change in weight (kg) | 4.68 | (3.37, 5.99) | <0.01 |
| BMI at baseline (kg/m2) | −1.26 | (−2.84, 0.31) | 0.12 |
| Estradiol at baseline (pmol/L) | −0.07 | (−0.26, 0.12) | 0.45 |
| Fasting plasma glucose at baseline (mmol/L) | −4.15 | (−11.87, 3.57) | 0.29 |
| (b) Change in intra-abdominal fat area over 10 years | |||
| Age (years) | −0.59 | (−1.05, −0.13) | 0.01 |
| Intra-abdominal fat area at baseline (cm2) | −0.18 | (−0.28, −0.08) | <0.01 |
| Subcutaneous abdominal fat area at baseline (cm2) | −0.03 | (−0.11, 0.05) | 0.42 |
| 10 year change in subcutaneous fat (cm2) | 0.40 | (0.28, 0.52) | <0.01 |
| Weight at baseline (kg) | −0.25 | (−0.78, 0.28) | 0.35 |
| 10 year change in weight (kg) | 4.67 | (3.72, 5.62) | <0.01 |
| BMI at baseline (kg/m2) | −1.27 | (−3.00, 0.45) | 0.15 |
| Estradiol at baseline (pmol/L) | −0.08 | (−0.29, 0.12) | 0.43 |
| Fasting plasma glucose at baseline (mmol/L) | −5.86 | (−14.45, 2.73) | 0.18 |
BMI: body mass index; BMI: body mass index.
Figure 1.
The change in intra-abdominal fat area (cm2) over 5 years by baseline estradiol concentration (pmol/L). The line represents the best fitting regression line (r = 0.05, p = 0.45).
Figure 2.
The change in intra-abdominal fat area (cm2) over 10 years by baseline estradiol concentration (pmol/L). The line represents the best fitting regression line (r = −0.05, p = 0.43).
Multivariate linear regression was used to examine the relationship between the change in intra-abdominal fat area over 5 and 10 years of follow-up and the baseline estradiol concentration (Table 3). Our multivariate linear regression model included the following variables: weight at baseline, change in weight over time, subcutaneous fat at baseline, change in subcutaneous fat area over time, BMI at baseline, and fasting plasma glucose at baseline. A priori, we forced age at baseline and intra-abdominal fat area at baseline into our model. The rest of the model was determined using backwards stepwise linear regression with P to enter <0.05. The results for the 5 and 10 year models were consistent, with no significant association between the change in intra-abdominal fat area and baseline estradiol concentration at either time point. Besides the a priori inclusion of age and intra-abdominal fat area at baseline, the change in weight over time was the only other variable that remained in the model as a significant predictor of change in intra-abdominal fat area at both time points. Multi-collinearity was not present in either of these multivariate models. Non-linear associations were not present as assessed using fractional polynomials.
Table 3.
Multiple linear regression analysis of the change in intra-abdominal fat area over (a) 5 years and (b) 10 years by baseline estradiol level with adjustment for baseline age, baseline intra-abdominal fat, and the change in weight over time.
| β coefficient | 95% confidence interval | p-Value | |
|---|---|---|---|
| (a) Multiple linear regression analysis of the change in intra-abdominal fat area over 5 years | |||
| Age (years) | 0.88 | (0.45, 1.30) | <0.01 |
| Intra-abdominal fat area at baseline (cm2) | −0.22 | (−0.31, −0.14) | <0.01 |
| Estradiol at baseline (pmol/L) | −0.05 | (−0.22, 0.11) | 0.52 |
| 5 year change in weight (kg) | 5.37 | (4.03, 6.71) | <0.01 |
| (b) Multiple linear regression analysis of the change in intra-abdominal fat area over 10 years | |||
| Age (years) | 0.55 | (0.09, 1.01) | 0.02 |
| Intra-abdominal fat area at baseline (cm2) | −0.16 | (−0.25, −0.07) | <0.01 |
| Estradiol at baseline (pmol/L) | −0.05 | (−0.23, 0.12) | 0.55 |
| 10 year change in weight (kg) | 4.94 | (3.92, 5.97) | <0.01 |
Discussion
In this prospective study of middle-aged to older community dwelling Japanese-American men, we found no association between the estradiol concentration at baseline and the accumulation of intra-abdominal fat over either 5 or 10 years. This lack of association persisted in our multivariate linear regression models after adjustment for age at baseline, intra-abdominal fat area at baseline, and the change in weight over time. This is a surprising finding, given the important role that estradiol appears to play in the maintenance of body composition, and central adiposity in particular, in men [6]. Additional potential confounding factors were considered for inclusion in our model, but did not meet criteria for inclusion in the final models. These included abdominal subcutaneous fat area at baseline, the change in abdominal subcutaneous fat area over time, weight at baseline, the change in weight over time, BMI, and fasting plasma glucose concentration. To our knowledge, ours is the first prospective longitudinal observational study B.M. Kocarnik et al. to examine the association between estradiol concentrations and directly measured intra-abdominal fat area in men. We also used LCMS to measure estradiol concentrations, a technique that has been shown to be more accurate than RIA, the method used by most of the prior studies [20].
Two previously published longitudinal studies of estradiol and central fat mass used surface measures instead of radiographic imaging techniques to examine this association. One study reported no association over an average follow-up of 4.8 years in 650 men [8], and the other reported a positive association over an average follow-up of 3 years in 2708 men [15]. Both of these studies used waist circumference to measure the change in adiposity over time, rather than imaging techniques. Waist circumference is a poor proxy for abdominal visceral fat area as it cannot discriminate between changes in subcutaneous versus visceral adiposity [25]. Multiple studies have assessed the correlation between waist circumference and abdominal visceral fat area with most finding correlation coefficients in the range of 0.4—0.7 [26,27]. In Japanese men specifically, the correlation between waist circumference and abdominal visceral fat was found to be 0.62 and 0.68 in two separate studies [28,29]. Moreover, the correlation between waist-hip ratio and abdominal visceral fat was no better in these analyses, at 0.54 [28]. Therefore, a strength of the analyses presented here is the accuracy and specificity of our quantification of intra-abdominal fat area over time.
A randomised controlled trial recently demonstrated a role for estradiol in the maintenance of body composition in men [6]. Based upon those results, we predicted that lower baseline estradiol concentrations would be associated with the accumulation of intra-abdominal fat mass, but our data did not demonstrate such a relationship. It is likely that these apparently conflicting results reflect differences in study design. Finkelstein et al. used pharmacological manipulation to suppress estradiol concentrations far below the normal physiologic range and in this setting demonstrated an accumulation of fat mass over a 16 week study period. Furthermore, the pharmacologic treatment given to reduce the peripheral conversion of testosterone to estradiol may have had an effect on intra-abdominal fat accumulation independent of estradiol concentration. In contrast, our data examined the relationship between endogenous estradiol concentrations in community-dwelling adult men, albeit across a broad range of estradiol levels. Our results suggest that endogenous serum estradiol concentrations play no role in the accumulation of intra-abdominal fat mass in men. In addition, our longitudinal analyses span a period of 10 years in contrast to the more acute effects a shorter term intervention trial demonstrates. Another potential explanation for the differences between our results and this interventional study are that the change seen in fat accumulation in the latter was due to the acute decline in estradiol concentration from the pharmacologic intervention. If this was shown to be the mechanism, it would be of biologic interest but would not be of practical clinical importance since an acute decline in estradiol concentration in men is unlikely to occur without pharmacologic or surgical intervention. Long term intervention trials would be needed to examine whether pharmacologic suppression of estradiol below physiologic levels in men results in a persistent change in the size of the intra-abdominal fat depot, as well as to examine the impact of estradiol replacement in men with low estradiol concentrations.
A strength of our analyses is our use of CT to directly quantify central adiposity. The accumulation of visceral adipose tissue has been shown to have multiple adverse health effects in a number of large, epidemiological studies. In the Framingham Heart Study, visceral adipose tissue was associated with cardiovascular disease and cancer [30]. The Dallas Heart Study found an association between visceral adiposity and insulin resistance, diabetes, dyslipidemia, hepatic steatosis, and aortic plaque [1]. An international, ethnically diverse study in 29 countries found an association between visceral adipose tissue and hypertension, type 2 diabetes, and hypertriglyceridemia and also found an increased predilection for the accumulation of visceral, as opposed to subcutaneous, adipose tissue among the East Asian population (Chinese, Japanese, and Korean) relative to the other populations included in the study [31]. Previously published work from our cohort has shown a positive correlation between the accumulation of intra-abdominal fat and the incidence of type 2 diabetes and hypertension over the 10 year study period [2,3].
Adipose tissue contains oestrogen receptors, so it is biologically plausible that estradiol may influence the accumulation and metabolic activity of intra-abdominal fat [32]. Two types of oestrogen receptors (ER) have been identified in adipose tissue, ERα and ERβ [32]. ERα knockout mice have been shown to develop increased white adipose tissue through both hyperplasia and hypertrophy of adipocytes [4]. The results of studies on ERβ knockout mice are less consistent, but this receptor is also thought to have a role in adipose tissue volume and distribution [32]. The concentration of ER receptors is higher in subcutaneous than visceral fat, and the specific effect of oestrogen on visceral fat remains unclear [4]. Adipose tissue also contains aromatase, an enzyme responsible for the conversion of testosterone to estradiol, which could additionally regulate the distribution and size of fat depots [5]. Aromatase expression is higher in adipose tissue in the thighs and buttocks than in the abdomen [5]. Increased intra-abdominal adiposity has been noted in humans with aromatase deficiency and male aromatase knockout mice [6,33]. In summary, the mechanisms through which circulating oestrogen regulates adipose tissue is an area of active investigation and the exact effect of circulating oestrogen on intra-abdominal fat at the level of the adipocyte remains unknown.
There are several limitations of our study. First, the study was limited to one measurement of estradiol at baseline. Changes in estradiol levels were not assessed at short or long intervals. Variation of estradiol concentration in men does occur but has been shown not to occur either seasonally or diurnally [34]. Furthermore, intercurrent illness is unlikely to have altered estradiol concentrations, as these men were healthy, community-dwelling individuals for the duration of the study. Our plasma samples were assayed after several years of storage in a freezer with a temperature of −70 °C, although the samples used for these assays had not been previously thawed and sex steroids are generally stable over time [35]. Lastly, our population included only Japanese-American men and may not be applicable to other ethnic populations.
In summary, we did not find an association between plasma estradiol concentration and the change in intra-abdominal fat as measured by serial CT imaging over 10 years in a cohort of healthy middle-aged to older Japanese-American men. These results argue against a role for oestrogen in long term accumulation of fat in the intra-abdominal depot in men.
Acknowledgments
Grant support
This study was supported in part by the Medical Research Service, Geriatric Research, Education and Clinical Center and Cooperative Studies Program of the Department of Veterans Affairs, Seattle, Washington as well as NIH Grants DK-031170, HL-049293, DK-002654, DK-017047, DK-035816 and RR-000037. Dr. Kocarnik was supported by a VA Advanced Fellowship in Geriatrics.
We dedicate this article to the memory of Marguerite J. McNeely who for many years played an important role as investigator in the Japanese American Community Diabetes Study. Her critical contributions will be missed. VA Puget Sound provided support for Drs. Kahn, Boyko, and Matsumoto’s participation in this research. Steven E. Kahn has requested to have his name withdrawn as an author on this article owing to his disagreement with the manner in which The Lancet and its publisher, Reed Elsevier, handled the recent publication of an “Open letter for the people in Gaza” by Manduca et al. Dr. Kahn participated in the interpretation of the data and critical editing of this article.
Footnotes
Conflicts of interest
None.
References
- 1.Neeland IJ, Ayers CR, Rohatgi AK, Turer AT, Berry JD, Das SR, et al. Associations of visceral and abdominal subcutaneous adipose tissue with markers of cardiac and metabolic risk in obese adults. Obesity (Silver Spring) 2013;21(9):E439–E447. doi: 10.1002/oby.20135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sullivan CA, Kahn SE, Fujimoto WY, Hayashi T, Leonetti DL, Boyko EJ. Change in intra-abdominal fat predicts the risk of hypertension in Japanese Americans. Hypertension. 2015;66(1):134–140. doi: 10.1161/HYPERTENSIONAHA.114.04990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wander PL, Boyko EJ, Leonetti DL, McNeely MJ, Kahn SE, Fujimoto WY. Change in visceral adiposity independently predicts a greater risk of developing type 2 diabetes over 10 years in Japanese Americans. Diabetes Care. 2013;36(2):289–293. doi: 10.2337/dc12-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayes JS, Watson GH. Direct effects of sex steroid hormones on adipose tissues and obesity. Obes Rev. 2004;5(4):197–216. doi: 10.1111/j.1467-789X.2004.00152.x. [DOI] [PubMed] [Google Scholar]
- 5.Belanger C, Luu-The V, Dupont P, Tchernof A. Adipose tissue intracrinology: potential importance of local androgen/estrogen metabolism in the regulation of adiposity. Horm Metab Res. 2002;34(11—12):737–745. doi: 10.1055/s-2002-38265. [DOI] [PubMed] [Google Scholar]
- 6.Finkelstein JS, Yu EW, Burnett-Bowie SA. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med. 2013;369(25):2457. doi: 10.1056/NEJMc1313169. [DOI] [PubMed] [Google Scholar]
- 7.Abate N, Haffner SM, Garg A, Peshock RM, Grundy SM. Sex steroid hormones, upper body obesity, and insulin resistance. J Clin Endocrinol Metab. 2002;87(10):4522–4527. doi: 10.1210/jc.2002-020567. [DOI] [PubMed] [Google Scholar]
- 8.Gates MA, Mekary RA, Chiu GR, Ding EL, Wittert GA, Araujo AB. Sex steroid hormone levels and body composition in men. J Clin Endocrinol Metab. 2013;98(6):2442–2450. doi: 10.1210/jc.2012-2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gautier A, Bonnet F, Dubois S, Massart C, Grosheny C, Bachelot A, et al. Associations between visceral adipose tissue, inflammation and sex steroid concentrations in men. Clin Endocrinol (Oxf) 2013;78(3):373–378. doi: 10.1111/j.1365-2265.2012.04401.x. [DOI] [PubMed] [Google Scholar]
- 10.Goh VH, Tong TY, Mok HP, Said B. Interactions among age, adiposity, bodyweight, lifestyle factors and sex steroid hormones in healthy Singaporean Chinese men. Asian J Androl. 2007;9(5):611–621. doi: 10.1111/j.1745-7262.2007.00322.x. [DOI] [PubMed] [Google Scholar]
- 11.Haffner SM, Karhapaa P, Mykkanen L, Laakso M. Insulin resistance, body fat distribution, and sex hormones in men. Diabetes. 1994;43(2):212–219. doi: 10.2337/diab.43.2.212. [DOI] [PubMed] [Google Scholar]
- 12.Ornstrup MJ, Kjaer TN, Harslof T, Stodkilde-Jorgensen H, Hougaard DM, Cohen A, et al. Adipose tissue, estradiol levels, and bone health in obese men with metabolic syndrome. Eur J Endocrinol. 2015;172(2):205–216. doi: 10.1530/EJE-14-0792. [DOI] [PubMed] [Google Scholar]
- 13.Sartorius G, Spasevska S, Idan A, Turner L, Forbes E, Zamojska A, et al. Serum testosterone, dihydrotestosterone and estradiol concentrations in older men self-reporting very good health: the healthy man study. Clin Endocrinol (Oxf) 2012;77(5):755–763. doi: 10.1111/j.1365-2265.2012.04432.x. [DOI] [PubMed] [Google Scholar]
- 14.Segal KR, Dunaif A, Gutin B, Albu J, Nyman A, Pi-Sunyer FX. Body composition, not body weight, is related to cardiovascular disease risk factors and sex hormone levels in men. J Clin Invest. 1987;80(4):1050–1055. doi: 10.1172/JCI113159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaidya D, Dobs A, Gapstur SM, Golden SH, Cushman M, Liu K, et al. Association of baseline sex hormone levels with baseline and longitudinal changes in waist-to-hip ratio: Multi-Ethnic Study of Atherosclerosis. Int J Obes (Lond) 2012;36(12):1578–1584. doi: 10.1038/ijo.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van den Beld AW, de Jong FH, Grobbee DE, Pols HA, Lamberts SW. Measures of bioavailable serum testosterone and estradiol and their relationships with muscle strength, bone density, and body composition in elderly men. J Clin Endocrinol Metab. 2000;85(9):3276–3282. doi: 10.1210/jcem.85.9.6825. [DOI] [PubMed] [Google Scholar]
- 17.Vandenput L, Mellstrom D, Karlsson MK, Orwoll E, Labrie F, Ljunggren O, et al. Serum estradiol is associated with lean mass in elderly Swedish men. Eur J Endocrinol. 2010;162(4):737–745. doi: 10.1530/EJE-09-0696. [DOI] [PubMed] [Google Scholar]
- 18.Vermeulen A, Kaufman JM, Goemaere S, van Pottelberg I. Estradiol in elderly men. Aging Male. 2002;5(2):98–102. [PubMed] [Google Scholar]
- 19.Wang F, Vihma V, Soronen J, Turpeinen U, Hamalainen E, Savolainen-Peltonen H, et al. 17beta-Estradiol and estradiol fatty acyl esters and estrogen-converting enzyme expression in adipose tissue in obese men and women. J Clin Endocrinol Metab. 2013;98(12):4923–4931. doi: 10.1210/jc.2013-2605. [DOI] [PubMed] [Google Scholar]
- 20.Stanczyk FZ, Clarke NJ. Advantages and challenges of mass spectrometry assays for steroid hormones. J Steroid Biochem Mol Biol. 2010;121(3—5):491–495. doi: 10.1016/j.jsbmb.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Fujimoto WY, Leonetti DL, Kinyoun JL, Newell-Morris L, Shuman WP, Stolov WC, et al. Prevalence of diabetes mellitus and impaired glucose tolerance among second-generation Japanese-American men. Diabetes. 1987;36(6):721–729. doi: 10.2337/diab.36.6.721. [DOI] [PubMed] [Google Scholar]
- 22.Shuman WP, Morris LL, Leonetti DL, Wahl PW, Moceri VM, Moss AA, et al. Abnormal body fat distribution detected by computed tomography in diabetic men. Invest Radiol. 1986;21(6):483–487. doi: 10.1097/00004424-198606000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Nelson RE, Grebe SK, OKane DJ, Singh RJ. Liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of estradiol and estrone in human plasma. Clin Chem. 2004;50(2):373–384. doi: 10.1373/clinchem.2003.025478. [DOI] [PubMed] [Google Scholar]
- 24.Royston P, Ambler G, Sauerbrei W. The use of fractional polynomials to model continuous risk variables in epidemiology. Int J Epidemiol. 1999;28(5):964–974. doi: 10.1093/ije/28.5.964. [DOI] [PubMed] [Google Scholar]
- 25.Despres JP. Body fat distribution and risk of cardiovascular disease: an update. Circulation. 2012;126(10):1301–1313. doi: 10.1161/CIRCULATIONAHA.111.067264. [DOI] [PubMed] [Google Scholar]
- 26.Chan DC, Watts GF, Barrett PH, Burke V. Waist circumference, waist-to-hip ratio and body mass index as predictors of adipose tissue compartments in men. QJM. 2003;96(6):441–447. doi: 10.1093/qjmed/hcg069. [DOI] [PubMed] [Google Scholar]
- 27.Grundy SM, Neeland IJ, Turer AT, Vega GL. Waist circumference as measure of abdominal fat compartments. J Obes. 2013;2013:454285. doi: 10.1155/2013/454285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Examination Committee of Criteria for ‘Obesity Disease’ in Japan, Japan Society for the Study of Obesity. New criteria for ‘obesity disease’ in Japan. Circ J. 2002;66(11):987–992. doi: 10.1253/circj.66.987. [DOI] [PubMed] [Google Scholar]
- 29.Kashihara H, Lee JS, Kawakubo K, Tamura M, Akabayashi A. Criteria of waist circumference according to computed tomography-measured visceral fat area and the clustering of cardiovascular risk factors. Circ J. 2009;73(10):1881–1886. doi: 10.1253/circj.cj-09-0183. [DOI] [PubMed] [Google Scholar]
- 30.Britton KA, Massaro JM, Murabito JM, Kreger BE, Hoffmann U, Fox CS. Body fat distribution, incident cardiovascular disease, cancer, and all-cause mortality. J Am Coll Cardiol. 2013;62(10):921–925. doi: 10.1016/j.jacc.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nazare JA, Smith JD, Borel AL, Haffner SM, Balkau B, Ross R, et al. Ethnic influences on the relations between abdominal subcutaneous and visceral adiposity, liver fat, and cardiometabolic risk profile: the International Study of Prediction of Intra-Abdominal Adiposity and Its Relationship With Cardiometabolic Risk/Intra-Abdominal Adiposity. Am J Clin Nutr. 2012;96(4):714–726. doi: 10.3945/ajcn.112.035758. [DOI] [PubMed] [Google Scholar]
- 32.Faulds MH, Zhao C, Dahlman-Wright K, Gustafsson JA. The diversity of sex steroid action: regulation of metabolism by estrogen signaling. J Endocrinol. 2012;212(1):3–12. doi: 10.1530/JOE-11-0044. [DOI] [PubMed] [Google Scholar]
- 33.Jones ME, Thorburn AW, Britt KL, Hewitt KN, Misso ML, Wreford NG, et al. Aromatase-deficient (ArKO) mice accumulate excess adipose tissue. J Steroid Biochem Mol Biol. 2001;79(1—5):3–9. doi: 10.1016/s0960-0760(01)00136-4. [DOI] [PubMed] [Google Scholar]
- 34.Andersson AM, Carlsen E, Petersen JH, Skakkebaek NE. Variation in levels of serum inhibin B, testosterone, estradiol, luteinizing hormone, follicle-stimulating hormone, and sex hormone-binding globulin in monthly samples from healthy men during a 17-month period: possible effects of seasons. J Clin Endocrinol Metab. 2003;88(2):932–937. doi: 10.1210/jc.2002-020838. [DOI] [PubMed] [Google Scholar]
- 35.Kley HK, Schlaghecke R, Kruskemper HL. Stability of steroids in plasma over a 10-year period. J Clin Chem Clin Biochem. 1985;23(12):875–878. [PubMed] [Google Scholar]