Abstract
Background
Varenicline has been found to decrease alcohol-motivated behaviors. Recent warnings regarding aversive events associated with varenicline used in conjunction with alcohol warrant further investigation into the safety of the drug when combined with alcohol. The purpose of this preliminary investigation was to examine the effect of combining varenicline with a high, fixed-dose of alcohol on subjective reactivity and cognitive function in adults with alcohol use disorders.
Methods
This double-blind, placebo-controlled preliminary investigation examined the effects of varenicline (0, 1, 2 mg/day) on subjective reactivity, cognition, perceptual motor function, and physiologic reactivity to a fixed-dose of alcohol (vs. non-alcohol control beverage) using an established laboratory paradigm in smokers and non-smokers meeting criteria for alcohol use disorders (AUDs; n=44). All participants had completed a parent varenicline study evaluating alcohol self-administration. Each subject completed two fixed-dose laboratory sessions assessing reactivity to a high-dose alcohol (0.08 g/dL) or a non-alcoholic control beverage, order counter-balanced.
Results
Varenicline attenuated alcohol-related increases in subjective intoxication and alcohol-related decreases in executive cognitive function. At baseline, varenicline reduced alcohol craving and diastolic blood pressure, and increased associative learning, working memory, and perceptual motor function. Varenicline produced non-specific effects on diastolic blood pressure and heart rate. Overall, there were few differences in effects between 1 mg/day and 2 mg/day varenicline versus placebo.
Conclusions
These preliminary results continue to support the safety and use of varenicline in combination with alcohol in individuals meeting criteria for AUDs.
Keywords: varenicline, alcohol, fixed-dose, nAChR, laboratory, cognition, perceptual motor
INTRODUCTION
Recent preclinical and human laboratory studies suggest that the nicotinic acetylcholine receptor (nAchR) system may be a promising therapeutic target for reducing alcohol-motivated behaviors. Varenicline (Chantix) (Pfizer, New York, USA), a partial agonist of α4β2 nAchRs and a Food and Drug Administration (FDA) - approved smoking cessation aid, has been found to reduce alcohol self-administration (Wouda et al., 2011, Steensland et al., 2007, Kamens et al., 2010) and cue-induced reinstatement to alcohol seeking (Wouda et al., 2011) in rodents. In humans, varenicline has primarily been tested at the 2 mg/day dose when assessing the effects of varenicline on drinking behaviors. We demonstrated that varenicline reduced alcohol consumption compared with placebo in heavy-drinking smokers, and decreased the subjective reinforcing effects of alcohol following a priming drink (McKee et al., 2009). Subsequent studies have also shown that varenicline decreased alcohol intake and alcohol craving in heavy drinking smokers and alcohol-dependent individuals (Mitchell et al., 2012, Plebani et al., 2013). More recently, a multi-site randomized, placebo-controlled clinical trial of 2 mg/day varenicline in smokers and non-smokers meeting criteria for alcohol dependence demonstrated that varenicline attenuated alcohol craving, number of heavy drinking days, and drinks per day compared with placebo (Litten et al., 2013).
While varenicline has demonstrated efficacy for the treatment of alcohol use disorders (AUDs), recent FDA warnings regarding aversive events associated with varenicline used in conjunction with alcohol (Food & Drug Administration, 2015) warrants further investigation into the safety of the drug for the treatment of AUDs. The new FDA warnings indicate that individuals who drink alcohol while taking varenicline may experience increased drunkenness and unusual or aggressive behavior (Food & Drug Administration, 2015). To date, there are no fixed-dose alcohol studies combining varenicline with alcohol in those with AUDs. The primary goal of the present investigation was to examine the effect of combining varenicline with a fixed, high-dose of alcohol on craving, intoxication, perceptual motor response, and executive cognitive function over the ascending and descending limbs of the blood alcohol curve in smokers and non-smokers who meet criteria for AUDs.
While the recent FDA warnings are indicative of increased impairment while taking varenicline in conjunction with alcohol, in studies of smokers, results demonstrate that 2 mg/day varenicline improves and quickens cognitive function. Abstinent smokers taking varenicline showed greater improved sustained attention and working memory compared with placebo (Patterson et al., 2009). Similarly, highly dependent smokers taking varenicline demonstrated greater performance on the visual N-back working memory task, and this was associated with an increase in working memory-related brain activity after three days of abstinence (Loughead et al., 2010). Varenicline has also been shown to speed reaction time on measures of attention in nicotine-deprived smokers (Ashare and McKee, 2012). Independent of smoking, healthy subjects also demonstrate enhanced effects on working and declarative memory following a short-term, low dose (up to 1 mg/day) varenicline regimen (Mocking et al., 2013).
However, some preclinical findings suggest that lower doses of varenicline may potentiate an alcohol effect. Lower doses (0.3 and 0.5 mg/kg) produced a slight, although non-significant increase in alcohol self-administration (Steensland et al., 2007, Wouda et al., 2011) and alcohol-seeking (Wouda et al., 2011). This effect was observed in a more recent study on alcohol-primed behaviors such that 0.3 mg/kg varenicline gave rise to a non-significant increase on alcohol responding (Randall et al., 2015). We have recently completed the first study on the effects of lowering the dose of varenicline on alcohol self-administration in individuals with AUDs (Verplaetse et al., in press), the parent study to the present investigation. We found a modest effect of varenicline (2 mg/day) on reduced alcohol consumption and craving, but no dose-ranging effect of varenicline at the doses tested (1 and 2 mg/day)(Verplaetse et al., in press).
For the current preliminary investigation, we examined the effect of varenicline (0, 1 mg/day, and 2 mg/day) in combination with alcohol (0.08 g/dL versus a no-alcohol beverage control) on craving, subjective intoxication, perceptual motor response, and executive cognitive function. We hypothesized that 1 and 2mg/day varenicline (versus placebo) would reduce craving and subjective intoxication following alcohol consumption (McKee et al., 2009). Based on work in smokers and non-smokers demonstrating that varenicline improves cognitive function (Patterson et al., 2009, Loughead et al., 2010, Mocking et al., 2013), we predicted that varenicline would improve cognitive function overall. It was unknown what effect varenicline would have on alcohol-related impairments in cognitive function and perceptual motor tasks.
MATERIALS AND METHODS
Participants
Participants were eligible if they were ≥ 21 years of age and were able to read and speak English. All participants met DSM-IV criteria for past 6 months alcohol abuse (n=18) or alcohol dependence (n=26), and met criteria for heavy drinking (>4 drinks per episode for women, >5 drinks per episode for men at least once per week). Exclusion criteria included illicit drug use (except for occasional cannabis use), past 30-day use of psychoactive drugs, treatment-seeking for alcohol or smoking, current Axis I disorders (except for nicotine dependence or alcohol abuse), current suicidal or homicidal ideation, pregnant or nursing, or medical conditions contraindicating alcohol use (e.g., liver enzymes ≥ 3× normal) or varenicline administration (e.g., known allergy to varenicline), or subjects likely to exhibit clinically significant alcohol withdrawal during the study (Clinical Institute Withdrawal Scale Score > 8).
Procedures
Eligibility Screening
Participants were consented for the parent study and the current protocol at the same time. Of the n=60 subjects who completed the parent study, n=44 elected to also complete the current protocol. Briefly, in the parent study, varenicline was titrated to steady-state levels over 7 days, and participants then completed a single laboratory session examining alcohol self-administration (Verplaetse et al., in press). Following the self-administration session, participants who enrolled in the present investigation were maintained at steady-state varenicline levels for an additional three week period. During this three week period participants completed two laboratory sessions as detailed below. The Human Investigation Committee of Yale University approved this study. Smokers and non-smokers meeting criteria for AUDs were included in the present investigation. Smokers were daily smokers, smoking more than 5 cigarettes per day over the past year. Non-smokers had not used any tobacco products over the past year.
Medication
The medication condition was double-blind and placebo-controlled, and was assigned in the parent varenicline study. Randomization to varenicline (1 or 2 mg/day) or a matching placebo (0 mg/day) was stratified by sex and smoking status. In the parent study, varenicline was titrated to steady-state levels over 7 days. Medication compliance was monitored with pill counts and riboflavin marker. Participants were maintained at steady-state levels for an additional three week period while they completed the two laboratory sessions (alcohol versus control beverage), scheduled one week apart during weeks 2 and 3. Further methodological details can be found in the parent study (Verplaetse et al., in press).
Laboratory Sessions
Participants attended a 2-hour practice session scheduled during the week before their first laboratory session. The purpose of this practice session was to familiarize the subjects with the assessment battery. After the practice session, each subject completed two randomly assigned fixed-dose laboratory sessions (alcohol versus control beverage). Sessions were approximately 9 hours in length. See Supplemental Table 1 for complete timeline of laboratory procedures. Reports of possible side effects from varenicline were evaluated in the parent study, which found all side effects to be minimal and mild (Verplaetse et al., in press).The laboratory session began at 9:00am, and baseline assessments of breath alcohol, breath CO, urine drug screen, urine pregnancy screen, vitals, height, and weight were obtained. Alcohol withdrawal was assessed, and pill counts and timeline follow-back information for alcohol and tobacco were obtained. Participants provided a urine sample to test for the riboflavin marker. If participants tested positive for alcohol, drug use, alcohol withdrawal, or pregnancy they did not complete the lab sessions. Varenicline (0.5 or 1.0 mg) or placebo was administered at 9:30am followed by a standardized breakfast. To ensure that participants were not nicotine deprived during the session, smokers were provided with 15-min smoke breaks every 2 hours for 15 minutes. Smoke breaks occurred at 11:15am, and after the assessments occurring at +60, +180, and +300 minutes after alcohol consumption. Regular smoke breaks were provided in order to test smokers in a non-nicotine deprived state, as nicotine deprivation in dependent smokers is known to reduce cognitive performance (Snyder et al., 1989, Shiffman et al., 1995).
Assessment Battery
At 11:30am, subjects completed the baseline assessment battery. The baseline assessment battery included breath alcohol concentration (BrAC), subjective reactivity, measures of cognitive function, perceptual motor response, physiologic reactivity, and adverse effects. Subjective reactivity was assessed using the Alcohol Urge Questionnaire (AUQ) (Bohn et al., 1995) and the Alcohol Effects Scale (AES) (Schuckit, 1984). AUQ is a self-report measure assessing the desire for a drink, the expectation of positive effects from drinking, and the inability to avoid drinking if alcohol was available. AES is a self-report measure assessing subjective intoxication, high, like, rush, and feel-good from alcohol. AUQ and AES used Visual Analogue Scale (VAS) with a range of 1 – 100. Cognitive function was measured using the Continuous Performance Task (CPT)(Rosvold et al., 1956), the Digit Symbol Substitution Task (DSST)(McLeod et al., 1982), and the N-Back task (Conway et al., 2005). The CPT assesses attention and response inhibition, and main outcomes were percent omission (the number of times the target was presented but the subject did not respond) and commission (the number of times the subject responded but no target was presented) errors in response to go and stop targets. The DSST assesses associative ability and learning, and main outcomes were the number of attempts and successes on a task in which a random digit appears on a computer screen and subjects use the numeric keypad to reproduce a geometric pattern associated with the digit. The N-Back task assesses working memory by requiring subjects to respond to whether a letter is identical to the one presented n letters back, and main outcomes were number correct and total time to complete the task. One and two n back versions were completed. The Pursuit Rotor task (Fillmore, 2003) assesses perceptual motor performance, and the main outcome was percent of time on target on a task in which subjects track a moving visual target on a computer screen by moving the computer mouse so that the crosshair sight is on the moving target. Heart rate and systolic and diastolic blood pressure were also measured using dinamap for continuous heart rate and blood pressure measures. The battery was provided in a set order and took 30 minutes to complete.
Alcohol Administration
Subjects were told that they were consuming either alcohol or a non-alcohol control beverage by research staff. Subjects were informed of the beverage condition to increase the ecological validity of the manipulation. The purpose of the non-alcoholic beverage control group was to control for the repeated administrations of each task in order to highlight effects specific to alcohol. A second blinded research staff member conducted the laboratory sessions. For the alcohol session (order counterbalanced), subjects were given a fixed dose of alcohol (0.08 g/dL) at 12:15pm. The alcohol beverage was designed to raise blood alcohol levels (BALs) to 0.08 g/dL of alcohol and consisted of 1 part 80 proof liquor of the subject’s choosing to 3 parts mixer chosen from a selection of equicaloric, non-caffeinated, non-carbonated drinks. The amount of alcohol in the drink was based on a formula that takes into account gender, age, height, and weight of each subject (Watson, 1989). The dose was divided into two glasses. Subjects had 5 minutes to consume each drink and a 5 minute rest period in between each drink. We have used this exact procedure previously (McKee et al., 2010) to successfully administer a dose of 0.08 g/dL. This procedure was originally adopted from King and colleagues (King et al., 2002, King et al., 2011a). Peak BALs are achieved within 60 minutes with levels declining over the next 5 hours. The non-alcohol control beverage used the same mixer and total volume as the alcohol beverage. Lunch was provided after the +120 timepoint. At 6:00pm, transportation home was provided if the subject’s BrAC did not reach 0.00%.
Time of Assessments
BrACs were assessed at baseline and at 15, 60, 120, 180, 240, and 360 min following alcohol consumption. CO levels, alcohol craving, subjective effects of alcohol (intoxication), objective effects of alcohol (cognitive function), physiologic measures (systolic and diastolic blood pressure, heart rate), and potential adverse effects were assessed at baseline, as previously described, and at 15, 60, 120, 180, 240, and 300 min following alcohol consumption.
Statistical Analysis
Baseline characteristics were compared across medication (0, 1, 2mg varenicline) with analyses of variance (ANOVA). If baseline differences existed, covariance adjustments were made as appropriate across planned analyses. Separate general linear models (GLM) were conducted for each measure of objective reactivity (cognitive function, perceptual motor response, physiologic reactivity, BrACs, adverse events) and for each measure of subjective reactivity (craving, intoxication). For each GLM, alcohol condition (0.08 g/dL, control beverage) and time (see study timepoints) were within subject factors and medication (0, 1, 2 mg/day varenicline) was a between subject factor. Contrasts examined 1 and 2 mg/day varenicline versus placebo within each timepoint and across beverage conditions. If the outcome differed by medication at the baseline timepoint (-45 min), the GLM was conducted with the -45 min values included as a covariate. Exploratory analyses were conducted to examine possible age, gender, smoking status, and alcohol use disorders identification test (AUDIT) effects across all planned analyses. These factors did not substantively change findings and were dropped from the final models for parsimony. A manipulation check was also conducted to determine if alcohol had an effect on outcomes across the placebo groups in the alcohol versus control beverage session. Separate GLMs were analyzed with the placebo subjects only to confirm significant beverage by time effects. Presentation of the results is focused on main effects of medication and interactive effects of medication with time and/or beverage condition (see Supplementary Materials for presentation of main effects of time and beverage, and interactions of time and beverage effects).
RESULTS
Baseline Characteristics
Baseline characteristics did not differ between subjects who participated in the current study and those that only completed the parent study. Varenicline (1 and 2 mg/day) and placebo groups were well matched for baseline demographic variables, and drinking and smoking behavior (Table 1).
Table 1.
Baseline Characteristics by Medication Condition, Mean (SD) or n (%)
| Placebo (n=17) | 1 mg/day Varenicline (n=12) | 2 mg/day Varenicline (n=15) | p | |
|---|---|---|---|---|
|
| ||||
| Age | 35.3 (9.47) | 33.9 (7.29) | 34.4 (12.6) | 0.93 |
| Sex (% male) | 65% | 58% | 73% | 0.71 |
| Race (% White) | 0.15 | |||
| White | 8 (47) | 8 (67) | 12 (80) | |
| Other | 9 (53) | 4 (33) | 3 (20) | |
| Education | 0.03 | |||
| ≤ High school | 8 (47) | 5 (42) | 1 (7) | |
| ≥ College | 9 (53) | 7 (58) | 14 (93) | |
| Marital Status | 0.09 | |||
| Not married | 13 (76) | 9 (75) | 15 (100) | |
| Married | 4 (24) | 3 (25) | 0 (0) | |
| Tobacco Use (smokers only) | ||||
| Cigarettes per day | 13.9 (7.26) | 13.5 (4.08) | 15.9 (6.39) | 0.68 |
| FTND scorea* | 4.30 (2.31) | 4.63 (1.85) | 4.0 (1.94) | 0.82 |
| Alcohol Use | ||||
| Weekly frequency (days)b | 4.94 (1.74) | 4.14 (1.60) | 4.39 (1.55) | 0.40 |
| Drinks per episode | 6.66 (3.46) | 6.66 (2.75) | 6.19 (3.26) | 0.90 |
| AUDIT scoresc | 13.5 (6.97) | 12.3 (4.58) | 10.1 (3.61) | 0.21 |
AUDIT, Alcohol Use Disorders Identification Test; FTND, Fagerström Test for Nicotine Dependence
Range: scores ≥ 4 for nicotine dependence.
Means calculated over 30 days before intake.
Scores ≥ 8 for alcohol misuse.
n=10, 8, 9 for placebo, 1 mg/day varenicline, and 2 mg/day varenicline, respectively.
Note: education (p=.03); otherwise, no difference between groups using chi-square or ANOVA where appropriate.
Fixed-Dose Alcohol versus Control Beverage Administration: Medication Effects
See Table 2 for a complete summary of GLM results and effect sizes. All effect sizes associated with significant and trend level GLM effects were calculated as Cohen’s d and all values were in the medium to large range (0.52 – 1.28). Within timepoint contrasts of significant medication results are presented below (all ps<0.05).
Table 2.
Summary of results and Cohen’s d effect sizes.
| Task | Outcome | Manipulation Check* | GLM Results
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Main Effects | Interactions | ||||||||
|
|
|
||||||||
| Med | Bev | Time | Med x Time | Bev x Time | M x B x T | ||||
| Subjective Reactivity | Craving Subjective | F=6.24, p=.02 | F=5.15, p=.03c,d | ||||||
| Intoxication | F=15.27, p<.01 | F=3.38, p=.04b | |||||||
|
| |||||||||
| Cognitive Function | CPT | Omissions | F=3.51, p=.08 | F=4.64, p=.04c | F=2.94, p=.07b | ||||
| Commissions | F=5.51, p=.03 | F=4.74, p=.02c | |||||||
| DSST | Attempts | F=3.40, p=.04b,d | |||||||
| Successes | F=3.14, p=.06b,d | ||||||||
| N-back 1 | Correct | F=4.34, p=.06 | F=3.29, p=.05b | ||||||
| Total Time | F=7.46, p=.02 | F=3.33, p=.05c,d | |||||||
|
| |||||||||
| Perceptual Motor Function | Pursuit Rotor | Average % on Target | F=10.07, p<.01 | F=2.50, p=.1b,d | |||||
|
| |||||||||
| Physiologic Reactivity | Systolic BP | F=3.62, p=.08 | F=5.77, p=.02c | ||||||
| Diastolic BP | F=5.48, p=.03 | F=4.26, p=.02c,d | |||||||
| Heart Rate | F=19.82, p<.01 | F=3.36, p=.05b | F=16, p<.01c | F=2.64, p=.08b | |||||
Note: Blank cells are n.s.
No significant medication by beverage interactions.
Manipulation Check for main effect of beverage or beverage by time in placebo only subjects. Cohen d Effect size:
Small (0.1 – 0.3),
medium (0.4 – 0.6),
large (0.7 - >0.9)
Controlled for baseline (−45 timepoint; for outcomes with baseline differences only).
Manipulation Check
Limiting analysis to placebo subjects only, a high fixed-dose of alcohol increased alcohol craving, subjective intoxication, and cognitive deficits during the alcohol session relative to the control beverage session (see Table 2).
Breath Alcohol Concentrations
As expected, BrACs increased after high fixed-dose alcohol consumption and steadily declined over the next 5 hours (F (1, 39) = 224.08, p < .001). Mean BrACs at the +60 timepoint were: grand mean = 0.058, SE = 0.002.
Alcohol craving
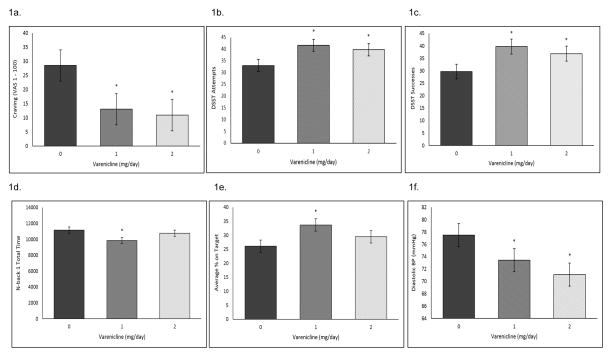
Both 1 and 2 mg/day varenicline decreased alcohol craving at the baseline timepoint (Figure 1a). Varenicline did not demonstrate any main or interactive effects after controlling for this baseline difference.
Figure 1.
Baseline differences (−45 timepoint; raw data) collapsed across the fixed-dose alcohol (0.08 g/dL) and control beverage sessions for (A) Mean (±SE) subjective alcohol craving ratings; (B) Mean (±SE) number of attempts on the DSST; (C) Mean (±SE) number of successes on the DSST (D) Total time (±SE) on the N-back 1; (E) Average (±SE) percent on target on the pursuit rotor task; (F) Mean (±SE) diastolic blood pressure. *Varenicline significantly different versus placebo.
Subjective intoxication
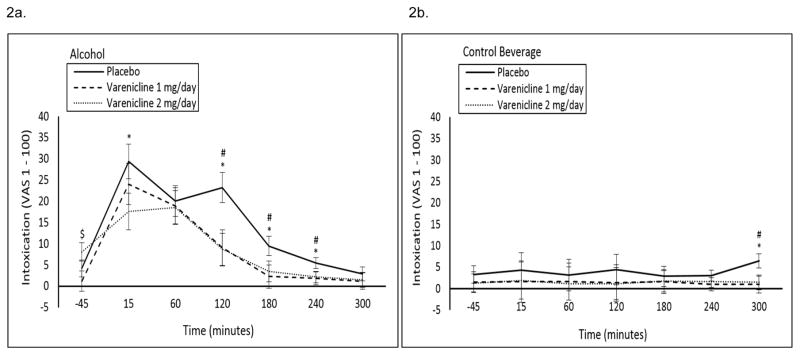
1 mg/day varenicline decreased subjective intoxication on the descending limb of the blood alcohol curve, and 2 mg/day varenicline decreased subjective intoxication at the +15 timepoint and over the course of the descending limb of the blood alcohol curve in the alcohol session only (medication by beverage by time interaction p=0.04; see Figure 2a, b).
Figure 2.
Mean (±SE) subjective intoxication ratings following a high fixed dose of (A) alcohol (0.08 g/dL) or (B) a control beverage by medication condition by time. #1 mg/day varenicline significantly different versus placebo; *2 mg/day varenicline significantly different versus placebo; $1 mg/day varenicline significantly different versus 2 mg/day varenicline.
Cognitive function
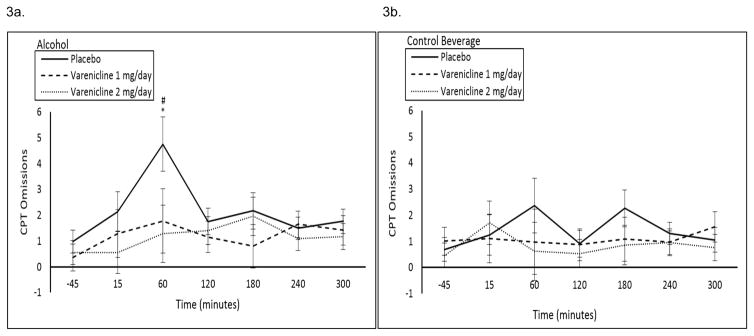
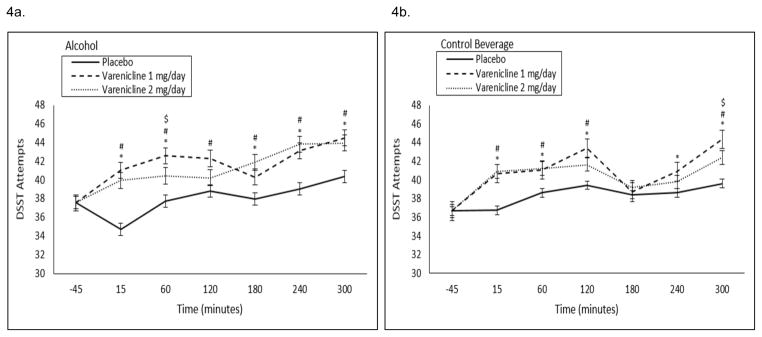
CPT: 1 and 2 mg/day varenicline decreased percent omissions at the +60 timepoint in the alcohol session only at a trend level (medication by beverage by time interaction p=0.07; see Figure 3a, b). CPT commissions demonstrated a significant beverage by time by medication interaction (p=0.02), but there were no significant within timepoint contrasts. DSST Attempts: At baseline, 1 and 2 mg/day varenicline increased DSST attempts (Figure 1b). Over the course of the alcohol and control beverage sessions, 1 and 2 mg/day varenicline increased DSST attempts relative to placebo (medication by time interaction p=0.04; see Figure 4a, b). DSST Successes: At baseline, 1 and 2 mg/day varenicline increased DSST successes (Figure 1c). Over the course of the alcohol and control beverage sessions, 1 and 2 mg/day varenicline increased DSST successes relative to placebo at a trend level (medication by time interaction p=0.06; see Supplemental Figure 1a, b).
Figure 3.
Mean (±SE) number of omissions on the CPT following a high fixed dose of (A) alcohol (0.08 g/dL) or (B) a control beverage by medication condition by time. #1 mg/day varenicline significantly different versus placebo; *2 mg/day varenicline significantly different versus placebo.
Figure 4.
Baseline (−45 timepoint) adjusted mean (±SE) number of attempts on the DSST following a high fixed dose of (A) alcohol (0.08 g/dL) or (B) a control beverage by medication condition by time. #1 mg/day varenicline significantly different versus placebo; *2 mg/day varenicline significantly different versus placebo. $1 mg/day varenicline significantly different versus 2 mg/day varenicline.
Working memory
Nback1 Correct: 1 and 2 mg/day varenicline increased the number correct on the descending limb of the blood alcohol curve in the alcohol session only compared with placebo (medication by beverage by time interaction p=0.05; see Supplemental Figure 2a, b). Nback1 Total Time: At baseline, 1 mg/day varenicline decreased total time on the Nback1 (Figure 1d). After equating for this baseline difference, 1 mg/day varenicline decreased Nback1 total time at the +15 minute timepoint in the alcohol session and over the course of the control beverage session. Varenicline increased Nback1 total time towards the end of the alcohol session only (medication by beverage by time interaction p=0.05; see Supplemental Figure 2c, d).
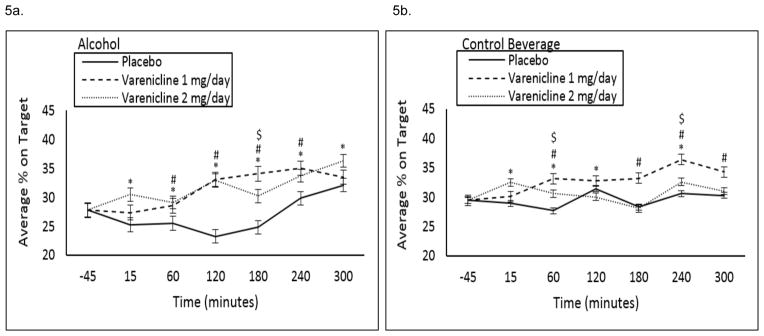
Perceptual motor function
At baseline, 1 and 2 mg/day varenicline increased average percent on target (Figure 1e). Over the course of the alcohol and control beverage sessions, 1 and 2 mg/day varenicline increased average percent on target relative to placebo at a trend level (medication by beverage by time interaction p=0.1, see Figure 5a, b).
Figure 5.
Baseline (−45 timepoint) adjusted average (±SE) percent on target on the pursuit rotor task following a high fixed dose of (A) alcohol (0.08 g/dL) or (B) a control beverage by medication condition by time. #1 mg/day varenicline significantly different versus placebo; *2 mg/day varenicline significantly different versus placebo; $1 mg/day varenicline significantly different versus 2 mg/day varenicline.
Physiologic Measures
Systolic blood pressure: There were no significant medication effects for this outcome (ps>0.05; see Supplemental Figure 3a, b). Diastolic blood pressure: At baseline, 1 and 2 mg/day varenicline decreased diastolic blood pressure (Figure 1f). After equating for this baseline difference, 1 and 2 mg/day varenicline increased diastolic blood pressure relative to placebo for some timepoints in both the alcohol and control beverage sessions (medication by beverage by time interaction p=0.02; see Supplemental Figure 3c, d). Heart rate: Varenicline increased heart rate over the course of the alcohol and control beverage sessions (medication by time interaction p=0.05, medication by beverage by time interaction p=0.08; see Supplemental Figure 3e, f). Differences were most pronounced at the +60 timepoint.
DISCUSSION
To our knowledge, this is the first preliminary fixed-dose investigation examining the effect of varenicline (1 and 2 mg/day) combined with high-dose alcohol on measures of subjective and objective reactivity in persons with AUDs. Overall, results demonstrated medication effects on subjective reactivity, cognitive function, and physiologic reactivity. Varenicline clearly attenuated alcohol-related increases in subjective intoxication and improved alcohol-related decrements in attention at a trend level. Prior to beverage administration, varenicline was found to reduce alcohol craving, and improve associative ability, working memory, and perceptual motor function. When these baseline medication differences were accounted for in the analysis, varenicline tended not to interact with alcohol to produce synergistic or additive effects for alcohol craving and some measures of cognitive function. There were few differences across the 1 and 2 mg/day doses.
With regard to subjective reactivity, varenicline reduced alcohol craving relative to placebo, and attenuated increases in subjective intoxication at the peak and descending limb of the blood alcohol curve. For alcohol craving, these differences were present prior to beverage administration, suggesting that varenicline targeted tonic craving, and these differences persisted throughout both the alcohol and control beverage conditions (data not shown). This is consistent with findings from our parent study indicating that 2 mg/day varenicline reduced tonic alcohol craving during a priming drink period in heavy drinkers (Verplaetse et al., in press), and other studies demonstrating a reduction in alcohol craving and subjective alcohol effects following 2 mg/day varenicline in heavy drinking smokers (McKee et al., 2009, Mitchell et al., 2012, Plebani et al., 2013, Litten et al., 2013).
Varenicline improved cognitive function relative to placebo, and this effect was generally more pronounced in the 1 mg/day varenicline group. Varenicline improved associative ability and learning, improved working memory, and trended towards enhanced attention and perceptual motor performance with moderate effect size values (all with Cohen’s d effect sizes in the moderate range). While varenicline attenuated alcohol-related decrements in attention, generally the effects of varenicline on improved cognitive function did not interact with alcohol. For associative ability and learning, working memory, and perceptual motor response, medication differences were present prior to beverage administration. However, varenicline tended to improve alcohol-related decrements in associative ability and perceptual motor function even after controlling for baseline differences. In contrast, varenicline increased total time on the Nback1 towards the end of the alcohol session only after controlling for baseline differences that varenicline reduced Nback1 total time. Further, smoking status did not significantly impact on the cognitive outcomes in the present investigation. Within our subset of smokers, a pattern of improved cognitive performance was not demonstrated based on time since last cigarette (data not shown). Overall, medication effects are consistent with prior work demonstrating that varenicline improves cognition in smokers and non-smokers (Patterson et al., 2009, Loughead et al., 2010, Mocking et al., 2013), and extends these findings by documenting that varenicline improves cognitive performance in individuals with alcohol use disorders and attenuates alcohol-related decrements in attention and perceptual motor response at a trend level.
Previous work with varenicline in smokers and non-smokers indicate that the cognitive enhancing effects of varenicline may be due to its cholinergic properties, specifically the high affinity of varenicline to α4β2 nAchRs or, with lower affinity, to the α7 nAchR subtype. There has been much work focusing on nAchRs as a target for cognitive enhancement, particularly α7 (Loughead et al., 2010, Mocking et al., 2013). Preclinical models support this hypothesis in that β2 and α7 knockout mice performed worse on cognitive tasks relative to their wildtype counterparts (Levin et al., 2009). Similarly, an imaging study found that varenicline activates brain regions associated with working memory (Loughead et al., 2010). It has been proposed (Mocking et al., 2013) that this activation may reduce cognitive deficits in working memory experienced as withdrawal symptoms during nicotine abstinence (Patterson et al., 2010). Future work should continue to examine varenicline-alcohol interactions with regard to alcohol-related safety, and the mechanism behind varenicline’s effects on cognitive function.
With regard to differences across active doses, 1 and 2 mg/day varenicline did not differ from each other on craving, subjective intoxication, and attention. There was a slight trend towards a dose-response for alcohol craving towards the end of the alcohol session but this effect was non-significant. Conversely, the 1 mg/day varenicline medication group performed better than the 2 mg/day medication group only at limited timepoints on measures of associative ability and perceptual motor function. The overall lack of differences between doses may indicate that varenicline may be equally efficacious at lower doses when given in combination with a fixed-dose of alcohol. However, the parent study to the present investigation (Verplaetse et al., in press) indicates that 2 mg/day but not 1 mg/day varenicline had modest effects on alcohol intake and craving, but demonstrated no evidence supporting an effect of 1 mg/day varenicline on alcohol consumption or craving during a 2-hour ad-libitum alcohol self-administration period. In the present investigation, there was no evidence for the lower, 1 mg/day varenicline dose to potentiate alcohol-related effects as suggested in some preclinical studies examining dose-ranging effects (Steensland et al., 2007, Wouda et al., 2011).
Our findings are of immediate clinical relevance for the use of varenicline for AUDs given the recent FDA warnings on adverse events (e.g. increased drunkenness) (Food & Drug Administration, 2015) associated with alcohol-varenicline interactions. Although we observed minimal side effects and non-specific effects of varenicline on physiologic reactivity, in the context of alcohol-related safety, our findings indicate that taking varenicline in combination with alcohol reduces alcohol-related cognitive impairment and decreases subjective intoxication. That is, individuals feel less intoxicated but also perform better on tasks measuring associative learning, attention, working memory, and perceptual motor function. From a clinical standpoint, our findings raise critical questions regarding the safety of individuals taking varenicline and drinking to intoxication. Individuals who feel less intoxicated may decide to continue drinking or underestimate the effects of alcohol on cognitive ability and decision-making, potentially leading to increased alcohol-related consequences (e.g., driving under the influence, legal risk). However, findings from the parent study and prior established findings demonstrate that varenicline is associated with reduced alcohol consumption (McKee et al., 2009, Fucito et al., 2011, Mitchell et al., 2012, Litten et al., 2013, Verplaetse et al., in press), reduced alcohol craving (Schacht et al., 2014), potentiated subjective aversive effects of alcohol (Childs et al., 2012), and reduced cue-elicited brain activation in the medial orbitofrontal cortex (OFC)(Schacht et al., 2014). Alternatively, individuals who experience less alcohol-related cognitive impairment may be less likely to make poor or risky decisions while intoxicated.
Varenicline was well-tolerated in our sample of heavy drinkers during the two fixed-dose laboratory sessions. Side effects were reported in the parent study (Verplaetse et al., in press), and mean severity ratings for each adverse event were reported as minimal to mild and did not differ across medication groups. Rates of dry mouth were greatest in the placebo group (35% placebo; 10% 1 mg/day varenicline; 10% 2 mg/day varenicline), whereas rates of insomnia were lowest in the 1 mg/day group (10% placebo; 0% 1 mg/day varenicline; 25% 2 mg/day varenicline). Further, no subject discontinued medication or required a dose adjustment as a result of adverse events. Overall, our preliminary findings indicate that combining varenicline with a high, fixed-dose of alcohol appears to be safe and well-tolerated in individuals meeting criteria for AUDs.
Similar to our parent study, there are study limitations. The present study sample consisted of non-treatment seeking adults meeting criteria for alcohol abuse or dependence. The results may not generalize to treatment seeking adults or drinkers without AUDs. Similarly, this investigation tested varenicline in combination with a single, fixed-dose of alcohol. We did not test the safety of varenicline in combination with other fixed-dose concentrations of alcohol. However, the parent study examined the dose-ranging effects of varenicline on alcohol self-administration, and varenicline was well-tolerated with minimal adverse events (Verplaetse et al., in press). Third, subjective and objective reactivity to alcohol while taking varenicline were only examined during the laboratory sessions. Thus, we do not know how craving, subjective intoxication, and cognitive function would be affected outside of the laboratory. Relatedly, we examined subjective intoxication as a single visual analog item. This may be problematic in that subjective intoxication may have bimodal (positive/drunk or bad/toxic) interpretations among study participants (King et al., 2011b). Fourth, there were a large number of outcomes in this study for a limited sample size. We examined these outcomes as preliminary and, thus, the reported results were not adjusted for multiple comparisons. However, effect sizes for significant and trend level effects were all in the medium to large range. Fifth, the order of tasks within the assessment battery was provided in a fixed task order during each performance of the assessment battery and it is unknown what effect this may have had on our results. Sixth, it is likely that BrAC levels peaked before the +60min timepoint. Our prior study using the same administration methods demonstrated that peak BrAC levels (0.078 g/dL) were achieved 45 minutes from the end of drinking (McKee et al., 2010). Ideally, we would have assessed BrAC levels every 15 minutes over the first hour after alcohol intake to capture peak BrACs. The first assessment battery was administered during the ascending limb of the blood alcohol curve and repeated BrAC measurements would have interfered with cognitive testing. However, we believe our dose manipulation was effective in capturing the effects of varenicline in combination with a fixed, high-dose of alcohol. Finally, subjects were aware of the beverage condition prior to consuming either alcohol or the control beverage which may have impacted on our outcomes. In a realistic setting, subjects know if they are consuming an alcoholic beverage or not, and we informed subjects of the beverage condition in order to increase the ecological validity of the study. Relatedly, we were not interested in examining effects in response to a placebo alcohol beverage, which is nearly impossible to mask at the dose used in this study (0.08 g/dL). The purpose of the non-alcoholic control beverage was to control for the repeated administrations of each task.
Collectively, our preliminary results support the clinical utility and safety of varenicline for the treatment of AUDs. In drinkers meeting criteria for AUDs, we found that 1 and 2 mg/day varenicline attenuated alcohol craving and subjective intoxication. We also found that varenicline increased cognitive functioning on tasks specific to attention, associative learning, and perceptual motor function. With the exception of subjective intoxication and attention, these effects were present prior to the consumption of alcohol, and effects of varenicline were generally not additive or synergistic with alcohol. Adverse events associated with varenicline-alcohol interactions were minimal and did not differ from the placebo group. These data suggest that varenicline continues to be a viable candidate for the treatment of AUDs.
Supplementary Material
Acknowledgments
Funding: NIH grants R01AA017976 (SAM), NIAAA T32AA015496 (TLV) and NIDA T32DA007238 (TLV). Investigator-initiated grant from Pfizer (SAM).
This work was supported by NIH grants R01AA017976 (SAM), NIAAA T32AA015496 (TLV), NIDA T32DA007238 (TLV), and an investigator-initiated grant from Pfizer (SAM).
References
- Ashare RL, McKee SA. Effects of varenicline and bupropion on cognitive processes among nicotine-deprived smokers. Experimental and clinical psychopharmacology. 2012;20:63–70. doi: 10.1037/a0025594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res. 1995;19:600–606. doi: 10.1111/j.1530-0277.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Childs E, Roche DJ, King AC, de Wit H. Varenicline potentiates alcohol-induced negative subjective responses and offsets impaired eye movements. Alcohol Clin Exp Res. 2012;36:906–914. doi: 10.1111/j.1530-0277.2011.01675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway ARA, Kane MJ, Bunting MF, Hambrick DZ, Wilhelm O, Engle RW. Working memory span tasks: A methodological review and user's guide. Psychonomic Bulletin & Review. 2005;12:769–786. doi: 10.3758/bf03196772. [DOI] [PubMed] [Google Scholar]
- Fillmore MT. Reliability of a computerized assessment of psychomotor performance and its sensitivity to alcohol-induced impairment. Perceptual and motor skills. 2003;97:21–34. doi: 10.2466/pms.2003.97.1.21. [DOI] [PubMed] [Google Scholar]
- Food & Drug Administration. FDA Drug Safety Communication: FDA updates label for stop smoking drug Chantix (varenicline) to include potential alcohol interaction, rare risk of seizures, and studies of side effects on mood, behavior, or thinking. [Accessed April 7, 2015]; Available at: http://www.fda.gov/Drugs/DrugSafety/ucm436494.htm.
- Fucito LM, Toll BA, Wu R, Romano DM, Tek E, O'Malley SS. A preliminary investigation of varenicline for heavy drinking smokers. Psychopharmacology. 2011;215:655–663. doi: 10.1007/s00213-010-2160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamens HM, Andersen J, Picciotto MR. Modulation of ethanol consumption by genetic and pharmacological manipulation of nicotinic acetylcholine receptors in mice. Psychopharmacology. 2010;208:613–626. doi: 10.1007/s00213-009-1759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, de Wit H, McNamara PJ, Cao D. Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Archives of general psychiatry. 2011a;68:389–399. doi: 10.1001/archgenpsychiatry.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Houle T, de Wit H, Holdstock L, Schuster A. Biphasic alcohol response differs in heavy versus light drinkers. Alcohol Clin Exp Res. 2002;26:827–835. [PubMed] [Google Scholar]
- King AC, Roche DJ, Rueger SY. Subjective responses to alcohol: a paradigm shift may be brewing. Alcoholism: Clinical and Experimental Research. 2011b;35:1726–1728. doi: 10.1111/j.1530-0277.2011.01629.x. [DOI] [PubMed] [Google Scholar]
- Levin ED, Petro A, Rezvani AH, Pollard N, Christopher NC, Strauss M, Avery J, Nicholson J, Rose JE. Nicotinic alpha7- or beta2-containing receptor knockout: effects on radial-arm maze learning and long-term nicotine consumption in mice. Behavioural brain research. 2009;196:207–213. doi: 10.1016/j.bbr.2008.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litten RZ, Ryan ML, Fertig JB, Falk DE, Johnson B, Dunn KE, Green AI, Pettinati HM, Ciraulo DA, Sarid-Segal O, Kampman K, Brunette MF, Strain EC, Tiouririne NA, Ransom J, Scott C, Stout R. A double-blind, placebo-controlled trial assessing the efficacy of varenicline tartrate for alcohol dependence. Journal of addiction medicine. 2013;7:277–286. doi: 10.1097/ADM.0b013e31829623f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughead J, Ray R, Wileyto EP, Ruparel K, Sanborn P, Siegel S, Gur RC, Lerman C. Effects of the alpha4beta2 partial agonist varenicline on brain activity and working memory in abstinent smokers. Biological psychiatry. 2010;67:715–721. doi: 10.1016/j.biopsych.2010.01.016. [DOI] [PubMed] [Google Scholar]
- McKee SA, Harrison EL, O'Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, Picciotto MR, Petrakis IL, Estevez N, Balchunas E. Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biological psychiatry. 2009;66:185–190. doi: 10.1016/j.biopsych.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Harrison EL, Shi J. Alcohol expectancy increases positive responses to cigarettes in young, escalating smokers. Psychopharmacology. 2010;210:355–364. doi: 10.1007/s00213-010-1831-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod DR, Griffiths RR, Bigelow GE, Yingling J. An automated version of the digit symbol substitution test (DSST) Behavior Research Methods & Instrumentation. 1982;14:463–466. [Google Scholar]
- Mitchell JM, Teague CH, Kayser AS, Bartlett SE, Fields HL. Varenicline decreases alcohol consumption in heavy-drinking smokers. Psychopharmacology. 2012;223:299–306. doi: 10.1007/s00213-012-2717-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocking RJ, Patrick Pflanz C, Pringle A, Parsons E, McTavish SF, Cowen PJ, Harmer CJ. Effects of short-term varenicline administration on emotional and cognitive processing in healthy, non-smoking adults: a randomized, double-blind, study. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38:476–484. doi: 10.1038/npp.2012.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Loughead J, Perkins K, Strasser AA, Siegel S, Frey J, Gur R, Lerman C. Working memory deficits predict short-term smoking resumption following brief abstinence. Drug and alcohol dependence. 2010;106:61–64. doi: 10.1016/j.drugalcdep.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Strasser AA, Loughead J, Perkins KA, Gur RC, Frey JM, Siegel S, Lerman C. Varenicline improves mood and cognition during smoking abstinence. Biological psychiatry. 2009;65:144–149. doi: 10.1016/j.biopsych.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plebani JG, Lynch KG, Rennert L, Pettinati HM, O'Brien CP, Kampman KM. Results from a pilot clinical trial of varenicline for the treatment of alcohol dependence. Drug and alcohol dependence. 2013;133:754–758. doi: 10.1016/j.drugalcdep.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall PA, Jaramillo AA, Frisbee S, Besheer J. The role of varenicline on alcohol-primed self-administration and seeking behavior in rats. Psychopharmacology. 2015 doi: 10.1007/s00213-015-3878-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosvold HE, Mirsky AF, Sarason I, Bransome ED, Jr, Beck LH. A continuous performance test of brain damage. Journal of consulting psychology. 1956;20:343. doi: 10.1037/h0043220. [DOI] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Randall PK, Li X, Henderson S, Myrick H. Varenicline effects on drinking, craving and neural reward processing among non-treatment-seeking alcohol-dependent individuals. Psychopharmacology. 2014;231:3799–3807. doi: 10.1007/s00213-014-3518-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA. Subjective responses to alcohol in sons of alcoholics and control subjects. Archives of general psychiatry. 1984;41:879–884. doi: 10.1001/archpsyc.1984.01790200061008. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JD, Elash C. Nicotine withdrawal in chippers and regular smokers: subjective and cognitive effects. Health Psychology. 1995;14:301. doi: 10.1037//0278-6133.14.4.301. [DOI] [PubMed] [Google Scholar]
- Snyder FR, Davis FC, Henningfield JE. The tobacco withdrawal syndrome: performance decrements assessed on a computerized test battery. Drug and alcohol dependence. 1989;23:259–266. doi: 10.1016/0376-8716(89)90090-2. [DOI] [PubMed] [Google Scholar]
- Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:12518–12523. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verplaetse TL, Pittman BP, Shi JM, Tetrault JM, Coppola S, McKee SA. Effect of Lowering the Dose of Varenicline on Alcohol Self-Administration in Drinkers with Alcohol Use Disorders. Journal of addiction medicine. doi: 10.1097/ADM.0000000000000208. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson PE. Total body water and blood alcohol levels: Updating the fundamentals. Human metabolism of alcohol. 1989;1:41–58. [Google Scholar]
- Wouda JA, Riga D, De Vries W, Stegeman M, van Mourik Y, Schetters D, Schoffelmeer AN, Pattij T, De Vries TJ. Varenicline attenuates cue-induced relapse to alcohol, but not nicotine seeking, while reducing inhibitory response control. Psychopharmacology. 2011;216:267–277. doi: 10.1007/s00213-011-2213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.