Abstract
Background
Statins may promote vasodilation following subarachnoid hemorrhage (SAH) and improve the response to blood pressure elevation. We sought to determine whether simvastatin increases cerebral blood flow (CBF) and alters the response to induced hypertension after SAH.
Methods
Statin-naïve patients admitted <72 hours after WFNS ≥2 aneurysmal SAH were randomly assigned to 80 mg simvastatin/day or placebo for 21 days. Regional cerebral blood flow (CBF) was measured with quantitative 15O PET on SAH day 7–10 before and after raising mean arterial pressure (MAP) 20–25%. Autoregulatory index (AI) was calculated as the ratio of % change in resistance (MAP/CBF) to % change in MAP. Angiography was performed within 24 hours of PET. Results are presented as simvastatin vs. placebo.
Results
Thirteen patients received simvastatin and 12 placebo. Clinical characteristics were similar. Moderate or severe angiographic vasospasm occurred in 42% vs.45% and delayed cerebral ischemia in 14% vs. 55% (p=0.074). During PET studies MAP (110±10 vs. 111±12), global CBF (41±12 vs. 43±13) and CVR (2.95±1.0 vs. 2.81±1.0) did not differ at baseline. When MAP was raised to 135±7 mm Hg vs. 137±15, global CBF did not change. Global AI did not differ (107±59 % vs 0. 89±52 %, p=0.68). CBF did not change in regions with low baseline flow or in regions supplied by vessels with angiographic vasospasm in either group. Six month modified Rankin Scale scores did not differ.
Conclusions
Our data indicate that initiation of therapy with high-dose simvastatin does not alter baseline CBF or response to induced hypertension.
Keywords: induced hypertension, blood pressure, vasopressors, positon emission tomography, delayed cerebral ischemia, vasospasm
Introduction
The rupture of an intracranial aneurysm is a devastating cerebrovascular event that results in both acute and delayed cerebral injury. The sudden egress of blood at arterial pressure into the subarachnoid space produces a sudden dramatic rise in intracranial pressure, transient circulatory arrest and initiates a cascade of events that may culminate in delayed cerebral ischemia (DCI). Delayed ischemia, engendering neurological deficits and cerebral infarction, is related to critical reductions in cerebral blood flow (CBF) and is a leading cause of morbidity in those that survive the initial hemorrhage[1]. DCI may be potentiated by abnormalities in cerebrovascular autoregulation, the ability to maintain a stable CBF in the face of changing perfusion pressures.
Several preliminary experimental and clinical studies suggested that early administration of HmG-Co Reductase Inhibitors (statins) can reduce the incidence of DCI, [2–5] although this was not borne out when 40 mg of simvastatin was recently tested in a larger multi-center trial. [6] Statins are pleotropic and the mechanisms through which they act in the brain remain incompletely understood. It has been proposed that, in addition to their cholesterol lowering and anti-inflammatory properties, they act via hemodynamic mechanisms. In animal models of stroke, statins raise resting cerebral blood flow (CBF), enhance the response to vasodilators and increase endothelial nitric oxide activity, responses that are absent in eNOS deficient mice [7, 8] [9]. In experimental subarachnoid hemorrhage (SAH) simvastatin administration resulted in augmented eNOS expression.[10] In SAH patients, 40 mg/d of pravastatin resulted in shorter duration of impaired dynamic autoregulation which was assessed using the transient hyperemic responses following carotid artery compression.[4] However, whether high-dose statin administration can improve CBF after SAH and alter static autoregulation has not been evaluated.
Further clarification of the effects of high-dose statins on cerebral autoregulatory function, and their relationship to vasospasm and DCI could help refine their use, influence future drug development and provide insight into the hemodynamic management of DCI. We chose to study statin-naïve patients who were at risk for DCI following acute aneurysmal SAH to determine if the initiation of statin therapy induces cerebrovascular hemodynamic effects that increase CBF and alter static autoregulatory function during the peak period of risk for delayed cerebral ischemia (DCI).
Methods
Patient Population
All SAH patients admitted to our institution were screened for eligibility based on the following criteria: age > 18 years, SAH from a ruptured cerebral aneurysm, enrolled within 72 hours of hemorrhage, WNFS ≥2, modified Fisher grade ≥2 on initial CT scan, planned surgical or endovascular aneurysm repair, and approval of the attending neurosurgeon. They were excluded if they had SAH secondary to traumatic or mycotic aneurysms, had additional untreated aneurysms, were receiving pre-ictal statin therapy, had contraindications to statin therapy (hypersensitivity; active liver disease or hepatic dysfunction, elevated aspartate aminotransferase (AST), or alanine aminotransferase (ALT); severe renal dysfunction; elevated creatine phosphokinase or had contraindications to induced hypertension (ongoing cardiac ischemia, worsening congestive heart failure, aortic dissection, hypertensive encephalopathy, malignant hypertension, acute renal failure or were pregnant). Patients were randomized by the hospital research pharmacist using a computerized system to in a blinded fashion receive either 80 mg of simvastatin or placebo once a day orally or via nasogastric tube for 21 days. The protocol was approved by the Human Research Protection Office and the Radioactive Drug Research Committee of Washington University. Written consent was obtained from patients or their legally authorized surrogate.
Routine care of SAH patients at our institution during the study period consisted of close monitoring of fluid balance to maintain a euvolemic state (with isotonic saline administered at a rate to maintain daily fluid intake equal or slighter greater than urine output), administration of nimodipine 60 mg every 4 hours for 21 days, an anticonvulsant (typically levetiracetam 500–1,000 mg twice daily) for 3–7 days following admission, and routine 4-vessel digital subtraction angiography to assess for vasospasm on day 7–9 following initial hemorrhage. A ventriculostomy was performed if hydrocephalus was believed to contribute to poor neurological status. DCI was defined as new focal deficit or global decline in consciousness after exclusion of other causes of neurological deterioration. If none was identified patients were treated with induced hypertension and underwent angiography. In the presence of moderate-severe angiographic vasospasm, angioplasty and/or intra-arterial infusion of vasodilators were performed. Modified Rankin scale (mRS) was scored in person 6 months after hemorrhage by a certified research coordinator who was blinded to treatment allocation.
Experimental Procedure
All subjects underwent 15O-PET on post-hemorrhage day 7–10 for measurement of CBF, cerebral blood volume (CBV), cerebral metabolic rate for oxygen (CMRO2) and oxygen extraction fraction (OEF). Blood pressure (BP) was then raised by 20–25% with infusion of phenylephrine or norepinephrine. Once BP was stable for 10 minutes, PET studies were repeated. An attending neurointensivist was present in the room at all times. Electrocardiography, blood pressure, oxygen saturation, and, when indicated, intracranial pressure were continuously monitored and recorded in all patients. Arterial blood gas analysis was performed for measurement of partial pressure of CO2 and arterial oxygen content (CaO2). At the time of each CBF measurement, clinical and physiological data including heart rate, blood pressure, temperature, and arterial blood gas results were recorded. Weekly measurements of liver function tests and creatine phosphokinase were performed to monitor for statin toxicity.
All patients were studied on the same PET scanner (ECAT Exact HR+ 47; Siemens/CTI, Knoxville, TN) located in the neurocritical care unit. A transmission scan was obtained in each patient and used for subsequent attenuation correction of emission scan data. The PET scans were obtained in the two-dimensional mode. Images were reconstructed with filtered back-projection by using measured attenuation and scatter correction to a resolution of 4.3-mm FWHM. All PET scans were calibrated for conversion of PET counts to quantitative radiotracer concentrations, as previously described. [11, 12] Radioactivity in arterial blood was measured using an automated blood sampler. The arterial time radioactivity curve recorded by the sampler was corrected for delay and dispersion by using previously determined parameters. CBF was measured by bolus injection of 50–60 mCi 15O-labeled water using an adaptation of the Kety autoradiographic method[12–14]. CBV was measured after a brief inhalation of 40–50 mCi15O-labeled CO [15]. CMRO2 and OEF were calculated using the CBF and CBV measurements and those collected after inhalation of 40–50 mCi 15O-labeled O2[11, 14].
After completion of the first set of PET measurements, an infusion of phenylephrine or norepinephrine was started and the dose increased until the target MAP (20–25% increase from blood pressure during the first PET measurements). The MAP was maintained at target for a minimum of 10 minutes before PET measurements were repeated. All patients underwent diagnostic arteriography, nineteen within 12 hours before or after the PET study.
Data Processing
For each patient, all emission scans were co-registered and aligned to the initial baseline CBV scan using Automated Image Registration software.[16, 17] All images were then co-registered to a reference brain image and resliced so that data could be localized in Talairach atlas space [18]
For each PET scan, a total of 36 spherical regions of 10-mm diameter were placed in the anterior, middle and posterior cerebral, basilar and border zone territories bilaterally. [14, 19, 20] For each patient, we reviewed a computerized tomography scan obtained within 24 hours of the PET study and compared it with the PET scans. PET regions that corresponded to locations within an intracerebral hematoma, retraction edema, or the ventricular system were excluded from evaluation. Each patient had a median of 3.2 regions (range 2–9 regions) excluded from analysis. Regions with low baseline CBF were defined as those with CBF <25 ml/100g/min. Cerebrovascular resistance (CVR) was calculated as CVR = MAP/CBF.
Static autoregulatory index (AI) was calculated as the percentage change in CVR in relation to the percentage change in MAP where AI = (%ΔCVR/%ΔMAP) × 100%. Using this method AI is expressed as a percentage of full autoregulatory capacity. Thus a change in CVR that would fully compensate for the change in MAP (i.e. intact autoregulation) would yield an AI of 100%, and no change in CVR would yield a static AI of 0% signifying absence of autoregulation. [21]
Angiograms were retrospectively reviewed as previously described.[22] The diameter of each large intracranial artery was measured and quantitative percent stenosis was calculated relative to baseline angiograms. Distal segments of the ACA, MCA, and PCA were qualitatively assigned a vasospasm severity of none, mild, moderate, or severe based on visual inspection. Significant vasospasm was considered present if proximal stenosis was at least 50% or if severity of distal vasospasm was moderate or severe
Data Analysis
Continuous variables are presented as mean and standard deviation, categorical variables as median and range. A two-way ANOVA with repeated measures was used to compare CBF in the two groups. Categorical variables including proportion of were compared using Fisher’s exact test. Since there was no effect of simvastatin, the groups were combined to conduct exploratory analyses of the effect of induced hypertension. Two-tailed paired t-tests were used to analyze the effect of hypertension on blood pressure, global CBF and oxygen delivery. Change in CBF in regions with and without low CBF (<25 ml/100g/min) and regions with and without moderate or severe angiographic vasospasm were compared using 2-tailed unpaired t-tests. To take into account the multiple comparisons performed, a p value of < 0.001 was considered statistically significant.
Results
Thirty-four patients were enrolled; PET studies could not be completed in seven due to logistical difficulties (n=4) or patient refusal (n=3). Two of the completed studies could not be analyzed for technical reasons. The final PET analyses included 13 patients in the simvastatin group and 12 in the placebo group. All had normal HDL and LDL cholesterol levels at the time of admission (see Table 1). Presenting characteristics did not differ between groups.
Table 1.
Patient characteristics
| Simvastatin | Placebo | |
|---|---|---|
|
| ||
| N | 13 | 12 |
|
| ||
| Age | 59 ± 12 | 60 ± 10 |
|
| ||
| Gender – Female % | 62% | 67% |
|
| ||
| Race – Caucasian % | 86% | 92% |
|
| ||
| Smoker (%) | 38% | 50% |
|
| ||
| Hypertension (%) | 23% | 50% |
|
| ||
| Coronary artery disease (%) | 0 | 8% |
|
| ||
| *HDL cholesterol (mean ± SD) | 57 ± 18.3 | 63 ± 19.8 |
|
| ||
| *LDL cholesterol (mean ± SD) | 115 ± 51.9 | 81 ± 26.8 |
|
| ||
| *Anti-platelet therapy (%) | 0 | 8% |
|
| ||
| Heavy alcohol use (%) | 7% | 8% |
|
| ||
| Admitted on SAH day (%) | Day of hemorrhage – 10 | Day of hemorrhage – 9 |
| 1 day post bleed - 3 | 1 day post bleed - 2 | |
| 2 days post bleed - 1 | ||
|
| ||
| * Mean blood pressure (mean ± SD) | 107 ± 11.9 | 102 ± 23.2 |
|
| ||
| Intubated (%) | 7% | 16% |
|
| ||
| *WFNS Grade(N) | 2 – 4 | 2 – 2 |
| 3 – 6 | 3 – 3 | |
| 4 – 1 | 4 – 5 | |
| 5 – 2 | 5 – 2 | |
|
| ||
| *Modified Fisher Scale (N) | 1 – 0 | 1 – 1 |
| 2 – 2 | 2 – 2 | |
| 3 – 7 | 3 – 6 | |
| 4 – 4 | 4 – 3 | |
|
| ||
| *Intraventricular hemorrhage(%) | 46% | 67% |
|
| ||
| Moderate/severe angiographic vasospasm (%) | 42% | 45% |
|
| ||
| Delayed cerebral ischemia with rescue therapy (%) | 14% | 55% |
|
| ||
| Modified Rankin Scale (6-month) | N | N |
| 0 | 3 | 0 |
| 1 | 3 | 2 |
| 2 | 4 | 4 |
| 3 | 0 | 4 |
| 4 | 0 | 2 |
| 5 | 1 | 0 |
| 6 | 2 | 0 |
On admission
All patients were administered oral nimodipine and anticonvulsants on admission; one control patient was treated with dexamethasone and 2 patients in each group received mannitol. Serum troponin I levels were mildly elevated in 5 control and 7 simvastatin patients; none were considered to have had myocardial infarction. External ventricular drains were placed in 16 (9 control and 7 simvastatin); 2 patients in each group required mechanical ventilation.
Clinical Endpoints
Clinical DCI developed in 14% of simvastatin patients and 55% of control patients (p=0.074). Four control patients were being treated for clinical DCI with vasopressors at the time of the PET study. Moderate or severe angiographic vasospasm developed in 42% of the simvastatin patients and 45% of controls. Functional outcome on the mRS did not differ between groups (Table 1). There were 2 deaths in the simvastatin group (both due to decisions to withdraw life-sustaining interventions) and none in the control group.
PET Data
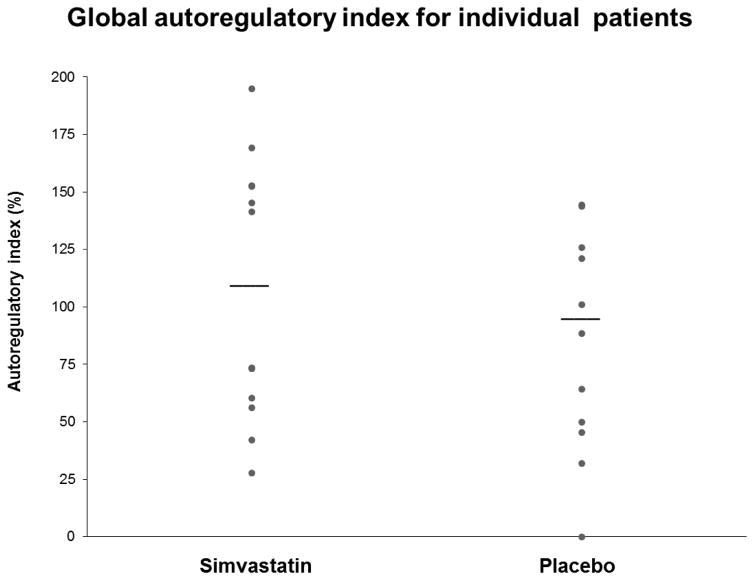
PET studies were performed 8.6 ± 1.3 days after SAH in the simvastatin group and 8.0 ± 1.8 days in the control group. Baseline MAP, arterial partial pressure of carbon dioxide (PaCO2), arterial oxygen content (CaO2), and other physiologic variables were similar between groups at baseline (Table 2). Global baseline CBF, OEF CMRO2, and CVR did not differ between groups. MAP was raised 20–25% over a mean of 18 ± 4 minutes and maintained at that level at least 10 minutes before repeating PET measurements. PaCO2, CaO2, average global CBF, OEF, and CMRO2 did not change when MAP was raised in either group while CVR rose by 17% in both groups. The average global AI was normal in both groups and they did not differ significantly (95% confidence interval for the difference between means of −39.1 – 56.3, see Table 2 and Figure 1). When groups were combined global AI did not differ in those with or without angiographic vasospasm (92 ± 54% compared with 105 ± 58.8%, p=0.59).
Table 2.
Average global response to induced hypertension
| Simvastatin | Placebo | p values * | p values + | |||
|---|---|---|---|---|---|---|
| Baseline | Hypertension | Baseline | Hypertension | |||
| Post SAH day | 8.6 ± 1.3 | 8.0 ± 1.8 | 0.32 | |||
| AI | 107 ± 59 % | 89 ± 52 % | 0.73 | |||
| MAP | 109.7 ± 9.4 | 135.4 ± 6.5 | 110.8 ± 12.3 | 136.7 ± 15.4 | 0.79 | <0.0001 |
| Global CBF | 41 ± 12 | 41 ± 13 | 43 ± 13 | 44 ± 12 | 0.55 | 0.67 |
| Global OEF | 0.34 ± 0.10 | 0.28 ± 0.07 | 0.30 ± 0.18 | 0.29 ± 0.17 | 0.85 | 0.04 |
| Global CVR | 2.95 ± 0.99 | 3.62 ± 1.13 | 2.81 ± 1.00 | 3.38 ± 1.25 | 0.66 | <0.0001 |
| PaCO2 | 35.5 ± 6.0 | 36.3 ± 5.9 | 35.8 ± 3.8 | 34.4 ± 3.5 | 0.87 | 0.33 |
| CaO2 | 13.8 ± 2.1 | 14.0 ± 2.1 | 12.8 ± 2.3 | 13.0 ± 2.4 | 0.42 | 0.48 |
baseline between groups;
baseline vs. hypertension groups combined;
AI = autoregulatory index; MAP = mean arterial pressure in mm Hg; CBF = cerebral blood flow in ml/100g/min; OEF = oxygen extraction fraction; CVR = cerebrovascular resistance (mmHg/ml/l00g/min); PaCO2 = arterial partial pressure of carbon dioxide; CaO2 = arterial oxygen content
Figure 1.
Each point represents an individual patient. Global autoregulatory index was calculated as AI = (%ΔCVR/%ΔMAP) × 100%. A change in CVR that would fully compensate for the change in MAP would yield an AI of 100%, and no change in CVR would yield a static an AI of 0%. Horizontal bar indicated group mean.
On regional analysis 19 regions (5.1%) and in simvastatin patients and 65 regions (15.8%) in placebo patients were supplied by vessels with moderate or severe angiographic vasospasm. CBF in those regions in simvastatin and placebo patients was 42.8 ± 15.2 vs 38.0 ± 12.3 ml/100g/min and during hypertension was 37.7 ± 17.0 and 41.3 ± 13.6 ml/100g/min, respectively. Contrariwise, there tended to be more regions with low CBF at baseline (<25 ml/100g/min) in the simvastatin group (11.6% vs. 7.8%, p=0.09) but the mean CBF did not change after hypertension (Table 3).
Table 3.
Regional response to induced hypertension across patients
| Simvastatin | Placebo | ||||
|---|---|---|---|---|---|
| Regions with low baseline CBF | Baseline | Hypertension | Baseline | Hypertension | |
| % of regions | 10.7 | 6.6& | |||
| CBF | 22 ± 5 | 24 ± 3 | 22 ± 2 | 24 ± 5 | |
| CVR | 4.98 ± 0.63 | 5.71 ± 1.34+ | 5.21 ± 0.644 | 6.35 ± 1.14+ | |
| AI | 101 ± 34 % | 96 ± 55 % | |||
| Regions with angiographic vasospasm | % of regions | 5.1 | 15.8& | ||
| CBF | 43 ± 15.2 | 38 ± 17.0 | 38 ± 12.3 | 41 ± 13.6 | |
| CVR | 3.45 ± 0.71 | 3.80 ± 0.74 | 3.82 ± 0.68 | 3.50 ± 0.79 | |
| AI | 91 ± 36 % | 83 ± 36 % | |||
p< 0.001 compared to baseline paired T-test;
P=0.03 Fisher’s exact;
p=0.08 Fisher’s exact;
CBF = cerebral blood flow (ml/100g/min); AI = autoregulatory index; CVR = cerebrovascular resistance (mm Hg/ml/l00g/min)
Safety
Liver function tests and creatine kinase measured on day 7 and 14 did not exceeded 2 standard deviations above the upper limit of normal except in one patient in the simvastatin group. The study drug was discontinued on Day 15 and the values subsequently returned to normal. That patient’s data were included in the PET analysis.
Discussion
In this prospective randomized controlled study of statin- naïve patients following acute aneurysmal SAH, we sought to determine if the initiation of therapy with 80 mg of simvastatin results in increased CBF and altered autoregulation during the peak period of risk for DCI. We found no effect of simvastatin on resting global CBF or static autoregulation. While the rates of angiographic vasospasm were similar in both groups, there was a trend toward less DCI in those receiving simvastatin; however, this did not result in any difference in six-month mRS between groups. These data indicate that despite the suggestive findings of pre-clinical studies, high-dose simvastatin does not improve resting CBF or change autoregulation in SAH patients at high-risk for DCI.
The only prior study to assess the impact of statins on autoregulation after SAH was performed by Tseng et al.[23] They utilized the transcranial Doppler transient hyperemic response test (THRT) to temporary carotid occlusion to assess dynamic autoregulation in SAH patients randomized to 40 mg of pravastatin or placebo. A daily THRT was performed and the duration of impaired autoregulation was found to be shorter in the pravastatin group. No data were provided regarding the incidence of abnormal autoregulation or the degree of impairment. In contrast, we assessed autoregulation at only one time point using a static rather than dynamic methodology.
Recently two large prospective randomized controlled trials were completed addressing the clinical utility of statins in SAH. The STASH trial compared 40 mg of simvastatin to placebo in 803 SAH patients[24]. The primary ordinal analysis of the mRS, adjusted for age and WFNS grade on admission, showed no benefit. In another study Wong et al. compared low and high dose simvastatin, 40 vs. 80 mg. [25] The groups did not differ in the incidence of delayed ischemic deficits (27% versus 24%) or in the rate of favorable outcomes (mRS score 0–2) at 3 months (73% versus 72%). Taken together these data indicate that simvastatin likely does not improve functional outcome in SAH. Still, it has been suggested that the failure to detect a benefit may be due to the insensitivity of the mRS to cognitive outcome or that a subset of patients might respond. [26]
This study also provides insight into the physiologic response to induced hypertension and capacity for autoregulation in SAH patients. Since the 1970s a number studies assessed vascular reactivity in SAH patients reporting a diversity of findings including: preserved static autoregulation in the absence of intracerebral haemorrhage, hydrocephalus or vasospasm,[27] altered static autoregulation and CO2 reactivity during early surgery, [28] impaired vasodilation during hypotension, [28, 29] correlation between impaired autoregulation and severe neurological deficits, severe angiographic vasospasm and marked depression of global CBF. [30] In a recent review Budohoski noted that the nature of autoregulatory failure varied considerably across studies and argued that such variability in outcomes suggests that autoregulatory failure is heterogeneous and depends on the prevailing pathophysiological mechanism.[31] Of note all of these studies assessed autoregulation by lowering blood pressure, thus assessing vasodilatory capacity. How these finding relate to testing autoregulation by raising blood pressure, which assesses vasoconstrictive function, is unknown.
More recently, numerous studies have reported abnormal dynamic autoregulation in SAH patients.[32–36] This approach differs from static autoregulation in that it either requires a physiologic maneuver to induce a transient blood pressure stimulus (leg or carotid compression) or more typically relies on spontaneous fluctuations of blood pressure. In addition, unlike static autoregulatory testing which provides a snapshot, it allows for continuous assessment over days. About three-quarters of studies that assessed dynamic autoregulation reported autoregulatory dysfunction as a result of SAH, and disturbed autoregulation was consistently associated with DCI and poor outcome. Interpretation of results using these methods, however, is complicated by the heterogeneity of the monitored parameter, physical locations of the sensors and lack of a standardized method for calculation of dynamic autoregulation.[31, 37]
In 1976 Kosnik and Hunt proposed to capitalize on impaired autoregulation in SAH by elevating blood pressure to reverse neurologic symptoms.[38] Their thesis was that “autoregulation is poor or absent in these patients, and that cerebral blood flow therefore follows the mean arterial pressure.” Over time induced hypertension evolved to be the mainstay of medical therapy to treat DCI.[39] Very few studies, however, have directly assessed the CBF response to induced hypertension. Darby et al. measured regional CBF using Xenon-CT in 13 patients with DCI before and after raising mean blood pressure from 90 to 111 mm Hg with dopamine. [40] Global CBF did not change. CBF rose to above ischemic levels in two-thirds of the regions with low baseline CBF but fell in other regions raising concerns about cerebral vasoconstriction from dopamine. In a retrospective review of clinical data, another group reported on five patients with severe angiographic vasospasm treated with phenylephrine to increase MAP from 102.4 to 132.1 mm Hg. They reported that in regions of diminished CBF, mean CBF increased from 19.2 to 33.7 ml/100 g/min.[41] No additional details are provided about global CBF, how regions were selected, or what occurred to CBF in other regions. A recent study reported on 25 SAH patients randomized to induced hypertension or control and used CT perfusion to measure the change in CBF. They found that raising BP an average of 12 mm Hg did not change global or CBF or CBF asymmetry in the region with the lowest baseline flow.[42]
Several points must be considered when interpreting these data. First, the autoregulatory response acts through both vasodilation and vasoconstriction. In SAH patients, testing both arms of the response in a single patient is logistically extremely difficult and has not been reported. The majority of static autoregulation studies tested the response to lowering pressure whereas only a handful of SAH patients have been reported testing the response to elevating pressure.
Second, autoregulation is a complex response whereby, under normal and stable conditions, CVR changes in response to changes in perfusion pressure to maintain stable oxygen delivery. Thus the definition of autoregulation assumes normal and stable physiological conditions such that oxygen demand and delivery are well matched. In disease states, however, several parameters may be disturbed. During ischemia, then cerebral vessels have already been subject to strong signals to dilate and thus unable to compensate for a fall in perfusion. While some may refer to this as impaired autoregulation, in fact the vasculature is responding appropriately but unable to dilate enough to response to the conditions. Third, despite our attempt to enrol patients a high risk of DCI the outcome is both groups was quite good. How these findings apply to more severely affected patients in uncertain.
In summary, our data indicate that initiation of therapy with high dose simvastatin does not alter average baseline CBF or induced hypertension.
Acknowledgments
Support: NIH/NINDS: P50 NS055977
References
- 1.Rosengart AJ, et al. Prognostic factors for outcome in patients with aneurysmal subarachnoid hemorrhage. Stroke. 2007;38:2315–2321. doi: 10.1161/STROKEAHA.107.484360. [DOI] [PubMed] [Google Scholar]
- 2.Tseng MY, et al. Effects of acute treatment with pravastatin on cerebral vasospasm, autoregulation, and delayed ischemic deficits after aneurysmal subarachnoid hemorrhage: a phase II randomized placebo-controlled trial. Stroke. 2005;36:1627–1632. doi: 10.1161/01.STR.0000176743.67564.5d. [DOI] [PubMed] [Google Scholar]
- 3.Lynch JR, et al. Simvastatin reduces vasospasm after aneurysmal subarachnoid hemorrhage: results of a pilot randomized clinical trial. Stroke. 2005;36:2024–2026. doi: 10.1161/01.STR.0000177879.11607.10. [DOI] [PubMed] [Google Scholar]
- 4.Tseng MY, et al. Effects of acute treatment with statins on cerebral autoregulation in patients after aneurysmal subarachnoid hemorrhage. Neurosurg Focus. 2006;21:E10. doi: 10.3171/foc.2006.21.3.10. [DOI] [PubMed] [Google Scholar]
- 5.Tseng MY, et al. Biological effects of acute pravastatin treatment in patients after aneurysmal subarachnoid hemorrhage: a double-blind, placebo-controlled trial. J Neurosurg. 2007;107:1092–1100. doi: 10.3171/JNS-07/12/1092. [DOI] [PubMed] [Google Scholar]
- 6.Kirkpatrick PJ, et al. Simvastatin in aneurysmal subarachnoid haemorrhage (STASH): a multicentre randomised phase 3 trial. pp. 1474–4465. Electronic. [DOI] [PubMed] [Google Scholar]
- 7.Endres M, et al. Stroke protection by 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors mediated by endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 1998;95(15):8880–5. doi: 10.1073/pnas.95.15.8880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamada M, et al. Endothelial nitric oxide synthase-dependent cerebral blood flow augmentation by L-arginine after chronic statin treatment. J Cereb Blood Flow Metab. 2000;20(4):709–17. doi: 10.1097/00004647-200004000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Amin-Hanjani S, et al. Mevastatin, an HMG-CoA reductase inhibitor, reduces stroke damage and upregulates endothelial nitric oxide synthase in mice. Stroke. 2001;32(4):980–6. doi: 10.1161/01.str.32.4.980. [DOI] [PubMed] [Google Scholar]
- 10.McGirt MJ, et al. Simvastatin increases endothelial nitric oxide synthase and ameliorates cerebral vasospasm resulting from subarachnoid hemorrhage. Stroke. 2002;33(12):2950–6. doi: 10.1161/01.str.0000038986.68044.39. [DOI] [PubMed] [Google Scholar]
- 11.Mintun MA, et al. Brain oxygen utilization measured with O-15 radiotracers and positron emission tomography. J Nucl Med. 1984;25(2):177–187. [PubMed] [Google Scholar]
- 12.Raichle ME, et al. Brain blood flow measured with intravenous H2(15)O. II. Implementation and validation. J Nucl Med. 1983;24(9):790–798. [PubMed] [Google Scholar]
- 13.Herscovitch P, Markham J, Raichle ME. Brain blood flow measured with intravenous H2(15)O. I. Theory and error analysis. J Nucl Med. 1983;24(9):782–789. [PubMed] [Google Scholar]
- 14.Videen TO, et al. Brain blood volume, flow, and oxygen utilization measured with 15O radiotracers and positron emission tomography: revised metabolic computations. J Cereb Blood Flow Metab. 1987;7(4):513–516. doi: 10.1038/jcbfm.1987.97. [DOI] [PubMed] [Google Scholar]
- 15.Martin WR, Powers WJ, Raichle ME. Cerebral blood volume measured with inhaled C15O and positron emission tomography. J Cereb Blood Flow Metab. 1987;7(4):421–426. doi: 10.1038/jcbfm.1987.85. [DOI] [PubMed] [Google Scholar]
- 16.Woods RP, Cherry SR, Mazziotta JC. Rapid automated algorithm for aligning and reslicing PET images. J Comput Assist Tomogr. 1992;16(4):620–33. doi: 10.1097/00004728-199207000-00024. [DOI] [PubMed] [Google Scholar]
- 17.Woods RP, Mazziotta JC, Cherry SR. MRI-PET registration with automated algorithm. J Comput Assist Tomogr. 1993;17(4):536–46. doi: 10.1097/00004728-199307000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- 19.Yundt KD, et al. Autoregulatory vasodilation of parenchymal vessels is impaired during cerebral vasospasm. J Cereb Blood Flow Metab. 1998;18(4):419–424. doi: 10.1097/00004647-199804000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Powers WJ, et al. Cerebral blood flow and cerebral metabolic rate of oxygen requirements for cerebral function and viability in humans. J Cereb Blood Flow Metab. 1985;5(4):600–608. doi: 10.1038/jcbfm.1985.89. [DOI] [PubMed] [Google Scholar]
- 21.Tiecks FP, et al. Comparison of static and dynamic cerebral autoregulation measurements. Stroke. 1995;26(6):1014–9. doi: 10.1161/01.str.26.6.1014. [DOI] [PubMed] [Google Scholar]
- 22.Dhar R, et al. Relationship between Angiographic Vasospasm and Regional Hypoperfusion In Aneurysmal Subarachnoid Hemorrhage. Stroke. 2012 doi: 10.1161/STROKEAHA.111.646836. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tseng M-Y, et al. Effects of acute treatment with statins on cerebral autoregulation in patients after aneurysmal subarachnoid hemorrhage. Neurosurgical focus. 2006;21:E10. doi: 10.3171/foc.2006.21.3.10. [DOI] [PubMed] [Google Scholar]
- 24.Kirkpatrick PJ, et al. Simvastatin in aneurysmal subarachnoid haemorrhage (STASH): a multicentre randomised phase 3 trial. Lancet Neurol. 2014;13(7):666–75. doi: 10.1016/S1474-4422(14)70084-5. [DOI] [PubMed] [Google Scholar]
- 25.Wong GK, et al. High-dose simvastatin for aneurysmal subarachnoid hemorrhage: multicenter randomized controlled double-blinded clinical trial. Stroke. 2015;46(2):382–8. doi: 10.1161/STROKEAHA.114.007006. [DOI] [PubMed] [Google Scholar]
- 26.Macdonald RL. Are statins to be STASHed in subarachnoid haemorrhage? Lancet Neurol. 2014;13(7):639–41. doi: 10.1016/S1474-4422(14)70095-X. [DOI] [PubMed] [Google Scholar]
- 27.Powers WJ. Acute hypertension after stroke: the scientific basis for treatment decisions. Neurology. 1993;43(3 Pt 1):461–7. doi: 10.1212/wnl.43.3_part_1.461. [DOI] [PubMed] [Google Scholar]
- 28.Heilbrun MP, Olesen J, Lassen NA. Regional cerebral blood flow studies in subarachnoid hemorrhage. J Neurosurg. 1972;37:36–44. doi: 10.3171/jns.1972.37.1.0036. [DOI] [PubMed] [Google Scholar]
- 29.Voldby B, Enevoldsen EM, Jensen FT. Cerebrovascular reactivity in patients with ruptured intracranial aneurysms. J Neurosurg. 1985;62(1):59–67. doi: 10.3171/jns.1985.62.1.0059. [DOI] [PubMed] [Google Scholar]
- 30.Ishii R. Regional cerebral blood flow in patients with ruptured intracranial aneurysms. J Neurosurg. 1979;50:587–594. doi: 10.3171/jns.1979.50.5.0587. [DOI] [PubMed] [Google Scholar]
- 31.Budohoski KP, et al. Cerebral autoregulation after subarachnoid hemorrhage: comparison of three methods. J Cereb Blood Flow Metab. 2013;33(3):449–56. doi: 10.1038/jcbfm.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Budohoski KP, et al. Impairment of cerebral autoregulation predicts delayed cerebral ischemia after subarachnoid hemorrhage: a prospective observational study. Stroke. 2012;43(12):3230–7. doi: 10.1161/STROKEAHA.112.669788. [DOI] [PubMed] [Google Scholar]
- 33.Jaeger M, et al. Continuous monitoring of cerebrovascular autoregulation after subarachnoid hemorrhage by brain tissue oxygen pressure reactivity and its relation to delayed cerebral infarction. Stroke. 2007;38(3):981–6. doi: 10.1161/01.STR.0000257964.65743.99. [DOI] [PubMed] [Google Scholar]
- 34.Ratsep T, Asser T. Cerebral hemodynamic impairment after aneurysmal subarachnoid hemorrhage as evaluated using transcranial doppler ultrasonography: relationship to delayed cerebral ischemia and clinical outcome. J Neurosurg. 2001;95(3):393–401. doi: 10.3171/jns.2001.95.3.0393. [DOI] [PubMed] [Google Scholar]
- 35.Lam JM, et al. Prediction of cerebral ischaemia during carotid endarterectomy with preoperative CO2-reactivity studies and angiography. Br J Neurosurg. 2000;14(5):441–8. doi: 10.1080/02688690050175238. [DOI] [PubMed] [Google Scholar]
- 36.Giller CA. A bedside test for cerebral autoregulation using transcranial Doppler ultrasound. Acta Neurochir (Wien) 1991;108(1–2):7–14. doi: 10.1007/BF01407660. [DOI] [PubMed] [Google Scholar]
- 37.Tzeng YC, et al. Assessment of cerebral autoregulation: the quandary of quantification. Am J Physiol Heart Circ Physiol. 2012;303(6):H658–71. doi: 10.1152/ajpheart.00328.2012. [DOI] [PubMed] [Google Scholar]
- 38.Kosnik EJ, Hunt WE. Postoperative hypertension in the management of patients with intracranial arterial aneurysms. J Neurosurg. 1976;45:148–154. doi: 10.3171/jns.1976.45.2.0148. [DOI] [PubMed] [Google Scholar]
- 39.Diringer MN, et al. Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the Neurocritical Care Society’s Multidisciplinary Consensus Conference. Neurocrit Care. 2011;15(2):211–40. doi: 10.1007/s12028-011-9605-9. [DOI] [PubMed] [Google Scholar]
- 40.Darby JM, et al. Acute cerebral blood flow response to dopamine-induced hypertension after subarachnoid hemorrhage. J Neurosurg. 1994;80:857–864. doi: 10.3171/jns.1994.80.5.0857. [DOI] [PubMed] [Google Scholar]
- 41.Joseph M, et al. Increases in cardiac output can reverse flow deficits from vasospasm independent of blood pressure: a study using xenon computed tomographic measurement of cerebral blood flow. Neurosurgery. 2003;53(5):1044–1051. doi: 10.1227/01.neu.0000088567.59324.78. [DOI] [PubMed] [Google Scholar]
- 42.Gathier CS, et al. Effects of Induced Hypertension on Cerebral Perfusion in Delayed Cerebral Ischemia After Aneurysmal Subarachnoid Hemorrhage: A Randomized Clinical Trial. Stroke. 2015;46(11):3277–81. doi: 10.1161/STROKEAHA.115.010537. [DOI] [PubMed] [Google Scholar]