SUMMARY
Neural inputs from internal organs are essential for normal autonomic function. The vagus nerve is a key body-brain connection that monitors the digestive, cardiovascular, and respiratory systems. Within the gastrointestinal tract, vagal sensory neurons detect gut hormones and organ distension. Here, we investigate the molecular diversity of vagal sensory neurons and their roles in sensing gastrointestinal inputs. Genetic approaches allowed targeted investigation of gut-to-brain afferents involved in homeostatic responses to ingested nutrients (GPR65 neurons) and mechanical distension of the stomach and intestine (GLP1R neurons). Optogenetics, in vivo ganglion imaging, and genetically guided anatomical mapping provide direct links between neuron identity, peripheral anatomy, central anatomy, conduction velocity, response properties in vitro and in vivo, and physiological function. These studies clarify the roles of vagal afferents in mediating particular gut hormone responses. Moreover, genetic control over gut-to-brain neurons provides a molecular framework for understanding neural control of gastrointestinal physiology.
INTRODUCTION
In addition to our external senses of sight, smell, sound, touch, and taste, internal sensory systems within our body relay vital information to the brain about physiological state. The vagus nerve is a major information highway from the periphery that innervates, surveys, and controls several principal physiological systems. Within the gastrointestinal tract, vagal sensory neurons monitor stomach volume and intestinal contents, and responsive neural circuits regulate digestive physiology (Brookes et al., 2013). However, a molecular and genetic classification of gastrointestinal fibers within the vagus nerve is not available, and would facilitate mechanistic studies of gut-to-brain signaling in health and disease.
The mouse vagus nerve contains ~2,300 sensory neurons with cell bodies in ganglia at the base of the skull, as well as a smaller group of motor neurons with soma in the brainstem. Each sensory neuron has both a peripheral terminal that interfaces with an internal organ and a central brainstem terminal. Classical anatomical tracing studies revealed a variety of terminal types within the gastrointestinal tract (Berthoud et al., 2004). Stomach terminals include mucosal endings, intraganglionic laminar endings (IGLEs), and intramuscular arrays. Gastric IGLEs contact enteric ganglia between layers of stomach muscle (Fox et al., 2000), and are proposed to sense stomach stretch, as they are near sites of mechanosensation (Zagorodnyuk et al., 2001). In the intestine, vagal afferents form IGLEs, endings near intestinal crypts, and free terminals embedded within the lamina propria of intestinal villi (Berthoud et al., 2004).
Vagal afferents have been proposed to detect nutrients at several locations, including in proximal and distal intestine, and the hepatic portal system (Maljaars et al., 2008; Ruttimann et al., 2009). Because vagal afferents do not directly contact the intestinal lumen, vagal nutrient detectors are likely to be second-order chemosensory neurons. Terminals in intestinal villi are excellent candidates to contribute to nutrient detection; however, specific characterization of sensory neurons with villous terminals has been technically challenging. Lumen-proximal endings were defined in some studies by sensitivity to mucosal stroking or luminal anesthetics (Blackshaw and Grundy, 1990; Richards et al., 1996); however, these manipulations may not selectively impact all villous terminals. Additional insights could be provided by genetically defining nutrient-responsive vagal afferents, and subsequently examining their terminal fields in the periphery.
Different models have been raised for how the vagus nerve receives and encodes information about ingested nutrients. Some studies reported polymodal responses of single vagal afferents to intestinal nutrients, as well as to changes in osmolarity and pH (Mei and Garnier, 1986; Zhu et al., 2001). Other studies concluded that vagal afferents are highly tuned for specific nutrients, with different fibers dedicated for sugars, amino acids, and fats (Jeanningros, 1982; Lal et al., 2001; Mei, 1978). Ingested nutrients activate enteroendocrine cells, sparsely distributed sentinel cells in the intestinal epithelium. Enteroendocrine cells respond to nutrients by releasing a myriad of gut hormones, including serotonin, glucagon-like peptide 1 (GLP1), cholecystokinin, peptide YY, and others (Chambers et al., 2013). Detection of different nutrients, perhaps through taste receptors (Jang et al., 2007), may evoke differential hormone secretion. Release of serotonin by enterochromaffin cells has also been linked to nausea-inducing toxins, inflammatory cues, mechanical forces, and commensal microflora (Bertrand and Bertrand, 2010; Yano et al., 2015). Once released, gut hormones orchestrate powerful systemic responses to nutrient intake, and can act locally as paracrine signals and distally after entering circulation. Vagus nerve terminals in intestinal villi occupy a privileged anatomical location to detect gut hormones.
Basic questions remain about the extent of functional overlap between different gut hormones, and whether they encode specific or redundant messages about intestinal stimuli to the vagus nerve. Perhaps best studied are serotonin and cholecystokinin, which activate different vagal afferents (Hillsley and Grundy, 1998). In some electrophysiological studies, each of these gut hormones reportedly activates lumen-proximal or nutrient-responsive vagal afferents (Blackshaw and Grundy, 1990; Zhu et al., 2001), and in other studies, the same gut hormones reportedly activate mechanoreceptors (Mazda et al., 2004; Schwartz et al., 1991). Different studies also claimed important roles for each hormone in various nutrient-evoked physiological responses (Chambers et al., 2013). A limitation of in vivo pharmacology is that injection of CCK and serotonin causes indirect responses, such as changes in gastrointestinal motility and tone. Genetic tools that enable selective labeling of villous-projecting neurons would enable precise characterization of gut hormone responsiveness in vitro.
GLP1 is another gut hormone proposed to mediate aspects of nutrient detection by the vagus nerve (Holst, 2007). Incretin therapies that involve mimicry or stabilization of GLP1 provide an important strategy for treatment of metabolic disease. GLP1 is detected by a dedicated G protein-coupled receptor (GLP1R) expressed in many cell types (Thorens, 1992). Studies involving localized injection of GLP1R agonists have concluded important roles for GLP1 reception in various locations, including the brain and periphery (Hayes et al., 2010). Some vagal afferents express GLP1R and some vagal afferents are positioned within intestinal villi near enteroendocrine cells. It has been presumed that these are the same sensory neurons, and as such, proposed that GLP1R contributes to vagal detection of intestinal nutrients. Surgical vagotomy reportedly impairs GLP1-evoked physiological responses in some studies but not others (Abbott et al., 2005; Zhang and Ritter, 2012), and effects could be due to loss of sensory or motor neurons. However, genetic deletion of GLP1R from PHOX2B-expressing vagal afferents does not impact GLP1R agonist-induced changes in body weight and glucose homeostasis (Sisley et al., 2014). Taken together, basic questions persist about the anatomy, responses, and functions of vagal GLP1R neurons.
Here, we used genetic approaches to study processing of gastrointestinal inputs by the vagus nerve. For selective targeting of vagal sensory neurons with terminals in intestinal villi, we sought to exploit the presumed role for vagal GLP1R in nutrient detection. We generated Glp1r-ires-Cre mice, and adapted genetic approaches to map, image, and control vagal GLP1R neurons. Surprisingly, vagal GLP1R neurons do not densely target intestinal villi but instead display characteristic IGLE terminals and function as gastrointestinal mechanoreceptors. Most sensory neurons that innervate intestinal villi instead contain another receptor, GPR65. Vagal GPR65 neurons are insensitive to GLP1 or cholecystokinin, but instead detect serotonin, and in vivo imaging reveals broad responses to meal-associated stimuli in the intestinal lumen. The central projections of GPR65 neurons are remarkably specific, and optogenetic activation of GPR65 neurons causes a powerful blockade of gastric contractions without impacting breathing or heart rate, which are also under vagal control. Genetic access to different neural populations that monitor and control gastrointestinal physiology has provided direct links between neuronal identities, peripheral terminal morphologies, central projection fields, response properties, hormone sensitivities, and physiological functions.
RESULTS
Imaging single neuron responses in vagal ganglia in vivo
We developed an in vivo calcium imaging approach in vagal ganglia to study the peripheral representation of autonomic inputs. Genetically encoded calcium indicators, such as GCaMP3, have provided an optical proxy for activity-dependent calcium transients in many neuron types, including peripheral sensory neurons (Barretto et al., 2015; Kim et al., 2014). In vivo calcium imaging enables a massively parallel analysis of single neuron responses, and is compatible with genetic marking techniques for neuron identification.
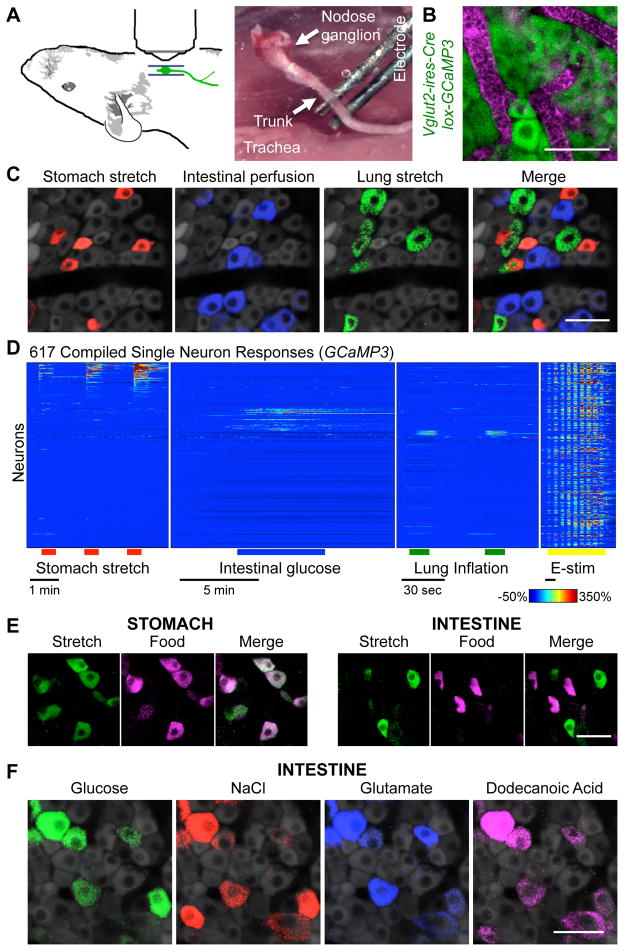
In initial experiments, GCaMP3 expression was driven using a Cre driver line (Vglut2-ires-Cre) that targets all vagal sensory neurons (Chang et al., 2015) and a Cre-dependent reporter allele (lox-GCaMP3); in some experiments, an alternate Cre driver line (E2a-Cre) was used that resulted in a constitutive GCamp3 allele (Rosa26-GCaMP3). Vagal ganglia were surgically exposed for in vivo imaging through a ventral incision in the neck with connections to peripheral organs intact (Figures 1A, 1B). About 150 neurons were analyzed in parallel per imaging field by confocal microscopy, with neurons remaining viable and stably imaged for over six hours. Neuron viability was determined after each session by electrical stimulation of the nerve trunk, applied as a series of increasing voltage steps (Movie S1). Ganglion imaging was performed during various end-organ stimulations, including 1) gastric distension, 2) duodenal nutrient application, 3) intestinal distension, and 4) lung inflation (Figures 1C–1E).
Figure 1. In vivo imaging of vagal sensory neurons.
(A) Cartoon and photograph of imaging preparation. (B) Wholemount image of GCaMP3 fluorescence (green) and blood vessels (magenta, intravenous Evans Blue) in vagal ganglia. (C) GCaMP3 fluorescence signal in vagal ganglia during stomach stretch (red), intestinal perfusion (blue), and lung stretch (green). (D) Time-resolved responses (ΔF/F, color scale) of 617 neurons (1 neuron per row) to stimuli indicated. GCaMP3 fluorescence signal in vagal ganglia during (E) organ stretch (nitrogen, saline), food injection (liquid diet), or (F) intestinal perfusion of glucose (1 M, saline), sodium chloride (500 mM, saline), sodium glutamate (500 mM, saline) and dodecanoic acid (25 mM, saline and conjugated mouse bile). Scale bars: 50 μm. See also Figures S1, S2, and Movie S1.
First, gastric mechanoreceptors were activated by stretching the stomach with a surgically implanted balloon or by infusion of nitrogen gas. Single neurons responded rapidly and reproducibly, and response magnitude correlated with the extent of gastric distension (Figure S1). Volume expansion by 300 μl mimicked meal-induced distension and activated 16.8% (198/1181, 10 mice) of imaging-accessible vagal sensory neurons. Gastric mechanoreceptors accounted for nearly all neurons (92%, 56/62) responsive to liquid diet infusion into the stomach after sealing the pyloric sphincter (Figures 1E, S1), consistent with prior evidence that the stomach lacks chemoreceptors for nutrients (Powley and Phillips, 2004).
Second, intestinal chemoreceptors were activated by either perfusing or injecting liquid diet into the proximal small intestine near the gastro-duodenal junction. Perfused stimuli were administered through a surgically implanted cannula in the duodenal bulb with a stimulus exit port located ~11 centimeters distally. Injection of liquid diet (200 μl) activated 11.1% of imaging-accessible sensory neurons (87/780 neurons, 6 mice). Isotonic saline (200 μl) did not evoke calcium transients, suggesting that these responses were not due to mechanosensation (Figure S1). Most individual neurons displayed broad responses to many stimuli, including intestinal glucose, glutamate, fatty acids, salt, and low pH, consistent with polymodal signal integration (Figures 1F, S1). Responses were not observed to the artificial sweetener saccharin at concentrations that activate the sweet taste receptor complex (Figure S1).
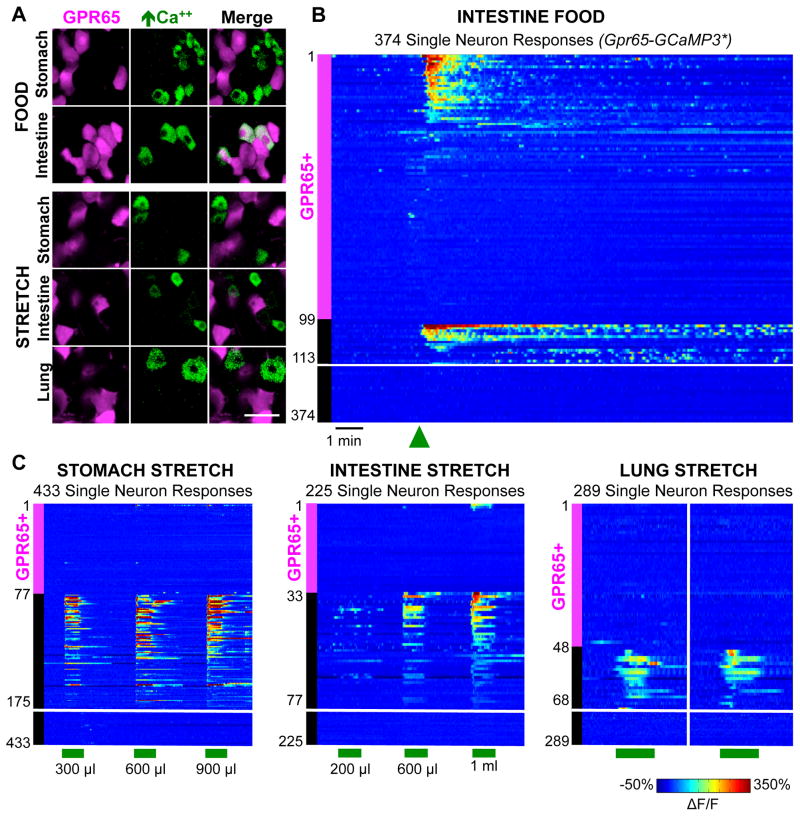
Third, mechanical stretch of the intestine was evoked by injecting a larger bolus of isotonic saline into the proximal intestine after clamping the exit port to prevent fluid release. This preparation resulted in a controlled distension of the intestine that was visually observed and alleviated by unclamping the exit port. Intestinal distension activated a subset of vagal sensory neurons (26%; 131/503), and in control experiments, the bolus of isotonic saline did not activate vagal afferents in the absence of an exit port clamp. Intestinal stretch and intestinal nutrients predominantly activated discrete sensory neuron cohorts (Figures 1E, S1).
Fourth, airway mechanoreceptors were activated by introducing nitrogen or ambient air through a tracheal cannula to inflate the lung. Lung inflation evoked rapid, robust, and reproducible calcium transients in 3.6% (28/762, 6 mice) of imaging-accessible sensory neurons. Lung inflation by ambient air, oxygen, and nitrogen activated the same neurons, suggesting a mechanosensory response indifferent to airway oxygen levels (Figure S2). Responses were typically sustained for the entire stimulus duration, and terminated abruptly when airway pressure was reduced. Consistent with prior studies, lung stretch-responsive neurons were cyclically active during tidal breathing, detecting lung expansion with each breath (Figure S2). These findings indicate that in vivo calcium imaging in vagal ganglia can reliably report on physiological stimuli over a time scale as rapid as tidal breathing.
Different vagal sensory neurons responded to stomach stretch, intestinal nutrients, and lung inflation, indicating that individual vagal sensory neurons have specific response properties. In vivo imaging also provides positional information about responsive neurons. Sensory neurons were intermingled without any apparent spatial clustering based on response properties (Figure S2), consistent with a salt-and-pepper organization of vagal inputs.
Vagal GLP1R neurons are gastrointestinal mechanoreceptors
Vagal GLP1R neurons co-express multiple gut hormone receptors (Figure S3) based on two-color fluorescence in situ hybridization (FISH), raising the possibility that these neurons provide an integrated nutrient response. To examine whether these neurons sense gastrointestinal inputs, we tagged them genetically during in vivo imaging experiments. We generated Glp1r-ires-Cre knock-in mice in which Cre recombinase is expressed from the endogenous Glp1r locus using an internal ribosome entry site (IRES) sequence (Figures 2A, S3). Expression of Cre-dependent reporters in Glp1r-ires-Cre mice was observed in many cell types that contain GLP1R (Baggio and Drucker, 2007), and two-color analysis validated that appropriate cells were targeted (Figure S3). We noted an increase in fluorescent cells visualized in vagal ganglia of Glp1r-ires-Cre; lox-tdTomato mice, likely due to inefficient detection of low-level or transient Glp1r transcript by FISH.
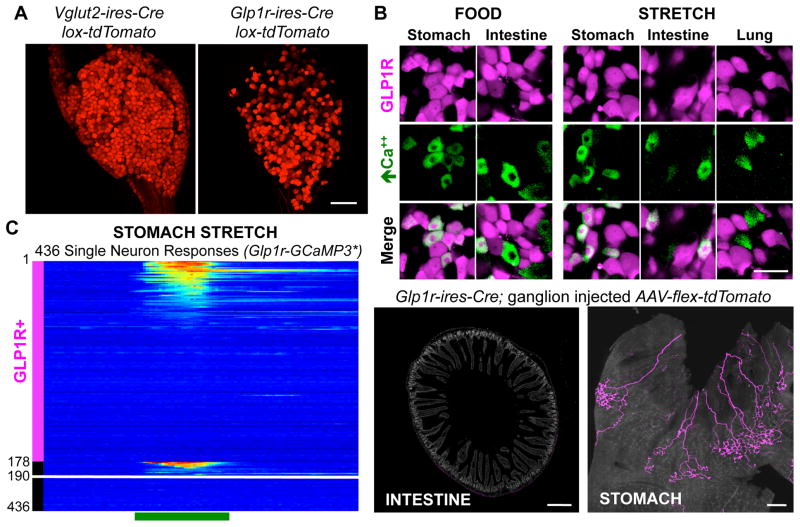
Figure 2. Vagal GLP1R neurons are mechanoreceptors.
(A) Whole mount tdTomato fluorescence in vagal ganglia from knock-in mice, scale bar: 100 μm. (B) Tomato fluorescence indicating GLP1R neurons (magenta) and GCaMP3 fluorescence responses to stimuli indicated (green) in vagal ganglia of Glp1r-GCaMP3* mice. (C) Time-resolved responses (ΔF/F, color scale) of 178 GLP1R neurons, and 258 other neurons (12 depicted which responded) to gastric distension induced by nitrogen perfusion. (D) AAV mapping of GLP1R neuron projections in intestine and stomach. scale bars: 500 μm (left), 1 mm (right). See also Figures S3, S4.
To measure the response properties of vagal GLP1R neurons, we generated a triple knock-in mouse line: Glp1r-ires-Cre; lox-tdTomato; Rosa26-GCaMP3 (Glp1r-GCaMP3*) in which all sensory neurons expressed GCaMP3 from a constitutive allele, and GLP1R neurons were visualized by tdTomato expression. Responses of vagal GLP1R neurons to gastric distension, intestinal nutrients, intestinal distension, and lung inflation were queried by in vivo ganglion imaging in Glp1r-GCaMP3* mice (Figures 2B, 2C, S4). We observed only rare vagal GLP1R neurons that responded to lung inflation (1%, 2/204, 4 mice), and some that detected liquid diet (200 μl) injected in the duodenal bulb (9.2%, 18/195, 3 mice). Instead, unexpectedly, GLP1R neurons accounted for most neurons responsive to gastric distension (81%, 46/57, 3 mice). Furthermore, a separate cohort of GLP1R neurons accounted for most neurons responsive to saline-induced intestinal distension (67.7%, 88/130, 2 mice). Stomach and intestinal stretch activated discrete subsets of vagal GLP1R neurons, with other vagal GLP1R neurons likely detecting different physiological stimuli. GLP1R neurons thus account for most vagal mechanoreceptors in stomach and intestine, while nutrient responses occur predominantly in GLP1R-negative neurons.
GLP1R neurons form IGLEs in stomach muscle
Next, we asked where vagal GLP1R neurons project in the gastrointestinal tract. We previously developed a genetic approach involving adeno-associated viruses (AAVs) to map vagal sensory neuron anatomy (Chang et al., 2015). Cre-dependent AAVs encoding a fluorescent reporter (AAV-flex-tdTomato) were injected into vagal ganglia of knock-in mice, and labeled fibers were visualized in peripheral tissues by whole mount fluorescence and/or immunohistochemistry. AAV infections occurred in ~50% of vagal sensory neurons, without apparent preference for particular neuron classes or targeting of passing motor fibers (Chang et al., 2015).
We infected vagal ganglia of Glp1r-ires-Cre mice with AAV-flex-tdTomato, and visualized tdTomato-containing fibers in the periphery (Figures 2D, S4). In the intestine, vagal GLP1R neurons were largely confined to intestinal muscle (Figure S4) and surprisingly did not densely innervate villi in the proximal duodenum. Quantitative analysis involving normalization with a Cre-independent GFP reporter virus (AAV-GFP) revealed that the vast majority of vagal afferents innervating intestinal villi (94.2 ± 2.2%, n=6) did not contain GLP1R. Vagal GLP1R neurons instead densely innervated stomach muscle. The extent of innervation was quantified by counting the number of enteric ganglia contacted by labeled IGLEs in Vglut2-ires-Cre and Glp1r-ires-Cre mice. AAV infections occurred unilaterally in the left ganglion, which innervates the ventral half of the stomach. We counted 101 ± 17 enteric ganglia innervated by labeled IGLEs per ventral stomach in Vglut2-ires-Cre mice, and 131 ± 38 in Glp1r-ires-Cre mice, suggesting that vagal GLP1R neurons account for most vagal IGLEs in stomach muscle.
Optogenetic control of gut motility
Vagal GLP1R neurons do not densely innervate intestinal villi, so we sought to identify other neuron types that do and might be relevant for gastrointestinal function. We previously used a genome-based strategy to identify G Protein-Coupled Receptors (GPCRs) expressed in vagal sensory neurons (Chang et al., 2015), and found two other GPCRs that mark small neuronal subsets: the purinergic receptor P2RY1 and the orphan receptor GPR65. P2RY1 neurons target the lung, forming stereotyped candelabra terminals at neuroepithelial bodies, and optogenetic activation of vagal P2RY1 neurons acutely silences breathing (Chang et al., 2015). In contrast, vagal neurons containing GPR65 do not densely innervate the lung (Chang et al., 2015), and their physiological function remained unknown.
We used optogenetics to ask whether any of these neurons might control gastrointestinal physiology (Figure 3). We crossed Glp1r-ires-Cre, Gpr65-ires-Cre, P2ry1-ires-Cre, and Vglut2-ires-Cre mice with a Cre-dependent channelrhodopsin allele (lox-ChR2; offspring of Marker-Cre mice are called Marker-ChR2). Neuron activity was evoked in anesthetized mice by focal illumination of the vagus nerve trunk, and robust light-induced action potentials were observed in Vglut2-ChR2, P2ry1-ChR2, Glp1r-ChR2 and Gpr65-ChR2 mice. Most vagal GLP1R and GPR65 neurons (>95%, >97%) are slow-conducting C fibers, as revealed by measuring neuron conduction velocities at fixed intervals from the illumination site (Figure S5).
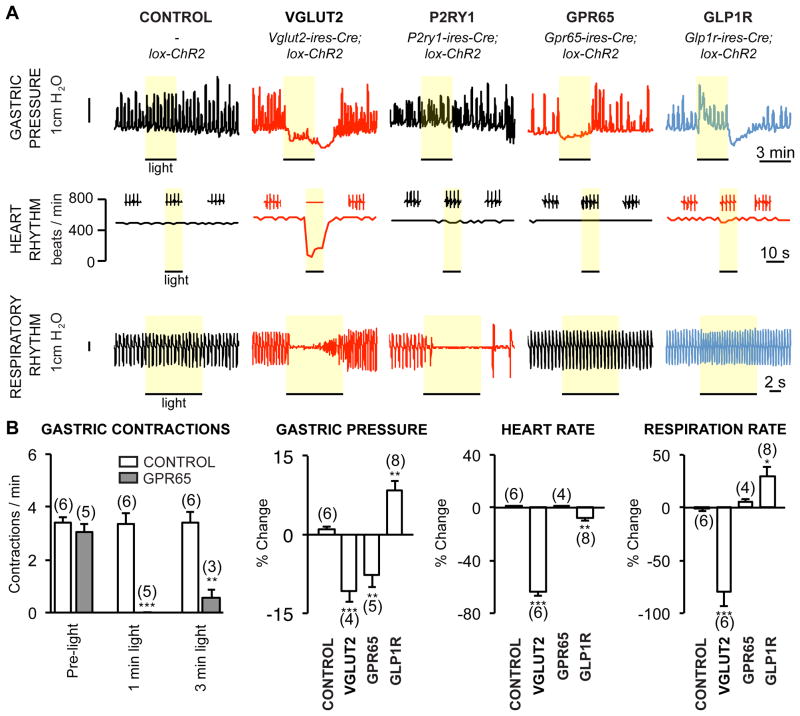
Figure 3. Optogenetic control of gut motility.
(A) Physiological responses to optogenetic activation (yellow bar) of vagal sensory neuron subtypes. (B) Quantifying physiological changes to neuron subtype stimulation (mean ± sem, n=3–8, *p<.05, **p<.01, ***p<.001). See also Figure S5.
Activating all vagal sensory neurons in Vglut2-ChR2 mice caused profound and immediate drops in breathing rate, heart rate, and gastric pressure (Chang et al., 2015). In contrast, activating vagal GPR65 neurons caused a striking light-induced blockade of gastric contractions, without impacting breathing or heart rate. Gastric contractions occurred at a frequency of 3.4 ± 0.3 per minute in control lox-ChR2 mice, and this frequency was not altered by vagus nerve illumination (3.4 ± 0.6 per minute, 6 mice). In Gpr65-ChR2 mice, gastric contractions occurred at a similar frequency at rest (3.1 ± 0.3 per minute, 5 mice), but optogenetic activation stopped or reduced gastric contractions during a 1 minute (0 ± 0.0 per minute, 5 mice) and 3 minute (0.6 ± 0.3 per minute, 3 mice) photostimulation period. Activating GPR65 neurons decreased both tonic and phasic measurements of gastric pressure. GPR65 is not expressed in vagal motor neurons, indicating that these effects are due to sensory neuron stimulation. Activating vagal GLP1R neurons instead caused a different response characterized by increased gastric pressure, and also produced a small but significant change in breathing and heart rate, suggesting that some vagal GLP1R neurons communicate with organ systems other than the gut. The strikingly selective response to GPR65 sensory neuron activation strengthens the conclusion that the vagus nerve consists of several co-fasciculating classes of sensory neurons (so-called ‘labeled lines’), each of which conveys a highly specific signal relevant for autonomic physiology.
GPR65 neurons target intestinal villi
Optogenetic studies suggested that GPR65 neurons receive inputs from the gastrointestinal tract. GPR65, P2RY1, and GLP1R each mark subsets of neurons, with FISH revealing expression in 10.2%, 11.6%, and 11.5% of sensory neurons (Chang et al., 2015); the Glp1r probe provided lower signal-to-noise and may not reveal all neurons containing Glp1r mRNA. Two-color FISH indicated that GPR65 neurons are distinct from GLP1R and P2RY1 neurons (Figure 4A). Nearly all GPR65 neurons lacked GLP1R (99.5%, 220/221) and nearly all GLP1R neurons lacked GPR65 (99.5%, 198/199); similarly orthogonal results were obtained in pairwise analyses involving P2RY1 (Figure S5).
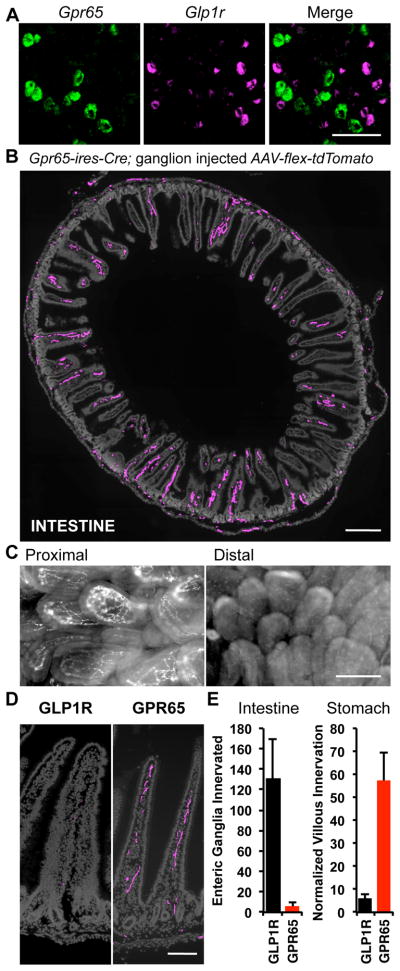
Figure 4. GPR65 neurons target intestinal villi.
(A) Two-color FISH in vagal ganglia reveals expression of Gpr65 and Glp1r in different sensory neurons, scale bar: 100 μm. (B) Vagal sensory neuron projections were mapped by infecting vagal ganglia of Gpr65-ires-Cre mice with AAV-flex-tdTomato. Terminals were visualized by immunofluorescence of duodenum (cryosections) and intestinal architecture visualized with DAPI (grey), scale bar: 500 μm. (C) Wholemount fluorescence of nerve terminals in an en face preparation of proximal (< 1 cm from pylorus) and distal (4 cm from pylorus) intestinal villi after injecting vagal ganglia of Vglut2-ires-Cre mice with AAV-flex-tdTomato. (D) High magnification image of villi innervation, scale bar: 100 μm. (E) Numbers of intestinal villi and gastric enteric ganglia innervated by vagal sensory neuron types were counted, and for villi, normalized using a Cre-independent reporter (mean ± sem, n=6, **p<.01). See also Figure S5.
The peripheral projections of vagal GPR65 neurons were mapped by infecting vagal ganglia of Gpr65-ires-Cre mice with AAV-flex-tdTomato. GPR65 neurons display extensive innervation of intestinal villi in the duodenal bulb immediately adjacent to the pyloric sphincter (Figure 4B). Innervation density of GPR65 neurons decreases dramatically beyond the duodenal bulb, with only sparse innervation of the rest of the duodenum and small intestine. Consistent with these findings, bulk labeling of vagal sensory neurons reveals maximal innervation of villi within the first 1–3 centimeters of duodenum (Figure 4C). Quantitative analysis (Figure 4E) revealed that GPR65 neurons accounted for most vagal innervation of duodenal villi (57.4 ± 11.9%, n=6) while as described before, GLP1R neurons did not. Orthogonal results were obtained in stomach muscle, where GPR65 neurons only accounted for 6 ± 4 enteric ganglia innervated by labeled IGLEs per ventral stomach. Thus, GLP1R and GPR65 neurons display strikingly distinct anatomical projections within the gastrointestinal tract, with GPR65 terminals enriched in villi of intestinal mucosa and GLP1R neurons largely confined to stomach and intestinal muscle.
GPR65 neurons detect serotonin in vitro and intestinal nutrients in vivo
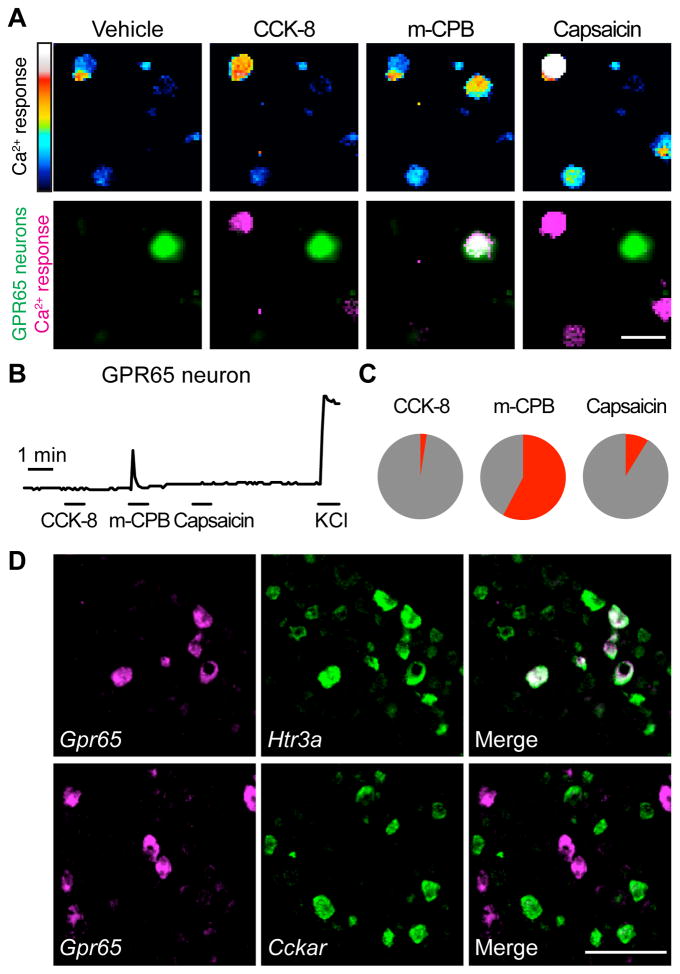
Genetically defining vagal sensory neurons in intestinal villi allows for a controlled analysis of neuron response properties. We next asked whether GPR65 neurons detect various gut hormones (Figure 5). Responses were imaged using the calcium indicator Fura-2 in acute vagal ganglia cultures from heterozygous knock-in/knock-out mice containing a GFP allele at the endogenous Gpr65 locus (Gpr65GFP/+) (Radu et al., 2006). For comparison, GLP1R neurons were analyzed using Glp1r-ires-Cre; lox-L10-GFP mice (Figure S6). Serotonin (or a specific agonist for the serotonin receptor HTR3A) activated most GPR65 neurons (58%, 15/26), while cholecystokinin did not (2%, 1/41). Two-color FISH revealed that GPR65 neurons predominantly represent a subset of HTR3A-containing neurons, and that most do not express the cholecystokinin receptor CCKAR (Figure 5D). In contrast, cholecystokinin activated many vagal GLP1R neurons (62%, 53/85), consistent with co-expression of these gut hormone receptors (Figure S3), while serotonin activated some (19%, 16/85). Likewise, the TRPV1 agonist capsaicin activated most GLP1R neurons (68%, 58/85) but only rare GPR65 neurons (9%, 3/34). We did not observe acute responses to GLP1R agonists in any vagal sensory neurons by in vitro calcium imaging, or by in vivo ganglion imaging following intraperitoneal injection (Figure S6), suggesting a modulatory or developmental role for GLP1R in vagal afferents. Prior studies reporting vagal responses to GLP1R agonists required intravenous agonist administration, and responses were delayed for minutes, consistent with indirect rather than direct activation of afferents or efferents (Bucinskaite et al., 2009). Taken together, these findings indicate a prominent role for serotonin sensation but not CCK or GLP1 sensation by GPR65-expressing vagal afferents, which represent the major species in intestinal villi. GPR65 afferents likely communicate with enterochromaffin cells, the major source of serotonin in the body.
Figure 5. GPR65 neurons respond in vitro to an HTR3A agonist.
(A) Calcium responses by Fura-2 imaging of dissociated vagal sensory neurons from Gpr65GFP/+ mice to CCK-8 (10 nM), the HTR3A agonist m-chlorophenylbiguanide (mCPB, 100 μM) and capsaicin (1 μM), scale bar: 40 μm. Top: Fura-2 excitation; bottom: GFP fluorescence (green) and calcium responses (magenta). (B) Representative responses of a GPR65 neuron. (C) Pie chart indicating percentage of GPR65 neurons activated (red) by each ligand. (D) Two color FISH in vagal ganglia. Scale bar: 100 μm. See also Figure S6.
Responses of GPR65 neurons to physiological stimuli were measured by in vivo calcium imaging in vagal ganglia of Gpr65-GCaMP3* mice (Figure 6). GPR65 neurons did not account for most neurons detecting lung inflation (0/48, 3 mice), saline-induced intestinal distension (6.3%, 3/47, 3 mice), or balloon-induced stomach stretch (5.6%, 4/72, 3 mice). Instead, GPR65 neurons accounted for most neurons responsive to liquid diet (200 μl) injected into the duodenal bulb (66%, 27/41, 4 mice). Only rare responses were observed to nutrients perfused through distal regions of the duodenum that did not include the duodenal bulb (Figure S6). Taken together with anatomical data, GPR65 neurons with terminals embedded in villi adjacent to the pyloric sphincter account for most vagal chemoreceptors responsive to intestinal nutrients.
Figure 6. GPR65 neurons detect intestinal nutrients in vivo.
(A) In vivo imaging of vagal ganglia in Gpr65-GCaMP3* mice showing GCaMP3 responses (green) of GPR65 neurons (magenta) to stimuli indicated. (B) Rows indicate time-resolved responses (ΔF/F, color coded) of single neurons in Gpr65-GCaMP3* mice to stimuli (green bars: 15 seconds). Magenta and black bars represent tdTomato-positive and negative neurons. Only some unresponsive tdTomato-negative neurons are depicted; numbers at Y-axis base indicate total number of viable imaged neurons. See also Figure S6.
Central representations of vagal inputs from the gastrointestinal tract
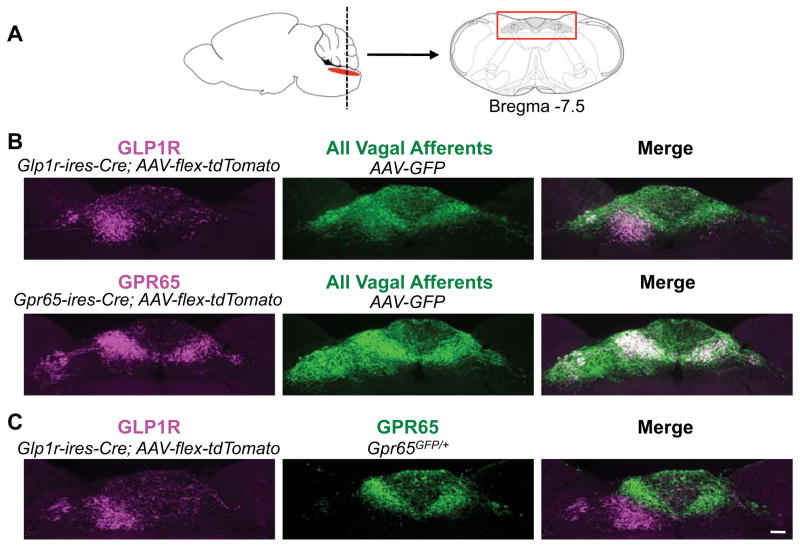
Next we used AAV mapping to ask whether GPR65 and GLP1R inputs are segregated centrally (Figure 7). We simultaneously infected vagal ganglia of Gpr65-ires-Cre and Glp1r-ires-Cre mice with AAV-flex-tdTomato and AAV-GFP. GFP signal revealed axons of all vagal sensory neuron types in the nucleus of the solitary tract (NTS) and area postrema, while tdTomato signal specifically labeled GPR65 and GLP1R axons. GLP1R and GPR65 axons segregate to topographically distinct regions of the posterior NTS (Figure 7; Figure S7 for entire rostral-caudal series). Vagal GLP1R neurons predominantly target the medial NTS subnucleus, a region that receives input from gastric mechanoreceptors (Willing and Berthoud, 1997). In contrast, GPR65 neurons projected more medially to the NTS commissural zone, just beneath the area postrema. For a direct comparison of GLP1R neuron (tdTomato) and GPR65 neuron (GFP) projection patterns (Figure 7C), vagal ganglia of Glp1r-ires-Cre; Gpr65GFP/+ mice were infected with AAV-flex-tdTomato. This strategy revealed that vagal GLP1R and GPR65 neurons target adjacent but distinct NTS subregions, suggesting engagement of different neural circuits.
Figure 7. Visualizing brainstem innervation.
(A) Vagal sensory neuron axons were analyzed in a brainstem region (red box) containing the NTS and area postrema. (B) Vagal ganglia of Glp1r-ires-Cre and Gpr65-ires-Cre mice were infected with AAV-flex-tdTomato and AAV-GFP for immunofluorescence-based detection of Cre-containing (magenta) and all (green) vagal sensory neuron axon types. (C) Vagal ganglia of Glp1r-ires-Cre; Gpr65GFP/+ mice were infected with AAV-flex-tdTomato for simultaneous visualization of GLP1R (magenta) and GPR65 (green) axons, scale bar: 100 μm. See also Figure S7.
DISCUSSION
Internal sensory neurons of the vagus nerve survey the state of several major physiological systems. Within the gastrointestinal tract, sensation of gastric distension and intestinal nutrients are long-appreciated signals that activate vagal afferents and impact physiology and behavior. Here, we genetically define sensory neurons that detect these cues, and use Cre-based anatomical mapping, in vivo imaging, and optogenetics to decipher aspects of gut-to-brain signaling.
One small group of vagal afferents marked by expression of the receptor GPR65 (~230 neurons per ganglion) innervates villi in the proximal small intestine close to the gastro-duodenal junction. GPR65 neurons respond to serotonin, but not other gut hormones such as GLP1 and cholecystokinin. In vivo calcium imaging revealed acute responses of GPR65 neurons to food introduced into the intestinal lumen, providing a direct functional link between sensory neurons with terminal fields in intestinal villi and nutrient detection. Responses of vagal GPR65 neurons to nutrients were rapid and transient, presumably turning off as peristaltic movements removed stimuli from the duodenal bulb. Artificial sweeteners perfused through the duodenum did not evoke a response in vagal sensory neurons, suggesting that any metabolic responses mediated by intestinal sweet receptors involve alternative pathways (Jang et al., 2007). Vagal GPR65 neurons accounted for most but not all nutrient-responsive neurons, indicating at least one other class of nutrient-responsive vagal afferent.
The ability of vagal GPR65 neurons to slow gastric motility suggests a two-pronged response to nutrient-evoked serotonin release in the duodenal bulb. During a meal, food is released through the pyloric sphincter into the duodenal bulb. When a critical level is reached in the duodenal bulb, as detected by a deflection in osmolarity, pH, and/or mechanical brushing, a burst of serotonin is released. Serotonin is a classic signal that promotes gut motility through the enteric nervous system, propelling resident contents distally to sites of enzyme secretion and absorption (Bertrand and Bertrand, 2010). Simultaneously, serotonin-responsive GPR65 neurons of the vagus nerve initiate an intestine-brain-stomach circuit that causes a striking feedback blockade of gastric motility, decreasing entry of new content into the duodenal bulb. This dual activity of serotonin should purge the proximal intestine of contents. After the first bolus has migrated, the system presumably re-sets and re-fills to prepare the next bolus. Based on these findings, we propose an important role for vagal GPR65 neurons in controlling the pulsatile rhythm of food entry into the intestine. Optogenetics enables a specific analysis of vagal chemosensors in the intestine that was not possible with sham feeding, which triggers a complex response involving multiple vagal afferent types but also enteric neurons, spinal neurons, circulating hormones, and direct nutrient effects. Whole nerve stimulations also do not distinguish contributions from villous neurons, gastrointestinal mechanoreceptors, motor neurons, or other fiber types. Future studies are needed to determine the role of GPR65 itself in intestinal homeostasis, as these studies reveal it to be a prime candidate for regulating gastrointestinal physiology. Specific targeting of GPR65 neurons may impact disorders of nutrient absorption and gut motility, such as dyspepsia and ileus.
Genetically guided anatomical tracing showed the central representation of nutrient-responsive vagal afferents containing GPR65. Anterograde tracing studies from the intestine using bulk tracing techniques are technically challenging and have not enabled differential analysis of fiber type-specific projection fields, such as those from chemoreceptors and mechanoreceptors. Immediate early gene (IEG) analysis in the NTS suggested a relatively broad topographical domain responsive to intestinal nutrients (Phifer and Berthoud, 1998). However, nutrient-evoked IEG induction potentially includes direct or indirect contributions from multiple vagal afferent types, as well as enteric neurons, spinal neurons, hormones, and circulating nutrients themselves. Experiments here instead reveal strikingly restricted central projections of vagal GPR65 neurons that are confined to the commissural NTS. This projection field is distinct from that of gastrointestinal mechanoreceptors (Figure 7) and apnea-promoting pulmonary afferents (Chang et al., 2015), consistent with a topographical NTS map linked to physiological input. Revealing the spatially confined projections of vagal GPR65 neurons highlights the power of using genetic tools for selective visualization of afferent subtype-specific terminal fields in the brainstem.
Four findings suggest that villous nutrient detection by the vagus nerve occurs primarily through GLP1R-independent mechanisms. Vagal GLP1R sensory neurons 1) do not account for most nutrient-responsive neurons, 2) do not densely innervate intestinal villi, 3) do not respond to GLP1R agonists in vitro, and 4) do not respond to GLP1R agonists administered intraperitioneally by in vivo ganglion imaging. Instead a cohort of vagal GLP1R neurons forms IGLE terminals in stomach, and accounts for most gastric stretch receptors by in vivo imaging. Furthermore, vagal GLP1R neurons project centrally to medial NTS regions that show IEG induction following gastric distension (Willing and Berthoud, 1997). The same genetically defined neuron type forms IGLEs and senses stomach stretch, supporting the model that IGLEs are mechanosensitive terminals (Zagorodnyuk et al., 2001). A second cohort of vagal GLP1R neurons responds to intestinal distention, indicating that vagal GLP1R neurons generally account for several classes of gastrointestinal mechanoreceptors.
Intriguingly, these studies add to the list of gut hormone receptors expressed by gastric mechanoreceptors. In some studies, but not all, cholecystokinin was reported to activate the same sensory neurons that detect gastric distension (Blackshaw and Grundy, 1990; Schwartz et al., 1991). One caveat is that cholecystokinin exerts profound effects on gastric motility and tone, effects which might secondarily impact stretch sensitivity. Here, analyzing responses of genetically defined gastrointestinal mechanoreceptors in cell culture reveals acute and direct cholecystokinin-evoked calcium transients that are independent of secondary physiological effects. Prior studies also reported that leptin activates gastric mechanoreceptors (Li et al., 2011) while ghrelin inhibits them (Page et al., 2007). One model is that gut hormones relay convergent state-dependent information about ingested and stored nutrients to modulate the sensitivity of gastric stretch sensors. When nutrients are abundant, subthreshold sensitization of gastric mechanoreceptors would promote satiety at lower distension levels; in contrast, when nutrients are scarce, a larger sized meal would be required for the same sensory neuron response. Here, we reveal that GLP1R is also expressed by mechanoreceptors in stomach, as well as intestine. Unlike CCKAR agonists, GLP1R agonists do not acutely activate vagal afferents, suggesting a modulatory role. Intriguingly, introduction of GLP1R agonists directly into the brainstem can gate NTS responses to stomach distension, and a model was proposed involving GLP1R expression in intrinsic NTS neurons (Hayes et al., 2009). Our studies raise the possibility that GLP1R agonists instead, or in addition, directly modulate vagal sensory neuron axons in the brainstem to control presynaptic neurotransmitter release. Together, these findings suggest that gut hormones exert multi-tiered control over gastric stretch sensitivity at different processing levels in the same neuron.
Sensory systems use different strategies to encode peripheral information. For example, the olfactory system can generate a myriad of odor perceptions. To achieve this, odors are encoded by combinations of receptors and sensory neuron types in the periphery. Olfactory sensory neuron inputs are subsequently mixed without apparent topography in olfactory cortex (Wilson and Sullivan, 2011). This organization allows individual cortical neurons to integrate responses from multiple receptors, which is relevant for generating diverse perceptions. In contrast, the gustatory system is more streamlined, with different sensory cells and peripheral neural circuits devoted to perception of sweet, salty, sour, umami, and bitter taste modalities (Barretto et al., 2015). These separate and parallel processing streams for taste inputs in ascending gustatory circuits are termed ‘labeled lines’. Our data indicate that the vagus nerve uses a coding logic that shares many similarities with gustatory nerves. Individual vagal sensory neurons transmit highly specific information from peripheral organs- such as stomach stretch during feeding and lung inflation during breathing. Furthermore, optogenetic stimulation of vagal GPR65 neurons inhibits gastric contractions without impacting breathing or heart rate, suggesting that individual sensory neurons not only monitor but also control particular organ systems. The sensory arm of the vagus nerve thus consists of several co-fasciculating labeled lines dedicated for particular sensory modalities. Moreover, the cell bodies of neurons responsive to different pulmonary and gastrointestinal inputs are intermingled within vagal ganglia in a salt-and-pepper manner, suggesting that spatial information from the periphery is largely not apparent at the level of the ganglion.
Genetically identifying neuron subtypes relevant for physiology and behavior is a major goal of the neuroscience field. Recent advances revealed neuron types involved in numerous perceptions and behaviors, such as touch, itch, hunger, and aggression (Aponte et al., 2011; Bai et al., 2015; Han et al., 2013; Lee et al., 2014). Genetic approaches help paint a comprehensive picture of neuron function that includes gene expression, peripheral anatomy, central anatomy, in vivo and in vitro responsiveness, and physiological function. Here, we genetically define two discrete classes of gut-to-brain afferents that differentially monitor and control the digestive system, providing a pivotal molecular foundation for exploring the sensory biology and neural circuitry associated with gut-to-brain signaling.
EXPERIMENTAL PROCEDURES
Glp1r-ires-Cre mice were prepared by BAC recombineering. Rosa26-GCaMP3 mice were generated by breeding lox-GCaMP3 with E2a-Cre mice (Jackson, 003314) to achieve germline excision, and then breeding out the E2a-Cre allele. Genotyping, FISH, in vitro calcium imaging, electrophysiology, optogenetics, and physiological measurements were as described previously (Chang et al., 2015), with reagents, minor modifications, and other mouse lines in Extended Experimental Procedures.
For anatomical mapping, AAV-flex-tdTomato and AAV-GFP were injected into vagal ganglia of Gpr65-ires-Cre and Glp1r-ires-Cre mice (Chang et al., 2015). IGLE density was determined in stomach muscle wholemounts with enteric ganglia labeled by Fluorogold (30 mg/kg IP). Normalized villus innervation is the ratio of tdTomato fibers to GFP fibers in duodenal cryosections (12 μm, sampled every mm over the first cm).
For in vivo imaging, vagal ganglia were surgically exposed and immobilized on a stable platform. GCaMP3 fluorescence was measured by confocal microscopy (Leica TCS SP5 II) in single ganglion neurons. Electrical stimulation involved steps (10 sec, 2 ms pulses at 5 Hz) of increasing voltage (1 to 70 V). Airway gases were introduced (1 l/min) by a tracheal cannula. Gastric distension was by nitrogen gas (3–6 ml/min, 15 sec) or a volume-controlled and surgically implanted balloon. Liquid diet (200 μl, TestDiet LD101) was injected (Figures 1E, 2, 6, S1B, S4, S6) after pyloric sphincter sealing. Other stimuli (Figures 1C, 1D, 1F, S1C–F) were introduced (4–5 min) during continuous perfusion (saline or stimulus, 125 μl/min) of the intestine (first ~11 cm). Cells were coded as responsive based on stimulus-evoked changes in GCaMP3 fluorescence. See Extended Experimental Procedures for more information about surgery, microscopy, introduction of test stimuli, and data analysis related to in vivo imaging.
For data analysis, sample sizes are indicated in main text, figure legends, or bar graphs (numbers in parentheses). Significance was determined by comparisons to the indicated control group using a two-tailed Student’s t test (Fig. 2, 3) or between indicated groups using a two-tailed Mann-Whitney test (Fig. 4).
Supplementary Material
Acknowledgments
We thank David Ginty, Ardem Patapoutian, and John Flanagan for manuscript comments, the Nikon Imaging Center at Harvard Medical Center for microscopy assistance, and the Boston Area Diabetes Research Center Transgenic Core (P30 DK046200) and Boston Area Nutrition Research Center Transgenic Core (P30 DK057521) for generating knock-in mice. Funding was provided by NIDDK (SDL, RO1 DK103703), an NSF pre-doctoral fellowship (DES), an F30 NIH training grant (EKW), a T32 training grant for the Harvard MSTP program (EKW), and the Harvard-MIT Joint Research Grants Program in Basic Neuroscience (SDL, BBL).
Footnotes
AUTHOR CONTRIBUTIONS
SDL, DES, RBC, BDU, and EKW designed and conceived the study; RBC and EKW made knock-in mice; DES, RBC, and EKW did FISH; DES and BDU did anatomical studies; RBC did optogenetics and in vitro imaging; EKW did in vivo imaging; BBL provided Vglut2-ires-Cre mice; and SDL, DES, RBC, BDU, and EKW wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott CR, Monteiro M, Small CJ, Sajedi A, Smith KL, Parkinson JR, Ghatei MA, Bloom SR. The inhibitory effects of peripheral administration of peptide YY(3-36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain research. 2005;1044:127–131. doi: 10.1016/j.brainres.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nature neuroscience. 2011;14:351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- Bai L, Lehnert BP, Liu J, Neubarth NL, Dickendesher TL, Nwe PH, Cassidy C, Woodbury CJ, Ginty DD. Genetic Identification of an Expansive Mechanoreceptor Sensitive to Skin Stroking. Cell. 2015;163:1783–1795. doi: 10.1016/j.cell.2015.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barretto RP, Gillis-Smith S, Chandrashekar J, Yarmolinsky DA, Schnitzer MJ, Ryba NJ, Zuker CS. The neural representation of taste quality at the periphery. Nature. 2015;517:373–376. doi: 10.1038/nature13873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR, Blackshaw LA, Brookes SJ, Grundy D. Neuroanatomy of extrinsic afferents supplying the gastrointestinal tract. Neurogastroenterol Motil. 2004;16(Suppl 1):28–33. doi: 10.1111/j.1743-3150.2004.00471.x. [DOI] [PubMed] [Google Scholar]
- Bertrand PP, Bertrand RL. Serotonin release and uptake in the gastrointestinal tract. Auton Neurosci. 2010;153:47–57. doi: 10.1016/j.autneu.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Blackshaw LA, Grundy D. Effects of cholecystokinin (CCK-8) on two classes of gastroduodenal vagal afferent fibre. J Auton Nerv Syst. 1990;31:191–201. doi: 10.1016/0165-1838(90)90185-l. [DOI] [PubMed] [Google Scholar]
- Brookes SJ, Spencer NJ, Costa M, Zagorodnyuk VP. Extrinsic primary afferent signalling in the gut. Nature reviews Gastroenterology & hepatology. 2013;10:286–296. doi: 10.1038/nrgastro.2013.29. [DOI] [PubMed] [Google Scholar]
- Bucinskaite V, Tolessa T, Pedersen J, Rydqvist B, Zerihun L, Holst JJ, Hellstrom PM. Receptor-mediated activation of gastric vagal afferents by glucagon-like peptide-1 in the rat. Neurogastroenterol Motil. 2009;21:978–e978. doi: 10.1111/j.1365-2982.2009.01317.x. [DOI] [PubMed] [Google Scholar]
- Chambers AP, Sandoval DA, Seeley RJ. Integration of satiety signals by the central nervous system. Curr Biol. 2013;23:R379–388. doi: 10.1016/j.cub.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang RB, Strochlic DE, Williams EK, Umans BD, Liberles SD. Vagal Sensory Neuron Subtypes that Differentially Control Breathing. Cell. 2015;161:622–633. doi: 10.1016/j.cell.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox EA, Phillips RJ, Martinson FA, Baronowsky EA, Powley TL. Vagal afferent innervation of smooth muscle in the stomach and duodenum of the mouse: morphology and topography. J Comp Neurol. 2000;428:558–576. doi: 10.1002/1096-9861(20001218)428:3<558::aid-cne11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Han L, Ma C, Liu Q, Weng HJ, Cui Y, Tang Z, Kim Y, Nie H, Qu L, Patel KN, et al. A subpopulation of nociceptors specifically linked to itch. Nature neuroscience. 2013;16:174–182. doi: 10.1038/nn.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes MR, Bradley L, Grill HJ. Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology. 2009;150:2654–2659. doi: 10.1210/en.2008-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes MR, De Jonghe BC, Kanoski SE. Role of the glucagon-like-peptide-1 receptor in the control of energy balance. Physiology & behavior. 2010;100:503–510. doi: 10.1016/j.physbeh.2010.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillsley K, Grundy D. Serotonin and cholecystokinin activate different populations of rat mesenteric vagal afferents. Neurosci Lett. 1998;255:63–66. doi: 10.1016/s0304-3940(98)00690-9. [DOI] [PubMed] [Google Scholar]
- Holst JJ. The physiology of glucagon-like peptide 1. Physiological reviews. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, Zhou J, Kim HH, Xu X, Chan SL, Juhaszova M, et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:15069–15074. doi: 10.1073/pnas.0706890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanningros R. Vagal unitary responses to intestinal amino acid infusions in the anesthetized cat: a putative signal for protein induced satiety. Physiology & behavior. 1982;28:9–21. doi: 10.1016/0031-9384(82)90094-4. [DOI] [PubMed] [Google Scholar]
- Kim YS, Chu Y, Han L, Li M, Li Z, Lavinka PC, Sun S, Tang Z, Park K, Caterina MJ, et al. Central terminal sensitization of TRPV1 by descending serotonergic facilitation modulates chronic pain. Neuron. 2014;81:873–887. doi: 10.1016/j.neuron.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal S, Kirkup AJ, Brunsden AM, Thompson DG, Grundy D. Vagal afferent responses to fatty acids of different chain length in the rat. American journal of physiology Gastrointestinal and liver physiology. 2001;281:G907–915. doi: 10.1152/ajpgi.2001.281.4.G907. [DOI] [PubMed] [Google Scholar]
- Lee H, Kim DW, Remedios R, Anthony TE, Chang A, Madisen L, Zeng H, Anderson DJ. Scalable control of mounting and attack by Esr1+ neurons in the ventromedial hypothalamus. Nature. 2014;509:627–632. doi: 10.1038/nature13169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wu X, Zhou S, Owyang C. Low-affinity CCK-A receptors are coexpressed with leptin receptors in rat nodose ganglia: implications for leptin as a regulator of short-term satiety. American journal of physiology Gastrointestinal and liver physiology. 2011;300:G217–227. doi: 10.1152/ajpgi.00356.2010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Maljaars PW, Peters HP, Mela DJ, Masclee AA. Ileal brake: a sensible food target for appetite control. A review. Physiology & behavior. 2008;95:271–281. doi: 10.1016/j.physbeh.2008.07.018. [DOI] [PubMed] [Google Scholar]
- Mazda T, Yamamoto H, Fujimura M, Fujimiya M. Gastric distension-induced release of 5-HT stimulates c-fos expression in specific brain nuclei via 5-HT3 receptors in conscious rats. American journal of physiology Gastrointestinal and liver physiology. 2004;287:G228–235. doi: 10.1152/ajpgi.00373.2003. [DOI] [PubMed] [Google Scholar]
- Mei N. Vagal glucoreceptors in the small intestine of the cat. J Physiol. 1978;282:485–506. doi: 10.1113/jphysiol.1978.sp012477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei N, Garnier L. Osmosensitive vagal receptors in the small intestine of the cat. J Auton Nerv Syst. 1986;16:159–170. doi: 10.1016/0165-1838(86)90022-6. [DOI] [PubMed] [Google Scholar]
- Page AJ, Slattery JA, Milte C, Laker R, O’Donnell T, Dorian C, Brierley SM, Blackshaw LA. Ghrelin selectively reduces mechanosensitivity of upper gastrointestinal vagal afferents. American journal of physiology Gastrointestinal and liver physiology. 2007;292:G1376–1384. doi: 10.1152/ajpgi.00536.2006. [DOI] [PubMed] [Google Scholar]
- Phifer CB, Berthoud HR. Duodenal nutrient infusions differentially affect sham feeding and Fos expression in rat brain stem. Am J Physiol. 1998;274:R1725–1733. doi: 10.1152/ajpregu.1998.274.6.R1725. [DOI] [PubMed] [Google Scholar]
- Powley TL, Phillips RJ. Gastric satiation is volumetric, intestinal satiation is nutritive. Physiology & behavior. 2004;82:69–74. doi: 10.1016/j.physbeh.2004.04.037. [DOI] [PubMed] [Google Scholar]
- Radu CG, Cheng D, Nijagal A, Riedinger M, McLaughlin J, Yang LV, Johnson J, Witte ON. Normal immune development and glucocorticoid-induced thymocyte apoptosis in mice deficient for the T-cell death-associated gene 8 receptor. Molecular and cellular biology. 2006;26:668–677. doi: 10.1128/MCB.26.2.668-677.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards W, Hillsley K, Eastwood C, Grundy D. Sensitivity of vagal mucosal afferents to cholecystokinin and its role in afferent signal transduction in the rat. J Physiol. 1996;497(Pt 2):473–481. doi: 10.1113/jphysiol.1996.sp021781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruttimann EB, Arnold M, Hillebrand JJ, Geary N, Langhans W. Intrameal hepatic portal and intraperitoneal infusions of glucagon-like peptide-1 reduce spontaneous meal size in the rat via different mechanisms. Endocrinology. 2009;150:1174–1181. doi: 10.1210/en.2008-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz GJ, McHugh PR, Moran TH. Integration of vagal afferent responses to gastric loads and cholecystokinin in rats. Am J Physiol. 1991;261:R64–69. doi: 10.1152/ajpregu.1991.261.1.R64. [DOI] [PubMed] [Google Scholar]
- Sisley S, Gutierrez-Aguilar R, Scott M, D’Alessio DA, Sandoval DA, Seeley RJ. Neuronal GLP1R mediates liraglutide’s anorectic but not glucose-lowering effect. J Clin Invest. 2014;124:2456–2463. doi: 10.1172/JCI72434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorens B. Expression cloning of the pancreatic beta cell receptor for the gluco-incretin hormone glucagon-like peptide 1. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:8641–8645. doi: 10.1073/pnas.89.18.8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willing AE, Berthoud HR. Gastric distension-induced c-fos expression in catecholaminergic neurons of rat dorsal vagal complex. Am J Physiol. 1997;272:R59–67. doi: 10.1152/ajpregu.1997.272.1.R59. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Sullivan RM. Cortical processing of odor objects. Neuron. 2011;72:506–519. doi: 10.1016/j.neuron.2011.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, Hsiao EY. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Chen BN, Brookes SJ. Intraganglionic laminar endings are mechano-transduction sites of vagal tension receptors in the guinea-pig stomach. J Physiol. 2001;534:255–268. doi: 10.1111/j.1469-7793.2001.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ritter RC. Circulating GLP-1 and CCK-8 reduce food intake by capsaicin-insensitive, nonvagal mechanisms. Am J Physiol Regul Integr Comp Physiol. 2012;302:R264–273. doi: 10.1152/ajpregu.00114.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JX, Zhu XY, Owyang C, Li Y. Intestinal serotonin acts as a paracrine substance to mediate vagal signal transmission evoked by luminal factors in the rat. J Physiol. 2001;530:431–442. doi: 10.1111/j.1469-7793.2001.0431k.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.