Abstract
Here we consider the value of neural population analysis as an approach to understanding how information is represented in the hippocampus and cortical areas and how these areas might interact as a brain system to support memory. We argue that models based on sparse coding of different individual features by single neurons in these areas (e.g., place cells, grid cells) are inadequate to capture the complexity of experience represented within this system. By contrast, population analyses of neurons with denser coding and mixed selectivity reveal new and important insights into the organization of memories. Furthermore, comparisons of the organization of information in interconnected areas suggest a model of hippocampal-cortical interactions that mediates the fundamental features of memory.
Large brains benefit from localization of function in which distinct regions extract and process different information to create a representation of experience. Systems neuroscience seeks to explain which neural patterns constitute a memory representation and, further, to understand how neural representations in different brain regions interact to support cognition. Here, we will explore how neural population analysis can support these goals.
Until recently, the field of neurophysiology has tackled the problem of how brain areas represent information by characterizing tuning curves that define the receptive fields for neurons in each region of a system and then model how successive regions employ the information coded in earlier stages. Within the study of the hippocampal system, this strategy has led the discovery of place cells in the hippocampus (O’Keefe and Dostrovsky, 1971), grid cells in the medial entorhinal cortex (Hafting et al., 2005), and other simple receptive field properties of neurons in neighboring regions (Kropff et al., 2015; Lever et al., 2009; Solstad et al., 2008; Taube et al., 1990; Zhang et al., 2013). Together, the identification of these putative cell types have been interpreted as evidence of a spatial navigation system (Hartley et al., 2014; McNaughton et al., 2006; Moser et al., 2015). However, we will argue that this approach has, at times, failed to capture the full range of information represented within the hippocampal system and, instead, we suggest that the analysis of neural populations in the hippocampus and associated cortical areas can provide much richer understanding of how this brain system supports memory.
First, we consider some basic theoretical issues central to population coding and the analytical techniques used to address these issues. We then turn to a series of experiments conducted in our lab in which population analysis has revealed new insights into the nature of associative memory representation in connected regions of the hippocampal system. Finally, we will consider how results from these experiments challenge current theoretical views and instead support a new model for how the hippocampus, rhinal cortices, and prefrontal cortex might coordinate to define complex and flexible representational spaces that are useful for both memory and action.
What the ensemble code reveals about the nature of neural representation
It is widely believed that the rate in which a neuron fires is the key determinant in representing information (Ferster and Spruston, 1995; Histed and Maunsell, 2014; Shadlen and Newsome, 1994). The application of this principle to identify the information coded by neurons historically involves a characterization of the receptive field of a neuron, defined as a change in the rate of action potentials associated with parametric manipulations of a stimulus, action, or cognitive variable (Adrian and Zotterman, 1926; Georgopoulos et al., 1986; Hubel and Wiesel, 1962; Kennerley et al., 2011). The neuron is said to “encode” or “represent” those variables that define the receptive field. Identifying tuning curves within a region works well when the dimensionality of the representational space is small and best when its unidimensional. For example, V1 has simple cells tuned to oriented edges (Hubel and Wiesel, 1959), A1 has a tonotopy with frequency-tuned cells (Merzenich et al., 1975), and thalamic nucleus AD has cells tuned to head direction (Peyrache et al., 2015; Taube, 1995). Narrow receptive fields with Gaussian tuning to a single dimension are often considered the hallmark of ‘efficient coding’ (Barlow, 1961, 1972; Martin, 1994; Olshausen and Field, 1996). This approach was extended from sensory areas to the hippocampus in the pioneering work of John O’Keefe, Robert Muller, David Olton (Muller et al., 1987; O’Keefe and Dostrovsky, 1971; Olton et al., 1978), and others (McNaughton et al., 1983), in their success in identifying receptive fields of hippocampal principal cells. These neurons were called “place cells” because, under circumstances where the representational space was limited to the single dimension of location, they showed narrow, approximately Gaussian tuning to the location of rats in an environment where behavior was controlled (and therefore not considered) across space (Muller et al., 1987). Since the discovery of place cells, the same approach has identified cells in neighboring regions associated with a systematic array of positions (grid cells), head direction, and environmental borders (Hartley et al., 2014).
Though great progress has been made with analyzing single cell tuning curves, there are limitations to this approach that can misguide our understanding of information coding in the hippocampus and associated cortical areas. The first limitation has to do with how information is distributed across a population of cells, even in simplified behavioral conditions where unidimensional coding is prominent. The second arises when neural activity reflects conjunctive coding to multiple dimensions of information in more complex behavioral conditions.
Sparse vs dense population coding
To illustrate the first limitation of the single cell approach, we consider the coding of two-dimensional space by cells in the dorsal hippocampus (dHPC), the ventral hippocampus (vHPC), and the medial entorhinal cortex (MEC) in rats foraging in open fields. Dorsal hippocampal cells have smaller place fields than those of cells recorded in the vHPC (Jung et al., 1994; Kjelstrup et al., 2008; Komorowski et al., 2013; Royer et al., 2010). Idealized grid cells in the MEC have place fields that tessellate the environment in hexagonal grids (Hafting et al., 2005). Based on the receptive field properties in these areas, a key question is which area provides the most accurate spatial code?
One approach to addressing this question involves using the observed neural signal to decode what the subject was doing. Many classification algorithms exist for this type of analyses (Brown et al., 2004; Haxby et al., 2014; Li, 2014), however, Bayesian decoding has emerged as a favorite since it theoretically provides the best possible classification given certain assumptions (Zhang et al., 1998). Without knowing how the brain decodes itself, the Bayesian approach is therefore a reasonable starting place to determine what could be represented.
As compared to the dHPC, cells in the vHPC and grid cells fire in a larger portion of the environment, which might suggest that these regions provide an ambiguous localization signal. However, analyses from ensembles of cells in the dHPC and vHPC show that these regions in fact store the same information but with different coding strategies. In a recent experiment, cells in the dHPC carried information about a limited region of space, due to the smaller spatial extent of the firing field (Keinath et al., 2014). In the vHPC, cells carried less information per unit of space, but were informative over a larger extent of the environment; representation of a single place was therefore distributed across a larger ensemble of cells. Considering ensembles of cells in each area, the dorsal and ventral hippocampus provided equally accurate Bayesian position estimates (Keinath et al., 2014) and therefore theoretically could convey equal spatial information. The dHPC code is known to be sparse (McNaughton and Nadel, 1990) while the distributed code in the vHPC is said to be more dense, both in the sense that single cells are active more of the time and that more cells are simultaneously active at any given time. Dense codes allow for more unique states to be represented across a population of cells (Barak et al., 2013). Since the goal of decoding is to match a unique neural state to a stimulus (position in this example), the preservation of decoding accuracy across the hippocampus is less surprising. A mathematical analysis of the coding space of MEC ensembles reached a similar conclusion: fewer grid cells are needed to uniquely code for a set of positions than in dHPC due to the density of the MEC code achieved by the repetitive firing fields (Fiete et al., 2008).
There are many known benefits of maintaining a sparse network, such as metabolic efficiency, ease of decoding, and less learning related interference in artificial neural networks (Amari, 1989; Buhmann et al., 1989; Graham and Willshaw, 2009; Mitchison and Durbin, 1989; Rolls and Treves, 2015). The relative value of dense versus sparse coding has had a profound influence on theoretical accounts of the hippocampus (McClelland et al., 1995; McNaughton and Morris, 1987; Rolls, 1990, 2013) that will be discussed below.
In a distributed code, the joint pattern of neuronal activity can theoretically convey information beyond that available from the independent information provided by each cell of an ensemble (Johnson, 1980; Panzeri et al., 1999). Trial-to-trial variability in how neurons fire to the same stimulus tends to be correlated (Averbeck and Lee, 2003; Deweese and Zador, 2004; Lin et al., 2015; Shadlen and Newsome, 1998) and these noise correlations may increase or decrease the ability to decode information from ensemble activity (Averbeck and Lee, 2006; Averbeck et al., 2006), and may or may not affect overall coding capacity of the network (Abbott and Dayan, 1999; Narayanan et al., 2005; Shamir and Sompolinsky, 2006; Zohary et al., 1994). Typically, the importance of these noise correlations is assessed by shuffling each cell’s trial-by-trial activity and testing whether there are any differences in the ability to discriminate stimuli based off of the observed versus shuffled ensemble activity patterns (Averbeck and Lee, 2006; Latham and Nirenberg, 2005). The empirical contributions of noise correlations to coding appears modest (Averbeck and Lee, 2004, 2006; Nirenberg et al., 2001; Oram et al., 2001), though theoretical work has shown that such contributions scale with network size (Zohary et al., 1994) and therefore conclusions based off of small ensembles may underestimate the role of these correlations to computation. From a theoretical perspective, there may be an interesting relationship between the sparsity and density of a code and the relative importance of noise correlations, since dense codes would offer more pairwise (and higher order) interactions. The existence of noise correlations in the hippocampal system is well documented (place cells: Harris et al., 2003; grid cells: Tocker et al., 2015; head direction cells: Peyrache et al., 2015), but the relationship of these correlations to information coding awaits further study.
Unidimensional receptive fields versus mixed selectivity
A second challenge to the single cell receptive field approach occurs when cells are sensitive to multiple streams of information that do not map neatly onto an observable continuous variable. This mixed selectivity is the more typical situation in everyday behavior and is also known to be particularly dependent upon computations within the hippocampal system (Eichenbaum et al., 2007; Ergorul and Eichenbaum, 2004; Gaffan, 1994; Parkinson et al., 1988). Furthermore, brain regions dedicated to coding of a single dimension may be exception rather than the rule (Pouget et al., 2002), especially in higher order brain areas, as demonstrated below.
In the case where cells show such mixed selectivity for conjunctions of task features, Rigotti et al (2013) have argued that analysis of neural population activity patterns can reveal the nature and organization of dimensions represented. In their study, firing properties of cells in the prefrontal cortex were analyzed as monkeys learned the order in which objects were presented (Rigotti et al., 2013). In general, the receptive fields were jointly conditional, in a non-linear fashion, on the interaction of object identity, the order of object presentation, and the nature of the memory demands. By artificially removing the main effect differences from how each cell differentiated task conditions, while leaving intact the non-linear interaction terms, the authors were able to test whether these non-linearities contained important task information. Indeed, the conjunctive code was highly informative on the ensemble level as decoding based on the ensemble of non-linear, interaction terms was accurate despite the inability to extract information from the modified single cell responses. Furthermore, on the ensemble level, use of conjunctive coding greatly expanded the dimensionality of the representational space thus allowing for a greater computational complexity that correlated with task performance.
Estimating the dimensionality of a coding space is a difficult challenge. Rigotti et al.’s estimate was based on an analysis of the number of binary, linear classifications that can be made between pairs stimuli based on ensemble firing patterns. However, the existence of curved planar (low dimensional) spaces have been suggested in other systems (Khan et al., 2007; Koulakov et al., 2011) which would have incorrectly been assessed as high dimensional in Rigotti and colleagues’ experiments due to their assumption of linear classification.
Given the success in identifying unidimensional spatial receptive fields in principle cells of the hippocampus, how is the problem of mixed selectivity, and the application of population analyses, relevant to our current interest in this brain area? In fact, hippocampal neuronal activity is correctly characterized as unidimensional spatial coding only in situations where only space is the relevant dimension. In any situation where behavior becomes salient and systematic, place cells are strongly influenced by the velocity and the direction of movement (Markus et al., 1995; McNaughton et al., 1983). Furthermore, when the animal must learn about important non-spatial stimuli or differential significance of different paths through the same space, the neurons also encode all of the relevant stimuli, contingencies, and accompanying behaviors (e.g., Wood et al., 1999, 2000). These observations challenge the simplistic approach in which single spatial receptive fields, along with other unidimensional spatial firing patterns, are combined to localize the animal in space or to perform navigational calculations of the “inner GPS” (e.g., Barry and Burgess, 2014). Instead, as we will show below, most hippocampal neurons have high-dimensional mixed selectivity in behavioral situations that emphasize multiple dimensions of experience (Eichenbaum and Cohen, 2014).
The coding of non-metric stimuli by hippocampal cells complicates any straightforward analysis of a receptive field, as there is no obvious continuous axis with which to plot firing rate. A similar challenge exists in the olfactory domain as continuous changes to an odorant do not map on to continuous changes in perception or neural activity (Koulakov et al., 2011; see also Stettler and Axel, 2009). The lack of a continuous coding variable for objects and odors is in contrast to the relationship between sound frequency and pitch, light wavelength and color, and space and time. It is notable that topographies often arise in systems in which cells encode a low dimensional stimulus space such that neurons with similar coding properties tend to be physically nearby (Knudsen et al., 1987). The benefits of such computational maps would be severely diminished for cells coding more dimensions than there are physical axes in the brain (n = 3). Whether high dimensional coding of non- metric information drives the lack of topography in both olfactory system and hippocampal system is an open question, though other possibilities for the lack of topography have been offered (Haberly, 2001; Samsonovich and McNaughton, 1997; Schultz and Rolls, 1999).
Can population analyses resolve that nature of neuronal representations in situations where behavior depends upon associating metric and non-metric information? We will show that, as rats perform such a task, hippocampal neurons show varying levels of mixed selectivity and sparsity: from extremely conjunctive, ‘grandmother cells’, that fire uniquely as animals sample a particular object in a specific place, to broadly tuned cells that fire in response to multiple objects in a single location or to a single object in multiple locations. The multimodal, and varied nature of the tuning profiles of individual cells suggests a high dimensional representational space on the population level, similar to that observed in the aforementioned study of prefrontal neurons (Rigotti et al., 2013), and different in kind from networks that generate simple receptive fields.
To probe how multiple dimensions of experience are represented by ensemble firing patterns, we employed a Representational Similarity Analysis (RSA; Kriegeskorte et al., 2008) to identify features of events that generate similar ensemble firing patterns and interpreted ensemble similarity as a metric by which different events are related in a brain area. Such an analysis requires an operationalization of what is meant by a memory representation and for the sake of our analyses we define such representations as the instantaneous pattern of firing rates observed across neurons. It is also necessary to consider how one defines a similar pattern of activity among a population of neurons. There are several metrics available to probe the similarity of population firing rate vectors. The choice of the similarity metric depends upon the conceptualization of what information is important in a neural read out. For example, when using correlation coefficients, the magnitude of the distance metric is normalized and only the relative differences in firing rates are important. This normalization implies that the brain has some way to account for the total amount of activity arriving from the pre-synaptic inputs, an assumption that has some experimental support (Carandini and Heeger, 2012; Louie et al., 2011; Pouille et al., 2009). On the other hand, Euclidean and cosine distance metrics preserve aspects of the absolute rate values. It is important to recognize the strengths and limitations of these and other assumptions in development of population analyses.
In our analyses, we normalize firing rates and calculated pairwise similarities by correlating population firing rate vectors between pairs of distinct behavioral events. The correlation coefficients across many such pairings are amassed in a similarity matrix that we subsequently query to identify the underlying structure of the correlations. Often, there are clearly interpretable meanings of each pairwise comparison, though this may not always be the case.
In our experiments, each ensemble representation (population vector) was recorded in trials that differed by the spatial context in which a trial was performed, the position of an object within a context, the identity of a presented object, and the reward potential of that object. Therefore, we could use these four dimensions of the animals’ experience to study the organization of the representational similarity among the memories the animal formed. The goal of the analysis was to determine whether the similarity of the ensemble representation is higher for memories that share common features as opposed to those that differ by that feature. To use object coding as an example, we can pool pairwise population correlations for events with common objects and compare those to events in which the objects differed. If the difference is larger than expected from a bootstrap distribution in which event labels are shuffled, then we can conclude that object coding was present. We can quantify the degree of object coding as the magnitude of the difference in ensemble similarity recorded after the subject samples the same object twice versus sampling events in which the objects differ. These analyses inform us about the representational distances, and the significance of those distances, between different conditions of each task dimension.
In addition, dendrograms are useful in revealing the organization of representations for the set of memories the animal acquires in this task. The construction of the dendrogram begins with a set of trial-averaged population vectors, one for each type of event that the animal must remember (i.e., a particular object with a specific reward value in a specific location and context). Then, the events are grouped into pairwise clusters according to which pairs of representations are most similar. Next, these pairwise clusters are compared and merged again according to the correlations between pairs of clusters, and so on, until only two clusters remain. The dendrogram provides a read out of what causes pattern separation in the data, as the top branching points explains the largest separation in the representations and, moving downwards, each subsequent branch point reflects smaller degrees of separation in the underlying neuronal representations. This hierarchical clustering algorithm necessarily splits and groups data points and therefore it is necessary to consider the magnitude of separation and the significance of the separation, which can be assessed indirectly through the bootstrap analysis described above or directly from similar Monte Carlo procedures developed for the analysis of dendrograms (Park et al., 2009).
Next, we will show how this RSA reveals the organization of memory representations in several components of the hippocampal system as well as striking differences in the organization of memories across brain regions.
The hippocampus, MEC, LEC, OFC and perirhinal cortex create distinct organizations of the memories
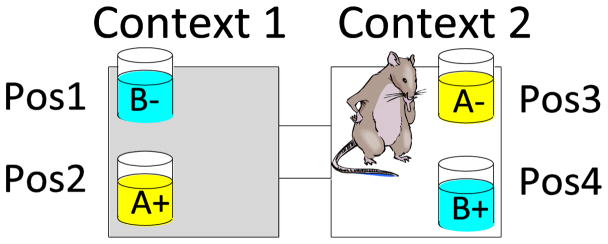
Our use of RSA to interrogate information coding has focused on rats performing a task where they employ the current spatial context to retrieve specific object memories. This task models the everyday way we use spatial and meaningful contexts to retrieve memories that are appropriate for that context. For example, when we arrive at work we remember the projects and meetings that are scheduled for that workday, and when we return home we remember chores and other home activities. In our model of context-guided memory, mice (Rajji et al., 2006) and rats (Komorowski et al., 2009) learn to use the current spatial context to guide memory for object-reward associations. Animals move between two environmental contexts where they are presented with a pair of objects distinguished by olfactory, visual, and tactile cues (Figure 1). In Context 1, one of the objects (A+) contains a buried reward and the other (B−) does not, whereas in Context 2, the contingency is reversed (A−/B+) regardless of the positions of the objects within each context. Normal learning in this task is hippocampal dependent (Komorowski et al., 2013; Rajji et al., 2006) and we have extended this paradigm to add, on alternative trials, two additional objects (C & D) presented under the same rules and in the same contexts (McKenzie et al., 2014). In our recent study we used multiple tetrodes to identify a large fraction of CA1/3 pyramidal cells that fire associated with multiple dimensions of events in this task (McKenzie et al., 2014).
Figure 1.
Context-guided object-association task. On each trial, rewarded (+) and nonrewarded (−) stimuli are presented in the positions shown (Pos1–4) or in the reverse positions within each context. Animals were either trained with two objects A vs B or with four (A vs B, C vs D), and then tested on both problems concurrently.
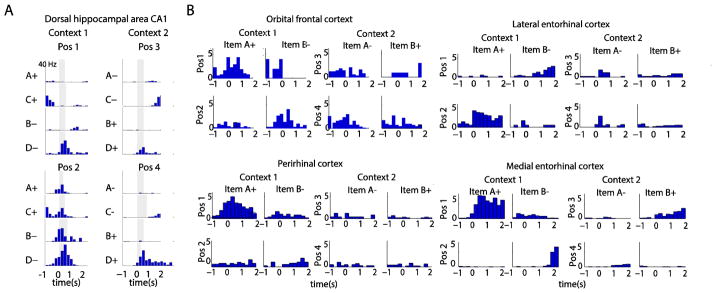
Across brain regions, single cell responses show receptive fields jointly modulated by the identity of the sampled object, the position and context occupied by the rat, and the anticipated reward potential (Figure 2). Such conjunctive mixed selectivity prevents analyses of receptive fields that rely upon an assumption of unimodal tuning on continuous axes. The heterogeneity of responses within a region and the qualitative similarity of responses across regions add to the challenge of understanding computational function based off of evoked responses. These challenges can be circumvented through the use of representational similarity analysis which compares the similarity of ensemble firing rate vectors from neurons recorded during the period of object sampling prior to the behavioral response. By considering which events evoke similar neural responses, at the population level, a hierarchy can be established amongst task dimensions that elicit similar neural activity versus those that evoke divergent responses. Such analysis reveals different organizational strategies across brain regions.
Figure 2.
Firing rate histograms illustrating example neurons with mixed selectivity in different brain regions. Each panel shows the response pattern of a neuron during the sampling of a specific object stimulus at a particular location in one of the two contexts. In each panel neural activity is time-locked to the onset of stimulus sampling (time = 0). (A) An example CA1 cell on the four object (A,B,C,D) version of the task. Gray bars indicate minimum object sampling period used in RSAs. (B) Example cortical cells recorded on the two object (A,B) version of the task.
Dorsal hippocampus
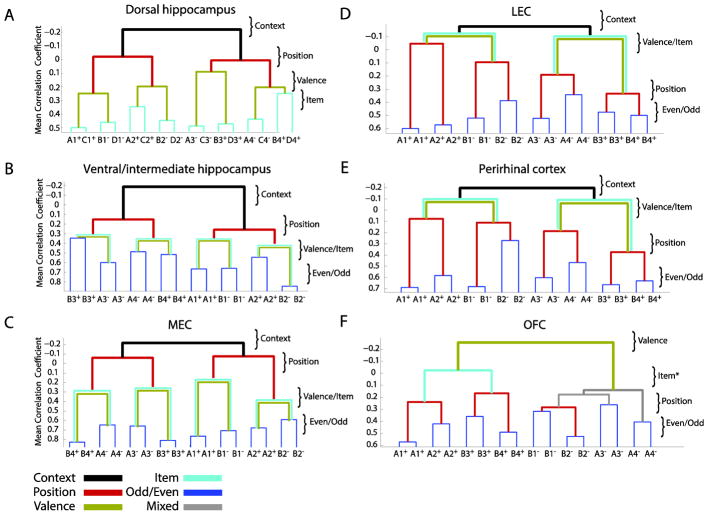
In recordings from dorsal CA1 & CA3 principal cells (Figure 3A), RSA revealed a systematic hierarchical organization of ensemble representations of distinct events - we call it a “neural schema” (McKenzie et al., 2014). Figure 3A illustrates the relationships between representations of each type of event (x-axis) as linked (y-axis) by specific task dimensions (right). At the top of this neural schema, events that occur in different contexts are widely separated in representational space (black lines), indicated by anti-correlation (mean correlation coefficient = −0.21) between events that occur in different contexts, putting context as the highest superordinate dimension. Within each context-based network, events are then separated by positions (dark red lines; mean correlation coefficient ~= 0.0) within a context (i.e., positions are subordinate to contexts. Next, events that occur within the same positions are separated according to the associated reward outcome (green lines, mean correlation coefficient ~ 0.3), i.e., reward association is subordinate to position. Finally, closest together in this neural schema are events that involve different objects that have the same reward association in the same location and context, i.e., object identity (light blue lines, mean correlation coefficient = 0.3 to 0.5) is subordinate to reward values. Two additional aspects of this neural schema are notable. First, the basic organization of the schema (except the lowest level) is fully established when animals initially learn the A vs B objects. When C vs D are learned the next day, the schema rapidly assimilates the new memories by elaborating the lowest level of the schema (McKenzie et al., 2014). Second, events that are closest together (A & C, B & D in each position) are never experienced in the same trial. Yet the proximity of their representations in the schema is likely to support strong indirect associations between these items, potentially mirroring inference and generalization properties of a schema.
Figure 3.
Dendrograms illustrating the hierarchical organization of memories across different neural schemas. X-axis indicates either 16 distinct events (A) or the even/odd trials of 8 distinct events (B–F). Lines indicate mean correlation coefficients (r) between events and clusters of events. Labels at right indicate task dimensions that correspond to levels of the schema that demarcate superordinate and subordinate dimensions that link and relate distinct memories. In the four item version of the task (Panel A), item and valence coding could be differentiated as there were pairs of items that shared the same context/reward association, unlike the two-item version of the task (Panels B–F) in which the observed coding hierarchy left some ambiguity as to which dimension drove pattern separation (except in the case of OFC). *Note that the Item coding in the OFC is not perfectly maintained across events of different valence, likely due to the more stereotyped behavior for rewarded vs unrewarded sampling events.
Notably, many previous studies have identified spatial and non-spatial coding by single hippocampal neurons (e.g., reviewed in Eichenbaum, 1999a, 2004). But these studies do not identify how these dimensions of representation are integrated to link related memories. By contrast, RSA reveals an emergent network representation of the organization of memories. Furthermore, the observations revealed through this analysis strongly support the notion that the hippocampus develops an organized representation of a network of related memories that reflects key properties of a schema, including a prominent separation of sub-schemas for each context, rapid assimilation of new memories within each sub-schema, and a network structure that would support inference between indirectly related memories.
Ventral hippocampus
In RSA analyses of neuronal firing patterns in the ventral hippocampus (Figure 3B), we observe a similar hierarchical organization as that observed in the dHPC. However, the dendrogram for the vHPC reveals that positions within a context are associated with more correlated representations than those in the dHPC (vHPC r ~ 0.2 vs dHPC r ~ 0.0), suggesting that the ventral hippocampus generalizes across specific memories that occur within the same context and, simultaneously, strongly pattern-separates events that occur between the two contexts (Komorowski et al., 2013).
Rhinal cortex
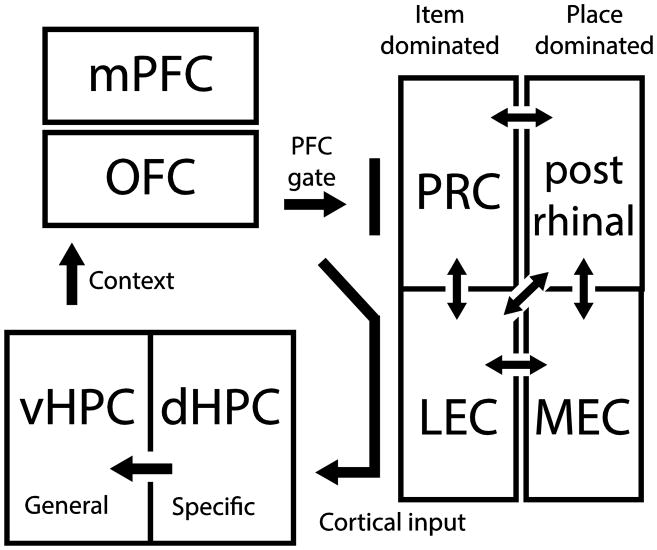
We have also used the same behavioral task to examine population firing patterns of neurons in cortical areas that interact with the hippocampus, including the lateral entorhinal cortex (LEC), medial entorhinal cortex (MEC), and perirhinal cortex (PRC). These areas are key components that are bidirectionally connected with the hippocampus in service of memory functions (Figure 4). The LEC and MEC are the end points of the classic cortical “what” and “where” streams wherein it is commonly conceived that the LEC selectively processes information about objects and events and the MEC selectively processing spatial information (Davachi, 2006; Eichenbaum et al., 2007; Ranganath, 2010). Contrary to the view that the PRC and LEC are specialized for object processing and MEC is specialized for spatial processing (Van Cauter et al., 2013; Deshmukh et al., 2012; Hargreaves et al., 2005; Henriksen et al., 2010), in animals performing our task that demands association of object with the places they are rewarded, single neurons in the PRC, LEC and MEC, including those in both superficial and deep cortical areas and in grid and border cells of MEC, have a highly similar range of selectivity to object and spatial dimensions of the task. Furthermore, contrary to the common emphasis on unidimensional spatial coding in MEC grid and border cells (Witter and Moser, 2006), we observed that grid cells, border cells, and head direction cells exhibited greater selectivity for all task dimensions, including those involving object selectivity, relative to the remaining MEC population (Keene et al., 2014).
Figure 4.
Circuit model for context-guided memory retrieval and schema formation. Cortical inputs arrive to the hippocampus via the rhinal cortices in which there are partially segregated “what” and “where” processing streams, labeled ‘item dominated’ and ‘place dominated’, respectively. In general, there is a large degree of inhibition preventing the flow of information into the hippocampus. However, when the rhinal activity is coincident with inputs from the prefrontal cortex, the ‘prefrontal gate’, information can arrive into the hippocampus and therefore shape the hippocampal memory space. In the dorsal hippocampus, the sparse representations favor representations for more specific episodes, while the denser representations observed more ventrally are more suitable for generalization – this is the context code. The strong monosynaptic connections from the ventral hippocampus to the prefrontal cortex provide a contextual signal which can then trigger activation of the context-appropriate rules for memory retrieval stored in the prefrontal cortices.
By contrast to similar mixed selectivity in single PRC, LEC and MEC neurons, RSA of population activity revealed a key distinction in the organization of information in these areas, such that LEC and PRC populations prioritized object over location information whereas MEC populations prioritized location over object information (Figure 3C,D,E). Specifically, at the top of the hierarchy for PRC, LEC and MEC, representations were strongly separated by events that occurred in different contexts, similar to the hippocampal schema. Next, however, in both PRC and LEC (Figure 3D, E), events within each context were separated by the object presented, then positions were separated within each object representation. By contrast, in MEC (Figure 3C), the second level of pattern separation is defined by events within each context that occurred in different positions. Finally, objects were separated within each position representation. In the three areas, representations of identical events are most closely associated (correlation of odd/even trials). Thus, although PRC, LEC and MEC exhibit similarity in mixed selectivity at the single neuron level, RSA reveals that information is organized in distinct but complementary ways (see also Ahn and Lee, 2015; Beer et al., 2013; Deshmukh and Knierim, 2011; Ohyama et al., 2012).
Orbitofrontal cortex
We have also examined population activity in the orbitalfrontal cortex (OFC), a prefrontal area thought to be particularly important for coding reward associations of stimuli, which are prominent in our task (Farovik et al., 2015). Like neurons in each of the medial temporal areas, single neurons in OFC also showed mixed selectivity encompassing each of the task dimensions. OFC neurons encoded the full range of dimensions that characterize each object-sampling event. Substantial proportions of the neurons fired differentially in response to object, position, or context dimensions, although showed firing that could be explained by any single dimension. Most notably, the largest proportion of OFC neurons distinguished combinations of objects and the context in which they were sampled, and the firing pattern of most of these neurons was characterized by activation during sampling of different objects in the opposing contexts, thus reflecting the common reward value of these events. However, reward associations did not fully account for differences in the activity across object-context combinations in many other cells.
RSA showed that OFC neuronal ensembles represented distinct value-based schemas, each composed of a systematic organization of the representations of objects in the contexts and positions where they were associated with reward or non-reward (Figure 3F). Events associated with opposite reward value involved strongly pattern-separated representations. This observation indicates the establishment of distinct OFC neural schemas that encode events that were associated with reward and non-reward. The separation of these schemas for opposing object-reward associations could support reduction of interference between events that share object, position, or context features but lead to opposite outcomes. Within each distinct value-based schema, objects in different contexts that predict the same outcome are differentially represented, whereas distinct objects that were not associated with reward were coded as similar across contexts, suggesting that object-context representation depended on reward status. Within each context representation, events were separated by position within the context, and finally within each position representation, events having the same object identity and value were encoded most similarly. The hierarchical coding revealed by our RSA is consistent with a mapping of contextual cues and specific stimuli to behavioral responses and the values of consequent reinforcement envisioned by Wilson et al., (2014).
This representational organization is strikingly different than the representation developed by the hippocampus in animals performing the same context-guided object association task (McKenzie et al., 2014). As described above, the hippocampal network strongly pattern separates events by the context in which they occur, thus, establishing distinct representational networks that include both rewarded and non-rewarded events that occur in different contexts. Within each distinct context-defined schema, events are separated by the locations where they occur within a context. Within representations of locations, events were separated by reward value, and within representations of reward values, events were separated by object identity. Thus, whereas the hippocampus develops a context-based schema, the OFC develops a value-based schema. The distinctive organizations of task dimensions in OFC and the hippocampus suggest commonality in the information contained in these brain areas, and complementary organization of information processing relevant to this memory task.
How are schemas composed during learning?
An influential theory of hippocampal function suggests that the hippocampus learns new associations quickly and then, through reactivations of hippocampal patterns, transmits these new associations to the cortex to be gradually woven into the stable attractor space that defines our semantic memory or schema (McClelland et al., 1995). In McClelland and colleagues’ seminal analysis, the exact mechanism of learning in the hippocampus was omitted but subsequent computational models have filled in the gaps. The common thread across this work is that the role of the hippocampus is to store representations of dense, correlated cortical inputs in independent sparse networks that can be queried with partial input to reinstate, via pattern completion, the activity that was present during initial learning (Hasselmo et al., 1996; Marr, 1971; McNaughton and Morris, 1987; McNaughton and Nadel, 1990; O’Reilly and McClelland, 1994; Treves and Rolls, 1994). In these models, pattern completion is thought to be limited to recreating a pattern of activity during encoding. Novel conjunctions of familiar elements would not associatively activate prior traces but instead create a new representation that is ideally independent of that for the originally experienced conjunctions (Kumaran and McClelland, 2012; O’Reilly and McClelland, 1994). Accordingly, these theoretical transforms on the signal within the hippocampus “by nature tend to support episodic memory at the expense of capturing the higher order structure of a set of experiences” (Kumaran and McClelland, 2012).
The observation that place cells generate independent spatial mappings in different contexts (Alme et al., 2014; Hayman et al., 2003; Kubie and Ranck, 1983; Leutgeb et al., 2004; Paz-Villagrán et al., 2004; Spiers et al., 2013) and the random nature in which new place fields are laid down within an environment (Rich et al., 2014) have been used as evidence for a random orthogonalization process. In a majority of place cell studies, a foraging task is employed in which reward is evenly distributed across the environment and behavior is randomized over space. This task guarantees that the only organizing principal of the experience is the spatial layout of the environment; the subject is tested in a low dimensional space and the data are analyzed assuming low dimensional coding.
Our findings are also at odds with key predictions of the model described above, as can be seen clearly in the hippocampal dendrogram that shows that the degree of similarity and pattern separation depends on task demands. We found that the higher order structure for a set of experiences defines the organization of the hippocampal representation, just as it does in the cortex, though different areas emphasize different dimensions of the task structure. There was no greater degree of ‘orthogonalization’ in the hippocampus as compared to upstream cortical regions, as predicted by the classic model. Instead, all medial temporal areas strongly separate representations of events in different contexts. Different medial temporal areas prioritized position and object dimensions differently, but all manifest a range of separations. Instead, the systematic hierarchical organization of memories is most striking and offers a different way to think about memory representation as organized schemas throughout this system.
These findings parallel previous observations of hippocampal neurons that link memories in humans and animals. As pointed out before (Cohen and Eichenbaum, 1993; Eichenbaum, 1999b) place cells represent the set of experiences within a particular location, which may be a particularly important feature in linking episodic memories that have shared common locations. A similar phenomenon has been observed in rats where cells fire at behaviorally equivalent positions on different arms of a maze or different mazes (Singer et al., 2010). Other striking examples of semantic representations that link specific experiences in the hippocampus comes from studies in human epileptic patients in which cells increase firing rate in response to pictures all from the same category of stimuli such as houses or faces (Kreiman et al., 2000) or, most remarkably, from cells that respond only to symbols that represent an individual, such as a cell that responded to colored photographs, line drawing, and the written name of the actor Halle Barry (Quiroga et al., 2005). Similar category cells have been reported in the monkey during a delayed matching task (Hampson et al., 2004). Our studies have shown that hippocampal system areas link memories in highly systematic organizations that are specific to each area.
The data presented here show that the hippocampal representation can reflect a highly organized hierarchical structure that matches the relationship between learned associations and the structure of representation in some cortical areas. However, the theoretical pattern separation function of the dentate and CA3 was critical in computational models of episodic memory to prevent ‘catastrophic interference’. How can the hippocampus rapidly encode episodic memories in a representational space that preserves the long-term structure that defines memory schemas?
The considerations of sparsity and density that have shaped many memory models assumed that the hippocampus would adopt the same coding principal to all stimuli. This may not the case. When rats learn to associate reward with a location, the goal locations begin to accumulate place fields (Dupret et al., 2010). Similarly, when rats stop on a track to gaze at the surroundings, a new place field is laid down (Monaco et al., 2014). This experience-dependent overrepresentation increases the density of the neural code for the associated events. A cognitive model of learning nicely illustrates the dual value of this overrepresentation. First, these overrepresentations are easier to pattern match with a subsequent repetition. Second, it is more difficult to retrieve false positives with other patterns being incorrectly mistaken for the target representation (Criss et al., 2011). Indeed, when rats learn that a location possesses a hidden reward, the representation of that location becomes decorrelated with those of the surrounding areas (McKenzie et al., 2013). The overrepresentation and conjunctive firing fields may act together to increase the dimensionality of the representational space, which allows more unique patterns to be observed, though the precise relationship between sparsity and memory capacity depends upon how the network learns and how the neural code is read out (Barak et al., 2013; Rigotti et al., 2013; Treves and Rolls, 1991; Willshaw and Dayan, 1990; Zhang and Sejnowski, 1999).
Below we present a model of how the hippocampus, rhinal cortices and prefrontal cortex might interact to bias which aspects of an experience define the representation space embodied by the interactions of these regions.
Cortical-hippocampal dialog is essential for maintaining high dimensional, multi-modal coding spaces
Our experiments have revealed a network of connected brain regions that code for overlapping events in the animal’s experience. Recent intervention studies suggest that the mixed selectivity observed in each region is supported by an anatomical loop between the prefrontal cortex, the rhinal cortices and the hippocampus. Here we review evidence for a systems-level model for context guided memory (Preston and Eichenbaum, 2013) and put forward the hypothesis that the bidirectional connections between the hippocampus and the cortex generate a high dimensional coding space tuned to the behaviorally relevant stimuli.
The model focuses on the interaction between the prefrontal cortex, the rhinal cortices and the hippocampus (Figure 4). Most anatomical studies have shown that the prefrontal cortex does not project directly to the hippocampus, but instead projects indirectly either through the rhinal cortices or the nucleus reuniens (Cenquizca and Swanson, 2007; Herkenham, 1978; Swanson and Cowan, 1977; Varela et al., 2014; Witter and Amaral, 2004), however, a recent report shows that such a connection may exist (Rajasethupathy et al., 2015). Our model focuses on the well-established indirection prefrontal cortex to hippocampus connection. The dorsal hippocampus also lacks direct connections to the prefrontal cortex, but can influence prefrontal activity through the ventral hippocampus which does show strong monosynaptic efferents (Burwell and Witter, 2002; Verwer et al., 1997).
The multimodal, conjunctive properties of the dorsal hippocampus depend upon integrity of the prefrontal and rhinal cortices. When rats were trained on the context guided memory task described above, muscimol inactivation of the medial prefrontal cortex (mPFC) caused a decrease in the degree to which objects could influence firing rates, though place fields remained intact (Navawongse and Eichenbaum, 2013). The loss of the object coding dimension of the representational space should cause deficits in segregating memories that depend on object recognition. Indeed, this is exactly what we found, as task performance was significantly impaired after mPFC inactivation. Over generalization was also observed in a contextual fear paradigm after mPFC projections were silenced at the level of the nucleus reuniens (Xu and Südhof, 2013). In another study, silencing of the nucleus reuniens reduced a place-by-trajectory conjunctive code in CA1 (Ito et al., 2015), further showing a necessary role for prefrontal-hippocampal interactions in maintaining a high dimensional code that depends on these conjunctive firing properties.
Although these recent reports show a clear role for the nucleus reuniens in coordinating a hippocampal-prefrontal network, we emphasize the role of the rhinal cortices in mediating this interaction due to the size of the reuniens. The small number of neurons in this nucleus puts a limit on the number of possible discernable states and therefore places a bottleneck on how the mPFC could drive pattern separation in the hippocampus. Therefore, our model instead focuses on the role of the rhinal cortices.
There is accumulating evidence that the rhinal cortices play this intermediary role. For example, the conjunctive code between room color and geometry found in area CA3 has been shown to depend upon LEC, as lesions to this region diminish ensemble discrimination of testing chambers that differ solely by color (Lu et al., 2013). In another study, cells in dorsal CA1 developed a conjunctive odor and place code alongside those recorded LEC which coordinated with one another on a fast (beta rhythm) time scale (Igarashi et al., 2014) that is a hallmark of inter-region communication (Rangel and Eichenbaum, 2014). In our studies, muscimol inactivation of MEC did not disrupt item coding (Navawongse and Eichenbaum, 2013), consistent with the stronger item coding seen in the LEC (Deshmukh and Knierim, 2011; Tsao et al., 2013; Figure 3C,D). Perirhinal inactivation, on the other hand, has been shown to disrupt CA1 object coding in an object-place association task (Lee and Park, 2013), suggesting a prefrontal-perirhinal-LEC-dHPC network in creating a conjunctive code for item and place in the hippocampus.
It is notable that position coding in the hippocampus is relatively intact after disrupted processing in MEC, LEC, PRC, the prefrontal cortex, and many other regions (Brun et al., 2008; Calton et al., 2003; Van Cauter et al., 2008; Hales et al., 2014; Hok et al., 2013; Kyd and Bilkey, 2005; Lu et al., 2013; Miller and Best, 1980; Navawongse and Eichenbaum, 2013; Suh et al., 2011). The redundant information about space observed across several regions (Figure 3) appears sufficient to drive location specific firing in the dorsal hippocampus. The necessary MEC contribution to the nature of the hippocampal representation remains somewhat of a mystery, though recent evidence suggests a role in establishing temporal relationships amongst hippocampal cells (Schlesiger et al., 2015). The two regions show an impressively similar coding hierarchy for which information drives ensemble similarity, yet MEC lesions do not disrupt the ability of the hippocampus to encode places (Brandon et al., 2014; Van Cauter et al., 2008; Hales et al., 2014; Miller and Best, 1980; Navawongse and Eichenbaum, 2013) or objects that occur in those places (Navawongse and Eichenbaum, 2013). Changes in MEC activity, either endogenous (Fyhn et al., 2007) or experimental (Hales et al., 2014; Navawongse and Eichenbaum, 2013; Rueckemann et al., 2015), reliably cause reconfigurations of the hippocampal firing properties (remapping) with preserved information coded by a different set of cells. This reconfiguration suggests that the MEC provides a broader contextual signal (Eichenbaum and Lipton, 2008) that defines the space of representations with which to encode the specifics of a new memory. This contextual signal may be detected by the similarity analyses outlined here.
As described above, a fundamental difference in coding between the dorsal and ventral hippocampus is the density of the code, which manifests as larger place fields in the ventral hippocampus. It remains to be seen whether coding of non-spatial information shows the same shift in coding level. A computational benefit of a denser code is better generalization between related cases for a downstream reader (Barak et al., 2013; Keinath et al., 2014). This implies that, when moving down the longitudinal axis of the hippocampus, there is a systematic shift in the generalization gradient (not the spatial resolution), from narrow to broad. This shift in generalization constitutes the contextual code where, in the spatial domain, places represented by correlated inputs can easily be mapped onto common downstream patterns. This same argument could be applied to the grid cell code providing the hippocampus with an evenly sampled generalization signal across a given context. Note that the generalization gradient does not mean that the correlated vHPC patterns must be mapped to a common output pattern, as shown by the Bayesian decoding results (Keinath et al., 2014).
The context signal that is available to the mPFC can be used as cue for recalling appropriate rules. It is commonly believed that the ‘executive’ functions of the PFC derive from its role as a top down hub (Cole et al., 2012; van den Heuvel and Sporns, 2013). In this capacity, the mPFC is thought to sustain the coordinated activity of multiple separate brain areas that are not strongly interconnected (Miller and Cohen, 2001). Here, we propose that the contextual signal from the vHPC activates mPFC ensembles that are associated with a specific cortico-cortico network – this association is the context dependent rule. This prefrontal signal can also act to bias specific hippocampal representations through the rhinal cortices as described above (Figure 4).
In support of this idea, recent experiments have shown the coordination of prefrontal ensembles to the hippocampal theta rhythm as rats learn a set shifting task (Benchenane et al., 2010). Other studies have also shown coordination of rhythms between the regions that tracks learning in rats (Fujisawa et al., 2008; Hyman et al., 2005; Spellman et al., 2015) and monkeys (Brincat and Miller, 2015) that depend upon the integrity of the ventral hippocampus (O’Neill et al., 2013). Furthermore, silencing vHPC terminals to mPFC disrupts spatial working memory and the ability to decode the location of a goal from mPFC ensemble activity, though other, non-spatial, aspects of the mPFC conjunctive code remain intact (Spellman et al., 2015). Therefore, aspects of the mixed selectivity of prefrontal cells are dependent upon hippocampal function.
The hippocampus is often seen as a passive recorder of all attended life events (Morris, 2006). However, salient events define memory more than the inconsequential ones (Dunsmoor et al., 2015; Lisman and Grace, 2005; Morris, 2006; Westmacott and Moscovitch, 2003). Just as the vHPC may select the appropriate rules in the mPFC, there may be a similar operation occurring in prefrontal areas that track valence, such as the OFC. A series of elegant studies in vitro provide an interesting system’s level hypothesis for how the OFC could interact with the perirhinal cortex to gate information flow into the hippocampus. These studies show that stimulating the perirhinal cortex is insufficient to cause propagation of activity into the entorhinal cortex and the hippocampus due to the high degree of inhibition at the rhinal sulcus (de Curtis and Paré, 2004; Kajiwara et al., 2003). These results suggest a large degree of information filtering takes places in the rhinal cortices. However, when perirhinal stimulation was paired with coincident activity of the amygdala, activity spread to the entorhinal cortex and the dentate (Iijima et al., 1996; Kajiwara et al., 2003; Koganezawa et al., 2008). Similarly, spike transmission efficacy in vivo increases between perirhinal and entorhinal cortex when spikes are also observed in the mPFC or amygdala (Paz et al., 2006, 2007, 2009). These results suggest that salience is signaled by the amygdala, and potentially by the OFC, to gate the passage of sensory information in the entorhinal cortex and hippocampus. We propose that the strong value coding seen in our OFC study gates information flow into the hippocampus via its connectivity with the rhinal cortices and the perirhinal cortex in particular.
It is noteworthy that communication between brain regions is often associated with synchrony of brain rhythms at various frequencies (Benchenane et al., 2010; Dickson and de Curtis, 2002; Engel et al., 2001; Fries, 2005; Igarashi et al., 2014; Scheffzük et al., 2011). A separate body of work has showed that such slower timescale synchrony, when observed, is associated with a fast-timescale decorrelation of local activity (Mizuseki and Buzsaki, 2014; Renart et al., 2010; Tan et al., 2014). Theoretically, such network decorrelation has been related to expansions in representational capacity (Hennequin et al., 2014; Kumar et al., 2008; Sompolinsky et al., 2001; Wang et al., 2004), and may be a signature of transient increases in the dimensionality of the coding space. In several studies the synchrony of brain rhythms across regions has been directly related to the formation of mixed selectivities (Igarashi et al., 2014; Ito et al., 2015; Tort et al., 2009) suggesting a close relationship between inter-regional communication and an increase in coding capacity.
Gating of information allows the dimensionality of the hippocampal space to depend upon the behaviorally relevant stimuli. According to this view, ensembles pattern-separate similar stimuli during learning when rewards depend upon discriminating previously neutral stimuli (e.g. Tort et al., 2011). The increased pattern separation may reflect the emergence of a new organization of information. It is important to keep in mind that no two learning trials are identical, so a subject must learn the necessary and sufficient features of a trial that constitute a linked set of memories that predict a reliable outcome. Separations may be supported by cells that discriminate the dimensions of an experience that differentially predict reward, or other differences and commonalities of individual events that have significance. Indeed, cortical representation of objects have been shown to change according to the dimensions in which they are categorized (De Baene et al., 2008; Engel et al., 2001; Goldstone et al., 2001; Sigala and Logothetis, 2002), consistent with modification of a neural schema to reflect new perceptual demands (Meeter et al., 2009).
According to our model, the prefrontal cortex biases retrieval of the context-appropriate memory representations in the dorsal hippocampus (Navawongse and Eichenbaum, 2013) through its action on the upstream cortical areas. Appropriate retrieval in the hippocampus is accomplished by selective disinhibition of representations that are appropriate for the current context, consistent with the finding that the PRC influences LEC via inhibitory regulation that is modulated by the prefrontal cortex (de Curtis and Paré, 2004). Thus the OFC and PRC together may support cognitive control over memory retrieval by biasing the selection of context-based schemas and through inhibition of competing schemas in other medial temporal structures (Bunce et al., 2013; Fernández and Tendolkar, 2006).
These considerations do not resolve how prefrontal mechanisms influence processing in the entorhinal cortex and hippocampus, but put the focus on interactions between very different organizations of information in the OFC and perirhinal and lateral entorhinal cortex as a strong candidate for where the critical interactions occur. Future studies that examine the simultaneous activity patterns, along with potentially mediating oscillatory synchronization, during the course of schema formation may offer new insights about how these areas and their representations interact.
Future Directions
We have emphasized the importance of probing neural representations using complex tasks and analyses suited to multidimensional coding spaces. This line of work is in its infancy and many basic questions remain.
The analyses described throughout this paper have been focused on the rate code, however, it is known that the relative timing between cells conveys information in several brain systems (Malhotra et al., 2012; Montemurro et al., 2008; O’Keefe and Recce, 1993; Panzeri et al., 2001; Saitoh and Suga, 1995). In our representational similarity analyses, we ignored this temporal code and it is possible that patterns that are considered similar by a rate analysis can be highly decorrelated if cells are sensitive to the permutations of cell activity in addition to the combinations that we have considered (Branco et al., 2010). The only way to address this issue is to test the sensitivity of postsynaptic areas to temporal permutations in cell activity, as has been done in the olfactory system (Haddad et al., 2013).
Another limitation to the RSA presented here is that there is no account of trial-to-trial variability. This variability may be particularly important in the hippocampus which is known to support episodic memory – memory for events that happen once. Our previous similarity analyses revealed substantial trial-to-trial differences in coding strength by using the instantaneous firing rates observed on each trail rather than the trial-averaged mean firing rate vectors (McKenzie et al., 2014). Dendrogram analysis requires large simultaneous ensembles to ensure adequate coverage of the representational space by the sampled ensemble, though with sufficient sampling, this too can be done on a trial-to-trial basis and statistical methods exist for assessing the reliability of the clustering structure (Park et al., 2009). The more important issue missed in RSA, however, relates to the underlying assumption that neurons encode stimuli by firing at a particular rate and variations from that set-point reflect noise. The possibility that pair-wise (or higher order) ‘noise’ correlations may provide information, as discussed earlier, is one violation of this implicit assumption. Additionally, there is a growing body of work showing the firing variability may reflect uncertainty. Under this interpretation, the instantaneous firing rate is a consequence of what is present and the probability of that presence (Fiser et al., 2010; Ma et al., 2006). This is an important departure from the classic coding perspective embodied by the RSA analysis which assumes that rate is an estimate of some world variable and not the likelihood of that estimate (Ma et al., 2008). The extension of this probabilistic view point to RSA requires storage of the representational organizations, as operationalized as the correlation structure of a set of population rate vectors, and the probability of observing such correlation structures. A recent theoretical paper has proposed just such an approach (Johnson et al., 2012), though empirical validation, and a mechanistic description for how such probability structures become stored is lacking.
Electrophysiology places limitations on the ability to track the same network over many days. New calcium imaging technology (Ghosh et al., 2011) now enables the observation of the full construction of a memory schema from the naïve state to the highly organized memory spaces that we observed. These studies will tell us whether the same cells remain involved in the memory representation over long periods of time. Recent studies have emphasized the transient nature of the hippocampal representation (Attardo et al., 2015; Ziv et al., 2013). However, we (Komorowski et al., 2009; McKenzie et al., 2014), and others (Hattori et al., 2015), have observed long term stability in the rules that dictate network organization. How do these rules get passed from one group of cells to another? One exciting possibility is that multiple high dimensional ensemble representations can be mapped onto a common lower dimensional space that embodies the rules carried from one ensemble to the next.
Our observation of coding of both objects and places in both PRC-LEC and MEC challenges the conventional model where separate object and place pathways converge only within the hippocampus to support conjunctive coding (Eichenbaum et al., 2007). Furthermore, the consistent failure to observe long term place field deficits following MEC lesions or inactivations (Brandon et al., 2014; Van Cauter et al., 2008; Hales et al., 2014; Miller and Best, 1980; Navawongse and Eichenbaum, 2013; Rueckemann et al., 2015; Schmidt et al., 2013) is also contrary to predictions that grid cells are required to form place cells (Moser et al., 2008). It is possible that the multidimensional approach laid out here may reveal the essential contribution of MEC input to hippocampal function, perhaps in the coding of context.
Our studies have shown that, over the course of seconds, there can be shifts in the dominance of place, object, and reward coding (McKenzie et al., 2014). Our context guided memory model (Figure 4) predicts that the shifts in coding dominance observed in the hippocampus is due to OFC or mPFC gating different afferents to the hippocampus at different times. Simultaneous, large-scale recordings between these brain areas may reveal evidence for this gating process (Paz et al., 2009) which may be evidenced by searching for moments of inter-regional oscillatory synchrony (Bieri et al., 2014; Fernández-Ruiz et al., 2012). More generally, it remains an open question as to what determines the hierarchy in the coding regimes that we have observed in each of the brain areas studied.
Conclusion
A major concern in research on the hippocampus and related cortical areas is how to reconcile the role of these areas in spatial mapping versus memory. Here we show that, in situations where memories involve both spatial and non-spatial information, the hippocampus, as well as a set of interconnected cortical areas, systematically organizes all of the information incorporated into the relevant memories. Within the closely interconnected hippocampal and entorhinal areas, ensemble representations reflect a separation of schemas based on spatially and meaningfully distinct contexts, wherein spatial and non-spatial information are integrated. This observation suggests that the prominent role of space in hippocampal neural activity should be viewed as one of several organizing dimension of events. Furthermore, our findings on the orbitofrontal cortex suggest that other cortical areas may strongly influence the memory schemas within the hippocampus, likely controlling both the establishment of the organization of memories and the retrieval of appropriate memories that are relevant in different contexts. While much remains to be revealed about the mechanisms of cortical-hippocampal interactions, the findings discussed here lead us towards a new understanding of how this brain system supports our capacity for memory.
Highlights.
Here we consider the value of neural population analysis as an approach to understanding how information is represented in the hippocampus and cortical areas.
We argue that population analyses of neurons with denser coding and mixed selectivity reveal new and important insights into the organization of memories.
Furthermore, comparisons of the organization of information in interconnected areas suggest a model of hippocampal-cortical interactions that mediates the fundamental features of memory
Acknowledgments
NIH MH094263, MH51570, MH052090, and MH107159
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott LF, Dayan P. The effect of correlated variability on the accuracy of a population code. Neural Comput. 1999;11:91–101. doi: 10.1162/089976699300016827. [DOI] [PubMed] [Google Scholar]
- Adrian ED, Zotterman Y. The impulses produced by sensory nerve-endings: Part II. The response of a Single End-Organ. J Physiol. 1926;61:151–171. doi: 10.1113/jphysiol.1926.sp002281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn JR, Lee I. Neural Correlates of Object-Associated Choice Behavior in the Perirhinal Cortex of Rats. J Neurosci. 2015;35:1692–1705. doi: 10.1523/JNEUROSCI.3160-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alme CB, Miao C, Jezek K, Treves A, Moser EI, Moser MB. Place cells in the hippocampus: Eleven maps for eleven rooms. Proc Natl Acad Sci. 2014;111:201421056. doi: 10.1073/pnas.1421056111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amari S. Characteristics of sparsely encoded associative memory. Neural Networks. 1989;2:451–457. [Google Scholar]
- Attardo A, Fitzgerald JE, Schnitzer MJ. Impermanence of dendritic spines in live adult CA1 hippocampus. Nature. 2015;523:592–596. doi: 10.1038/nature14467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averbeck BB, Lee D. Neural noise and movement-related codes in the macaque supplementary motor area. J Neurosci. 2003;23:7630–7641. doi: 10.1523/JNEUROSCI.23-20-07630.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averbeck BB, Lee D. Coding and transmission of information by neural ensembles. Trends Neurosci. 2004;27:225–230. doi: 10.1016/j.tins.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Averbeck BB, Lee D. Effects of noise correlations on information encoding and decoding. J Neurophysiol. 2006;95:3633–3644. doi: 10.1152/jn.00919.2005. [DOI] [PubMed] [Google Scholar]
- Averbeck BB, Latham PE, Pouget A. Neural correlations, population coding and computation. Nat Rev Neurosci. 2006;7:358–366. doi: 10.1038/nrn1888. [DOI] [PubMed] [Google Scholar]
- De Baene W, Ons B, Wagemans J, Vogels R. Effects of category learning on the stimulus selectivity of macaque inferior temporal neurons. Learn Mem. 2008;15:717–727. doi: 10.1101/lm.1040508. [DOI] [PubMed] [Google Scholar]
- Barak O, Rigotti M, Fusi S. The Sparseness of Mixed Selectivity Neurons Controls the Generalization-Discrimination Trade-Off. J Neurosci. 2013;33:3844–3856. doi: 10.1523/JNEUROSCI.2753-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. Possible principles underlying the transformations of sensory messages. 1961:217–234. [Google Scholar]
- Barlow HB. Single units and sensation: A neuron doctrine for perceptual psychology? Perception. 1972;1:371–394. doi: 10.1068/p010371. [DOI] [PubMed] [Google Scholar]
- Barry C, Burgess N. Neural mechanisms of self-location. Curr Biol. 2014;24:R330–R339. doi: 10.1016/j.cub.2014.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer Z, Chwiesko C, Kitsukawa T, Sauvage MM. Spatial and stimulus-type tuning in the LEC, MEC, POR, PrC, CA1, and CA3 during spontaneous item recognition memory. Hippocampus. 2013;23:1425–1438. doi: 10.1002/hipo.22195. [DOI] [PubMed] [Google Scholar]
- Benchenane K, Peyrache A, Khamassi M, Tierney PL, Gioanni Y, Battaglia FP, Wiener SI. Coherent theta oscillations and reorganization of spike timing in the hippocampal- prefrontal network upon learning. Neuron. 2010;66:921–936. doi: 10.1016/j.neuron.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Bieri KW, Bobbitt KN, Colgin LL. Slow and fast gamma rhythms coordinate different spatial coding modes in hippocampal place cells. Neuron. 2014;82:670–681. doi: 10.1016/j.neuron.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branco T, Clark BA, Häusser M. Dendritic discrimination of temporal input sequences in cortical neurons. Science. 2010;329:1671–1675. doi: 10.1126/science.1189664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon MP, Koenig J, Leutgeb JK, Leutgeb S. New and distinct hippocampal place codes are generated in a new environment during septal inactivation. Neuron. 2014;82:789–796. doi: 10.1016/j.neuron.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brincat SL, Miller EK. Frequency-specific hippocampal-prefrontal interactions during associative learning. Nat Neurosci. 2015;18:576–581. doi: 10.1038/nn.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EN, Kass RE, Mitra PP. Multiple neural spike train data analysis: state-of-the-art and future challenges. Nat Neurosci. 2004;7:456–461. doi: 10.1038/nn1228. [DOI] [PubMed] [Google Scholar]
- Brun VH, Leutgeb S, Wu HQ, Schwarcz R, Witter MP, Moser EI, Moser MB. Impaired spatial representation in CA1 after lesion of direct input from entorhinal cortex. Neuron. 2008;57:290–302. doi: 10.1016/j.neuron.2007.11.034. [DOI] [PubMed] [Google Scholar]
- Buhmann J, Divko R, Schulten K. Associative memory with high information content. Phys Rev A. 1989;39:2689–2692. doi: 10.1103/physreva.39.2689. [DOI] [PubMed] [Google Scholar]
- Bunce JG, Zikopoulos B, Feinberg M, Barbas H. Parallel prefrontal pathways reach distinct excitatory and inhibitory systems in memory-related rhinal cortices. J Comp Neurol. 2013;521:4260–4283. doi: 10.1002/cne.23413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burwell R, Witter M. Basic anatomy of the parahippocampal region in monkeys and rats 2002 [Google Scholar]
- Calton JL, Stackman RW, Goodridge JP, Archey WB, Dudchenko PA, Taube JS. Hippocampal place cell instability after lesions of the head direction cell network. J Neurosci. 2003;23:9719–9731. doi: 10.1523/JNEUROSCI.23-30-09719.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carandini M, Heeger DJ. Normalization as a canonical neural computation. Nat Rev Neurosci. 2012;13:51–62. doi: 10.1038/nrn3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cauter T, Poucet B, Save E. Unstable CA1 place cell representation in rats with entorhinal cortex lesions. Eur J Neurosci. 2008;27:1933–1946. doi: 10.1111/j.1460-9568.2008.06158.x. [DOI] [PubMed] [Google Scholar]
- Van Cauter T, Camon J, Alvernhe A, Elduayen C, Sargolini F, Save E. Distinct roles of medial and lateral entorhinal cortex in spatial cognition. Cereb Cortex. 2013;23:451–459. doi: 10.1093/cercor/bhs033. [DOI] [PubMed] [Google Scholar]
- Cenquizca LA, Swanson LW. Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain Res Rev. 2007;56:1–26. doi: 10.1016/j.brainresrev.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen NJ, Eichenbaum HB. Memory, amnesia, and the hippocampal system. Cambridge, MA: The MIT Press; 1993. [Google Scholar]
- Cole MW, Yarkoni T, Repovs G, Anticevic A, Braver TS. Global connectivity of prefrontal cortex predicts cognitive control and intelligence. J Neurosci. 2012;32:8988–8999. doi: 10.1523/JNEUROSCI.0536-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criss AH, Malmberg KJ, Shiffrin RM. Output interference in recognition memory. J Mem Lang. 2011;64:316–326. [Google Scholar]
- De Curtis M, Paré D. The rhinal cortices: a wall of inhibition between the neocortex and the hippocampus. Prog Neurobiol. 2004;74:101–110. doi: 10.1016/j.pneurobio.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Curr Opin Neurobiol. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Deshmukh SS, Knierim JJ. Representation of non-spatial and spatial information in the lateral entorhinal cortex. Front Behav Neurosci. 2011;5:69. doi: 10.3389/fnbeh.2011.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh SS, Johnson JL, Knierim JJ. Perirhinal cortex represents nonspatial, but not spatial, information in rats foraging in the presence of objects: comparison with lateral entorhinal cortex. Hippocampus. 2012;22:2045–2058. doi: 10.1002/hipo.22046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deweese MR, Zador AM. Shared and private variability in the auditory cortex. J Neurophysiol. 2004;92:1840–1855. doi: 10.1152/jn.00197.2004. [DOI] [PubMed] [Google Scholar]
- Dickson CT, de Curtis M. Enhancement of temporal and spatial synchronization of entorhinal gamma activity by phase reset. Hippocampus. 2002;12:447–456. doi: 10.1002/hipo.10013. [DOI] [PubMed] [Google Scholar]
- Dunsmoor JE, Murty VP, Davachi L, Phelps EA. Emotional learning selectively and retroactively strengthens memories for related events. Nature. 2015;520:345–348. doi: 10.1038/nature14106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupret D, O’Neill J, Pleydell-Bouverie B, Csicsvari J. The reorganization and reactivation of hippocampal maps predict spatial memory performance. Nat Neurosci. 2010;13:995–1002. doi: 10.1038/nn.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. The hippocampus and mechanisms of declarative memory. Behav Brain Res. 1999a;103:123–133. doi: 10.1016/s0166-4328(99)00044-3. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. The hippocampus and mechanisms of declarative memory. Behav Brain Res. 1999b;103:123–133. doi: 10.1016/s0166-4328(99)00044-3. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. Hippocampus : Cognitive Processes and Neural Representations that Underlie Declarative Memory The hippocampus serves a critical role in declarative. Neuron. 2004;44:109–120. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. Can We Reconcile the Declarative Memory and Spatial Navigation Views on Hippocampal Function? Neuron. 2014;83:764–770. doi: 10.1016/j.neuron.2014.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Lipton PA. Towards a functional organization of the medial temporal lobe memory system: role of the parahippocampal and medial entorhinal cortical areas. Hippocampus. 2008;18:1314–1324. doi: 10.1002/hipo.20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel AK, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci. 2001;2:704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- Ergorul C, Eichenbaum H. The hippocampus and memory for “what,” “where,” and “when”. Learn Mem. 2004;11:397–405. doi: 10.1101/lm.73304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farovik A, Place RJ, McKenzie S, Porter B, Munro CE, Eichenbaum H. Orbitofrontal Cortex Encodes Memories within Value-Based Schemas and Represents Contexts That Guide Memory Retrieval. J Neurosci. 2015;35:8333–8344. doi: 10.1523/JNEUROSCI.0134-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez G, Tendolkar I. The rhinal cortex: “gatekeeper” of the declarative memory system. Trends Cogn Sci. 2006;10:358–362. doi: 10.1016/j.tics.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Fernández-Ruiz A, Makarov VA, Benito N, Herreras O. Schaffer-specific local field potentials reflect discrete excitatory events at gamma frequency that may fire postsynaptic hippocampal CA1 units. J Neurosci. 2012;32:5165–5176. doi: 10.1523/JNEUROSCI.4499-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferster D, Spruston N. Cracking the Neuronal Code. Science (80-) 1995;270:756–757. doi: 10.1126/science.270.5237.756. [DOI] [PubMed] [Google Scholar]
- Fiete IR, Burak Y, Brookings T. What grid cells convey about rat location. J Neurosci. 2008;28:6858–6871. doi: 10.1523/JNEUROSCI.5684-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiser J, Berkes P, Orbán G, Lengyel M. Statistically optimal perception and learning: from behavior to neural representations. Trends Cogn Sci. 2010;14:119–130. doi: 10.1016/j.tics.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9:474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Fujisawa S, Amarasingham A, Harrison MT, Buzsáki G. Behavior-dependent short-term assembly dynamics in the medial prefrontal cortex. Nat Neurosci. 2008;11:823–833. doi: 10.1038/nn.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyhn M, Hafting T, Treves A, Moser MB, Moser EI. Hippocampal remapping and grid realignment in entorhinal cortex. Nature. 2007;446:190–194. doi: 10.1038/nature05601. [DOI] [PubMed] [Google Scholar]
- Gaffan D. Dissociated effects of perirhinal cortex ablation, fornix transection and amygdalectomy: evidence for multiple memory systems in the primate temporal lobe. Exp Brain Res. 1994;99 doi: 10.1007/BF00228977. [DOI] [PubMed] [Google Scholar]
- Georgopoulos A, Schwartz A, Kettner R. Neuronal population coding of movement direction. Science (80-) 1986;233:1416–1419. doi: 10.1126/science.3749885. [DOI] [PubMed] [Google Scholar]
- Ghosh KK, Burns LD, Cocker ED, Nimmerjahn A, Ziv Y, El Gamal A, Schnitzer MJ. Miniaturized integration of a fluorescence microscope. Nat Methods. 2011;8:871–878. doi: 10.1038/nmeth.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstone RL, Lippa Y, Shiffrin RM. Altering object representations through category learning. Cognition. 2001;78:27–43. doi: 10.1016/s0010-0277(00)00099-8. [DOI] [PubMed] [Google Scholar]
- Graham B, Willshaw D. Capacity and information efficiency of the associative net 2009 [Google Scholar]
- Haberly LB. Parallel-distributed processing in olfactory cortex: new insights from morphological and physiological analysis of neuronal circuitry. Chem Senses. 2001;26:551–576. doi: 10.1093/chemse/26.5.551. [DOI] [PubMed] [Google Scholar]
- Haddad R, Lanjuin A, Madisen L, Zeng H, Murthy VN, Uchida N. Olfactory cortical neurons read out a relative time code in the olfactory bulb. Nat Neurosci. 2013;16:949–957. doi: 10.1038/nn.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafting T, Fyhn M, Molden S, Moser MB, Moser EI. Microstructure of a spatial map in the entorhinal cortex. Nature. 2005;436:801–806. doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- Hales JB, Schlesiger MI, Leutgeb JK, Squire LR, Leutgeb S, Clark RE. Medial entorhinal cortex lesions only partially disrupt hippocampal place cells and hippocampus-dependent place memory. Cell Rep. 2014;9:893–901. doi: 10.1016/j.celrep.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson RE, Pons TP, Stanford TR, Deadwyler SA. Categorization in the monkey hippocampus: a possible mechanism for encoding information into memory. Proc Natl Acad Sci U S A. 2004;101:3184–3189. doi: 10.1073/pnas.0400162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves EL, Rao G, Lee I, Knierim JJ. Major dissociation between medial and lateral entorhinal input to dorsal hippocampus. Science. 2005;308:1792–1794. doi: 10.1126/science.1110449. [DOI] [PubMed] [Google Scholar]
- Harris KD, Csicsvari J, Hirase H, Dragoi G. Organization of cell assemblies in the hippocampus. Nature. 2003;424:552–556. doi: 10.1038/nature01834. [DOI] [PubMed] [Google Scholar]
- Hartley T, Lever C, Burgess N, O’Keefe J. Space in the brain: how the hippocampal formation supports spatial cognition. Philos Trans R Soc Lond B Biol Sci. 2014;369:20120510. doi: 10.1098/rstb.2012.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Wyble BP, Wallenstein GV. Encoding and retrieval of episodic memories: role of cholinergic and GABAergic modulation in the hippocampus. Hippocampus. 1996;6:693–708. doi: 10.1002/(SICI)1098-1063(1996)6:6<693::AID-HIPO12>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Hattori S, Chen L, Weiss C, Disterhoft JF. Robust hippocampal responsivity during retrieval of consolidated associative memory. Hippocampus. 2015;25:655–669. doi: 10.1002/hipo.22401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Connolly AC, Guntupalli JS. Decoding Neural Representational Spaces Using Multivariate Pattern Analysis. Annu Rev Neurosci. 2014 doi: 10.1146/annurev-neuro-062012-170325. [DOI] [PubMed] [Google Scholar]
- Hayman RMA, Chakraborty S, Anderson MI, Jeffery KJ. Context-specific acquisition of location discrimination by hippocampal place cells. Eur J Neurosci. 2003;18:2825–2834. doi: 10.1111/j.1460-9568.2003.03035.x. [DOI] [PubMed] [Google Scholar]
- Hennequin G, Vogels TP, Gerstner W. Optimal control of transient dynamics in balanced networks supports generation of complex movements. Neuron. 2014;82:1394–1406. doi: 10.1016/j.neuron.2014.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen EJ, Colgin LL, Barnes CA, Witter MP, Moser MB, Moser EI. Spatial Representation along the Proximodistal Axis of CA1. Neuron. 2010;68:127–137. doi: 10.1016/j.neuron.2010.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M. The connections of the nucleus reuniens thalami: evidence for a direct thalamo-hippocampal pathway in the rat. J Comp Neurol. 1978;177:589–610. doi: 10.1002/cne.901770405. [DOI] [PubMed] [Google Scholar]
- Van den Heuvel MP, Sporns O. Network hubs in the human brain. Trends Cogn Sci. 2013;17:683–696. doi: 10.1016/j.tics.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Histed MH, Maunsell JHR. Cortical neural populations can guide behavior by integrating inputs linearly, independent of synchrony. Proc Natl Acad Sci U S A. 2014;111:E178–E187. doi: 10.1073/pnas.1318750111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hok V, Chah E, Save E, Poucet B. Prefrontal cortex focally modulates hippocampal place cell firing patterns. J Neurosci. 2013;33:3443–3451. doi: 10.1523/JNEUROSCI.3427-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields of single neurones in the cat’s striate cortex. J Physiol. 1959;148:574–591. doi: 10.1113/jphysiol.1959.sp006308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. J Physiol. 1962;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman JM, Zilli EA, Paley AM, Hasselmo ME. Medial prefrontal cortex cells show dynamic modulation with the hippocampal theta rhythm dependent on behavior. Hippocampus. 2005;15:739–749. doi: 10.1002/hipo.20106. [DOI] [PubMed] [Google Scholar]
- Igarashi KM, Lu L, Colgin LL, Moser MB, Moser EI. Coordination of entorhinal-hippocampal ensemble activity during associative learning. Nature. 2014;510:143–147. doi: 10.1038/nature13162. [DOI] [PubMed] [Google Scholar]
- Iijima T, Witter MP, Ichikawa M, Tominaga T, Kajiwara R, Matsumoto G. Entorhinal-hippocampal interactions revealed by real-time imaging. Science. 1996;272:1176–1179. doi: 10.1126/science.272.5265.1176. [DOI] [PubMed] [Google Scholar]
- Ito HT, Zhang SJ, Witter MP, Moser EI, Moser MB. A prefrontal thalamo hippocampal circuit for goal-directed spatial navigation. Nature. 2015;522:50–55. doi: 10.1038/nature14396. [DOI] [PubMed] [Google Scholar]
- Johnson KO. Sensory discrimination: decision process. J Neurophysiol. 1980;43:1771–1792. doi: 10.1152/jn.1980.43.6.1771. [DOI] [PubMed] [Google Scholar]
- Johnson A, Varberg Z, Benhardus J, Maahs A, Schrater P. The hippocampus and exploration: dynamically evolving behavior and neural representations. Front Hum Neurosci. 2012;6:216. doi: 10.3389/fnhum.2012.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung MW, Wiener SI, McNaughton BL. Comparison of spatial firing characteristics of units in dorsal and ventral hippocampus of the rat. J Neurosci. 1994;14:7347–7356. doi: 10.1523/JNEUROSCI.14-12-07347.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiwara R, Takashima I, Mimura Y, Witter MP, Iijima T. Amygdala input promotes spread of excitatory neural activity from perirhinal cortex to the entorhinal-hippocampal circuit. J Neurophysiol. 2003;89:2176–2184. doi: 10.1152/jn.01033.2002. [DOI] [PubMed] [Google Scholar]
- Keene C, Bladon JH, McKenzie S, Eichenbaum H. Unique contributions of medial and lateral entorhinal cortices to episodic memory in a context-guided object-association task. Abstr. SfN 44th Annu. Meet 560.09 2014 [Google Scholar]
- Keinath AT, Wang ME, Wann EG, Yuan RK, Dudman JT, Muzzio IA. Precise spatial coding is preserved along the longitudinal hippocampal axis. Hippocampus. 2014;24:1533–1548. doi: 10.1002/hipo.22333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerley SW, Behrens TEJ, Wallis JD. Double dissociation of value computations in orbitofrontal and anterior cingulate neurons. Nat Neurosci. 2011;14:1581–1589. doi: 10.1038/nn.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan RM, Luk CH, Flinker A, Aggarwal A, Lapid H, Haddad R, Sobel N. Predicting odor pleasantness from odorant structure: pleasantness as a reflection of the physical world. J Neurosci. 2007;27:10015–10023. doi: 10.1523/JNEUROSCI.1158-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelstrup KB, Solstad T, Brun VH, Hafting T, Leutgeb S, Witter MP, Moser EI, Moser MB. Finite scale of spatial representation in the hippocampus. Science. 2008;321:140–143. doi: 10.1126/science.1157086. [DOI] [PubMed] [Google Scholar]
- Knudsen EI, du Lac S, Esterly SD. Computational maps in the brain. Annu Rev Neurosci. 1987;10:41–65. doi: 10.1146/annurev.ne.10.030187.000353. [DOI] [PubMed] [Google Scholar]
- Koganezawa N, Taguchi A, Tominaga T, Ohara S, Tsutsui KI, Witter MP, Iijima T. Significance of the deep layers of entorhinal cortex for transfer of both perirhinal and amygdala inputs to the hippocampus. Neurosci Res. 2008;61:172–181. doi: 10.1016/j.neures.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Komorowski RW, Manns JR, Eichenbaum H. Robust conjunctive item-place coding by hippocampal neurons parallels learning what happens where. J Neurosci. 2009;29:9918–9929. doi: 10.1523/JNEUROSCI.1378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komorowski RW, Garcia CG, Wilson A, Hattori S, Howard MW, Eichenbaum H. Ventral hippocampal neurons are shaped by experience to represent behaviorally relevant contexts. J Neurosci. 2013;33:8079–8087. doi: 10.1523/JNEUROSCI.5458-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulakov AA, Kolterman BE, Enikolopov AG, Rinberg D. In search of the structure of human olfactory space. Front Syst Neurosci. 2011;5:65. doi: 10.3389/fnsys.2011.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiman G, Koch C, Fried I. Category-specific visual responses of single neurons in the human medial temporal lobe. Nat Neurosci. 2000;3:946–953. doi: 10.1038/78868. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Mur M, Bandettini P. Representational similarity analysis - connecting the branches of systems neuroscience. Front Syst Neurosci. 2008;2:4. doi: 10.3389/neuro.06.004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropff E, Carmichael JE, Moser MB, Moser EI. Speed cells in the medial entorhinal cortex. Nature. 2015;523:419–424. doi: 10.1038/nature14622. [DOI] [PubMed] [Google Scholar]
- Kubie J, Ranck J. Sensory-behavioral correlates in individual hippocampus neurons in three situations: Space and context. Neurobiol Hippocampus 1983 [Google Scholar]
- Kumar A, Rotter S, Aertsen A. Conditions for propagating synchronous spiking and asynchronous firing rates in a cortical network model. J Neurosci. 2008;28:5268–5280. doi: 10.1523/JNEUROSCI.2542-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D, McClelland JL. Generalization through the recurrent interaction of episodic memories: a model of the hippocampal system. Psychol Rev. 2012;119:573–616. doi: 10.1037/a0028681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyd RJ, Bilkey DK. Hippocampal place cells show increased sensitivity to changes in the local environment following prefrontal cortex lesions. Cereb Cortex. 2005;15:720–731. doi: 10.1093/cercor/bhh173. [DOI] [PubMed] [Google Scholar]
- Latham PE, Nirenberg S. Synergy, redundancy, and independence in population codes, revisited. J Neurosci. 2005;25:5195–5206. doi: 10.1523/JNEUROSCI.5319-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Park S-B. Perirhinal cortical inactivation impairs object-in-place memory and disrupts task-dependent firing in hippocampal CA1, but not in CA3. Front. Neural Circuits. 2013;7 doi: 10.3389/fncir.2013.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Treves A, Moser MB, Moser EI. Distinct ensemble codes in hippocampal areas CA3 and CA1. Science (80-) 2004;305:1295–1298. doi: 10.1126/science.1100265. [DOI] [PubMed] [Google Scholar]
- Lever C, Burton S, Jeewajee A, O’Keefe J, Burgess N. Boundary vector cells in the subiculum of the hippocampal formation. J Neurosci. 2009;29:9771–9777. doi: 10.1523/JNEUROSCI.1319-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. Decoding methods for neural prostheses: where have we reached? Front Syst Neurosci. 2014;8:129. doi: 10.3389/fnsys.2014.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin IC, Okun M, Carandini M, Harris KD. The Nature of Shared Cortical Variability. Neuron. 2015;87:644–656. doi: 10.1016/j.neuron.2015.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Louie K, Grattan LE, Glimcher PW. Reward value-based gain control: divisive normalization in parietal cortex. J Neurosci. 2011;31:10627–10639. doi: 10.1523/JNEUROSCI.1237-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Leutgeb JK, Tsao A, Henriksen EJ, Leutgeb S, Barnes CA, Witter MP, Moser MB, Moser EI. Impaired hippocampal rate coding after lesions of the lateral entorhinal cortex. Nat Neurosci. 2013;16:1085–1093. doi: 10.1038/nn.3462. [DOI] [PubMed] [Google Scholar]
- Ma WJ, Beck JM, Latham PE, Pouget A. Bayesian inference with probabilistic population codes. Nat Neurosci. 2006;9:1432–1438. doi: 10.1038/nn1790. [DOI] [PubMed] [Google Scholar]
- Ma WJ, Beck JM, Pouget A. Spiking networks for Bayesian inference and choice. Curr Opin Neurobiol. 2008;18:217–222. doi: 10.1016/j.conb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Malhotra S, Cross RWA, van der Meer MAA. Theta phase precession beyond the hippocampus. Rev Neurosci. 2012;23:39–65. doi: 10.1515/revneuro-2011-0064. [DOI] [PubMed] [Google Scholar]
- Markus E, Qin Y, Leonard B, Skaggs W, McNaughton B, Barnes C. Interactions between location and task affect the spatial and directional firing of hippocampal neurons. J Neurosci. 1995;15:7079–7094. doi: 10.1523/JNEUROSCI.15-11-07079.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr D. Simple memory: a theory for archicortex. Philos Trans R Soc Lond B Biol Sci. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- Martin KAC. A Brief History of the “Feature Detector. Cereb Cortex. 1994;4:1–7. doi: 10.1093/cercor/4.1.1. [DOI] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O’Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- McKenzie S, Robinson NTM, Herrera L, Churchill JC, Eichenbaum H. Learning Causes Reorganization of Neuronal Firing Patterns to Represent Related Experiences within a Hippocampal Schema. J Neurosci. 2013;33:10243–10256. doi: 10.1523/JNEUROSCI.0879-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie S, Frank AJ, Kinsky NR, Porter B, Rivière PD, Eichenbaum H. Hippocampal Representation of Related and Opposing Memories Develop within Distinct, Hierarchically Organized Neural Schemas. Neuron. 2014 doi: 10.1016/j.neuron.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton BL, Morris RG. Hippocampal synaptic enhancement and information storage within a distributed memory system. Trends Neurosci. 1987;10:408–415. [Google Scholar]
- McNaughton BL, Nadel L. Hebb-Marr networks and the neurobiological representation of action in space. In: Gluck M, David Rumelhart, editors. Neuroscience and Connectionist Theory. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1990. pp. 1–63. [Google Scholar]
- McNaughton BL, Barnes CA, O’Keefe J. The contributions of position, direction, and velocity to single unit activity in the hippocampus of freely-moving rats. Exp Brain Res. 1983;52:41–49. doi: 10.1007/BF00237147. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser MB. Path integration and the neural basis of the “cognitive map”. Nat Rev Neurosci. 2006;7:663–678. doi: 10.1038/nrn1932. [DOI] [PubMed] [Google Scholar]
- Meeter M, Shohamy D, Myers CE. Acquired equivalence changes stimulus representations. J Exp Anal Behav. 2009;91:127–141. doi: 10.1901/jeab.2009.91-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merzenich MM, Knight PL, Roth GL. Representation of cochlea within primary auditory cortex in the cat. J Neurophysiol. 1975;38:231–249. doi: 10.1152/jn.1975.38.2.231. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miller VM, Best PJ. Spatial correlates of hippocampal unit activity are altered by lesions of the fornix and endorhinal cortex. Brain Res. 1980;194:311–323. doi: 10.1016/0006-8993(80)91214-7. [DOI] [PubMed] [Google Scholar]
- Mitchison GJ, Durbin RM. Bounds on the learning capacity of some multi-layer networks. Biol Cybern. 1989;60 [Google Scholar]
- Mizuseki K, Buzsaki G. Theta oscillations decrease spike synchrony in the hippocampus and entorhinal cortex. Philos Trans R Soc Lond B Biol Sci. 2014;369:20120530. doi: 10.1098/rstb.2012.0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco JD, Rao G, Roth ED, Knierim JJ. Attentive scanning behavior drives one-trial potentiation of hippocampal place fields. Nat Neurosci. 2014;17:725–731. doi: 10.1038/nn.3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montemurro MA, Rasch MJ, Murayama Y, Logothetis NK, Panzeri S. Phase-of-firing coding of natural visual stimuli in primary visual cortex. Curr Biol. 2008;18:375–380. doi: 10.1016/j.cub.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Morris RGM. Elements of a neurobiological theory of hippocampal function: the role of synaptic plasticity, synaptic tagging and schemas. Eur J Neurosci. 2006;23:2829–2846. doi: 10.1111/j.1460-9568.2006.04888.x. [DOI] [PubMed] [Google Scholar]
- Moser EI, Kropff E, Moser MB. Place cells, grid cells, and the brain’s spatial representation system. Annu Rev Neurosci. 2008;31:69–89. doi: 10.1146/annurev.neuro.31.061307.090723. [DOI] [PubMed] [Google Scholar]
- Moser MB, Rowland DC, Moser EI. Place cells, grid cells, and memory. Cold Spring Harb Perspect Med. 2015;5:a021808. doi: 10.1101/cshperspect.a021808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller RU, Kubie JL, Ranck JB. Spatial firing patterns of hippocampal complex-spike cells in a fixed environment. J Neurosci. 1987;7:1935–1950. doi: 10.1523/JNEUROSCI.07-07-01935.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Kimchi EY, Laubach M. Redundancy and synergy of neuronal ensembles in motor cortex. J Neurosci. 2005;25:4207–4216. doi: 10.1523/JNEUROSCI.4697-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navawongse R, Eichenbaum H. Distinct Pathways for Rule-Based Retrieval and Spatial Mapping of Memory Representations in Hippocampal Neurons. J Neurosci. 2013;33:1002–1013. doi: 10.1523/JNEUROSCI.3891-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirenberg S, Carcieri SM, Jacobs AL, Latham PE. Retinal ganglion cells act largely as independent encoders. Nature. 2001;411:698–701. doi: 10.1038/35079612. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Recce ML. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus. 1993;3:317–330. doi: 10.1002/hipo.450030307. [DOI] [PubMed] [Google Scholar]
- O’Neill PK, Gordon JA, Sigurdsson T. Theta oscillations in the medial prefrontal cortex are modulated by spatial working memory and synchronize with the hippocampus through its ventral subregion. J Neurosci. 2013;33:14211–14224. doi: 10.1523/JNEUROSCI.2378-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly RC, McClelland JL. Hippocampal conjunctive encoding, storage, and recall: avoiding a trade-off. Hippocampus. 1994;4:661–682. doi: 10.1002/hipo.450040605. [DOI] [PubMed] [Google Scholar]
- Ohyama K, Sugase-Miyamoto Y, Matsumoto N, Shidara M, Sato C. Stimulus-related activity during conditional associations in monkey perirhinal cortex neurons depends on upcoming reward outcome. J Neurosci. 2012;32:17407–17419. doi: 10.1523/JNEUROSCI.2878-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olshausen BA, Field DJ. Emergence of simple-cell receptive field properties by learning a sparse code for natural images. Nature. 1996;381:607–609. doi: 10.1038/381607a0. [DOI] [PubMed] [Google Scholar]
- Olton DS, Branch M, Best PJ. Spatial correlates of hippocampal unit activity. Exp Neurol. 1978;58:387–409. doi: 10.1016/0014-4886(78)90096-1. [DOI] [PubMed] [Google Scholar]
- Oram MW, Hatsopoulos NG, Richmond BJ, Donoghue JP. Excess synchrony in motor cortical neurons provides redundant direction information with that from coarse temporal measures. J Neurophysiol. 2001;86:1700–1716. doi: 10.1152/jn.2001.86.4.1700. [DOI] [PubMed] [Google Scholar]
- Panzeri S, Schultz SR, Treves A, Rolls ET. Correlations and the encoding of information in the nervous system. Proc Biol Sci. 1999;266:1001–1012. doi: 10.1098/rspb.1999.0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzeri S, Petersen RS, Schultz SR, Lebedev M, Diamond ME. The role of spike timing in the coding of stimulus location in rat somatosensory cortex. Neuron. 2001;29:769–777. doi: 10.1016/s0896-6273(01)00251-3. [DOI] [PubMed] [Google Scholar]
- Park PJ, Manjourides J, Bonetti M, Pagano M. A permutation test for determining significance of clusters with applications to spatial and gene expression data. Comput Stat Data Anal. 2009;53:4290–4300. doi: 10.1016/j.csda.2009.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson JK, Murray EA, Mishkin M. A selective mnemonic role for the hippocampus in monkeys: memory for the location of objects. J Neurosci. 1988;8:4159–4167. doi: 10.1523/JNEUROSCI.08-11-04159.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz R, Pelletier JG, Bauer EP, Paré D. Emotional enhancement of memory via amygdala-driven facilitation of rhinal interactions. Nat Neurosci. 2006;9:1321–1329. doi: 10.1038/nn1771. [DOI] [PubMed] [Google Scholar]
- Paz R, Bauer EP, Paré D. Learning-related facilitation of rhinal interactions by medial prefrontal inputs. J Neurosci. 2007;27:6542–6551. doi: 10.1523/JNEUROSCI.1077-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz R, Bauer EP, Paré D. Measuring correlations and interactions among four simultaneously recorded brain regions during learning. J Neurophysiol. 2009;101:2507–2515. doi: 10.1152/jn.91259.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz-Villagrán V, Save E, Poucet B. Independent coding of connected environments by place cells. Eur J Neurosci. 2004;20:1379–1390. doi: 10.1111/j.1460-9568.2004.03570.x. [DOI] [PubMed] [Google Scholar]
- Peyrache A, Lacroix MM, Petersen PC, Buzsáki G. Internally organized mechanisms of the head direction sense. Nat Neurosci. 2015;18:569–575. doi: 10.1038/nn.3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouget A, Deneve S, Duhamel JR. A computational perspective on the neural basis of multisensory spatial representations. Nat Rev Neurosci. 2002;3:741–747. doi: 10.1038/nrn914. [DOI] [PubMed] [Google Scholar]
- Pouille F, Marin-Burgin A, Adesnik H, Atallah BV, Scanziani M. Input normalization by global feedforward inhibition expands cortical dynamic range. Nat Neurosci. 2009;12:1577–1585. doi: 10.1038/nn.2441. [DOI] [PubMed] [Google Scholar]
- Preston AR, Eichenbaum H. Interplay of hippocampus and prefrontal cortex in memory. Curr Biol. 2013;23:R764–R773. doi: 10.1016/j.cub.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroga RQ, Reddy L, Kreiman G, Koch C, Fried I. Invariant visual representation by single neurons in the human brain. Nature. 2005;435:1102–1107. doi: 10.1038/nature03687. [DOI] [PubMed] [Google Scholar]
- Rajasethupathy P, Sankaran S, Marshel JH, Kim CK, Ferenczi E, Lee SY, Berndt A, Ramakrishnan C, Jaffe A, Lo M, et al. Projections from neocortex mediate top-down control of memory retrieval. Nature. 2015;526:653–659. doi: 10.1038/nature15389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajji T, Chapman D, Eichenbaum H, Greene R. The role of CA3 hippocampal NMDA receptors in paired associate learning. J Neurosci. 2006;26:908–915. doi: 10.1523/JNEUROSCI.4194-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C. A unified framework for the functional organization of the medial temporal lobes and the phenomenology of episodic memory. Hippocampus. 2010;20:1263–1290. doi: 10.1002/hipo.20852. [DOI] [PubMed] [Google Scholar]
- Rangel LM, Eichenbaum H. Brain rhythms: towards a coherent picture of ensemble development in learning. Curr Biol. 2014;24:R620–R621. doi: 10.1016/j.cub.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renart A, de la Rocha J, Bartho P, Hollender L, Parga N, Reyes A, Harris KD. The asynchronous state in cortical circuits. Science. 2010;327:587–590. doi: 10.1126/science.1179850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich PD, Liaw HP, Lee AK. Place cells. Large environments reveal the statistical structure governing hippocampal representations. Science. 2014;345:814–817. doi: 10.1126/science.1255635. [DOI] [PubMed] [Google Scholar]
- Rigotti M, Barak O, Warden MR, Wang XJ, Daw ND, Miller EK, Fusi S. The importance of mixed selectivity in complex cognitive tasks. Nature. 2013;497:585–590. doi: 10.1038/nature12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. Theoretical and neurophysiological analysis of the functions of the primate hippocampus in memory. Cold Spring Harb Symp Quant Biol. 1990;55:995–1006. doi: 10.1101/sqb.1990.055.01.095. [DOI] [PubMed] [Google Scholar]
- Rolls ET. A quantitative theory of the functions of the hippocampal CA3 network in memory. Front Cell Neurosci. 2013;7 doi: 10.3389/fncel.2013.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Treves A. The relative advantages of sparse versus distributed encoding for associative neuronal networks in the brain. Netw Comput Neural Syst 2015 [Google Scholar]
- Royer S, Sirota A, Patel J, Buzsáki G. Distinct representations and theta dynamics in dorsal and ventral hippocampus. J Neurosci. 2010;30:1777–1787. doi: 10.1523/JNEUROSCI.4681-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueckemann JW, DiMauro AJ, Rangel LM, Han X, Boyden ES, Eichenbaum H. Transient optogenetic inactivation of the medial entorhinal cortex biases the active population of hippocampal neurons. Hippocampus. 2015 doi: 10.1002/hipo.22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh I, Suga N. Long delay lines for ranging are created by inhibition in the inferior colliculus of the mustached bat. J Neurophysiol. 1995;74:1–11. doi: 10.1152/jn.1995.74.1.1. [DOI] [PubMed] [Google Scholar]
- Samsonovich A, McNaughton BL. Path integration and cognitive mapping in a continuous attractor neural network model. J Neurosci. 1997;17:5900–5920. doi: 10.1523/JNEUROSCI.17-15-05900.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffzük C, Kukushka VI, Vyssotski AL, Draguhn A, Tort ABL, Brankačk J. Selective Coupling between Theta Phase and Neocortical Fast Gamma Oscillations during REM-Sleep in Mice. PLoS One. 2011;6:e28489. doi: 10.1371/journal.pone.0028489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesiger MI, Cannova CC, Boublil BL, Hales JB, Mankin EA, Brandon MP, Leutgeb JK, Leibold C, Leutgeb S. The medial entorhinal cortex is necessary for temporal organization of hippocampal neuronal activity. Nat Neurosci. 2015;18:1123–1132. doi: 10.1038/nn.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt B, Papale A, Redish AD, Markus EJ. Conflict between place and response navigation strategies: effects on vicarious trial and error (VTE) behaviors. Learn Mem. 2013;20:130–138. doi: 10.1101/lm.028753.112. [DOI] [PubMed] [Google Scholar]
- Schultz SR, Rolls ET. Analysis of information transmission in the Schaffer collaterals. Hippocampus. 1999;9:582–598. doi: 10.1002/(SICI)1098-1063(1999)9:5<582::AID-HIPO12>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Shadlen MN, Newsome WT. Noise, neural codes and cortical organization. Curr Opin Neurobiol. 1994;4:569–579. doi: 10.1016/0959-4388(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Shadlen MN, Newsome WT. The variable discharge of cortical neurons: implications for connectivity, computation, and information coding. J Neurosci. 1998;18:3870–3896. doi: 10.1523/JNEUROSCI.18-10-03870.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamir M, Sompolinsky H. Implications of neuronal diversity on population coding. Neural Comput. 2006;18:1951–1986. doi: 10.1162/neco.2006.18.8.1951. [DOI] [PubMed] [Google Scholar]
- Sigala N, Logothetis NK. Visual categorization shapes feature selectivity in the primate temporal cortex. Nature. 2002;415:318–320. doi: 10.1038/415318a. [DOI] [PubMed] [Google Scholar]
- Singer AC, Karlsson MP, Nathe AR, Carr MF, Frank LM. Experience-dependent development of coordinated hippocampal spatial activity representing the similarity of related locations. J Neurosci. 2010;30:11586–11604. doi: 10.1523/JNEUROSCI.0926-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solstad T, Boccara CN, Kropff E, Moser MB, Moser EI. Representation of geometric borders in the entorhinal cortex. Science. 2008;322:1865–1868. doi: 10.1126/science.1166466. [DOI] [PubMed] [Google Scholar]
- Sompolinsky H, Yoon H, Kang K, Shamir M. Population coding in neuronal systems with correlated noise. Phys Rev E. 2001;64:051904. doi: 10.1103/PhysRevE.64.051904. [DOI] [PubMed] [Google Scholar]
- Spellman T, Rigotti M, Ahmari SE, Fusi S, Gogos JA, Gordon JA. Hippocampal-prefrontal input supports spatial encoding in working memory. Nature. 2015;522:309–314. doi: 10.1038/nature14445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiers HJ, Hayman RMA, Jovalekic A, Marozzi E, Jeffery KJ. Place Field Repetition and Purely Local Remapping in a Multicompartment Environment. Cereb Cortex. 2013 doi: 10.1093/cercor/bht198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stettler DD, Axel R. Representations of odor in the piriform cortex. Neuron. 2009;63:854–864. doi: 10.1016/j.neuron.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Suh J, Rivest AJ, Nakashiba T, Tominaga T, Tonegawa S. Entorhinal cortex layer III input to the hippocampus is crucial for temporal association memory. Science (80-) 2011;334:1415–1420. doi: 10.1126/science.1210125. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Cowan WM. An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. J Comp Neurol. 1977;172:49–84. doi: 10.1002/cne.901720104. [DOI] [PubMed] [Google Scholar]
- Tan AYY, Chen Y, Scholl B, Seidemann E, Priebe NJ. Sensory stimulation shifts visual cortex from synchronous to asynchronous states. Nature. 2014;509:226–229. doi: 10.1038/nature13159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JS. Head direction cells recorded in the anterior thalamic nuclei of freely moving rats. J Neurosci. 1995;15:70–86. doi: 10.1523/JNEUROSCI.15-01-00070.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube J, Muller R, Ranck J. Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J Neurosci. 1990 doi: 10.1523/JNEUROSCI.10-02-00420.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tocker G, Barak O, Derdikman D. Grid cells correlation structure suggests organized feedforward projections into superficial layers of the medial entorhinal cortex. Hippocampus. 2015 doi: 10.1002/hipo.22481. n/a – n/a. [DOI] [PubMed] [Google Scholar]
- Tort ABL, Komorowski RW, Manns JR, Kopell NJ, Eichenbaum H. Theta-gamma coupling increases during the learning of item-context associations. Proc Natl Acad Sci. 2009;106:20942–20947. doi: 10.1073/pnas.0911331106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tort ABL, Komorowski R, Kopell N, Eichenbaum H. A mechanism for the formation of hippocampal neuronal firing patterns that represent what happens where. Learn Mem. 2011;18:718–727. doi: 10.1101/lm.2307711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treves A, Rolls ET. What determines the capacity of autoassociative memories in the brain ? Network. 1991;2:371–397. [Google Scholar]
- Treves A, Rolls ET. Computational analysis of the role of the hippocampus in memory. Hippocampus. 1994;4:374–391. doi: 10.1002/hipo.450040319. [DOI] [PubMed] [Google Scholar]
- Tsao A, Moser MB, Moser EI. Traces of experience in the lateral entorhinal cortex. Curr Biol. 2013;23:399–405. doi: 10.1016/j.cub.2013.01.036. [DOI] [PubMed] [Google Scholar]
- Varela C, Kumar S, Yang JY, Wilson MA. Anatomical substrates for direct interactions between hippocampus, medial prefrontal cortex, and the thalamic nucleus reuniens. Brain Struct Funct. 2014;219:911–929. doi: 10.1007/s00429-013-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwer R, Meijer R, Van Uum H, Witter M. Collateral projections from the rat hippocampal formation to the lateral and medial prefrontal cortex. Hippocampus. 1997;7:397–402. doi: 10.1002/(SICI)1098-1063(1997)7:4<397::AID-HIPO5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Wang S, Liu F, Wang W, Yu Y. Impact of spatially correlated noise on neuronal firing. Phys Rev E. 2004;69:011909. doi: 10.1103/PhysRevE.69.011909. [DOI] [PubMed] [Google Scholar]
- Westmacott R, Moscovitch M. The contribution of autobiographical significance to semantic memory. Mem Cognit. 2003;31:761–774. doi: 10.3758/bf03196114. [DOI] [PubMed] [Google Scholar]
- Willshaw D, Dayan P. Optimal Plasticity from Matrix Memories: What Goes Up Must Come Down. Neural Comput. 1990;2:85–93. [Google Scholar]
- Wilson RC, Takahashi YK, Schoenbaum G, Niv Y. Orbitofrontal cortex as a cognitive map of task space. Neuron. 2014;81:267–279. doi: 10.1016/j.neuron.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter MP, Amaral D. The Hippocampal Formation. In: Paxinos G, editor. Th Rat Nervous System. San Diego, CA: Elselvier; 2004. pp. 635–704. [Google Scholar]
- Witter MP, Moser EI. Spatial representation and the architecture of the entorhinal cortex. Trends Neurosci. 2006;29:671–678. doi: 10.1016/j.tins.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Wood ER, Dudchenko PA, Eichenbaum H. The global record of memory in hippocampal neuronal activity. Nature. 1999;397:613–616. doi: 10.1038/17605. [DOI] [PubMed] [Google Scholar]
- Wood ER, Dudchenko PA, Robitsek RJ, Eichenbaum H. Hippocampal neurons encode information about different types of memory episodes occurring in the same location. Neuron. 2000;27:623–633. doi: 10.1016/s0896-6273(00)00071-4. [DOI] [PubMed] [Google Scholar]
- Xu W, Südhof TC. A neural circuit for memory specificity and generalization. Science (80-) 2013;339:1290–1295. doi: 10.1126/science.1229534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Sejnowski TJ. Neuronal tuning: To sharpen or broaden? Neural Comput. 1999;11:75–84. doi: 10.1162/089976699300016809. [DOI] [PubMed] [Google Scholar]
- Zhang K, Ginzburg I, McNaughton BL, Sejnowski TJ. Interpreting neuronal population activity by reconstruction: unified framework with application to hippocampal place cells. J Neurophysiol. 1998;79:1017–1044. doi: 10.1152/jn.1998.79.2.1017. [DOI] [PubMed] [Google Scholar]
- Zhang SJ, Ye J, Miao C, Tsao A, Cerniauskas I, Ledergerber D, Moser MB, Moser EI. Optogenetic dissection of entorhinal-hippocampal functional connectivity. Science. 2013;340:1232627. doi: 10.1126/science.1232627. [DOI] [PubMed] [Google Scholar]
- Ziv Y, Burns LD, Cocker ED, Hamel EO, Ghosh KK, Kitch LJ, El Gamal A, Schnitzer MJ. Long-term dynamics of CA1 hippocampal place codes. Nat Neurosci. 2013;16:264–266. doi: 10.1038/nn.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohary E, Shadlen MN, Newsome WT. Correlated neuronal discharge rate and its implications for psychophysical performance. Nature. 1994;370:140–143. doi: 10.1038/370140a0. [DOI] [PubMed] [Google Scholar]