Abstract
Background and Objective
Prior studies have suggested that after stroke there is a time-limited period of increased responsiveness to training due to heightened plasticity; a sensitive period thought to be induced by ischemia itself. Using a mouse model we have previously shown that most training-associated recovery after a caudal forelimb area (CFA) stroke occurs in the first week and is attributable to reorganization in a medial premotor area (AGm). The existence of a stroke-induced sensitive period leads to the counterintuitive prediction that a second stroke should reopen this window and and promote full recovery from the first stroke. To test this prediction, we induced a second stroke in AGm of mice with incomplete recovery after a first stroke in CFA.
Methods
Mice were trained to perform a skilled prehension (reach-to-grasp) task to an asymptotic level of performance after which they underwent photocoagulation-induced stroke in CFA. After a 7 day post-stroke delay, the mice were then retrained to asymptote. We then induced a second stroke in AGm and after only a one day delay retrained the mice.
Results
Recovery of prehension was incomplete when training was started after a 7-day post-stroke delay and continued for 19 days. However, a second focal stroke in AGm led to a dramatic response to 9 days of training with full recovery to normal levels of performance.
Conclusions
New ischemia can re-open a sensitive period of heightened responsiveness to training and mediate full recovery from a previous stroke.
Introduction
Most training-associated recovery at both an impairment and functional level occurs in the first month after stroke in rodent models [1, 2] and in the first three months after stroke in humans [3–5]. We have previously shown that the medial agranular cortex (AGm), a medial premotor area, reorganizes after a focal caudal forelimb area (CFA), rodent primary motor cortex, stroke and mediates recovery if post-stroke training is initiated after a one-day post-stroke delay (i.e. 48 hours after stroke) but not if initiation of training is delayed for a week [6, 7]. We refer to this period of increased responsiveness to training as the post-stroke “sensitive period,” which represents a unique, time-limited environment of heightened plasticity [8]. That is to say, the sum of molecular, physiological, and structural changes that lead to greater behavioral gains for the same amount of training inside as compared to outside the sensitive period [2, 8].
Experiments in rodents suggest a causal link between the unique short-lived plasticity milieu after stroke and the amount of recovery from hemiparesis in this same period. For example, manipulation of plasticity in the sensitive period either by increasing [9, 10] or decreasing BDNF [11], augments or prevents recovery, respectively. Furthermore, Nudo and colleagues demonstrated that training monkeys on skilled digital retrieval of food pellets from small wells after an infarct in the hand area of the primary motor cortex prevented loss of hand representation in the peri-infarct cortex. In contrast, withholding motor training led to a decrease in digit representations by more than 50% [12, 13]. Thus, motor training directs reorganization in remaining cortical areas, including premotor areas, presumably enabled by the unique post-stroke plasticity milieu.
A counterintuitive implication of a stroke-induced sensitive period is that a second stroke should reopen a sensitive period and thereby trigger recovery from a prior stroke. That is to say, if ischemia heightens plasticity, motor deficits induced from a first-stroke could be reversed by repeat ischemic damage if rehabilitative training is initiated at an appropriate time. To test this idea, we induced a second focal stroke in the medial premotor area of mice that had only partially recovered prehension (reach-to-grasp) performance after a first CFA stroke because motor training had been delayed. We then induced a second stroke and retrained the mice after a 1-day delay (i.e. 48 hours after stroke). The prediction was that training within the sensitive period induced by the second stroke would lead to full recovery from the first stroke.
Material and Methods
Subjects
Adult male C57bl/6 mice 100 to 150 days old were singly housed in custom made chambers and kept on a 12/12-hour light/dark cycle. Behavioral tasks were carried out in the same room and same chambers in which the mice were housed to reduce the stress of new surroundings. Two to three days prior to learning the prehension task, mice were placed on a scheduled administration of 2.5 g Bio-serv dustless precision pellet mouse chow per day with water ad libitum. Mice were food restricted to 85% of their starting weight. We studied a total of 15 mice based upon effect sizes in previous studies [1, 6, 14]. Three mice were excluded from the analysis: two died prior to completion of the study; the other because of failure to induce a stroke in AGm. Mice were randomized to, and investigators blinded to, training condition. All animal handling and use was performed according to the protocols set by the Johns Hopkins University Animal Care and Use Committee.
Skilled prehension task
Training was conducted as described previously [6]. Briefly, standard mouse cages were modified with a sealable, vertical 16 cm X 0.9 cm slit through which the mice would stick their paw as well as with a standing steel stage measuring 1.5 cm×11.5 cm×8 cm directly in front of the slit. Once the mice were familiarized to the pellets and had lost 15% of their body weight, they were trained on the prehension task. 45 mg Bio-serv dustless precision pellets were placed on sticky tape on a movable steel bar and maintained at the same height as the standing steel cage. The pellets were positioned 0.5 cm away from the standing steel stage and aligned with the edge of the cage slit contralateral to the preferred paw. This configuration required the mouse to reach and grasp with its preferred paw for pellets one at a time. Prehension was scored as a success when the mouse reached its forelimb through the slit, grabbed the pellet, and ate it without knocking it from its resting space, dropping it, or in any other way losing control of it. If not, the attempt was recorded as a miss. Our behavioral outcome measure was percent of successful prehension attempts, which was determined per pellet; thus, if the mouse did not touch the pellet, it was not counted as an attempt. Paw preference was determined in a series of preliminary reaching blocks that were not scored. Once paw preference was determined and the mice were familiar with the task, the space between the opening of the cage and bar loaded with food pellets was increased to a maximum distance of 1 cm to increase the difficulty of the task. The mice then underwent 2 blocks of 30 reaching attempts per training day. The animals had one training day off per week (including the day after stroke induction). During training, mice were also fed at the end of each day with additional food pellets placed in their cage, in order to maintain their weight at 85% of baseline. Training after stroke began either 48 hours or 8 days after stroke induction and followed the same protocol as described above. All investigators were blinded to training condition after stroke induction.
Stroke Induction
The location of motor areas was identified based upon prior anatomic [15] and functional [16] mapping. These areas are geographically consistent within a given strain and we have used them with prior success [6, 7]. Focal cortical infarction was induced by photothrombosis of the cortical microvessels with some modification to previously described protocols [17]. Each mouse was anesthetized with 4.5 ml/kg of a Ketamine (21 mg/ml) plus Xylazine (3.2 mg/ml) mixture and placed in a stereotaxic frame (Stoelting, Wood Dale, Ill). Temperature was monitored and maintained at 36.5 to 37.5 8C with the help of a heating pad. At the dorsal aspect of the head, the skull was exposed by a median incision of the skin, the periostium was removed and the bregma point identified. The skull was thinned using a fine dremmel. A fiber optic bundle of a cold light source (Zeiss 1500 electronic, Jena, Germany) with a 20 gauge aperture was centered at 2 mm lateral and 0.5 mm anterior from bregma (caudal forelimb area-CFA[16]), 0 mm laterally and 0.5 mm anteriorly from bregma (AGm), or 2.5 mm laterally and 1 mm anterior of lambda (visual cortex) and placed against the skull. The brains were then illuminated through the intact skull for 15 min starting 5 min after the IP injection of 150 µl of a 10 mg/ml rose Bengal solution in sterile normal saline. The scalp was then sutured and mice were allowed to awaken while still on the heating pad. Dual location and size (≈0.25 mm3) of stroke was pathologically confirmed in each mouse included in the analysis; there was no difference in size of the strokes (data not shown).
Statistics
The effect of the second stroke was analyzed with a repeated-measures ANOVA, with 3 relevant time points (just prior to stroke #1, just prior to stroke #2, and the last training day) as within-group factors and the second stroke location as the between-group factor. Post-hoc comparisons made with Sidak’s correction.
Results
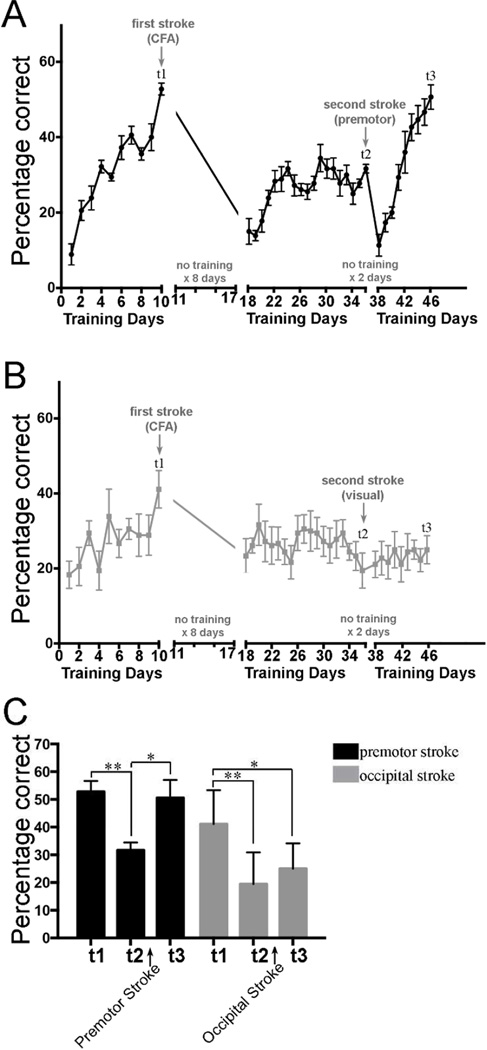
To test if a second stroke could re-open a sensitive period and allow full recovery from a first CFA stroke, we followed the experimental paradigm shown in Figure 1A. Specifically, using custom built cages (schematized in Figure 1B – E) we trained wild-type adult male mice to perform a skilled prehension task to an asymptotic level of performance, photothrombotically induced a focal CFA infarction (t1; Figure 2 A and B), and retrained them after a 7-day delay. Assessment on the prehension task on day 18 (eight days after stroke) revealed that there was little spontaneous recovery of performance, which is in agreement with our prior results [7], and subsequent training over 19 days total led to only mild performance gains that never returned to pre-stroke levels (Figure 3A and B days 18 – 36). A second focal stroke was then induced in the ipsilesional medial premotor area (AGm) at time point t2 (figure 2 A and B). Training was started after a 1-day post-stroke delay (i.e. 48 hours after stroke). After t2, prehension performance was initially worse, as would be expected from a second stroke in an ipsilesional premotor area [6], but then returned to the normal level seen before the first CFA stroke (figure 2A – t3). As a control, another group of mice was given a second stroke in the ipsilesional occipital cortex. This group showed neither significant worsening nor subsequent improvement. There was no significant between-group difference in pre-stroke performance at t1 (two tailed, paired t-test), although this could be due to lack of power. This is not a problem, however, because the control group started, if anything, at a higher level of performance than the premotor group (although again, this difference did not reach significance via a two tailed, paired t-test). Nevertheless, despite starting from this higher level of performance, the control group did not show any recovery after a second stroke in occipital cortex, whereas a second stroke in the medial premotor area led to a dramatic reversal of the motor deficit due to the first stroke (Figure 2B and 2C).
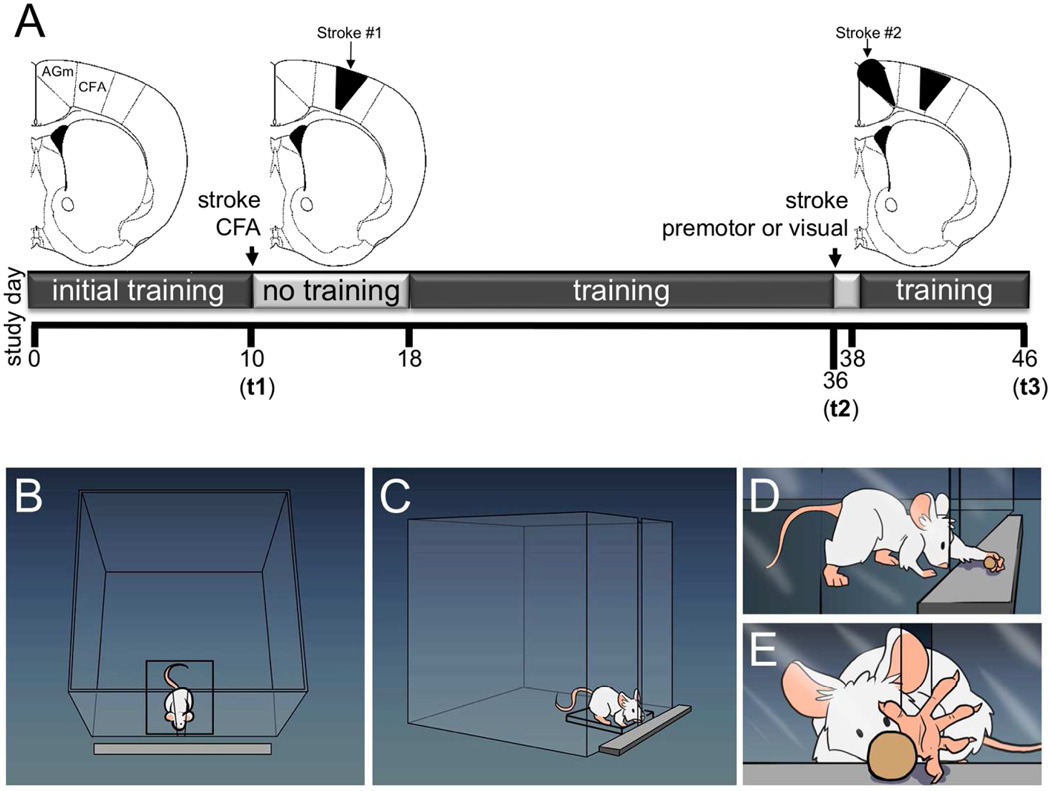
Figure 1.
(A) Schematic of experimental timeline. Initial CFA stroke at t1; Second stroke which occurred in either the medial premotor area (AGm) or in the visual cortex (occipital lobe) at t3; day of sacrifice at t3. (B – E) drawings of prehension task and training apparatus.
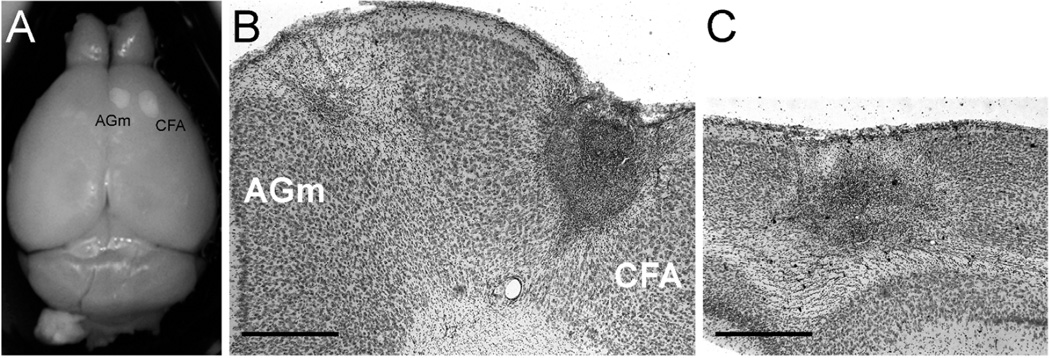
Figure 2.
Representative images of CFA, AGm, and occipital strokes. (A) PFA-fixed brain with strokes in CFA and AGm. (B, C) Representative 50 µm Cresyl violet-stained coronal sections through frontal lobe showing strokes in both CFA and AGm (B) or through the occipital lobe showing a stroke in visual cortex (C). Scale bar = 200 µm.
Figure 3.
Mice were trained to perform the skilled prehension task to an asymptotic level of performance (t1) after which they underwent photocoagulation-induced stroke in the CFA. After a 7 day post-stroke delay (t2), the mice were then retrained for 19 days. A second photocoagulation-induced stroke was then induced in either ipsilesional medial premotor cortex (A) or in ipsilesional visual cortex (B). The mice were re-trained after only a one-day delay (i.e. 48 hours later) and sacrificed at t3. Each group had n=6. (C) Prehension performance at time points t1, t2, and t3. A repeated-measures ANOVA showed a significant interaction between group and time points t1, t2 and t3 (p = 0.015). Asterisks indicate significant post-hoc differences compared using Sidak’s multiple comparisons test (* < 0.001; ** < 0.0001).
Discussion
Here we show that a second stroke in ipsilesional premotor cortex was sufficient to re-open a sensitive period, restore responsiveness to training after it had reached plateau following a first stroke in primary motor cortex, with subsequent full recovery. This is the first example of a double-stroke paradigm being used to re-open a post-stroke sensitive period and induce motor recovery.
The post-stroke sensitive period
Recent work in rodent models has shown that there are unique molecular, structural and physiological changes in the peri-infarct cortex during the first four weeks post-stroke that likely serve as the substrate for increased plasticity in response to training [2, 8]. Many of these peri-infarct changes peak within the first 7 days [8, 18, 19] and then begin to normalize. The lack of responsiveness to further training (e.g. during days 18–36) that we saw here in the mouse is consistent with studies in the rat that failed to show a benefit of late tune-ups despite a response to training early after stroke [20]. That the impact of training falls off rapidly within one week post-stroke is consistent with our prior results [7] and also consistent with results in rats showing only a modest response to training and enrichment given 2 weeks post-stroke, compared to when given early [1]. Human data also suggest a short-lived plasticity window after stroke with most spontaneous recovery, which follows a predictable proportionality rule [3, 21], occurring in the first 3 months [4, 22].
We showed that mice had the capacity to recover back to normal even when they had hit a performance plateau and were no longer responding to training after the first CFA stroke (i.e. days 22–36). Post-stroke performace reached plateau by approximately day 23, i.e., after 4 days of training. There was no further improvement despite 14 extra days of training. After a second stroke in AGm, there was a marked transient worsening in performance but it then improved beyond the previous plateau within 4 days (day 42) and continued to improve for the next 5 days after this. Thus whereas the mice showed a flat training response for 14 days before the second stroke, after it they showed an immediate and sustained steep training response for 9 days. In stark contrast, a second stroke in visual cortex led to no training response over 9 days. These observations make it highly implausible that the mice, in the absence of the second motor stroke, would have suddenly started to dramatically respond to training on days 38 and 39 despite a complete lack of responsiveness for the previous 2 weeks, or that the mice with a second stroke in visual cortex would have suddenly started to respond dramatically had they continued beyond 9 days. Instead, the only plausible explanation of our results is that the renewed responsiveness to training after a plateau was reached is attributable to a second stroke in AGm. We chose AGm because in prior work we showed that it reorganizes after CFA stroke [6]. It is possible that a stroke in the rostral forelimb area or another premotor area may have produced similar results.
These results have important implications for understanding mechanisms of recovery early after stroke. In a primate model of stroke, the amount of reorganization in ventral premotor cortex was proportional to the size of the ischemic lesion in primary motor cortex, suggesting a dose-like response to factor release after ischemia [23]. In a rodent model it has been shown that AMPA receptor modulation starting at day 5 after stroke further augments the increases in BDNF expression seen in peri-infarct cortex and is associated with improved motor recovery [24]. Conversely, antagonizing tonic GABA inhibition in peri-infarct cortex early after stroke enhances motor recovery [25]. Thus manipulation of the peri-infarct milieu during the sensitive period can augment and/or prolong the heightened plasticity that transiently occurs and mitigate those factors that begin to normalize it.
There are obvious similarities between the post-stroke sensitive period and developmental critical periods during which environmental stimuli are most likely to influence brain reorganization [26]. Perhaps the best examples of environment-induced plasticity come from studies of visual system, in which there are limited time windows within which specific visual stimuli can alter gene expression, dendritic spine dynamics, neuronal tuning, and ultimately circuit connectivity [27, 28]. Of particular interest are those studies that show how visual cortical critical periods can be reinstated in the adult rodent [29–32]. Similar to our results, these studies indicate that the adult brain has the capacity to undergo large-scale change in response to environmental stimuli, but requires some additional stimulus (e.g. fluoxetine) to overcome a inhibitory mechanisms [7].
Double lesions and recovery
The double-lesion approach has been used in previous studies to identify those regions mediating recovery after a first stroke. In these cases the second lesion is used to reinstate the original deficit, not reverse it as we did here. For example, in a previous study we showed that the medial premotor area undergoes reorganization after training-induced recovery from CFA stroke [6]. Similarly, a second stroke placed in the rostral forelimb area (RFA) after rats had been rehabilitated from an initial CFA stroke, reinstates the initial stroke-induced phenotype [33, 34]. Conversely, it has also been shown that CFA can mediate recovery after a focal RFA stroke [34]. Thus previous double-lesion experiments have been used to probe reorganization rather than induce it. In addition, these prior studies did not directly test the importance of rehabilitation timing after stroke. Specifically, there was either no rehabilitation after the second stroke [33] or rehabilitation was initiated with the same delay used after the first stroke [34].
The double-lesion effect we observed is also distinct from those paradoxical lesions that physiologically ameliorate symptoms from a primary neurological condition. For example, bradykinesia due to degeneration of the nigrostriatal pathway (caused by ischemia or otherwise) can be improved by lesions (including stroke) in the internal pallidal segment or in the subthalamic nucleus [35]. In this case the mechanism of such improvement is fast, independent of rehabilitation, and is due to a reduction of excessive and disordered activity in the inhibitory pallido-thalamic pathway [36–38]. This physiological mechanism is quite distinct from a slower training-dependent effect in a period of heightened plasticity.
Finally, the double-lesion effect we observed is distinct from ischemic pre-conditioning, in which initial sub-lethal cerebral ischemia results in the up-regulation of certain genes, which conveys tolerance to later, otherwise lethal, ischemia. Thus, ischemic pre-conditioning lessens the impact of a stroke by decreasing infarct size [39–41]. In contrast, our second stroke increased infarct volume and initially worsened the residual deficit from the first stroke. It was only after training that behavioral recovery was seen.
Implications for recovery in humans
Whereas previous work has shown that the sensitive period can be pharmacologically modulated once it is already in progress [7, 42], we show that the post-stroke sensitive period can be reset once it is over. Although this proves the existence of a post-ischemic sensitive period, inducing a second stroke is not a tenable therapeutic option for patients. Recent work, however, has shown that there may be other ways to reset critical periods in the healthy adult rodent using certain molecules, medications, and/or modification of inhibitory/excitatory balance [29–32]. Such approaches may provide insight into to how to reset the sensitive period in the absence of an additional ischemic insult.
Acknowledgments
Sources of funding
Dr S.R.Z. is currently supported by 1K08NS085033-01, the Richard S. Ross Clinician Scientist Award as well as by a startup fund from the Johns Hopkins department of Neurology. Dr J.W.K. is currently supported by R01 NS052804– 05, R01 120 86264, R01 HD073147, James S. McDonnell Foundation 220020220, and two Brain Science Institute grants.
Footnotes
The authors have no conflicts of interest to disclose.
Contributor Information
Steven R. Zeiler, Email: sz@jhmi.edu.
Robert Hubbard, Email: robbhubbard@gmail.com.
Ellen M. Gibson, Email: ellenmichellegibson@gmail.com.
Tony Zheng, Email: tzheng2@jhu.edu.
Kwan Ng, Email: Kwan528@gmail.com.
Richard O’Brien, Email: richard.obrien@duke.edu.
John W. Krakauer, Email: jkrakau1@jhmi.edu.
References
- 1.Biernaskie J, Chernenko G, Corbett D. Efficacy of rehabilitative experience declines with time after focal ischemic brain injury. J Neurosci. 2004 Feb 4;24(5):1245–1254. doi: 10.1523/JNEUROSCI.3834-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krakauer JW, Carmichael ST, Corbett D, Wittenberg GF. Getting neurorehabilitation right: what can be learned from animal models? Neurorehabilitation and neural repair. 2012 Oct;26(8):923–931. doi: 10.1177/1545968312440745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prabhakaran S, Zarahn E, Riley C, Speizer A, Chong JY, Lazar RM, et al. Inter-individual variability in the capacity for motor recovery after ischemic stroke. Neurorehabil Neural Repair. 2008 Jan-Feb;22(1):64–71. doi: 10.1177/1545968307305302. [DOI] [PubMed] [Google Scholar]
- 4.Jorgensen HS, Nakayama H, Raaschou HO, Olsen TS. Stroke. Neurologic and functional recovery the Copenhagen Stroke Study. Phys Med Rehabil Clin N Am. 1999 Nov;10(4):887–906. [PubMed] [Google Scholar]
- 5.Hankey GJ, Spiesser J, Hakimi Z, Bego G, Carita P, Gabriel S. Rate, degree, and predictors of recovery from disability following ischemic stroke. Neurology. 2007 May 8;68(19):1583–1587. doi: 10.1212/01.wnl.0000260967.77422.97. [DOI] [PubMed] [Google Scholar]
- 6.Zeiler SR, Gibson EM, Hoesch RE, Li MY, Worley PF, O'Brien RJ, et al. Medial premotor cortex shows a reduction in inhibitory markers and mediates recovery in a mouse model of focal stroke. Stroke. 2013 Feb;44(2):483–489. doi: 10.1161/STROKEAHA.112.676940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ng KL, Gibson EM, Hubbard R, Yang J, Caffo B, O'Brien RJ, et al. Fluoxetine Maintains a State of Heightened Responsiveness to Motor Training Early After Stroke in a Mouse Model. Stroke. 2015 Aug 20; doi: 10.1161/STROKEAHA.115.010471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeiler SR, Krakauer JW. The interaction between training and plasticity in the poststroke brain. Curr Opin Neurol. 2013 Dec;26(6):609–616. doi: 10.1097/WCO.0000000000000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muller HD, Hanumanthiah KM, Diederich K, Schwab S, Schabitz WR, Sommer C. Brain-derived neurotrophic factor but not forced arm use improves long-term outcome after photothrombotic stroke and transiently upregulates binding densities of excitatory glutamate receptors in the rat brain. Stroke. 2008 Mar;39(3):1012–1021. doi: 10.1161/STROKEAHA.107.495069. [DOI] [PubMed] [Google Scholar]
- 10.Schabitz WR, Berger C, Kollmar R, Seitz M, Tanay E, Kiessling M, et al. Effect of brain-derived neurotrophic factor treatment and forced arm use on functional motor recovery after small cortical ischemia. Stroke. 2004 Apr;35(4):992–997. doi: 10.1161/01.STR.0000119754.85848.0D. [DOI] [PubMed] [Google Scholar]
- 11.Ploughman M, Windle V, MacLellan CL, White N, Dore JJ, Corbett D. Brain-derived neurotrophic factor contributes to recovery of skilled reaching after focal ischemia in rats. Stroke. 2009 Apr;40(4):1490–1495. doi: 10.1161/STROKEAHA.108.531806. [DOI] [PubMed] [Google Scholar]
- 12.Nudo RJ, Wise BM, SiFuentes F, Milliken GW. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science. 1996 Jun 21;272(5269):1791–1794. doi: 10.1126/science.272.5269.1791. [DOI] [PubMed] [Google Scholar]
- 13.Nudo RJ, Milliken GW. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. J Neurophysiol. 1996 May;75(5):2144–2149. doi: 10.1152/jn.1996.75.5.2144. [DOI] [PubMed] [Google Scholar]
- 14.Farr TD, Whishaw IQ. Quantitative and qualitative impairments in skilled reaching in the mouse (Mus musculus) after a focal motor cortex stroke. Stroke. 2002 Jul;33(7):1869–1875. doi: 10.1161/01.str.0000020714.48349.4e. [DOI] [PubMed] [Google Scholar]
- 15.Paxinos G, Franklin KBJ. Plates. In: Paxinos G, Franklin KBJ, editors. The mouse brain in stereotaxic coordinates. 2nd ed. San Diego: Academic Press; 2001. pp. 49–350. [Google Scholar]
- 16.Tennant KA, Adkins DL, Donlan NA, Asay AL, Thomas N, Kleim JA, et al. The organization of the forelimb representation of the C57BL/6 mouse motor cortex as defined by intracortical microstimulation and cytoarchitecture. Cereb Cortex. 2011 Apr;21(4):865–876. doi: 10.1093/cercor/bhq159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JK, Park MS, Kim YS, Moon KS, Joo SP, Kim TS, et al. Photochemically induced cerebral ischemia in a mouse model. Surgical neurology. 2007 Jun;67(6):620–625. doi: 10.1016/j.surneu.2006.08.077. discussion 5. [DOI] [PubMed] [Google Scholar]
- 18.Carmichael ST, Archibeque I, Luke L, Nolan T, Momiy J, Li S. Growth-associated gene expression after stroke: evidence for a growth-promoting region in peri-infarct cortex. Experimental neurology. 2005 Jun;193(2):291–311. doi: 10.1016/j.expneurol.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Carmichael ST. Cellular and molecular mechanisms of neural repair after stroke: making waves. Ann Neurol. 2006 May;59(5):735–742. doi: 10.1002/ana.20845. [DOI] [PubMed] [Google Scholar]
- 20.Clarke J, Mala H, Windle V, Chernenko G, Corbett D. The effects of repeated rehabilitation "tune-ups" on functional recovery after focal ischemia in rats. Neurorehabil Neural Repair. 2009 Nov;23(9):886–894. doi: 10.1177/1545968309341067. [DOI] [PubMed] [Google Scholar]
- 21.Winters C, van Wegen EE, Daffertshofer A, Kwakkel G. Generalizability of the Proportional Recovery Model for the Upper Extremity After an Ischemic Stroke. Neurorehabil Neural Repair. 2014 Dec 11; doi: 10.1177/1545968314562115. [DOI] [PubMed] [Google Scholar]
- 22.Duncan PW, Lai SM, Keighley J. Defining post-stroke recovery: implications for design and interpretation of drug trials. Neuropharmacology. 2000 Mar 3;39(5):835–841. doi: 10.1016/s0028-3908(00)00003-4. [DOI] [PubMed] [Google Scholar]
- 23.Frost SB, Barbay S, Friel KM, Plautz EJ, Nudo RJ. Reorganization of remote cortical regions after ischemic brain injury: a potential substrate for stroke recovery. J Neurophysiol. 2003 Jun;89(6):3205–3214. doi: 10.1152/jn.01143.2002. [DOI] [PubMed] [Google Scholar]
- 24.Clarkson AN, Overman JJ, Zhong S, Mueller R, Lynch G, Carmichael ST. AMPA receptor-induced local brain-derived neurotrophic factor signaling mediates motor recovery after stroke. J Neurosci. 2011 Mar 9;31(10):3766–3775. doi: 10.1523/JNEUROSCI.5780-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clarkson AN, Huang BS, Macisaac SE, Mody I, Carmichael ST. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature. 2010 Nov 11;468(7321):305–309. doi: 10.1038/nature09511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cramer SC, Chopp M. Recovery recapitulates ontogeny. Trends in neurosciences. 2000 Jun;23(6):265–271. doi: 10.1016/s0166-2236(00)01562-9. [DOI] [PubMed] [Google Scholar]
- 27.Espinosa JS, Stryker MP. Development and plasticity of the primary visual cortex. Neuron. 2012 Jul 26;75(2):230–249. doi: 10.1016/j.neuron.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hensch TK. Controlling the critical period. Neuroscience research. 2003 Sep;47(1):17–22. doi: 10.1016/s0168-0102(03)00164-0. [DOI] [PubMed] [Google Scholar]
- 29.Maya Vetencourt JF, Sale A, Viegi A, Baroncelli L, De Pasquale R, O'Leary OF, et al. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science. 2008 Apr 18;320(5874):385–388. doi: 10.1126/science.1150516. [DOI] [PubMed] [Google Scholar]
- 30.Silingardi D, Scali M, Belluomini G, Pizzorusso T. Epigenetic treatments of adult rats promote recovery from visual acuity deficits induced by long-term monocular deprivation. Eur J Neurosci. 2010 Jun;31(12):2185–2192. doi: 10.1111/j.1460-9568.2010.07261.x. [DOI] [PubMed] [Google Scholar]
- 31.Beurdeley M, Spatazza J, Lee HH, Sugiyama S, Bernard C, Di Nardo AA, et al. Otx2 binding to perineuronal nets persistently regulates plasticity in the mature visual cortex. J Neurosci. 2012 Jul 4;32(27):9429–9437. doi: 10.1523/JNEUROSCI.0394-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis MF, Figueroa Velez DX, Guevarra RP, Yang MC, Habeeb M, Carathedathu MC, et al. Inhibitory Neuron Transplantation into Adult Visual Cortex Creates a New Critical Period that Rescues Impaired Vision. Neuron. 2015 May 20;86(4):1055–1066. doi: 10.1016/j.neuron.2015.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Conner JM, Chiba AA, Tuszynski MH. The basal forebrain cholinergic system is essential for cortical plasticity and functional recovery following brain injury. Neuron. 2005 Apr 21;46(2):173–179. doi: 10.1016/j.neuron.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 34.Gharbawie OA, Karl JM, Whishaw IQ. Recovery of skilled reaching following motor cortex stroke: do residual corticofugal fibers mediate compensatory recovery? Eur J Neurosci. 2007 Dec;26(11):3309–3327. doi: 10.1111/j.1460-9568.2007.05874.x. [DOI] [PubMed] [Google Scholar]
- 35.Duval C, Panisset M, Strafella AP, Sadikot AF. The impact of ventrolateral thalamotomy on tremor and voluntary motor behavior in patients with Parkinson's disease. Experimental brain research Experimentelle Hirnforschung Experimentation cerebrale. 2006 Apr;170(2):160–171. doi: 10.1007/s00221-005-0198-4. [DOI] [PubMed] [Google Scholar]
- 36.Miller WC, DeLong MR. Parkinsonian symptomatology. An anatomical and physiological analysis. Annals of the New York Academy of Sciences. 1988;515:287–302. doi: 10.1111/j.1749-6632.1988.tb32998.x. [DOI] [PubMed] [Google Scholar]
- 37.DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends in neurosciences. 1990 Jul;13(7):281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- 38.Filion M, Tremblay L. Abnormal spontaneous activity of globus pallidus neurons in monkeys with MPTP-induced parkinsonism. Brain Res. 1991 Apr 26;547(1):142–151. [PubMed] [Google Scholar]
- 39.Stetler RA, Leak RK, Gan Y, Li P, Zhang F, Hu X, et al. Preconditioning provides neuroprotection in models of CNS disease: paradigms and clinical significance. Prog Neurobiol. 2014 Mar;114:58–83. doi: 10.1016/j.pneurobio.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu XQ, Sheng R, Qin ZH. The neuroprotective mechanism of brain ischemic preconditioning. Acta pharmacologica Sinica. 2009 Aug;30(8):1071–1080. doi: 10.1038/aps.2009.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Narayanan SV, Dave KR, Perez-Pinzon MA. Ischemic preconditioning and clinical scenarios. Curr Opin Neurol. 2013 Feb;26(1):1–7. doi: 10.1097/WCO.0b013e32835bf200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chollet F, Tardy J, Albucher JF, Thalamas C, Berard E, Lamy C, et al. Fluoxetine for motor recovery after acute ischaemic stroke (FLAME): a randomised placebo-controlled trial. Lancet Neurol. 2011 Feb;10(2):123–130. doi: 10.1016/S1474-4422(10)70314-8. [DOI] [PubMed] [Google Scholar]