Abstract
Aims/hypothesis
Adipose tissue dysfunction is a prime risk factor for the development of metabolic disease. Bone morphogenetic proteins (BMPs) have previously been implicated in adipocyte formation. Here, we investigate the role of BMP signalling in adipose tissue health and systemic glucose homeostasis.
Methods
We employed the Cre/loxP system to generate mouse models with conditional ablation of BMP receptor 1A in differentiating and mature adipocytes, as well as tissue-resident myeloid cells. Metabolic variables were assessed by glucose and insulin tolerance testing, insulin-stimulated glucose uptake and gene expression analysis.
Results
Conditional deletion of Bmpr1a using the aP2 (also known as Fabp4)-Cre strain resulted in a complex phenotype. Knockout mice were clearly resistant to age-related impairment of insulin sensitivity during normal and high-fat-diet feeding and showed significantly improved insulin-stimulated glucose uptake in brown adipose tissue and skeletal muscle. Moreover, knockouts displayed significant reduction of variables of adipose tissue inflammation. Deletion of Bmpr1a in myeloid cells had no impact on insulin sensitivity, while ablation of Bmpr1a in mature adipocytes partially recapitulated the initial phenotype from aP2-Cre driven deletion. Co-cultivation of macrophages with pre-adipocytes lacking Bmpr1a markedly reduced expression of proinflammatory genes.
Conclusions/interpretation
Our findings show that altered BMP signalling in adipose tissue affects the tissue’s metabolic properties and systemic insulin resistance by altering the pattern of immune cell infiltration. The phenotype is due to ablation of Bmpr1a specifically in pre-adipocytes and maturing adipocytes rather than an immune cell-autonomous effect. Mechanistically, we provide evidence for a BMP-mediated direct crosstalk between pre-adipocytes and macrophages.
Electronic supplementary material
The online version of this article (doi:10.1007/s00125-016-3990-8) contains peer-reviewed but unedited supplementary material, which is available to authorised users.
Keywords: Adipose tissue, Ageing, Bone morphogenetic proteins, Insulin sensitivity, Macrophage infiltration
Introduction
Obesity is recognised as a significant risk factor for several of our most common medical conditions, such as type 2 diabetes mellitus and diseases associated with cardiovascular complications [1–3]. The majority of adipose tissue in the body is white adipose tissue (WAT), which stores energy as triacylglycerols and secretes adipokines [4]. The second type of fat, brown adipose tissue (BAT), expends energy in a process known as thermogenesis [5].
Normal adipose tissue displays a low-grade inflammation, which is presumably due to removal of apoptotic adipocytes. In obese individuals, WAT becomes a significant source of proinflammatory cytokines, which are known to promote systemic insulin resistance [6]. Specifically, increased infiltration of macrophages that surround the dead adipocytes, forming the so-called crown-like structures, is a source of these proinflammatory signals [7–9]. Recently, other immune cell populations, such as regulatory T cells and neutrophils, have also been implicated in these processes [10]. Adipose tissue-resident macrophages (ATMs) assume either proinflammatory or anti-inflammatory phenotypes termed M1 and M2, respectively. A general shift from a predominantly M2-like phenotype in healthy, lean WAT towards an M1 phenotype in inflamed, obese WAT is well documented [10, 11]. Generally, it should be noted that obesity leads to increased infiltration of all macrophage types, although accumulation of proinflammatory M1 ATMs greatly exceeds that of alternatively activated M2 ATMs [12, 13].
Bone morphogenetic proteins (BMPs) are members of the TGFβ protein superfamily. The role of BMPs in the regulation of adipose biology and energy metabolism has only recently become a field of interest [14–20]. Several BMPs are known to induce adipogenesis in a concentration-dependent manner; low concentrations promote adipogenesis while high concentrations are anti-adipogenic and, instead, promote osteochondrogenesis [21–24]. We recently discovered that BMP signalling plays an important role in the formation of brown adipocytes [15, 16, 25]. However, the role of BMPs in the physiological function of mature, adult WAT has not been addressed in detail. In our previous study, conditional deletion of the type 1A BMP receptor (Bmpr1a) using the Myf5-Cre driver led to a specific atrophy of interscapular BAT and compensatory browning of WATs, altogether establishing the metabolic equivalence of brite/beige adipose tissue and classical BAT [15]. To investigate BMP signalling in a broader spectrum of adipocytes, we deleted Bmpr1a in pre-adipocytes and adipocytes, targeting both BAT and WAT. Unexpectedly, the development of insulin resistance with increased age was prevented in knockout mice, suggesting that the role of BMP signalling in adipocyte function is highly context-dependent.
Methods
A detailed description of the methods is included in the electronic supplementary material (ESM).
Animals
All animal procedures were performed according to the Guide for the Care and Use of Laboratory Animals (http://grants.nih.gov/grants/olaw/Guide-for-the-Care-and-Use-of-Laboratory-Animals.pdf) and were approved by the Institutional Animal Care and Use Committee at Joslin Diabetes Center. Mice with aP2-Cre-driven deletion of the floxed Bmpr1a allele were generated and maintained as described previously [15, 26].
Insulin tolerance testing
For the insulin tolerance test (ITT), mice were fasted for 2 h on the morning of the experiment before receiving an i.p. dose of 1.5 IU/(kg body weight) of recombinant human insulin (Humalog; Lilly, Indianapolis, IN, USA). Blood was collected from the tail vein for measurement of blood glucose levels before and 15, 30 and 60 min after injections.
Glucose tolerance testing
Mice were fasted overnight (16 h) prior to i.p. injection of 2 g/(kg body weight) of glucose using a 20% (w/v) solution. Blood glucose was measured before and 15, 30, 60 and 120 min after injection.
Serum analysis
Analyses of serum insulin, leptin, triacylglycerols, NEFA, TNFα and IL-6 were performed using standard colorimetric assays and ELISA procedures.
Insulin-stimulated glucose uptake
The procedure was performed as described previously, with minor modifications (see ESM Methods) [27].
Protein expression analysis
Analysis of gene expression on the protein level was performed as described previously [15]. Antibodies are specified in ESM Methods.
Gene expression analysis
Total RNA isolation and gene expression analysis was conducted as described previously [15]. Primer sequences are listed in ESM Table 1.
Analysis of adipocyte size
Adipocytes were analysed using ImageJ software (U.S. National Institutes of Health, Bethesda, MD) [28].
Analysis of tissue-resident macrophages and blood monocytes
ATMs were analysed using FACS of freshly isolated stromal-vascular fractions of WAT as described previously [15].
Analysis of physiology
Body composition, activity levels and energy expenditure were assessed as described previously [15].
Cell culture
Pre-adipocytes were cultured as described previously [15]. Macrophages were collected from the peritoneal cavity of untreated, healthy mice.
Statistical analysis
The data are presented as means ± SEM. Statistical significance was defined as p < 0.05 and determined by Student’s t test or two-way ANOVA when comparing multiple groups. In cases of unequal variance and non-normal distribution, non-parametric testing was conducted (Mann–Whitney U test).
Results
Loss of BMP receptor 1A in adipose tissue prevents age-related decline in insulin sensitivity
BMP signalling regulates early and late stages of adipocyte differentiation [20]. Therefore, we chose to use the aP2 promoter to drive adipose-specific expression of Cre recombinase to generate a tissue-specific deletion of Bmpr1a in mouse adipose tissues (aP2-Bmpr1a-KO) [26]. As previously described, these mice displayed significantly reduced expression of Bmpr1a in BAT and WAT and a significant depletion of brown and brite/beige adipocytes [15]. Knockout mice were born smaller, had reduced bone length and maintained a trend of reduced body weight, lean mass and fat mass when body composition was analysed at 6 months of age on normal diet and after high-fat diet (HFD) feeding (ESM Fig. 1). Activity levels were not altered and energy expenditure tended to be reduced in aP2-Bmpr1a-KOs, but the latter was no longer apparent when normalised to body weight or lean mass (ESM Fig. 1). Histological evaluation of WAT revealed no changes in morphology, white adipocyte size or accumulation of fibrosis (ESM Fig. 2). Somewhat unexpectedly, we observed reduced expression and lower circulating levels of leptin, while expression of adiponectin remained unchanged (ESM Fig. 3). These findings suggest that lower leptin expression may be a direct effect of reduced BMP signalling rather than be due to reduced adipocyte size. In the absence of exogenous ligand treatment, we observed reduced phosphorylation of one of the main BMP target pathways, p38 mitogen-activated protein kinase (p38MAPK), but no changes of mothers against DPP homolog (SMAD)-1/5 phosphorylation in epididymal WAT (eWAT), whereas no changes in either pathway were observed in inguinal WAT (iWAT) (ESM Fig. 4).
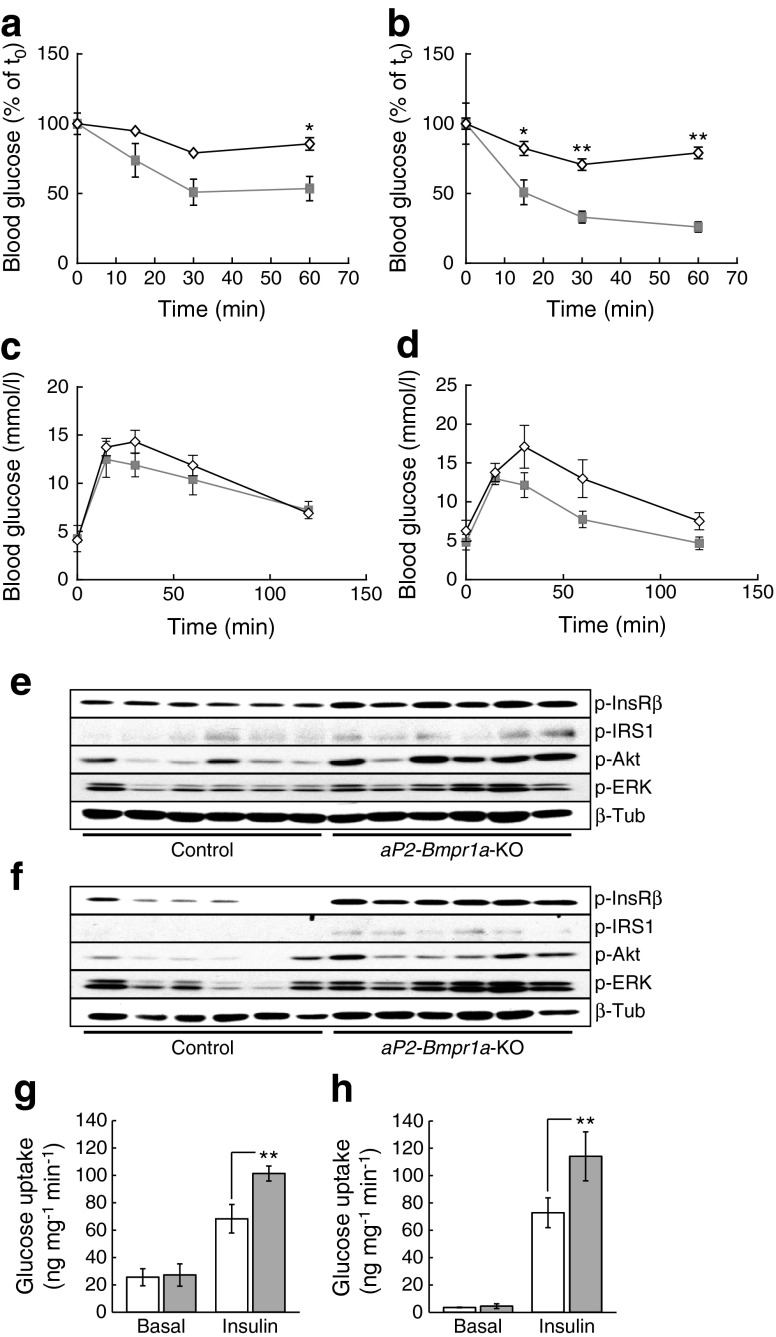
To analyse glucose homeostasis in more detail, we conducted ITTs and GTTs in mice either maintained on a normal diet or on an HFD containing 45% of energy from fat (45%HFD). Interestingly, aP2-Bmpr1a-KO mice on both diets displayed improved insulin sensitivity (Fig. 1a, b) and similar results were obtained for aged, but not young, mice maintained on 60%HFD (ESM Fig. 5). Glucose tolerance, on the other hand, showed a trend towards (but not significant) improvement on either diet when assessed at 52 weeks of age (Fig. 1c, d). Blood glucose, serum insulin and lipid levels remained unchanged at this age, although insulin levels tended to be lower in knockout mice on both diets (ESM Fig. 6).
Fig. 1.
Loss of Bmpr1a in adipose tissue improves insulin sensitivity. (a, b) ITT in 38-week-old mice maintained on a normal chow diet (a) (AUC: p = 0.0286) or in 40-week-old mice maintained on 45%HFD from 4–5 weeks of age (b) (AUC: p = 0.0043). Diamonds, control mice; squares, aP2-Bmpr1a-KO mice. Data are shown as means ± SEM (n = 4 for both groups in a; n = 5 for control and n = 6 for knockout in b). *p < 0.05 and **p < 0.01 compared with control mice (c, d) GTT in 50-week-old mice fed either a normal chow diet (c) (AUC: p = 0.3429) or 45%HFD (d) (AUC: p = 0.6095). Diamonds, control mice; squares, aP2-Bmpr1a-KO mice. Data are shown as means ± SEM (n = 4 for both groups in c; n = 5 for control and n = 6 for knockout in d). (e, f) Western blot analysis of insulin-stimulated activation of the insulin signalling pathway in iWAT (e) and eWAT (f). Levels of the phosphorylated forms of insulin receptor-β (p-InsRβ), insulin receptor substrate (p-IRS)1, protein kinase B (p-Akt) and extracellular-signal regulated kinase (p-ERK) were detected and normalised to basal expression of β-tubulin (β-Tub). Quantification is shown in ESM Fig. 7. (g, h) Unstimulated (Basal) and insulin-stimulated glucose uptake (Insulin) in BAT (g) and tibialis anterior skeletal muscle (h). White bars, control mice; grey bars, aP2-Bmpr1a-KO mice. Data are shown as means ± SEM (n = 7 for basal control; n = 6 for basal knockout; n = 8 for insulin control; n = 6 for insulin knockout). **p < 0.01 compared with control mice
To further explore this phenotype, we assessed the activation of the insulin signalling cascade following insulin stimulation. In this cohort, mice were maintained on 60%HFD until approximately 32 weeks of age. Consistent with the improved insulin sensitivity phenotype, phosphorylation of several members of the insulin signalling cascade was significantly enhanced in iWAT or eWAT of the knockout mice (Fig. 1e, f and ESM Fig. 7). BAT has recently been recognised as a significant glucose sink upon exposure to cold [29]. Despite the previously reported atrophy of BAT [15], aP2-Bmpr1a-KO mice displayed significant elevation of glucose uptake in the residual brown fat and skeletal muscle in response to insulin stimulation compared with their control littermates (Fig. 1g, h).
Loss of Bmpr1a reduces proinflammatory gene expression and attenuates macrophage infiltration into adipose tissue
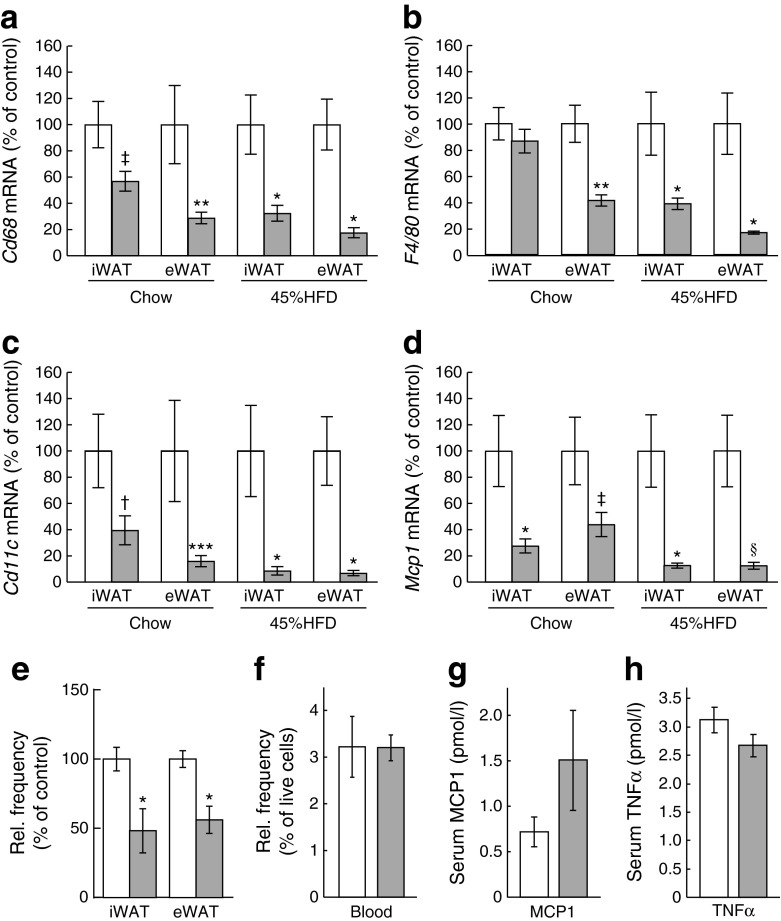
The link between insulin resistance and obesity-related adipose tissue immune cell infiltration is well established [30]. Therefore, we investigated whether expression of proinflammatory markers was reduced in aP2-Bmpr1a-KO mice. Indeed, gene expression of typical macrophage markers, such as Cd68, F4/80 (also known as Adgre1), Cd11c (Itgax) and Mcp1 (Ccl2), were significantly reduced in both inguinal and epididymal fat pads of aP2-Bmpr1a-KO mice maintained on either standard chow or a 45%HFD (Fig. 2a–d). Similar trends were also observed in mice maintained on a 60%HFD until 1 year of age, while no differences in inflammatory gene expression were observed in young mice on normal diet (ESM Fig. 8). To address the role of macrophages in this phenotype, we next quantified macrophage infiltration. Infiltration with CD45+/CD11b+/F4/80+ macrophages in WAT of knockout mice was significantly diminished whereas frequencies of peripheral blood monocytes were unchanged, suggesting that reduced macrophage infiltration occurred within the adipose tissue (Fig. 2e, f). This was consistent with the unchanged levels of the circulating proinflammatory cytokines monocyte chemotactic protein 1 (MCP1) and TNFα (Fig. 2g, h).
Fig. 2.
Loss of Bmpr1a protects adipose tissue from macrophage infiltration. (a–d) Gene expression analysis of macrophage markers Cd68 (a), F4/80 (b), Cd11c (c) and Mcp1 (d) in iWAT and eWAT of mice maintained on chow diet or 45%HFD until 52 weeks of age. White bars, control mice; grey bars, aP2-Bmpr1a-KO mice. Data are shown as means ± SEM (n = 4–8 mice/group). (e, f) Flow-cytometric analysis of tissue-resident macrophages (surface markers: CD45+;CD11b+; F4/80+;CD3e−;CD19−;CD49b−;Ter119−) in iWAT and eWAT (e) (n = 6 for control and n = 5 for knockout) and circulating monocytes from whole blood (f) (n = 3 for both groups). (g, h) ELISA quantification of serum levels of MCP1 (g) and TNFα (h) (n = 6 for control; n = 4 for knockout). *p < 0.05, **p < 0.01, ***p < 0.001,† p = 0.05, ‡ p = 0.065, § p = 0.067 compared with control mice of the same treatment group and/or tissue type
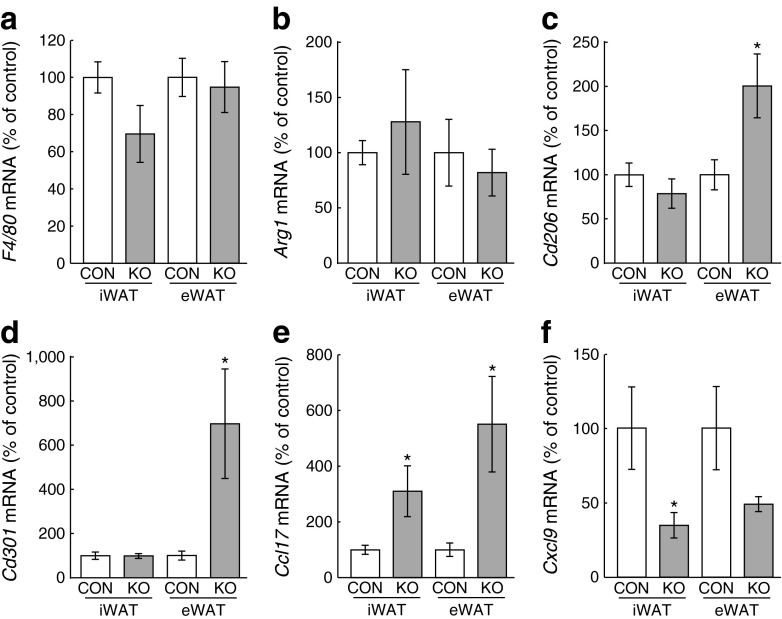
To determine whether macrophage activation was altered in aP2-Bmpr1a-KO mice, we next quantified expression of typical macrophage activation markers [31, 32]. To this end, we isolated macrophages from both WAT depots by flow cytometry. While mRNA levels of a general macrophage marker (F4/80) and a well-established M2 marker (Arg1) were unchanged (Fig. 3a, b), expression of other M2 markers (Cd206 [Mrc1] and Cd301 [Clec10a]) was upregulated in eWAT, but not iWAT, of aP2-Bmpr1a-KO mice (Fig. 3c, d). Accordingly, expression levels of the M2-related Ccl17 and the M1-related Cxcl9 were upregulated and downregulated, respectively (Fig. 3e, f) [32]. Expression of other established M1 markers, such as Il1b or inducible nitric oxide synthase, were not altered (data not shown).
Fig. 3.
Anti-inflammatory polarisation of ATMs in Bmpr1a-deficient adipose tissue. Gene expression analysis of F4/80 (a), Arg1 (b), Cd206 (c), Cd301 (d), Ccl17 (e) and Cxcl9 (f) in FACS-purified macrophages (surface markers: CD45+;CD11b+;F4/80+;CD3e−;CD19−;CD49b−;Ter119−) isolated from mouse iWAT and eWAT. White bars, control mouse macrophages; grey bars, aP2-Bmpr1a-KO mouse macrophages. Data are shown as means ± SEM. Macrophage isolation experiments were repeated with two or three mice/genotype and two or three independent experiments were carried out for mice maintained on an HFD. mRNA yield from sorted macrophages was limited and gene expression data from the individual experiments were pooled for statistical analysis (n = 9 for control and n = 8 for knockout in a, e, and f; control: n = 6 for control and n = 5 for knockout in b–d). *p < 0.05 compared with control mice of the same tissue type
Loss of BMP signalling in myeloid cells does not affect insulin sensitivity
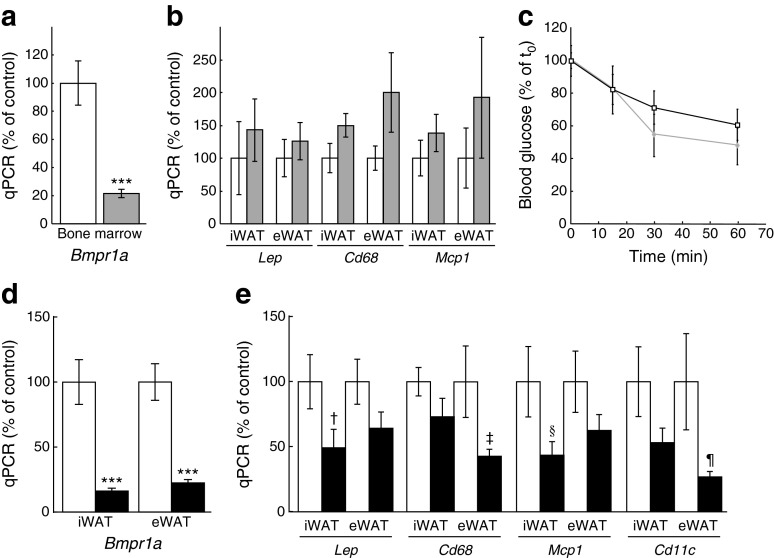
Previous studies have demonstrated that aP2 is also expressed in cell types other than adipocytes. Specifically, it is also expressed in macrophages, where aP2 plays a role in foam cell formation [33]. These findings raise the possibility that use of aP2-Cre may also result in gene deletion in macrophages infiltrating the adipose tissue. While this possibility is still valid, a recent study using the same aP2-Cre strain as us showed no Cre-mediated recombination in adipose tissue macrophages [34]. Consistent with this report, Bmpr1a mRNA levels were not changed in macrophages sorted from WAT of aP2-Bmpr1a-KO mice when compared with WAT from control mice (ESM Fig. 9). Nevertheless, to determine whether loss of BMP signalling in macrophages could still be responsible for reduced adipose tissue macrophage infiltration and improved insulin sensitivity, we generated a mouse model with myeloid-specific ablation of BMP receptor 1A (BMPR1A) using the LysM (also known as Lyz2)-Cre mouse strain [35]. Efficient ablation of BMPR1A expression was observed in tissues with a high content of myeloid cells, such as bone marrow, in LysM-Bmpr1a-KO mice (Fig. 4a). In this strain, body and tissue weights were unchanged (data not shown) and gene expression levels of Lep, Cd68 and Mcp1, which were significantly decreased in the aP2-Bmpr1a-KO mice, were unchanged in iWAT and eWAT of 5-month-old mice (Fig. 4b). Moreover, insulin sensitivity was not altered in 12-month-old LysM-Bmpr1a-KO mice compared with control mice under high-fat feeding (Fig. 4c). Hence, the improved insulin sensitivity in aP2-Bmpr1a-KO mice cannot be attributed to deletion of Bmpr1a in macrophages.
Fig. 4.
Loss of Bmpr1a in adipocytes, but not myeloid cells, reduces macrophage infiltration. (a) Gene expression analysis of Bmpr1a mRNA in bone marrow of mice with LysM-Cre-driven deletion of Bmpr1a (LysM-Bmpr1a-KO). White bars, control mice; grey bars, LysM-Bmpr1a-knockout mice. Data are shown as means ± SEM (n = 3/group). (b) mRNA levels of leptin and macrophage infiltration markers Cd68 and Mcp1 in WAT depots of LysM-Bmpr1a-KO mice. White bars, control mice; grey bars, LysM-Bmpr1a-knockout mice. Data are shown as means ± SEM (n = 3/group). (c) ITT in HFD-fed LysM-Bmpr1a-KO mice at 52 weeks of age. Squares, control mice; diamonds, LysM-Bmpr1a-KO mice. Data are shown as means ± SEM (n = 7 mice/group). (d) Bmpr1a mRNA levels in WAT depots of knockout mice with Adipoq-Cre-driven deletion of Bmpr1a (Adipoq-Bmpr1a-KO). White bars, control mice; black bars, Adipoq-Bmpr1a-KO mice. Data are shown as means ± SEM (n = 7 for control and n = 6 for knockout). (e) mRNA levels of leptin and macrophage infiltration markers Cd68, Mcp1 and Cd11c in WAT of Adipoq-Bmpr1a-KO mice. Data are shown as means ± SEM (n = 7 for control and n = 6 for knockout). ***p < 0.001, † p = 0.078, ‡ p = 0.084, § p = 0.096, ¶ p = 0.097 compared with control mice of the same treatment group and/or tissue type. qPCR, quantitative real-time PCR
Loss of Bmpr1a in mature adipocytes improves the inflammatory gene expression profile
To determine whether the improved insulin sensitivity in aP2-Bmpr1a-KO mice can be directly linked to adipocyte-specific changes, we generated a third mouse model using the Adipoq-driven Cre mouse strain (Adipoq-Cre). Unlike aP2-Cre, which also causes recombination in adipogenic progenitor cells [36], Adipoq-Cre is expressed exclusively in mature adipocytes, thus targeting a more restricted population of cells within WAT [34]. In Adipoq-Bmpr1a-KO mice, decreased Bmpr1a mRNA levels were observed in WAT (Fig. 4d). Further analysis revealed a trend towards decreased gene expression of Lep and macrophage markers Cd68, Mcp1 and Cd11c (Fig. 4e). However, body weight, adipose tissue weight and insulin sensitivity analysed by GTT or ITT remained unaltered in Adipoq-Bmpr1a-KO mice on a normal diet or after 5 months of high-fat feeding (ESM Fig. 10).
Loss of Bmpr1a in pre-adipocytes directly affects activation and cytokine expression patterns in macrophages
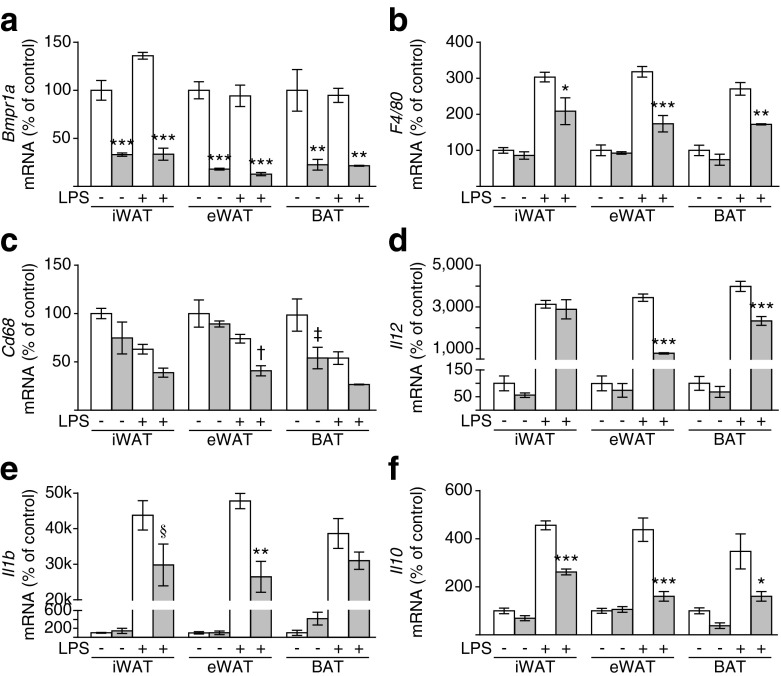
To determine whether interactions between macrophages and adipocyte progenitors could be responsible for the more pronounced phenotype of the aP2-Bmpr1a-KO mice, we used a co-culture approach (see ESM Fig. 11a for experimental scheme). Macrophages were isolated from wild-type C57BL/6J mice and pre-adipocytes were isolated from mice carrying a homozygous floxed Bmpr1a allele. Isolated pre-adipocytes were infected with adenoviruses either expressing green fluorescent protein or Cre recombinase to generate pre-adipocytes with intact or impaired BMP signalling, respectively. Lipopolysaccharide (LPS) was added to the co-culture to activate expression of inflammatory cytokines from macrophages (ESM Fig. 11). Four days post infection, control or Bmpr1a-deficient progenitor cells isolated from white and brown adipose depots were co-cultured with macrophages for 24 h. Measurement of Bmpr1a mRNA levels showed a significant reduction in co-cultures with Cre-infected pre-adipocytes and pure cultures of Cre-infected pre-adipocytes (Fig. 5a and ESM Fig. 11b). Importantly, expression of macrophage-specific marker genes (such as F4/80 and Cd68) and LPS-induced cytokines (Il1b, Il10 and Il12) was significantly reduced in co-cultures of knockout pre-adipocytes and macrophages (Fig. 5b–f). These data provide a potential cellular mechanism for the phenotypes observed in aP2-Bmpr1a-KO mice.
Fig. 5.
Co-cultivation of macrophages with Bmpr1a-KO pre-adipocytes reduces macrophage activation. Gene expression analysis of Bmpr1a (a), F4/80 (b), Cd68 (c), Il12 (d), Il1b (e) and Il10 (f) was carried out in co-cultures of macrophages with pre-adipocytes isolated by flow cytometry from iWAT, eWAT and BAT of Bmpr1a-KO mice. White bars, pre-adipocytes infected with adenovirus expressing the gene for green fluorescent protein (control); grey bars, pre-adipocytes infected with adenovirus expressing Cre to cause deletion of the floxed Bmpr1a allele. Macrophages were isolated from non-floxed wild-type mice and co-cultured with pre-adipocytes for 24 h alone (−) or in the presence of LPS (+). Data are shown as means ± SEM (n = 3).*p < 0.05, **p < 0.01, ***p < 0.001, † p = 0.07, ‡ p = 0.06, § p = 0.09 compared with control cells of the same treatment group as assessed by ANOVA for each tissue type separately
Discussion
In the present study, we address the physiological effects of adipose tissue BMP signalling on glucose homeostasis and insulin sensitivity. We report that WAT displays a marked reduction in macrophage infiltration and improved insulin sensitivity, which develops with increased age in mice with adipose-specific deletion of Bmpr1a. In those mice, the response to insulin stimulation is enhanced locally within the adipose tissue, as well as at the systemic level, as signified by improved insulin sensitivity and elevated insulin-stimulated glucose uptake in skeletal muscle. This phenotype can be explained, at least in part, by reduced macrophage infiltration into WAT and reduced proinflammatory polarisation of ATMs due to loss of BMP signalling in the adipocytic lineage. Importantly, myeloid-specific deletion of Bmpr1a does not affect WAT inflammation or insulin sensitivity, indicating that reduced BMP signalling in macrophages does not contribute to this phenotype.
We previously demonstrated that loss of BMPR1A specifically impairs brown adipogenesis [15]. This occurred in classical interscapular BAT using a Myf5-Cre driver and, similarly, in aP2-Bmpr1a-KO mice where an impaired formation of brown and brite/beige adipocytes was observed [15]. The novel findings presented here are surprising since it is commonly assumed that brown adipocytes confer beneficial metabolic features and promote an insulin-sensitive state. For instance, transplantation of BAT to the visceral cavity of mice resulted in a marked improvement of glucose tolerance [37]. Consistent with these data, the residual BAT in aP2-Bmpr1a-KO mice appears to be more insulin sensitive and this could offset the overall effects of BAT atrophy to some degree. On the other hand, it is well known that immune cells and proinflammatory processes play a major role in the development of insulin resistance [38]. Interestingly, increased expression of Bmpr1a in WAT correlates with insulin resistance in human obesity, as reported by Boettcher et al [39]. This study also reports that individuals with impaired glucose tolerance or overt diabetes show increased expression levels of BMPR1A in WAT [39]. These findings support the notion that changed expression levels of BMPR1A in WAT could regulate insulin sensitivity.
Since aP2-Cre potentially deletes Bmpr1a in macrophages in addition to adipogenic cells, an important aspect of our study is to determine whether loss of Bmpr1a in either the adipogenic or myeloid lineages leads to improved insulin sensitivity. However, consistent with a previous report [34], we found expression of Bmpr1a in macrophages isolated from WAT of aP2-Bmpr1a-KO mice to be unchanged. Additionally, deletion of Bmpr1a in myeloid cells, which include macrophages, does not recapitulate the phenotype of the aP2-driven knockouts. The role of BMPs in inflammatory processes is rather complex. Some studies have reported anti-inflammatory effects on macrophages and other immune cells [40, 41], while others show that active BMP signalling may promote inflammation, a process that seems to be highly ligand-specific [42].
In a previous study it was reported that aP2-Cre is active in the heart and interstitial cells of the skeletal muscle [34]. Muscle-resident interstitial cells are known to possess high adipogenic potential and are involved in myogenic regeneration [25, 43]. This could therefore help explain the improved insulin sensitivity observed in muscle of aP2-Bmpr1a-KO mice. Alternatively, an endocrine effect of a healthier adipose tissue releasing different adipokines that affect muscle insulin sensitivity is possible. As the Adipoq-Bmpr1a-KO strain only partially recapitulates the phenotype of the aP2-Cre driven knockout mice, these findings, taken together, suggest that reduced BMP signalling in adipogenic progenitor cells is a key factor in this process. This supposition is strongly supported by our observation that co-cultivation with Bmpr1a-deficient pre-adipocyte blunts expression of proinflammatory markers. It is thus conceivable that the phenotype observed in aP2-Bmpr1a-KO mice is due to loss of BMP signalling within the adipogenic lineage, comprised of pre-adipocytes and mature adipocytes. It is also conceivable that the differences in manifestation of the phenotype using the two adipose-specific Cre-lines are related to differences in the timing of Cre expression in both models that occurs later (i.e. only in mature adipocytes in the Adipoq-Cre strain). Thus, alterations of signalling through BMPR1A during the earlier stages of white adipocyte differentiation, rather than in fully mature adipocytes, could be critical to the reduction of proinflammatory signals and improved insulin sensitivity.
‘Inflamm-ageing’ is a concept encompassing age-related deterioration of the innate immune system response, low-grade chronic inflammation and the onset of age-related pathologies such as insulin resistance [44]. In a well-described vicious circle, chemoattractants originating from senescent adipocytes and pre-adipocytes promote increased infiltration by proinflammatory immune cells, which in turn exacerbate the negative metabolic properties of adipocytes [45]. Thus, altered BMP signalling in adipogenic cells might affect the release of adipokines that regulate recruitment to and function of immune cells within adipose tissue. In aged animals, predominantly proinflammatory immune cells (i.e. M1 macrophages) are recruited and reducing infiltration with these immune cells would, therefore, attenuate the development of insulin resistance [46]. In addition, ageing is accompanied by a switch from M2 anti-inflammatory macrophages towards proinflammatory M1 macrophages [47]. Hence, a model such as the aP2-Bmpr1a-KO mouse, where overall macrophage infiltration into adipose tissue is reduced, could retain a healthier metabolic profile due to a general lack of infiltrating immune cells.
In summary, our study provides new insight into the role of BMP signalling in maturing white adipocytes. In brown adipocytes, BMPs are critical for formation and thermogenic activity, whereas in white adipocytes, BMP signalling appears to regulate the endocrine interaction between cells of the adipose lineage and immune cells. A better understanding of these processes could help decipher the intricate crosstalk between adipocytes and other adipose tissue-resident cell types and this could provide novel avenues to counter the progression and pathology of insulin resistance.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(PDF 584 kb)
Acknowledgements
We thank J. LaVecchio and G. Buruzula of Joslin’s Flow Cytometry Core and M. Mulvey and A. Clermont of the Joslin’s Animal Physiology Core, as well as N. Dittberner, S. Richter and S. Trautwein from the German Institute of Human Nutrition for expert technical assistance. We thank H. Zhang from University of Copenhagen, Denmark, K. L. Townsend from University of Maine, USA and A. Slack and A. Pan from Joslin Diabetes Center for critical reading and editing of the manuscript.
Abbreviations
- ATM
Adipose tissue-resident macrophage
- BAT
Brown adipose tissue
- BMP
Bone morphogenetic protein
- BMPR1A
BMP receptor 1A
- eWAT
Epididymal WAT
- HFD
High-fat diet
- ITT
Insulin tolerance test
- iWAT
Inguinal WAT
- LPS
Lipopolysaccharide
- MCP1
Monocyte chemotactic protein 1
- WAT
White adipose tissue
Funding
This work was supported by National Institutes of Health grants NIDDK, R01DK077097 (to Y-HT) and P30DK036836 (to Joslin Diabetes Center’s Diabetes Research Center). Y-HT was also supported by a grant from the Harvard Stem Cell Institute and the American Diabetes Association (ADA7-12-BS-191). TJS was supported by a research fellowship from the Mary K. Iacocca Foundation, the German Research Foundation (SCHU 2445/1-1 and SCHU 2445/2-1), the European Research Council (ERC-StG-2012-311082) and the German Center for Diabetes Research (DZD). LJG was supported by a National Institutes of Health grant, R01 DK099511.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
All the authors provided substantial contributions to the conception and design of this study and/or to acquisition and analysis of the data. All the authors participated in drafting the article and approved the version to be published. Specifically, TJS performed experiments, analysed data and wrote the manuscript. AG, TLH, RX, DA, SP-K, AT and LEO performed experiments and analysed data. MDL performed experiments and reviewed the manuscript. MFH, MS, LJG and YM contributed to conception and design and reviewed the manuscript. Y-HT analysed data and wrote the manuscript. Y-HT and TJS are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Contributor Information
Tim J. Schulz, Email: Tim.Schulz@dife.de
Yu-Hua Tseng, Email: Yu-Hua.Tseng@joslin.harvard.edu.
References
- 1.Haslam DW, James WP. Obesity. Lancet. 2005;366:1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 2.LeRoith D, Novosyadlyy R, Gallagher EJ, Lann D, Vijayakumar A, Yakar S. Obesity and type 2 diabetes are associated with an increased risk of developing cancer and a worse prognosis; epidemiological and mechanistic evidence. Exp Clin Endocrinol Diabetes. 2008;116(suppl. 1):S4–S6. doi: 10.1055/s-2008-1081488. [DOI] [PubMed] [Google Scholar]
- 3.Tseng YH, Cypess AM, Kahn CR. Cellular bioenergetics as a target for obesity therapy. Nat Rev Drug Discov. 2010;9:465–482. doi: 10.1038/nrd3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bluher M. Adipokines—removing road blocks to obesity and diabetes therapy. Mol Metab. 2014;3:230–240. doi: 10.1016/j.molmet.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 6.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 7.Cinti S, Mitchell G, Barbatelli G, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 8.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI200319246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI200319451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathis D. Immunological goings-on in visceral adipose tissue. Cell Metab. 2013;17:851–859. doi: 10.1016/j.cmet.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herrero L, Shapiro H, Nayer A, Lee J, Shoelson SE. Inflammation and adipose tissue macrophages in lipodystrophic mice. Proc Natl Acad Sci U S A. 2010;107:240–245. doi: 10.1073/pnas.0905310107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56:16–23. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- 13.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuo MM, Kim S, Tseng CY, Jeon YH, Choe S, Lee DK. BMP-9 as a potent brown adipogenic inducer with anti-obesity capacity. Biomaterials. 2014;35:3172–3179. doi: 10.1016/j.biomaterials.2013.12.063. [DOI] [PubMed] [Google Scholar]
- 15.Schulz TJ, Huang P, Huang TL, et al. Brown-fat paucity due to impaired BMP signalling induces compensatory browning of white fat. Nature. 2013;495:379–383. doi: 10.1038/nature11943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tseng YH, Kokkotou E, Schulz TJ, et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008;454:1000–1004. doi: 10.1038/nature07221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang W, Yang Y, Meng Y, Shi Y. GDF-3 is an adipogenic cytokine under high fat dietary condition. Biochem Biophys Res Commun. 2004;321:1024–1031. doi: 10.1016/j.bbrc.2004.07.058. [DOI] [PubMed] [Google Scholar]
- 18.Whittle AJ, Carobbio S, Martins L, et al. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell. 2012;149:871–885. doi: 10.1016/j.cell.2012.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xue R, Wan Y, Zhang S, Zhang Q, Ye H, Li Y. Role of bone morphogenetic protein 4 in the differentiation of brown fat-like adipocytes. Am J Physiol Endocrinol Metab. 2014;306:E363–E372. doi: 10.1152/ajpendo.00119.2013. [DOI] [PubMed] [Google Scholar]
- 20.Zamani N, Brown CW. Emerging roles for the transforming growth factor-{beta} superfamily in regulating adiposity and energy expenditure. Endocr Rev. 2011;32:387–403. doi: 10.1210/er.2010-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taha MF, Valojerdi MR, Mowla SJ. Effect of bone morphogenetic protein-4 (BMP-4) on adipocyte differentiation from mouse embryonic stem cells. Anat Histol Embryol. 2006;35:271–278. doi: 10.1111/j.1439-0264.2006.00680.x. [DOI] [PubMed] [Google Scholar]
- 22.Asahina I, Sampath TK, Hauschka PV. Human osteogenic protein-1 induces chondroblastic, osteoblastic, and/or adipocytic differentiation of clonal murine target cells. Exp Cell Res. 1996;222:38–47. doi: 10.1006/excr.1996.0005. [DOI] [PubMed] [Google Scholar]
- 23.Bachner D, Ahrens M, Schroder D, et al. Bmp-2 downstream targets in mesenchymal development identified by subtractive cloning from recombinant mesenchymal progenitors (C3H10T1/2) Dev Dyn. 1998;213:398–411. doi: 10.1002/(SICI)1097-0177(199812)213:4<398::AID-AJA5>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 24.Chen TL, Shen WJ, Kraemer FB. Human BMP-7/OP-1 induces the growth and differentiation of adipocytes and osteoblasts in bone marrow stromal cell cultures. J Cell Biochem. 2001;82:187–199. doi: 10.1002/jcb.1145. [DOI] [PubMed] [Google Scholar]
- 25.Schulz TJ, Huang TL, Tran TT, et al. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc Natl Acad Sci U S A. 2011;108:143–148. doi: 10.1073/pnas.1010929108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mishina Y, Hanks MC, Miura S, Tallquist MD, Behringer RR. Generation of Bmpr/Alk3 conditional knockout mice. Genesis. 2002;32:69–72. doi: 10.1002/gene.10038. [DOI] [PubMed] [Google Scholar]
- 27.Stanford KI, Middelbeek RJ, Townsend KL, et al. A Novel role for subcutaneous adipose tissue in exercise-induced improvements in glucose homeostasis. Diabetes. 2015;64:2361–2368. doi: 10.2337/db15-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bartelt A, Bruns OT, Reimer R, et al. Brown adipose tissue activity controls triglyceride clearance. Nat Med. 2011;17:200–205. doi: 10.1038/nm.2297. [DOI] [PubMed] [Google Scholar]
- 30.McNelis JC, Olefsky JM. Macrophages, immunity, and metabolic disease. Immunity. 2014;41:36–48. doi: 10.1016/j.immuni.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 31.Martinez FO, Gordon S (2014) The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000 Prime Reports 6:13 [DOI] [PMC free article] [PubMed]
- 32.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Makowski L, Boord JB, Maeda K, et al. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat Med. 2001;7:699–705. doi: 10.1038/89076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee KY, Russell SJ, Ussar S, et al. Lessons on conditional gene targeting in mouse adipose tissue. Diabetes. 2013;62:864–874. doi: 10.2337/db12-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265–277. doi: 10.1023/A:1008942828960. [DOI] [PubMed] [Google Scholar]
- 36.Shan TZ, Liang XR, Bi PP, Zhang PP, Liu WY, Kuang SH. Distinct populations of adipogenic and myogenic Myf5-lineage progenitors in white adipose tissues. J Lipid Res. 2013;54:2214–2224. doi: 10.1194/jlr.M038711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stanford KI, Middelbeek RJ, Townsend KL, et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest. 2013;123:215–223. doi: 10.1172/JCI62308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell. 2014;156:20–44. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bottcher Y, Unbehauen H, Kloting N, et al. Adipose tissue expression and genetic variants of the bone morphogenetic protein receptor 1A gene (BMPR1A) are associated with human obesity. Diabetes. 2009;58:2119–2128. doi: 10.2337/db08-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rocher C, Singla R, Singal PK, Parthasarathy S, Singla DK. Bone morphogenetic protein 7 polarizes THP-1 cells into M2 macrophages. Can J Physiol Pharmacol. 2012;90:947–951. doi: 10.1139/y2012-102. [DOI] [PubMed] [Google Scholar]
- 41.Tan HC, Poh CK, Cai Y, Soe MT, Wang W. Covalently grafted BMP-7 peptide to reduce macrophage/monocyte activity: an in vitro study on cobalt chromium alloy. Biotechnol Bioeng. 2013;110:969–979. doi: 10.1002/bit.24756. [DOI] [PubMed] [Google Scholar]
- 42.Grgurevic L, Christensen GL, Schulz TJ, Vukicevic S. Bone morphogenetic proteins in inflammation, glucose homeostasis and adipose tissue energy metabolism. Cytokine Growth Factor Rev. 2016;27:105–118. doi: 10.1016/j.cytogfr.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 43.Joe AW, Yi L, Natarajan A, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cevenini E, Caruso C, Candore G, et al. Age-related inflammation: the contribution of different organs, tissues and systems. How to face it for therapeutic approaches. Curr Pharm Des. 2010;16:609–618. doi: 10.2174/138161210790883840. [DOI] [PubMed] [Google Scholar]
- 45.Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112:1785–1788. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tchkonia T, Morbeck DE, Von Zglinicki T, et al. Fat tissue, aging, and cellular senescence. Aging Cell. 2010;9:667–684. doi: 10.1111/j.1474-9726.2010.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lumeng CN, Liu J, Geletka L, et al. Aging is associated with an increase in T cells and inflammatory macrophages in visceral adipose tissue. J Immunol. 2011;187:6208–6216. doi: 10.4049/jimmunol.1102188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 584 kb)