Abstract
Background
Platelet-rich plasma (PRP) contains high concentrations of autologous growth factors that originate from platelets. Intra-articular injections of PRP have the potential to ameliorate the symptoms of osteoarthritis in the knee. Superficial zone protein (SZP) is a boundary lubricant in articular cartilage and plays an important role in reducing friction and wear and therefore is critical in cartilage homeostasis.
Purpose
To determine if PRP influences the production of SZP from human joint-derived cells and to evaluate the lubricating properties of PRP on normal bovine articular cartilage.
Study Design
Controlled laboratory study.
Methods
Cells were isolated from articular cartilage, synovium, and the anterior cruciate ligament (ACL) from 12 patients undergoing ACL reconstruction. The concentrations of SZP in PRP and culture media were measured by enzyme-linked immunosorbent assay. Cellular proliferation was quantified by determination of cell numbers. The lubrication properties of PRP from healthy volunteers on bovine articular cartilage were investigated using a pin-on-disk tribometer.
Results
In general, PRP stimulated proliferation in cells derived from articular cartilage, synovium, and ACL. It also significantly enhanced SZP secretion from synovium- and cartilage-derived cells. An unexpected finding was the presence of SZP in PRP (2.89 ± 1.23 µg/mL before activation and 3.02 ± 1.32 µg/mL after activation). In addition, under boundary mode conditions consisting of high loads and low sliding speeds, nonactivated and thrombin-activated PRP decreased the friction coefficient (μ = 0.012 and μ = 0.015, respectively) compared with saline (μ = 0.047, P < 0.004) and high molecular weight hyaluronan (μ = 0.080, P < 0.006). The friction coefficient of the cartilage with PRP was on par with that of synovial fluid.
Conclusion
PRP significantly stimulates cell proliferation and SZP secretion by articular cartilage and synovium of the human knee joint. Furthermore, PRP contains endogenous SZP and, in a functional bioassay, lubricates bovine articular cartilage explants.
Clinical Relevance
These findings provide evidence to explain the biochemical and biomechanical mechanisms underlying the efficacy of PRP treatment for osteoarthritis or damage in the knee joint.
Keywords: platelet-rich plasma, superficial zone protein, PRG4, lubricin, osteoarthritis, lubrication
INTRODUCTION
Normal articular cartilage maintains a well-lubricated surface with an extremely low coefficient of friction to minimize wear and promote lifelong painless joint mobility.8 Superficial zone protein (SZP), also known as lubricin or PRG4,17,18 is synthesized and secreted into synovial fluid (SF) by the surface zone articular chondrocytes and synoviocytes in the synovium.31,37 In addition, SZP acts as a chondroprotective barrier against direct solid-to-solid contact in joints when kinematic conditions, such as high loading and low sliding speeds, are conducive to surface sliding in the boundary lubrication mode.27,30 Finally, SZP is encoded by the PRG4 gene. A mutation in this gene has been shown to result in CACP (camptodactyly-arthropathy-coxa vara-pericarditis) syndrome in humans. This syndrome results in early-onset noninflammatory joint damage and failure with a loss of superficial zone chondrocytes, fouling of the articular surface, and synovial hyperplasia.5,26 Mice lacking the PRG4 gene are born with normal joints but develop a CACP-like phenotype during maturation with an attendant increase in friction and decrease in cartilage stiffness.9,35 The pathophysiology of joints lacking functional copies of the PRG4 gene demonstrates the importance of SZP to synovial joint development and homeostasis.27
Cartilage lubrication also plays a role in the progression of osteoarthritis (OA). Studies of induced and posttraumatic OA in small and large animal models have shown that SZP production and SF lubricity decrease after injury.12,20 These results have been observed and corroborated in humans with early and chronic knee OA.11,25 Animal studies suggest that intra-articular administration of recombinant or purified SZP can reduce cartilage degeneration after knee injury.15,19 Interestingly, in humans with advanced OA who required total knee replacement surgery, SZP expression in the arthritic cartilage is elevated relative to age-matched controls, suggestive of a late-stage compensation mechanism.29 Overall, animal models suggest that maintaining or restoring cartilage lubrication may be important for the prevention or treatment of OA.
Concentrates of autologous platelet-rich plasma (PRP) have been utilized with increasing frequency in the treatment of musculoskeletal maladies, such as chronic sports-related injuries of the muscles and tendons, owing to their degenerative nature and the tissues’ limited capacity for self-repair.28 The appeal of stimulating tissue regeneration by PRP is based on the presence of growth factors and cytokines in the platelets, which induce cellular proliferation, migration, differentiation, and matrix synthesis.2,3,14 More recently, several clinical studies showed significant improvement with PRP treatment for OA compared with hyaluronan/hyaluronic acid injection and placebo.7,13,32,36,40 A systematic review concluded that multiple intra-articular PRP injections might have beneficial effects in the treatment of mild to moderate knee OA at 6 months.22
In addition to cell proliferation, differentiation, and matrix synthesis, the functional mechanisms of PRP in OA treatment have been explained by its effect on modulating inflammation and angiogenesis, as well as maintaining joint homeostasis.2,14 However, despite the increased interest in PRP use for the treatment of OA, the precise mechanisms and effects of PRP on knee joint tissues remain unclear. To elucidate how PRP might be effective in the treatment of OA of the knee, this investigation sought to examine the effects of PRP on SZP production by synovium-, articular cartilage–, and anterior cruciate ligament (ACL)–derived cells. In addition, the lubrication properties of PRP and its effects on articular cartilage friction were also evaluated.
METHODS
Samples
Articular cartilage, synovium, and torn ACL remnants were obtained from 12 patients undergoing ACL reconstructive surgery (mean age, 26.7 ± 7.1 years). Articular cartilage was obtained from a standard notchplasty during the ACL reconstruction, and synovial tissue was obtained from the area of the fat pad. None of the patients had evidence of articular cartilage injury or degeneration in the knee at the time of arthroscopy. All subjects provided informed consent to have their discarded tissues used for the experimental assays. The study was approved by the Institutional Review Board of our institution.
Cell Isolation and Expansion
Tissues were minced into small pieces and digested with 0.2% collagenase P (Roche) and 3% fetal bovine serum (FBS; Gibco) in Medium A consisting of DMEM/F12 (Gibco) supplemented with 50 µg/mL of ascorbate-2-phosphate (Sigma-Aldrich), 0.1% bovine serum albumin (Sigma-Aldrich), and antibiotics at 37°C. The digestion time was 2 hours for synovium tissues and 3 hours for cartilage and ACL tissues. Cells released from the tissues were filtered through a 70-µm cell strainer (BD Biosciences) and rinsed with DMEM/F-12. The isolated cells were seeded onto a culture plate for expansion with Medium A supplemented with 10% FBS at 37°C in a humidified 5% CO2 incubator. Culture media was changed twice a week. Proliferated cells from the second passage were used in experiments.
PRP Preparation
Whole blood samples were collected from 7 healthy donors after informed consent was obtained. The healthy blood donors were different from the patients who donated the tissues, articular cartilage, synovium, and ACL. The Autologous Conditioned Plasma Double Syringe System (Arthrex) was used to prepare PRP in accordance with the manufacturer’s instructions. Two previously published methods were utilized for activation: (1) 100 U/mL of bovine thrombin was added to PRP and incubated for 30 minutes at room temperature1; (2) PRP was frozen with liquid nitrogen for 1 minute and thawed at 37°C for 10 minutes for 3 cycles (freeze/thaw).41 Activated PRP was centrifuged for 5 minutes at 8000 revolutions per minute, and the supernatant was harvested. Nonactivated PRP aliquots were also centrifuged.
Cell Proliferation Assay
Cells were seeded in a monolayer at a density of 1 × 105 cells/well (2.5 × 104 cells/cm2) in 12-well culture plates (Corning) in Medium A with 10% FBS and incubated for 24 hours. Next, the medium was changed to fresh Medium A with 1% ITS+ Premix (BD Biosciences) containing 10% PRP and incubated for 3 days. As the untreated control group, the cells were cultured in the Medium A with 1% ITS+ Premix without 10% PRP. Triplicate cell samples were then enumerated by 2 independent observers manually with a hemocytometer with blinded condition, and the numbers were averaged. The culture media was harvested for protein analysis.
Enzyme-Linked Immunosorbent Assay for SZP
Since the majority of SZP is secreted into the culture medium,37 the media was harvested after the 3-day PRP treatment, and SZP media accumulation was measured by an enzyme-linked immunosorbent assay (ELISA) with purified bovine SZP as a standard.31 Levels of SZP from activated and nonactivated PRP were also measured. Briefly, each well of 96-well MaxiSorp plates (Nalge Nunc) was coated with 1 mg/mL of peanut lectin (EY Laboratories) in 50 mM sodium carbonate buffer (pH 9.5). The wells were then blocked with 1% bovine serum albumin in the same buffer for at least 2 hours. Aliquots of culture medium were incubated in the wells. Thereafter, the wells were incubated overnight with monoclonal antibody S6.79 (1:5000; a generous gift from Dr T. Schmid, Rush Medical College) as the primary antibody at 4°C and then incubated at room temperature for 1 hour with goat anti-mouse IgG conjugated with horseradish peroxidase (1:3000; Bio-Rad) as the secondary antibody. SuperSignal ELISA Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific) was added, and the results were quantified in a luminometer. The wells were washed with phosphate buffered saline containing 0.05% Tween 20 after each step. Concentrations of SZP were calculated using a bovine SZP standard, which was purified by affinity chromatography on a peanut lectin column. Purity was verified by immunoblot analysis. The concentration of the SZP standard was quantified using a Micro BCA Protein Assay Kit (Thermo Fisher Scientific).24,31
Friction Testing
Cartilage tissue samples for determining the lubrication properties of PRP were obtained from the lateral femoral condyle of 3-month-old bovine stifle joints. The 4 cartilage samples were harvested from each animal, and the samples examined were obtained from a pair of joints from 1 calf. Before the initiation of each friction experiment, the cylindrical cartilage explants (5 mm diameter × 4 mm thick) were equilibrated in 5 mL of the test lubricant for 2 minutes before measurement to minimize any fluid effects during tests. For friction coefficient measurement, cylindrical explants slide against a glass plate in a pin-on-disk tribometer at a speed of 0.5 mm/second and an average contact pressure of 100 kPa for 5 minutes. For comparison, saline, high molecular weight hyaluronan (Orthovisc; Anika Therapeutics), and bovine SF (harvested by micropipette from bovine joint during dissection of the articular cartilage plug) were also examined. The data were analyzed using a standard software package (Excel; Microsoft).
Statistical Analysis
To investigate the effect of PRP on cell proliferation and SZP production from each cell source, a sample size of 10 to 12 was used. For determining the SZP content of PRP, 7 healthy donors were used. In the cell proliferation assay, the values were calculated relative to their respective control groups. Values are presented as the mean + SD. A 1-way analysis of variance (ANOVA) was performed for the cell proliferation and SZP content of PRP results. For analysis of SZP accumulation due to PRP treatment, a 2-way ANOVA was used. If a significant difference was found, a Tukey-Kramer post hoc test was conducted. For the cartilage friction test, a pairwise Student t test was employed. P values < 0.05 were considered significant. Treatment groups (a, b, c, d) and factors (cell source: α, β, γ; treatment: A, B, C) not connected by the same letter were found to be significantly different. All statistical analyses were performed using the JMP statistical software package (SAS).
RESULTS
Stimulation of Cell Proliferation by PRP in Knee Joint Tissues
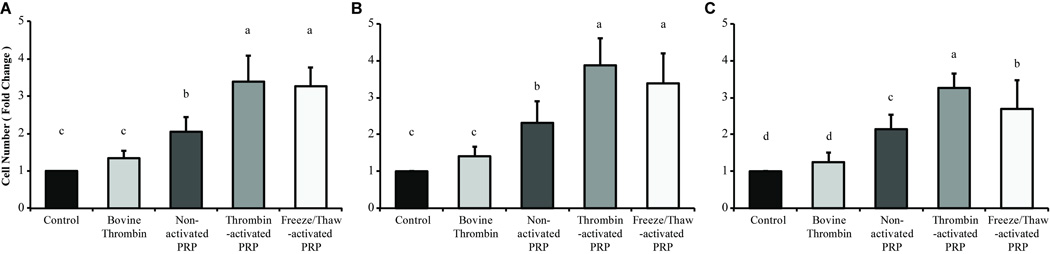
Treatment with PRP significantly influenced cell proliferation in all cells examined (P < 0.0001). For all cell sources, nonactivated and activated PRP treatment significantly stimulated cell proliferation when compared with control and thrombin-treated groups (Figure 1). In addition, PRP activation methods had no effect on proliferation in articular cartilage–derived cells (Figure 1A), synovium-derived cells (Figure 1B), and ACL-derived cells (Figure 1C). Only in ACL-derived cells, freeze/thaw-activated PRP did not show significant difference from nonactivated PRP (Figure 1C). Since bovine thrombin was used as a method of PRP activation, the effects of thrombin alone were also examined. As expected, thrombin did not significantly affect cell proliferation. Notably, thrombin-activated PRP increased cell proliferation around 4-fold greater than control cultures in synovium-derived cells.
Figure 1.
Platelet-rich plasma (PRP) treatment stimulates the proliferation of cells derived from synovial joint components. Joint tissue-derived cells were cultured with or without PRP treatment for 3 days in monolayer culture. Triplicate cell cultures were manually counted with a hemocytometer. The values were calculated relative to the control group as fold change in each cell source: (A) articular cartilage-derived cells, (B) synovium-derived cells, and (C) anterior cruciate ligament–derived cells. Among the cell sources, bars sharing the same letter (a, b, c, d) indicate no statistically significant differences, and ab indicates no significant differences with a or b. PRP resulted in greater cell proliferation than bovine thrombin-treated and control cultures, with activated PRP (thrombin- and freeze/thaw-activated PRP) producing the greatest increases. The activation method had no effect, except in anterior cruciate ligament–derived cell cultures. Values are presented as mean + SD for 12 samples per group.
Stimulation of SZP Synthesis by PRP in Knee Joint Tissues
To determine if PRP contained SZP before activation, an ELISA was performed to quantify the amount of SZP in culture medium supplemented with 10% PRP. After 10% PRP media was incubated without cells for 3 days under in vitro culture conditions, the SZP level of PRP in the incubated culture medium was 95.7 ± 3.7 µg/mL in nonactivated PRP, 108.6 ± 13.1 µg/mL in thrombin-activated PRP, and 98.2 ± 18.8 µg/mL in freeze/thaw-activated PRP. To account for this baseline concentration from endogenous SZP in incubated PRP in culture media, these concentrations were subtracted from the measurements of SZP in the culture media obtained from the cells in this investigation. The difference was considered to be newly synthesized SZP that accumulated in the medium from the monolayer cell cultures.
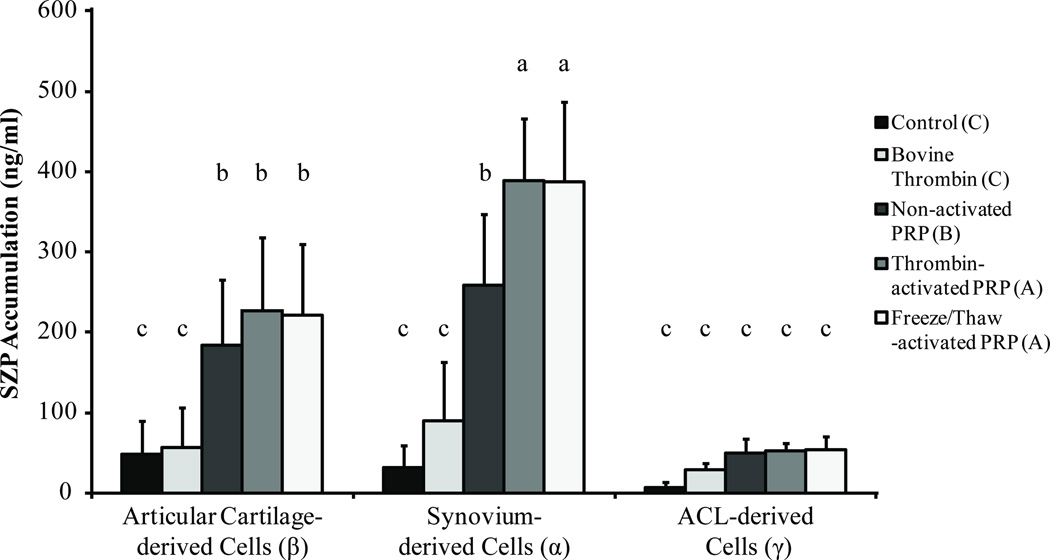
A 2-way ANOVA was used to compare the effects of cell source and PRP treatment on SZP production (Figure 2). Both cell source and treatment conditions had significant effects on SZP synthesis (P < 0.0001). Among all cell sources, synovium-derived cells produced the highest amounts of SZP, followed by articular cartilage–derived cells, which produced almost 58% as much SZP when stimulated with activated PRP. The ACL-derived cells synthesized the lowest amount SZP of the 3 tissues examined.
Figure 2.
Superficial zone protein (SZP) synthesis in synovial joint tissues is stimulated by platelet-rich plasma (PRP) treatment. Joint tissue-derived cells were cultured with or without PRP treatment for 3 days in monolayer culture, and SZP accumulation in the cell culture supernatants was quantified by enzyme-linked immunosorbent assay (ELISA). Both cell source and treatment condition had significant effects on SZP synthesis (P < 0.0001). Among all cell sources and treatments, groups of symbols— α, β, γ and A, B, C—indicate statistically significant differences. The post hoc test was conducted with each group, and a, b, and c indicate statistically significant differences with those not sharing the same letter. Treatment with PRP resulted in greater production of SZP from synovium-derived and articular cartilage–derived cells, with activated PRP (thrombin- and freeze/thaw-activated PRP) eliciting a greater increase in SZP synthesis as compared with nonactivated PRP. Bovine thrombin had no effect on SZP synthesis. Values are presented as mean + SD for 12 samples per group. ACL, anterior cruciate ligament.
With regard to the treatment, PRP significantly stimulated SZP accumulation compared with control and bovine thrombin treatment (P < 0.0001). Bovine thrombin alone had no effect on SZP synthesis (P = 0.27). Overall, activated PRP, whether by thrombin or freeze/thaw methods, induced significantly higher SZP accumulation than did nonactivated PRP (P < 0.001). No statistical differences were observed between activation methods (P = 0.99). Specifically, synovium-derived cells treated with activated PRP showed a ten-fold increase in SZP synthesis. Nonactivated PRP produced a 7-fold increase compared with untreated synovium-derived cells. Among articular cartilage–derived cells, PRP treatment induced a 4- to 5-fold increase in SZP accumulation compared with untreated control. The ACL-derived cells did not significantly increase SZP production in response to any of the employed treatments.
Presence of SZP in the PRP
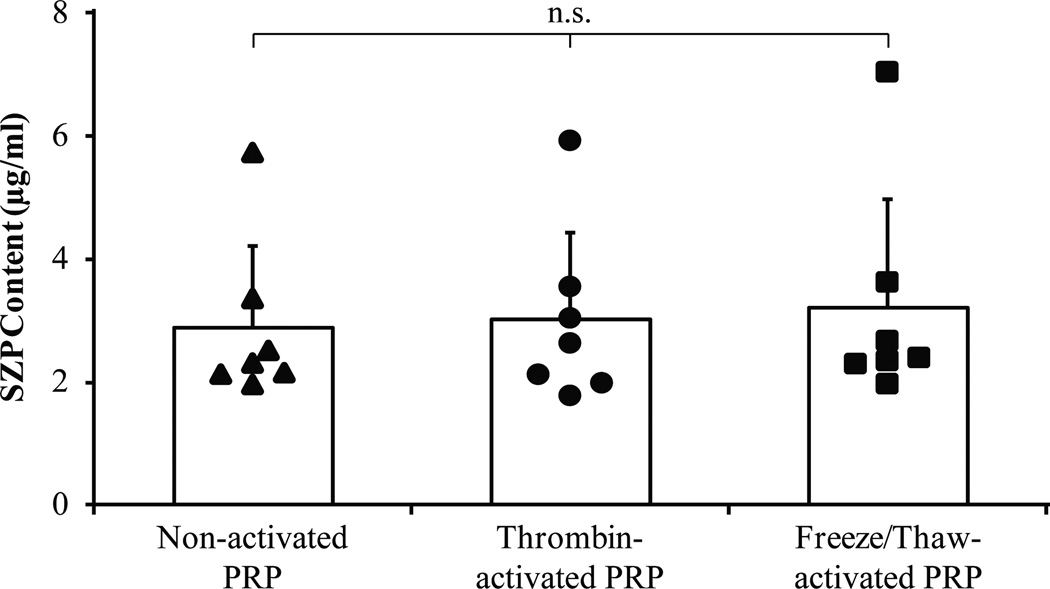
The SZP concentrations of nonactivated PRP, thrombin-activated PRP, and freeze/thaw-activated PRP were determined to be 2.89 ± 1.23 µg/mL, 3.02 ± 1.32 µg/mL, and 3.20 ± 1.64 µg/mL, respectively (Figure 3). When these PRP activation methods were compared using a 1-way ANOVA, the SZP content of PRP was not found to be influenced by the method of activation (P = 0.927).
Figure 3.
Activation does not affect endogenous levels of superficial zone protein (SZP) in platelet-rich plasma (PRP). Whole blood samples were collected from 7 healthy donors. Endogenous SZP concentration of nonactivated and activated PRP was quantified using enzyme-linked immunosorbent assay. The SZP content of PRP was not influenced by the 2 activation methods employed (P = .927). Values are presented as the mean + SD for 7 samples per group. ns, not significant.
Lubrication Properties of PRP
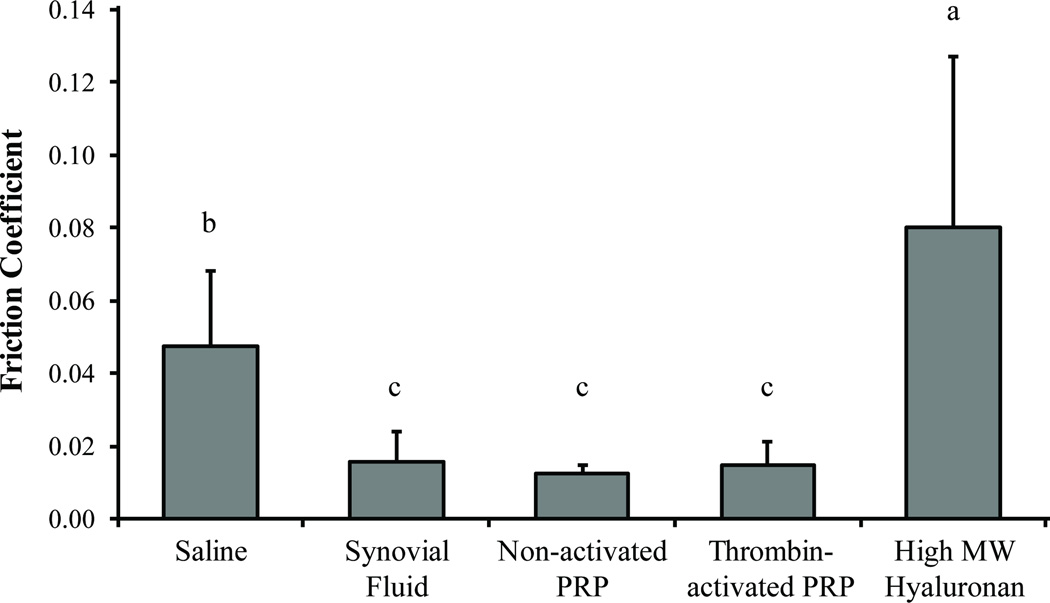
In general, PRP was found to lubricate cartilage equal to SF under boundary mode conditions (Figure 4). There were no significant differences (P > 0.77) in cartilage friction coefficient (μ) among nonactivated PRP (μ = 0.012), thrombin-activated PRP (μ = 0.015), and bovine SF (μ = 0.016). Saline (μ = 0.047) elicited significantly greater (P < 0.004) cartilage friction values than those of PRP or SF, with high molecular weight hyaluronan generating the greatest cartilage friction coefficient (μ = 0.080) of the lubricants examined (P < 0.006).
Figure 4.
Platelet-rich plasma lubricates a cartilage-glass interface as well as synovial fluid (SF) under boundary mode conditions. The lubrication properties of saline, bovine SF, nonactivated PRP, thrombin-activated PRP, and high molecular weight (MW) hyaluronan were tested in a pin-on-disk tribometer with a cartilage-glass interface operating under boundary mode lubrication conditions. High MW hyaluronan resulted in the greatest cartilage friction coefficient, followed by saline. Nonactivated PRP and thrombin-activated PRP lubricated cartilage as well as the positive control: bovine SF. Activation had no discernible effect on the lubrication properties of PRP. Bars bearing the same letter signify no statistically significant differences. Values are presented as mean + SD for 10 samples per group.
DISCUSSION
The treatment of synovial joint tissues with PRP elicits both biochemical and biophysical responses. The paramount finding of this study was that PRP treatment of synoviocytes and articular chondrocytes in vitro induced a significant increase in SZP synthesis: 10-fold increase in SZP produced by synovium-derived cells and a nearly 5-fold increase in SZP secreted by articular cartilage– derived cells, as compared with untreated controls. Furthermore, PRP unexpectedly contained a significant amount of endogenous SZP, suggesting that PRP could function as an effective boundary lubricant for articular cartilage. This inference was confirmed when we determined that nonactivated and thrombin-activated PRP produced cartilage friction coefficients equivalent to bovine SF in a cartilage friction coefficient assay. These results suggest that PRP may function to promote regeneration through enhanced cell proliferation, as well as inhibit cartilage degeneration by reducing friction and wear.
There are several reports on the effect of PRP on proliferation of the chondrocytes and synoviocytes. In chondrocytes, all previous reports demonstrated that PRP stimulates cell proliferation.2,14,22,32,39 However, some of these studies demonstrated that PRP only stimulates cell proliferation but does not enhance chondrogenic differentiation.10,16 Most others reported an increase in chondrocyte proliferation without affecting maintenance of the chondrogenic phenotype with addition of PRP as compared with a control.13,39 In addition, earlier work demonstrated that chondrocytes cultured in the presence of PRP synthesized significantly more proteoglycan and type II collagen and stimulated expression of Sox9 and aggrecan, as opposed to that cultured with human serum or FBS.1,41 In synoviocytes, Anitua et al4 reported that PRP induced a significant proliferative response in synoviocytes and secretion of extracellular matrix as compared with nonstimulated synoviocytes. In this study, we found that PRP enhanced cell proliferation of each cell source. Although the effect of PRP on extracellular matrix synthesis or cell metabolism was not measured, we found that PRP stimulates the SZP synthesis in this study.
Platelet activation increases the potency of PRP by leading to degranulation, or the release of platelet α-granule contents, and by supplying growth factors such as transforming growth factor β, vascular endothelial growth factor, and fibroblast growth factor.3 Two methods were employed to induce platelet degranulation: thrombin initiation of the clotting cascade1 and the mechanical rupture of the platelet membrane by freezing and thawing.41 There were no significant differences in efficacy of SZP production (Figure 2) or cellular proliferation (Figure 1) between these activation methods for synovium- and articular cartilage–derived cells. Activation of platelets also did not affect the concentration of SZP in the PRP, demonstrating that the SZP resides in the plasma rather than within the platelet granules (Figure 3). While bovine thrombin was employed in this study, previous studies have shown it to be similarly effective to human thrombin in initiating the clotting cascade.38 These results show that PRP activation is required for the maximal effect and suggest that a granules are the source of the biochemical signals in PRP.
In addition to stimulating SZP synthesis, PRP contains SZP and functions as a boundary lubricant; PRP contains a relatively modest concentration of SZP in comparison to normal human SF (Figure 3). Although SZP was reported to be present in plasma and serum,42 this study is the first to identify the presence and efficacy of SZP in PRP preparations. The reported concentration of SZP in human SF ranges from 250 to 287 µg/mL.11,25 In cases of primary and secondary OA, SZP levels and the boundary lubrication properties of human SF were found to be reduced as compared with SF from uninjured knees.11,19,25 Despite possessing a lower level of SZP, PRP was found to be an optimal lubricant for the cartilage (Figure 4) and may provide an autologous and renewable source for replenishing the diminished boundary-lubricating ability of SF in injured and arthritic knees. In addition to the SZP content in PRP, nonactivated PRP and thrombin-activated PRP showed similar friction coefficient. This result suggests that in terms of lubrication properties of PRP, plasma components in the PRP rather than growth factors may be the major contributor to boundary lubrication. While hyaluronan improves the hydrodynamic lubrication properties of SF through enhanced viscosity, the current study and others have shown that hyaluronan does not, in solo, contribute to cartilage lubrication under boundary mode conditions.27 In combination with SZP, hyaluronan contributes to synergistically reducing friction and dissipating shear forces within SF.21 Additional studies are needed to elucidate the lubricating components and biotribological properties of PRP and to determine any possible combinatorial effects with hyaluronan.
Activated PRP has been shown to contain transforming growth factor β1,3 a critical anabolic regulator of SZP synthesis.31 On the basis of this evidence, we hypothesized and demonstrated that administration of PRP would stimulate SZP synthesis from knee-derived cells. Our results support the concept that through inducing and supplementing SZP production in intra-articular cells, PRP may play an important role in maintaining the joint surface and overall joint homeostasis. It would seem plausible that enhanced production of SZP may prove beneficial as well in retarding degeneration of the articular surface and may account for the mechanism whereby improvement in symptoms in patients with knee arthritis has been observed after treatment with PRP.
Systematic reviews of in vitro studies showed an overall positive effect of PRP on cartilage tissue.2,14 Most findings support the role of PRP in increasing chondrocyte proliferation and matrix synthesis. In previous reports, PRP was found to influence the entire joint environment by attracting mesenchymal stem cells,23 modulating inflammation,33,43 maintaining joint homeostasis, and reducing pain.6 However, there is still a debate over the efficacy and use of PRP in joint tissues, and the mechanism of action is still unclear. For example, a standardized protocol for the preparation and characterization of PRP is needed. This is also a limitation of this study, as only 1 commercially available PRP preparation system was employed. It is plausible that PRP with higher platelet concentrations may stimulate greater SZP production. Future studies are needed to study and optimize a preparation method for PRP with respect to optimizing the potential benefits of PRP.
While we demonstrated the positive effects that PRP had on cell proliferation and SZP synthesis, we did not assess the effect of the PRP on cell migration or matrix synthesis and chondrocyte regeneration. In the previous reports, several publications (including reviews) demonstrated the positive effect of PRP on cell migration.2,23 However, the effect of PRP on the matrix synthesis and chondrogenic differentiation has not been fully understood and is still controversial. Spreafico et al41 suggested that chondrocytes expanded with PRP maintain their chondrogenic phenotype, while Drengk et al10 showed that reduced type II collagen production in PRP expanded chondrocytes. The difference in these findings may be related to different techniques in PRP preparation.
To our knowledge, this study is the first to demonstrate the biochemical and biophysical effects of PRP on SZP synthesis and cartilage boundary lubrication. In sum, PRP stimulated cell proliferation in ACL-, articular cartilage–, and synovium-derived cells, and SZP synthesis was enhanced in the latter 2 cell types. Of note, synovium was the most potent source of SZP production in response to PRP treatment. Furthermore, PRP contains endogenous SZP and may function as a boundary lubricant of articular cartilage. These findings are consistent with previous basic science and clinical studies that demonstrated the potential benefits of PRP in regenerative medicine,1,23,34,41 and they provide evidence for a mechanism of the clinical efficacy of PRP in the treatment of OA of the knee. In conclusion, PRP may serve as a cost-effective and autologous source for replenishing the diminished boundary-lubricating ability of SF in injured as well as arthritic knees.
Acknowledgments
The authors thank Dr. Thomas Schmid of Rush University for his gift of the antibody S6.79. This investigation was supported by the Lawrence J. Ellison Chair in Musculoskeletal Molecular Biology at the University of California, Davis, and the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award AR061496.
REFERENCES
- 1.Akeda K, An HS, Okuma M, et al. Platelet-rich plasma stimulates porcine articular chondrocyte proliferation and matrix biosynthesis. Osteoarthritis Cartilage. 2006;14(12):1272–1280. doi: 10.1016/j.joca.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Andia I, Maffulli N. Platelet-rich plasma for managing pain and inflammationin osteoarthritis. Nat Rev Rheumatol. 2013;9(12):721–730. doi: 10.1038/nrrheum.2013.141. [DOI] [PubMed] [Google Scholar]
- 3.Anitua E, Andia I, Ardanza B, Nurden P. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91(1):4–15. doi: 10.1160/TH03-07-0440. [DOI] [PubMed] [Google Scholar]
- 4.Anitua E, Sanchez M, Zalduendo MM, et al. Fibroblastic response to treatment with different preparations rich in growth factors. Cell Prolif. 2009;42(2):162–170. doi: 10.1111/j.1365-2184.2009.00583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bahabri SA, Suwairi WM, Laxer RM, Polinkovsky A, Dalaan AA, Warman ML. The camptodactyly-arthropathy-coxa vara-pericarditis syndrome: clinical features and genetic mapping to human chromosome 1. Arthritis Rheum. 1998;41(4):730–735. doi: 10.1002/1529-0131(199804)41:4<730::AID-ART22>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 6.Bendinelli P, Matteucci E, Dogliotti G, et al. Molecular basis of anti-inflammatory action of platelet-rich plasma on human chondrocytes: mechanisms of NF-kappaB inhibition via HGF. J Cell Physiol. 2010;225(3):757–766. doi: 10.1002/jcp.22274. [DOI] [PubMed] [Google Scholar]
- 7.Cerza F, Carni S, Carcangiu A, et al. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med. 2012;40(12):2822–2827. doi: 10.1177/0363546512461902. [DOI] [PubMed] [Google Scholar]
- 8.Charnley J. The lubrication of animal joints in relation to surgical reconstruction by arthroplasty. Ann Rheum Dis. 1960;19:10–19. doi: 10.1136/ard.19.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coles JM, Zhang L, Blum JJ, et al. Loss of cartilage structure, stiffness, and frictional properties in mice lacking PRG4. Arthritis Rheum. 2010;62(6):1666–1674. doi: 10.1002/art.27436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drengk A, Zapf A, Sturmer EK, Sturmer KM, Frosch KH. Influence of platelet-rich plasma on chondrogenic differentiation and proliferation of chondrocytes and mesenchymal stem cells. Cells Tissues Organs. 2009;189(5):317–326. doi: 10.1159/000151290. [DOI] [PubMed] [Google Scholar]
- 11.Elsaid KA, Fleming BC, Oksendahl HL, et al. Decreased lubricin concentrations and markers of joint inflammation in the synovial fluid of patients with anterior cruciate ligament injury. Arthritis Rheum. 2008;58(6):1707–1715. doi: 10.1002/art.23495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elsaid KA, Jay GD, Warman ML, Rhee DK, Chichester CO. Association of articular cartilage degradation and loss of boundary-lubricating ability of synovial fluid following injury and inflammatory arthritis. Arthritis Rheum. 2005;52(6):1746–1755. doi: 10.1002/art.21038. [DOI] [PubMed] [Google Scholar]
- 13.Filardo G, Kon E, Di Martino A, et al. Platelet-rich plasma vs hyaluronic acid to treat knee degenerative pathology: study design and preliminary results of a randomized controlled trial. BMC Musculoskelet Disord. 2012;13:229. doi: 10.1186/1471-2474-13-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filardo G, Kon E, Roffi A, Di Matteo B, Merli ML, Marcacci M. Plateletrich plasma: why intra-articular? A systematic review of preclinical studies and clinical evidence on PRP for joint degeneration. [published online November 26, 2013];Knee Surg Sports Traumatol Arthrosc. doi: 10.1007/s00167-013-2743-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flannery CR, Zollner R, Corcoran C, et al. Prevention of cartilage degeneration in a rat model of osteoarthritis by intraarticular treatment with recombinant lubricin. Arthritis Rheum. 2009;60(3):840–847. doi: 10.1002/art.24304. [DOI] [PubMed] [Google Scholar]
- 16.Gaissmaier C, Flitz J, Krackhardt T, Flesch I, Aicher WK, Ashammakhi N. Effect of human platelet supernatant on proliferation and matrix synthesis of human articular chondrocytes in monolayer and three dimensional alginate cultures. Biomaterials. 2005;26(14):1953–1960. doi: 10.1016/j.biomaterials.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 17.Ikegawa S, Sano M, Koshizuka Y, Nakamura Y. Isolation, characterization and mapping of the mouse and human PRG4 (proteoglycan 4) genes. Cytogenet Cell Genet. 2000;90(3–4):291–297. doi: 10.1159/000056791. [DOI] [PubMed] [Google Scholar]
- 18.Jay GD, Elsaid KA, Zack J, et al. Lubricating ability of aspirated synovial fluid from emergency department patients with knee joint synovitis. J Rheumatol. 2004;31(3):557–564. [PubMed] [Google Scholar]
- 19.Jay GD, Fleming BC, Watkins BA, et al. Prevention of cartilage degeneration and restoration of chondroprotection by lubricin tribosupplementation in the rat following anterior cruciate ligament transection. Arthritis Rheum. 2010;62(8):2382–2391. doi: 10.1002/art.27550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jay GD, Tantravahi U, Britt DE, Barrach HJ, Cha CJ. Homology of lubricin and superficial zone protein (SZP): products of megakaryocyte stimulating factor (MSF) gene expression by human synovial fibroblasts and articular chondrocytes localized to chromosome 1q25. J Orthop Res. 2001;19(4):677–687. doi: 10.1016/S0736-0266(00)00040-1. [DOI] [PubMed] [Google Scholar]
- 21.Jay GD, Torres JR, Warman ML, Laderer MC, Breuer KS. The role of lubricin in the mechanical behavior of synovial fluid. Proc Natl Acad Sci U S A. 2007;104(15):6194–6199. doi: 10.1073/pnas.0608558104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khoshbin A, Leroux T, Wasserstein D, et al. The efficacy of platelet-rich plasma in the treatment of symptomatic knee osteoarthritis: a systematic review with quantitative synthesis. Arthroscopy. 2013;29(12):2037–2048. doi: 10.1016/j.arthro.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Kruger JP, Hondke S, Endres M, Pruss A, Siclari A, Kaps C. Human platelet-rich plasma stimulates migration and chondrogenic differentiation of human subchondral progenitor cells. J Orthop Res. 2012;30(6):845–852. doi: 10.1002/jor.22005. [DOI] [PubMed] [Google Scholar]
- 24.Lee SY, Niikura T, Reddi AH. Superficial zone protein (lubricin) in the different tissue compartments of the knee joint: modulation by transforming growth factor beta 1 and interleukin-1 beta. Tissue Eng Part A. 2008;14(11):1799–1808. doi: 10.1089/ten.tea.2007.0367. [DOI] [PubMed] [Google Scholar]
- 25.Ludwig TE, McAllister JR, Lun V, Wiley JP, Schmidt TA. Diminished cartilage-lubricating ability of human osteoarthritic synovial fluid deficient in proteoglycan 4: Restoration through proteoglycan 4 supplementation. Arthritis Rheum. 2012;64(12):3963–3971. doi: 10.1002/art.34674. [DOI] [PubMed] [Google Scholar]
- 26.Marcelino J, Carpten JD, Suwairi WM, et al. CACP, encoding a secreted proteoglycan, is mutated in camptodactyly-arthropathy- coxa vara-pericarditis syndrome. Nat Genet. 1999;23(3):319–322. doi: 10.1038/15496. [DOI] [PubMed] [Google Scholar]
- 27.McNary SM, Athanasiou KA, Reddi AH. Engineering lubrication in articular cartilage. Tissue Eng Part B Rev. 2012;18(2):88–100. doi: 10.1089/ten.teb.2011.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moraes VY, Lenza M, Tamaoki MJ, Faloppa F, Belloti JC. Platelet-rich therapies for musculoskeletal soft tissue injuries. Cochrane Database Syst Rev. 2013;12:CD010071. doi: 10.1002/14651858.CD010071.pub2. [DOI] [PubMed] [Google Scholar]
- 29.Neu CP, Khalafi A, Komvopoulos K, Schmid TM, Reddi AH. Mechanotransduction of bovine articular cartilage superficial zone protein by transforming growth factor beta signaling. Arthritis Rheum. 2007;56(11):3706–3714. doi: 10.1002/art.23024. [DOI] [PubMed] [Google Scholar]
- 30.Neu CP, Reddi AH, Komvopoulos K, Schmid TM, Di Cesare PE. Increased friction coefficient and superficial zone protein expression in patients with advanced osteoarthritis. Arthritis Rheum. 2010;62(9):2680–2687. doi: 10.1002/art.27577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niikura T, Reddi AH. Differential regulation of lubricin/superficial zone protein by transforming growth factor beta/bone morphogenetic protein superfamily members in articular chondrocytes and synoviocytes. Arthritis Rheum. 2007;56(7):2312–2321. doi: 10.1002/art.22659. [DOI] [PubMed] [Google Scholar]
- 32.Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41(2):356–364. doi: 10.1177/0363546512471299. [DOI] [PubMed] [Google Scholar]
- 33.Pereira RC, Scaranari M, Benelli R, et al. Dual effect of platelet lysate on human articular cartilage: a maintenance of chondrogenic potential and a transient proinflammatory activity followed by an inflammation resolution. Tissue Eng Part A. 2013;19(11–12):1476–1488. doi: 10.1089/ten.TEA.2012.0225. [DOI] [PubMed] [Google Scholar]
- 34.Petrera M, De Croos JN, Iu J, Hurtig M, Kandel RA, Theodoropoulos JS. Supplementation with platelet-rich plasma improves the in vitro formation of tissue-engineered cartilage with enhanced mechanical properties. Arthroscopy. 2013;29(10):1685–1692. doi: 10.1016/j.arthro.2013.07.259. [DOI] [PubMed] [Google Scholar]
- 35.Rhee DK, Marcelino J, Baker M, et al. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J Clin Invest. 2005;115(3):622–631. doi: 10.1172/JCI200522263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanchez M, Fiz N, Azofra J, et al. A randomized clinical trial evaluating plasma rich in growth factors (PRGF-Endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy. 2012;28(8):1070–1078. doi: 10.1016/j.arthro.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 37.Schumacher BL, Block JA, Schmid TM, Aydelotte MB, Kuettner KE. A novel proteoglycan synthesized and secreted by chondrocytes of the superficial zone of articular cartilage. Arch Biochem Biophys. 1994;311(1):144–152. doi: 10.1006/abbi.1994.1219. [DOI] [PubMed] [Google Scholar]
- 38.Semple E, Speck ER, Aslam R, Kim M, Kumar V, Semple JW. Evaluation of platelet gel characteristics using thrombin produced by the thrombin processing device: a comparative study. J Oral Maxillofac Surg. 2008;66(4):632–638. doi: 10.1016/j.joms.2007.06.623. [DOI] [PubMed] [Google Scholar]
- 39.Smyth NA, Murawski CD, Fortier LA, Cole BJ, Kennedy JG. Platelet-rich plasma in the pathologic processes of cartilage: review of basic science evidence. Arthroscopy. 2013;29(8):1399–1409. doi: 10.1016/j.arthro.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 40.Spakova T, Rosocha J, Lacko M, Harvanova D, Gharaibeh A. Treatment of knee joint osteoarthritis with autologous platelet-rich plasma in comparison with hyaluronic acid. Am J Phys Med Rehabil. 2012;91(5):411–417. doi: 10.1097/PHM.0b013e3182aab72. [DOI] [PubMed] [Google Scholar]
- 41.Spreafico A, Chellini F, Frediani B, et al. Biochemical investigation of the effects of human platelet releasates on human articular chondrocytes. J Cell Biochem. 2009;108(5):1153–1165. doi: 10.1002/jcb.22344. [DOI] [PubMed] [Google Scholar]
- 42.Su JL, Schumacher BL, Lindley KM, et al. Detection of superficial zone protein in human and animal body fluids by cross-species monoclonal antibodies specific to superficial zone protein. Hybridoma. 2001;20(3):149–157. doi: 10.1089/027245701750293475. [DOI] [PubMed] [Google Scholar]
- 43.van Buul GM, Koevoet WL, Kops N, et al. Platelet-rich plasma releasate inhibits inflammatory processes in osteoarthritic chondrocytes. Am J Sports Med. 2011;39(11):2362–2370. doi: 10.1177/0363546511419278. [DOI] [PubMed] [Google Scholar]