Abstract
Penicillium expansum is among the most ubiquitous fungi disseminated worldwide, that could threaten the fruit sector by secreting patulin, a toxic secondary metabolite. Nevertheless, we lack sufficient data regarding the growth and the toxigenesis conditions of this species. This work enables a clear differentiation between the favorable conditions to the P. expansum growth and those promising for patulin production. A mathematical model allowing the estimation of the P. expansum growth rate according to temperature, a W, and pH, was also developed. An optimal growth rate of 0.92 cm/day was predicted at 24°C with pH level of 5.1 and high a W level of 0.99. The model's predictive capability was tested successfully on artificial contaminated apples. This model could be exploited by apple growers and the industrialists of fruit juices in order to predict the development of P. expansum during storage and apple processing.
Keywords: Growth rate, patulin production, Penicillium expansum, pH, predictive mycology, temperature, water activity
Introduction
Filamentous fungi are broadly dispersed throughout the environment and are responsible for the spoilage and poisoning of several food matrices. The most common and widespread mycotoxigenic fungi are mainly triggered by the genera: Aspergillus, Fusarium, and Penicillium (Sweeney and Dobson 1999; Binder et al. 2007). Within the latter genus, Penicillium expansum is one of the most studied species (Andersen et al. 2004). Penicillium expansum is a wound parasite fungus that invades fruits via injuries, caused by unfavorable weather conditions before harvest (hail, strong wind) or by rough handling, harvesting, and transport (Sanderson and Spotts 1995). This ubiquitous fungus commonly found on pome fruits causes a serious postharvest disease known as blue mold rot and produces significant amounts of patulin, giving rise to substantial fruit losses and serious public health issues (Moake et al. 2005). Patulin is known to have potent cytotoxic, genotoxic as well as immunotoxic effects even at relatively low exposure levels (Puel et al. 2010). Therefore, the European Union has fixed a maximum tolerated level of 50 μg/kg for fruit juices and derived products and 25 μg/kg for solid apple products. The maximum level allowed for apple products intended for infants and young children was set at 10 μg/kg (European C, 2003, 2006).
The understanding of P. expansum physiology under controlled experimental conditions may help forecast its behavior in natural conditions and predict its potential risks on the fruit sector and consumer health. In the last decades, predictive microbiology has emerged to be a useful tool in food industry used to predict the behavior of microorganisms through the development of several mathematical models capable of describing the responses of these pathogenic organisms to particular environmental conditions (Ross and McMeekin 1994; Fakruddin et al. 2011). Although it was more commonly used to control the bacterial growth (Gibson et al. 1988; Baranyi and Roberts 1994; Gaillard et al. 1998; Juneja et al. 2007), the situation has changed and this tool was lately employed in the modeling of fungal growth as well. The fungal proliferation and mycotoxin synthesis in foodstuffs are subject to multiple physicochemical parameters. The water activity (a W), and the temperature adopted during the storage period deemed as the most imperative ones (Holmquist et al. 1983; Dantigny et al. 2005; Bryden 2007). Likewise, other intrinsic factors, particularly the pH of the product, can largely affect the mold development (Rousk et al. 2009). The combination of these physicochemical parameters along with the usage of modeling techniques might be helpful to control the fungal growth and subsequently the biosynthesis of mycotoxins.
A growing number of studies are available in the literature dealing with the predictive modeling approach of fungi (Valık et al. 1999; Panagou et al. 2003; Parra and Magan 2004; Tassou et al. 2007; Garcia et al. 2011). For P. expansum in particular, few studies have been conducted to characterize the growth and the toxigenesis conditions of this species despite its large implication in foodstuff contamination. The growth rate of P. expansum has been studied as function of the storage temperature, the a W and the oxygen levels (Lahlali et al. 2005; Marín et al. 2006; Baert et al. 2007a; Judet‐Correia et al. 2010).Moreover, its patulin production capacity has been independently assessed as a function of temperature, pH, and fruit varieties (Morales et al. 2008; Salomao et al. 2009). All these studies lack sufficient information about the simultaneous effects of such parameters on P. expansum growth and its patulin production capability. In this regard, it is worth mentioning that the most suitable conditions for the fungal growth may not be the optimal conditions for mycotoxin production, thus it is not possible to predict the latter from the kinetic growth data. Moreover, the interactive effects of different sets of abiotic factors cannot be predicted by such types of studies.
With these perspectives, this study was undertaken to firstly determine in vitro the individual effects of three major physicochemical parameters; the temperature, pH, and a W on both the growth and patulin production by the blue‐rot ascomycetous fungus, P. expansum. These data were subsequently invested in the development of a mathematical model which enables accurate prediction of optimal and marginal conditions for P. expansum growth.
Experimental
Fungal isolate
This study was carried out on one strain of P. expansum, initially isolated from grapes in the Languedoc Roussillon region of France. The strain was previously characterized by DNA sequencing of the ITS gene region and deposited in ARS collection (USDA, Peoria, IL) as NRRL 35695. The strain was formerly confirmed as a patulin‐producer (Tannous et al. 2014).
Experimental setup
Inoculum preparation
The investigated strain was subcultured on Potato Dextrose Agar (PDA) medium (Biolife, Milano, Italy) and incubated at 25°C to obtain a heavily sporulating culture. The conidial suspension was prepared by washing the surface of the fresh, mature (7‐day‐old colony) culture with 10 mL of sterile distilled water amended with Tween 80 (0.05%, v:v) and by gently rubbing with a sterile loop. The spores' concentration was reckoned by microscopy using a Neubauer counting chamber, and then adjusted to 105 spores/μL.
Growth media and incubation conditions
All the assays were conducted on the synthetic Czapek Glucose agar medium in order to minimize other sources of variation that could be encountered on natural media and to identify clearly the effects of temperature, pH, and a W. This medium has already been proven to be a favorable substrate for P. expansum growth and patulin production (data not shown).
The overall assayed conditions were five temperatures, three pH levels, and four a W values. Six separate replicate Petri plates were used for each temperature, a W, and pH value, three of which were overlaid with sterilized cellophane disks to ensure a good separation between mycelium and agar. This will allow an accurate estimation of the mycelial mass and the amount of patulin produced on agar medium (Reeslev and Kjoller 1995; Tannous et al. 2014).
In all the experimental conditions, media were centrally inoculated with 106 spores from the spore suspension. For temperature investigations, the synthetic Czapek glucose agar medium was prepared based on the formulation reported by Puel et al. (2005). The inoculated Petri plates were incubated at 30, 25, 16, 8, and 4°C in high precision (±0.1°C) for 2 weeks.
The synthetic Czapek glucose agar medium was also used for assessing the effect of a W on the growth and patulin production by P. expansum. The unmodified medium (a W 0.99) was adjusted to a W levels of 0.95, 0.90, and 0.85 by adding increasing amounts of glycerol. Water activities were subsequently determined with the HygroLab 2 water activity indicator (Rotronic, Hauppauge, NY). Petri plates of the same a W value were separately enclosed in polyethylene bags to prevent water loss. The inoculated Petri plates were incubated at 25°C for 2 weeks.
The pH surveys were also conducted on Czapek glucose agar incubated at a constant temperature of 25°C for only 7 days. The pH of the medium was adjusted to 2.5, 4, and 7 using two buffer solutions (Citric acid (0.5 mol/L): Potassium Hydrogen Phosphate (0.5 mol/L)) in the respective combinations 49 mL: 2 mL, 30.725 mL: 38.55 mL, and 8.825 mL: 82.35 mL, for a total volume of 250 mL of medium. These pH values were chosen as they cover the pH range found in different eating‐apple and cider apple varieties. The final pH of the medium was verified using a pH‐meter with special probe for alimentary articles by Hanna instruments (Tanneries, France).
Growth and lag phase assessment
After inoculation, agar plates, harvested without cellophane disks, were checked on a daily basis to perceive if visible growth had started. As soon as a visible growth has begun, P. expansum growth was monitored by diameter measurements along two perpendicular directions, at regular time intervals. The lag phase (time required for growth) was evaluated and the radial growth rate (cm/day) was obtained from linear regression slopes of the temporal growth curves. Measurements were carried for an overall period of 14 days for the temperature and a W surveys and 7 days only for the pH surveys.
Fungal growth was also evaluated with regard to biomass (mg dry weight). After the appropriate incubation period, the mycelia developed on the surface of agar plates topped with cellophane disks were scratched with a scalpel, collected, and dried at 80°C until a constant weight, corresponding to the dry biomass weight, was obtained.
Patulin extraction and HPLC analysis
After the appropriate incubation period (7 days for the pH assays and 14 days for the temperature and a W assays), the agar medium was scraped off the Petri dishes overlaid with sterile cellophane, cut into strips, mixed with 50 mL of ethyl acetate (Sigma‐Aldrich, Saint‐Quentin Fallavier, France) and macerated with agitation (250 rpm) at room temperature on an orbital shaker (Ningbo Hinotek Technology, Zhejiang, China). The contact time was 2 days. The organic phase was then filtered through Whatman Grade 413 filter paper (Merck, Darmstadt, Germany) and evaporated to dryness under liquid nitrogen. The dried residue was dissolved in 2 mL of methanol and then filtered through a 0.45 μm syringe filter (Navigator, Huayuan Tianjin, China) into a clean 2 mL vial. One hundred microliter aliquots of these extracts were injected onto the Waters Alliance HPLC system (Saint‐Quentin‐en‐Yvelines, France) for the quantitative determination of the patulin concentration. The patulin was detected with a Waters 2998 Photodiode Array Detector, using a 25 cm × 4.6 mm Supelco 5 μm Discovery C18 HPLC Column (Sigma‐Aldrich) at a flow rate of 1 mL/min. A gradient program was used with water (Eluent A) and acetonitrile HPLC grade (eluent B) and the following elution conditions: 0 min 5% B, 16 min 2% B, 20 min 60% B, 32 min 5% B. The presence of patulin was monitored at a 277 nm wavelength. A calibration curve was constructed with patulin standard (Sigma‐Aldrich) at concentrations ranging from 0.05 to 10 μg/mL. Accordingly, the patulin concentrations were determined and results were expressed in ppm. The LOD and LOQ of the method were calculated using the slope (S) of the calibration curve, obtained from linearity assessment, and the standard deviation of the response (SD). These values were determined as follows: LOD = 3.3 × SD/S, LOQ = 10 × SD/S.
Model development
Growth rate experimental data were implemented in a home developed C++ language program that is able to interpolate between various points in different or multiple dimensions. Effects of the different parameters (temperature, pH, and a W) on the P. expansum growth rate were taken into account according to the experimental points already obtained. Thus, the effect of each of these parameters was considered on its own calculating the growth ratio factor effect obtained from the experimental data. The program proceeds as a simultaneous interpolator between the different data points and the effect ratios of each parameter on the growth rate. Therefore, the program allows us to estimate the growth rates (expressed in cm/day) for fixed temperature, a W, and pH values depending on the variations defined by the input data. The model took into account the latency phase versus the temperature, which was modeled by a 4th degree polynomial equation:
| (1) |
where T is the temperature parameter expressed in Degree Celsius (°C).
Likewise, the latency versus the a W was taken into account to fit the following power equation:
| (2) |
where a w represents the water activity of the medium.
In the both latency phase fits, the correlation parameter R 2 was higher than 0.97 showing a good accuracy of the fitting procedure.
The P. expansum diameter growth (cm) versus time should theoretically follow a linear regression while considering the variation in each of the temperature, a W, and pH parameters. Therefore, a Pearson chi‐squared test was performed confirming that our hypothesis is true for over 99.9%. Thus, the slope and the intercept dependencies on each of the previously mentioned parameters were calculated according to the available experimental points.
Growth slope and intercept dependencies on temperature were fit into the following 4th degree polynomial equations:
| (3) |
| (4) |
The growth slope and intercept dependencies on a W were fit into the following equations:
| (5) |
| (6) |
And the growth slope dependency on pH was fit into the following equation, considering the value for intercept as null:
| (7) |
All slope and intercept fits were convergent to more than 99% with the experimental points.
In order to analyze the simultaneous effect of the different parameters, growth rate values were calculated for an a W level of 0.99 and a pH of 4 (reference values) using the temperature's formula given by equations (3) and (4). Using these equations the growth rate (cm/day) can be calculated for different temperature values. Using the same method of proceeding we can use equations (5) and (6) to calculate the effect of the a W on the growth rate and the equation (7) to analyze the effect of the pH. If two or more parameters are to be changed at the same time, the temperature effect is taken into account first, and then the obtained growth rate is further modified by the second parameter effect. The modification is a simple ratio factor that is applied to the growth rate following temperature change. Therefore, the effects of the a W and the pH were implemented as a diameter ratio factor. Each factor was calculated by dividing the diameter obtained for a desired parameter value by that obtained for the experimental values that we considered as a reference.
Finally, a routine test of the different values combinations of pH, T, and a W was carried out in order to retrieve the highest growth rate and its relative optimal parameters to obtain such a result.
Validation of the predictive model in vivo
In order to assess the validity of the predictive model in natural conditions, three apple varieties (Golden Delicious, Granny Smith, and Royal Gala) with different initial pH values (Table 1) were used. As previously described by Sanzani et al. (2012), apples were surface‐sterilized using a 2% sodium hypochlorite solution and rinsed with water. Apples were then injured using a sterile toothpick to a depth of approximately 0.5 cm, and the wounded sites were inoculated with a 10 μL droplet of the P. expansum conidial suspension at a concentration of 105 conidia/μL. The infected apples were then incubated for 2 weeks under three different temperatures (4°C, 25°C, and 30°C). The set of experimental conditions used to check the predictive capability of the model are given in Table 1. Duplicate analyses were performed on each set of conditions.
Table 1.
Validation set of conditions of the predictive model for Penicillium expansum growth and patulin production
| Apple variety | Temperature (°C) | Water activity | pH |
|---|---|---|---|
| Golden Delicious | 4 | 0.98 | 3.5–3.6 |
| 25 | 0.98 | 3.5–3.6 | |
| 30 | 0.98 | 3.5–3.6 | |
| Granny Smith | 4 | 0.98 | 3.1–3.2 |
| 25 | 0.98 | 3.1–3.2 | |
| 30 | 0.98 | 3.1–3.2 | |
| Royal Gala | 4 | 0.99 | 4.1–4.2 |
| 25 | 0.99 | 4.1–4.2 | |
| 30 | 0.99 | 4.1–4.2 |
The diameters of the rotten spots were measured daily and the experimental growth rates were estimated. To evaluate the performances of the developed model, the observed and predicted values were compared by plotting predicted growth rates against the experimental values.
Statistical analysis
All values are stated as mean ± SEM unless otherwise indicated. For statistical analysis, the one‐way analysis of variance (ANOVA) was used (*P < 0.05; **P < 0.01; ***P < 0.001).
Results and Discussion
Studies on the growth and patulin production by P. expansum under different conditions
Colony diameters were measured on a daily basis and plotted against time. For all the tested conditions, the growth curves based on colony diameters were typical of a linear fungal growth after a short lag period ranged from 1 to 7 days (Baert et al. 2007a). However, it is worth mentioning that the fungal growth was in some cases limited by the Petri plates' dimension. In such cases, growth curves lose their linear appearance just after reaching the limiting diameter value (~7 cm) (Figs. 1A, 3A). Under each culture condition, the patulin content was quantified by HPLC and expressed in ppm.
Figure 1.
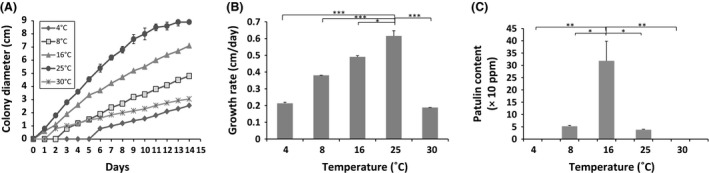
Growth curves (A), radial growth rates (cm/day) (B), and patulin production (ppm) of Penicillium expansum NRRL 35695 on Czapek glucose agar medium under different temperatures. Five different temperatures were tested (4°C, 8°C, 16°C, 25°C, and 30°C) with pH and a W values fixed to 5.2 and 0.99, respectively. The results shown are the mean of three technical replicates for each condition. The standard errors of the mean (SEM) are represented by error bars: *P < 0.05; **P < 0.01; ***P < 0.001.
Figure 3.
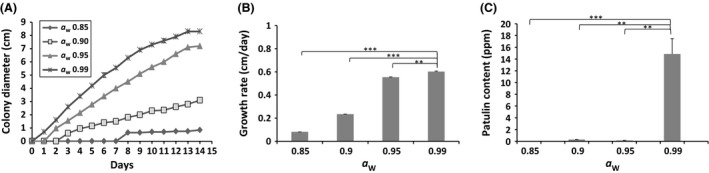
Growth curves (A), radial growth rates (cm/day) (B), and patulin production (ppm) of Penicillium expansum NRRL 35695 on Czapek glucose agar medium under modified water activity. Four different water activities were tested (0.85, 0.90, 0.95, and 0.99) with pH and temperature values fixed to 5.2°C and 25°C, respectively. The results shown are the mean of three technical replicates for each condition. The standard errors of the mean (SEM) are represented by error bars: *P < 0.05; **P < 0.01; ***P < 0.001.
Temperature effect
Since in many cases, apples and other fruits are stored in refrigerators (at 4°C) or in plastic barrels outdoors where temperatures of 25–30°C are very common, the temperature analysis were performed within a 4 to 30°C range. The investigated strain of P. expansum was able to grow in the temperature range studied at unmodified a W and pH (Fig. 1A). Interestingly, the strain displayed a different colonial morphology under the five tested temperatures. At 8°C and 16°C, green colony with white margins and yellow to cream reverse side was observed, whereas at 25°C, the fungus showed green conidia with dull‐brown color on reverse. An unusual morphology of the fungus was perceived at 30°C; the colonies grew vertically and stayed smaller than 3 cm, with serrated edges (Fig. 2). These morphological changes noticed following the incubation under various temperatures have been linked to stress response in other filamentous fungi (Verant et al. 2012).
Figure 2.
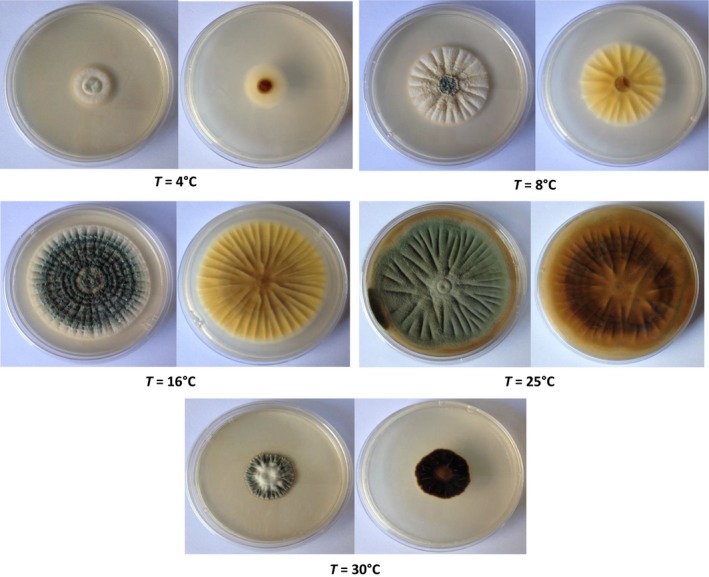
The colony appearance (surface and reverse) of Penicillium expansum isolate NRRL 35695 after 14 days of growth on Czapek glucose agar media under a wide range of temperatures.
The optimal temperature for the growth of this strain of P. expansum was around 25°C, at which the fungus exhibited the shortest lag phase and the most important colony growth (8.9 cm), at the end of the incubation period (Fig. 1A). This observation is in accordance with the literature data that describes also an optimum growth temperature for this species near 25°C (Pitt et al. 1991; Lahlali et al. 2005; Baert et al. 2007a; Pitt and Hocking 2009). Lag phases prior to growth increased when temperature varied from optimum to marginal conditions; a lag phase of 6 and 3 days was noticed at the lowest temperatures of 4°C and 8°C, respectively, besides a 2‐day‐latency period perceived at the highest studied temperature (30°C) (Fig. 1A). This result supports the prediction of Baert et al. (2007a) that cold storage does not prevent the fruit deterioration by P. expansum, but just delays it.
The colony growth rates were calculated as the slope of the linear segment of each growth curve. The growth rate of P. expansum as a function of temperature appears to have a bell‐shaped distribution with an optimum at 25°C and an experimentally determined value of 0.67 cm per day (Fig. 1B). The growth features of P. expansum were also evaluated in terms of fungal biomass development. The highest mycelia dry weight of 160 ± 15 mg per 20 mL of Czapek glucose medium was obtained at 25°C, followed by 16°C (130 ± 0 mg), 30°C (120 ± 10 mg) and 8°C (60 ± 10 mg). The lowest mycelia dry weight of 10 ± 0 mg was perceived at 4°C.
Patulin was identified by its retention time (9 min) and its UV spectra according to an authentical standard and quantified by measuring peak area according to the constructed standard curve of 0.919% coefficient of variation. The values of limit of detection (LOD) and limit of quantification (LOQ) for patulin were 0.04 μg/mL and 0.1 μg/mL, respectively. The patulin production by P. expansum exhibited also a marked temperature‐dependent variability. The histogram of patulin production versus temperature seen in Figure 1C has a characteristic bell shape. The highest patulin concentrations were attained at 16°C. However, a further increase in temperature to 25°C and 30°C caused a decrease in patulin production. These data matched a previous study of Paster et al. (1995) that compared patulin production on apples kept at various storage temperatures of 0, 3, 6, 17, and 25°C. In this study, the highest patulin concentration was found at 17°C. Our results are also in perfect agreement with those of Baert et al. (2007b) that showed a higher patulin production at low temperatures. However, they contradict many other studies that have reported a stimulation of the patulin production by this fungus by increasing the temperature (McCallum et al. 2002; Salomao et al. 2009). These results proved that temperature plays a role in patulin accumulation but not in a determinant way, other extrinsic and intrinsic factors appear to interact.
A comparison of the obtained bell‐shaped dependencies of the P. expansum growth rates and patulin levels as a function of temperature (Fig. 1B and C) revealed that the temperature ranges required to produce patulin were different and more restrictive than those for growth. Similar results have been reported for other fungal species. The Fusarium molds associated with the production of trichothecene (T‐2 and HT‐2 toxins) have been reported to grow prolifically at temperatures ranging between 25 and 30°C with a low production of mycotoxins. However, high levels of mycotoxins were produced at low temperatures (10 to 15°C), associated with a reduced fungal growth (Nazari et al. 2014). Similarly, the optimal temperature for Fumonisin B1 production was lower than the optimal temperature for the growth of Fusarium verticillioides and Fusarium proliferatum (Marin et al. 1999). Another example of a narrower range of temperatures for toxin production when compared with fungal growth is shown by the accumulation of ochratoxin in barley by Penicillium verrucosum. The growth of this species was conceivable at temperatures fluctuating between 0°C and 31°C, whereas the ochratoxin A production was only detected in the temperature range 12–24°C (Northolt et al. 1979).
Effect of water activity
The a W of fresh fruits falls in the range 0.97–0.99. Though that patulin was also detected in dried fruits (Karaca and Nas 2006; Katerere et al. 2008) with a W values less than 0.90, analyses were carried out over an a W range 0.85–0.99. The Figure 3A shows the mean diameters of P. expansum, measured at different controlled a W, along culturing time. This species displayed an optimum growth at the highest a W of 0.99, with the shortest lag phase and the most important colony growth (8.3 cm) after incubation period. For this highest value of a W, the fungus recorded the highest growth rate (0.6 cm per day). A drastic decrease in the P. expansum growth rate was observed by lowering the a W from 0.99 to 0.85, using glycerol as humectant (Fig. 3B). The P. expansum isolate displays a different mycelial mass production in the Czapek glucose medium with modified a W. After 14 days, the highest production of fungal dry mass was obtained at the a W of 0.95 (400 ± 50 mg dry weight per 20 mL of medium), followed by 0.99 (201 ± 10 mg) and 0.90 (105 ± 15 mg). A weak mycelium growth (1.1 ± 0 mg fungal dry weight) was reported at the minimal a W tested. In literature, the minimal a W for the germination of this species ranges between 0.83 (Mislivec and Tuite 1970) and 0.85 (Judet‐Correia et al. 2010), depending on the strain. As it can be observed, the mycelial dry weight estimated at the 0.95 a W is approximately twice the value found at 0.99 a W. However, the fresh mycelial weight was significantly greater at the highest a W value (data not shown).
The patulin production was also significantly affected by the water availability in the medium. No patulin was produced at an a W of 0.85 throughout the incubation period. On the other hand, traces of patulin were detected after 14 days of culture, when the fungus was grown at the two a W values of 0.90 and 0.95. A significant increase in the patulin production by P. expansum was perceived at the a W of 0.99 (Fig. 3C). These findings on the impact of a W on the patulin production by P. expansum are consistent with the two ancient studies reporting that the minimal a W that allows patulin production by this fungus is of 0.95 (Lindroth et al. 1978; Patterson and Damoglou 1986).
As previously outlined for the temperature analysis, the a W conditions that promote patulin production were also more restrictive than those allowing growth. Although there were no significant differences in terms of P. expansum growth at both water activities 0.95 and 0.99, the patulin production was significantly stimulated at 0.99, whereas only traces of patulin were detected at 0.95 (Fig. 3B and C).
Effect of pH
As several previous studies have reported a decrease in the pH of the medium during P. expansum growth, the pH assays were conducted on an overall incubation period of 7 days, in order to reduce pH fluctuations. This pH decrease is due to organic acids (gluconic acid) production, that lower the pH to values in which patulin is more stable (Baert et al. 2007b; Morales et al. 2008; Barad et al. 2013). In our study, the pH of the medium was recorded at the end of the experiment. The initial pH 7 slightly decreased to 6 along 7 days experiment; however, the two pH 2.5 and 4 were maintained constant at the initial value throughout the incubation period. The ability to change the ambient pH in order to generate a more suitable growing environment has been described for other fungal species and was shown to occur in either direction. Some necrotrophic species like Alternaria alternata (Eshel et al. 2002) or Colletotrichum gloeosporioides (Kramer‐Haimovich et al. 2006) can alkalize the host tissue by secreting ammonium, whereas other species can acidify the medium by secreting organic acids, like oxalic acid in the case of Botrytis cinerea (Manteau et al. 2003).
Under the different pH tested in our study, the lag phase periods were estimated to 1 day of culture from the linear regression curves for colony radius plotted versus time (Fig. 4A). It was also found that the growth rate of P. expansum as a function of pH is bell‐shaped with a maximum estimated value of 0.9 cm per day at pH 4 (Fig. 4B). Regarding, the mycelium dry weights, there were no significant differences between the three tested pH levels. Similarly, Morales et al. (2008) found that the P. expansum growth, estimated in terms of fungal biomass, was unaffected by the fruit juice initial pH.
Figure 4.
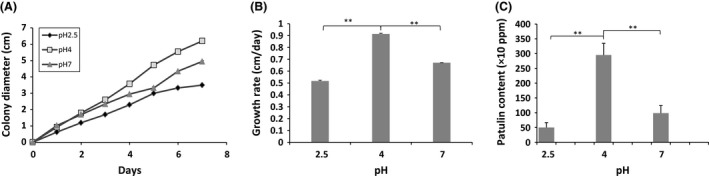
Growth curves (A), radial growth rates (cm/day) (B), and patulin production (ppm) of Penicillium expansum NRRL 35695 on Czapek glucose agar medium under modified pH. Three different pH values were tested (2.5, 4, and 7) with temperature and a W values fixed to 25°C and 0.99, respectively. The results shown are the mean of three technical replicates for each condition. The standard errors of the mean (SEM) are represented by error bars: *P < 0.05; **P < 0.01; ***P < 0.001.
The pH of the medium showed a significant effect on the ability of this fungus to produce patulin. The lowest patulin production was reported at pH 2.5, whereas the highest patulin level was detected at pH 4. The patulin‐producing capacity of P. expansum decreased when the pH of the medium increased from 4 to 7 (Fig. 4C). These results are comparable with those presented in previous studies. Damoglou and Campbell (1986) have previously reported that the pH range 2.8–3.2 resulted in less patulin accumulation by P. expansum compared to the pH range 3.4–3.8. While assessing the patulin accumulation in both apple and pear juices at different pH, Morales et al. (2008) have also observed an increase in patulin production by raising the pH from 2.5 to 3.5. The small amounts of patulin found at pH 2.5 are most probably due to a low production rather than to low stability of patulin. In the study of Drusch et al. (2007), the patulin stability was assessed over a wide pH range. Data from this study indicate that patulin is highly stable in the range pH 2.5–5.5. However, a greater decrease in the patulin concentration to 36% of the initial concentration was observed at neutral pH (Drusch et al. 2007).
Mathematical modeling of P. expansum growth
The growth data modeled in this work comprised the latency phase and the growth curves of P. expansum strain NRRL 3565 at five temperatures, four a W, and three pH. Mycelial extension of colonies against time was almost invariable showing a straight line, after an initial lag period. The growth rates expressed in cm per day were calculated as the slope of the regression curves. The growth rates recorded under the different conditions (data presented above) were used as inputs to calculate the design parameters of equations (3), (4), (5), (6), (7).
The presented calculation approach has undergone a first mathematical validation, confirming that the differences between the theoretical values predicted by the model and the data obtained under the conditions used to build the model are not significant. The Figure 5 shows the effects of temperature, a W and pH on growth rate (expressed in cm/day) obtained using the approach described in this study. The surfaces generated by the model data summarizes all the growth rate values predicted under combined temperature and a W (at a fixed pH 4), combined pH and temperature (at a fixed a W of 0.99), and combined pH and a W (at a fixed temperature of 25°C). The fixed values are those for which the effect of the other combination of factors on P. expansum growth is visualized the best. The model predicts that the optimal conditions for P. expansum growth were a temperature of 24°C, an a W value of 0.99, and a pH value of 5.1. The predicted growth rate at optimal conditions was 0.92 cm/day. The minimal conditions for P. expansum growth as predicted by the model were a temperature of 3°C, an a W value of 0.85, and a pH value of 2. Under these combined set of conditions, a slowdown of growth to almost zero level is predicted by the model.
Figure 5.
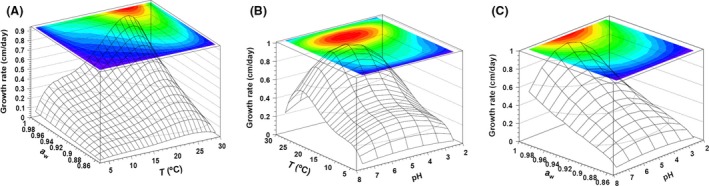
Three‐dimensional response surfaces showing the expected growth rates (cm/day) determined by the developed model as a function between temperature and a W (A), temperature and pH (B), and pH and a W (C). The graph A correspond to a fixed pH value of 4, the graph B to a fixed a W value of 0.99 and the graph C to a fixed temperature of 25°C.
The experimental validation of the P. expansum growth model was carried out on apples. The objective was to test whether the performance of the predictive model may be low in a realistic situation or not. This validation led to acceptable results under most of the conditions. The growth of the fungus on apples was in general slower than predicted by the model (Fig. 6). However, using the Pearson product‐moment correlation coefficient, a value of 0.96 was found between the experimental and predicted growth rate values. The difference between the predicted and observed growth rates on apple is most probably due to the apple itself, which might be a stress factor for the fungus. Such stress factors include the intact tissue structure of the apple, which must be degraded in order to enable mold development to occur and which causes a reduced O2 availability within the fruit. A similar result was observed in a previous study investigating the effect of temperature on P. expansum growth in both Apple Puree Agar Medium (APAM) and apples (Baert et al. 2007a). Our results obtained from in vivo experiments indicate that the employment of the developed modeling approach, to assess the combined effect of temperature, a W and pH on the growth responses of P. expansum could be satisfactory. However, the risk in using the described model in real situations may lie in the difference in the initial inoculum size and the unrealistic constant conditions of temperature and moisture content.
Figure 6.
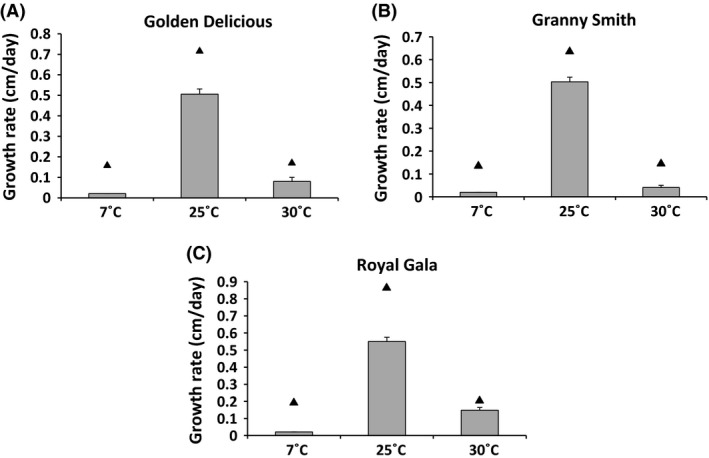
Comparison of the predicted and the observed Penicillium expansum growth responses on apples. The experimental growth rate values are shown in histograms ( ), whereas the predicted values are represented by black triangles (▲). The experimental growth rate values are the average of two replicates for each condition with the standard deviations shown as error bars.
), whereas the predicted values are represented by black triangles (▲). The experimental growth rate values are the average of two replicates for each condition with the standard deviations shown as error bars.
A review of the literature reveals that certain mathematical models were developed to describe and predict the P. expansum growth under different environmental conditions. The combined effects of temperature and a W on the growth rate of P. expansum were previously studied and modeled by Lahlali et al. (2005) on PDA medium. In their study, the data obtained with both sorbitol and glycerol as humectant were modeled by means of the quadratic polynomial model. In agreement with our findings, it was shown that P. expansum grows best at temperatures ranging from 15 to 25°C and at an a w ranging from 0.960 to 0.980. The growth rate and the lag time for six P. expansum strains were modeled as a function of temperature by Baert et al. (2007a) on APAM. In accordance with our results, the optimal temperature for growth varied between 24°C and 27°C depending on the strain. A similar modeling study was later conducted on both malt extract agar (with a pH value of 4.2 and an a W of 0.997) and on simulating yogurt medium (Gougouli and Koutsoumanis 2010), where a Cardinal Model with Inflection (CMI) was used. The optimal temperature for P. expansum growth was determined as 22.08°C, which was close to that predicted by our model. Moreover, the predicted growth rate (0.221 mm/h, the equivalent of 0.55 cm/day), was lower than that expected in our study. Another predictive study was conducted by Judet‐Correia et al. (2010) using the Cardinal Model with Inflection. The objective of the latter was to develop and validate a model for predicting the combined effect of temperature and a W on the radial growth rate of P. expansum on PDA medium. The optimal conditions estimated by this study on Potato Dextrose were 23.9°C for temperature and 0.981 for a W. These estimated values are close to those predicted in the present work. However, the optimal growth rate expected was remarkably lower. This difficulty in comparing the growth rates is obviously due to the fact that the isolates in the study of Judet‐Correia et al. (2010) were grown on PDA, whose composition differs from that of Czapek glucose agar.
The importance of this study resides in the fact that it takes into account the three key growth factors (Temperature, a W and pH) unlike the previously conducted studies that did not consider more than two exogenous factors. It is also worth mentioning that the effect of the latter factor on P. expansum growth has never been modeled before. In addition to its growth modeling approach, this study considers the distinction between the favorable conditions for growth and those for toxigenesis of P. expansum.
It remains to note that this predictive model is built up based on the data on one P. expansum strain (NRRL 35695). As previously reported by McCallum et al. (2002), P. expansum isolates exhibit different growth rates. In this regard, it will be interesting to evaluate the ability of this model to extrapolate to other strains within the same species. Ultimately, the extent to which the model can be applied to other inoculum sizes, other growth media and fluctuating temperatures is to be determined in future validation studies for extrapolation.
Conclusion
The findings in this study provide a considerable insight and a very interesting and informative comparison of the growth rate and patulin production of P. expansum regarding three eco‐physiological factors mainly involved in the proliferation of pathogenic fungi.
In the present work, a predictive model was also developed as a tool to be used for the interpretation of P. expansum growth rate data. Within the experimental limits of temperature, pH and a W, this model was able to predict the colony radial growth rates (cm/day) along a wide combination of culture conditions. Furthermore, the validation showed that the model can predict the growth of P. expansum under natural conditions on apples, with an acceptable accuracy. To conclude, the developed mathematical model for predicting the P. expansum growth on a laboratory scale can be used as a tool to assess the risk of P. expansum in fruit juices industry by predicting conditions over which the P. expansum growth in food matrices might be a problem.
Conflict of Interest
None declared.
Acknowledgments
The authors express their gratitude to C. Afif from the Department of Chemistry at Saint Joseph's University for his kind cooperation in achieving the HPLC analyses. The research work was financially supported by the National Council for Scientific Research (CNRS), Lebanon and the Research Council of Saint‐Joseph University (Lebanon).
References
- Andersen, B. , Smedsgaard J., and Frisvad J. C.. 2004. Penicillium expansum: consistent production of patulin, chaetoglobosins, and other secondary metabolites in culture and their natural occurrence in fruit products. J. Agric. Food Chem. 52:2421–2428. [DOI] [PubMed] [Google Scholar]
- Baert, K. , Valero A., De Meulenaer B., Samapundo S., Ahmed M. M., Bo L., et al. 2007a. Modeling the effect of temperature on the growth rate and lag phase of Penicillium expansum in apples. Int. J. Food Microbiol. 118:139–150. [DOI] [PubMed] [Google Scholar]
- Baert, K. , Devlieghere F., Flyps H., Oosterlinck M., Ahmed M. M., Rajković A., et al. 2007b. Influence of storage conditions of apples on growth and patulin production by Penicillium expansum . Int. J. Food Microbiol. 119:170–181. [DOI] [PubMed] [Google Scholar]
- Barad, S. , Horowitz S. B., Kobiler I., Sherman A., and Prusky D.. 2013. Accumulation of the mycotoxin patulin in the presence of gluconic acid contributes to pathogenicity of Penicillium expansum . Mol. Plant Microbe Interact. 27:66–77. [DOI] [PubMed] [Google Scholar]
- Baranyi, J. , and Roberts T. A.. 1994. A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 23:277–294. [DOI] [PubMed] [Google Scholar]
- Binder, E. M. , Tan L. M., Chin L. J., Handl J., and Richard J.. 2007. Worldwide occurrence of mycotoxins in commodities, feeds and feed ingredients. Anim. Feed Sci. Technol. 137:265–282. [Google Scholar]
- Bryden, W. L. 2007. Mycotoxins in the food chain: human health implications. Asia Pac. J. Clin. Nutr. 16:95–101. [PubMed] [Google Scholar]
- Damoglou, A. P. , and Campbell D. S.. 1986. The effect of pH on the production of patulin in apple juice. Lett. Appl. Microbiol. 2:9–11. [Google Scholar]
- Dantigny, P. , Guilmart A., and Bensoussan M.. 2005. Basis of predictive mycology. Int. J. Food Microbiol. 100:187–196. [DOI] [PubMed] [Google Scholar]
- Drusch, S. , Kopka S., and Kaeding J.. 2007. Stability of patulin in a juice‐like aqueous model system in the presence of ascorbic acid. Food Chem. 100:192–197. [Google Scholar]
- Eshel, D. , Miyara I., Ailing T., Dinoor A., and Prusky D.. 2002. pH regulates endoglucanase expression and virulence of Alternaria alternata in persimmon fruit. Mol. Plant Microbe Interact. 15:774–779. [DOI] [PubMed] [Google Scholar]
- European C . 2003. Commission regulation (EC) No 1425/2003 of 11 August 2003 amending regulation (EC) No 466/2001 as regards patulin. Off. J. Eur. Union L 203:1–3. [Google Scholar]
- European C . 2006. Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union L 364:5–24. [Google Scholar]
- Fakruddin, M. , Mazumdar R. M., and Mannan K. S. B.. 2011. Predictive microbiology: modeling microbial responses in food. Ceylon J. Sci. Biol. Sci. 40:121–131. [Google Scholar]
- Gaillard, S. , Leguérinel I., and Mafart P.. 1998. Model for combined effects of temperature, pH and water activity on thermal inactivation of Bacillus cereus spores. J. Food Sci. 63:887–889. [Google Scholar]
- Garcia, D. , Ramos A. J., Sanchis V., and Marín S.. 2011. Modelling the effect of temperature and water activity in the growth boundaries of Aspergillus ochraceus and Aspergillus parasiticus . Food Microbiol. 28:406–417. [DOI] [PubMed] [Google Scholar]
- Gibson, A. M. , Bratchell N., and Roberts T. A.. 1988. Predicting microbial growth: growth responses of Salmonellae in a laboratory medium as affected by pH, sodium chloride and storage temperature. Int. J. Food Microbiol. 6:155–178. [DOI] [PubMed] [Google Scholar]
- Gougouli, M. , and Koutsoumanis K. P.. 2010. Modelling growth of Penicillium expansum and Aspergillus niger at constant and fluctuating temperature conditions. Int. J. Food Microbiol. 140:254–262. [DOI] [PubMed] [Google Scholar]
- Holmquist, G. U. , Walker H. W., and Stahr H. M.. 1983. Influence of temperature, pH, water activity and antifungal agents on growth of Aspergillus flavus and A. parasiticus . J. Food Sci. 48:778–782. [Google Scholar]
- Judet‐Correia, D. , Bollaert S., Duquenne A., Charpentier C., Bensoussan M., and Dantigny P.. 2010. Validation of a predictive model for the growth of Botrytis cinerea and Penicillium expansum on grape berries. Int. J. Food Microbiol. 142:106–113. [DOI] [PubMed] [Google Scholar]
- Juneja, V. K. , Valenzuela Melendres M., Huang L., V. Gumudavelli , Subbiah J., and Thippareddi H.. 2007. Modeling the effect of temperature on growth of Salmonella in chicken. Food Microbiol. 24:328–335. [DOI] [PubMed] [Google Scholar]
- Karaca, H. , and Nas S.. 2006. Aflatoxins, patulin and ergosterol contents of dried figs in Turkey. Food Addit. Contam. 23:502–508. [DOI] [PubMed] [Google Scholar]
- Katerere, D. R. , Stockenström S., and Shephard G. S.. 2008. HPLC‐DAD method for the determination of patulin in dried apple rings. Food Control 19:389–392. [Google Scholar]
- Kramer‐Haimovich, H. , Servi E., Katan T., Rollins J., Y. Okon , and Prusky D.. 2006. Effect of ammonia production by Colletotrichum gloeosporioides on pelB activation, pectate lyase secretion, and fruit pathogenicity. Appl. Environ. Microbiol. 72:1034–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahlali, R. , Serrhini M. N., and Jijakli M. H.. 2005. Studying and modelling the combined effect of temperature and water activity on the growth rate of P. expansum . Int. J. Food Microbiol. 103:315–322. [DOI] [PubMed] [Google Scholar]
- Lindroth, S. , Niskanen A., and Pensala O.. 1978. Patulin production during storage of blackcurrant, blueberry and strawberry jams inoculated with Penicillium expansum mould. J. Food Sci. 43:1427–1429. [Google Scholar]
- Manteau, S. , Abouna S., Lambert B., and Legendre L.. 2003. Differential regulation by ambient pH of putative virulence factor secretion by the phytopathogenic fungus Botrytis cinerea . FEMS Microbiol. Ecol. 43:359–366. [DOI] [PubMed] [Google Scholar]
- Marin, S. , Magan N., Serra J., Ramos A. J., Canela R., and Sanchis V.. 1999. Fumonisin B1 production and growth of Fusarium moniliforme and Fusarium proliferatum on maize, wheat, and barley grain. J. Food Sci. 64:921–924. [Google Scholar]
- Marín, S. , Morales H., Ramos A. J., and Sanchis V.. 2006. Evaluation of growth quantification methods for modelling the growth of Penicillium expansum in an apple‐based medium. J. Sci. Food Agric. 86:1468–1474. [Google Scholar]
- McCallum, J. L. , Tsao R., and Zhou T.. 2002. Factors affecting patulin production by Penicillium expansum . J. Food Prot. 65:1937–1942. [DOI] [PubMed] [Google Scholar]
- Mislivec, P. B. , and Tuite J.. 1970. Temperature and relative humidity requirements of species of Penicillium isolated from yellow dent corn kernels. Mycologia 62:75–88. [PubMed] [Google Scholar]
- Moake, M. M. , Padilla‐Zakour O. I., and Worobo R. W.. 2005. Comprehensive review of patulin control methods in foods. Compr. Rev. Food Sci. Food Saf. 4:8–21. [DOI] [PubMed] [Google Scholar]
- Morales, H. , Barros G., Marín S., Chulze S., Ramos A. J., and Sanchis V.. 2008. Effects of apple and pear varieties and pH on patulin accumulation by Penicillium expansum . J. Sci. Food Agric. 88:2738–2743. [Google Scholar]
- Nazari, L. , Pattori E., Terzi V., Morcia C., and Rossi V.. 2014. Influence of temperature on infection, growth, and mycotoxin production by Fusarium langsethiae and F. sporotrichioides in durum wheat. Food Microbiol. 39:19–26. [DOI] [PubMed] [Google Scholar]
- Northolt, M. D. , Van Egmond H. P., and Paulsch W. E.. 1979. Ochratoxin A production by some fungal species in relation to water activity and temperature. J. Food Prot. 42:485–490. [DOI] [PubMed] [Google Scholar]
- Panagou, E. Z. , Skandamis P. N., and Nychas G.‐J.. 2003. Modelling the combined effect of temperature, pH and a W on the growth rate of Monascus ruber, a heat‐resistant fungus isolated from green table olives. J. Appl. Microbiol. 94:146–156. [DOI] [PubMed] [Google Scholar]
- Parra, R. , and Magan N.. 2004. Modelling the effect of temperature and water activity on growth of Aspergillus niger strains and applications for food spoilage moulds. J. Appl. Microbiol. 97:429–438. [DOI] [PubMed] [Google Scholar]
- Paster, N. , Huppert D., and Barkai‐Golan R.. 1995. Production of patulin by different strains of Penicillium expansum in pear and apple cultivars stored at different temperatures and modified atmospheres. Food Addit. Contam. 12:51–58. [DOI] [PubMed] [Google Scholar]
- Patterson, M. , and Damoglou A. P.. 1986. The effect of water activity and pH on the production of mycotoxins by fungi growing on a bread analogue. Lett. Appl. Microbiol. 3:123–125. [Google Scholar]
- Pitt, J. I. , and Hocking A. A. D.. 2009. Fungi and food spoilage, 3rd edition Springer, United States. [Google Scholar]
- Pitt, J. I. , Spotts R. A., Holmes R. J., and Cruickshank R. H.. 1991. Penicillium solitum revived, and its role as a pathogen of pomaceous fruit. Phytopathology 81:1108–1112. [Google Scholar]
- Puel, O. , Tadrist S., Galtier P., Oswald I. P., and M. Delaforge . 2005. Byssochlamys nivea as a source of mycophenolic acid. Appl. Environ. Microbiol. 71:550–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel, O. , Galtier P., and Oswald I. P.. 2010. Biosynthesis and toxicological effects of patulin. Toxins 2:613–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeslev, M. , and Kjoller A.. 1995. Comparison of biomass dry weights and radial growth rates of fungal colonies on media solidified with different gelling compounds. Appl. Environ. Microbiol. 61:4236–4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross, T. , and McMeekin T. A.. 1994. Predictive microbiology. Int. J. Food Microbiol. 23:241–264. [DOI] [PubMed] [Google Scholar]
- Rousk, J. , Brookes P. C., and Bååth E.. 2009. Contrasting soil pH effects on fungal and bacterial growth suggest functional redundancy in carbon mineralization. Appl. Environ. Microbiol. 75:1589–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomao, B. , Aragão G. M. F., Churey J. J., Padilla‐Zakour O. I., and Worobo R. W.. 2009. Influence of storage temperature and apple variety on patulin production by Penicillium expansum . J. Food Prot. 72:1030–1036. [DOI] [PubMed] [Google Scholar]
- Sanderson, P. G. , and Spotts R. A.. 1995. Postharvest decay of winter pear and apple fruit caused by species of Penicillium . Phytopathology 85:103–110. [Google Scholar]
- Sanzani, S. M. , Reverberi M., Punelli M., Ippolito A., and Fanelli C.. 2012. Study on the role of patulin on pathogenicity and virulence of Penicillium expansum . Int. J. Food Microbiol. 153:323–331. [DOI] [PubMed] [Google Scholar]
- Sweeney, M. J. , and Dobson A. D. W.. 1999. Molecular biology of mycotoxin biosynthesis. FEMS Microbiol. Lett. 175:149–163. [DOI] [PubMed] [Google Scholar]
- Tannous, J. , El Khoury R., Snini S. P., Lippi Y., El Khoury A., Atoui A., et al. 2014. Sequencing, physical organization and kinetic expression of the patulin biosynthetic gene cluster from Penicillium expansum . Int. J. Food Microbiol. 189:51–60. [DOI] [PubMed] [Google Scholar]
- Tassou, C. C. , Panagou E. Z., Natskoulis P., and Magan N.. 2007. Modelling the effect of temperature and water activity on the growth of two ochratoxigenic strains of Aspergillus carbonarius from Greek wine grapes. J. Appl. Microbiol. 103:2267–2276. [DOI] [PubMed] [Google Scholar]
- Valık, L. , Baranyi J., and Görner F.. 1999. Predicting fungal growth: the effect of water activity on Penicillium roqueforti . Int. J. Food Microbiol. 47:141–146. [DOI] [PubMed] [Google Scholar]
- Verant, M. L. , Boyles J. G., Waldrep W. Jr, Wibbelt G., and Blehert D. S.. 2012. Temperature‐dependent growth of Geomyces destructans, the fungus that causes bat white‐nose syndrome. PLoS One 7:e46280. [DOI] [PMC free article] [PubMed] [Google Scholar]


